Abstract
The precise role of nitric oxide (NO) in cutaneous active vasodilatation in humans is unknown. We tested the hypothesis that NO is necessary to permit the action of an unknown vasodilator. Specifically, we investigated whether a low-dose infusion of exogenous NO, in the form of sodium nitroprusside (SNP), would fully restore vasodilatation in an area of skin in which endogenous NO was inhibited during hyperthermia. This finding would suggest a ‘permissive’ role for NO in active vasodilatation. Eight subjects were instrumented with three microdialysis fibres in forearm skin. Sites were randomly assigned to (1) Site A: control site; (2) Site B: NO synthase (NOS) inhibition during established hyperthermia; or (3) Site C: NOS inhibition throughout the protocol. Red blood cell flux was measured using laser-Doppler flowmetry (LDF) and cutaneous vascular conductance (CVC; LDF/mean arterial pressure) was normalized to maximal vasodilatation at each site. In Site B, NG-nitro-l-arginine methyl ester (l-NAME) infusion during hyperthermia reduced CVC by ∼32 % (65 ± 4 % CVCmaxvs. 45 ± 4 % CVCmax; P < 0.05). Vasodilatation was not restored to pre-NOS inhibition values in this site following low-dose SNP infusion (55 ± 4 % CVCmaxvs. 65 ± 4 % CVCmax; P < 0.05). CVC remained significantly lower than the control site with low-dose SNP infusion in Site C (P < 0.05). The rise in CVC with low-dose SNP (ΔCVC) was significantly greater in Site B and Site C during hyperthermia compared to normothermia (P < 0.05). No difference in ΔCVC was observed between hyperthermia and normothermia in the control site (Site A). Thus, NO does not act permissively in cutaneous active vasodilatation in humans but may directly mediate vasodilatation and enhance the effect of an unknown active vasodilator.
Exposure to heat stress reflexively increases skin blood flow, directing heated blood from the body core to the cooler skin surface. These blood flow changes in human skin are controlled by the sympathetic nervous system through an adrenergic vasoconstrictor mechanism and an active vasodilator mechanism (Grant & Holling, 1938). During heat stress, the initial increase in skin blood flow is due to a passive withdrawal of vasoconstrictor tone. As core temperature continues to rise, the active vasodilator mechanism mediates further increases in skin blood flow (Grant & Holling, 1938). This active vasodilator system is known to be associated with cholinergic nerves, where pre-synaptically blocking cutaneous cholinergic nerves with botulinum toxin abolishes both sweating and active vasodilatation (Kellogg et al. 1995). One theory suggests that a vasoactive neurotransmitter may be co-released with acetylcholine from sympathetic cholinergic nerves, where acetylcholine mediates the sweat response and the unknown neurotransmitter mediates active vasodilatation. However, there is evidence to suggest that sweating and active vasodilatation are mediated by separate nerves, which are both sensitive to blockade with botulinum toxin (Crandall et al. 1995). More recent studies have demonstrated a role for NO in active cutaneous vasodilatation in humans (Kellogg et al. 1998; Shastry et al. 1998).
In an elegant series of experiments, Bishop and collegues sought to determine the precise role of nitric oxide (NO) in active vasodilatation. Taylor & Bishop (1993) used the rabbit ear as a model and demonstrated that active vasodilatation was abolished when endogenous production of NO by NO synthase (NOS) was inhibited during hyperthermia. Further, Farrell & Bishop (1995) completely restored active vasodilatation in the rabbit ear with the presence of a very low dose of the exogenous NO donor sodium nitroprusside (SNP). Specifically, the dose of SNP given did not change ear blood flow during normothermia. However, the same dose given during hyperthermia, following NOS inhibition, fully restored active vasodilatation. Farrell & Bishop (1995) defined the complete restoration of ear blood flow by this dose of SNP as a ‘permissive’ role for NO in active vasodilatation. This permissive role for NO has not been sufficiently explored in human skin. In humans, NO is an important component in active vasodilatation as NOS inhibition during hyperthermia attenuates skin blood flow by approximately 30 % (Kellogg et al. 1998; Shastry et al. 1998). That is, the presence of NO is required for the full expression of the active vasodilator system. Since the inhibition of NO production in humans does not completely abolish active vasodilatation, the role of NO in the rabbit ear and human skin may differ. The role of NO in human skin is important to our understanding of the control of skin blood flow. The NO mechanism may be affected by various pathologies (Clough & Church, 2002) as well as aging (Holowatz et al. 2003), thus affecting overall thermoregulatory control.
Using the available evidence as background, at least three hypotheses exist for the role of NO in active vasodilatation in humans. First, there may be more than one unknown vasodilator, and the action of at least one unknown vasodilator is dependent on the presence of NO (i.e. NO is ‘permissive’ for this substance). Second, NO is acting independently of other neurotransmitters during active vasodilatation and directly mediates a portion of active vasodilatation. Third, NO interacts in a ‘synergistic’ manner with a single unknown neurotransmitter. That is, NO enhances the vasodilator action of this substance. Specifically, our goal was to test the hypothesis that NO is permissive for an unknown vasoactive substance in cutaneous active vasodilatation in human skin.
METHODS
Subjects
Five men (mean age 25.6 ± 6.3) and three women (mean age 21.0 ± 1.0 years) volunteered for this study. Further, we recruited an additional six subjects (3 men and 3 women) to participate in a follow-up protocol. Institutional Review Board approval was obtained and each subject gave informed consent prior to participation. All subjects underwent a standard health screening and were healthy, normotensive, non-smokers. All experiments conformed with the guidelines contained in the Declaration of Helsinki.
Instrumentation
Subjects were instrumented for an electrocardiogram which was monitored throughout the protocol with a CardioCap monitor (Datex-Ohmeda, Tewksbury, MA, USA), and arterial pressure was assessed by brachial auscultation every 5 min. A water-perfused suit that covers the entire body except the face, hands, and area of skin being studied was used to control whole-body skin temperature. This suit was covered with a water impermeable rain suit to limit evaporative heat loss. Whole-body skin temperature was determined by the average of four copper-constantan thermocouples placed on the thigh, calf, abdomen and chest. An index of core body temperature was measured continuously with a thermistor placed in the sublingual sulcus (Tor).
Three microdialysis fibres (MD 2000; Bioanalytical Systems, West Lafayette, IN, USA) with 10-mm-long, 20 kDa molecular mass cut-off membranes were placed in the skin of the ventral aspect of the non-dominant forearm. The microdialysis probe was placed with a 25-gauge needle inserted through the dermis of the skin using sterile techniques in the absence of anaesthesia. The probe was then threaded through the internal lumen of the needle and the needle was withdrawn, leaving the membrane in place. The fibre was taped in place and perfused with lactated Ringer solution at a rate of 2 μl min−1 with a microinfusion pump (Harvard Apparatus, Holliston MA and CMA/102; CMA Microdialysis, Stockholm, Sweden). Sites were at least 5 cm apart.
To obtain an index of skin blood flow, cutaneous red blood cell flux was measured directly over the three microdialysis sites by laser-Doppler flowmetry (MoorLAB, Moor Instruments, UK) with integrated laser-Doppler probes. Each integrated probe has one optic fibre emitting a laser light surrounded by eight receiving fibres in a 2 mm ring. The integrated probe provides a greater surface area measured by the laser-Doppler system than single point laser-Doppler probes. After placement of the microdialysis fibres, skin blood flow was monitored to determine that the insertion trauma had resolved before beginning the protocol (between 90 and 150 min).
Protocol
Our general protocol was based on the model developed by Farrell & Bishop (1995) for studies performed in the rabbit ear. Subjects underwent a protocol designed to examine the skin blood flow responses to a 15 min low-dose infusion (0.5 μm at 2.0 μl min−1) of the exogenous NO donor SNP (Nitropress, Abbott labs, Chicago, IL, USA) during normothermia and with NOS inhibition during established hyperthermia. If low-dose SNP infusion fully restores active vasodilatation, in a skin site where NOS is inhibited during hyperthermia, we would conclude that NO is necessary to permit the action of at least one vasoactive neurotransmitter. If active vasodilatation is not fully restored, and the rise in skin blood flow to low-dose SNP is similar in normothermia and hyperthermia, we would conclude that NO directly mediates a portion of the active vasodilatation. Lastly, if active vasodilatation is not fully restored and the rise in skin blood flow to low-dose SNP is greater in hyperthermia than in normothermia, we would conclude that, in addition to its direct effects, NO interacts and enhances the dilatation of a vasoactive neurotransmitter. This result would support a ‘synergistic’ role for NO in active vasodilatation.
Thermoneutral water (33 °C) was perfused through the suit during normothermic conditions, and baseline measurements of at least 15 min preceded the infusion of low-dose SNP. The hyperthermic condition involved perfusing water through the suit at 50 °C for ∼90 min. This was sufficient to raise Tor by ∼1.0 °C, which was sustained for the remainder of the heating protocol. The low-dose SNP was again infused during the hyperthermic condition in each microdialysis site. Following hyperthermia, 28 mm SNP was infused (4 μl min−1) to maximally vasodilate the skin at each microdialysis site to enable comparison of relative skin blood flow values. This dose has been previously shown to elicit maximal skin blood flow (Kellogg et al. 1999; Minson et al. 2002). The three microdialysis sites were randomly assigned to serve as a control site (Site A), a site receiving NOS inhibitor during established hyperthermia (Site B), or a site receiving NOS inhibitor throughout normothermia and hyperthermia (Site C). A schematic diagram of the specific experimental and control protocols at each microdialysis site is presented in Fig. 1.
Figure 1. A schematic drawing of the experimental protocol depicting specific infusions at each site during normothermic and hyperthermic conditions.
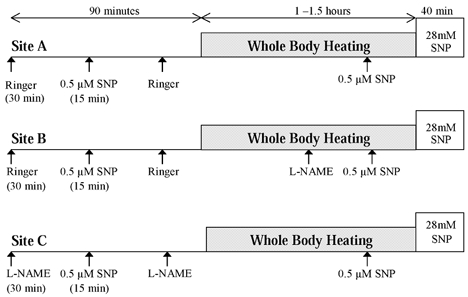
Site A, control, received infusion of low-dose SNP during both conditions. Site B received low dose SNP under normothermic conditions, and following NOS inhibition after established heating. Site C received NOS inhibitor throughout the protocol, prior to normothermic infusion of low-dose SNP.
Site A
This site served as the control site to ensure that the 15 min low-dose SNP was sufficient to cause only minimal dilation in human skin during both normothermic and hyperthermic conditions, and for comparison with the experimental sites. This site received only the low-dose SNP infusions during the normothermic and hyperthermic conditions.
Site B
This site served as an experimental site, receiving the 15 min low-dose SNP infusion following baseline measurements in the normothermic condition. During established hyperthermia, defined as a rise in Tor of at least 0.8 °C, a 10 mm concentration of the NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME; CalBiochem, San Diego, CA, USA) dissolved in Ringer solution was infused at a rate of 2.0 μl min−1. The hyperthermic infusion of low-dose SNP followed the drop in skin blood flow with NOS inhibition.
Site C
This site was infused with the NOS inhibitor l-NAME 30 min prior to the 15 min low-dose SNP in the normothermic condition. The infusion of l-NAME continued throughout hyperthermia. This was to inhibit endogenous NO produced under normothermic conditions and thereby control for possible effects of SNP on the rise in skin blood flow in an l-NAME-treated site. In addition, this site controlled for any effect of endogenous NO production during the initial rise in skin blood flow (before an increase in Tor of 0.8 °C) that may have an impact on vasodilatation later in the heat stress.
Data acquisition and analysis
Data were digitized and stored at 20 Hz on a computer and were analysed offline using signal processing software (Windaq, Dataq Instruments, Akron, OH, USA). Skin blood flow was assessed by averaging laser-Doppler flux (LDF) values over a stable 5 min period. For data analysis, skin blood flow was expressed as cutaneous vascular conductance (CVC), calculated as LDF (mV)/mean arterial pressure (MAP; mmHg), and normalized to the maximal levels achieved during infusion of 28 mm SNP.
To compare the changes in CVC under normothermic conditions to low-dose SNP, values were obtained at baseline and from the rise during the low-dose SNP infusion at each site. These values were compared using a two-way repeated measures analysis of variance (ANOVA). In the hyperthermic condition, CVC values were obtained in all microdialysis sites at three points: (1) following a rise in Tor of ∼0.8 °C and prior to l-NAME infusion in Site B, (2) just prior to low-dose SNP infusion following the drop in CVC with l-NAME, and (3) during low-dose SNP infusion. CVC values from the hyperthermic condition were also compared by two-way repeated measures ANOVA. Tukey's post hoc analysis was used to determine when significant differences occured. To compare the dilatation to exogenous NO infusion between normothermia and hyperthermia, ΔCVC was calculated by subtracting values just prior to the low-dose SNP infusion from those values obtained during the low-dose infusion. The ΔCVC values were compared by Student's paired t test between the normothermic and hyperthermic condition within each site. Associated P values of < 0.05 were considered statistically significant. All values are presented as means ±s.e.m.
Follow-up study
Moncada et al. (1991) demonstrated that NOS inhibition enhanced the relaxation responses to SNP in vascular smooth muscle. That is, the NO pathway is ‘super-sensitive’ to exogenous NO when endogenous NO production was previously inhibited. Therefore, we performed a follow-up study to determine if a ‘super-sensitivity’ to our low-dose NO infusion could explain our findings from the first protocol. We designed the follow-up protocol to provide a continuous supply of NO to the smooth muscle in an area of skin where the production of endogenous NO was inhibited during hyperthermia. This continuous infusion addresses the possible super-sensitivity from NOS inhibition. Subject monitoring (ECG, blood pressure, skin temperature and Tor) and instrumentation for the heating protocol was the same as the original protocol. Two microdialysis fibres were placed in the skin of the ventral forearm and skin blood flow was assessed by laser-Doppler flowmetry as discussed above. As in the initial protocol, whole-body heating consisted of perfusing the suit with 50 °C water for ∼1 h. Site 1 was infused with lactated Ringer solution until hyperthermia was established. Site 1 then received an infusion of 10 mm l-NAME at a rate of 2 μl min−1 to inhibit NOS. Site 2 received a continuous infusion of low-dose SNP (0.5 μm) prior to and throughout hyperthermia. This provided a constant level of NO available to the vascular smooth muscle throughout the protocol. Low-dose SNP and l-NAME diluted in Ringer solution were infused in this site during established hyperthermia (final concentrations of 0.5 μm SNP and 10 mm l-NAME). Maximal skin blood flow values were obtained by the infusion of 28 mm SNP through each microdialysis site following hyperthermia.
RESULTS
Presented in Fig. 2 is a representative tracing of each site from one subject demonstrating the normothermic and hyperthermic condition and the drug infusion in each microdialysis site.
Figure 2. Representative tracings from one subject displaying CVC repsonses at each microdialysis site.
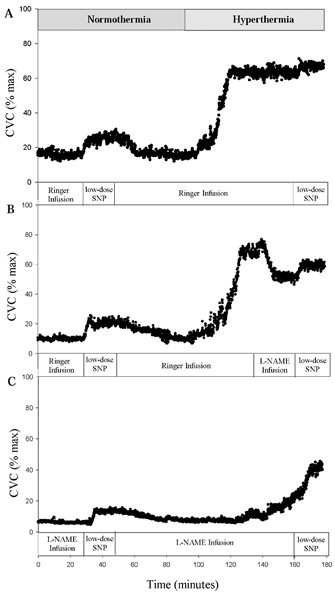
A, representative tracing of cutaneous vascular conductance in Site A with low-dose SNP infusion during normothermic and hyperthermic conditions. B, representative tracing from Site B, showing the response to low-dose SNP infusion during normothermic conditions and following NOS inhibition with established hyperthermia. C, representative tracing from Site C where NOS inhibitor was present throughout the normothermic and hyperthermic infusions of low-dose SNP.
Normothermic condition
Baseline CVC values were 11 ± 2 % CVCmax, 10 ± 2 % CVCmax and 7 ± 1 % CVCmax in Site A, Site B and Site C, respectively. With the low-dose SNP infusion, CVC values rose to 16 ± 4 % CVCmax (Δ5 ± 2 % CVCmax from baseline) in Site A, 17 ± 5 % CVCmax (Δ7 ± 2 % CVCmax from baseline) in Site B, and 12 ± 2 % CVCmax (Δ5 ± 2 % CVCmax from baseline) in Site C. There was a significant main effect during the normothermic condition, where low-dose SNP infusion was sufficient to increase CVC during the normothermic condition in the three microdialysis sites (P < 0.05). There were no significant differences in CVC between the sites during baseline or from low-dose SNP infusion.
Hyperthermic condition
Figure 3 presents the group data during the hyperthermic condition. Figure 4 contains group data from ΔCVC values to low-dose SNP infusion during normothermic and hyperthermic conditions. Hyperthermia increased CVC in Site A to 68 ± 6 % CVCmax. Low-dose SNP infusion in this site increased CVC to 72 ± 6 % CVCmax. The ΔCVC to low-dose SNP infusion during hyperthermia was not significantly different from ΔCVC during the normothermic condition (Fig. 4).
Figure 3. Mean (+s.e.m.) CVC responses from each microdialysis site.
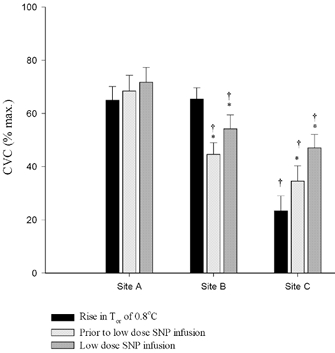
Responses were measured at Tor∼0.8 ° C, just prior to low-dose SNP infusion, and the during low-dose SNP infusion. * Significantly different from previous CVC measurement within the microdialysis site (P < 0.05); † significantly different from the control microdialysis site (P < 0.05).
Figure 4. Mean (+s.e.m.) ΔCVC responses (CVC during low-dose SNP - CVC prior to the infusion) to exogenous NO infusion during the normothermia and hyperthermia.
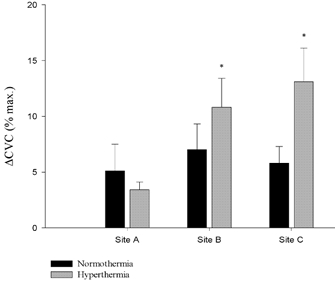
*Significant difference from the normothermic condition (P < 0.05).
Hyperthermia increased CVC in Site B to 65.4 ± 4.3 prior to NOS inhibition. The inhibition of NOS significantly reduced CVC by ∼32 % to 45 ± 4 % CVCmax (P < 0.05). This CVC value was not significantly different from the same time period in Site C, where NOS was inhibited throughout hyperthermia. Low-dose SNP infusion significantly increased CVC in Site B to 55 ± 4 % CVCmax (P < 0.05). Importantly, the increase in CVC with low-dose SNP infusion remained significantly lower than CVC values obtained prior to NOS inhibition (P < 0.05). The ΔCVC to low-dose SNP infusion during hyperthermia in Site B was significantly greater than the low-dose infusion in this site under the normothermic condition (P < 0.05; Fig. 4).
Hyperthermia increased CVC in Site C to 35 ± 6 % CVCmax prior to the low-dose SNP infusion, which was significantly lower than Site A at the same time period (P < 0.05). Low-dose SNP infusion significantly increased CVC to 47 ± 5 % CVCmax (P < 0.05), which was not significantly different from the low-dose SNP infusion during hyperthermia in Site B. The ΔCVC to low-dose SNP infusion during hyperthermia was significantly greater than ΔCVC in the normothermic condition (P < 0.05; Fig. 4).
Follow-up study
Presented in Fig. 5 are the group data from both microdialysis sites showing the reduction in CVC with NOS inhibition during established hyperthermia. Baseline CVC values in Site 1 were 12 ± 6 % CVCmax and increased to 63 ± 5 % CVCmax during hyperthermia. With NOS inhibition, CVC decreased to 40 ± 5 % CVCmax, falling 38 ± 6 % from CVC values obtained prior to l-NAME infusion. Baseline CVC in Site 2 (in which low-dose SNP was continuously infused) was 19 ± 4 % CVCmax. During hyperthermia, CVC rose to 68 ± 6 % CVCmax prior to NOS inhibition. Inhibiting endogenous NO production, along with the continued exogenous source of NO, caused CVC to fall to 51 ± 5 % CVCmax, which is a reduction of 24 ± 4 % from pre NOS inhibition values. The reduction in CVC with NOS inhibition was significantly less with continuous low-dose SNP infusion (P < 0.05; Fig. 5).
Figure 5. The fall in CVC (mean +s.e.m.) with NOS inhibition following established hyperthermia (follow-up study).
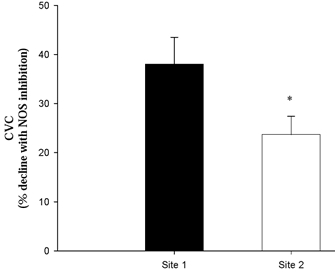
Site 1 received an infusion of Ringer solution prior to NOS inhibition, whereas Site 2 received a continuous infusion of 0.5 μm SNP prior to and during NOS inhibiton. * Significant difference from Site 1(P < 0.05).
DISCUSSION
The primary finding of our study was that, in humans, exogenous NO does not completely restore cutaneous active vasodilatation when NOS is inhibited. Therefore, we conclude that NO does not play a ‘permissive’ role for an unknown vasoactive substance released during hyperthermia. This finding is contrary to our hypothesis, which was based on the studies of Farrell & Bishop (1995). These authors demonstrated that the infusion of low doses of NO donor (SNP) completely restored active vasodilatation in the rabbit ear when endogenous NO production was inhibited (Farrell & Bishop, 1995). The findings in the rabbit ear suggest that the presence of a critical level of NO is necessary to permit the action of an unknown neurotransmitter released in response to increased core temperature. However, several studies have demonstrated a reduction of approximately 30 % in active vasodilatation with NOS inhibition in humans (Kellogg et al. 1998; Shastry et al. 1998, 2000), suggesting that the mechanism of active vasodilatation in the rabbit ear and human skin differs.
Our results suggest that NO directly causes a portion of vasodilatation during hyperthermia. This was demonstrated in Site B, where CVC remained significantly lower (55 ± 4 % CVCmax) than pre-NOS inhibition values (65 ± 4 % CVCmax) upon infusion of an endothelium-independent source of NO (Fig. 3). If low-dose SNP infusion completely restored CVC to pre-NOS inhibition levels, our data would suggest that NO acts in a permissive manner during active vasodilatation in human skin. In no case did the low-dose SNP infusion restore full vasodilatation during hyperthermia. Consistent with our findings, Kellogg et al. (2002) were able to measure increases in NO concentration in the cutaneous interstitium during heat stress, providing further evidence for the direct role of NO. In contrast, Crandall & MacLean (2001) reported that NO concentration in the interstitium does not significantly increase during hyperthermia. The discrepancy in these results may be due to the different analysis methods employed in the two studies. Crandall & Maclean (2001) indirectly determined NO levels by measuring the by-products of NO metabolism, nitrite and nitrate from dialysate samples, which may be insensitive to small changes in NO concentration. Kellogg et al. (2002) directly measured changes in NO levels via a NO-sensitive probe inserted into the cutaneous interstitium.
The source of NO in the skin during hyperthermia is still unknown. One possible source is the stimulation of NO from the endothelium by the release of a neuropeptide from sympathetic active vasodilator nerves. Vasoactive intestinal peptide, histochemically identified as co-existing with acetycholine in cutaneous cholinergic nerves (Hokfelt et al. 1980; Schulze et al. 1997), has been shown to stimulate NO release in isolated smooth muscle cells (Grider et al. 1992). This neuropeptide remains a strong candidate for the unknown vasodilator co-released with acetylcholine during heating. Therefore, its release during hyperthermia may stimulate NO production from the vascular endothelium in human skin.
Another possible source for NO is from mechanical sheer stress, a mechanism known to initiate the release of NO from the vascular endothelium (Rubanyi et al. 1986). In this scenario, the unknown vasodilator increases skin blood flow which then stimulates NO production by a shear stress mechanism. However, the role of flow-mediated NO production in human skin is unclear.
A third possible source for NO could be acetylcholine release from sudomotor nerves, which may be important early in heating. It has been reported that active vasodilatation is delayed with muscarinic blockade by atropine infusion (Kellogg et al. 1995). However, atropine infusion late in hyperthermia does not affect the vasodilatory response (Shastry et al. 2000). A recent study by Shibasaki et al. (2002) demonstrated that early in heating, vasodilatation was greater in the presence of a cholinesterase inhibitor but not when NOS was inhibited, suggesting more available NO from the acetylcholine-generated source.
In addition to the direct role of NO, our data further suggest that NO interacts to some degree with a neurotransmitter in a ‘synergistic’ fashion. In this construct, the dilatation produced in the presence of NO and another vasoactive substance is greater than the sum of their independent actions. Our data demonstrate that the increase in CVC (ΔCVC) to exogenous NO during hyperthermia was ∼11 % CVCmax compared to ∼7 % CVCmax during normothermia, at a site in which NOS was inhibited (Site B). Similar responses were observed in Site C, in which NOS was inhibited throughout the protocol, suggesting that the production of NO early in hyperthermia does not affect this response (Fig. 4). Taken together, these results demonstrate a potentially important interaction between exogenous NO and a vasoactive substance released during hyperthermia that does not occur under normothermic conditions. In other words, the response to exogenous NO is greater in the presence of substances released from sympathetic active vasodilator nerves.
The mechanism of this synergistic interaction may occur at two levels. First, NO production may pre-junctionally enhance the release of a neurotransmitter. This elevated level of neurotransmitter in turn stimulates the release of more NO (Grider et al. 1992). A second possible interaction is at the level of the second messenger. NO initiates vasodilatation by activating the soluble guanylate cyclase pathway and, through a cGMP second-messenger cascade, ultimately leads to smooth muscle relaxation and dilatation. This cGMP from the NO pathway, may interact with the cAMP produced from the adenylate cyclase pathway (Farrell & Bishop, 1995; Carvajal et al. 2000) initiated by the release of a neurotransmitter. Applying the above concepts to active vasodilatation, the unknown neurotransmitter may interact with NO, either pre-junctionally or at the level of the second messenger, thereby enhancing the vasodilatory response during hyperthermia. Of the possible candidates for the unknown vasodilator, vasoactive intestinal peptide (Grider et al. 1992) and calcitonin gene-related peptide (Fiscus et al. 1994) have been shown to behave in this manner. However, the interaction between NO and these neuropeptides has not been examined in human skin.
In isolated vascular rings, Moncada et al. (1991) found enhanced sensitivity of the smooth muscle to exogenous NO following NOS inhibition. Thus, it is possible that the elevated response to exogenous NO noted during hyperthermia is from a ‘super-sensitivity’ of the NO pathway due to NOS inhibition. To address this issue we performed the follow-up protocol to ensure that the higher vasodilatation in response to exogenous NO during hyperthermia was not due to this ‘super-sensitivity’. In this protocol, the continuous infusion of exogenous NO provided the vascular smooth muscle with a constant source of NO, where endogenous NO production was inhibited. As the soluble guanylate cyclase enzyme is thought to be the source of the ‘super-sensitivity’ (Moncada et al. 1991), providing a constant source of NO reduces this possibility. In our follow-up study, inhibiting endogenous NO production in a site with a constant low-dose SNP infusion (Site 2) reduced CVC by 24 ± 4 % CVCmax. NOS inhibition in the absence of a constant source of low-dose SNP (Site 1) reduced CVC by 38 ± 6 % CVCmax (P < 0.05; Fig. 5). If a super-sensitivity had occurred in the original protocol, we would expect a similar reduction in CVC with l-NAME infusion at both microdialysis sites during the follow-up protocol. This finding provides further evidence that exogenous NO interacts with an unknown neurotransmitter during hyperthermia, supporting our conclusion that the enhanced response to exogenous NO noted with hyperthermia in the original protocol was not due to a ‘super-sensitivity’ of the NO pathway.
Proposed model
From our results, and the work of others, we propose a working model for the role of NO in cutaneous active vasodilatation. Early in hyperthermia, acetylcholine and one or more unknown vasoactive substances are released from sympathetic cholinergic active vasdodilator nerves in response to increasing core temperature. Acetylcholine binding to muscarinic receptors causes the release of NO from the vascular endothelium. This NO-mediated dilatation contributes to the steep increase in skin blood flow noted early in hyperthermia. Once skin blood flow begins to rise, NO is also produced from the action of the unknown neurotransmitter(s) or from other sources and interacts in a synergistic manner with the unknown vasoactive substance(s) to maintain vasodilatation. This synergistic interaction may occur at the level of the nerve, where NO pre-junctionally enhances the release of the unknown neurotransmitter, or downstream at the level of the second messenger systems.
Acknowledgments
This study was conducted by Brad W. Wilkins in partial fulfillment of the requirements for the degree of Doctor of Philosophy at the University of Oregon. This study was supported by a foundation research grant from the American College of Sports Medicine and the National Institutes of Health (Minson, RO1HL70928). We would especially like to thank the subjects who volunteered for this study, and we thank Belinda Houghton for her assistance with data collection.
REFERENCES
- Carvajal JA, Germain AM, Huidobro-Toro JP, Weiner CP. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Clough GF, Church MK. Vascular responses in the skin: an accessible model of inflammation. News Physiol Sci. 2002;17:170–174. doi: 10.1152/nips.01378.2001. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Maclean DA. Cutaneous interstitial nitric oxide concentration does not increase during heat stress in humans. J Appl Physiol. 2001;90:1020–1024. doi: 10.1152/jappl.2001.90.3.1020. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Musick J, Hatch JP, Kellogg DL, Jr, Johnson JM. Cutaneous vascular and sudomotor responses to isometric exercise in humans. J Appl Physiol. 1995;79:1946–1950. doi: 10.1152/jappl.1995.79.6.1946. [DOI] [PubMed] [Google Scholar]
- Farrell DM, Bishop VS. Permissive role for nitric oxide in active thermoregulatory vasodilation in rabbit ear. Am J Physiol. 1995;269:H1613–1618. doi: 10.1152/ajpheart.1995.269.5.H1613. [DOI] [PubMed] [Google Scholar]
- Fiscus RR, Hao H, Wang X, Arden WA, Diana JN. Nitroglycerin (exogenous nitric oxide) substitutes for endothelium-derived nitric oxide in potentiating vasorelaxations and cyclic AMP elevations induced by calcitonin gene-related peptide (CGRP) in rat aorta. Neuropeptides. 1994;26:133–144. doi: 10.1016/0143-4179(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Grant RT, Holling HE. Further observations on the vascular responses of the human limb to body warming; evidence for sympathetic vasodilator nerves in the normal subject. Clin Sci. 1938;3:273–285. [Google Scholar]
- Grider JR, Murthy KS, Jin JG, Makhlouf GM. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am J Physiol. 1992;262:G774–778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Ljungdahl A, Lundberg JM, Schultzberg M. Peptidergic neurones. Nature. 1980;284:515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, Minson CT. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003 doi: 10.1152/ajpheart.00871.2002. 10.1152/ajpheart.00871. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Liu H, Roman LJ. Bioavailable nitric oxide and cutaneous active vasodilation in humans. FASEB J. 2002;5 A824 (Abstract) [Google Scholar]
- Kellogg DL, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide and axon reflex mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- Moncada S, Rees DD, Schulz R, Palmer RM. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci U S A. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250:H1145–1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Schulze E, Witt M, Fink T, Hofer A, Funk RH. Immunohistochemical detection of human skin nerve fibers. Acta Histochem. 1997;99:301–309. doi: 10.1016/S0065-1281(97)80024-4. [DOI] [PubMed] [Google Scholar]
- Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and l-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol. 2000;88:467–472. doi: 10.1152/jappl.2000.88.2.467. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine release from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol. 2002;93:1947–1952. doi: 10.1152/japplphysiol.00036.2002. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Bishop VS. A role for nitric oxide in active thermoregulatory vasodilation. Am J Physiol. 1993;264:H1355–1359. doi: 10.1152/ajpheart.1993.264.5.H1355. [DOI] [PubMed] [Google Scholar]


