Abstract
While there is indisputable evidence supporting the beneficial role of aerobic exercise in reducing cardiovascular risk factors, there are few dose-response studies of this relationship. Increasingly, it is thought that the cardiovascular benefits of exercise are significantly influenced by adaptations within skeletal muscle and its vasculature. However, little is known about the molecular mechanisms underlying these adaptations. To address this need, we initiated a study utilizing longitudinal, microarray-based gene expression profiling of serial skeletal muscle biopsies obtained from the study of targeted risk reduction intervention through defined exercise (STRRIDE). STRRIDE participants were overweight and exhibited symptoms characteristic of the metabolic syndrome that typically precedes type II diabetes such as insulin resistance, abnormal lipids and glucose intolerance. Expression data were statistically filtered and sorted into exercise training-responsive clusters based on gene product knowledge. One such cluster included genes that promote the degradation of fibrin clots such as tissue plasminogen activator (t-PA), connective tissue activation peptide III (CTAP III) and tetranectin. The fibrinolytic activity and protein levels of tetranectin, and t-PA and its endogenous inhibitor PAI-1, were subsequently shown to change significantly in both skeletal muscle and serum in response to exercise training. Our data show that the rigors of exercise directly induce fibrinolytic genes and protein cascades, both within muscle, and in the systemic circulation. This finding is particularly significant given that the metabolic syndrome is an independent risk factor for peripheral vascular disease and thrombotic events within the heart and brain. We conclude that aerobic exercise training induces both local and systemic changes in fibrinolysis and vascular homeostasis that are probably protective against cardiovascular disease.
Cardiovascular disease is a major public health problem worldwide despite the favourable health trends in mortality and morbidity seen over the past several decades (Pandolfi et al. 2000; Kohler, 2002). The ‘metabolic syndrome’ is a strong predictor of symptomatic cardiovascular disease and is characterized by an idiosyncratic combination of elevated triglycerides, insulin resistance and impaired fibrinolysis (Sakkinen et al. 2000; Kraus et al. 2001, 2002; Kohler, 2002). Metabolic syndrome affects over 20 % of the population of Western industrialized countries and typically precedes the onset of type II diabetes mellitus (Siconolfi & Seeds, 2001; Womack et al. 2001). The recent rapid increase in newly diagnosed type II diabetes has occurred too quickly to be the result of increased gene frequency within the population, emphasizing the predominant role of environmental factors such as obesity and physical inactivity (Flegal, 1999). Particularly striking is the rapid emergence of type II diabetes in children and adolescents, representing from 8 to 45 % of newly diagnosed cases (Flegal, 1999; American Diabetes Association, 2000). This trend is expected to continue as obesity and physical inactivity are at an all time high in the USA and abroad. Many of the cardiovascular risk factors associated with the metabolic syndrome can be reduced or eliminated through weight control, diet and, most importantly, regular exercise (Flegal, 1999; Whaley et al. 1999; Sakkinen et al. 2000; Kraus et al. 2001). However, there is little known concerning the cause-effect relationship between the observed increased rate of thrombotic events and the associated risk factors of weight, diet and exercise. The study of targeted risk reduction intervention through defined exercise (STRRIDE) is a controlled clinical trial of various doses and intensities of exercise in a structured exercise programme (Kraus et al. 2001). The goal of this study is to more clearly define the dose-response relationships between the amount and intensities of exercise and improvements in risk parameters of cardiovascular health, particularly those associated with the metabolic syndrome. The driving hypothesis is that the benefits of chronic exercise are mediated primarily through adaptations within skeletal muscle (Kraus et al. 2001). Muscle typically comprises one-third of body mass and is among the most highly vascularized and metabolically active tissues of the body. However, the effects of muscle metabolism on systemic circulation and fibrinolytic state are wholly unknown.
Adaptation and remodelling of muscle after exercise involves a large series of gene expression and protein modification changes. A number of candidate gene and protein studies have been conducted with exercise in humans and rodents; however little is known concerning the direct contribution of muscle adaptation to the systemic changes observed in exercised metabolic syndrome subjects. An effective technology for highly parallel assessment of gene usage throughout the genome is expression profiling using microarrays. The bioinformatics-driven reproducibility and redundancy of the Affymetrix GeneChip platform has been particularly effective in producing high quality gene expression data from skeletal muscle and subsequently in generating new hypotheses about the regenerative capacity and pathophysiology of this dynamic tissue (Chen et al. 2000, 2002; Tezak et al. 2002; Zhao et al. 2002).
Here we report a novel expression profile analysis of longitudinal STRRIDE skeletal muscle biopsies (four biopsies per subject) and identify clusters of genes that reflect exercise-responsive adaptive changes in muscle. We go on to show a link between muscle adaptation, systemic modulation of blood haemostasis and vascular remodelling through a series of blood serum and muscle assays of fibrinolytic activity. Our finding of the muscle's effect on systemic fibrinolysis is particularly significant given the observed association of physical fitness with reduction of the risk factors associated with cardiovascular events and other diseases of increased thrombosis (el-Sayed, 1996; Rauramaa & Vaisanen, 1999; Whaley et al. 1999; Imhof & Koenig, 2001; Kohler, 2002).
METHODS
STRRIDE study design and considerations
All subjects provided written, informed consent. This study was performed in accordance with the Declaration of Helsinki and approved by an ethics committee (Kraus et al. 2001). The skeletal muscle biopsies used in this study were obtained from three men participating in a high dose, high intensity aerobic exercise group (2000 kcal week−1 at 65–80 % peak oxygen consumption, V̇O2). Subjects were 64, 58 and 44 years of age, inactive (exercised less than once weekly; peak V̇O2 25.8, 31.5 and 34.6 ml kg−1 min−1, respectively), overweight (body mass index (BMI) 36.1, 29.4 and 27.9, respectively) and exhibited signs of fasting hyperinsulinaemia (18.8, 12.2 and 12.3 i.u. ml−1, respectively) with mild to moderate lipid abnormalities (low density lipoprotein (LDL) 163, 126 and 132 mg dl−1 and high density lipoprotein (HDL) 27, 40 and 24 mg dl−1, respectively). Skeletal muscle biopsies were obtained using the percutaneous needle biopsy technique using 1 % lignocaine (lidocaine) without adrenaline (epinephrine) (Kraus et al. 2001). Four serial vastus lateralis muscle biopsies (100–200 mg) were obtained with a triple pass of the needle: one upon entry into the study, one after 9 months of exercise training (24 h after the last bout of exercise), and two at detraining time points (96 h and 2 weeks after the last exercise bout). Tissue was flash frozen and approximately 20 mg used for expression profiling. Blood samples were taken at the time of each biopsy, and serum stored for analysis. All serum and muscle samples were stored at −80 °C.
RNA isolation, two-round amplification and expression profiling
For the isolation of total RNA, 15–25 mg of muscle biopsy material was homogenized using the TRIZOL reagent (Gibco-BRL) according to the manufacturer's protocol. Total RNA was extracted from the muscle biopsies of three male participants at entry, 9 months exercise-trained (24 h after last bout) and 2 week detraining time points (Kraus et al. 2001). A two-round cRNA amplification protocol was used to produce sufficient biotinylated cRNA target for Affymetrix analysis of the small (10–20 mg) muscle biopsy material. Briefly, 0.5–1 μg of total RNA was converted to double-stranded cDNA using a T7-promoter primer. This cDNA was then used as a template to drive the production of cRNA using the Megascript T7 in vitro transcription kit (Ambion, Austin, TX, USA). A small amount of the first round amplified cRNA (< 300 ng) was used to synthesize double-stranded cDNA first using a random primer to drive the first strand synthesis, and then the T7 promoter/primer to drive the second strand synthesis. This amplified cDNA was used to drive the synthesis of biotinylated cRNA from the T7 promoter using the standard Affymetrix protocol described previously (Chen et al. 2002). Biotinylated cRNAs for each exercise time point, for each individual were hybridized to three separate Human U95Av2 GeneChips for each time point so that each individual STRRIDE participant had their own three chip analysis. Procedures for cRNA preparation and GeneChip processing were performed as previously described (Bakay et al. 2002; Chen et al. 2002).
Gene expression data analysis
Absolute analysis of Affymetrix ‘raw’ data was conducted using Affymetrix suite 4.0 as previously described (Bakay et al. 2002; Chen et al. 2002). Of the nine chips analysed, the average scaling factor ranged from 0.89 to 1.8, the number of genes called ‘present’ ranged from 35 to 39 % and the average 3′/5′ ratios for GAPDH were consistently below 3.0. Briefly, each gene was queried with 16 ‘perfect match’ 25 bp oligonucleotides (PM) and paired ‘mismatched’ oligonucleotides (MM) designed with a single mismatch in the centre position. Comparison of the hybridization signal from the PM and MM probes allows for a specificity measure of signal intensity and elimination of most non-specific cross-hybridization signal. Values of intensity differences as well as ratios of each probe pair were used to determine whether a gene was called ‘present’ or ‘absent’ within the analysis. The lists of genes generated by Affymetrix software were subsequently analysed by GeneSpring software (Silicon Genetics, Redwood City CA, USA).
Genes called ‘present’ in at least three chips (of nine total) were selected and the remaining genes filtered out from subsequent analysis. This step was intended to increase the stringency and quality control of the analysis. The Welch t test/Welch ANOVA parametric test was performed to calculate the probabilities of significant exercise-responsive gene expression changes shared by the three subjects. We used a statistical analysis including both a GeneSpring P value cut-off (P < 0.05) and an at least 2-fold change to further increase the stringency of the analysis.
Quantitative multiplex fluorescent reverse transcriptase PCR (QMF RT-PCR)
RT-PCR was used to confirm the expression levels of connective tissue activation peptide III (CTAP III; Accession Nos. M54995, M38441), and tissue plasminogen activator (t-PA; Accession No. M15518) relative to that of the control leucine-rich, glioma-inactivated 1 gene (LGT1; Accession No. NM-005097). This control gene exhibited similar expression or ‘average difference’ levels to those of the test genes and its expression levels were relatively invariant and called ‘present’ across all exercise time points, in all three individuals. Primers were designed to produce small (100–200 bp) multiplex PCR products that could be easily resolved from one another (10–20 bp) on a polyacrylamide-Tris-borate-EDTA (TBE)-resolving gel. For all PCR products analysed, the reverse primer was designed with a covalently coupled near infrared (NIR) dye that allows detection by fluorescence scanning and avoids the background fluorescence. Primers for tissue plasminogen activator were: forward 5-GAC ACT CAG AAC GCC TAC TT-3 and reverse 5-TGC TTC ACT GCG TAC ACA TC-3. Primers for CTAP III were: forward, 5-CAG AAC AGT CAC CGA CAA CA-3 and reverse, 5-ATG CGG CCA TCG TTC AGC CA-3. Primers for the control leucine-rich, glioma inactivated 1 gene were: forward, 5-GAA CTC CGC TGC ATG TGT AT-3 and reverse, 5-GTG GCT ATC ACT TCG ACT TG-3.
Primers were added to equal amounts of unamplified primary cDNA and PCR conducted for 14–17 cycles using conditions optimized for each multiplex reaction (control plus experimental amplicons). PCR products were subsequently heat-denatured and size-fractionated by electrophoresis through a 7 % TBE-acrylamide gel. Gels were run and scanned using a LiCor Global IR 2 DNA Analyzer (LiCor, Lincoln, NE, USA). Expression levels were quantified using SCANALYTICS Gene Profiler (Fairfax, VA, USA).
Western blot analysis
Skeletal muscle protein extracts were obtained from 10 serial 14 μm cryostat sections of frozen biopsy and homogenized in SDS-PAGE loading buffer (10 % SDS, 0.1 m Tris-HCl (pH 8.0), 50 mm DTT, 5 mm EDTA). Each heat-denatured protein sample (20 μg) was loaded onto a 4–20 % SDS-PAGE gradient gel. After electrophoretic size fractionation, proteins were transferred to a nitrocellulose membrane (Amersham). Membranes were blocked for 1 h at room temperature in 5 % non-fat powdered milk in TBST (tris-buffered saline + 0.1 % Tween 20). Membranes were then incubated for 2 h at room temperature with primary antibodies (t-PA and PAI-1 antibodies (Santa Cruz Biotech); β-thromboglobulin/CTAP III antibody (Affiland, Liége, Belgium); tetranectin antibody (AntibodyShop, Copenhagen, Denmark)), washed and incubated for 1 h at room temperature with peroxidase-conjugated secondary antibodies. Signal was detected using an enhanced chemiluminescence (ECL) detection kit (Amersham). Each nitrocellulose membrane and gel was subsequently stained with Coomassie brilliant blue to confirm equal sample loading and transfer based upon the density of the predominant myosin heavy chain band. Expression levels were quantified from scanned X-ray film images using UNSCANIT automated digitizing system (Silk Scientific, Orem UT, USA).
Fibrin plate assay
Plasma was obtained from the same three male participants at the same exercise time points as described previously (Kraus et al. 2001). Plasma fibrinolytic activity was measured using the fibrin plate method (Pandolfi et al. 2000). Briefly, euglobulins were prepared by acidification of 1:10 diluted plasma to pH 5.9 with 0.25 % (v/v) glacial acetic acid at 4 °C. The euglobulin precipitate was then resuspended in an EDTA-gelatin-barbital buffer (pH 7.8) and 30 μl of each sample placed in (duplicate) identical depressions in a fibrin-agarose plate. The area of the lysis zone was measured after 18 h of incubation at 37 °C.
Plasminogen activator fibrin gel zymography
A 12 % acrylamide-fibrin gel was prepared by co-polymerizing the gel with fibrin and plasminogen (1 NIH unit ml−1, Sigma) as described previously (Choi & Kim, 2001; Siconolfi & Seeds, 2001). Briefly, equal numbers of 14 μm cryostat-sectioned tissue were homogenized in 10 mm Tris-Cl (pH 6.8), quantified (BioRad, Hercules, CA, USA) and equal amounts of protein loaded onto the gels and electrophoresed. After electrophoresis, gels were incubated for 1 h in 2.5 % Triton X-100 to remove SDS. Gels were subsequently incubated for 16 h in 0.1 m Tris (pH 8.1) at 37 °C, followed by staining with Coomassie Blue and destaining until zones of lysis appeared against the dark protein background.
ELISA and EUI functional PAI-1 ASSAY
Relative tetranectin antigen levels were assayed in plasma from three STRRIDE participants by enzyme-linked immunosorbent assay (ELISA) using serial dilutions of serum as described previously (Kamper et al. 1998). PAI-1 activity was assayed using a Chromogenix Coatest kit (Milano, Italy) according to the manufacturer's protocol.
Immunofluorescent staining
Serial 8-μm-thick frozen muscle sections were cut with a HM 505E Microm cryostat, mounted to Superfrost Plus Slides (Fisher Scientific, PA, USA), and fixed in cold (−20 °C), anhydrous acetone. Sections were subsequently blocked for 30 min in 10 % horse serum and 1 × PBS and incubated with a 1:500 dilution of a tetranectin monoclonal antibody (Antibodyshop) for 1 h at room temperature. After three washes with 10 % horse serum and 1 × PBS, sections then incubated with a 1:1000 dilution of a Cy3-conjugated secondary rabbit anti-mouse IgG (Jackson ImmunoResearch Laboratories, PA, USA) for 1 h and washed an additional three times. Sections were then mounted with Gel/Mount (Biomeda, Foster City, CA, USA), and analysed with a Nikon FXA microscope (Melville, NY, USA).
RESULTS
Expression profiling
Affymetrix GeneChip analysis was used to expression profile longitudinal muscle biopsies obtained from three overweight male subjects with characteristics of metabolic syndrome, who participated in a high dose, high intensity (2000 kcal week−1; 65–80 % peak V̇O2) 9 month aerobic training-detraining programme (Kraus et al. 2001). Analysis of the RNA expression patterns in the three muscle biopsies from each individual over the exercise training-detraining time course identified genes whose expression levels were exercise responsive and shared by all three individuals.
Approximately 12 000 full-length genes were analysed using 32 oligonucleotide probes (16 probe pairs) for each gene. Data analysis included an assessment of ‘present’ and ‘absent’ calls, and a requirement of > 33 % of profiles to show a ‘present’ call for each gene. In addition, potential exercise-responsive genes were filtered for statistical significance (P < 0.05; Welch t test/ANOVA for shared direction and magnitude of change between two or more time points) and a change in expression greater than or equal to 2.0-fold at the training and/or detraining time point relative to the control at study entry. We found that 37 % of all genes tested were present in at least three of nine microarrays. The rationale for this statistical approach to data management is to select the most robust candidate genes for subsequent confirmation. Using these parameters 505 genes were selected, 116 of which increased 2-fold or more and 42 of which decreased 2-fold or more in response to exercise training and detraining. The remaining 347 genes were filtered out using an arbitrary 2-fold cut-off. These candidate genes were then grouped for subsequent analysis into arbitrary clusters based on gene product knowledge and/or temporal expression patterns. A full list of these exercise responsive genes is available for download from: http://microarray.cnmcresearch.org/pgadatatable.asp
Fibrinolysis and haemostasis genes
A group of functionally related genes involved with vascular and blood haemostasis was selected from Affymetrix expression analysis for subsequent confirmation (Table 1). All of these genes were confirmed by RT-PCR, Western blot and/or functional analysis. In particular, we focused on two exercise-responsive genes, t-PA and tetranectin, whose expression levels increased sharply and similarly after exercise training and then decreased after two weeks of detraining (Table 1). t-PA and tetranectin are known to activate and mediate, respectively, fibrinolysis (Kamper et al. 1998; Iba et al. 2001). By way of contrast the fibrinolysis-mediating gene PAI-1 (Pandolfi et al. 2000), whose Affymetrix mRNA expression levels did not change significantly with exercise training, was also included in this analysis (Table 1).
Table 1.
Affymetrix and GeneSpring expression analysis of haemostasis genes
| 9 month | t test | 2 week | t test | |||
|---|---|---|---|---|---|---|
| Probe set | Accession | trained* | P value | detrained* | P value | Gene name |
| 39209-r-at | M54995 | 9.79 | 0.01 | 0.50 | 0.049 | Pro-platelet basic protein, CTAP III |
| 33452-at | M15518 | 3.60 | 0.008 | 1.57 | 0.59 | Tissue plasminogen activator (t-PA) |
| 36569-at | X64559 | 3.41 | 0.05 | 0.30 | 0.012 | Tetranectin (TN) |
| 38125-at | M14083 | 0.66 | 0.048 | 1.14 | 0.64 | Plasminogen activator inhibitor 1 (PA-I1) |
The differential expression of these genes has been confirmed at the mRNA, protein and or functional level.
Values represent average change relative to the control at study entry (n = 3).
Tissue plasminogen activator expression in skeletal muscle
Since t-PA is potent activator of plasmin-driven fibrinolysis, we further characterized its expression at the mRNA and protein level. The increased levels of t-PA mRNA and its protein product in exercised skeletal muscle were confirmed by QMF-RT-PCR (Fig. 1A), and also by Western immunoblot analysis (Fig. 1B). RT-PCR data demonstrated a striking increase in t-PA mRNA after exercise training (10.6-fold ± 2.4; P < 0.05) and a return to control levels after detraining relative to control. Western immunoblot analysis (Fig. 1C) showed a statistically significant increase in t-PA protein in skeletal muscle after exercise training (5.6-fold ± 1.1) and 96 h detraining (5.7-fold ± 0.7) relative to control levels. After 2 weeks of detraining the expression levels of the t-PA protein were highly variable among the three individuals, reflecting perhaps, the loss of coordinated gene expression provided by repeated exercise bouts and differences in the production and secretion of t-PA protein in different individuals.
Figure 1. Tissue plasminogen activator (t-PA) mRNA and protein is produced by exercised skeletal muscle from overweight men with characteristics of metabolic syndrome.
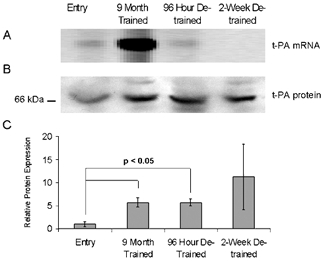
Representative QMF RT-PCR quantitation of mRNA (A) and Western blot of protein (B) of t-PA expression in a single metabolic syndrome patient sequentially biopsied over the times indicated. C, the average, normalized (to myosin), relative expression levels of t-PA protein in skeletal muscle after exercise training and detraining. Data are longitudinal samples from 3 subjects ±s.e.m.; P value was determined using Student's t test.
Plasminogen activator inhibitor 1 expression in skeletal muscle
Plasminogen activator inhibitor 1 (PAI-1) is the primary regulator of t-PA activity in the serum where it forms a tight 1:1 complex, effectively blocking t-PA activity and hastening its clearance from the blood (Pandolfi et al. 2000). Expression profile analysis indicated a small, non-statistically significant drop in the expression level of PAI-1 mRNA in exercise-trained muscle (Table 1). Western immunoblot analysis demonstrated a persistent and significant drop (98 ± 10 %) in PAI-1 protein level after exercise training, over the 96 h and 2 week detraining time points (Fig. 2). This result suggests that exercise training dramatically reduces PAI-1 protein levels in muscle, further enhancing the local fibrinolytic state.
Figure 2. Plasminogen activator inhibitor 1 (PAI-1) protein is reduced in muscle of longitudinally sampled overweight men with characteristics of metabolic syndrome.
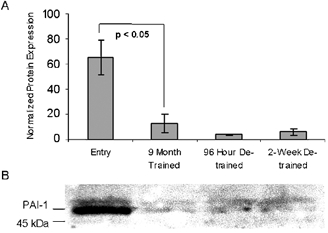
A, normalized (to myosin) expression levels of PAI-1 in skeletal muscle after exercise training and detraining as shown by Western blot quantification. B, representative PAI-1 Western blot with the molecular weight marker shown on the left. Data are longitudinal samples from 3 subjects ±s.e.m.; P value was determined using Student's t test.
The activities of t-PA and PAI-1 in serum
The activities of t-PA and PAI-1 were assessed in sera collected from the same three male STRRIDE participants, at the same training and detraining time points as the muscle biopsies were obtained (Kraus et al. 2001) (Fig. 3). The fibrin plate assay is an acccepted indirect assay for total plasminogen activator activity in the serum (Pandolfi et al. 2000). We found a statistically significant increase in mean fibrinolysis area (2.2-fold ± 0.2 increase) after exercise training (Fig. 3A). This rapidly reversed during the subsequent 96 h and 2 week detraining time points. The pre-incubation of the euglobulin fraction with an excess (1:100 dilution) of anti-t-PA antibody eliminated the differences in fibrin lysis area between the different time points (data not shown), whereas pre-incubation with a similar dilution of an unrelated antibody had no such inhibitory effect, confirming the sensitivity and specificity of the assay. These data show that the changes in fibrinolytic activity associated with exercise training are directly related to the plasma concentration or activity of t-PA and further, that muscle tissue is a likely source of circulating t-PA.
Figure 3. Exercise induces systemic fibrinolysis in STRRIDE participants.
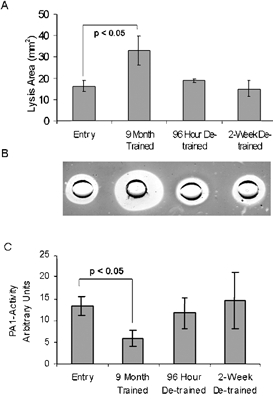
A, longitudinal analysis of fibrin plate assays of sera collected at entry, exercise-trained and detraining time points in three male overweight subjects. B, photograph of a fibrin plate assay conducted on sera taken from time points shown in panel A. C, the average results of three chromogenic PAI-1 activity assays conducted on the same sera samples. Data are longitudinal samples from 3 subjects ±s.e.m.; P value was determined using Student's t test.
As mentioned above, PAI-1 is a major thrombotic compound, capable of completely inhibiting the fibrinolytic activity of t-PA. We therefore measured the average PAI-1 activity (Fig. 3C) by a chromogenic assay based upon the inhibition of t-PA activation of plasminogen into plasmin (Pandolfi et al. 2000). A statistically significant decrease (56 ± 16 %) in PAI-1 activity was observed at the exercise-trained time point followed by an increase in activity back to or above control levels by 2 weeks of detraining. Interestingly, this mirrors the changes in fibrinolytic activity described by the fibrin plate assay; however, it does not reflect the changes in PAI-1 expression observed in skeletal muscle (Fig. 2). This indicates perhaps, a considerable decrease in PAI-1 expression and secretion by other tissues in the body or a blocking and/or clearance effect due to high t-PA serum levels. Together, these data demonstrate a dramatic change in the fibrinolytic state of both muscle and blood in the three individuals that were expression profiled.
To test if our findings were more broadly observed in a larger cohort of exercise-trained, overweight subjects with characteristics of metabolic syndrome, fibrin plate assays were also conducted on plasma from 15 overweight men also participating in the high dose, high intensity aerobic exercise training described previously and a group of six overweight men who were randomized into a non-exercising control group (Kraus et al. 2001) (Fig. 4). In the larger exercise-trained population there was a statistically significant (P < 0.05) increase (2.1-fold ± 0.1) in mean fibrinolysis area as compared with an insignificant change (P = 0.38) in the parallel, unexercised control group (1.7-fold ± 0.4). The variability in the data, particularly that of the non-exercised control group, highlights the potential difficulties in selecting a wholly appropriate ‘control’ group in this type of study, particularly since the individuals selected for this study are not considered entirely ‘healthy’. There are also known circadian, dietary and stress-induced variations in t-PA levels in the body, all of which are more easily controlled in the highly monitored, exercise-trained group as opposed to the non-exercised control group (Pandolfi et al. 2000). Taken together, our data demonstrate what is probably a direct molecular effect of t-PA production in muscle with consequent increases in serum t-PA, and corresponding decreases in both muscle and serum PAI-1. The result is a significant increase in fibrinolytic state induced by exercise training. This heightened fibrinolytic activity returns to the pre-exercise pro-thrombotic state with 2 weeks of detraining, suggesting that continued exercise is required to sustain fibrinolysis.
Figure 4. Fibrinolysis is increased in serum from exercise-trained but not parallel control untrained subjects.
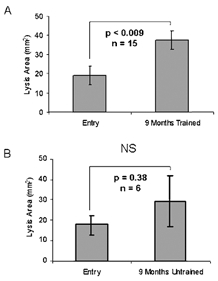
A, serum fibrin plate assay results of longitudinal entry and exercise-trained individuals. B, data from a randomized control group. Data are represented ±s.e.m.; P value was determined using Student's t test. NS, not significant.
Connective tissue activation peptide III expression in skeletal muscle
Analysis of array data revealed connective tissue activation peptide III (CTAP III), also called pro-platelet basic protein (PBP), whose longitudinal expression levels mirrored that of t-PA (Table 1). This gene is known to induce the expression and secretion of t-PA from endothelial cells (Hoogewerf et al. 1995). QMF-RT-PCR data (Fig. 5A) indicate that the mRNA for CTAP III increases significantly (7.6-fold ± 1.1) after exercise training and then rapidly decreases during the de-training time points.
Figure 5. CTAP III mRNA and protein are strongly induced in exercised muscle.
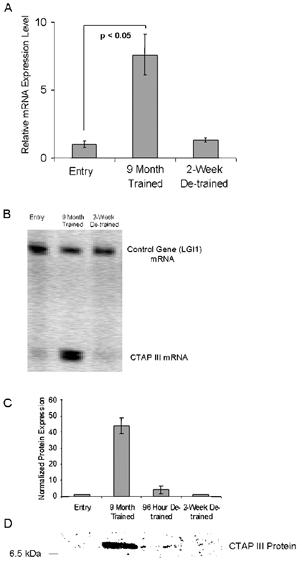
A, QMF RT-PCR analysis of CTAP III versus a control gene (LG1) shows strong induction of mRNA in longitudinal biopsies from an exercised male metabolic syndrome patient. B, quantification of CTAP III mRNA in longitudinal biopsies from three males shows increased CTAP III mRNA expression levels in skeletal muscle after exercise training then a rapid decrease by 2 weeks detraining. C and D, results of CTAP III protein immunoblots of longitudinal samples from two subjects normalized (to myosin). CTAP III protein expression paralleled mRNA expression, with loss of CTAP III protein within 96 h of detraining. Data are ±s.e.m.; P value was determined using Student's t test.
Western immunoblot analysis of skeletal muscle (Fig. 5D) showed an increase in CTAP III protein (using a β-thromboglobulin antibody) in two individuals tested, while no protein was detected in the third. These data are consistent with exercise inducing t-PA production and secretion through CTAP III protein production in muscle.
Tetranectin expression in skeletal muscle and serum
Tetranectin is a C-type lecithin originally identified as a contaminant in the purification of anti-plasmin (Kamper et al. 1998, 1999). Its plasminogen and fibrin-binding capacity and high relative concentration in the serum (> 10 mg l−1) suggests a role in mediating fibrinolysis (Kamper et al. 1998, 1999). The expression pattern of tetranectin mirrored that of t-PA and CTAP III. Interestingly, Western immunoblot analysis did not consistently detect tetranectin protein in muscle (data not shown) despite the relatively high level of tetranectin mRNA detected by Affymetrix analysis. Immunostaining of biopsy material (Fig. 6) showed an exercise-responsive increase in both cytoplasmic fluorescence, and the numbers of muscle fibres that stained in a distinct, punctate pattern at or near the plasma membrane (Fig. 6B). This pattern of immunostaining was not consistently observed at subsequent detraining time points. Relative levels of plasma tetranectin also increased after exercise training as shown by ELISA (1.6 ± 0.2) (Fig. 7A) and immunoblot (1.6 ± 0.1) (Fig. 7C) analysis.
Figure 6. Tetranectin immunostaining of skeletal muscle shows increased punctate staining in muscle following exercise training.
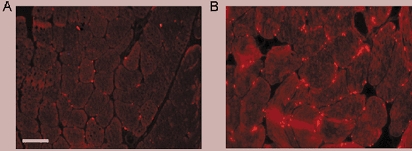
Representative acetone-fixed cryosections of skeletal muscle from entry (A) and exercise-trained (B) STRRIDE participants. Given the variable nature of tetranectin immunostaining, images were taken in regions empirically presenting the ‘strongest’ fluorescent signals in each biopsy. Bar = 50 μm.
Figure 7. Relative tetranectin protein level is increased in plasma of exercise-trained, overweight men with characteristics of metabolic syndrome.
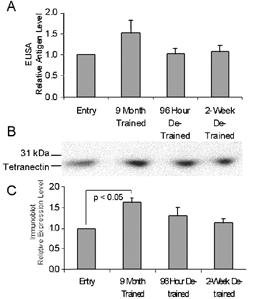
A, the relative expression levels of tetranectin in subject plasma as determined by ELISA. B, a representative immunoblot of subject plasma. C, quantification of plasma immunoblots. Panels A and C show the mean ±s.e.m. of results from three subjects normalized to the entry sample.
Gel zymography of skeletal muscle extracts
To investigate the hypothesis that chronic activation of muscle matrix metalloproteinases and plasmin by urokinase-type plasminogen activator (u-PA) was linked to increase in plasma fibrinolysis, we analysed muscle protein extracts obtained from biopsies using SDS-fibrin zymography (Choi & Kim, 2001; Siconolfi & Seeds, 2001). At all time points, we observed only a single fibrinolytic band that was approximately the same molecular weight as t-PA (data not shown). This suggests that the pathway involving u-PA activation of plasminogen in response to acute, exercise-induced muscle damage does not significantly influence muscle or plasma fibrinolysis.
DISCUSSION
Dramatic increases in obesity and inactivity in Western industrialized countries over the past several decades, particularly among the young, are linked to the rapid increase in newly diagnosed cases of type II diabetes (Flegal, 1999). This epidemic is not caused by an increase in gene frequency within the population but changes in diet and lifestyle that probably unmask the effects of genetic susceptibility (Flegal, 1999). Despite overwhelming evidence in support of the positive benefits of aerobic exercise on cardiovascular health, there is a lack of basic research directed towards defining the molecular basis of these benefits. Microarray-based gene expression analysis has been recently used to define the underlying molecular pathologies of several muscular dystrophies in humans (Chen et al. 2000; Bakay et al. 2002; Tezak et al. 2002) and the response of damaged muscle in the mouse and rat (Chen et al. 2002; Zhao et al. 2002). Here we present microarray studies of longitudinal muscle biopsies from a series of human volunteers showing characteristics of the metabolic syndrome that typically precedes type II diabetes (Kraus et al. 2001). Our longitudinal design allowed us to eliminate the substantial inter-individual noise in human subjects (Bakay et al. 2002), and thus to detect more subtle changes in gene expression, namely those underlying the adaptation of skeletal muscle to exercise in each individual.
Our analysis of muscle expression data identified clusters of functionally related genes that were similarly responsive to exercise training. One such cluster contains genes whose protein products are known to activate, or co-activate blood fibrinolytic activity (Table 1). The observed expression changes in skeletal muscle suggest a dynamic and probably significant role for muscle in regulating micro- and macrovascular haemostasis. This finding may be particularly significant given the high incidence of peripheral vascular disease and critical limb ischaemia ultimately associated with diseases of inactivity, such as metabolic syndrome and type II diabetes (Mustonen et al. 1998; Womack et al. 2001). Consistent with this model, we showed both protein abundance and protein activity measurements of t-PA, tetranectin, PAI-1 and CTPA III that were consistent with muscle driving a systemic fibrinolytic state. Indeed, our data suggest that muscle tissue is directly responsible for altering both local and systemic blood flow and that exercise of muscle could, in and of itself, prove modulatory to some of the major morbidity factors in type II diabetes.
We propose a model in which the mechanical rigors of repeated exercise stimulate capillary beds within the muscle, resulting in the repeated activation of fibrinolytic cascades to maintain the flow of blood to the active muscle. Emerging evidence suggests that mild tissue and cell damage is part of the normal physiology of muscle tissue, permitting rapid remodelling of myofibres, microvasculature and other physiological properties based upon the type and frequency of activity (Chen et al. 2002). However, our report is the first to begin to define the molecular events of exercise and their effects on the systemic circulation. Further, we propose that skeletal muscle is innately designed to be active, and that prolonged periods of inactivity, perhaps exasperated by age, result in the dysregulation of vascular endothelium, leading in turn to increased thrombosis and morbidity associated with type II diabetes.
Changes in serum t-PA and PAI-1 expression have been noted in some previous exercise studies; the results were often inconclusive if not contradictory (Carr, 2001; Imhof & Koening, 2001; Rauramaa & Vaisanan, 2001; Koller, 2002). We feel our longitudinal study design, with access to paired muscle and serum samples, has allowed us to conclusively address this issue. We also show that t-PA, along with the activating proteins CTAP III and tetranectin, are produced by skeletal muscle, and that production quickly drops after cessation of exercise. We show that the observed decreases of PAI-1 in both muscle and serum are not due to significant changes in transcript level, but instead are probably due to binding and clearance by high levels of muscle t-PA.
Alternatively, post-transcriptional control of PAI-1 protein translation or enhanced degradation of PAI-1 mRNA may occur. There is precedent for translational control in response to exercise and other types of physiological adaptations within skeletal muscle (Chen et al. 2002; Kimball et al. 2002).
An additional novel and significant finding is the production of CTAP III by exercised muscle (Fig. 5). CTAP III is considered to be a platelet-derived growth factor that stimulates a variety of specific metabolic and cellular activities including mitogenesis, extracellular matrix synthesis, glucose metabolism, and plasminogen activator synthesis (t-PA) in human fibroblast cultures (Hoogewerf et al. 1995). CTAP III is a member of the platelet basic protein (PBP) family all of which are heparin sulfate binding/degrading enzymes and members of the chemokine (chemoattractant and cytokine) superfamily that stimulate inflammation, wound healing and growth regulation (Hoogewerf et al. 1995). It is thought that the highly localized biological activity of CTAP III is derived from its ability to degrade immobilized heparin sulfate proteoglycans (HSPG) that are found abundantly on cell surfaces and in the extracellular matrix (ECM), releasing bound growth factors to act on adjacent cells (Hoogewerf et al. 1995). Pro-platelet basic protein is the precursor of two platelet α-granule proteins, PBP and CTAP III. Upon platelet activation they are released and further processed in plasma to neutrophil-activating peptide-2 (NAP-2) and β-thromboglobulin (β-TG), a common serum marker of platelet activation (Hoogewerf et al. 1995). We propose that CTAP III is released from exercise-activated platelets or perhaps the muscle itself, and facilitates the rapid degradation of any thrombi produced by damage to the muscle vascular bed by stimulating the synthesis and release of t-PA from the vascular endothelium or surrounding cells. It would be interesting to conduct a prospective study on the long-term effects of exercise-induced platelet activation on the incidence of peripheral artery disease and critical muscle ischaemia. The presence of CTAP III/PBP mRNA in skeletal muscle (Fig. 5B) either indicates increased numbers of platelets adhering to vascular surfaces within the muscle or expression of CTAP III by the muscle itself in response to exercise. Future studies will try to ascertain the cell type and location of CTAP III expression within exercise-stimulated muscle.
We found an exercise-responsive increase in the tissue and serum expression of tetranectin (Fig. 6 and Fig. 7). Tetranectin was initially described as a contaminant in the purification of plasminogen kringle 4-binding proteins (Wewer et al. 1998). Tetranectin was subsequently shown to be present in quite high concentrations in the plasma (≈10 mg l−1) where it forms a homo-trimer with a C-type lecithin domain that is common in many secreted and cell surface proteins (Hoogewerf et al. 1995; Wewer et al. 1998).
Tetranectin also has also been shown to bind heparin and while tetranectin mRNA has been detected in lung, spleen, heart and muscle, the cellular source(s) of the serum protein has not been unambiguously defined (Wewer et al. 1998). Of particular interest is the ability of tetranectin to anchor plasminogen to fibrin and other extracellular matrix molecules where it participates in the regulation of local proteolysis (Kamper et al. 1998; Iba et al. 2001). Levels of circulating tetranectin are known to decrease during heart attack and certain types of cancer (Kamper et al. 1998, 1999), suggesting that it may have a hitherto undescribed protective role in these conditions. It would therefore be interesting to incorporate the screening of plasma tetranectin levels into future prospective studies of cardiovascular fitness. The distinct exercise-responsive punctate pattern of tetranectin expression in skeletal muscle (Fig. 6), is consistent with its description in the literature as a matricellular protein (Wewer et al. 1998). Tetranectin is probably exported to the extracellular matrix, perhaps in concert with CTAP III-mediated degradation of chondroitin sulfate and other heparin sulfate proteoglycans, thus freeing tetranectin to bind to fibrin and co-activate plasminogen along with t-PA.
Tetrenectin expression has also been observed in damaged, regenerating muscle; however, our fibrin and gelatin zymography data and expression profile data showed no evidence of muscle damage and regeneration. We believe therefore that t-PA, CTAP III and tetranectin upregulation are not associated with acute muscle damage but instead with more subtle adaptations of muscle vascular beds to exercise training.
We are currently generating gene expression data on additional male and female STRRIDE subjects. The inclusion of additional subjects will reduce the false-positive rate and allow us to compare exercise-responsive gene expression patterns between men and women.
In summary, we have used microarray and protein analysis in both muscle and serum from metabolic syndrome subjects to identify exercise training-responsive genes in skeletal muscle that influence the systemic fibrinolytic state. As thrombotic events are a primary clinical complication of type II diabetes, our data suggest that muscle activity can modulate the pro-coagulative state associated with metabolic syndrome and diabetes through exercise-induced production of potent fibrinolytic proteins. Importantly, we found the effects of exercise training to be temporary with most fibrinolytic gene mRNAs and proteins, returning to baseline levels within 2 weeks of detraining. This has obvious implications for the prevalence of peripheral artery disease and critical muscle ischaemia associated with metabolic syndrome and type II diabetes (Mustonen et al. 1998; Rauramaa & Vaisanen, 1999; Carr, 2001; Kohler, 2002). Our data indicate that both repeated bouts of exercise and an exercise training routine should have beneficial effects on the mortality and morbidity associated with insulin resistance, and that muscle activity and exercise can directly drive much of the systemic improvement. Finally, this study demonstrates the utility of longitudinal gene expression analysis as a hypothesis-generating tool and for elucidating the molecular mechanisms underlying the adaptations of skeletal muscle to exercise training.
Acknowledgments
This work was supported by NIH grant Hl-57354 to WEK, the A. James Clark foundation to E.P.H. and D.S.H., and an NIH Programs in Genomic Applications grant NHLBI U01 HL66614-01; HOPGENE to E.P.H.
REFERENCES
- American Diabetes Association. Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- Bakay M, Chen YW, Borup R, Zhao P, Nagaraju K, Hoffman EP. Sources of variability and effect of experimental approach on expression profiling data interpretation. BMC Bioinformatics. 2002;3:4. doi: 10.1186/1471-2105-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Nader GA, Baar KR, Fedele MJ, Hoffman EP, Esser KA. Response of muscle to acute resistance exercise defined by transcriptional and translational profiling. J Physiol. 2002;545:27–41. doi: 10.1113/jphysiol.2002.021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Zhao P, Borup R, Hoffman EP. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J Cell Biol. 2000;151:1321–1336. doi: 10.1083/jcb.151.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr ME. Diabetes mellitus: a hypercoagulable state. J Diabetes Complications. 2001;15:44–54. doi: 10.1016/s1056-8727(00)00132-x. [DOI] [PubMed] [Google Scholar]
- Choi NS, Kim SH. Two fibrin zymography methods for analysis of plasminogen activators on gels. Anal Biochem. 2001;281:236–238. doi: 10.1006/abio.2000.4572. [DOI] [PubMed] [Google Scholar]
- el-Sayed MS. Effects of exercise on blood coagulation, fibrinolysis and platelet aggregation. Sports Med. 1996;22:282–298. doi: 10.2165/00007256-199622050-00002. [DOI] [PubMed] [Google Scholar]
- Flegal KM. The obesity epidemic in children and adults: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S509–S514. doi: 10.1097/00005768-199911001-00004. [DOI] [PubMed] [Google Scholar]
- Hoogewerf AJ, Leone JW, Reardon IM, Howe WJ, Asa D, Heinrikson RL, Ledbetter SR. CXC chemokines connective tissue activating peptide-III and neutrophil activating peptide-2 are heparin/heparan sulfate-degrading enzymes. J Biol Chem. 1995;270:3268–3277. doi: 10.1074/jbc.270.7.3268. [DOI] [PubMed] [Google Scholar]
- Imhof A, Koenig W. Exercise and thrombosis. Cardiol Clin. 2001;19:389–400. doi: 10.1016/s0733-8651(05)70224-1. [DOI] [PubMed] [Google Scholar]
- Iba K, Durkin ME, Johnsen L, Hunziker E, Damgaard-Pedersen K, Zhang H, Engvall E, Albrechtsen R, Wewer UM. Mice with a targeted deletion of the tetranectin gene exhibit a spinal deformity. Mol Cell Biol. 2001;21:7817–7825. doi: 10.1128/MCB.21.22.7817-7825.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamper EF, Kopeikina L, Mantas A, Stefanadis C, Toutouzas P, Stavridis J. Tetranectin levels in patients with acute myocardial infarction and their alterations during thrombolytic treatment. Ann Clin Biochem. 1998;35:400–407. doi: 10.1177/000456329803500309. [DOI] [PubMed] [Google Scholar]
- Kamper EF, Papaphilis AD, Angelopoulou MK, Kopeikina LT, Siakantaris MP, Pangalis GA, Stavridis JC. Serum levels of tetranectin, intercellular adhesion molecule-1 and interleukin-10 in B-chronic lymphocytic leukemia. Clin Biochem. 1999;32:639–645. doi: 10.1016/s0009-9120(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Kherif S, Lafuma C, Dehaupas M, Lachkar S, Fournier JG, Verdiere-Sahuque M, Fardeau M, Alameddine HS. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev Biol. 1999;205:158–170. doi: 10.1006/dbio.1998.9107. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Farrell PA, Jefferson LS. Invited Review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol. 2002;93:1168–1180. doi: 10.1152/japplphysiol.00221.2002. [DOI] [PubMed] [Google Scholar]
- Kohler HP. Insulin resistance syndrome: interaction with coagulation and fibrinolysis. Swiss Med Wkly. 2002;132:241–522. doi: 10.4414/smw.2002.09856. [DOI] [PubMed] [Google Scholar]
- Koller A. Hemostasis and exercise. Am J Cardiol. 2002;89:1242. doi: 10.1016/s0002-9149(02)02184-7. [DOI] [PubMed] [Google Scholar]
- Kraus WE, Torgan CE, Duscha BD, Norris J, Brown SA, Cobb FR, Bales CW, Annex BH, Samsa GP, Houmard JA, Slentz CA. Studies of a targeted risk reduction intervention through defined exercise (STRRIDE) Med Sci Sports Exerc. 2001;33:1774–1784. doi: 10.1097/00005768-200110000-00025. [DOI] [PubMed] [Google Scholar]
- Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- Mustonen P, Lepantalo M, Lassila R. Physical exertion induces thrombin formation and fibrin degradation in patients with peripheral atherosclerosis. Arterioscler Thromb Vasc Biol. 1998;18:244–249. doi: 10.1161/01.atv.18.2.244. [DOI] [PubMed] [Google Scholar]
- Pandolfi A, Giaccari A, Polishuck R, Alberta MM, Pellegrini G, Morviducci L, Vitacolonna E, Buongiorno AM, Capani F, Consoli A. Diabetis mellitus induces decreased plasma fibrinolytic activity and increased tissue synthesis of plasminogen activator inhibitor-1 (PAI-1). in the rat. Fibrinol Proteol. 2000;14:261–267. [Google Scholar]
- Rauramaa R, Vaisanen SB. Physical activity in the prevention and treatment of a thrombogenic profile in the obese: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S631–S634. doi: 10.1097/00005768-199911001-00023. [DOI] [PubMed] [Google Scholar]
- Rauramaa R, Vaisanen SB. Dose-response and coagulation and hemostatic factors. Med Sci Sports Exerc. 2001;33:S516–S520. doi: 10.1097/00005768-200106001-00022. [DOI] [PubMed] [Google Scholar]
- Sakkinen PA, Wahl P, Cushman M, Lewis MR, Tracy RP. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am J Epidemiol. 2000;152:897–907. doi: 10.1093/aje/152.10.897. [DOI] [PubMed] [Google Scholar]
- Siconolfi LB, Seeds NW. Mice lacking tPA, uPA, or plasminogen genes showed delayed functional recovery after sciatic nerve crush. J Neurosci. 2001;21:4348–4355. doi: 10.1523/JNEUROSCI.21-12-04348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezak Z, Hoffman EP, Lutz JL, Fedczyna TO, Stephan D, Bremer EG, Krasnoselska-Riz I, Kumar A, Pachman LM. Gene expression profiling in DQA1*0501+ children with untreated dermatomyositis: a novel model of pathogenesis. J Immunol. 2002;68:4154–4163. doi: 10.4049/jimmunol.168.8.4154. [DOI] [PubMed] [Google Scholar]
- Wewer UM, Iba K, Durkin ME, Nielsen FC, Loechel F, Gilpin BJ, Kuang W, Engvall E, Albrechtsen R. Tetranectin is a novel marker for myogenesis during embryonic development, muscle regeneration, and muscle cell differentiation in vitro. Dev Biol. 1998;200:247–259. doi: 10.1006/dbio.1998.8962. [DOI] [PubMed] [Google Scholar]
- Whaley MH, Kampert JB, Kohl HW 3rd, Blair SN. Physical fitness and clustering of risk factors associated with the metabolic syndrome. Med Sci Sports Exerc. 1999;31:287–293. doi: 10.1097/00005768-199902000-00013. [DOI] [PubMed] [Google Scholar]
- Womack CJ, Ivey FM, Gardner AW, Macko RF. Fibrinolytic response to acute exercise in patients with peripheral arterial disease. Med Sci Sports Exerc. 2001;33:214–219. doi: 10.1097/00005768-200102000-00007. [DOI] [PubMed] [Google Scholar]
- Zhao P, Iezzi S, Carver E, Dressman D, Gridley T, Sartorelli V, Hoffman EP. Slug is a novel downstream target of MyoD. Temporal profiling in muscle regeneration. J Biol Chem. 2002;277:30091–30101. doi: 10.1074/jbc.M202668200. [DOI] [PubMed] [Google Scholar]


