Abstract
This study was designed to investigate the effect of ageing on the mechanical and electromyographic (EMG) characteristics of the soleus motor units (MUs) activated by the maximal Hoffmann reflex (Hmax) and by the direct muscle compound action potential (Mmax). Eleven young (mean age 25 ± 4 years) and ten elderly (mean age 73 ± 5 years) males took part in this investigation. The senior group presented lower amplitudes of Mmax (−57 %, P < 0.001) and Hmax (−68 %, P < 0.001) waves compared to the younger population. These were associated with a depression of relative twitch torque of the plantar flexors. The average values of the Hmax/Mmax ratio did not statistically differ between the two populations, despite a tendency for lower values (∼23 %) in the senior group. However, the older adults showed a greater relative amplitude of the sub-maximal M wave evoked at Hmax (MatHmax) than did the younger males (young 5 % vs. elderly 29 % of the Mmax, P < 0.01). This finding suggests an increased homogeneity between the excitability threshold of sensory and motor axons. The twitch torque at Hmax (PtH–M) was subsequently calculated by subtraction from the total twitch torque of the mechanical contamination associated with MatHmax. The resulting PtH–M was significantly lower in the elderly (−59 %, P < 0.001). Despite a discrepancy of 20 % between the two groups, the mechanical ratio (PtH–M/PtM; PtM, twitch tension related to the Mmax compound action potential), like the EMG ratio, did not statistically differ between the young and older individuals. Nevertheless, the senior subjects exhibited a higher twitch/EMG ratio for the reflexively activated MUs (PtH–M/Hmax) than the younger individuals (+40 %, P < 0.05). This finding suggests an on-going neuromuscular remodelling, resulting in an increased innervation ratio. The neural rearrangement may be viewed as a compensatory adaptation of the motor system to preserve the mechanical efficiency of the surviving MUs, despite the age-related impairment of the segmental reflex system. This phenomenon is confirmed by the maintenance, with senescence, of the approximately constant values of the twitch/EMG ratio for the entire motor pool (PtM/Mmax).
The neuromuscular system is known to undergo structural and functional changes in old age (Doherty et al. 1993; Lexell, 1995; Roos et al. 1997; Luff, 1998; Vandervoort, 2002). The quantitative evaluation of the peripheral neural excitability and the associated contractile features may be assessed by measuring the electromyographic (EMG) activity, evoked by an electrical stimulus applied on a peripheral nerve, along with the associated mechanical response. It has been established that the maximal Hoffmann reflex (Hmax) of the soleus muscle produced by a sub-maximal electrical stimulation of the posterior tibial nerve may essentially be related to the activation of the slowest-twitch units in the examined motor pool, which are easily excited by an Ia afferent volley (Buchthal & Schmalbruch, 1970; Schieppati, 1987; Callancie & Bawa, 1990; Pierrot-Deseilligny & Mazevet, 2000; Zehr, 2002). Instead the compound muscle action potential (Mmax), due to the direct depolarisation of the α-motoneurons, is the electrical response to the activation of the entire motor pool, including fast-twitch units. The ratio between the Hmax and Mmax waves (EMG ratio) is commonly used as an index of peripheral reflex excitability (Schieppati, 1987; Maffiuletti et al. 2001). However, since it depends on the facilitation of the transmission between the Ia fibres and the α-motoneurons (Schieppati, 1987), the effects of pre-synaptic inhibitions and, possibly, of oligosynaptic contributions on modulation of the reflex response cannot be neglected (Burke et al. 1984; Zehr, 2002). It is well known that ageing is associated with impaired neural function, which peripherally seems to lead to decreased motoneuron excitability. Although several investigations reported a significant decrease in reflex response with ageing (Sabbahi & Sedgwick, 1982; Schmidt et al. 1982; Vandervoort & Hayes, 1989; Koceja & Mynark, 2000; Scaglioni et al. 2002), an attenuation of its normalised index (Hmax/Mmax) does not necessarily follow. Indeed, some studies showed no effect of age on the proportion of reflexively recruited motor units (deVries et al. 1985; Vandervoort & Hayes, 1989) while others reported a reduction of the EMG ratio pointing to a defective reflex function (Sabbahi & Sedgwick, 1982; Koceja et al. 1995; Angulo-Kinzler et al. 1998; Scaglioni et al. 2002). Hence, if ageing can be associated with a reduced neural excitability, it might be speculated that the kinetics of the twitches associated with the Hmax and Mmax waves are affected to a similar extent as their related compound muscle potentials. As reported previously, the contractile features of the twitch evoked by Mmax provide some indications of differences between young and elderly muscles. Several studies reported a lower maximal twitch force together with a slowing in triceps surae contractile kinetics with advancing age (Davies & White, 1983; Davies et al. 1986; Vandervoort & McComas, 1986; Klein et al. 1988; Vandervoort & Hayes, 1989; Winegard et al. 1997). Despite these reports, very little is known on the force output capacity of those motor units (MUs) contributing to the H reflex, even though this might represent a way to ascertain how senescence affects the various MU types. The report by Vandervoort & Hayes (1989) is, to our knowledge, the only one to address the effect of ageing on contractile properties associated with Hmax. This previous investigation showed a similar slowing in contraction kinetics of the reflex and direct evoked twitch force. However, these studies did not take into account that the mechanical response associated with Hmax does not represent the pure mechanical expression of the MUs reflexively activated. In fact, the Hmax stimulus intensity frequently evokes a sub-maximal M wave (Sabbahi & Sedgwick, 1982) which corresponds to the activation of the units innervated by the largest diameter motor axons (Schieppati, 1987), being the most excitable in response to artificial electrical stimulation. Nevertheless, a recent investigation conducted in healthy young individuals, in our laboratory (Maffiuletti et al. 2000), has identified a procedure for determining the real mechanical contribution of the MUs reflexively recruited, by subtracting the contribution of those MUs activated by the sub-maximal M wave. This method makes it possible to couple the EMG ratio with its effectual mechanical output, and thus may provide a way to explore the central and peripheral adaptations induced by senescence on soleus force production.
The Hoffmann reflex pathway seems to be affected by several age-related changes, involving both interneurons (Morita et al. 1995) and the afferent and efferent tracks. The result is a reorganisation of neural structures (Jacobs & Love, 1985), involving the progressive loss of the largest fibres (Wang et al. 1999) and the increased incidence of demyelinisation/remyelinisation, responsible for the axonal diameter shrinkage (Ochoa & Mair, 1969; Rao & Krinke, 1983) and the reduced internodal length (Lascelles & Thomas, 1966). In keeping with morphological findings, neurophysiological measurements also reveal a depression in the compound action potential size and a reduction in the conduction velocity of the neural response (Buchthal & Rosenfalck, 1966; Sabbahi & Sedgwick, 1982). Since ageing appears to be associated with various neurogenic alterations at the peripheral and spinal level, leading to attenuated neural excitability, the reduction of the EMG ratio with increasing age may be regarded as an obvious consequence. However, due to old age, MUs undergo considerable remodelling. Typically, this phenomenon involves the reinnervation of denervated type II fibres by sprouting axons of the surviving motoneurons of slow-twitch units, inducing a shift of the reinnervated type II fibres towards type I in terms of physiological and biomechanical properties (Doherty et al. 1993; Carmeli & Reznick, 1994; Roos et al. 1997; Connelly et al. 1999; Vandervoort, 2002). Given the greater excitability of the slow MUs by the Ia afferent volley, the presence of ‘giant and slower’ units in aged muscles may be viewed as a compensatory mechanism aimed at preserving their mechanical efficiency, despite the impairment of the segmental reflex system. Therefore, in view of these considerations, the present study was designed to investigate how senescence affects the electrical and mechanical properties of the soleus MUs activated by H reflex and direct motor responses.
METHODS
Subjects
Ten young (aged 22 ± 3 years, mass 72 ± 6 kg, height 180 ± 7 cm) and nine elderly (aged 73 ± 4 years; mass 76 ± 11 kg; height 172 ± 8 cm) male adults gave written informed consent to participate to the study, after being fully informed about the investigation and the possible related risks and discomfort. The older adults underwent a complete medical examination and only individuals free from muscular, neurological, cardiovascular, metabolic and inflammatory diseases took part in the present investigation. To select moderately active individuals, an activity pattern profile was defined for each subject, taking into account the type and number of hours of exercise per week. Only individuals, taking part in recreational, non-competitive, physical activities with a frequency of no more than twice a week, were admitted to the study.
Approval for the experimental procedures was obtained from the Committee on Human Research of the ‘S. Maugeri Foundation’ (Institute of Occupational Medicine and Rehabilitation, Pavia, Italy) and all experimental procedures were performed in accordance with the Declaration of Helsinki.
Electromyographic and mechanical recordings
The tests were performed in the sitting position with the trunk inclined at 60 deg with respect to the vertical. All measurements were carried out on the dominant lower limb fixed at about 90 deg of flexion at the hip, knee and ankle joints. The flexed position at the knee joint was chosen to reduce the mechanical contribution of the gastrocnemii to the plantar flexor twitch torque (Cresswell et al. 1995). Furthermore, particular care was taken in monitoring the posture of the subject and avoiding head rotations during the test to maintain constant cortico-vestibular influences on the excitability of the motor pool and limit afferent feedback from other peripheral receptors, i.e. Golgi tendon organs, cutaneous and joint afferences (Schieppati, 1987; Zehr, 2002).
Surface EMG activities of the soleus and the tibialis anterior muscles were recorded. Since the mechanical response induced by tibial nerve stimulation is generated by the plantar flexors and eventually reduced by concomitant activation of the tibialis anterior (TA), the EMG activity of the antagonist muscle and palpation of the muscle/tendon allowed the exclusion of the activation of the TA. EMG was recorded using bipolar surface electrodes (Neuroline, Medicotest, Denmark) with an inter-electrode (centre-to-centre) distance of 2 cm. For the soleus muscle the recording electrodes were placed along the mid-dorsal line of the leg at ∼4 cm below the distal insertion of the gastrocnemii into the Achille's tendon and for the TA these were positioned in the upper third of the muscle belly. The reference electrode was placed in a central position between the gastrocnemii bellies. Inter-electrode resistance was kept < 5 kΩ by rubbing the skin with fine emery paper and cleaning with ether. The EMG responses were associated with plantar flexion mechanical recordings by using a custom-made pedal equipped with strain gauges, specifically developed for the study by the mechanical workshop of the local engineering school. A strap was placed around the foot to secure it firmly to the pedal. The mechanical signals were sampled at a frequency of 2 kHz and amplified by five. EMG signals were band-pass filtered (10 Hz–1 kHz) using an A/D board (Biopac, MP100, USA) and stored with a commercially available software (Acqknowledge MP 100 USA) for off-line analysis.
Electrical stimulation
One millisecond square-wave stimuli were applied to the posterior tibial nerve via a cathode ball electrode (0.5 cm diameter) pressed in the popliteal fossa and an anode electrode of large size (12.5 cm × 7.5 cm, VersaStim, ConMed, USA) was placed on the anterior surface of the knee. The percutaneous stimulation was delivered by a constant-current stimulator (DS7, Digitimer Ltd, Welwyn Garden City, UK). Subjects were familiarised with sub-maximal electrical stimuli over a period of 10 min before the beginning of the testing session. The stimulus intensity was then progressively increased (by 1 mA increments) from the level required to elicit the H reflex, up to the level for evoking a maximal plantar flexor twitch torque and soleus M wave amplitude. The largest H reflex response not preceded by a sub-maximal M wave was called Hfree (Maffiuletti et al. 2000). Four stimuli were delivered at each intensity interspaced by a 5 s interval.
Data analysis
For each subject, we measured the peak-to-peak amplitude and duration (i.e. the peak-to-peak time) of the soleus Hmax and Mmax. The Hmax wave was normalised to the amplitude of the Mmax wave (Hmax/Mmax) in order to assess the proportion of MUs activated by the Ia afferents and also to minimise the influences of peripheral factors. The amplitude of the sub-maximal M wave evoked at Hmax intensity (MatHmax) was also recorded. In addition, we measured the latency of the H (ΔtH) and M (ΔtM) waves (i.e. the time interval between the onset of the stimulus artefact up to the first deflection of the H or the M waves) normalised to the subject height (ΔtH/height; ΔtM/height), since the length of the reflex loop is a function of the height of each subject (Guiheneuc & Bathien, 1976). Concerning the twitch torque associated with Hmax and Mmax we assessed: (1) the peak torque (Pt), namely the highest plantar flexor twitch tension related to the Hmax wave (PtH) and the Mmax compound action potential (PtM). For the twitch produced at Mmax the following were also determined: (2) the twitch contraction time (CT), the time to maximal twitch tension; (3) the twitch half-relaxation time (HRT), the time to recover half of the maximal twitch tension; and consequently (4) the mean rate of twitch tension development (Pt/CT).
As previously mentioned, the Hmax wave frequently occurs with a sub-maximal M response (MatHmax, Fig. 1). A procedure to determine the relative contribution of the Hmax and MatHmax waves to the compound electrically evoked twitch torque, in a population of young healthy subjects, has been described in a previous study carried out in our laboratory (Maffiuletti et al. 2000). These authors postulated that different MUs are associated with each wave and that the mechanical response is the result of total EMG activity. Thus plotting the amplitude of the twitches evoked by stimulus intensities < Hfree against the amplitude of the corresponding potentials (see examples in Fig. 2) may allow us to determine the mechanical response strictly related to the MUs reflexively activated. For each subject, the regression which was found to best fit the whole range of torque values was linear and the equation was used to estimate the twitch torque values not contaminated by the sub-maximal M wave. The twitch torque thus obtained was then subtracted from the total twitch torque produced by the H and M waves. The resulting twitch values were plotted against the corresponding M waves (see examples in Fig. 2), for the range of stimulus intensities from H > Hfree to M = Mmax. Even in this case, a linear correlation was observed. Starting from the relationships between the twitch torque and the H reflex or the M wave amplitude, an equation has been proposed for estimating for each subject the relative contribution to the Pt of the units activated by the H wave:
where PtH is the total twitch torque evoked at the Hmax intensity of stimulation and SR is the mean ratio of the slopes of the H and the M regressions, namely the ratio between the amplitude of the twitches selectively evoked by equal H and M wave amplitudes.
Figure 1. Hoffmann (H) reflex and sub-maximal M wave (MatHmax): electrical and mechanical responses recorded in one young and one elderly subject.
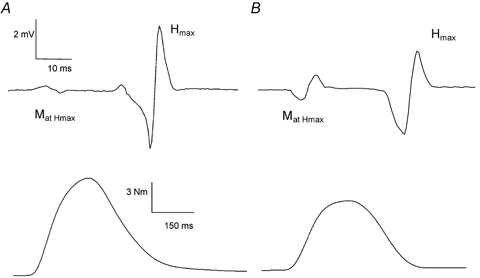
Typical traces of the electrical (at the top) and mechanical responses (at the bottom) recorded in a young subject (A) and an elderly subject (B) in response to maximal reflex stimulation of the posterior tibial nerve. The myoelectrical signal from the soleus muscle shows the maximal reflex response (Hmax) and the concomitantly evoked sub-maximal M wave (MatHmax). The higher MatHmax shown by the elderly subject indicates the substantial contribution of directly activated motor units to the total twitch torque (PtH) produced by the motor units activated at Hmax stimulus intensity.
Figure 2. Relationship between the twitch torque and the H-reflex or the M-wave amplitude in one young and one elderly subject.
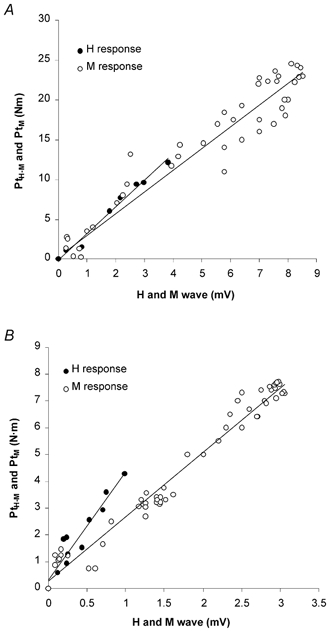
•, peak twitch torque (PtH–M), evoked by stimulus intensities equal to or lower than those evoking the Hfree wave (i.e. H responses not preceded by any sub-maximal M wave), plotted against the amplitude of the corresponding reflex electrical signals (H wave). ○, peak twitch (PtM), obtained for stimulus intensities exceeding those evoking the Hfree potential, plotted against the amplitude of the related direct motor potential (M wave). A shows the results obtained from one young subject. The equations of the linear regressions are: y = 2.72x+ 0.29 (r = 0.96) and y = 3.30x− 0.10 (r = 0.99) for the H reflex and the M response, respectively. B illustrates the data from one older subject. The equations of the linear regressions are: y = 2.40x+ 0.28 (r = 0.94) and y = 4.03x+ 0.33 (r = 0.95) for the H reflex and the M response, respectively. The regression lines indicate a significant association between changes in the amplitude of the electrical and mechanical signals (P < 0.01).
To validate the equation for the elderly population, the average SR value was calculated using the regression equations for those elderly subjects in which Hfree responses could be evoked.
Statistics
The critical level for statistical significance was set at 5 %. The data are presented as means ±s.d. Age-related differences for all measurements were analysed with Student's unpaired t test. In cases where the data did not meet the criteria of normality (Shapiro-Wilks' W test, P < 0.05) a non-parametric Mann-Whitney rank-sum test was applied. Linear regression analysis (Pearson product-moment correlation) was used to compare the degree of association between variables. The one-sample Student's t test was applied to assess whether the PtH–M/Hmax and PtM/Mmax ratios were significantly different from ratio values equal to 1.
RESULTS
Mmax wave: amplitude, duration, latency and twitch contractile properties
Characteristics of the soleus compound action potential and the related plantar flexor twitch contractile properties are presented in Table 1. As can be observed, the amplitude of the Mmax wave was significantly lower in the older group (−57.3 %, P < 0.001), while the peak-to-peak duration was 41.2 % (P < 0.01) longer in the elderly than in the younger population. The ΔtM/height, measured to detect possible changes in the signal conduction velocity through the direct motor pathway, was similar in both groups.
Table 1.
Amplitude, duration, latency (Δtm/height) of soleus compound actionpotential(Mmax) and plantar-flexor peak twitch torque(Ptm),contraction time (CT),half-relaxation time (HRT) and mean rate of tension development (Pt/CT)
| Young | Elderly | P | |
|---|---|---|---|
| Mmax amplitude (mV) | 9.29 ± 1.49 | 3.97 ± 1.27*** | 0.001 |
| Mmax duration (ms) | 2.57 ± 0.46 | 3.63 ± 0.86** | 0.010 |
| Δtm/height (ms cm−1) | 0.038 ± 0.016 | 0.039 ± 0.005 | 0.450 |
| PtM (N m) | 17.66 ± 5.08 | 9.08 ± 1.87*** | 0.001 |
| CT (ms) | 139.33 ± 17.32 | 145.00 ± 15.09 | 0.242 |
| HRT (ms) | 109.96 ± 18.35 | 109.47 ± 18.25 | 0.478 |
| Pt/CT (N m ms−1) | 0.127 ± 0.034 | 0.063 ± 0.014*** | 0.001 |
Data are means ±s.d.; n for young = 10, n for elderly = 9.
P < 0.01
P < 0.001, young vs. elderly.
Likewise for the EMG recording, the peak twitch amplitude produced by the Mmax wave was significantly depressed in the senior group compared to the younger population (−48.6 %, P < 0.001), whereas no significant differences in CT and HRT were observed. Taken together, the peak twitch torque and the contraction time account for the lower Pt/CT value in the older adults (−50.4 %, P < 0.001).
Hmax wave: amplitude, duration and latency
When the peak-to-peak amplitude of the soleus Hmax wave for the elderly subjects was compared with that of the younger adults the mean difference was 67.6 % (P < 0.001, Table 2). However, no statistical differences in the Hmax peak-to-peak duration were observed between young and elderly individuals. The H wave latency time (ΔtH/height), measured to detect possible changes in the signal conduction velocity through the reflex arc, proved to be significantly longer in the older than the younger group (+20.0 %, P < 0.001).
Table 2.
Amplitude, duration and latency(ΔtH/height) of soleus reflex response (Hmax) and amplitude of sub-maximal M wave (MatHmax), and plantar flexor peak twitch torque (PtH) and estimated peak twitch torque not contaminated by the M wave mechanical contribution (ptH-M)
| Young | Elderly | P | |
|---|---|---|---|
| Hmax amplitude (mV) | 5.37 ± 1.47 | 1.74 ± 1.15*** | 0.001 |
| Hmax duration (ms) | 2.93 ± 0.45 | 3.42 ± 0.82 | 0.093 |
| ΔtH/height (ms cm−1) | 0.176 ± 0.007 | 0.211 ± 0.014*** | 0.001 |
| MatHmax(%Mmax) | 4.89 ± 3.28 | 28.57 ± 19.66** | 0.002 |
| ptH(N m) | 12.21 ± 3.96 | 6.81 ± 1.85** | 0.002 |
| ptH-M(Nm) | 11.42 ± 3.61 | 4.62 ± 2.07***§ | 0.001 |
Data are means ±s.d.; n for young = 10, n for elderly = 9. *P < 0.05
P < 0.01
P < 0.001 young vs. elderly
P < 0.05 Pthvs.pth-m.
It is likely that the reflex-electrical and -contractile characteristics are essentially the result of the depolarisation of the reflex loop. However, the twitch related to Hmax was usually associated with an M response (Fig. 1). The amplitude of the MatHmax was significantly different between groups (P < 0.01), the average value for the young individuals being 4.9 % of Mmax and for the older subjects it was 28.6 %. These results indicate that the influence of the antidromic volley on the H response and its contribution to Pt at Hmax is not negligible, especially with increasing age. Hence, particular care should be taken when analysing both the Hmax EMG and its associated mechanical properties, and thus analysis of parameters concerning the kinetics of the twitch, such as the time course of the twitch associated with Hmax, which cannot be corrected for direct contamination, is precluded. Results regarding the rectified amplitudes of reflexively evoked twitch torque are presented below.
Estimation of the relative Hmax twitch torque value (PtH–M)
The regression lines that were found to best fit the whole range of torque values associated with the H and M waves and the amplitude of the corresponding potentials were linear (see examples in Fig. 2) for older (H regression 0.82 < r < 0.94, P < 0.01 vs. M regression 0.80 < r < 0.98, P < 0.001) and young (H regression 0.92 < r < 0.99, P < 0.001 vs. M regression 0.96 < r < 0.99, P < 0.001) subjects. For the elderly population, the average slope of the H and M regressions was determined in six out of nine subjects, since the other three individuals did not present Hfree responses. The mean slope of the H regressions proved to be significantly higher in the elderly group (+66.0 %, P < 0.05), suggesting that the reflex activation generates a greater mechanical response in comparison with the younger population (Fig. 3). On the other hand, the average slope of the M regressions did not appear to be significantly different between groups.
Figure 3. Slope of the linear regression for the H reflex and the M response in young and elderly subjects.
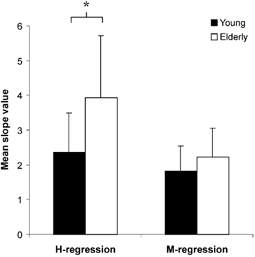
The amplitude of the histograms represents the slope of the linear regressions between the electrical and mechanical H signals (H regression), and the electrical and mechanical M responses (M regression). The H regression slope for the elderly group (□) was determined on 6 out of 9 subjects, since it was not possible to evoke Hfree responses on the remaining 3 older adults. The value thus obtained (3.9 ± 1.8) was significantly higher (*P < 0.05) than the value shown by the 10 younger subjects (2.4 ± 1.1, ▪). No statistical difference between groups was observed for the M regression slope value (young 1.8 ± 0.7 vs. elderly 2.2 ± 0.8). Data are presented as means ±s.d.
The individual fitting also allowed calculation of the SR coefficient of the formula for estimation of PtH–M (see Methods) for each subject. The value of 0.8 ± 0.1, found in the control group, may be considered typical for a population of normal young subjects, being in line with the results of Maffiuletti et al. (2000). The SR was significantly lower in the senior group than in the younger subjects (0.6 ± 0.2, P < 0.05). This result allowed us to ‘tailor’ the equation for a population of healthy elderly individuals. Hence it was possible to estimate the PtH–M of young and elderly subjects using the following equations:
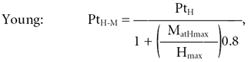 |
(1) |
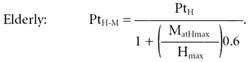 |
(2) |
It is worth noting that eqn (2) enables the determination of PtH–M in an older population as a whole, that is to say, even for those subjects for whom it was not possible to construct the relationship between PtH–M and the related H wave, due to the recurrent difficulty of evoking ‘free reflex responses’ in old age.
The estimation of PtH–M by: (i) equations (1) and (2) or (ii) direct extrapolation from the H regression did not show any significant difference within the two groups (Fig. 4). Hence, subsequently only the values determined using the equations were taken into consideration. The estimated peak twitch proved to be significantly lower in the older then in the young subjects (−59.5 %, P < 0.001; see also Table 2). The difference between the measured PtH (see values in Table 2) and the estimated PtH–M, expressed as a percentage of PtM, is an index of the relative mechanical contribution of MatHmax to PtH. This value was 23.5 ± 17.5 % for the elderly subjects and 4.3 ± 2.7 % for the young group, which is approximately in line with the relative amplitude of MatHmax, as confirmed by statistical analysis. It has also to be noted that the discrepancy between PtH and PtH–M reached statistical significance only in the older group (−32.0 ± 22.9 %, P < 0.05), while the younger population presented a non-statistical difference of 6.2 ± 3.8 %. This confirms, once more, the increasing mechanical influence of MatHmax with advancing age.
Figure 4. Evaluation of the relative mechanical contribution of the motor units activated by the H wave using the equation PtH–M = PtH/(1 + MatHmax/Hmax× SR) and by direct extrapolation.
Comparison of two methods of estimation of the twitch torque evoked by the maximal H wave (PtH–M). ○, individual data obtained from the equation: PtH–M= PtH/(1 + MatHmax/Hmax× SR); •, data extrapolated from the relationship between the mechanical response and the related electrical response evoked for stimulus intensities below or equal to those evoking the Hfree wave. ▵, values determined using the equations for the 10 young and 9 elderly subjects; ▴, values determined by extrapolation for the 9 young and the 6 elderly individuals for which was possible to evoke Hfree responses. The PtH–M values calculated with the equation were not statistically different from the PtH–M values determined by the extrapolation method, either in the young or in the elderly groups. *** Significant difference between populations, P < 0.001. Data are presented as means ±s.d.
Hmax/Mmax (EMG ratio) and PtH–M/PtM (mechanical ratio)
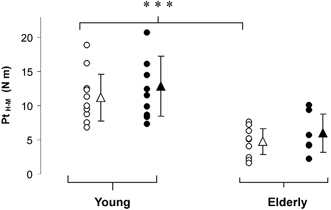
Although the average value of the Hmax/Mmax ratio did not statistically discriminate between the two populations, a 22.7 % difference was found between the young and the senior clusters (Fig. 5A). The absence of a significant gap might be related to the great inter-subject variability of the EMG ratio (see s.d. in Fig. 5A).
Figure 5. Electromyographic (EMG) ratio and mechanical ratio in young and elderly subjects.
A, the amplitude of the columns correspond to the values of the EMG ratio (Hmax/Mmax ratio) and the mechanical ratio (the ratio between the twitch torque associated with Hmax and the peak twitch associated with Mmax) for the young (n = 10, ▪) and elderly subjects (n = 9, □). No statistical differences were observed between the two groups and within ratios. B, the individual values of the mechanical ratio are plotted against EMG ratio measurements. •, data points for young individuals; ○, individual values for the elderly subjects. A linear correlation was found between the two variables; the equations are y = 0.64x+ 0.28 (r = 0.82, P < 0.01) and y = 0.94x+ 0.10 (r = 0.97, P < 0.001) for the young and the older groups, respectively. The best-fit line is indicated by the continuous line for young subjects and the dashed line for older individuals. The dotted line marks the line of identity and corresponds to the values which would be achieved when the twitches evoked by either wave were equal for equal wave amplitudes. Data are presented as means ±s.d.
PtH–M was further employed to define the ratio of the pure mechanical response associated with Hmax to that related to Mmax, i.e. the mechanical ratio. We expected that comparison of the two populations might enable detection of eventual differences in force production in response to reflex activation. However, despite a disparity of 20.2 % between young and elderly individuals, the difference was not statistically significant (Fig. 5A), indicating that the Hmax wave produced a mechanical contribution to the whole muscle twitch torque that was equivalent in both populations.
Given that the mechanical ratio is more difficult to obtain than the EMG ratio, it may be of interest to investigate whether both values change pari passu, thus providing similar outcomes. To this aim the individual PtH–M/PtM values were plotted against the Hmax/Mmax ratio. It was found that the best-fit regression was linear for both groups (r = 0.82, P < 0.01 for the young vs. r = 0.97, P < 0.001 for elderly subjects) but not coincident with the identity (represented with the dashed line in Fig. 5B). The identity is the value, which would be achieved if the twitches evoked by either wave were equal for equal wave amplitudes (Maffiuletti et al. 2001). The senior group in our study presented a regression line above the line of identity but parallel to it, suggesting that the Hmax/Mmax ratio is in a nearly constant relation with the mechanical ratio (slope 0.94). Therefore, it could be viewed as a possible index providing simultaneous information on the electrical and mechanical features. On the other hand, the slope of 0.63 observed for the control group did not enable us to draw the same conclusion for the younger population.
PtH–M/Hmax (reflex ratio) and PtM/Mmax (maximal ratio)
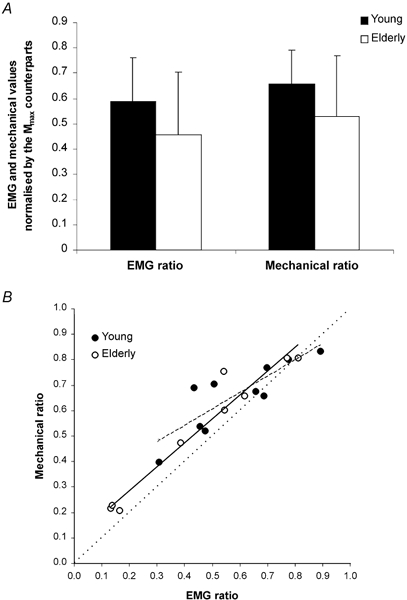
The ratio of the real mechanical contribution of Hmax to the Hmax potential (reflex ratio) is compared with the ratio for the total motor pool (maximal ratio). As depicted in Fig. 6A, there was no significant difference between the reflex and maximal ratios within groups. Although both ratios were higher in the older population, only the reflex ratio exhibited a significant difference in comparison with the younger group (+40.3 %, P < 0.05).
Figure 6. Ratio of PtH–M to the Hmax wave (reflex ratio) and ratio of PtM to the Mmax response (maximal ratio) in young and elderly subjects.
A, the histograms represent the average ratio of the mechanical and the EMG amplitudes, associated with Hmax (□) and Mmax (▪), in the young (n = 10) and in the elderly (n = 9) populations, respectively. While the reflex ratio appeared to be significantly higher in the elderly population (*P < 0.05), no statistical difference between groups was observed for the maximal ratio. B, the columns correspond to the quotient of the reflex and the maximal ratios in the young (n = 10) and the older (n = 9) populations. The dashed line represents the identity value (indicated by a dotted line in Fig. 5B) and corresponds to the case in which the twitches evoked by the Hmax and Mmax waves are identical. The mean quotient values of both populations are statistically different from the identity value: §P < 0.05. Data are presented as means ±s.d.
To effectively compare the reflex responses with respect to the whole muscle, the quotient of the reflex and maximal ratios was calculated for each subject (Fig. 6B). Despite the absence of statistically significant differences between groups, it is noteworthy that both populations presented a mean quotient significantly higher than the identity value (+16 %, P < 0.05 for the young vs.+26 %, P < 0.05 for the older subjects). This indicates a relatively higher mechanical efficiency during the reflex than direct muscle activation. Coincidence with the identity line would be expected if the Hmax/Mmax ratio was equal to 1, which would indicate recruitment of the whole motor pool by either wave type and suggest that MUs activated by both waves had a similar fibre type composition.
DISCUSSION
In the present study we describe the effect of ageing on the mechanical and electromyographic characteristics of the soleus MUs activated by maximal H reflex and direct motor responses. In order to investigate the impact of the neurogenic adaptations on the mechanical output evoked by the reflex and the direct efferent responses, and eventually make inferences regarding the characteristics of the age-remodelled MUs associated with both waves, we coupled the analysis of the EMG response with the twitch torque evoked by the soleus Hmax and Mmax in older and younger adults.
Mmax EMG and mechanical properties
In agreement with the literature on Mmax characteristics of lower limb muscles (Vandervoort & McComas, 1986; Vandervoort & Hayes, 1989; Cupido et al. 1992; Scaglioni et al. 2002), the present analysis has shown age-related changes in both the amplitude and duration of the muscle compound action potential. These modifications may, to some extent, be attributable to peripheral myopathic and neurogenic adaptations such as: (i) a slower electrical propagation related to impaired muscle membrane excitability and (ii) a desynchronisation of the soleus motoneurons, which is consistent with an increased duration of the muscle compound action potential, associated with a reduced peak-to-peak amplitude, as presently observed. However, no alteration of the direct motor latency (ΔtM/height) was noticed in the elderly group, attesting the apparent preservation of efferent functionality, namely the effectiveness of the neuromuscular junction and the efferent conduction speed. Therefore, the age-related adaptations responsible for a modified direct motor wave seem to be mainly present at muscle tissue level.
The plantar flexor contractile properties of the senior group in our study showed the typical traits of senile muscle — reduced twitch torque and slower contraction kinetics — in accordance with data from previous studies (Davies & White, 1983; Davies et al. 1986; Vandervoort & McComas, 1986; Klein et al. 1988; Vandervoort & Hayes, 1989; Winegard et al. 1997). Our analysis points to an absence of modification in CT and HRT with age, which can be accounted for by the greater difference in PtM observed between our two age groups (49 %) than was found in the above investigations (from 13 % (not significant) to 26 %). In other words, a simple scaling phenomenon seems to explain the age-related stability in the contractile time values fairly well. Vandervoort & McComas (1986) observed slower contraction kinetics in the plantar flexor than we did, but they also demonstrated that the CT and the HRT of the soleus muscle are not affected by age. In view of this, it has to be mentioned that the test position of the subjects might have affected these results. Since in the present experiments, the knee was placed at a right angle, the contribution of the gastronemii, which are bi-articulate, was considerably reduced (Cresswell et al. 1995). As a result, it seems likely that most of the mechanical response recorded in these experiments was generated by the soleus. Given that this muscle comprises mostly slow-twitch fibres, it is likely to be less affected by ageing, which would explain the lack of change in CT and HRT. This hypothesis seems to be further confirmed by the findings of Winegard et al. (1997), who, in subjects in a similar age group (60–80 years), with an equivalent ankle position, also observed a weaker plantar flexor Pt but no modification of CT and HRT. In our study, because of the consistent difference in peak twitch torque observed between the two age groups, the expected slower contraction kinetics in older subjects is clearly shown by the remarkable reduction in the mean rate of twitch tension development.
Taken together, the foregoing studies indicate a general slowing in the twitch contractile characteristics with ageing, even though this is less evident for the predominantly slow-twitch muscles. These findings suggest an age-related reorganisation of the motor unit pool, implying a selective loss of fast-twitch MUs (Coggan et al. 1992; Doherty et al. 1993; Lexell, 1995; Roos et al. 1997; Connelly et al. 1999; Wang et al. 1999; Nikolic et al. 2001; Vandervoort, 2002), an increase in the relative number of MUs with slow and transitional properties (Brown et al. 1988; Galea et al. 1991; Doherty et al. 1993; Luff, 1998) and a slowing of the contractile speed of the muscle fibres, regardless of their physiological type. Other factors that may be held responsible for the slowing down of the twitch kinetics with age could be related to alterations in the excitation-contraction coupling process (Klitgaard et al. 1989; Delbono et al. 1997), a possible decrease in tendon stiffness with ageing, as recently observed by Narici et al. (2003), and changes in the spread of excitation through the transverse tubular system, as attested by the longer duration of the Mmax compound action potential.
Hmax EMG and mechanical properties
The present results confirm the progressive age-related impairment of the neural system at the peripheral and spinal level. Various mechanisms, affecting interneurons (modified presynaptic inhibition; Morita et al. 1995), as well as both the afferent and efferent pathways, may be responsible for the age-related decrease in reflex responses. The significantly longer reflex latency exhibited by our older subjects allows us to put forward the hypothesis of neural structure remodelling, including drop-out of the largest fibres, and a segmental demyelinisation and remyelinisation process with a consequent reduction in internodal length (Jacobs & Love, 1985; Wang et al. 1999). Yet since the longer reflex latency (ΔtH/height) in older subjects was accompanied by an unchanged direct motor contractile time (ΔtM/height) it seems plausible that the alterations are essentially focused at the afferent level. These findings imply a modified conduction velocity of Ia afferents and/or a decreased synaptic transmission efficacy, as previously observed (Scaglioni et al. 2002). However, in view of the progressive slowing of motor conduction velocity with ageing, which has been frequently reported in the literature (Borg, 1981; Wang et al. 1999), the maintenance of the ΔtM/height in our study appears as an unexpected result. However, the size of the decrease generally reported is typically very small and seems preferentially to affect the fast-conducting nerve fibres (Wang et al. 1999). The M latency obviously gives only an indication of a global change in MU conduction velocity; therefore this measure is probably not sensitive enough to detect such small and localised alterations.
To our knowledge, Vandervoort & Hayes (1989) are the only authors, to date, to have addressed the problem of the contractile characteristics of the twitch torque associated with the Hmax response during senescence. In contrast to our results, Vandervoort & Hayes (1989) found no significant alteration in PtH with increasing age. This is quite surprising in view of the 57 % reduction in Hmax amplitude found in their study. It is likely that the discrepancy between the present and earlier finding may be ascribed to the mechanical influence of the MUs activated by MatHmax, which has not been taken into account in this previous investigation. This implies that the mechanical response associated with Hmax is the result of the torque produced by both the units reflexively recruited (i.e. slowest-twitch units) and those directly activated (i.e. fastest-twitch units). Hence, if it is accepted that direct mechanical contamination cannot be ignored, no particular conclusion can be drawn from the results of Vandervoort & Hayes (1989). However, the possible impact of subject sample differences should also be taken into consideration. The elderly group tested previously was composed of female subjects from geriatric care facilities, who were older (mean age 82 years; Vandervoort & Hayes, 1989) than the subjects in the present study, although they were nevertheless still capable of independently performing the activities involved in daily living and were free from diagnosed neurological disease.
As observed from Table 1 and Fig. 1 in this report, the influence of MatHmax appears to increase with increasing age. On the basis of this observation, it is speculated that the lower amplitude of the Hmax wave with senescence may be related to an increased homogeneity of the neural trunk (i.e. a possible decrease in the diameter of the more excitable Ia afferent fibres) and consequently to an increased similarity between the excitability thresholds of the afferent and efferent axons. In fact, Ochoa & Mair (1969) revealed an extensive fibre atrophy of the sural nerve (pure sensory nerve) with increasing age, and a more recent study by Rao & Krinke (1983) in aged lower mammals points to axonal shrinkage following a process of demyelinisation and remyelinisation of the dorsal spinal roots. Our findings of a higher MatHmax in the senior group, and the greater difference between groups in the Hmax than Mmax amplitudes, seem to corroborate the hypothesis of an enhanced neural homogeneity between sensory and motor axons with age. Moreover, the longer reflex latency in aged muscle, associated with a maintained direct motor contractile time, also bears out this assumption. Indeed, the good correlation between the conduction velocity and the fibre diameter with senescence, observed by Norris et al. (1953), supports the hypothesis that, in our study, the process of neurogenic degeneration preferentially affects the afferent pathway.
It may be asked why no reference has, so far, been made to the role of age-related modulation of tonic presynaptic inhibition (PI) of Ia afferent terminals in the reduction of the maximal reflex response and the occurrence of a greater MatHmax with ageing. The reason is that the literature contains conflicting results. Morita et al. (1995) found an increase in PI on Ia terminals with senescence, while recent and more direct measurements (Earles et al. 2001) revealed a lower level of PI at rest in the elderly subjects. In conclusion, it appears that the effect of this specific mechanism in modulating the reflex response still remains to be fully explored.
Estimation of the relative Hmax twitch torque value
The present investigation showed that the presence of a contaminant sub-maximal M wave preceding Hmax led to overestimation of the peak twitch value evoked by the Hmax wave, and it proved that MatHmax, expressed as a percentage of Mmax, was significantly greater in the older group. This suggests that the influence on mechanical properties of PtH by directly activated MUs is more pronounced with increasing age. In several investigations evidence that direct stimulation has a preferential effect on the larger motor units has been provided (see Enoka, 1988 for review). Previous studies have shown that the order of motor unit activation during electrically induced contraction depends on the diameter of the motor axon (Erlanger & Gasser, 1937). The effect of axon diameter is such that the largest axons have the lowest activation threshold on electrical stimulation (Eccles et al. 1958; Clamann et al. 1974). In a recent review, Pierrot-Deseilligny & Mazevet (2000) referred to the difference in motoneuron recruitment by the H and the M waves. Following the described sequence of orderly recruitment (Hugon, 1973; Schieppati, 1987), the small motor axons are depolarised first during H reflex stimulation, whereas electrical stimulation, which gives rise to the M wave, initially activates axons with a large diameter, innervating fast motor units. In the light of these observations, it seems plausible that MatHmax might be responsible for the depolarisation of the largest diameter axons of the motor pool examined in our study. For these reasons, we speculate that at Hmax stimulus intensities a greater percentage of the fastest surviving twitch units is activated by a direct volley in the aged plantar flexor muscles. This seems to be an advantage in counteracting their selective impairment with increasing age. Therefore, when testing older individuals it is essential to deduce the mechanical contribution of the sub-maximal M wave. Equation (1) (see Methods and Maffiuletti et al. 2000 for references) was used to correct the M wave contamination of PtH in young individuals. However, given the significantly greater influence of MatHmax with senescence, a second equation (eqn (2); see Methods) was fitted for the older population. A discrepancy between PtH and PtH–M was observed in both groups, but the difference was found to be statistically significant only in the older population. This suggests that the mechanical influence of MatHmax on reflex twitch torque may be negligible in young subjects, but not in an aged study group. The subtraction of this contribution has the effect of exacerbating the mechanical difference between the two groups, suggesting that reflex recruitment of MUs is even more difficult in aged muscle than has been thought hitherto.
EMG and mechanical ratios
Our findings point to a virtually unchanged efficiency of the reflex transmission between the Ia spindle afferent input and the soleus α-motoneuron pool with ageing. Indeed, no significant modification of Hmax/Mmax was found in the senior group tested. Vandervoort & Hayes (1989) and deVries et al. (1985) reported similar findings, suggesting that there is no effect of age on the proportion of reflexively recruited MUs, despite an expected decrease in the absolute number of units with ageing. Even though there was no significant difference in the EMG ratio between the two populations, a gap of 23 % between the young and elderly groups was found. The reason for the lack of a significant discrepancy may be, at least partially, ascribed to the great variability of the reflex response, which seems, as observed in previous studies (Sabbahi & Sedgwick, 1982; Falco et al. 1994; Scaglioni et al. 2002) and confirmed by the present results (Fig. 5), to increase with increasing age.
Earlier findings of a significant decline in the Hmax/Mmax ratio with ageing (Sabbahi & Sedgwick, 1982; Schmidt et al. 1982; Koceja et al. 1995; Scaglioni et al. 2002) support the hypothesis of a substantial age-related attenuation in the excitability of the spinal reflex pathway and/or in the modulation of the interneuronal activity mediating presynaptic inhibition. It is likely that the discrepancy between previous and present results may have arisen partially from methodological differences in test conditions (e.g. prone/supine position, with/without foot constraints) and stimulation procedures (e.g. different types of stimulation electrode: monopolar or bipolar electrodes with the anode proximal to the cathode). Differences in the training background of the populations tested may also have played a role. In fact, there is evidence that the excitability of the spinal reflex pathway may be modulated by physical activity (Perot et al. 1991; Maffiuletti et al. 2001). Although all the subjects selected for the present investigation were regarded as moderately active, it was obviously not possible to account for the impact of lifespan physical exercise on neural adaptations.
Given the assumption that slow MUs are more easily excited by the Ia afferent volley than fast-twitch ones (Buchthal & Schmalbruch, 1970; Calancie & Bawa, 1990; Zehr, 2002), the influence of the subjects' physical training genre on Hmax/Mmax supports the hypothesis that a different distribution of motor unit types would alter the efficiency of the Ia α-motoneuron reflex loop (Munson, 1990). It is now widely accepted that ageing is characterised by a significant loss of MUs, accompanied by remodelling of the surviving units (Doherty et al. 1993; Roos et al. 1997; Luff, 1998; Connelly et al. 1999; Vandervoort, 2002). This age-related reorganisation seems preferentially to involve type II fibres (Coggan et al. 1992; Doherty et al. 1993; Lexell, 1995; Wang et al. 1999; Nikolic et al. 2001) in a process of selective denervation and reinnervation by collateral sprouting of axons from fibres of slow MUs (Doherty et al. 1993; Carmeli & Reznick, 1994; Roos et al. 1997; Connelly et al. 1999; Vandervoort, 2002). Consequently, it seems plausible that the elderly subjects, as the endurance-trained athletes (Perot et al. 1991; Maffiuletti et al. 2001), exhibit a higher soleus Hmax/Mmax ratio. However, in the light of the present results, which point to a trend towards a decrease in the EMG ratio, it is apparent that the age-related rearrangement of the neural system, namely atrophy and loss of the largest neural fibres, associated with a demyelinisation and remyelinisation process (Lascelles & Thomas, 1966; Ochoa & Mair, 1969; Jennekens et al. 1971; Rao & Krinke, 1983), plays a primary role compared with the shift in muscle characteristics towards the slower contractile properties.
PtH–M/Hmax (reflex ratio) and PtM/Mmax (maximal ratio)
The older population showed a higher reflex ratio compared with the younger group (+40 %). This means that for equivalent EMG responses, the associated mechanical response was greater in older adults. The finding agrees with the assumption of a larger mechanical output in response to reflex activation with ageing, as was indicated by the steeper slope of the relationship between the twitch torque and the H wave amplitudes observed in the elderly population (Fig. 3). Comparison of the slopes shows an even greater discrepancy between the young and the elderly groups (+66 %). This may be due to the fact that the average slope for the older population was calculated for six out of nine subjects. The age-related higher ‘reflex mechanical response’ is in keeping with the assumption of an age-related peripheral reorganisation of the MUs, such that decreases in the number of MUs appear in conjunction with an increase in the number of muscle fibres innervated, on average, by each motoneuron (Doherty et al. 1993; Roos et al. 1997; Connelly et al. 1999; Wang et al. 1999). The higher innervation ratio may be viewed as a strategy of ‘strengthening’ each surviving unit adopted by the ageing neuromuscular system in an attempt to counterbalance the attenuation of the reflex excitability and the loss of MUs. Therefore, the combined effect of a higher reflex ratio and a maintained maximal ratio may be to preserve the entire motor pool efficiency during senescence, despite a reduction in the number of functioning MUs.
In conclusion, whereas in the young adult the peripheral reflex response appears to be related to the muscle phenotype, as indicated by the plasticity of the Hmax/Mmax ratio in response to training, in the elderly the attenuation of the EMG ratio is basically the consequence of neural adaptive rearrangements. This study has shown a trend towards a decrease in neural peripheral functionality with senescence related to a progressive increase in MatHmax. Nevertheless, the higher reflex ratio found in the older population probably reflects an increase in the MU innervation ratio due to ongoing neuromuscular remodelling. This phenomenon may be viewed as a compensatory/adaptive mechanism to preserve the mechanical efficiency of the surviving motor units, despite the impairment of the segmental reflex system. However, it appears that the higher mechanical efficiency of the reflexively activated MUs does not manage to counterbalance the age-related impairment of neural excitability and loss of MUs. In fact, it has been observed that older adults present a reduced reflex mechanical response (PtH–M) in comparison with their younger counterparts. From a functional point of view, the attenuated neural excitability associated with a weaker reflex twitch torque may be regarded as a possible cause of the reduced ability to maintain an upright posture during sudden balance perturbation with increasing age.
Acknowledgments
The authors are particularly grateful to the ‘S. Maugeri Foundation’ of Pavia (Italy), for allowing access to the CSAM Laboratory where the experiments were performed, and to Dr Paolo Capodaglio for the medical screening of the participants and his support to the study. Also, we are very much indebted to the senior citizens of Pavia who volunteered to take part in this study.
REFERENCES
- Angulo-Kinzler RM, Mynark RG, Koceja DM. Soleus H-reflex gain in elderly and young adults: modulation due to body position. J Gerontol A Biol Sci Med Sci. 1998;53:M120–M125. doi: 10.1093/gerona/53a.2.m120. [DOI] [PubMed] [Google Scholar]
- Borg J. Properties of single motor units of the extensor digitorum brevis in elderly humans. Muscle Nerve. 1981;4:429–434. doi: 10.1002/mus.880040513. [DOI] [PubMed] [Google Scholar]
- Brown WF, Strong MJ, Snow R. Methods for estimating numbers of motor units in biceps-brachialis muscles and losses of motor units with aging. Muscle Nerve. 1988;11:423–432. doi: 10.1002/mus.880110503. [DOI] [PubMed] [Google Scholar]
- Buchthal F, Rosenfalck P. Spontaneous electrical activity of human muscle. Electroencephalogr Clin Neurophysiol. 1966;20:321–336. doi: 10.1016/0013-4694(66)90001-0. [DOI] [PubMed] [Google Scholar]
- Buchthal F, Schmalbruch H. Contraction times of twitches evoked by H-reflexes. Acta Physiol Scand. 1970;80:378–382. doi: 10.1111/j.1748-1716.1970.tb04801.x. [DOI] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, McKeon B. Monosynaptic and oligosynaptic contributions to human ankle jerk and H-reflex. J Neurophysiol. 1984;52:435–448. doi: 10.1152/jn.1984.52.3.435. [DOI] [PubMed] [Google Scholar]
- Calancie B, Bawa P. Motor units recruitment in humans. In: Binder MD, Mendell LM, editors. The Segmental Motor Systems. Oxford, UK: Blackwell Science Inc; 1990. pp. 75–95. [Google Scholar]
- Carmeli E, Reznick AZ. The physiology and biochemistry of skeletal muscle atrophy as a function of age. Proc Soc Exp Biol Med. 1994;206:103–113. doi: 10.3181/00379727-206-43727. [DOI] [PubMed] [Google Scholar]
- Clamann HP, Gillies JD, Skinner RD, Henneman E. Quantitative measures of output of a motoneuron pool during monosynaptic reflexes. J Neurophysiol. 1974;37:1328–1337. doi: 10.1152/jn.1974.37.6.1328. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol A Biol Sci Med Sci. 1992;47:B71–B76. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. J Appl Physiol. 1999;87:843–852. doi: 10.1152/jappl.1999.87.2.843. [DOI] [PubMed] [Google Scholar]
- Cresswell AG, Loscher WN, Thorstensson A. Influence of gastrocnemius muscle length on triceps surae torque development and electromyographic activity in man. Exp Brain Res. 1995;105:283–290. doi: 10.1007/BF00240964. [DOI] [PubMed] [Google Scholar]
- Cupido CM, Hicks AL, Martin J. Neuromuscular fatigue during repetitive stimulation in elderly and young adults. Eur J Appl Physiol Occup Physiol. 1992;65:567–572. doi: 10.1007/BF00602367. [DOI] [PubMed] [Google Scholar]
- Davies CT, Thomas DO, White MJ. Mechanical properties of young and elderly human muscle. Acta Med Scand Suppl. 1986;711:219–226. doi: 10.1111/j.0954-6820.1986.tb08954.x. [DOI] [PubMed] [Google Scholar]
- Davies CT, White MJ. Contractile properties of elderly human triceps surae. Gerontology. 1983;29:19–25. doi: 10.1159/000213090. [DOI] [PubMed] [Google Scholar]
- Delbono O, Renganathan M, Messi ML. Excitation-Ca2+ release-contraction coupling in single aged human skeletal muscle fiber. Muscle Nerve Suppl. 1997;5:S88–S92. doi: 10.1002/(sici)1097-4598(1997)5+<88::aid-mus21>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- deVries HA, Wiswell RA, Romero GT, Heckathorne E. Changes with age in monosynaptic reflexes elicited by mechanical and electrical stimulation. Am J Phys Med. 1985;64:71–81. [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit: a brief review. Can J Appl Physiol. 1993;18:331–358. doi: 10.1139/h93-029. [DOI] [PubMed] [Google Scholar]
- Earles D, Vardaxis V, Koceja D. Regulation of motor output between young and elderly subjects. Clin Neurophysiol. 2001;112:1273–1279. doi: 10.1016/s1388-2457(01)00571-5. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Eccles RM, Lundberg A. The action potentials of the alpha motoneurons supplying fast and slow muscles. J Physiol. 1958;142:275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM. Muscle strength and its development. New perspectives. Sports Med. 1988;6:146–168. doi: 10.2165/00007256-198806030-00003. [DOI] [PubMed] [Google Scholar]
- Erlanger J, Gasser HS. Electrical Signs of Nervous Activity. Philadelphia: Blackwell Science Inc; 1937. [Google Scholar]
- Falco FJ, Hennessey WJ, Goldberg G, Braddom RL. H reflex latency in the healthy elderly. Muscle Nerve. 1994;17:161–167. doi: 10.1002/mus.880170205. [DOI] [PubMed] [Google Scholar]
- Galea V, de Bruin H, Cavasin R, McComas AJ. The numbers and relative sizes of motor units estimated by computer. Muscle Nerve. 1991;14:1123–1130. doi: 10.1002/mus.880141114. [DOI] [PubMed] [Google Scholar]
- Guiheneuc P, Bathien N. Two patterns of the results in polyneuropathies investigated with the H reflex. Correlation between proximal and distal conduction velocities. J Neurol Sci. 1976;30:83–94. doi: 10.1016/0022-510x(76)90257-4. [DOI] [PubMed] [Google Scholar]
- Hugon M. Methodology of the Hoffmann reflex in man. In: Desmedt JE, editor. New Developments in Electromyography and Clinical Neurophysiology. Vol. 3. Basle: Blackwell Science Inc; 1973. pp. 713–729. [Google Scholar]
- Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain. 1985;108:897–924. doi: 10.1093/brain/108.4.897. [DOI] [PubMed] [Google Scholar]
- Jennekens FG, Tomlinson BE, Walton JN. Histochemical aspects of five limb muscles in old age. An autopsy study. J Neurol Sci. 1971;14:259–276. doi: 10.1016/0022-510x(71)90216-4. [DOI] [PubMed] [Google Scholar]
- Klein C, Cunningham DA, Paterson DH, Taylor AW. Fatigue and recovery of contractiles properties of young and elderly men. Eur J Appl Physiol Occup Physiol. 1988;57:684–690. doi: 10.1007/BF01075989. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Ausoni S, Damiani E. Sarcoplasmic reticulum of human skeletal muscle: age-related changes and effect of training. Acta Physiol Scand. 1989;137:23–31. doi: 10.1111/j.1748-1716.1989.tb08717.x. [DOI] [PubMed] [Google Scholar]
- Koceja DM, Markus CA, Trimble MH. Postural modulation of the soleus H reflex in young and old subjects. Electroencephalogr Clin Neurophysiol. 1995;97:387–393. doi: 10.1016/0924-980x(95)00163-f. [DOI] [PubMed] [Google Scholar]
- Koceja DM, Mynark RG. Comparison of heteronymous monosynaptic Ia facilitation in young and elderly subjects in supine and standing positions. Int J Neurosci. 2000;103:1–17. doi: 10.3109/00207450009035005. [DOI] [PubMed] [Google Scholar]
- Lascelles RG, Thomas PK. Changes due to age in internodal length in the sural nerve in man. J Neurol Neurosurg Psychiatry. 1966;29:40–44. doi: 10.1136/jnnp.29.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann N Y Acad Sci. 1998;854:92–101. doi: 10.1111/j.1749-6632.1998.tb09895.x. [DOI] [PubMed] [Google Scholar]
- Maffiuletti AN, Martin A, Babault N, Pensini M, Lucas B, Schieppati M. Electrical and mechanical Hmax-to-Mmax ratio in power- and endurance-trained athletes. J Appl Physiol. 2001;90:3–9. doi: 10.1152/jappl.2001.90.1.3. [DOI] [PubMed] [Google Scholar]
- Maffiuletti NA, Martin A, Van Hoecke J, Schieppati M. The relative contribution to the plantar-flexor torque of the soleus motor units activated by the H reflex and M response in humans. Neurosci Lett. 2000;14(288):127–130. doi: 10.1016/s0304-3940(00)01212-x. [DOI] [PubMed] [Google Scholar]
- Morita H, Shindo M, Yanagawa S, Yoshida T, Momoi H, Yanagisawa N. Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Exp Brain Res. 1995;104:167–170. doi: 10.1007/BF00229867. [DOI] [PubMed] [Google Scholar]
- Munson JB. Synaptic inputs to type-identified motor units. In: Binder MD, Mendell LM, editors. The Segmental Motor System. Oxford, UK: Blackwell Science Inc; 1990. pp. 291–307. [Google Scholar]
- Narici MV, Maganaris CN, Reeves N. Muscle and tendon adaptations to ageing and spaceflight. J Gravitat Physiol. 2003;9:137–138. [PubMed] [Google Scholar]
- Nikolic M, Malnar-Dragojevic D, Bobinac D, Bajek S, Jerkovic R, Soic-Vranic T. Age-related skeletal muscle atrophy in humans: an immunohistochemical and morphometric study. Coll Antropol. 2001;25:545–553. [PubMed] [Google Scholar]
- Norris AH, Shock NW, Wagman IH. Age changes in maximum conduction velocity of motor fibers of human ulnar nerves. J Appl Physiol. 1953;5:589–593. doi: 10.1152/jappl.1953.5.10.589. [DOI] [PubMed] [Google Scholar]
- Ochoa J, Mair Wg. The normal sural nerve in man. II Changes in the axons and Schwann cells due to ageing. Acta Neuropathol (Berl) 1969;13:217–239. doi: 10.1007/BF00690643. [DOI] [PubMed] [Google Scholar]
- Perot C, Goubel F, Mora I. Quantification of T- and H-responses before and after a period of endurance training. Eur J Appl Physiol Occup Physiol. 1991;63:368–375. doi: 10.1007/BF00364464. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Mazevet D. The monosynaptic reflex: a tool to investigate motor control in humans. Neurophysiol Clin. 2000;30:67–80. doi: 10.1016/s0987-7053(00)00062-9. [DOI] [PubMed] [Google Scholar]
- Rao RS, Krinke G. Changes with age in the number and size of myelinated axons in the rat L4 dorsal spinal root. Acta Anat (Basel) 1983;117:187–192. doi: 10.1159/000145786. [DOI] [PubMed] [Google Scholar]
- Roos MR, Rice CL, Vandervoort AA. Age-related changes in motor unit function. Muscle Nerve. 1997;20:679–690. doi: 10.1002/(sici)1097-4598(199706)20:6<679::aid-mus4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Sabbahi MA, Sedgwick EM. Age-related changes in monosynaptic reflex excitability. J Gerontol A Biol Sci Med Sci. 1982;37:24–32. doi: 10.1093/geronj/37.1.24. [DOI] [PubMed] [Google Scholar]
- Scaglioni G, Ferri A, Minetti AE, Martin A, Van Hoecke J, Capodaglio P, Sartorio A, Narici MV. Plantar flexor activation capacity and H reflex in older adults: adaptations to strength training. J Appl Physiol. 2002;92:2292–2302. doi: 10.1152/japplphysiol.00367.2001. [DOI] [PubMed] [Google Scholar]
- Schieppati M. The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol. 1987;28:345–376. doi: 10.1016/0301-0082(87)90007-4. [DOI] [PubMed] [Google Scholar]
- Schmidt JM, Penin F, Cuny G, Brichet B, Weber M, Gehin P. Electrophysiologic study of Achilles areflexia of aged subjects. Rev Electroencephalogr Neurophysiol Clin. 1982;12:357–360. doi: 10.1016/s0370-4475(82)80026-9. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, Hayes KC. Plantarflexor muscle function in young and elderly women. Eur J Appl Physiol Occup Physiol. 1989;58:389–394. doi: 10.1007/BF00643514. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA, McComas AJ. Contractile changes in opposing muscles of human ankle joint with aging. J Appl Physiol. 1986;61:361–367. doi: 10.1152/jappl.1986.61.1.361. [DOI] [PubMed] [Google Scholar]
- Wang FC, de Pasqua V, Delwaide PJ. Age-related changes in fastest and slowest conducting axons of thenar motor units. Muscle Nerve. 1999;22:1022–1029. doi: 10.1002/(sici)1097-4598(199908)22:8<1022::aid-mus3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Winegard KJ, Hicks AL, Vandervoort AA. An evaluation of the length-tension relationship in elderly human plantarflexor muscles. J Gerontol A Biol Sci Med Sci. 1997;52:B337–B343. doi: 10.1093/gerona/52a.6.b337. [DOI] [PubMed] [Google Scholar]
- Zehr P. Considerations for use of the Hoffman reflex in exercise studies. Eur J Appl Physiol. 2002;86:455–468. doi: 10.1007/s00421-002-0577-5. [DOI] [PubMed] [Google Scholar]


