Abstract
Activation of the vestibular system changes ventilation in humans. The purpose of the present study was to investigate whether aging alters the vestibulorespiratory reflex in humans. Because aging attenuates the vestibulosympathetic reflex, it was hypothesized that aging would attenuate the vestibulorespiratory reflex. Changes in ventilation during engagement of the semicircular canals and/or the otolith organs were measured in fourteen young (26 ± 1 years) and twelve older subjects (66 ± 1 years). In young subjects, natural engagement of the semicircular canals and the otolith organs by head rotation increased breathing frequency during dynamic upright pitch at 0.25 Hz (15 cycles min−1) and 0.5 Hz (30 cycles min−1) (Δ2 ± 1 and Δ4 ± 1 breaths min−1, respectively; P < 0.05) and during dynamic upright roll (Δ2 ± 1 and Δ4 ± 1, respectively; P < 0.05). In older subjects, the only significant changes in breathing frequency occurred during dynamic pitch and roll at 0.5 Hz (Δ2 ± 1 and Δ2 ± 1 for pitch and roll, respectively). Stimulation of the horizontal semicircular canals by yaw rotation increased minute ventilation in young but not older subjects. Selective engagement of the otolith organs during static head-down rotation did not alter breathing frequency in either the young or older subjects. The results of this study indicate that the vestibulorespiratory reflex is attenuated in older humans, with greater vestibular stimulation needed to activate the reflex.
Stimulation of the vestibular system has been reported to elicit cardiovascular and respiratory changes. In humans, stimulation of the otolith organs and not the semicircular canals has been shown to increase muscle sympathetic nerve activity and cause increases in peripheral vascular resistance (Shortt & Ray, 1997; Ray & Hume, 1998; Ray et al. 1998; Monahan & Ray, 2002a). In contrast, it has been observed that stimulation of the semicircular canals and not the otolith organs increases breathing frequency and ventilation in healthy young subjects (Monahan et al. 2002). In support of this concept, Jauregui-Renaud et al. (2000, 2001) reported that stimulation of the semicircular canals increased breathing frequency in normal subjects but not in vestibular-deficient patients. Animal studies have also demonstrated that activation of the vestibular system alters nerve activity to the respiratory pump and upper airway muscles (Rossiter & Yates, 1996; Mori et al. 2001).
Aging has been reported to alter normal vestibular function (Furman & Redfern, 2001; Tian et al. 2002a, b). It has been demonstrated that during engagement of the otolith organs the vestibulosympathetic reflex is attenuated with aging (Ray & Monahan, 2002). Moreover, it has been reported that during orthostatic stress the vestibulosympathetic reflex remains attenuated in older subjects (Monahan & Ray, 2002b). However, to our knowledge the influence of aging upon the vestibulorespiratory reflex has not been investigated. Because of known changes in the vestibulosympathetic reflex, it was hypothesized that respiratory responses would be attenuated in older subjects during activation of the vestibulorespiratory reflex.
METHODS
Subjects
Fourteen healthy young volunteers (eight men and six women; age: 26 ± 1 (s.e.m.) years; height: 170 ± 2 cm; weight: 65 ± 2 kg) and 12 healthy older volunteers (six men and six women; age: 66 ± 1 years; height: 169 ± 3 cm; weight: 66 ± 3 kg) participated in this study. All subjects were non-smokers, non-obese, normotensive and not taking any medications that would influence the results of this study. Written consent was obtained from all subjects after they were first verbally familiarized with the testing procedure. Each subject also received a physical examination from a physician before participation. The experimental protocol was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board of the Pennsylvania State University College of Medicine.
Experimental design
To examine the influences of the otolith organs and the semicircular canals on ventilation, subjects performed different head movements to naturally stimulate these sensory organs. The order of the interventions for all subjects was randomized and counterbalanced. All protocols contained a 2 min baseline period followed by a 1 min intervention and a 2 min recovery period. To eliminate any effect of visual inputs the subjects performed all trials with their eyes closed. Additionally, all head movements were conducted by an investigator to minimize central ‘volitional’ influences. During dynamic head movements, a metronome was used to control head rotation rates by the investigator. The investigator, because of headphones, was the only one to hear the metronome. On each auditory signal the subject's head was passively moved from endpoint to endpoint of the range of motion for each intervention. Two rotation rates were used during the study 0.25 Hz (15 cycles min−1) and 0.5 Hz (30 cycles min−1).
Experimental protocols
Dynamic upright pitch and dynamic upright roll
These trials examined whether simultaneous activation of both the semicircular canals and otolith organs elicited any changes in ventilation. During dynamic upright pitch, subjects sat in a chair and their heads were passively rotated by an investigator in the vertical axis from the point of maximal neck extension to the point of maximal neck flexion (i.e. ‘yes’ head rotation). Dynamic upright roll was performed with the subjects seated and by moving their heads in the frontal plane from the point of maximal roll to the left (i.e. left ear to shoulder) to the point of maximal roll to the right (i.e. right ear to shoulder). Two separate trials were performed during upright pitch and upright roll, one at a rotation frequency of 0.25 Hz and the other at 0.5 Hz.
Dynamic pitch in the lateral decubitus position
This trial examined the effect of isolated activation of the vertical semicircular canals on ventilation. During this trial the subject was placed in the lateral decubitus position with the head held in line with the dorsal aspect of the spine. The subject's head was then passively rotated in the horizontal axis from the neck extended position to neck flexed position (‘yes’ motion). Each subject performed pitch in the lateral decubitus position at rotation frequencies of 0.25 Hz and 0.5 Hz.
Dynamic yaw and dynamic chair rotation
These trials examined whether isolated activation of the horizontal semicircular canals evoked any ventilatory changes. During dynamic yaw, the subject was seated and their head was passively rotated from the point of maximal yaw on the left to the point of maximal yaw on the right (‘no’ motion) at a frequency of 0.25 Hz. Dynamic chair rotation eliminated any influence of the neck afferents on ventilation and was performed by rotating the subjects while they were securely fastened to a swivel chair. During chair rotation the subject was rotated through a range of motion similar to that used during yaw at a frequency of 0.25 Hz.
Static head-down rotation
The purpose of static head-down rotation (HDR) was to determine whether isolated activation of the otolith organs altered ventilation. At baseline, the subject was positioned in the prone position with the neck extended and chin supported as previously described (Shortt & Ray, 1997). After the 2 min baseline period, the support was removed and the head was passively rotated to the point of maximal neck flexion.
Measurements
Changes in ventilation were measured breath-by-breath using a metabolic cart (Sensormedics, Yorba Linda, CA, USA) interfaced with a Macintosh computer using a MacLab analog-to-digital converter (8S, ADInstruments, Castle Hill, Australia). During the experiment each subject was outfitted with a mask that securely covered the mouth and nose and allowed for easy breathing while minimizing dead space. A pneumotachograph was secured to a two-way valve on the mask to measure inspiratory, expiratory and whole breath characteristics. Specific respiratory variables that were measured included inspiratory time (ms), expiratory time (ms), tidal volume (ml), breathing frequency (breaths min−1) and minute ventilation (l min−1).
Finger arterial blood pressure (Finapres, Louisville, CO, USA) was used to continuously monitor blood pressure and heart rate throughout the length of the protocol. The right hand was used for blood pressure recordings and was maintained at heart level during all trials. Blood pressure and heart rate data were collected on-line (MacLab 8S) throughout each trial. An electrogoniometer was used to measure range of motion during upright pitch and yaw rotation.
Data analysis
All respiratory values were analysed off-line. Baseline data were averaged over the 2 min collection period. Interventional responses were compared using a one-between (age) and one-within (intervention) repeated measures analysis of variance. A least square means test was used to determine if responses for each age group were different from baseline. Statistical significance was set at P < 0.05 and the data are presented as the mean ±s.e.m.
RESULTS
Responses to dynamic upright pitch
The results of these trials are presented in Table 1. In young subjects, upright pitch at both frequencies significantly increased breathing frequency (Fig. 1) and decreased expiratory time. Upright pitch at a frequency of 0.5 Hz significantly increased minute ventilation and significantly lowered tidal volume. In older subjects, there were no significant increases in minute ventilation and breathing frequency during movement at 0.25 Hz, but there were at 0.5 Hz (Fig. 1). Tidal volume and expiratory and inspiratory times did not significantly change in the older subjects at either frequency.
Table 1.
Changes in ventilatory and cardiovascular variables from baseline during dynamic upright pitch at 0.25 Hz and 0.5 Hz in young and older subjects
| Variable | 0.25 Hz | 0.5 Hz | ||||
|---|---|---|---|---|---|---|
| Young | Older | P value | Young | Older | P value | |
| (n = 13) | (n = 12) | (n = 13) | (n = 12) | |||
| ▵Inspiratory time (ms) | −245 ± 58* | −47 ± 62 | 0.259 | −113 ± 71 | −158 ± 113 | 0.848 |
| ▵Expiratory time (ms) | −238 ± 114* | −139 ± 87 | 0.161 | −564 ± 112* | −228 ± 124 | 0.056 |
| ▵Ventilation (1 min−1) | 0.6 ± 0.3 | 0.3 ± 0.5 | 0.663 | 1.1 ± 0.6* | 1.1 ± 0.4* | 0.881 |
| ▵Tidal volume (ml) | −23 ± 16 | −9 ± 35 | 0.697 | −39 ± 13* | 3 ± 30 | 0.198 |
| ▵HR (beats min−1) | 2± 1* | −2 ± 1* | 0.634 | 1 ± 1 | −3 ± 2 | 0.414 |
| ▵MAP (mmHg) | 1 ± 1 | −3 ± 1* | 0.274 | −3 ± 1* | −2 ± 2 | 0.549 |
Values are mean ±s.e.m. HR, heart rate; MAP, mean arterial pressure. P value represents the interaction term from the ANOVA (age x interaction).
P < 0.05 for change from baseline.
Figure 1.
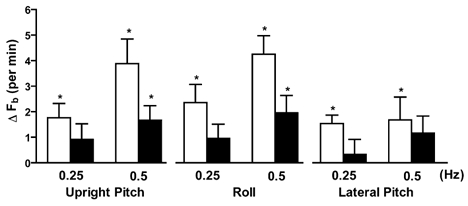
Changes in breathing frequency in the young and older subjects during dynamic upright pitch and roll and dynamic pitch in the lateral decubitus position (lateral pitch) at movement frequencies of 0.25 Hz and 0.5 Hz. *P < 0.05 for change from baseline.
Responses to dynamic upright roll
The results of these trials are presented in Table 2. In young subjects, breathing frequency increased significantly at both rates, but with significantly greater change occurring at 0.5 Hz than at 0.25 Hz (Fig. 1). Dynamic roll significantly increased minute ventilation at 0.5 Hz but not at 0.25 Hz. Tidal volume and inspiratory and expiratory times all significantly decreased at both rates. In older subjects, breathing frequency did not increase during head roll at 0.25 Hz, but did at 0.5 Hz (Fig. 1). In older subjects, dynamic roll at 0.5 Hz produced significant decreases in inspiratory and expiratory times.
Table 2.
Changes in ventilatory and cardiovascular variables from baseline during dynamic upright roll at 0.25 Hz and 0.5 Hz in the young and older subjects
| Variable | 0.25 Hz | 0.5 Hz | ||||
|---|---|---|---|---|---|---|
| Young | Older | P value | Young | Older | P value | |
| (n = 14) | (n = 12) | (n = 14) | (n = 12) | |||
| ▵Inspiratory time (ms) | −131 ± 44* | −28 ± 49 | 0.024 | −289 ± 90* | −159 ± 66* | 0.273 |
| ▵Expiratory time (ms) | −346 ± 58* | −48 ± 51 | 0.001 | −504 ± 79* | −209 ± 78* | 0.015 |
| ▵Ventilation (1 min−1) | 0.5 ± 0.4 | 0.3 ± 0.4 | 0.633 | 1.4 ± 0.6* | 0.7 ± 0.4* | 0.353 |
| ▵Tidal volume (ml) | −36 ± 13* | −7 ± 22* | 0.263 | −38 ± 15* | −40 ± 28 | 0.981 |
| ▵HR (beats min−1) | −2 ± 1 | −4 ± 1* | 0.201 | −1 ± 1 | −3 ± 1* | 0.157 |
| ▵MAP (mmHg) | −4 ± 2* | −3 ± 1* | 0.566 | −3 ± 1* | −4 ± 2* | 0.452 |
Values are mean ±s.e.m.P value represents the interaction term from the ANOVA (age x intervention).
P < 0.05 for change from baseline.
Responses to dynamic pitch in the lateral decubitus position
The results of these trials are presented in Table 3. In young subjects, breathing frequency significantly increased during movement at both frequencies (Fig. 1). Inspiratory time decreased during lateral pitch at 0.25 Hz, but not during 0.5 Hz. Pitch at 0.5 Hz significantly decreased expiratory time and tidal volume. In older subjects, the only significant ventilatory change was an increase in minute ventilation during lateral pitch at 0.5 Hz.
Table 3.
Changes in ventilatory and cardiovascular variables from baseline during pitch in the lateral decubitus position at 0.25 Hz and 0.5 Hz the young and older subjects
| Variable | 0.25 Hz | 0.5 Hz | ||||
|---|---|---|---|---|---|---|
| Young | Older | P value | Young | Older | P value | |
| (n = 13) | (n = 12) | (n = 13) | (n = 12) | |||
| ▵Inspiratory time (ms) | −193 ± 83* | −141 ± 120 | 0.028 | −120 ± 102 | −121 ± 79 | 0.997 |
| ▵Expiratory time (ms) | −215 ± 126 | 17 ± 15 | 0.241 | −264 ± 126* | −176 ± 122 | 0.623 |
| ▵Ventilation (1 min−1) | 0.6 ± 0.5 | 0.4 ± 0.4 | 0.723 | 0.6 ± 0.5 | 0.8 ± 0.4* | 0.723 |
| ▵Tidal volume (ml) | −30 ± 18 | 40 ± 51 | 0.182 | −11 ± 29* | 7 ± 23 | 0.915 |
| ▵HR (beats min−1) | −1 ± 2 | −2 ± 1* | 0.467 | 2 ± 1* | −1 ± 1 | 0.007 |
| ▵MAP (mmHg) | 2 ± 2 | −4 ± 1* | 0.589 | −2 ± 1 | −1 ± 2 | 0.435 |
Values are mean ±s.e.m.P value represents the interaction term from the ANOVA (age x intervention).
P < 0.05 for change from baseline.
Responses to dynamic yaw and dynamic chair rotation
The results of these trials are presented in Table 4. Breathing frequency was significantly greater during dynamic yaw in both the young (Δ3 ± 0 breaths min−1) and older subjects (Δ2 ± 1 breaths min−1), but only increased in the older subjects during chair rotation (Δ2 ± 0 breaths min−1). However, minute ventilation was significantly increased during both dynamic yaw (Δ1.6 ± 0.6 l min−1) and chair rotation (Δ1.1 + 0.4 l min−1) in the young, but did not change during either intervention in the older subjects. During dynamic yaw, inspiratory and expiratory times were significantly reduced in the young. Tidal volume was significantly decreased during chair rotation in the young but not older subjects.
Table 4.
Changes in ventilatory and cardiovascular variables from baseline during yaw and chair rotation in the young and older subjects
| Variable | Yaw | Chair | ||||
|---|---|---|---|---|---|---|
| Young | Older | P value | Young | Older | P value | |
| (n = 14) | (n = 12) | (n = 14) | (n = 12) | |||
| ▵Inspiratory time (ms) | −220 ± 74* | −125 ± 71 | 0.382 | −40 ± 62 | −22 ± 42 | 0.820 |
| ▵Expiratory time (ms) | −410 ± 76* | −252 ± 113* | 0.253 | −211 ± 143 | −100 ± 50 | 0.496 |
| ▵Ventilation (1 min−1) | 1.6 ± 0.6* | 0.4 ± 0.5 | 0.122 | 1.1 ± 0.4* | 0.4 ± 0.4 | 0.241 |
| ▵A Breathing frequency (breaths min−1) | 3 ± 0* | 2 ± 1* | 0.180 | 1 ± 1 | 2 ± 0* | 0.668 |
| ▵Tidal volume (ml) | 11 ± 30 | −37 ± 28 | 0.247 | 33 ± 15* | −30 ± 23 | 0.028 |
| ▵HR (beats min−1) | 1 ± 1 | −3 ± 1* | 0.354 | 1 ± 1 | 0 ± 0† | 0.322 |
| ▵MAP (mmHg) | −2 ± 1* | −2 ± 1 | 0.739 | 1 ± 1† | 1 ± 1 | 0.634 |
Values are mean ±s.e.m.P value represents the interaction term from the ANOVA (age x intervention).
P < 0.05 for change from baseline.
P < 0.05 significant difference in interaction term from ANOVA between yaw and chair rotation.
No significant differences were observed in measured ventilatory variables when yaw rotation was compared with chair rotation in both groups. This finding indicated that the horizontal semicircular canals were responsible for the observed changes during each respective intervention and not activation of the neck afferents.
Responses to static HDR
The results of these trials are presented in Table 5. All ventilatory variables were unchanged during HDR in the young subjects, whereas, minute ventilation decreased slightly from baseline in the older subjects. None of the observed changes in ventilation during HDR indicated an age interaction.
Table 5.
Changes in ventilatory and cardiovascular variables from baseline during head down rotation (HDR) in the young and older subjects
| Variable | HDR | ||
|---|---|---|---|
| Young | Older | P value | |
| (n = 14) | (n = 10) | ||
| ▵Inspiratory time (ms) | −25 ± 67 | 72 ± 91 | 0.386 |
| ▵Expiratory time (ms) | 2 ± 120 | 46 ± 12 | 0.789 |
| ▵Ventilation (1 min−1) | 0.2 ± 0.5 | −0.9 ± 0.3* | 0.102 |
| ▵A Breathing frequency (breaths min−1) | 1 ± 1 | 0 ± 1 | 0.394 |
| ▵Tidal volume (ml) | −8 ± 50 | 56 ± 27 | 0.137 |
| ▵HR (beats min−1) | 3 ± 1* | −3 ± 1* | 0.002 |
| ▵MAP (mmHg) | 0 ± 1 | −5 ± 2* | 0.027 |
Values are mean ±s.e.m.P value represents the interaction term from the ANOVA (age x intervention).
P < 0.05 for change from baseline.
Range of motion
Average range of motion during upright pitch in the young and older subjects was 135 ± 7 deg and 124 ± 4 deg, respectively (P = 0.18). During yaw rotation, the average range of motion was 153 ± 5 deg and 141 ± 6 deg for the young and older subjects, respectively (P = 0.14). These results indicate that possible differences in the range of motion between the two age groups were not responsible for altered ventilatory responses during head rotations. To further explore this issue, we examined a subset of four young and older subjects with comparable range of motion for pitch and yaw (128 deg and 155 deg for pitch and yaw, respectively). We observed similar results to that reported for the entire group. For example, breathing frequency was increased at both rotation frequencies (1.3 and 2.7 breaths min−1 for 0.25 and 0.5 Hz, respectively) for upright pitch in the young subjects but only changed at 0.5 Hz in the older subjects (1.6 breaths min−1).
DISCUSSION
The major finding of this study is that aging impairs vestibular-mediated activation of ventilation in humans. This finding is supported by the absence or attenuated increases in breathing frequency in the older subjects during dynamic upright pitch and roll and dynamic pitch in the lateral decubitus position as compared to the young. These results suggest that aging attenuates the vestibulorespiratory reflex in humans.
Significant increases in breathing frequency were observed in the young subjects during both dynamic upright pitch and roll during both stimulation frequencies, whereas, the only significant changes in breathing frequency in the older subjects during the same interventions occurred during the higher frequency of head movement. Moreover, the changes in breathing frequency during these trials were greater in the young subjects than in the older. Additionally, pitch in the lateral decubitus position produced significant changes in breathing frequency at both stimulation frequencies in the young, but not in the older subjects. These results indicate that to elicit vestibular-mediated increases in ventilation in older humans, greater vestibular stimulation is needed. Furthermore, these results clearly demonstrate that the ventilatory changes observed during vestibular activation in older humans are reduced relative to the young.
Unlike dynamic pitch and roll, isolated activation of the horizontal semicircular canals produced no age-related differences between the young and older subjects with respect to breathing frequency. However, minute ventilation was significantly increased in the young but not older subjects during both yaw and chair rotation. These findings provide additional evidence that aging alters vestibular-mediated ventilatory responses. Chair rotation, which stimulates only the semicircular canals, produced changes in ventilation that were not significantly different from yaw rotation, indicating that ventilatory changes were mediated by the vestibular system and not the neck afferents. These results are in agreement with the findings of our previous study (Monahan et al. 2002). In contrast with horizontal semicircular canal stimulation, isolated otolith engagement in both the young and older subjects did not alter breathing frequency. Because of our previous finding that otolithic stimulation did not increase breathing frequency in the young (Monahan et al. 2002), we did not expect that otolith engagement would alter breathing frequency in older humans. Collectively, these data indicate that the semicircular canals, but not the otolith organs or neck afferents, mediate increased ventilation and this supports the concept that vestibular activation alters respiration in humans.
During vestibular stimulation in animals, changes in ventilation, respiratory drive and respiratory pump muscle activity have been reported. Xu et al. (2002) found that during selective activation of the three subnuclei of the vestibular nucleus in the rat, ventilation was either inhibited or excited. These responses might be related to differential vestibular inputs that the three nuclei would receive from the vestibular organs. Electrical stimulation of the vestibular nerve has been shown to increase phrenic, abdominal and intercostal nerve activity in the cat (Yates et al. 1993; Miller et al. 1995). Mori et al. (2001) found that stimulation of the vestibular nerve can inhibit or excite motor neuron activity to the diaphragm and abdominal muscles in the cat. These cat studies, even though they did not measure ventilation directly, demonstrate that the vestibular system is able to elicit functional changes in respiratory muscle activity, which in turn could influence ventilation.
Several studies have demonstrated a decrease in age related function of vestibular-mediated reflexes. The vestibulo-ocular reflexes and the vestibulospinal reflexes have been reported to be impaired by age (Sloane et al. 1989; Hirvonen et al. 1997; Furman & Redfern, 2001; Tian et al. 2002b). Ray & Monahan (2002) found that when the otoliths are engaged, older subjects have an attenuation in muscle sympathetic nerve responses relative to young subjects. In relation to the current study these findings indicate that aging attenuates vestibular-mediated activation of the cardiorespiratory system. The mechanism(s) behind age-mediated impairment of these vestibular reflexes remains unclear. However, significant morphological changes in the vestibular organs occur with age (i.e. loss of hair cells). Rauch et al. (2001) studied 67 temporal bones in humans ranging in age from birth to 100 years and found a highly significant decrease in hair cells as age increased. Moreover, hair cell density has been reported to decline with age also (Merchant et al. 2000). Hair cells can also undergo morphological changes with aging such as cilia disarrangement, increased cilia fragility and development of giant cilia (Rosenhall & Rubin, 1975). Velazquez-Villasenor et al. (2000) studied 106 temporal bones and found a significant decrease in Scarpa's ganglion cells with age. Animals demonstrate similar changes with aging as microscopic examination of older rat vestibular epithelium has revealed a reduction in the number of cilia in the vestibular organs, as well as giant cilia (Nakayama et al. 1994), and the number of cells in the vestibular nucleus has been shown to decrease with age (Sturrock, 1989; Lopez et al. 1997). These changes observed with aging may be responsible for the attenuated vestibulorespiratory reflex in older humans.
Similar to the vestibular system, morphological changes have been observed in the auditory system of older humans. These changes include loss of hair cells in the cochlea (McGill & Schuknecht, 1976; Schuknecht & Gacek, 1993; Felder & Schrott-Fischer, 1995), the occurrence of giant cilia (Soucek et al. 1986) and degeneration of the cochlear nuclei (Arnesen, 1982). These morphological changes with aging are associated with hearing loss. Based on these findings along with our previous finding of an attenuation of the vestibulosympathetic reflex, it is not surprising that older humans have an attenuation of the vestibulorespiratory reflex.
Jauregui-Renaud et al. (2000, 2001), using caloric stimulation and vertical axis rotation, found that stimulation of the vestibular organs increased breathing frequency in normal healthy adults; however, in vestibular-deficient patients breathing frequency remained unchanged. The vestibular-deficient patients were defined by Jauregui-Renaud et al. as patients with reduced bilateral vestibular function as indicated by absent nystagmic responses to caloric stimulation and horizontal rotation in the dark. The vertical axis rotation used by Jauregui-Renaud et al. (2001) selectively examined the influence of semicircular canal activation in a manner similar to the yaw and chair rotation used in the present study. The lack of ventilatory drive during vestibular activation in the deficient patients demonstrates that the vestibular system contributes to the regulation of ventilation. Although aging does not impair the vestibulorespiratory reflex to the same degree as seen in these patients, the reduced ventilatory responses in the present study clearly suggest a decline in vestibular function.
The functional implications of an attenuated vestibulorespiratory reflex with aging are not clear. However, it is possible that the immediate increase in ventilation during non-stationary exercise and standing may be blunted. With respect to the latter, the failure to activate the respiratory muscles may hinder venous return to the heart and arterial blood pressure regulation upon standing.
There are two inherent limitations with the current study. First, the study is a cross-sectional and not a longitudinal investigation. Second, it cannot be ascertained for certain that neck afferents did not participate in the ventilatory responses during pitch rotation. However, the failure for ventilation to change during static head-down (pitch) rotation, which activates neck afferents, argues against this possibility.
Summary
The present study examined the effect of aging upon the vestibulorespiratory reflex in humans. Vestibular-mediated ventilatory changes in older humans were less than those observed in the young. Moreover, older humans require greater vestibular stimulation to produce ventilatory changes during vestibular engagement. Therefore, these findings indicate that the vestibulorespiratory reflex is attenuated with aging.
Acknowledgments
Grants from the National Heart Lung and Blood Institute (HL-58503), National Aeronautics and Space Administration (NAG 9-1034), National Space Biomedical Research Institute (NCC 9-58-168) and an Established Investigator Grant from the American Heart Association awarded to Dr Ray supported this project. Additional support was provided by a NIH sponsored General Clinical Research Center with a National Center for Research Resources Grant M01 RR10732.
References
- Arnesen AR. Presbyacusis - loss of neurons in the human cochlear nuclei. J Laryngol Otol. 1982;96:503–511. doi: 10.1017/s002221510009277x. [DOI] [PubMed] [Google Scholar]
- Felder E, Schrott-Fischer A. Quantitative evaluation of myelinated nerve fibres and hair cells in cochleae of humans with age-related high-tone hearing loss. Hear Res. 1995;91:19–32. doi: 10.1016/0378-5955(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Furman JM, Redfern MS. Effect of aging on the otolith-ocular reflex. J Vestib Res. 2001;11:91–103. [PubMed] [Google Scholar]
- Hirvonen TP, Aalto H, Pyykko I, Juhola M, Jantti P. Structural changes in vestibulo-ocular reflex of elderly people. Acta Otolaryngol Suppl. 1997;529:108–110. doi: 10.3109/00016489709124097. [DOI] [PubMed] [Google Scholar]
- Jauregui-Renaud K, Gresty MA, Reynolds R, Bronstein AM. Respiratory responses of normal and vestibular defective human subjects to rotation in the yaw and pitch planes. Neurosci Lett. 2001;298:17–20. doi: 10.1016/s0304-3940(00)01731-6. [DOI] [PubMed] [Google Scholar]
- Jauregui-Renaud K, Yarrow K, Oliver R, Gresty MA, Bronstein AM. Effects of caloric stimulation on respiratory frequency and heart rate and blood pressure variability. Brain Res Bull. 2000;53:17–23. doi: 10.1016/s0361-9230(00)00304-x. [DOI] [PubMed] [Google Scholar]
- Lopez I, Honrubia V, Baloh RW. Aging and the human vestibular nucleus. J Vestib Res. 1997;7:77–85. [PubMed] [Google Scholar]
- McGill TJ, Schuknecht HF. Human cochlear changes in noise induced hearing loss. Laryngoscope. 1976;86:1293–1302. doi: 10.1288/00005537-197609000-00001. [DOI] [PubMed] [Google Scholar]
- Merchant SN, Velazquez-Villasenor L, Tsuji K, Glynn RJ, Wall C III, Rauch SD. Temporal bone studies of the human peripheral vestibular system. Normative vestibular hair cell data. Ann Otol Rhinol Laryngol Suppl. 2000;181:3–13. doi: 10.1177/00034894001090s502. [DOI] [PubMed] [Google Scholar]
- Miller AD, Yamaguchi T, Siniaia MS, Yates BJ. Ventral respiratory group bulbospinal inspiratory neurons participate in vestibular-respiratory reflexes. J Neurophysiol. 1995;73:1303–1307. doi: 10.1152/jn.1995.73.3.1303. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Ray CA. Limb neurovascular control during altered otolithic input in humans. J Physiol. 2002a;538:303–308. doi: 10.1113/jphysiol.2001.013131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan KD, Ray CA. Vestibulosympathetic reflex during orthostatic challenge in aging humans. Am J Physiol Regul Integr Comp Physiol. 2002b;283:R1027–1032. doi: 10.1152/ajpregu.00298.2002. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Sharpe MK, Drury D, Ertl AC, Ray CA. Influence of vestibular activation on respiration in humans. Am J Physiol Regul Integr Comp Physiol. 2002;282:R689–694. doi: 10.1152/ajpregu.00568.2001. [DOI] [PubMed] [Google Scholar]
- Mori RL, Bergsman AE, Holmes MJ, Yates BJ. Role of the medial medullary reticular formation in relaying vestibular signals to the diaphragm and abdominal muscles. Brain Res. 2001;902:82–91. doi: 10.1016/s0006-8993(01)02370-8. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Helfert RH, Konrad HR, Caspary DM. Scanning electron microscopic evaluation of age-related changes in the rat vestibular epithelium. Otolaryngol Head Neck Surg. 1994;111:799–806. doi: 10.1177/019459989411100617. [DOI] [PubMed] [Google Scholar]
- Rauch SD, Velazquez-Villasenor L, Dimitri PS, Merchant SN. Decreasing hair cell counts in aging humans. Ann N Y Acad Sci. 2001;942:220–227. doi: 10.1111/j.1749-6632.2001.tb03748.x. [DOI] [PubMed] [Google Scholar]
- Ray CA, Hume KM. Neck afferents and muscle sympathetic activity in humans: implications for the vestibulosympathetic reflex. J Appl Physiol. 1998;84:450–453. doi: 10.1152/jappl.1998.84.2.450. [DOI] [PubMed] [Google Scholar]
- Ray CA, Hume KM, Steele SL. Sympathetic nerve activity during natural stimulation of horizontal semicircular canals in humans. Am J Physiol. 1998;275:R1274–1278. doi: 10.1152/ajpregu.1998.275.4.R1274. [DOI] [PubMed] [Google Scholar]
- Ray CA, Monahan KD. Aging attenuates the vestibulosympathetic reflex in humans. Circulation. 2002;105:956–961. doi: 10.1161/hc0802.104289. [DOI] [PubMed] [Google Scholar]
- Rosenhall U, Rubin W. Degenerative changes in the human vestibular sensory epithelia. Acta Otolaryngol. 1975;79:67–80. doi: 10.3109/00016487509124657. [DOI] [PubMed] [Google Scholar]
- Rossiter CD, Yates BJ. Vestibular influences on hypoglossal nerve activity in the cat. Neurosci Lett. 1996;211:25–28. doi: 10.1016/0304-3940(96)12710-5. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Shortt TL, Ray CA. Sympathetic and vascular responses to head-down neck flexion in humans. Am J Physiol. 1997;272:H1780–1784. doi: 10.1152/ajpheart.1997.272.4.H1780. [DOI] [PubMed] [Google Scholar]
- Sloane PD, Baloh RW, Honrubia V. The vestibular system in the elderly: clinical implications. Am J Otolaryngol. 1989;10:422–429. doi: 10.1016/0196-0709(89)90038-0. [DOI] [PubMed] [Google Scholar]
- Soucek S, Michaels L, Frohlich A. Evidence for hair cell degeneration as the primary lesion in hearing loss of the elderly. J Otolaryngol. 1986;15:175–183. [PubMed] [Google Scholar]
- Sturrock RR. Age related changes in neuron number in the mouse lateral vestibular nucleus. J Anat. 1989;166:227–232. [PMC free article] [PubMed] [Google Scholar]
- Tian JR, Crane BT, Wiest G, Demer JL. Effect of aging on the human initial interaural linear vestibulo-ocular reflex. Exp Brain Res. 2002a;145:142–149. doi: 10.1007/s00221-002-1111-z. [DOI] [PubMed] [Google Scholar]
- Tian JR, Crane BT, Wiest G, Demer JL. Impaired linear vestibulo-ocular reflex initiation and vestibular catch-up saccades in older persons. Ann N Y Acad Sci. 2002b;956:574–578. doi: 10.1111/j.1749-6632.2002.tb02886.x. [DOI] [PubMed] [Google Scholar]
- Velazquez-Villasenor L, Merchant SN, Tsuji K, Glynn RJ, Wall C, III, Rauch SD. Temporal bone studies of hte human peripheral vestibular system. Normative Scarpa's ganglion cell data. Ann Otol Rhinol Laryngol Suppl. 2000;181:14–19. doi: 10.1177/00034894001090s503. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhuang J, Zhou TR, Gibson T, Frazier DT. Activation of different vestibular subnuclei evokes differential respiratory and pressor responses in the rat. J Physiol. 2002;544:211–223. doi: 10.1113/jphysiol.2002.022368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates BJ, Jakus J, Miller AD. Vestibular effects on respiratory outflow in the decerebrate cat. Brain Res. 1993;629:209–217. doi: 10.1016/0006-8993(93)91322-j. [DOI] [PubMed] [Google Scholar]


