Abstract
The modulatory effects of serotonin mediated by 5-HT1A receptors in adult spinal motoneurons were investigated by intracellular recordings in a slice preparation from the turtle. In current-clamp mode, activation of 5-HT1A receptors by 8-OH-DPAT led to depolarization and an increase in input resistance in most motoneurons but caused hyperpolarization and a decrease in input resistance in the remaining smaller fraction of cells. When slices were preincubated in medium containing the 5-HT1A receptor antagonist WAY-100635, 8-OH-DPAT had no effect. In voltage-clamp mode, with 1 mm CsCl in the bathing medium, 8-OH-DPAT consistently inhibited a leak current that was sensitive to extracellular acidification and anandamide, a TASK-1 channel blocker. In medium with a low pH, as in the presence of anandamide, 8-OH-DPAT had no effect. Our results show that activation of 5-HT1A receptors contributes to the excitatory effect of serotonin on spinal motoneurons by inhibition of a TASK-1 potassium channel leading to depolarization and increased input resistance.
The serotonergic raphé spinal projection is a major modulatory pathway in the spinal motor system. Motoneurons are contacted by serotonergic synaptic terminals (Kojima & Sano, 1983; Kiehn et al. 1992) and the level of activity in raphé neurons correlates with motor activity (Jacobs & Fornal, 1997). Serotonin (5-HT) activates 5-HT1A and 5-HT2 metabotropic receptors in motoneurons and regulates the intrinsic response properties via down-stream effects on ion channels (Rekling et al. 2000). The rich variety of ion channels expressed by motoneurons changes during development and differs between functionally distinct populations of motoneurons. Therefore, it is not surprising that 5-HT exerts a wide variety of actions ranging from inhibition associated with hyperpolarization and decreased input resistance to excitation associated with depolarization and increased input resistance (Rekling et al. 2000). Most detailed studies have been performed on motoneurons in isolated preparations from developing mammals. In spinal motoneurons in the neonatal rat, the excitatory effect of 5-HT is due in part to facilitation of a slowly activating non-specific cationic current, Ih, activated by hyperpolarization (Takahashi & Berger, 1990; Kjærulff & Kiehn, 2001). However, facilitation of Ih is not the only contributor since 5-HT also increases motoneuronal excitability by inhibiting a resting K+ conductance (Wang & Dun, 1990; Ziskind-Conhaim et al. 1993). A similar reduction of a leak potassium conductance in trigeminal motoneurons in juvenile guinea pigs is mediated by 5-HT2 receptors (Hsiao et al. 1997) while a seemingly similar effect is mediated by 5-HT1A receptors in spinal motoneurons of neonatal rats (Ziskind-Conhaim et al. 1993).
In the adult spinal cord of the cat, the excitatory effects of 5-HT on motoneurons include facilitation of plateau potentials (Hounsgaard et al. 1988). The properties of adult spinal motoneurons can be studied in detail in in vitro preparations from the turtle (Perrier & Hounsgaard, 2000). In turtle motoneurons, 5-HT, in addition to facilitation of plateau potentials, also caused depolarization from the resting membrane potential and increased excitability (Hounsgaard & Kiehn, 1989; Skydsgaard & Hounsgaard, 1996). In the present study we show that the depolarization and increased excitability at rest is due, at least in part, to a reduction of a voltage-insensitive K+ conductance following activation of 5-HT1A receptors. This effect of 5-HT1A receptor activation is occluded by extracellular acidification and anandamide. Our results show that adult spinal motoneurons express a TASK-1-like potassium conductance. This conductance is inhibited by activation of 5-HT1A receptors. We suggest that the diversity of channels modulated by a single subtype of 5-HT receptor allows the functional effect of serotonin to be conditioned over a wide range.
METHODS
Slices preparation
Experiments were performed on slice preparations (1.5 mm thick) from the lumbar enlargement of spinal cord from adult turtles (Chrysemys scripta elegans) anaesthetized by intraperitoneal injection of 100 mg sodium pentobarbitone and killed by decapitation. The surgical procedures complied with Danish legislation and were approved by the controlling body under The Ministry of Justice. Experiments were performed at room temperature (20–22 oC) in a solution containing (mm): 120 NaCl; 5 KCl; 15 NaHCO3; 2 MgCl2; 3 CaCl2; and 20 glucose; saturated with 98 % O2 and 2 % CO2 to obtain pH 7.6. For some of the experiments, the pH was lowered by addition of the necessary amount of HCl.
Recordings
Intracellular recordings in current- and voltage-clamp modes were performed with an Axoclamp 2B amplifier (Axon Instruments, CA, USA). Pipettes were filled with a mixture of 0.5 m KCl and 0.5 m potassium acetate. For voltage-clamp experiments, the capacitance of the pipette was reduced by coating its tip with Sylgard. Voltage-clamp recordings were performed in discontinuous service mode at a sample rate of 6.2 – 11.1 kHz, a gain of 0.7–1.5 nA mV−1 and a filter cutoff setting of 0.1 kHz. Voltage ramps were produced by a homemade signal generator. Motoneurons were selected for study if they had a stable membrane potential of more than −60 mV. Data were sampled at 10 kHz with a 12-bit analogue-to-digital converter (Digidata 1200 from Axon Instruments, CA, USA) and displayed by means of Axoscope software and stored on a hard disk for later analysis.
Drugs
All the drugs were applied by bath application. The non-specific cation current activated by hyperpolarization, Ih, was blocked by caesium chloride (1 mm) or by the selective antagonist ZD7288 (100 μm; Tocris Cookson Ltd, Bristol, UK). In current-clamp mode, ionotropic synaptic input was eliminated by a mixture of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 25 μm; Tocris), dl-2-amino-5-phosphonopentanoic acid (dl-AP5, 50 μm; Tocris) or d(−)-2-amino-7-phosphonoheptanoic acid (AP7, 25 μm; Tocris), and strychnine (10 μm) in a first series of experiments. In a second series of experiments, (+)-bicuculline (20 μm; Tocris) was also added to the extracellular medium. In voltage-clamp experiments, spike activity and synaptic transmission were eliminated by tetrodotoxin (TTX, 1 μm; Alomone, Israel).
Other drugs used were: serotonin (5-HT; 10 μm; Sigma); (±)-2-dipropylamino-8-hydroxy-1, 2, 3, 4-tetrahydronaphthalene hydrobromide (8-OH-DPAT; 10 μm; Sigma); nifedipine (10–20 μm; Sigma), arachidonylethanolamide (anandamide; 10 μm; Tocris); N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide (WAY-100635; 10 μm; Sigma); caesium chloride (CsCl; 1 mm).
Data quantification
The input resistance of cells recorded from was estimated as the voltage change induced by small hyperpolarizing current pulses applied from resting membrane potential divided by the amount of current injected.
Voltage-clamp protocols consisted either of 1 s pulses of increasing amplitude or voltage ramps from − 110 to − 60 mV with a rise time of 1.5–2.5 s. In this voltage range and with this rate of change, only instantaneous currents were present (Ih was blocked with ZD7288 or caesium). Therefore the currents induced by the pulses or by the ramp had the same amplitude (see Fig. 3A–D). I–V plots were made from data extracted from ramp protocols. To average different I–V plots, the current was normalized to the value measured in control conditions at − 110 mV.
Figure 3. 8-OH-DPAT blocks a leak conductance.
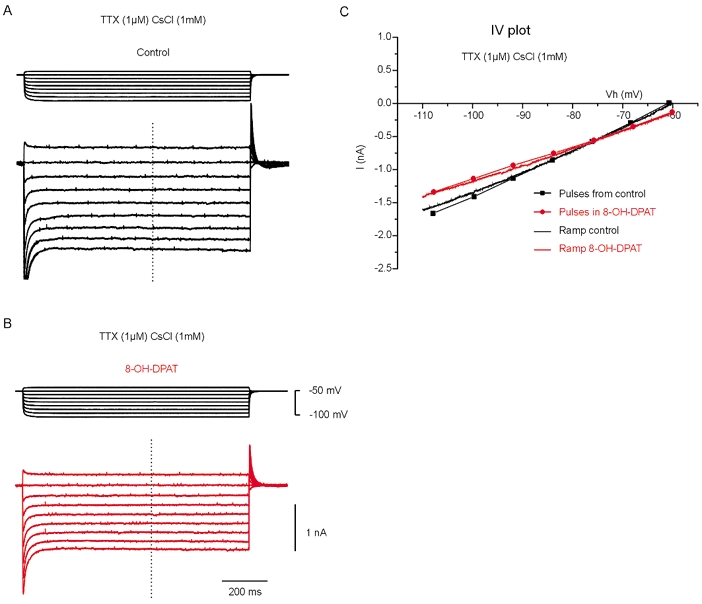
Experiments in voltage-clamp mode in the presence of TTX (1 μm) and CsCl (1 mm). A, current response to 1 s voltage steps of incrementing amplitude. B, response to the same protocol in the presence of 8-OH-DPAT (10 μm). Note the inhibition of a voltage-insensitive current (see the dots linked by lines in the I–V plot in C). C, I–V plot from the data from the step protocol (lines + dots) and from a voltage ramp before (black line) and after addition of 8-OH-DPAT (red line). The two protocols produced similar results.
Data were analysed statistically by using a two-population (paired) t test (Microcal Origin software, OriginLab Corp., Northampton, MA, USA). Significance was accepted when P < 0.05. Data are presented as means ±s.e.m.
RESULTS
Serotonin has a heterogeneous effect on spinal motoneurons
The effect of serotonin (5-HT) was tested on 28 motoneurons recorded in current-clamp mode after elimination of fast synaptic potentials (see Methods). As expected from previous reports (Rekling et al. 2000), 5-HT caused hyperpolarization in some cells and depolarization in others. Hyperpolarization (−3.6 ± 0.8 mV; n = 11; Fig. 1A) was associated with a decreased input resistance −12.2 ± 1.9 %; Fig. 1A) and a decreased excitability (Fig. 1B). The depolarizing effect of 5-HT (+3.6 ± 0.6 mV; n = 17) was associated with an increase in input resistance (+16.2 ± 3.9 %; Fig. 1C) and, for 16 cells out of 17, excitability (Fig. 1D), since the number of spikes generated by a given current pulse was increased. In one cell depolarized by 5-HT, the input resistance was reduced (−12 %). This indicates facilitation of an inward current and may be due to enhancement of Ih by 5-HT as in neonatal motoneurons (Takahashi & Berger, 1990). In 22 of the 28 cells, 5-HT also promoted the generation of plateau potentials (13 out of the 17 cells depolarized by 5-HT and 9 out of the 11 cells hyperpolarized by 5-HT). This array of effects evoked by 5-HT in spinal motoneurons may reflect variations in the relative weight of the signalling pathways activated by 5-HT1A and 5-HT2 receptors in different cells. Here we restrict our analysis to the effects of selective activation of 5-HT1A receptors. Since the presence of Ih varies in turtle motoneurons these channels were excluded from the analysis by addition of antagonists.
Figure 1. Activation of 5-HT receptors induces heterogeneous effects in spinal motoneurons.
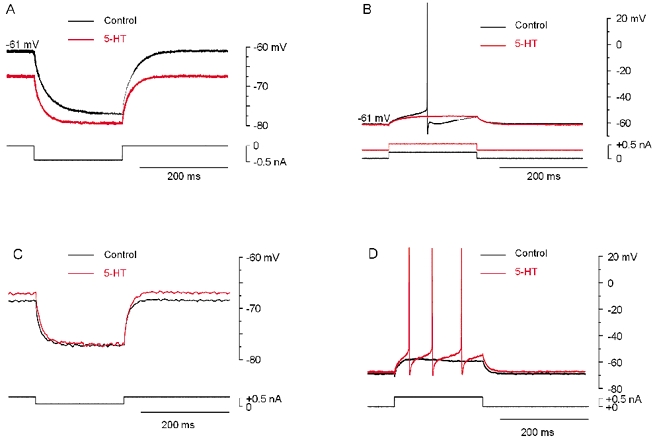
A, response of a motoneuron to a hyperpolarizing current pulse before (black trace) and after addition of 5-HT (10 μm; red trace). 5-HT hyperpolarized the cell from −61 to −68 mV and decreased the input resistance from 32 to 25 MΩ (−21.9 %). B, response to positive depolarizing current pulses. In 5-HT (red), a positive bias current was injected in the cell to adjust the membrane potential to the same value as in control. Note the decrease in excitability. C, response of another motoneuron to hyperpolarizing current pulses. 5-HT (red) depolarized the cell from −70 to −68 mV and increased the input resistance from 18 to 20 MΩ (+11.1 %). D, response to positive depolarizing current pulses. Note the increase in excitability induced by 5-HT (red). No bias current was injected. A and B from one motoneuron, C and D from another motoneuron. In all experiments synaptic potentials were blocked by CNQX (25 μm), AP7 (25 μm) and strychnine (10 μm).
Activation of postsynaptic 5-HT1A receptors increases motoneuron excitability
Activation of postsynaptic 5-HT1A receptors was first investigated during intracellular recordings from motoneurons in current-clamp mode, after elimination of synaptic potentials with CNQX, dl-AP5 and strychnine and of Ih with ZD7288. In most motoneurons recorded from, addition of 8-OH-DPAT to the extracellular medium induced depolarization (+2.4 ± 0.8 mV; n = 9/11; Fig. 2A) associated with an increase in input resistance (+7.7 ± 5.0 %). At the same time, the excitability of motoneurons increased, as seen by the number of spikes elicited by a depolarizing current pulse applied at the same membrane potential before and after application of 8-OH-DPAT (Fig. 2B and C). In a small fraction of motoneurons (2/11), however, 8-OH-DPAT had a hyperpolarizing effect (−2.5 ± 0.5 mV; Fig. 2D) concomitant with a decrease in input resistance (−15.6 ± 9.4 %). This inhibitory effect was associated with a decrease in excitability, since the same current pulse applied at the same potential induced fewer action potentials in the presence of 8-OH-DPAT (Fig. 2E and F). 8-OH-DPAT is the most selective 5-HT1A receptor agonist available (Hoyer & Martin, 1997). In high concentrations, however, it also activates 5-HT7 receptors (Markstein et al. 1999). To exclude an effect of 8-OH-DPAT on 5-HT7 receptors, slices were preincubated in WAY-100635, a selective antagonist for 5-HT1A receptors (Fletcher et al. 1996). Subsequent addition of 8-OH-DPAT did not change the membrane potential (+0.7 ± 0.6 mV; n = 5; inset in Fig. 2), which strongly suggests that the effects induced by 8-OH-DPAT were due to activation of 5-HT1A receptors. The depolarization induced by 8-OH-DPAT is compatible with inhibition of a channel mediating an outward current at the resting membrane potential. The experiments presented in the following were aimed at testing this hypothesis.
Figure 2. Activation of 5-HT1A receptors induces various effects in spinal motoneurons.
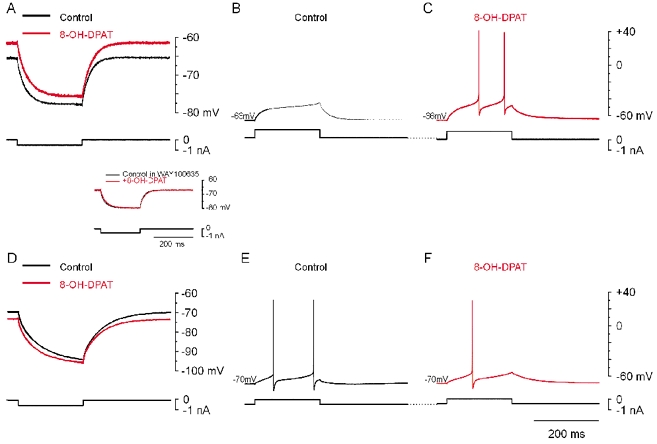
Data in A–C and D–F were from two different motoneurons. In all experiments synaptic potentials were blocked by CNQX (25 μm), AP5 (50 μm) and strychnine (10 μm). Ih was blocked by ZD7288 (100 μm). A, response to a hyperpolarizing current pulse. 8-OH-DPAT (10 μm; red trace) depolarized the cell from −66 to −61 mV and increased input resistance from 25 to 27.5 MΩ (+10 %). B, subthreshold response to a depolarizing current pulse in control medium. C, suprathreshold response to the same current pulse in the presence of 8-OH-DPAT; resting potential normalized with negative bias current (i.e. −66 mV). D, response of another motoneuron to a hyperpolarizing current pulse. Addition of 8-OH-DPAT (10 μm; red trace) hyperpolarized the cell from −70 to −73 mV, and decreased its input resistance from 49 to 46 MΩ (−6 %). E and F, response to a depolarizing current pulse before (E) and after (F) addition of 8-OH-DPAT. With the membrane potential normalized to −70 mV with depolarizing bias current, the same current pulse as in E generated one rather than two spikes. Inset: in a motoneuron from a slice preincubated in WAY-100635 (10 μm), 8-OH-DPAT (red trace) did not change the membrane potential or input resistance.
5-HT1A receptor activation inhibits a TASK-1-like conductance
To investigate the nature of the conductances modulated by 8-OH-DPAT, we performed voltage-clamp experiments. In order to simplify the analysis, the receptor agonist was tested while specific K+ conductances were blocked. The experiments were performed in the presence of 1 mm CsCl in the extracellular medium. At this concentration, caesium blocks several conductances including delayed rectifier K+ channels, Ca2+-dependent K+ channels, inward rectifier K+ channels and Ih (Hille, 2001). Under these conditions and in the voltage range tested (−110 to −60 mV), only instantaneous currents were detected (Fig. 3A). Therefore, the amplitude of the currents induced by voltage steps (Fig. 3A) and by voltage ramps (Fig. 3C) were the same. For this reason I–V relations were obtained directly from voltage-ramp experiments. Addition of 8-OH-DPAT (10 μm) inhibited a current (Figs 3B and C and 4A) that was voltage insensitive (Fig. 3C and Fig. 4A) and that showed mild outward rectification (Fig. 4A). This result was reproducible (average I–V plot in Fig. 4B; n = 4) and statistically significant (P < 0.05 for the current amplitude measured at −110 mV; paired t test performed on non-normalized data). The I–V relations before and after application of 8-OH-DPAT crossed at −80 mV (± 4.6 mV; n = 4), a value compatible with the reversal potential for K+ ions. Among the possible channels that could mediate this leak current, the TWIK-related acid-sensitive K+ channels (TASK) channels (Duprat et al. 1997), belonging to the two-pore domain K+ channel family, seemed to be good candidates. To test this possibility, the pH of the extracellular medium was shifted from 7.6 to 6.4. Extracellular acidification inhibited a voltage-insensitive current (Fig. 4C; n = 7; P < 0.05 for the current amplitude measured at −110 mV; paired t test on non-normalized data) that reversed at −78.2 mV (± 3.1 mV; n = 7). At low extracellular pH, 8-OH-DPAT had no significant effect on the I–V relationship (red dots in Fig. 4C). This suggests that acidification and 8-OH-DPAT inhibit the same current. We then tested the effect of the naturally occurring ligand of the cannabinoid receptor anandamide, which, among the two-pore domain K+ channel family, selectively blocks the TASK-1 channels (Maingret et al. 2001). Addition of anandamide to the medium (pH 7.6) also blocked a voltage-insensitive current (Fig. 4D, blue dots; n = 4; P < 0.05 for the current amplitude measured at −110 mV; paired t test on non-normalized data) and as in the case of low extracellular pH, subsequent addition of 8-OH-DPAT had no significant effect (Fig. 4C, red dots; n = 4). The results indicate that 8-OH-DPAT inhibits a TASK-1-like channel.
Figure 4. 8-OH-DPAT inhibits a TASK-1-like channel.
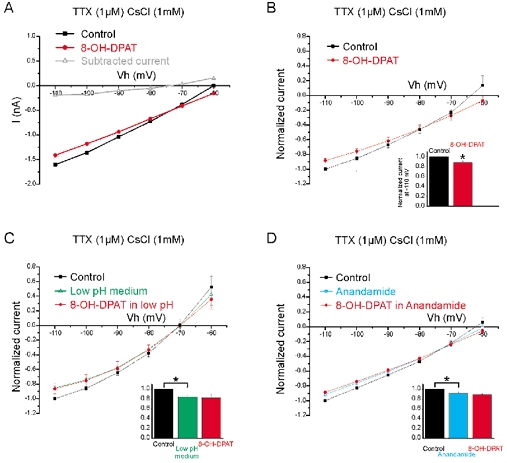
Experiments in voltage-clamp mode in the presence of TTX (1 μm) and CsCl (1 mm). A, I–V plot from the ramp protocol before (black) and after addition of 8-OH-DPAT (10 μm; red). The 8-OH-DPAT sensitive component (grey) showed mild outward rectification. B, normalized and averaged I–V plot from the ramp protocol before (black) and after addition of 8-OH-DPAT (10 μm; red) (n = 4). Inset: normalized current measured at −110 mV. The inhibition was statistically significant. C, normalized and averaged I–V plot before (black) and after changing to medium with low pH (green) (n = 7). The inhibition was significant (*). Subsequent addition of 8-OH-DPAT (10 μm; red) had no additional effect. D, normalized and averaged I–V plot before (black) and after addition of anandamide (blue) (n = 4). The inhibition was significant (*). Subsequent addition of 8-OH-DPAT (10 μm) had no additional effect (inset; red).
TASK-1 channels significantly contribute to the increase in excitability induced by 5-HT
In order to establish whether the excitatory effect induced by 5-HT depends on the modulation of TASK-1 channels, we have compared, the effect induced by 5-HT in normal, medium or low pH medium on the same cells. Motoneurons selected for this study were all depolarized by 5-HT (n = 4). In normal medium, addition of serotonin increased the mean input resistance of the motoneurons from 16.1 ±3.9 to 18.3 ±3.9 MΩ (Fig. 5A), i.e. an increase of 15.7 ± 7.4 % (Fig. 5B, left column). The effect of 5-HT was reversible (Fig. 5A, third column; the input resistance after washing out: 16.8 ± 4.7 MΩ). As expected from our previous observations, subsequent acidification (pH 6.4) also induced an increase in input resistance (Fig. 5A; 20.6 ± 5.6 MΩ). Finally, addition of 5-HT in low pH medium increased the mean input resistance to 22.0 ± 5.4 MΩ, i.e. an increase of 8.8 ± 6.6 %, (Fig. 5B, right column) which was significantly lower than the increase induced in normal medium (P < 0.01; paired t test on non-normalized data). This occlusion experiment shows that a significant fraction of the channels inhibited by 5-HT are sensitive to the extracellular pH. Altogether, our results suggest that the increase of motoneuron excitability by 5-HT is produced by activation 5-HT1A receptors which inhibit TASK-1 channels.
Figure 5. 5-HT increases motoneuron excitability by inhibiting TASK-1 channels.
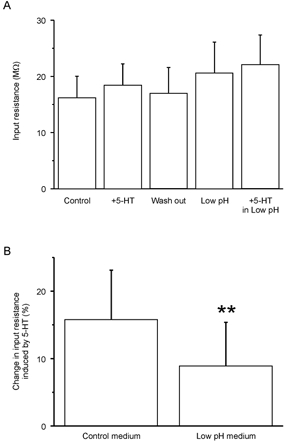
A, mean input resistance of motoneurons (n = 4). First column: control conditions. Second column: after addition of 5-HT (10 μm). Third column: after wash out of 5-HT. Fourth column: in low pH Ringer solution (6.4). Fifth column: after addition of 5-HT (10 μm) in the low pH Ringer solution. The input resistance was estimated as the voltage change induced by small hyperpolarizing current pulses applied from resting membrane potential divided by the amount of current injected. B, in normal medium, 5-HT induced an increase in input resistance of 15.7 ± 7.4 %. In low pH medium, 5-HT increased the input resistance by 8.8 ± 6.6 %, a value significantly lower than the one measured in normal medium (P < 0.01; paired t test). Synaptic potentials were blocked by CNQX (25 μm), AP7 (25 μm), strychnine (10 μm) and bicuculline (20 μm).
DISCUSSION
In this study we provide evidence for the presence of functional 5-HT1A serotonin receptors in somatic motoneurons in the adult spinal cord. We also show that activation of 5-HT1A receptors inhibits a leak current mediated by TASK-1-like potassium channels.
5-HT1A receptors in adult motoneurons
In the rat, the expression of 5-HT1A receptors in hypoglossal motoneurons peaks at postnatal day 7 only to diminish to low levels in later stages of development (Talley et al. 1997). In adult primates, however, discreet and intense expression was demonstrated in the axon hillock of brainstem and spinal motoneurons (Kheck et al. 1995; Azmitia et al. 1996). Our study confirms these anatomical data and demonstrates that functional 5-HT1A receptors are expressed in lumbar motoneurons in an adult vertebrate.
5-HT1A receptors inhibit TASK-1
The dominant effect of 5-HT1A receptor activation was excitation (9/11 cells), since 8-OH-DPAT induced depolarization and increased the excitability. Several lines of evidence support the hypothesis that this effect is mediated by inhibition of a TASK-1 channel. First, 8-OH-DPAT inhibited a current that reversed around −80 mV, i.e. compatible with the reversal potential for K+. Second, the current that was inhibited was a voltage-insensitive leak current. Third, extracellular acidification blocked this leak current and prevented the effect of 8-OH-DPAT. Taken together, these results suggest that the current is generated by a member of the TWIK-related acid-sensitive K+ (TASK) channels. Among the channels in this subfamily, only TASK-1 and TASK-3 generate leak currents (Patel & Honoré, 2001). In the family of two-pore domain K+ channels, only TASK-1 is blocked by anandamide (Maingret et al. 2001). In our experiments anandamide and 8-OH-DPAT seemed to block the same leak current. However, although the effect of anandamide on TASK-1 channels is thought to be direct (Maingret et al. 2001), anandamide also activates cannabinoid receptors, including type 1 (CB1) receptors which have been found in motoneurons (Ong & Mackie, 1999). Moreover, CB1 receptors are coupled to ion channels including inwardly rectifying K+ channels (Ho et al. 1999). In addition to the direct block of TASK-1 channels, anandamide undoubtedly affects other channels in motoneurons via CB1 receptors. In our experiments, however, we show that anandamide and 8-OH-DPAT have occlusive effects on a leak conductance. Taken together, the evidence supports the conclusion that anandamide, low pH and activation of 5-HT1A receptors reduce a leak conductance mediated by TASK-1 channels in spinal motoneurons.
Talley et al. (2000) previously reported the presence of mRNA for TASK-1 in spinal motoneurons of adult rats. Here we show that spinal motoneurons in the adult turtle express functional TASK-1-like channels. In their study, Talley et al. (2000) also found that 5-HT inhibited TASK-1 channels in hypoglossal motoneurons in neonatal rats and suggested that this was mediated by a Gαq/11 G protein. For this reason and in analogy with earlier findings in trigeminal motoneurons in juvenile guinea-pigs (Hsiao et al. 1997) serotonin was proposed to block TASK-1 channels by activating 5-HT2 receptors. In the present study, however, we show that TASK-1-like channels in spinal motoneurons are blocked by 5-HT1A receptor activation, supposedly via the Gαi/o signalling pathway. Although surprising, this is in agreement with the finding that 5-HT1A receptor activation blocks a leak conductance in spinal motoneurons in neonatal rats (Ziskind-Conhaim et al. 1993). It remains to be seen if the same TASK-1 channel is regulated by several G protein signalling pathways. This question and regulation of other channels in turtle motoneurons by 5-HT1A and 5-HT2 receptors need further study.
Functional considerations
5-HT had an excitatory effect on most of the motoneurons recorded from (17/28, i.e. 61 %). This effect was much reduced when 5-HT was applied in a low pH medium, i.e. when some of the TASK-1 channels were blocked. This result suggests that TASK-1 channels contribute significantly to the increase in excitability mediated by 5-HT.
In the absence of synaptic activity, spinal motoneurons are protected from spurious spike activity by a resting membrane potential 15–20 mV below threshold for action potentials. Synaptically regulated tonic depolarization induced by inhibition of TASK-1 channels may provide a functionally flexible mechanism for the central nervous system to facilitate recruitment of particular groups of motoneurons during motor actions. Inhibition of TASK-1 also enhances ionotropic synaptic responses in these cells by increasing input resistance and making them electrically compact. During fictive locomotion, rhythmic activity in motoneurons is often superimposed on a tonic depolarizing drive potential not produced by fast synaptic potentials (Roberts & Kahn, 1982; Shefchyk & Jordan, 1985; Hochman & Schmidt, 1998). Inhibition of TASK-1 channels due to the enhanced raphé spinal activity associated with motor activity might provide a plausible mechanism for this aspect of motor behaviour.
Acknowledgments
This work was kindly funded by the European Union, the Danish MRC, The Lundbeck Foundation, The Novo-Nordisk Foundation and The Foundation Agnes and Poul Friis. J.-F. P. is supported by a grant from the Danish MRC.
references
- Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, Jones DE, McLenachan A, Stanhope KJ, Critchley DJ, Childs KJ, Middlefell VC, Lanfumey L, Corradetti R, Laporte AM, Gozlan H, Hamon M, Dourish CT. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 3. MA USA: Blackwell Science Inc; 2001. [Google Scholar]
- Ho BY, Uezono Y, Takada S, Takase I, Izumi F. Coupling of the expressed cannabinoid CB1 and CB2 receptors to phospholipase C and G protein-coupled inwardly rectifying K+ channels. Receptors Channels. 1999;6:363–374. [PubMed] [Google Scholar]
- Hochman S, Schmidt BJ. Whole cell recordings of lumbar motoneurons during locomotor-like activity in the in vitro neonatal rat spinal cord. J Neurophysiol. 1998;79:743–752. doi: 10.1152/jn.1998.79.2.743. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Martin G. 5-HT receptor classification and nomenclature: towards a harmonization with the human genome. Neuropharmacology. 1997;36:419–428. doi: 10.1016/s0028-3908(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Hsiao CF, Trueblood PR, Levine MS, Chandler SH. Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. J Neurophysiol. 1997;77:2910–2924. doi: 10.1152/jn.1997.77.6.2910. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7:820–825. doi: 10.1016/s0959-4388(97)80141-9. [DOI] [PubMed] [Google Scholar]
- Kheck NM, Gannon PJ, Azmitia EC. 5-HT1A receptor localization on the axon hillock of cervical spinal motoneurons in primates. J Comp Neurol. 1995;355:211–220. doi: 10.1002/cne.903550205. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Rostrup E, Moller M. Monoaminergic systems in the brainstem and spinal cord of the turtle Pseudemys scripta elegans as revealed by antibodies against serotonin and tyrosine hydroxylase. J Comp Neurol. 1992;325:527–547. doi: 10.1002/cne.903250406. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. 5-HT modulation of multiple inward rectifiers in motoneurons in intact preparations of the neonatal rat spinal cord. J Neurophysiol. 2001;85:580–593. doi: 10.1152/jn.2001.85.2.580. [DOI] [PubMed] [Google Scholar]
- Kojima M, Sano Y. The organization of serotonin fibers in the anterior column of the mammalian spinal cord. An immunohistochemical study. Anat Embryol (Berl) 1983;167:1–11. doi: 10.1007/BF00304597. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lazdunski M, Honore E. The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. EMBO J. 2001;20:47–54. doi: 10.1093/emboj/20.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markstein R, Matsumoto M, Kohler C, Togashi H, Yoshioka M, Hoyer D. Pharmacological characterisation of 5-HT receptors positively coupled to adenylyl cyclase in the rat hippocampus. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:454–459. doi: 10.1007/pl00005375. [DOI] [PubMed] [Google Scholar]
- Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in the primate spinal cord. J Neurocytol. 1999;28:39–45. doi: 10.1023/a:1007011700677. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. Development and regulation of response properties in spinal cord motoneurons. Brain Res Bull. 2000;53:529–535. doi: 10.1016/s0361-9230(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Kahn JA. Intracellular recordings from spinal neurons during ‘swimming’ in paralysed amphibian embryos. Philos Trans R Soc Lond B Biol Sci. 1982;296:213–228. doi: 10.1098/rstb.1982.0003. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Jordan LM. Motoneuron input-resistance changes during fictive locomotion produced by stimulation of the mesencephalic locomotor region. J Neurophysiol. 1985;54:1101–1108. doi: 10.1152/jn.1985.54.5.1101. [DOI] [PubMed] [Google Scholar]
- Skydsgaard M, Hounsgaard J. Multiple actions of iontophoretically applied serotonin on motorneurones in the turtle spinal cord in vitro. Acta Physiol Scand. 1996;158:301–310. doi: 10.1046/j.1365-201X.1996.558326000.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. J Physiol. 1990;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Sadr NN, Bayliss DA. Postnatal development of serotonergic innervation, 5-HT1A receptor expression, and 5-HT responses in rat motoneurons. J Neurosci. 1997;17:4473–4485. doi: 10.1523/JNEUROSCI.17-11-04473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MY, Dun NJ. 5-Hydroxytryptamine responses in neonate rat motoneurones in vitro. J Physiol. 1990;430:87–103. doi: 10.1113/jphysiol.1990.sp018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Seebach BS, Gao BX. Changes in serotonin-induced potentials during spinal cord development. J Neurophysiol. 1993;69:1338–1349. doi: 10.1152/jn.1993.69.4.1338. [DOI] [PubMed] [Google Scholar]


