Abstract
The regional organization of the ventral respiratory group (VRG) was examined with respect to generation of respiratory rhythm (breathing frequency) versus control of the respiratory motor pattern on individual nerves. In urethane-anaesthetized, neuromuscularly blocked and vagotomized Sprague-Dawley rats, arterial blood pressure (ABP) and respiratory motor outputs (phrenic, pharyngeal branch of the vagus, or superior laryngeal nerves) were recorded. The VRG organization was mapped systematically using injections of the excitatory amino acid dl-homocysteic acid (DLH; 5–20 mm, 2–6 nl) from single- or double-barrel pipettes at 100–200 μm intervals between the facial nucleus and the calamus scriptorius. Recording of respiratory neurons through the injection pipette ensured that the pipette was located within the VRG. At the end of each experiment, the injection pipette was used to make an electrical lesion, thereby marking the electrode position for subsequent histological reconstruction of injection sites. Four rostrocaudal regions were identified: (1) a rostral bradypnoea area, at the level of the Bötzinger complex, in which respiratory rhythm slowed and ABP increased, (2) a tachypnoea/dysrhythmia area, at the level of the preBötzinger complex, in which breathing rate either increased or became irregular, with little or no change in ABP, (3) a caudal bradypnoea area at the level of the anterior part of the rostral VRG in which ABP decreased and (4) a caudal ‘no effect’ region in the posterior part of the rostral VRG. The peak amplitude of phrenic nerve activity decreased with injections into all three rostral regions. Changes in respiratory rhythm were associated with opposite changes in inspiratory (TI) and expiratory (TE) durations after injections into either the Bötzinger complex or anterior rostral VRG, while both TI and TE decreased after injections into the preBötzinger complex. Effects on selected cranial nerves were similar to those on the phrenic nerve except that tonic activity was elicited on the superior larygneal nerve ipsilateral to injections in the Bötzinger complex and on the pharyngeal branch of the vagus ipsilateral to injections in the preBötzinger complex. These data reinforce the subdivision of the VRG into functionally distinct compartments and suggest that a further subdivision of the rostral VRG is warranted. They also suggest that region-specific influences, especially on the pattern of cranial motor discharge, can be used to assist the identification of recording sites within the VRG. However, the postulated clear functional separation of rhythm- versus pattern-generating regions was not supported.
The ventral respiratory group (VRG) is commonly proposed to contain the essential circuitry generating the basic respiratory rhythm as well as the varying augmenting and decrementing patterns of respiratory motor output evident on cranial and spinal nerves (Richter et al. 1992; Bianchi et al. 1995; Ramirez et al. 1998, 2002; Feldman & McCrimmon, 2003). The VRG consists of a long, heterogeneous column of cells in the ventrolateral medulla extending rostrally from the first cervical segment to the caudal end of the facial nucleus. It is typically divided into four rostrocaudally segregated compartments. Beginning rostrally and proceeding caudally, these are designated the Bötzinger complex (BotC), preBötzinger complex (preBotC), rostral VRG (rVRG) and caudal VRG (Feldman & McCrimmon, 2003). The minimal circuitry capable of generating a respiratory rhythm appears to be localized within the region referred to as the preBotC (Smith et al. 1991; Bianchi et al. 1995; Koshiya & Guyenet, 1996; Ramirez et al. 1998, 2002; Gray et al. 1999; McCrimmon et al. 2000b). Interference with neuronal activity within the preBotC either in vivo or in vitro abolishes respiratory rhythm (Smith et al. 1991; Funk et al. 1993; Pierrefiche et al. 1998; Ramirez et al. 1998). Selective disruption of neurokinin-1 receptor-positive neuron activity by bilateral injection into the preBotC of the ribosomal toxin saporin conjugated to substance P results in a markedly ataxic breathing pattern in unanaesthetized animals, accompanied by abnormal arterial PO2 and PCO2 (Gray et al. 2001). These studies argue strongly for a necessary role of the preBotC in the generation of a normal breathing rhythm. Other in vitro studies have demonstrated that medullary slices including this region are sufficient for the generation of a basic respiratory rhythm (Smith et al. 1991; Funk et al. 1993; Johnson et al. 2001).
Heterogeneity in the neuronal organization of the VRG is supported by neuroanatomical data; for example, the preBotC region contains propriobulbar, but very few bulbospinal respiratory neurons (Ellenberger & Feldman, 1990; Dobbins & Feldman, 1994; Sun et al. 1998; Guyenet et al. 2002). The rostral and caudal VRG contain inspiratory and expiratory bulbospinal neurons, respectively, which provide excitatory drive to spinal motoneurons innervating inspiratory or expiratory muscles (Monteau & Hilaire, 1991; Feldman & McCrimmon, 2003). The BotC provides inhibitory inputs to spinal respiratory motoneurons (Tian et al. 1998) as well as to medullary respiratory neurons (Ezure & Manabe, 1988; Schreihofer et al. 1999). A modular organization of the VRG is also suggested by the finding that in cats and rats, chemical activation of neurons within the subregions of the VRG elicit differing excitatory or inhibitory respiratory responses depending on the specific injection site (McCrimmon et al. 1986, 1987; Tolentino-Silva et al. 1997; Chitravanshi & Sapru, 1999; Solomon et al. 1999; Solomon, 2002; Wang et al. 2002). The goal of the current study was to test the hypothesis that neurons in separate VRG regions differ with respect to their roles in generating respiratory rhythm versus pattern.
The VRG rostral to the calamus scriptorius was identified by extracellular recording of phasic respiratory activity and then systematically mapped in all three cardinal planes using injections of a short-acting excitatory amino acid (dl-homocysteic acid; DLH) while monitoring phrenic and, in a subset of experiments, cranial motor output. We postulated that activation of small VRG subregions with specialized roles either in the generation of respiratory rhythm (i.e. breathing frequency) or motor pattern (e.g. tidal volume or augmenting versus decrementing discharge patterns on motor nerves) would result in differential effects on rhythm versus pattern, as measured on cranial and motor nerves controlled by the VRG.
METHODS
All animals were handled in accordance with National Institutes of Health and Northwestern University Animal Care and Use Committee guidelines. Experiments were performed on 49 adult male Sprague-Dawley rats (vendor, Charles River) weighing 300–500 g. Anaesthesia was induced with isoflurane and maintained with 20 % urethane in saline (1.5 g kg−1, half of which was administered i.m. and half i.v.). Adequacy of anaesthesia was assessed at least every 30 min by suspending DLH injections (see below) and noting any changes in heart rate, arterial blood pressure (ABP), or respiratory rate in response to a noxious paw pinch. When such responses occurred supplemental anaesthetic (0.15 g kg−1, i.v.) was administered immediately. A femoral vein and artery were catheterized for intravenous drug delivery and ABP monitoring, respectively. Rats were tracheotomized, bilaterally vagotomized in the neck and mechanically ventilated with O2 through a tracheal cannula (70–90 breaths min−1; tidal volume 2.5–3 ml). Animals were subject to neuromuscular block with 0.3 % d-tubocurarine (0.2 ml initial dose and additional doses given every hour, or as needed). End-tidal PCO2 was estimated (Hayashi et al. 1996) from a continuous measurement of expired PCO2 with an infrared analyser (Puritan-Bennett, Datex 223). End-tidal PCO2 was maintained between 34 and 40 mmHg for all of the animals and within a range of 2–3 mmHg for a given animal, by adjusting the frequency and tidal volume of the ventilator. A continuous venous infusion was administered (saline with 25 mm sodium bicarbonate, 1–8 ml h−1) to help maintain arterial pressure and acid-base balance (Quintin et al. 1989). Rectal temperature was monitored and maintained between 37.5 and 38.5 °C by means of a thermistor-controlled heating pad and lamp.
A phrenic nerve was dissected using a dorsal approach in all animals. In some animals, the pharyngeal branch of the vagus nerve was dissected via a ventral approach (n = 10) and/or a superior laryngeal nerve (n = 9) was dissected by either a dorsal or a ventral approach. All nerves were cut and mounted on bipolar silver wire electrodes and either placed in mineral oil or covered with petroleum jelly to prevent drying. Nerve activities were amplified, filtered (band pass 300 Hz-3 kHz), full-wave rectified, and integrated (Paynter filter; time constant 15 ms).
The rats were placed in a stereotaxic frame with the head ventroflexed such that the dorsal brainstem was approximately horizontal, and an occipital craniotomy was performed to expose the dorsal surface of the brainstem. The area postrema was exposed by removing the dura and arachnoid membranes.
Recording respiratory neurons and DLH injections
Glass micropipettes (1.2 mm o.d., 0.68 mm i.d.) for both drug delivery and recording were pulled and broken to yield a tip with an outside diameter of 3–4 μm. The micropipettes were filled with DLH (for approximately 85 % of injections, DLH was at 5 mm in saline; in initial experiments, DLH was at 20 mm; Sigma, St Louis, MO, USA). A silver wire was introduced into the pipette for recording neuronal activity. For pressure injection, polyethylene tubing was sealed over the pipette end and connected to a regulated, solenoid-valve-controlled pressure source. Injection volumes were monitored by measuring the movement of the fluid meniscus in the pipette using a compound microscope equipped with a fine reticule. Using a dissecting microscope, the tip of the electrode was positioned on the dorsal surface of the brainstem at the level of the calamus scriptorius. This point defined a relative zero for rostrocaudal and lateral displacements.
Experimental protocol
The average rostrocaudal length of the VRG between the calamus and the caudal end of the facial nucleus, measured from serial histological sections, was 2400 ± 73 μm (n = 6). The injection volume was generally 3 nl (range = 1–6 nl). Extracellular recording of unit activity was used to locate the ventral respiratory group in every rat (n = 49). A systematic mapping of the response elicited by DLH injection into the VRG was undertaken in 29 of these animals. An initial electrode penetration of the brainstem was made at stereotaxic coordinates appropriate for targeting a particular rostrocaudal level of the VRG. Unit activity was recorded as the electrode was advanced in increments of 20 μm. The location of respiratory neurons was noted. Once the full dorsoventral extent of VRG respiratory activity was recorded, the electrode was withdrawn in steps of intervals of 100–200 μm. At each interval, small (∼90 % of injections were 3 nl; range < 1–6 nl) injections of DLH were made beginning 500 μm ventral to the respiratory activity and continuing to about 400 μm dorsal to the respiratory population. This approach ensured that the DLH responsive region was completely incorporated within the region covered by the injection protocol. Following completion of a track, the electrode was removed from the brainstem, displaced 200 μm in either the mediolateral or rostrocaudal direction, and the process repeated. Movement in the mediolateral plane was continued until the entire mediolateral dimension of the VRG was mapped at a given rostrocaudal level. The medial and lateral extent of the VRG was indicated by making penetrations in which no respiratory activity was encountered. Nevertheless, to ensure that the limits of the DLH-responsive regions were obtained, DLH injections were made at these non-respiratory boundary sites (at depths at which respiratory activity had been recorded on previous tracks). Since significant responses were not attained by injections either immediately medial or lateral to the VRG, the mapping was not extended further. An average of 12 injections per track and seven tracks per rat were made. In the remaining 20 rats, the number of injections per animal was reduced by targeting the injections at specific DLH-responsive regions within the VRG. As in the earlier studies, the VRG was identified by recording respiratory neuronal activity through the injection pipette. In six rats, control injections of saline (the DLH vehicle) or other bioactive agents (strychnine, picrotoxin or substance P) were made at DLH-responsive sites using double-barrel pipettes. The effect on respiratory motor output and ABP and the position of each DLH injection relative to the calamus (stereotaxic zero), and the population of recorded respiratory neurons were noted on both a chart recorder and magnetic tape for subsequent analysis.
Data analysis
The response to DLH injection was analysed on the phrenic motor output in terms of both rhythm-related variables (i.e. inspiratory duration (TI), expiratory duration (TE), respiratory cycle duration (TTOT)) and a pattern-related variable, peak amplitude of integrated phrenic nerve discharge. An average of 84 DLH injections per rat were made in the mapping studies. Qualitatively similar cardiorespiratory responses elicited from adjacent injection sites were grouped as a single response pattern. Of these grouped responses, the largest-magnitude response was used to represent the response pattern of a region. The extent of a given region within an animal was defined by the distribution of contiguous injection sites from which a qualitatively similar response pattern was elicited. The ‘n’ in these studies refers to the number of animals in which each response pattern was seen, not the number of injections eliciting a given response pattern.
Once the representative response was determined within a given region, the magnitude and time course of the response on the phrenic nerve were determined. The time of occurrence and amplitude of each breath were recorded beginning 10 breaths prior to an injection and continuing for at least 40 breaths (until recovery to control values) following the injection in each rat.
To determine the average response for a given response pattern across animals, the phrenic bursts were numbered consecutively and each burst of a given number was averaged across all animals, both with respect to the time of occurrence and the amplitude of the breath. This gave rise to a variance in both time and amplitude, but the s.e.m. with respect to time was small and not discernable in plots of the response pattern (e.g. see Figs 2, 4, 5 and 8). The statistical significance of changes from the 10 pre-injection control breaths were determined at the peak response and for breaths at 5 s intervals following the injection.
Figure 2. dl-Homocysteic acid (DLH) injection into the most rostral region of the VRG (the Bötzinger complex, BotC) elicits a bradypnoea.
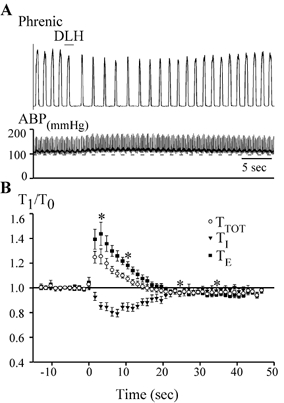
A, example of the bradypnoea obtained after 3 nl of 5 mm DLH injected into the VRG 500 μm caudal to the facial nucleus and 100 μm ventral to the dorsal aspect of the VRG. The upper trace shows integrated phrenic nerve activity and the bottom trace shows arterial blood pressure (ABP). The time of DLH injection is indicated by the bar. This injection elicited a bradypnoea and slight increase in ABP. B, the average inspiratory (TI), expiratory (TE) and total cycle (TTOT) times (n = 21) following DLH injection (3 nl, 5 mm) in the rostral bradypnoea area. Values are normalized to their control values (1 on the y-axis). DLH injection occurred at time = 0. *TTOT significantly different from control (determined only for measurements made at the peak effect and then at 5 s intervals from 10 to 35 s; P < 0.05).
Figure 4. DLH-induced tachypnoea.
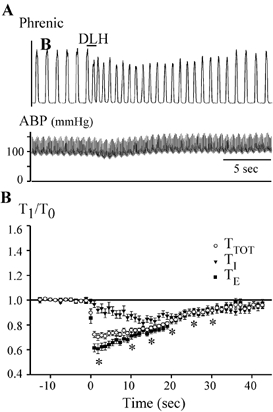
A, DLH injection (3 nl, 5 mm) administered at the bar, 300 μm ventral to the dorsal aspect of the VRG and 900 μm caudal to the facial nucleus. Top trace: integrated phrenic nerve activity. Bottom trace: ABP. Note the initial slight decrease in ABP, followed by a slight increase. B, average TI, TE and TTOT following DLH injection in the tachypnoea/dysrhythmia (preBötzinger complex, PreBotC) area (n = 28). TTOT, TE and TI are normalized to their control values in each animal. DLH injection occurred at time = 0. *TTOT significantly different from control (determined only for measurements made at the peak effect and then at 5 s intervals from 10 to 35 s; P < 0.05).
Figure 5. DLH-induced dysrhythmia.
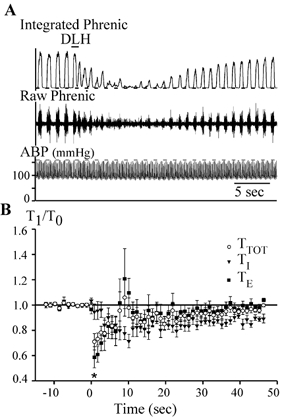
A, DLH injection (3 nl, 5 mm) injected at the bar, 300 μm ventral to the dorsal aspect of the VRG and 1 mm caudal to the facial nucleus (preBotC). Upper and middle traces: integrated and raw phrenic nerve activity, respectively. Bottom trace: ABP. Note the DLH-induced very irregular rhythm and pattern on the phrenic nerve. A slight tonic activation of the phrenic nerve is visible on both the raw and integrated traces. B, average TI, TE and TTOT following DLH injection in rats in which dysrhythmia was elicited (n = 13). TTOT, TE and TI are normalized to their control in each animal. DLH injection took place at time = 0. The error bars are much bigger than for the other evoked responses, showing the higher variability in the response for this specific dysrhythmia response. *TTOT significantly different from control (determined only for measurements made at the peak effect and then at 5 s intervals from 10 to 35 s; P < 0.05).
Figure 8. DLH-induced bradypnoea.
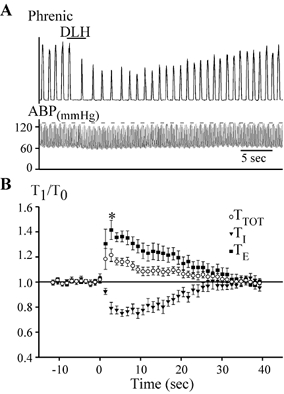
A, example of DLH (3 nl, 5 mm) injected 300 μm ventral to the dorsal aspect of the VRG in the caudal bradypnoea area (anterior portion of the rostral VRG, rVRG). Note the slight decrease in ABP following the DLH injection. Upper trace: integrated phrenic nerve activity; lower trace: ABP. B, average bradypnoea response (n = 13) elicited by injection in the caudal bradypnoea area. TTOT, TE and TI are normalized to their control in each animal. DLH injection took place at time = 0. *TTOT significantly different from control (determined only for measurements made at the peak effect and then at 5 s intervals from 10 to 35 s; P < 0.05).
The variations in ABP are expressed as the peak changes in mean ABP. Results are expressed as means ±s.e.m. The statistical significance (P < 0.05) of changes in measured variables was ascertained by Student's paired t test (two-tailed) with the correction for multiple comparisons suggested by Bonferroni (Wallenstein et al. 1980).
Histology
At the end of each experiment an electrical lesion (20 μA, 18 min, electrode negative) was made to mark the last electrode position. From this site, the relative position of the other injection sites was estimated. Each animal was perfused transcardially under deep anaesthesia with 4 % formaldehyde in 0.1 m phosphate buffer. The brainstem was removed and stored in the same fixative solution, but with 25 % sucrose added. Frozen coronal 50 μm sections were stained with thionin and the lesions localized with a microscope at low and medium magnifications and documented on drawings made with the aid of a camera lucida attachment, or at later stages of this experiment via digital photography.
RESULTS
Forty-nine rats (body mass, 407 ± 9 g) were included in this study. In 29 of these, a total of 2436 injections of DLH were made to systematically map the VRG between the facial nucleus and the calamus scriptorius in the rostrocaudal, mediolateral and dorsoventral dimensions. In the remaining 20 rats, DLH injections were targeted at specific regions within the VRG, thereby reducing the number of tracks and injections made in an individual animal. No significant differences in the responses to DLH were observed between the two groups of rats and the results were pooled.
DLH injection elicited four distinct respiratory responses. Each of these was reproducibly elicited from distinct VRG subregions between the facial nucleus and the calamus scriptorius (Fig. 1). The two most rostral regions appeared equivalent to the BotC and preBotC regions, with injections rostrally (in the BotC) eliciting bradypnoea, while injections into the caudally adjacent preBotC elicited a tachypnoea or dysrhythmia. Extracellular unit activity recorded in these two areas revealed a marked predominance of expiratory neurons in the rostral bradypnoea area and a transition to inspiratory and, especially, pre-inspiratory neurons in the tachypnoea/dysrhythmia region. In the rVRG (caudal to preBotC) two different response patterns were observed. Immediately caudal to the preBotC, DLH injections elicited bradypnoea, whereas injections into posterior portions of the rVRG did not elicit a detectable change in respiratory motor output. Inspiratory neurons were recorded throughout these last two regions.
Figure 1. Subregions of the ventral respiratory group (VRG) identified by the cardiorespiratory response patterns to DLH injection.
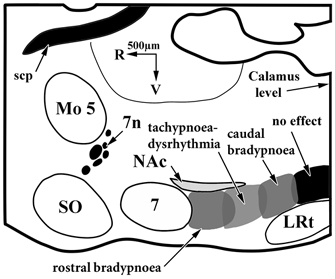
This schematic diagram illustrates a sagittal section through the brainstem of a rat 1.9 mm lateral to the midline. 7, facial nucleus; 7n, facial nerve; LRt, lateral reticular nucleus; Mo 5, motor nucleus of the trigeminal nerve; NAc, nucleus ambiguus, compact part; scp, superior cerebellar peduncle; SO, superior olive.
Injections into regions eliciting a change in respiratory rhythm did so primarily through an alteration in TE. A decrease in the peak amplitude of the phrenic nerve activity accompanied either increases or decreases in respiratory frequency. The effects elicited by DLH injection on respiratory rhythm and arterial pressure were short lasting (recovery in less than 2 min) and reproducible at a given injection site.
Effects of DLH injection on respiratory rhythm
The BotC: a rostral bradypnoea area
As shown in the example in Fig. 2A, a 3 nl DLH injection 500 μm caudal to the facial nucleus typically slowed respiratory frequency and modestly increased ABP. These changes were attained with injections in a region extending caudally from the caudal end of the facial nucleus for 700 ± 100 μm, which roughly corresponds to the BotC in the rat (Dobbins & Feldman, 1994). The peak respiratory response obtained by injections within this region for each of 21 rats was averaged and is presented in Fig. 2B. TTOT began to increase almost immediately, achieving a maximum increase of 25 ± 6 % (P < 0.05) in 2.9 ± 0.1 s (n = 21; Fig. 2). This slowing was due entirely to a change in TE, which exhibited a peak increase of 45 ± 9 % (P < 0.05). In contrast, TI decreased with a somewhat slower time course, shortening by 21 ± 3 % (P < 0.05) after 7.6 ± 0.3 s. Both TI and TE then progressively returned towards control values. In all animals in which ABP was measured in concert with injections into the rostral bradypnoea area (n = 16), small but consistent increases in ABP were associated with the rhythm changes, increasing 13 ± 2 % from a mean of 102 ± 4 mmHg (Fig. 2 and Fig. 3; P < 0.05). After DLH injections in the BotC, changes in the pattern of discharge on the phrenic nerve appeared to be a function of the altered respiratory rhythm. Specifically, the peak amplitude of the integrated phrenic nerve discharge decreased bilaterally by 10 ± 3 % (mean ±s.e.m.; n = 21; P < 0.05), while the ratio of peak amplitude of integrated phrenic nerve activity to TI did not change consistently among animals, increasing in 10, decreasing in five and not changing from control in six.
Figure 3. Tonic excitation of the superior laryngeal nerve (SLN) ipsilateral to a DLH injection in the BotC/rostral bradypnoea area.
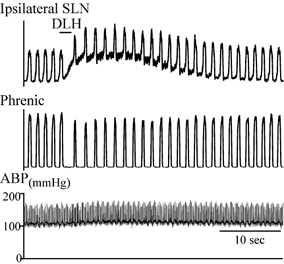
DLH (3 nl, 5 mm) was injected 100 μm dorsal to VRG respiratory neurons and 500 μm caudal to the facial nucleus. Traces from top to bottom: integrated SLN activity ipsilateral to the DLH injection, integrated phrenic nerve activity, and ABP.
Marked changes were also evident in the superior laryngeal nerve discharge (n = 2). Tonic activation was evident on the nerve ipsilateral to the BotC injection (Fig. 3), while the peak amplitude of the contralateral superior laryngeal nerve activity was reduced (data not shown) similar to the decrease in phrenic nerve activity.
The preBotC: a tachypnoea/dysrhythmia area
DLH injections (n = 36 rats) within the VRG from 700 to 1300 μm (± 100 μm) caudal to the facial nucleus (i.e. preBotC) elicited two distinct types of tachypnoeic responses. In one, termed tachypnoea (Fig. 4, n = 28), the respiratory frequency increased to a peak on the immediately succeeding phrenic bursts, and then progressively decreased back to control. In the second type, termed dysrhythmia (Fig. 5; n = 13), there was also an overall, immediate increase in respiratory frequency that was particularly evident in the first three to four respiratory cycles following the injection. Subsequent cycles exhibited considerable ‘breath-to-breath’ variability in TI, TE and TTOT. Despite a slight overall tachypnoea, the irregularity was large enough that for an individual respiratory cycle, TTOT and TE could be longer than control (Fig. 5B). In five animals, both tachypnoeic and dysrhythmic patterns were observed. Specifically, regions producing dysrhythmia were intermingled with those producing tachypnoea. The response pattern was reproducible at any one location, but an underlying anatomical basis for these differences was not immediately evident.
Within the tachypnoea region, the decrease in TTOT was due mainly to a decrease in TE. As shown in the averaged responses in Fig. 4B, TTOT rapidly (2.0 ± 0.1 s) decreased by 30 ± 3 % (Fig. 4B; n = 28; P < 0.05), primarily due to a peak decrease in TE of 39 ± 4 % (P < 0.05). Both TTOT and TE then progressively increased towards the pre-injection control value. DLH injection also caused a decrease in TI but with a slightly slower time course than TE, with a peak decrease of 19 ± 2 % (P < 0.05) in 13 ± 3 s. TI then returned towards control values at approximately the same rate as TE.
DLH injections usually had identical effects on respiratory rhythm, regardless of the nerve recorded (phrenic, pharyngeal branch of the vagus, or superior laryngeal nerve). The only exception to this occurred when injections into the dysrhythmia region elicited bursts on the superior laryngeal nerve during a silent period on the phrenic nerve. These additional bursts occurred bilaterally on the superior laryngeal nerve, but were markedly larger in amplitude on the side contralateral to the injection.
In addition to the changes in respiratory rhythm, there were marked changes in the peak amplitude of the integrated activity on all recorded nerves. Both the phrenic and superior laryngeal nerves exhibited bilateral decreases in the integrated peak amplitude of the phasic bursts. Injections eliciting tachypnoea were associated with an 18 ± 2 % decrease in amplitude on the phrenic nerve. On the other hand, the ratio of peak amplitude of integrated phrenic nerve activity divided by TI did not change consistently among animals, increasing in five, decreasing in 11 and not changing from control in 13. Injections that produced a dysrhythmic pattern tended to produce more marked reductions in peak amplitude, but, as with the change in rhythm, the magnitude of this decrease varied considerably on a burst-to-burst basis, thereby accentuating the dysrhythmic pattern. In many cases, a modest, tonic activation of the phrenic (n = 15/41) and contralateral superior laryngeal (n = 2 of 2) nerves was transiently induced along with either the dysrhythmia or tachypnoea (Fig. 5A and Fig. 6). Tonic activation was not evident on the ipsilateral superior laryngeal nerve (n = 3 of 3). As shown in Fig. 7, the response on the pharyngeal branch of the vagus depended on whether the ipsi- or contralateral nerves were considered. Peak responses on the pharyngeal branch of the vagus were elicited by VRG injections located dorsal to the injection sites that produced the greatest effects on respiratory rhythm (i.e. closer to the motoneuron soma in the semicompact division of the nucleus ambiguus). The ipsilateral pharyngeal branch of the vagus exhibited marked increases in both phasic and tonic discharge (Fig. 7A; n = 6 of 6). In contrast, the response pattern of the contralateral pharyngeal branch of the vagus consisted of a marked decrease in the amplitude of phasic bursts (Fig. 7B; n = 4 of 4).
Figure 6. Effect on bilateral SLN discharge of DLH injection in the dysrhythmia/tachypnoea area.
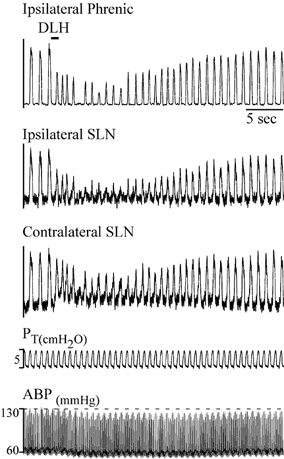
DLH (3 nl, 5 mm) was injected into preBotC 500 μm ventral to the dorsal aspect of the VRG (defined by recording respiratory neurons), and 750 μm caudal to the facial nucleus. DLH injection elicited a tachypnoeic pattern and a decrease in amplitude of the phasic activity on all nerves. Note the absence of tonic excitation on the SLN ipsilateral to the injection, whereas the contralateral SLN showed moderately increased level of tonic activity. Traces from top to bottom: ipsilateral integrated phrenic nerve discharge, ipsilateral and contralateral integrated SLN discharge, tracheal pressure (PT) and ABP.
Figure 7. Effect on bilateral pharyngeal branch of the vagus nerve (PhX) activity by a DLH injection in the dysrhythmia/tachypnoea area (preBotC) in one rat.
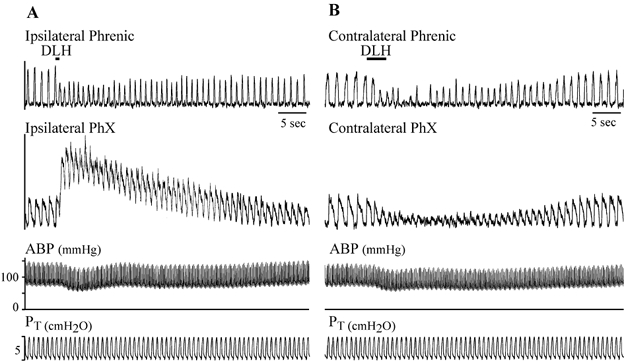
A, DLH injection (6 nl, 5 mm) into the tachypnoeic region produced a strong tonic activation of the ipsilateral PhX while maintaining the amplitude of the integrated phasic bursts. B, injection (3 nl) into the contralateral VRG (1.0 mm caudal to the facial nucleus) of the same rat elicited a dysrhythmia and a reduction in the amplitude of phasic activity on both nerves with no evident tonic activation. Traces from top to bottom: integrated phrenic nerve activity, integrated activity of the PhX, ABP and PT.
In animals in which ABP was measured in concert with injections into the tachypnoea/dysrhythmia area (n = 31), DLH injections modestly changed ABP, with an average decrease of 7 ± 2 % (Fig. 1) from a mean pressure of 103 ± 4 mmHg.
The anterior rVRG: a caudal bradypnoea area
Progressing caudally within the VRG, DLH injections from 1200 to 1700 μm (± 100 μm) caudal to the facial nucleus (n = 13) elicited a bradypnoea with a 21 ± 5 % increase in TTOT (Fig. 8; P < 0.05) occurring within 2.5 ± 0.2 s. This slowing in respiratory frequency was due to a 41 ± 8 % increase in TE (P < 0.05) that offset a decrease in TI of 25 ± 3 % (P < 0.05). As with more rostral injections, the time course of the change in TI was slower (peak effect at 5.3 ± 0.3 s) than for the change in TE. There was also a significant decrease in the peak amplitude of phrenic nerve discharge (24 ± 3 %; P < 0.05) and, as in the rostral bradypnoea and tachypnoea areas, the ratio of peak amplitude of integrated phrenic nerve activity to TI did not change consistently among animals, increasing in four, decreasing in six and not changing from control in three.
In all animals in which ABP was measured in concert with injections into the caudal bradypnoea area (n = 10), DLH injections decreased arterial pressure by an average of 12 ± 2 % from a pre-injection mean pressure of 92 ± 6 mmHg (P < 0.05).
The posterior rVRG: a caudal ‘no effect’ area
Immediately caudal to the caudal bradypnoea area and extending to the calamus level (i.e. from ∼1700–2400 μm caudal to the facial nucleus), DLH injections did not change either the respiratory rhythm or amplitude (n = 5), although injections into the caudal bradypnoea area (described above) of these same animals elicited a typical slowing of respiratory rhythm.
Mapping in the rostrocaudal plane
Given the large number of tracks required to completely map the VRG at 200 μm intervals in all three planes, a complete mapping was not performed in any single animal. To overcome this limitation and ensure that the rostrocaudally defined regions did not depend on the order in which injections were made (rostral to caudal vs. caudal to rostral) or on the number of injections made into an individual animal, in four animals mapping in the mediolateral dimension was restricted (one or two tracks at any given rostrocaudal level) so that an extensive mapping in the rostrocaudal dimension could be carried out. In two of these animals, mapping was begun rostrally and progressed caudally, while in the remaining two it was begun caudally and progressed rostrally. In all four animals, the approximate mediolateral centre of the VRG was identified by recording single respiratory neuron activity, and then DLH was injected in a series of tracks that progressed rostrocaudally in this plane. Mapping in each of these animals was complete enough to identify at least three of the four previously described areas and confirmed the distribution of the four VRG subregions. Figure 9 is an example from one rat, indicating the sites at which DLH injection elicited responses typical of each region. Rostrally, injection at position A in Fig. 9 produced bradypnoea. Injections on a single track immediately below the caudal part of the compact division of nucleus ambiguus elicited either a dysrhythmia (location B) or tachypnoea (location C). The most caudal injections in this animal (point D) produced a bradypnoea. The locations of transitions between two different effects on rhythm were highly predictable (within 200 μm rostrocaudally).
Figure 9. Example of the respiratory response patterns elicited by DLH injection in the VRG at different rostrocaudal levels in one rat.
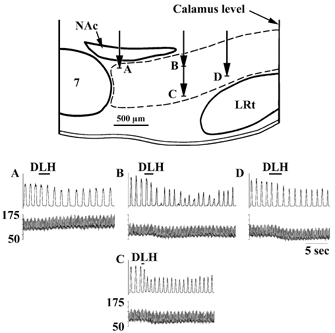
The schematic diagram illustrates a sagittal section through the brainstem of a rat 1.9 mm lateral to the midline. Each panel (A–D) shows the response obtained on the phrenic nerve (upper traces) and ABP (lower traces) at the corresponding injection sites in the top panel. A, BotC; DLH (2 nl, 5 mm) injected 100 μm ventral to the dorsal aspect of the VRG immediately caudal to the facial nucleus elicited a bradypnoea along with an increase in ABP. Dysrhythmia (B) and tachypnoea (C) elicited by DLH (3 nl) injections along the same track (preBotC; 1 mm caudal to the facial nucleus) at 200 and 600 μm, respectively, ventral to the dorsal aspect of the VRG. D, anterior rVRG; bradypnoea and decrease in ABP following DLH injection (3 nl) 500 μm ventral to the dorsal aspect of the VRG, 1.6 mm caudal to the facial nucleus.
Mapping in the dorsoventral and mediolateral planes
At any given rostrocaudal level within the VRG, moving the injection pipette in the mediolateral and dorsoventral planes did not produce systematic, qualitative differences in the response pattern. Within the dorsoventral plane of the VRG, the DLH effect on respiratory rhythm and pattern was confined to a region extending from ∼210 ± 20 μm ventral to the respiratory population to ∼70 ± 20 μm ventral to the dorsal edge of the VRG (recorded at the time of the injection with the same pipette). DLH injections made in a track either 200 μm lateral or medial to the respiratory population elicited no, or a minimal, respiratory response. Figure 10 shows an example, relating the histologically reconstructed VRG location, pipette position and the evoked response on three electrode tracks through the VRG at a rostrocaudal level within the tachypnoeic region. The maximal effect was elicited on the middle track through the VRG (location C; 1.7 mm lateral to the midline; 500 μm ventral to dorsal aspect of the VRG). An injection 300 μm dorsally on the same track (location B) elicited minimal respiratory responses. Separate tracks at the same depth, but 400 μm medial (location E) or 200 μm lateral (location A) similarly elicited only minimal effects. A reduced, but significant, response was elicited by an injection ∼200 μm ventral to the recorded VRG activity (location D). This site was 300 μm ventral to the site at which the maximal response was obtained.
Figure 10. Respiratory response patterns elicited by DLH injection (3 nl, 5 mm) into, or immediately proximal to, the preBotC/tachypnoea region in a single mediolateral and dorsoventral plane in one rat.
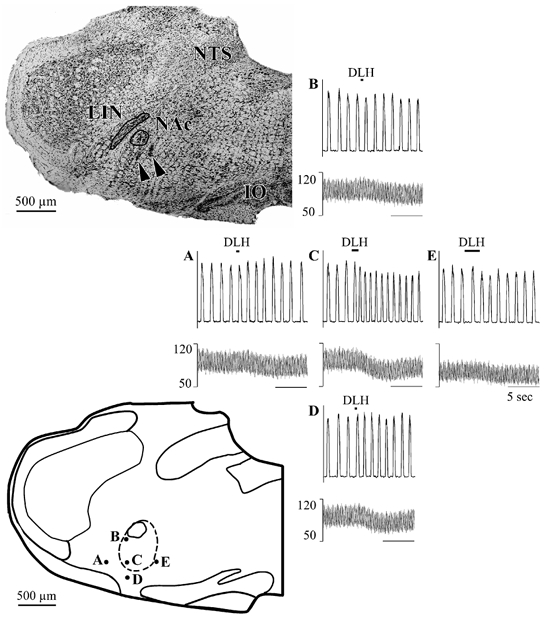
Top left: coronal section of the rat brainstem (1.3 mm caudal to the facial nucleus). Note electrode tracks/lesions (arrowheads) just ventral to the nucleus ambiguus. Bottom left: schematic representation of the injection sites on a coronal section traced from the brainstem section at the upper left. The dashed line outlines the VRG as reconstructed from electrode tracks and respiratory unit recording. Right: panels showing the responses of the phrenic nerve (top traces) and ABP (bottom traces) at each of the injection sites indicated on the schematic coronal section. A, C and E show responses obtained at the same depth but different mediolateral locations: A, 200 mm lateral to the VRG; C, lateral aspect of the VRG; E, medial edge of the VRG (400 mm medial to C). The maximum tachypnoea was obtained at C. B, C and D show responses obtained at the same mediolateral level but different dorsoventral positions. The maximum tachypnoea was obtained at C, 500 μm ventral to the dorsal aspect of the VRG and 100 μm dorsal to its ventral aspect. At B, 300 μm dorsal to C, there was no effect on respiratory rhythm and a slight decrease in ABP. An attenuated tachypnoea was obtained at D, 200 μm ventral to C (and 100 μm ventral to the VRG). NAc: nucleus ambiguus compact part; LIN linear nucleus; NTS: nucleus of the solitary tract.
For a given rostrocaudal level, a systematic variation in dorsoventral or mediolateral response pattern occurred only at the transitions between rostrocaudally defined regions. At these transitions (extending over 200–300 μm in the rostrocaudal direction) the response pattern sometimes changed from a tachypnoea to a bradypnoea, or vice versa, as either the depth or mediolateral position of the pipette was varied. These changes, at the interface between regions, were reproducible within an animal but varied sufficiently between animals, such that a systematic rule cannot be described.
Changes in ABP were also routinely evoked by injections within, or proximal to the VRG. Depending on the rostrocaudal level, either pressor or depressor responses were elicited. In the dorsoventral plane, the arterial-pressure-responsive region largely overlapped the respiratory responsive region, but was shifted slightly ventral. Arterial pressure changes were elicited by injections beginning about 100 μm ventral to those eliciting respiratory responses and extended dorsally to about 50 μm ventral to the dorsal edge of respiratory responsive region.
Control injections
Injections of saline (n = 1 rat) or neuroactive chemicals other than DLH, including substance P (50 μm, 3–21 nl; n = 2), picrotoxin (5 mm, 15–60 nl; n = 1) or strychnine (2 mm, 15–60 nl; n = 2) in six animals elicited no, or minimal, change in respiratory rhythm in any of the VRG regions. A typical example is shown in Fig. 11, where DLH (3 nl) caused a marked increase in respiratory frequency when injected into the tachypnoea region. Injection of a larger volume of 2 mm strychnine (15 nl) at the same site, from an adjacent pipette barrel of the same pipette, had no effect on respiratory rhythm.
Figure 11. Example of lack of response to strychnine injection (15 nl; 2 mm in 150 mm NaCl) in the preBotC tachypnoea/dysrhythmia area.
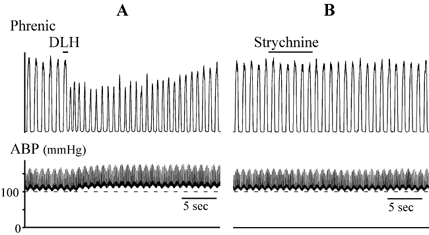
Top traces: integrated phrenic nerve activity. Bottom traces: ABP. A, DLH injection (3 nl, 5 mm) elicited a tachypnoea and decrease in phrenic nerve response amplitude and an increase in ABP. B, strychnine injection at the same site (from another barrel of the same electrode) did not elicit a discernable change in phrenic nerve activity or ABP.
DISCUSSION
The functional organization of the VRG rostral to the calamus scriptorius was examined by determining the cardiorespiratory responses to injections of the broad-spectrum excitatory amino acid DLH. The use of small volumes of DLH (typically 3 nl) and low concentrations (usually 5 mm in 150 mm NaCl) permitted a relatively fine-grained analysis of the rat VRG between the calamus scriptorius and the facial nucleus. Four distinct response patterns were observed from regions corresponding to the traditionally defined BotC, preBotC and two subregions of the rVRG. The responses consisted of: (1) bradypnoea and pressor responses within the BotC, (2) tachypnoea/dysrhythmia within the preBotC, (3) bradypnoea and depressor responses within the anterior component of the rVRG and (4) a region within the posterior aspect of the rVRG from which no alteration in respiratory response was observed. The functional heterogeneity of the VRG in response to injections of excitatory amino acids is consistent with previous studies in cats and rats (McCrimmon et al. 1986, 1987; Bonham & Jeske, 1989; Tolentino-Silva et al. 1997; Chitravanshi & Sapru, 1999; Solomon et al. 1999). However, there are some significant differences in the response patterns to excitatory amino acid injection between the present report and earlier studies (discussed below). Nevertheless, consistent with the findings of others, good between-animal reproducibility in response patterns makes it possible to use the DLH-elicited cardiorespiratory response patterns to discriminate the relative location of an electrode within identified functional VRG compartments (e.g. McCrimmon et al. 1986; Solomon et al. 1999; Solomon, 2002; Wang et al. 2002).
Pressure injection and chemical stimulation: technical considerations
As with any study employing stimulation or inactivation of CNS regions, the interpretation is dependent, in part, on the resolution of the technique. Chemical stimulation of neurons reduces the likelihood of activation of fibres of passage. However, the volume of tissue affected, and hence the number of neurons activated by the injection, must be taken into consideration. Our delivery system allows reliable injections of small, measured volumes of a relatively dilute solution of DLH, thereby reducing variability due to inconsistent injection volumes or inconsistent molar concentrations (McCrimmon et al. 1986; Bonham & McCrimmon, 1990; Bonham et al. 1993). The use of a relatively dilute solution of DLH permitted short-term, reversible and reproducible perturbations in the activity of subgroups of VRG neurons. This approach facilitated an extensive mapping of the VRG responsive sites within individual animals. While the precise diffusion distance is unknown, the minimum distance would be represented by the 90 μm radius of a sphere with a volume of 3 nl. Nevertheless, a relatively short effective diffusion distance for a 3 nl injection is consistent with previous findings in which marked, qualitative differences in drug-evoked responses were seen following relocation of a delivery pipette by as little as 50 μm (Bonham & McCrimmon, 1990; Bonham et al. 1993). In addition, equal or larger volumes of 150 mm NaCl (the vehicle for DLH) or other neuroactive agents (substance P, strychnine, picrotoxin) did not mimic the responses induced by DLH. A theoretical calculation of drug diffusion by Nicholson (1985) suggests that the concentration of drug present at a radius of 300 μm from the site of a 10 nl injection is no more than ∼20 % of that at the pipette tip. In the present study, the concentration of DLH in the pipette and the total amount injected were well below the amounts reported to give rise to depolarization block (Lipski et al. 1988). These observations suggest that the effects of DLH resulted from the activation of neurons with excitatory amino acid receptors within a radius of < 300 μm from the pipette tip.
It is unlikely that the DLH-induced changes in respiratory motor outputs were secondary to changes in ABP. Injections within the tachypnoea/dysrhythmia region, for example, could elicit respiratory responses with relatively little change in arterial pressure (Figs 3, 5A and 6). In addition, injections within different subregions of a given rat could elicit similar changes in respiration despite opposite changes in blood pressure. For example, bradypnoea following injection of DLH into the BotC was accompanied by a small pressor response (+13 % ABP), while bradypnoea following DLH injection into the anterior part of the rVRG resulted in a depressor response (−12 % ABP). In contrast, the +40 % increase in TE was comparable for both regions.
Taken together, these findings are consistent with the interpretation that the DLH-induced changes were consequent to activation of VRG neurons in close proximity to the tip of the injection pipette. That the DLH injections induced qualitatively different respiratory responses, the nature of which depended on the location of the pipette tip, suggests that neurons in different VRG subregions have different functional roles and, presumably, different postsynaptic connections. It should be kept in mind, however, that interpretations such as those raised in the following discussion are qualified by potential non-physiological aspects of the DLH stimulus; for example, neurons might discharge at supernormal rates and/or functionally distinct types of respiratory neurons that normally fire out of phase with one another may have been induced to fire in synchrony (see McCrimmon et al. 1986).
Effect of DLH injection on respiratory rhythm versus pattern
The working hypothesis was that neurons in separate VRG regions differ with respect to their roles in generating respiratory rhythm vs. pattern. This developed from the observations that preBotC neurons are required for respiratory rhythm generation (Smith et al. 1991), and that at least bulbospinal premotor neurons with little or no role in rhythm generation (Feldman et al. 1984) are largely located outside of the preBotC (Ellenberger & Feldman, 1990; Dobbins & Feldman, 1994). However, the finding that DLH injections into three of the four identified regions altered respiratory rhythm suggests that at least some aspects of rhythm generation are distributed across this larger region of the VRG. Nevertheless, the striking difference in the DLH effects on respiratory rhythm ranging from tachypnoea and dysrhythmia in the preBotC to bradypnoea in the BotC and anterior rVRG indicates that each of these regions provides a unique contribution to rhythm generation.
The DLH-induced changes in respiratory rhythm were primarily due to changes in TE, regardless of the region injected, with peak effects of about a 40 %. An increase in TE caused bradypnoea, a decrease caused tachypnoea. TI, on the other hand, decreased by about 20 % in the three effective regions, regardless of whether there was an overall bradypnoea or tachypnoea. This consistent shortening of TI for injections into the BotC, preBotC and anterior rVRG was surprising given the marked differences in the types of respiratory neurons found in these areas. For example, the BotC contains a large fraction of expiratory neurons, while the preBotC and rVRG contain mostly inspiratory neurons, and a substantial percentage of these in the preBotC have a phase-spanning discharge pattern (Sun et al. 1998; McCrimmon et al. 2001). Considering network models of rhythm generation, shortening of TI could occur as a result of activation of inhibitory populations of late-inspiratory neurons or expiratory-decrementing (postinspiratory) neurons (e.g. Rybak et al. 1997). Alternatively, a decrease in the activity of inhibitory inspiratory-decrementing neurons could also lead to a shortening of TI. This latter possibility is consistent with previous observations that DLH injections into the preBotC elicit a decrease in the peak discharge rate of inspiratory-decrementing neurons as well as an earlier termination of their discharge (McCrimmon et al. 2001 and the authors' unpublished observations), In contrast, TE could be lengthened consequent to increases in the activity of expiratory-decrementing (inhibitory) neurons (Hayashi et al. 1996; Rybak et al. 1997). Consistent with this possibility, injections of DLH or bicuculline into the preBotC elicit either shortening or lengthening of TE, respectively, and produce a proportional change in the peak frequency and duration of discharge of expiratory-decrementing neurons (McCrimmon et al. 2000a, 2001).
Interestingly, not only were the changes in TI and TE in opposite directions with injections into the bradypnoea-producing regions, but with injections into the BotC or preBotC regions, the nadir in TI was delayed relative to that in TE. The peak change in TE occurred within the first 2–3 s, while TI progressively decreased over ∼7–12 s (see Figs 2, 4 and 5). On the other hand, this delayed peak decrease in TI is not obvious for injections into the anterior rVRG. It seems unlikely that the differential time course for TE and TI results from activation of a single group of neurons (e.g. expiratory-decrementing) responsible for the opposing effects. However, determination of the neurons that are involved will require simultaneous recording of single-unit activity during DLH activation with a second electrode at some distance from the injection site.
The simultaneous effects of DLH injections on the pattern (as opposed to rhythm) of phrenic nerve activity were consistent across the injections into the three areas that produced respiratory effects. Each uniformly produced a decrease in the peak amplitude of integrated phrenic nerve activity. Given the truncation of TI, this decrease was not unexpected since the phrenic nerve has a generally augmenting or bell-shaped discharge pattern and, as a consequence, shortening the TI could prevent it from reaching the peak control level of activity. The lack of a significant change in the ratio of TI to the peak amplitude of phrenic nerve activity suggests, however, that there was little effect on the ‘pattern’ of phrenic nerve discharge, independent of the rhythm change for any of the injected regions. This was somewhat surprising given our initial expectation that regions containing bulbospinal premotor neurons, especially the rVRG, would have a predominant influence on respiratory motor pattern.
Changes in the phasic respiratory pattern of cranial nerve discharge were generally qualitatively similar to those elicited in the phrenic nerve. However, the decreases in peak amplitude of discharge from either the pharyngeal branch of the vagus or the superior laryngeal nerves tended to be greater than from the phrenic nerve (Fig. 6 and Fig. 7). The greater sensitivity of cranial nerve discharge is consistent with reported observations in which cranial nerve discharge tends to be more sensitive to changes in respiratory drive (e.g. Fenik et al. 1998). In addition, tonic discharge was elicited ipsilateral to injections at the same level as the cell bodies for either the pharyngeal branch of the vagus or the superior laryngeal nerve. While some of this tonic activity could be induced by activation of premotor neurons, it is likely that a substantial component was the result of direct activation of motoneurons with dendrites extending into the injection site. An intriguing additional observation was the production of tonic discharge on the contralateral superior laryngeal nerve with injections into the preBotC region, a region that is both caudal and contralateral to the main population of motoneurons in the recorded nerve. The latter response is likely to be due to the activation of premotor neurons. Hence, although changes in respiratory rhythm can account for many of the changes in motor pattern, there do appear to be changes in pattern that exceed those predicted by the changes in respiratory rhythm alone.
Rostral bradypnoea region
The BotC forms the most rostral division of the VRG and is identified by the presence of a relatively large concentration of expiratory neurons. The transition between the predominance of expiratory neurons in the BotC and the adjoining preBotC is fairly abrupt in the cat (Feldman, 1986). This transition is less pronounced in rats owing to the relatively greater number of preBotC expiratory neurons, many of which are likely to be pharyngeal motoneurons (Zheng et al. 1991; Hayashi et al. 1996). Nevertheless, both the location of the rostral bradypnoea region and predominance of expiratory neurons found in this study as well as others (Zheng et al. 1992; Hayashi et al. 1996) suggest that the rostral bradypnoea region is coextensive with the BotC.
The slowing of respiratory rhythm is consistent with the apnoea observed previously with larger injections in rats (Chitravanshi & Sapru, 1999; Wang et al. 2002), cats (Bongianni et al. 1993) and rabbits (Bongianni et al. 1997), and with electrical stimulation of the BotC in cats (Gang & Lei, 1996). It is also consistent with the widespread inhibition of VRG neurons mediated by many BotC expiratory neurons in both cats (Ezure & Manabe, 1988; Fedorko et al. 1989; Jiang & Lipski 1990; Tian et al. 1998) and rats (Tian et al. 1998, 1999). The proximity of this region to rostroventrolateral medullary pressor neurons is consistent with the modest increase in arterial pressure evoked from this region.
Tachypnoea and an increased amplitude in phrenic nerve discharge were sometimes observed when injections were made at the rostral limit of the rostral bradypnoea area and the pipette was positioned about 500 μm ventral to the main mass of phasic VRG neurons (i.e. near the ventral surface of the medulla). In tracks in which this effect was observed, a region in which DLH did not elicit a respiratory response was interposed between the VRG bradypnoea region and this deeper responsive area. This effect is similar to one noted by Nattie & Li (1994) for injection into the retrotrapezoid nucleus, albeit we observed a faster time course in the current study. It could also correspond to the rostral ventral medullary area in which Tolentino-Silva et al. (1997) noted that large injections of glutamate elicited an increase in tidal volume and breathing frequency in anaesthetized rats. Because the presumed retrotrapezoid region where we observed this response pattern was considerably ventral to the VRG and beyond the scope of our planned experiments, it was not systematically examined in the present study. This preliminary observation, however, may be useful insofar as this ventral zone appears to produce a unique response pattern compared with that elicited by our injections in the VRG, when activated under the same experimental conditions.
In addition to bradypnoea, injections into the BotC also produced a marked increase in tonic activity on the ipsilateral superior laryngeal nerve (Fig. 3). Thus, the associated motoneurons may provide reasonable anatomical landmarks for the rostrocaudal extent of the rostral bradypnoea region (BotC). The main population of these motoneurons extends caudally from the caudal level of the facial nucleus for about 500 μm (Bieger & Hopkins, 1987). This corresponds closely to the rostral bradypnoea region in the present report, as well as with the location of recorded expiratory neurons and the extent of the BotC in the rat as described by Sun et al. (1998) and Schreihofer et al. (1999).
Tachypnoea/dysrhythmia region
The anatomical localization as well as the response patterns to DLH injection and patterns of unit activity within the tachypnoea/dysrhythmia region are consistent with this region being coextensive with the preBotC as defined by Smith et al. (1991). The preBotC has been postulated to contain the kernel of the respiratory rhythm-generating circuitry (Smith et al. 1991; Ramirez et al. 1998; Gray et al. 1999, 2001). The production of a respiratory dysrhythmia is consistent with the concept that DLH injections into this region disrupted the activity within the circuitry generating respiratory rhythm.
We were not able to discriminate a separate region eliciting dysrhythmia from that producing tachypnoea. Tachypnoea could be elicited during almost all experiments, whereas dysrhythmia was observed less frequently, but in some animals it was elicited on several adjacent tracks 200 μm apart. Between-subject differences in the level of background excitatory drive to the preBotC rhythm-generating circuitry, or slight anatomical variations in the organization/distribution of the rhythm-generating neurons may make some rats more susceptible to disruption of breathing rhythm.
Smith et al. (1991) identified the preBotC in neonatal rats in vitro as extending for about 200 μm in the rostrocaudal dimension and located immediately caudal to the retrofacial nucleus (i.e. the compact division of the nucleus ambiguus). Neuroanatomical examination of the presumably homologous region in adult rats reveals a preponderance of propriobulbar neurons with few bulbospinal respiratory premotoneurons (Ellenberger & Feldman 1990; Smith et al. 1991; Dobbins & Feldman 1994; Sun et al. 1998; Wang et al. 2002). Based on transynaptic labelling using pseudorabies virus in rats, Dobbins & Feldman (1994) identified this region as beginning about 400 μm caudal to the facial nucleus and extending ∼400 μm further caudally in young adult (150–250 g) rats. In somewhat larger rats (250–350 g), Wang et al. (2002) depicted the preBotC as beginning about 500 μm caudal to the facial nucleus and extending approximately 600 μm further caudally. Sun et al. (1998) identified in 350–450 g rats a slightly smaller region, extending over a rostrocaudal distance of about 400 μm, based on the predominance of pre-inspiratory neurons. In the current study, using animals comparable in size to those used by Sun et al. (1998), the tachypnoea/dysrhythmia region covered approximately 500–700 μm in its rostrocaudal dimension.
Thus, within the accuracy of the measurements and differences between laboratories with respect to experimental set-ups and histological processing, there is reasonably good agreement as to the size and location of the preBotC in rats. In the current study, transitions between regions were defined with an accuracy of about ± 200 μm (based on the spacing between electrode tracks), and minor differences in localization could be largely accounted for by this variance. One might also predict a slightly smaller estimate for the extent of the preBotC when using the location of labelled cell bodies (Dobbins & Feldman, 1994) or extracellular unit recordings (Sun et al. 1998), than when using chemical stimulation (e.g. Chitravanshi & Sapru 1999; Wang et al. 2002, and the present report), since the rostrocaudal spread of the dendritic field may contribute to the response to the chemical injection. However, while this would tend to broaden the distribution curve for a given area, it would only change the crossover point between adjacent distributions if the population density and/or the dendritic length or orientation also varied between regions. For example, respiratory neurons appear to be less densely packed in the BotC, and this could contribute to a relative decrease in the estimated size of the region giving rise to bradypnoea when judging its extent based on small chemical injections.
Three recent studies have examined the respiratory response patterns elicited by injections of an excitatory amino acid into the preBotC of rats (Chitravanshi & Sapru 1999; Wang et al. 2002) and cats (Solomon et al. 1999). In all of these studies, as well as the present study, preBotC injections elicited an increase in the frequency of phrenic nerve bursts. Nevertheless, there were some notable differences in the findings. In the present study, preBotC DLH injections elicited a modest tonic activation of the phrenic nerve in about 50 % of animals. In contrast, Chitravanshi & Sapru (1999) reported a substantial tonic activation of the phrenic nerve in response to excitatory amino acid injection in all rats tested. These authors considered the tonic activation of phrenic nerve activity to be a unique, defining characteristic of the response to preBotC injections. Wang et al. (2002) also obtained a tonic activation of phrenic nerve (notice their Fig. 1), although the frequency of this occurrence was not commented on. Several differences in experimental paradigm could contribute to these discrepancies. Chitravanshi & Sapru (1999) injected N-methyl-d-aspartate, which tends to have a more potent and prolonged effect than DLH (e.g. McCrimmon et al. 1987). In addition, Chitravanshi & Sapru (1999) used rats with intact vagi and made larger injections (20 nl vs. 3 nl in the preBotC), presumably activating a larger population of neurons. Diffusion to regions outside the preBotC could also contribute to the difference. Bongianni et al. (1993) noted a substantial tonic activation in cats when the injection was targeted into the rostral ventrolateral medulla outside of the VRG. However, in the present study we did not observe alterations in respiratory responses from small injections placed just outside the medial to lateral extent of the VRG (see Methods). Interestingly, Zhang et al. (1991; working in rabbits) reported that only procaine injections into the medial area of the ‘retrofacial nucleus’ (presumably the preBotC) and the adjacent anterior part of the rostral VRG altered respiratory rhythm, with blockade of these cells leading to tonic activity on neurons in other portions of the VRG. In the same vein, it is possible that tonic activity reported in experiments with glutamate or DLH injections could reflect depolarization block of preBotC neurons.
Similar to the findings of Chitravanshi & Sapru (1999), we found that the tachypnoea was associated with a decrease in the peak amplitude of the phasic respiratory activity on the phrenic nerve as well as cranial nerve discharge. In the present study, the decrease in amplitude was related to the shortening of TI such that there was no change in their ratio, a parameter that can be an indicator of respiratory drive (von Euler, 1986). This contrasts with the finding of Wang et al. (2002), who noted a modest increase in the peak amplitude of phrenic nerve discharge. In cats, Solomon et al. (1999) found a decrease in some animals and an increase in others. The reasons for the partial differences among studies are not clear, but where larger injections and higher concentrations were used the differences could reflect a systematic involvement of neurons extrinsic to the VRG that provide tonic inputs to the VRG. Alternatively, differences in the anaesthetic used or the strain of rat are possible contributing factors.
Caudal bradypnoea region
DLH injection within a region extending 500–600 μm caudal to the preBotC, like injections in the BotC, slowed breathing rate and decreased the amplitude of phrenic nerve discharge. These findings are similar to the slowed respiratory frequency and decrease in the amplitude of diaphragm activity reported by Bonham & Jeske (1989). The slowing of respiration is consistent with the observed inhibition of some of the rostral VRG neurons by neurons in more posterior aspects of the rostral VRG (Segers et al. 1987). However, the response is markedly different than the tachypnoea and increased amplitude of phrenic nerve activity reported by Chitravanshi & Sapru (1999). The reasons for this discrepancy are not readily apparent, but several factors could contribute. The coordinates reported by Chitravanshi & Sapru (1999) suggest that the injections eliciting an increase in respiratory rate and amplitude overlap our dysrhythmia/tachypnoea region. Further, the use of a different excitatory amino acid analogue, a larger injection volume and apparently more lateral injection sites (Fig. 5E and F in Chitravanshi & Sapru, 1999) could all contribute to the differences.
Subdivision of the rVRG into anterior and posterior compartments
Our results suggest a functional distinction between the anterior and posterior parts of the rostral VRG. Recent observations on the colocalization of bulbospinal neurons with neurokinin-1 (NK1)-receptor-expressing neurons also provide at least indirect support for subdividing the rostral VRG. In particular, Liu & Wong-Riley (2001) observed two different sizes of NK1-receptive neurons (excluding nucleus ambiguus motoneurons) in the region of the preBotC. Guyenet et al. (2002) demonstrated that the larger NK1 receptive neurons were glutamatergic, projected to the spinal cord, and were restricted to the anterior half of the rostral VRG. In contrast, small NK1-receptive neurons were concentrated within the preBotC and represented glutamatergic propriobulbar neurons. We have recently confirmed that when compared to the preBotC, adjacent larger sized NK1-receptive neurons populate the anterior part of the rostral VRG (the authors' unpublished observations).
It has been demonstrated that stimulation of bulbospinal respiratory neurons or their axons does not reset respiratory rhythm (Feldman et al. 1984; Zhang et al. 1991), and at posterior levels of the rostral VRG only a few medullary-projecting (propriobulbar) neurons are found (Dobbins & Feldman, 1994). This is a reasonable explanation for the lack of effect on respiratory rhythm of DLH injections targeted at posterior levels of the rostral VRG.
Taken together, these findings reinforce the conclusion that physiological and anatomical distinctions exist between the anterior and posterior aspects of the rVRG, and that some further subdivision of this region may be warranted.
In summary, the findings presented here confirm the existence of VRG subregions with different functional roles. DLH-elicited cardiorespiratory response patterns were highly reproducible between animals with respect to their rostrocaudal position within the VRG. Bradypnoea and a decrease in arterial pressure are indicative of the caudal bradypnoea region, while bradypnoea, an increase in superior laryngeal nerve activity, and a pressor response are typical for DLH injections into the rostral bradypnoea (BotC) region. The tachypnoea/dysrhythmia region, which is located between the two bradypnoea regions, is localized by a tachypnoea, with or without a dysrhythmia, and an increase in ipsilateral pharyngeal nerve activity. The dysrhythmia is also consistent with the disruption of activity within the rhythm-generating circuitry. The presence of region-specific response patterns also implies a differential pattern of synaptic interactions among neurons in these different regions. It is also notable that the changes in respiratory rhythm were associated with opposing changes in TI and TE with injections into the BotC and anterior rVRG. Moreover, for the BotC and preBotC, the peak effect on TE occurred within the first few seconds, while the effect on TI was delayed. This independence of the modulation of inspiration and expiration will have to be incorporated within any valid model of respiratory rhythm generation. Clearly, DLH injections are a useful tool permitting functional localization within the VRG and probing phasic changes in the respiratory network in response to focal activation of VRG neurons.
Acknowledgments
This work was supported by NIH grants HL/NS-60097 and HL/NS-60696.
REFERENCES
- Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987;262:546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Corda M, Fontana GA, Pantaleo T. Excitatory and depressant respiratory responses to chemical stimulation of the rostral ventrolateral medulla in the cat. Acta Physiol Scand. 1993;148:315–325. doi: 10.1111/j.1748-1716.1993.tb09562.x. [DOI] [PubMed] [Google Scholar]
- Bongianni F, Mutolo D, Pantaleo T. Depressant effects on inspiratory and expiratory activity produced by chemical activation of Bötzinger complex neurons in the rabbit. Brain Res. 1997;749:1–9. doi: 10.1016/s0006-8993(96)01153-5. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Coles SK, McCrimmon DR. Pulmonary stretch receptor afferents activate excitatory amino acid receptors in the nucleus tractus solitarii in rats. J Physiol. 1993;464:725–745. doi: 10.1113/jphysiol.1993.sp019660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham AC, Jeske I. Cardiorespiratory effects of dl-homocysteic acid in caudal ventrolateral medulla. Am J Physiol. 1989;256:H688–696. doi: 10.1152/ajpheart.1989.256.3.H688. [DOI] [PubMed] [Google Scholar]
- Bonham AC, McCrimmon DR. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol. 1990;427:261–280. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitravanshi VC, Sapru HN. Phrenic nerve responses to chemical stimulation of the subregions of ventral medullary respiratory neuronal group in the rat. Brain Res. 1999;821:443–460. doi: 10.1016/s0006-8993(99)01139-7. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Subnuclear organization of the lateral tegmental field of the rat. I: Nucleus ambiguus and ventral respiratory group. J Comp Neurol. 1990;294:202–211. doi: 10.1002/cne.902940205. [DOI] [PubMed] [Google Scholar]
- Ezure K, Manabe M. Decrementing expiratory neurons of the Bötzinger complex. II. Direct inhibitory synaptic linkage with ventral respiratory group neurons. Exp Brain Res. 1988;72:159–166. doi: 10.1007/BF00248511. [DOI] [PubMed] [Google Scholar]
- Fedorko L, Duffin J, England S. Inhibition of inspiratory neurons of the nucleus retroambigualis by expiratory neurons of the Bötzinger complex in the cat. Exp Neurol. 1989;106:74–77. doi: 10.1016/0014-4886(89)90146-5. [DOI] [PubMed] [Google Scholar]
- Feldman JL. Neurophysiology of breathing in mammals. In: Bloom FE, editor. Handbook of Physiology, section 1, The Nervous System, vol. 4, Intrinsic Regulatory Systems of the Brain. Bethesda: American Physiological Society; 1986. pp. 463–524. [Google Scholar]
- Feldman JL, McCrimmon DR. Neural control of breathing. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, editors. Fundamental Neuroscience. 2. New York: Academic Press; 2003. pp. 967–990. [Google Scholar]
- Feldman JL, McCrimmon DR, Speck DF. Effect of synchronous activation of medullary inspiratory bulbo-spinal neurons on phrenic nerve discharge in cat. J Physiol. 1984;374:241–254. doi: 10.1113/jphysiol.1984.sp015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenik V, Davies RO, Pack AI, Kubin L. Differential suppression of upper airway motor activity during carbachol-induced REM sleep-like atonia. Am J Physiol. 1998;275:R1013–1024. doi: 10.1152/ajpregu.1998.275.4.R1013. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Gang S, Lei L. Reappraisal of the inspiratory effect of Bötzinger complex on phrenic nerve discharge. Respir Physiol. 1996;105:17–21. doi: 10.1016/0034-5687(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci. 2002;22:3806–3816. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. J Neurosci. 1996;16:6526–6536. doi: 10.1523/JNEUROSCI.16-20-06526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Bötzinger complex in the cat. Exp Brain Res. 1990;81:639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Koshiya N, Smith JC. Isolation of the kernel for respiratory rhythm generation in a novel preparation: the pre-Bötzinger complex ‘island’. J Neurophysiol. 2001;85:1772–1776. doi: 10.1152/jn.2001.85.4.1772. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. Tonic sympathetic chemoreflex after blockade of respiratory rhythmogenesis in the rat. J Physiol. 1996;491:859–869. doi: 10.1113/jphysiol.1996.sp021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Bellingham MC, West MJ, Pilowsky P. Limitations of the technique of pressure microinjection of excitatory amino acids for evoking responses from localized regions of the CNS. J Neurosci Methods. 1988;26:169–179. doi: 10.1016/0165-0270(88)90166-5. [DOI] [PubMed] [Google Scholar]
- Liu Y-Y, Ju G, Wong-Riley MTT. Distribution and colocalization of neurotransmitters and receptors in the pre-Bötzinger complex of rats. J Appl Physiol. 2001;91:1387–1395. doi: 10.1152/jappl.2001.91.3.1387. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Feldman JL, Speck DF. Respiratory motoneuronal activity is altered by injections of picomoles of glutamate into cat brain stem. J Neurosci. 1986;6:2384–2392. doi: 10.1523/JNEUROSCI.06-08-02384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Feldman JL, Speck DF, Ellenberger HH, Smith JC. Functional heterogeneity of dorsal, ventral, and pontine respiratory groups revealed by micropharmacological techniques. In: von Euler C, Lagercrantz H, editors. Neurobiology of the Control of Breathing. Karolinska Institute Nobel Conference Series. New York: Raven; 1987. pp. 201–208. [Google Scholar]
- McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clin Exp Pharmacol Physiol. 2000a;27:126–131. doi: 10.1046/j.1440-1681.2000.03193.x. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Monnier A, Ptak K, Zummo G, Zhang Z, Alheid GF. Respiratory rhythm generation: preBötzinger neuron discharge patterns and persistent sodium current. Adv Exp Med Biol. 2001;499:147–152. doi: 10.1007/978-1-4615-1375-9_23. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Ramirez JM, Alford S, Zuperku EJ. Unraveling the mechanism for respiratory rhythm generation. Bioessays. 2000b;22:6–9. doi: 10.1002/(SICI)1521-1878(200001)22:1<6::AID-BIES3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Monteau R, Hilaire G. Spinal respiratory motoneurons. Prog Neurobiol. 1991;37:83–144. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Retrotrapezoid nucleus glutamate injections: long-term stimulation of phrenic activity. J Appl Physiol. 1994;76:760–772. doi: 10.1152/jappl.1994.76.2.760. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Bötzinger complex in the cat suppresses respiratory rhythm generation in vivo. J Physiol. 1998;509:245–254. doi: 10.1111/j.1469-7793.1998.245bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin L, Gillon JY, Saunier CF, Chouvet G, Ghignone M. Continuous volume infusion improves circulatory stability in anesthetized rats. J Neurosci Methods. 1989;30:77–83. doi: 10.1016/0165-0270(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Schwarzacher SW, Pierrefiche O, Olivera BM, Richter DW. Selective lesioning of the cat pre-Bötzinger complex in vivo eliminates breathing but not gasping. J Physiol. 1998;507:895–907. doi: 10.1111/j.1469-7793.1998.895bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Zuperku EJ, Alheid GF, Lieske SP, Ptak K, McCrimmon DR. Respiratory rhythm generation: converging concepts from in vitro and in vivo approaches? Respir Physiol Neurobiol. 2002;131:43–56. doi: 10.1016/s1569-9048(02)00036-8. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Paton JF, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern. II. Network models of the central respiratory pattern generator. J Neurophysiol. 1997;77:2007–2026. doi: 10.1152/jn.1997.77.4.2007. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Bötzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol. 1999;407:583–597. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Segers LS, Shannon R, Saporta S, Lindsey BG. Functional associations among simultaneously monitored lateral medullary respiratory neurons in the cat. I. Evidence for excitatory and inhibitory actions of inspiratory neurons. J Neurophysiol. 1987;57:1078–1100. doi: 10.1152/jn.1987.57.4.1078. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon EC. Modulation of gasp frequency by activation of pre-Bötzinger complex in vivo. J Neurophysiol. 2002;87:1664–1668. doi: 10.1152/jn.00742.2001. [DOI] [PubMed] [Google Scholar]
- Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Bötzinger complex in vivo. J Neurophysiol. 1999;81:1150–1161. doi: 10.1152/jn.1999.81.3.1150. [DOI] [PubMed] [Google Scholar]
- Sun QJ, Goodchild AK, Chalmers JP, Pilowsky PM. The pre-Bötzinger complex and phase-spanning neurons in the adult rat. Brain Res. 1998;809:204–213. doi: 10.1016/s0006-8993(98)00872-5. [DOI] [PubMed] [Google Scholar]
- Tian G-F, Peever JH, Duffin J. Bötzinger-complex expiratory neurons monosynaptically inhibit phrenic motoneurons in the decerebrate rat. Exp Brain Res. 1998;122:149–156. doi: 10.1007/s002210050502. [DOI] [PubMed] [Google Scholar]
- Tian G-F, Peever JH, Duffin J. Mutual inhibition between Bötzinger-complex bulbospinal expiratory neurons detected with cross-correlation in the decerebrate rat. Exp Brain Res. 1999;125:440–446. doi: 10.1007/s002210050701. [DOI] [PubMed] [Google Scholar]
- Tolentino-Silva FP, Campos RR, Jr, Russo AK, Cravo SL, Lopes OU. Cardiorespiratory effects of L-glutamate microinjected into the rat ventral medulla. Respir Physiol. 1997;108:23–33. doi: 10.1016/s0034-5687(97)02530-9. [DOI] [PubMed] [Google Scholar]
- Von Euler C. Brain stem mechanisms for generation and control of breathing pattern. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, section 3, The Respiratory System, vol. II, Control of Breathing. Bethesda: American Physiological Society; 1986. pp. 1–67. [Google Scholar]
- Wallenstein S, Zucker CL, Fleiss JL. Some statistical methods useful in circulation research. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Wang H, Germanson TP, Guyenet PG. Depressor and tachypneic responses to chemical stimulation of the ventral respiratory group are reduced by ablation of neurokinin-1 receptor-expressing neurons. J Neurosci. 2002;22:3755–3764. doi: 10.1523/JNEUROSCI.22-09-03755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FT, Wu ZA, Li ZH, Li YR. Effect of blocking medial area of nucleus retrofacialis on respiratory rhythm. Respir Physiol. 1991;85:73–81. doi: 10.1016/0034-5687(91)90007-6. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Barillot JC, Bianchi AL. Are the post-inspiratory neurons in the decerebrate rat cranial motoneurons or interneurons? Brain Res. 1991;551:256–266. doi: 10.1016/0006-8993(91)90940-w. [DOI] [PubMed] [Google Scholar]


