Abstract
The effects of body loading and unloading on human postural responses elicited by 1 mA bilateral, bipolar galvanic vestibular stimulation (GVS) were investigated. Subjects stood symmetrically, and in separate experiments were either loaded by 16, 33 and 50 % of their body weight with weights attached to the trunk, or unloaded by 10, 20 and 30 % using a whole-body harness that partially lifted the body but was free to translate horizontally. Randomised blocks of stimuli for each loading/unloading condition were compared to a non-loaded control condition. The rate of lateral reaction force development over the period 200–350 ms poststimulus increased in both legs with loading and decreased with unloading. The rate of force development was always larger from the leg on the side of cathodal stimulation. Vertical force responses were equal and opposite in the two legs, increasing on the side of the cathode and decreasing on the side of the anode. The rate of vertical force development over the period 200–350 ms after stimulus onset was increased with loading and decreased with unloading. In the frontal plane, the rate of head and trunk tilt in space was increased and decreased with loading and unloading, respectively. However, the relative rate of head tilt with respect to the trunk was not affected by loading conditions. These experiments provide further evidence that load-related afferent feedback influences the processing of vestibular information for the control of balance.
We have shown that asymmetrical standing alters postural control in healthy subjects (Marsden et al. 2002). In those experiments, postural control was measured by the response to bilateral galvanic vestibular stimulation (GVS). GVS modulates the firing rate of vestibular nerve afferents, causing an increase in firing rate on the side of the cathode and a decrease on the side of the anode (Lowenstein, 1955; Goldberg et al. 1982; Courjon et al. 1987), resulting in a sway towards the anode (Day et al. 1997). Standing asymmetrically resulted in a redistribution of the GVS-evoked reaction forces generated through each leg compared to symmetrical standing, with the amplitude of the initial force response varying with the load taken by that leg (Marsden et al. 2002). Thus, the response in the leg that was more loaded increased whereas the response in the less-loaded leg decreased. We therefore proposed that there is an interaction between load-related afferent information and the descending GVS-elicited signal and that the asymmetrical response seen might contribute to the postural control asymmetries seen following stroke (Brunt et al. 1995; Di Fabio, 1997; Garland et al. 1997; Kirker et al. 2000). However, as well as varying the degree of loading, asymmetrical standing was also associated with an alteration of posture as subjects leant slightly to one side. Control experiments suggested that the effect was not due to the associated head tilt or to changes in muscle activation, but it was not possible to rule out some other indirect influence of the asymmetrical posture. Therefore, in the present experiments we investigated the effect of loading and unloading the body on the response to GVS while subjects stood symmetrically. If a true loading effect exists, then we expected to see similar load-dependent changes in both legs simultaneously during symmetrical stance.
METHODS
The effects of loading and unloading on a subject's response to bilateral GVS were tested on two occasions. Twelve subjects participated in experiment 1 (mean ±s.d.; age 35.3 ± 14 years; body mass 74.7 ± 10.2 kg) and 12 subjects participated in experiment 2 (mean ±s.d.; age 33.2 ± 14 years; body mass 74.2 ± 13.0 kg), with 10 subjects participating in both experiments. All subjects were male with no known neurological disease. They participated with informed consent and the approval of the local ethics committee, according to the Declaration of Helsinki.
Four conditions were assessed in each experiment. In experiment 1 subjects were loaded by 16, 33 and 50 % of their body weight, whilst in experiment 2 subjects were unloaded by 10, 20 and 30 % of their body weight. The responses were compared each time to a control condition where the subject was not unloaded/loaded. Loading was achieved using weights attached to two belts that hung from the shoulders of the subject. The weights were equally distributed both left/right and front/back. The subjects wore a padded shoulder harness and the weights were attached securely using an elastic corset to ensure that they moved with the subject during each trial. During the control condition, foam pads of identical appearance to the weights and the elastic corset were applied to the subject. Unloading in experiment 2 was achieved using a whole-body harness that was also worn during the control condition. An overhead bar connected the harness via a rope to a pulley. Securing the rope to the overhead bar allowed unloading of varying degrees with the aid of feedback from the vertical forces applied to the force plate. The pulley system was in turn attached to an overhead gantry that allowed low-friction movement of the pulley in the horizontal plane. The subject was therefore free to move horizontally and was not restricted to pivoting about a fixed point above the head.
For each loading condition, four blocks of five stimuli were presented, with the order of blocks being randomised using a latin squares design. The polarity of GVS was also randomised on a trial-by-trial basis. A 1.0 mA GVS was applied bilaterally via 2.5 cm diameter electrodes (PALS plus, Nidd Valley Medical, Knaresborough, North Yorkshire, UK) placed over the mastoid processes.
The stimuli were applied as follows. Subjects stood with their head facing forwards and their feet parallel, 5 mm apart on separate force plates (Kistler 9281B - left leg and 9287 – right leg, Kistler Instrumente, CH-8408 Winterthur, Switzerland). A 50 ms warning tone initiated each trial. The subjects then distributed their weight such that each leg supported 50 % of their total load, with the aid of a display that gave feedback in 5 % steps of the vertical force applied to the right force plate. The maximum excursion of the display that indicated 100 % of the subject's total weight was recalibrated prior to every block.
Once the subjects had achieved equal weight distribution they pressed a hand-held switch that triggered occlusion of vision via opaque spectacles (PLATO visual occlusion spectacles, Translucent Technologies, Toronto, Ontario, Canada). Data collection commenced following a random 0.5–2 s delay, and after a 3 s baseline period a 3 s 1.0 mA GVS was given. Data collection ceased and vision was restored after a further 3 s poststimulus period. A 1–3 min minimum rest separated each block, with longer rests being provided in experiment 1.
Ground reaction force data were collected over the 9 s recording period from the two force plates. The force plates measured the vertical (z), lateral horizontal (x) and anteroposterior horizontal (y) forces acting on the body. Three-dimensional body motion was measured by a CODA mpx30 system (Charnwood Dynamics, Rothley, Leicestershire, UK). Trunk movements were recorded from markers attached to the skin at the L3 and C7 vertebral spinous processes. Hip movements were recorded via markers attached to a rigid horizontal bar that was fixed to the posterior aspect of a belt worn level with the superior iliac crest. Head movements were recorded from markers that were attached to a helmet that was worn by the subject. The force and kinematic data were sampled at 200 Hz and stored for analysis off-line.
Analysis
Head, trunk and pelvis tilts in space and relative segment tilt in the frontal and sagittal plane were calculated as indicated in Fig. 1A. Prior to averaging, responses in the lateral direction were multiplied by −1 when the anode was on the left side. Trials were then averaged for each subject under the same loading conditions and the two polarities of stimulation. The average prestimulus segment tilt in the sagittal and frontal planes was calculated over the 3 s baseline period. The initial rate of reaction force development from 200–350 ms poststimulus and rate of segment tilt from 200–500 ms poststimulus were measured from the slope of the trace calculated using a least squares error linear regression (Fig. 1B).
Figure 1. Definition of kinematic measures and responses seen in control conditions.
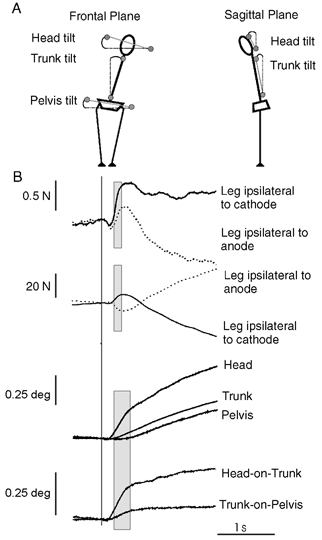
A, definition of the angles measured (circles indicate the position of the markers used). Subjects are shown schematically from behind in the frontal plane and from the left side in the sagittal plane. B, group mean control (no loading) response taken from experiment 2. From top to bottom: lateral reaction forces, vertical forces, frontal plane segment tilt and relative segment tilt. Reaction forces acting on the foot on the side of the stimulating anode or cathode are shown. Traces have been aligned to stimulus onset indicated by the vertical line. Shaded boxes indicate the regions over which linear regression was performed.
Rates of reaction force development, segment tilt and baseline measures were analysed using a repeated-measures general linear model (SPSS, version 10.0). The factor was loading/unloading (four levels), and an additional factor of polarity of stimulation (two levels) was included when assessing the data from each force plate. A Greenhouse-Geisser correction was used when necessary to deal with violations of sphericity (i.e. inequalities in the variance of the differences between factors; Greenhouse & Geisser, 1959). Unless indicated otherwise, data are presented as the mean ±s.d., and results were taken to be significant if P < 0.05.
RESULTS
The effect of loading and unloading on baseline measures prior to stimulation
Unloading and loading did not affect the degree of head, trunk or pelvis tilt in the frontal plane over the baseline period prior to stimulation. In the sagittal plane, the trunk angle increased on average by 3.8 ± 1.7 deg with 50 % loading and by 5.5 ± 2.8 deg with 30 % unloading (trunk tilt in space: effect of loading F(3,33) = 48.9, P < 0.001; effect of unloading F(3,33) = 39.2, P < 0.001). In contrast, the head tilted backwards relative to the trunk by an approximately equal and opposite amount such that the head tilt in space was unaffected by loading and unloading (head tilt in space: effect of loading F(3,33) = 1.4, P > 0.05; effect of unloading F(3,33) = 1.6, P > 0.05).
The average percentage of body weight for the three loading conditions (16, 33 and 50 %) in experiment 1 was 115.3 ± 1.8, 132.5 ± 2.2 and 148.4 ± 1.7 %, respectively (effect of loading F(2,22) = 2327, P < 0.001). In experiment 2, the percentage of body weight for the three unloading conditions (10, 20 and 30 %) was 89.2 ± 1.4 %, 81.5 ± 1.6 % and 73.9 ± 1.6 %, respectively (effect of unloading F(2,22) = 713, P < 0.001).
Effects of loading and unloading on GVS-evoked responses
Following GVS, subjects tilted towards the side of the anode. The frontal plane movement was produced by changes in both lateral and vertical reaction forces (Fig. 1). The lateral horizontal and vertical reaction force responses usually had three components. The first two components consisted of a small, short-lasting response, which peaked at around 150 ms, followed by an oppositely directed and larger response that peaked at approximately 500 ms. The third component started at around 700 ms and was characterised by a further reversal in the vertical forces from both legs. All subsequent analyses will focus on the second of these components from 200 to 350 ms after stimulus onset, since it was assumed to be responsible for the initial body sway response. Changes in anteroposterior forces were small (< 0.4 N) and there was no effect of loading or unloading on the early development of anteroposterior horizontal forces from 200 to 350 ms after stimulus onset (effect of load, experiment 1: F(3,33) = 1.4, P > 0.05; experiment 2: F(3,33) = 0.5, P > 0.05).
Lateral horizontal reaction forces
Bilateral lateral reaction forces developed in the direction of the anode with a latency of around 200 ms (Fig. 1B). The rate of lateral reaction force development at 200–350 ms was increased by loading (F(3,33) = 12.3, P < 0.001) and decreased by unloading (F(3,33) = 17.0, P < 0.001; Fig. 2A). In both experiments there was an effect of polarity, with a higher rate of force development being seen from the leg on the side of the cathode (effect of polarity, experiment 1: F(1,9) = 6.5, P < 0.05; experiment 2: F(1,8) = 10.0, P < 0.01; Fig. 2A).
Figure 2. Effects of loading and unloading on group mean ±s.e.m. galvanic vestibular stimulation (GVS)-evoked reaction forces and frontal plane segment tilt.
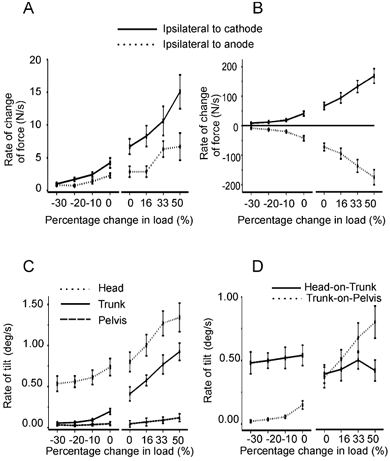
In all panels the effects of loading are shown on the right and the effects of unloading are shown on the left. A, change in the rate of lateral horizontal force development 200–350 ms after stimulus onset. B, change in the rate of vertical force development 200–350 ms after stimulus onset. C, change in the rate of segment tilt 250–500 ms after stimulus onset. D, change in the rate of relative head-on-trunk and trunk-on-pelvis tilt 250–500 ms after stimulus onset.
Vertical reaction forces
Equal and opposite changes in the vertical forces between the legs also contributed to the lateral sway. The main response peaked at ∼450 ms and consisted of an increase in vertical force from the leg on the side of the cathode and a decrease on the side of the anode (Fig. 1B). These oppositely directed changes in vertical forces in the two legs resulted in a significant effect of polarity in both experiments (effect of polarity, experiment 1: F(1,11) = 43.0, P < 0.001; experiment 2: F(1,11) = 45.2, P < 0.001; Fig. 2B). The rate of vertical force development at 200–350 ms was also affected by the degree of loading and unloading, as revealed by a significant loading × polarity interaction. The size of response was greater with greater loading (loading × polarity interaction, experiment 1: F(3,33) = 6.8, P < 0.001; Fig. 2B). In contrast, progressive unloading led to a diminution in response size (loading × polarity interaction, experiment 2: F(3,33) = 12.1, P < 0.002; Fig. 2B).
Segment tilt in the frontal plane
In keeping with the effect of loading and unloading on the net lateral and vertical reaction forces, there was a significant increase in the initial rate of head and trunk tilt in space with loading (experiment 1: head tilt F(3,33) = 10.5, P < 0.001; trunk tilt F(3,33) = 16.0, P < 0.001; Fig. 2C) and a decrease with unloading (experiment 2: head tilt F(3,33) = 3.9, P = 0.05; trunk tilt F(3,24) = 22.7, P < 0.001; Fig. 2C). There was no effect of loading or unloading on pelvis tilt (experiment 1: pelvis tilt F(3,33) = 1.0, P > 0.05; experiment 2: pelvis tilt F(3,33) = 1.1, P > 0.05; Fig. 2C).
As the tilt of the head in space is partly due to contributions from tilt of the pelvis and trunk, the relative tilts of the trunk-on-pelvis and the head-on-trunk were also measured. There was an effect of loading and unloading on the initial rate of trunk-on-pelvis tilt (experiment 1: F(3,33) = 10.6, P < 0.001; experiment 2: F(3,33) = 6.6, P < 0.05; Fig. 2D), which increased with greater load. However, there was no effect of either loading or unloading on the amplitude of head-on-trunk tilt (experiment 1: F(3,33) = 0.7, P > 0.05; experiment 2: F(3,33) = 0.7, P > 0.05; Fig. 2D).
In Fig. 2 it can be seen that the control condition (normal loading) did not yield identical response sizes in the two experiments. When the control responses from the 10 subjects who participated in both experiments were compared, the rate of trunk tilt in the frontal plane and the rate of vertical force development were significantly different (paired t test: rate of trunk tilt P < 0.05; rate of change of vertical force P < 0.01). The differences between the control conditions probably arise from the non-identical procedures used in the two experiments. Although the exact origin of this effect is unclear, it presumably remained constant within an experiment and so does not detract from the main result of the load sensitivity of vestibular-evoked responses.
DISCUSSION
The aim of the present experiments was to isolate the effect of load on the response to GVS, since in previous experiments changes in load co-varied with changes in posture (Marsden et al. 2002). There was no change in baseline posture in the frontal plane with loading or unloading, but in both situations the subjects' trunk tilted forwards in the sagittal plane. However, despite the similarity in the direction and magnitude of this tilt between loading and unloading conditions, there were differential effects on the reaction force response to GVS. Loading caused a bilateral increase, whilst unloading caused a bilateral decrease in lateral and vertical reaction force responses. Thus, it is unlikely that the small sagittal plane tilt of the trunk contributed to these changes in response to GVS.
Loading and unloading would also be accompanied by an increase and decrease, respectively, in baseline lower limb muscle activity. In theory, changes in the excitability of the motoneurone pool could result in modulation of the response to the descending GVS-elicited signal. However, we have demonstrated previously that matching lower-limb EMG levels during symmetrical stance to that seen in the more loaded leg during asymmetrical stance did not enhance either the reaction force response or the EMG response to GVS (Marsden et al. 2002).
We suggest that the changes in response size observed here are most likely to be due to alterations in load-related afferent feedback interacting with the processing of vestibular information. This supports the previous interpretation that the cause of response modulation when standing asymmetrically was not due to the accompanying alterations in posture or motorneuronal excitability, but rather was a direct effect of loading (Marsden et al. 2002). Other authors have shown that during gait or postural responses to platform perturbations, stretch-related soleus or gastrocnemius activity is modulated in a similar way by alterations in body loading (Berger et al. 1984; Dietz et al. 1989; Horstmann & Dietz, 1990). It remains to be determined which afferents contribute to the effect and where the interaction occurs.
The head and the trunk behaved differently to load changes. The rate of head tilt in the frontal plane with respect to the trunk remained constant across loading conditions, whereas the trunk tilt with respect to the pelvis changed with load. The load on the head was not altered in either experiment, but this was also the case for the trunk in experiment 2, in which only the legs were unloaded. In experiment 2 the progressive reduction in trunk tilt was unlikely to have been due to interference from the harness, since it lifted clear from the shoulders as the legs were unloaded, allowing even more freedom to tilt in the frontal plane. One explanation for the trunk-tilt modulation is that the responses of the lower limbs and the trunk are coupled such that a decrease in load-related information from the lower limbs interacts with the trunk control system. Thus the trunk and lower limbs may act as a functional unit, with the head being relatively independent.
In conclusion, we suggest that load-related afferent feedback from the lower limbs and/or pelvic region influences the processing of vestibular information for the control of balance. An increase in loading of 50 % and a decrease in loading of 30 % of a subject's weight can produce a threefold variation in the magnitude of lateral and vertical reaction forces and segment tilt. It may be possible to exploit such modulation during rehabilitation and locomotor retraining after stroke and incomplete spinal cord injury (Dietz et al. 1994; Chaudhuri & Aruin, 2000).
Acknowledgments
We would like to thank Mr R. Bedlington for technical assistance.
REFERENCES
- Berger W, Horstmann G, Dietz V. Tension development and muscle activation in the leg during gait in spastic hemiparesis: independence of muscle hypertonia and exaggerated stretch reflexes. J Neurol Neurosurg Psychiatry. 1984;47:1029–1033. doi: 10.1136/jnnp.47.9.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt D, Vander Linden DW, Behrman AL. The relation between limb loading and control parameters of gait initiation in persons with stroke. Arch Phys Med Rehabil. 1995;76:627–634. doi: 10.1016/s0003-9993(95)80631-8. [DOI] [PubMed] [Google Scholar]
- Chaudhuri S, Aruin AS. The effect of shoe lifts on static and dynamic postural control in individuals with hemiparesis. Arch Phys Med Rehabil. 2000;81:1498–1503. doi: 10.1053/apmr.2000.17827. [DOI] [PubMed] [Google Scholar]
- Courjon JH, Precht W, Sutherling WW. Vestibular nerve and nuclei unit responses and eye movement responses to repetitive galvanic vestibular stimulation of the labyrinth in the rat. Exp Brain Res. 1987;66:41–48. doi: 10.1007/BF00236200. [DOI] [PubMed] [Google Scholar]
- Day BL, Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol. 1997;500:661–672. doi: 10.1113/jphysiol.1997.sp022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Colombo G, Jensen L. Locomotor activity in spinal man. Lancet. 1994;344:1260–1263. doi: 10.1016/s0140-6736(94)90751-x. [DOI] [PubMed] [Google Scholar]
- Dietz V, Horstmann GA, Trippel M, Gollhofer A. Human postural reflexes and gravity - an under water simulation. Neurosci Lett. 1989;106:350–355. doi: 10.1016/0304-3940(89)90189-4. [DOI] [PubMed] [Google Scholar]
- DiFabio RP. Adaptation of postural stability following stroke. Top Stroke Rehabil. 1997;3:62–75. doi: 10.1080/10749357.1997.11781074. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Stevenson TJ, Ivanova T. Postural responses to unilateral arm perturbation in young, elderly and hemiplegic subjects. Arch Phys Med Rehabil. 1997;78:1072–1077. doi: 10.1016/s0003-9993(97)90130-1. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C, Smith CE. Responses of vestibular nerve afferents in the squirrel monkey to externally applied galvanic currents. Brain Res. 1982;252:156–160. doi: 10.1016/0006-8993(82)90990-8. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Horstmann GA, Dietz V. A basic posture control mechanism: the stabilization of the center of gravity. Electroencephalogr Clin Neurophysiol. 1990;76:165–176. doi: 10.1016/0013-4694(90)90214-5. [DOI] [PubMed] [Google Scholar]
- Kirker SGB, Jenner JR, Simpson DS, Wing A. Changing patterns of postural hip muscle activity during recovery from stroke. Clin Rehabil. 2000;14:618–626. doi: 10.1191/0269215500cr370oa. [DOI] [PubMed] [Google Scholar]
- Lowenstein O. The effect of galvanic polarization on the impulse discharge from sense endings in the isolated labyrinth of the thornback ray (Raja clavata) J Physiol. 1955;127:104–117. doi: 10.1113/jphysiol.1955.sp005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden JF, Castellote J, Day BL. Bipedal distribution of human vestibular-evoked postural responses during asymmetrical standing. J Physiol. 2002;542:323–331. doi: 10.1113/jphysiol.2002.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]


