Abstract
Neuronal activity regulated pentraxin (Narp) is a secreted, synaptic protein that has been implicated in modulating synaptic transmission. However, it is unclear how Narp secretion is regulated. Since we noted prominent Narp immunostaining in vasopressin neurons of the hypothalamus and in the posterior pituitary, we assessed whether it, like vasopressin, is released into the systemic circulation in an activity-dependent fashion. Consistent with this hypothesis, electron microscopic studies of the posterior pituitary demonstrated that Narp is located in secretory vesicles containing vasopressin. Using affinity chromatography, we detected Narp in plasma and found that these levels are markedly decreased by hypophysectomy. In addition, we confirmed that injection of a viral Narp construct into the hypothalamus restores plasma Narp levels in Narp knockout mice. In checking for activity-dependent secretion of Narp from the posterior pituitary, we found that several stimuli known to trigger vasopressin release, i.e. hypovolemia, dehydration and endotoxin, elevate plasma Narp levels. Taken together, these findings provide compelling evidence that Narp is secreted from vasopressin neurons in an activity-dependent fashion.
Keywords: pituitary, hypovolemia, dehydration, hypothalamus, plasma, hypophysectomy
INTRODUCTION
The pentraxin family of proteins is composed of two subfamilies: classical pentraxins, such as serum amyloid protein and C-reactive protein, thought to be involved in mediating the innate immune response, and neuronal pentraxins, a set of three secreted proteins (Narp, NP1 and NPR) that have been localized to synaptic zones and implicated in regulating synaptogenesis and synaptic function (Tsui et al., 1996; Kirkpatrick et al., 2000). Neuronal pentraxins form large, homo- and hetero-meric complexes that are thought to bind directly to AMPA type glutamate receptors and, in this way, regulate their clustering and/or trafficking (O’Brien et al., 1999; Xu et al., 2003). As neuronal pentraxins represent a novel class of synaptic mediators, it will be important to understand how their secretion is regulated. This question has been difficult to address in conventional neuronal culture preparations by monitoring the levels of neuronal pentraxins in the media, as the large neuronal pentraxin complexes appear to remain associated with cellular membranes following secretion. In preliminary studies, we noted intense Narp staining in vasopressin neurons of the hypothalamus which extends into the posterior pituitary. Accordingly, we reasoned that examining release of Narp from vasopressin neurons via the posterior pituitary into the systemic circulation might be advantageous for assessing how Narp secretion is regulated.
EXPERIMENTAL PROCEDURES
Animals
Sprague-Dawley rats (Charles River), Narp knockout (KO) or wild type mice were used for immunohistochemical and biochemical experiments. Some rats were hypophysectomized at the Charles River facility one week before being transported to Johns Hopkins. Animal ages are reported below. Rodents were maintained on a 12 hour light/dark cycle and were given access to food and water ad libitum. Hypophysectomized rats were provided water with 5% sucrose. Experiments were approved by the Johns Hopkins University Institutional Animal Care and Use Committee.
Generation of Narp KO mice is described elsewhere (Johnson et al., submitted). Briefly, 129 genomic DNA was prepared by screening of a mouse genomic DNA BAC library. Genomic DNA regions of the mouse Narp gene were subcloned into a pBluescript vector and the targeting vector construct was based on mouse genomic DNA databases. The entire exon 2 was replaced by a neo resistance gene cassette. ES cell screening and generation of the knock out mouse line were carried out as described previously (Kim et al., 2003; Gardner et al., 2005). Deletion of exon 2 was confirmed by Southern blot and absence of Narp protein in brain was confirmed by Western blot. All control and KO mice used in these studies were generated by heterozygote breeding pairs that had been backcrossed to C57BL/6 for 4 generations.
Human plasma
To check whether Narp is present in human plasma, Dr Reti provided a specimen in accordance with local IRB regulations.
Antibodies
In these studies we used rabbit Narp polyclonal antibodies raised against either a full-length (minus the signal sequence) GST-Narp fusion protein or the C-terminal peptide (O’Brien et al., 1999; Tsui et al., 1996). Other antibodies used are: guinea pig anti-vasopressin (Peninsula) and guinea pig anti-alpha subunit (NIDDK).
Experimental procedures
We examined the regulation of plasma Narp by a variety of stressors that have previously been evaluated for their effects on vasopressin release.
(1) Hypovolemia
Rats were administered polyethylene glycol (PEG; MW 3,000, Sigma) which is known to reduce plasma volume (Kondo et al., 2004). PEG was dissolved in isotonic saline (30% wt/vol) and was injected (2% body wt) ip. Ninety minutes later, animals were injected with hypertonic (900 mosmol/kg) saline at 2% body wt ip. Animals were killed 2 hours later.
(2) Dehydration
Access to water was removed 24 hours prior to being sacrificed. Food was available during this time.
(3) Endotoxin
Animals were administered lipopolysaccharide (E. coli 055:B5) at 2.5mg/kg ip and sacrificed 12 hours later.
(4) Restraint stress
Animals were enclosed in rodent restrainers (Plas-Labs) for 2 or 3 hours. Animals restrained for 3 hours were provided with water. All manipulations were performed on at least 3 animals and yielded similar results.
Immunohistochemistry
Sections from hypothalamus and pituitary were processed as previously described (Tsui et al., 1996; Reti et al., 2002b; Reti et al., 2002c; Reti and Baraban. 2003) for single and double-labeling studies. Primary antibody concentrations were as follows:- Narp (1:1500), vasopressin (1:100), alpha subunit (1:100). Secondary antibodies (Jackson) were conjugated to cy3 or FITC and were used at 1:200. To ensure that co-localization observed in double-labeling studies does not reflect “bleed-through” of one fluorochrome into the other channel, we routinely checked that omission of either primary antibody eliminated staining in the corresponding channel.
Electron microscopy
Immunogold studies of Narp localization were done as described previously (O’Brien et al., 1999; Petralia and Wenthold, 1999; Xu et al., 2003). Briefly, rats were perfused with 4% paraformaldehyde + 0.5% glutaraldehyde and sections of tissue were frozen in liquid propane in a Leica CPC cryopreparation chamber and then freeze-substituted into Lowicryl HM-20 in a Leica AFS. Ultrathin sections on grids were incubated in 0.1% sodium borohydride plus 50 mM glycine in Tris-buffered saline plus 0.1% Triton X-100 (TBST), followed by 10% normal goat serum (NGS) in TBST. Both sides of the sections were then incubated with both primary antibodies mixed together in 1% NGS/TBST overnight at 4C. After several washing and blocking steps, the secondary immunogold antibodies (BB International Gold, distributed by Ted Pella) in 1% NGS/TBST plus 0.5% polyethylene glycol (20,000 MW) were applied to both sides of the sections for one hour at room temperature. Finally, sections were stained with uranyl acetate and lead citrate. Figures were processed in Adobe Photoshop with minimal use of levels; brightness and contrast were employed uniformly over the images. For double-labeling studies, we used Narp rabbit polyclonal antibody (1/50-1/67) with either the guinea pig alpha subunit or vasopressin antibodies (1/100). Omission of primary antibodies yielded negligible staining.
Detection of plasma Narp by affinity chromatography
As we were unable to detect Narp in native plasma by immunoblotting, we used affinity purification of Narp from rat plasma using the snake venom taipoxin (Schlimgen et al., 1995), which binds Narp with high affinity in a calcium dependent fashion. Affinity beads were generated by conjugating Sepharose CH (GE Healthcare) to taipoxin, obtained from Alomone Labs, according to the manufacturer’s instructions. Narp was concentrated on the beads in the following manner. Blood was collected by cardiac puncture into heparinized tubes. After addition of protease inhibitors (aprotinin and leupeptin, each at 1μg/mL, and 1mM phenylmethylsulphonyl fluoride), blood was centrifuged for 1 minute at 14000g and plasma was collected and added to taipoxin-conjugated beads. Beads and plasma were rotated at 4C for 75 minutes. After briefly spinning down the beads on a table-top centrifuge, the supernatant was collected and discarded. Beads were washed three times with 1mL of PBS containing1mM CaCl2. Beads were then re-suspended and thoroughly mixed in 35 μL PBS+10mM EDTA. They were allowed to sit at room temp for 5 minutes. Beads were then centrifuged and 30μL of eluate was drawn and mixed with an equal amount of Laemmli buffer and analyzed by Western blot (Reti and Baraban, 2000). In all cases when comparing Narp expression in wild type and Narp KO mice and when examining regulation of plasma Narp, samples for all lanes of a gel were prepared in parallel and equal volumes were loaded onto the gel.
Virus
A cDNA containing wild-type Narp was subcloned into the adeno-associated virus (AAV) plasmid (pAM/CBA-pl-WPRE-BGH) and packaged by Drs Bland and During to produce recombinant AAV1 (this serotype is also known as AAV2/1; Burger et al., 2004). We chose AAV type 1 as it is highly effective in delivering genes to neurons with minimal or no glial infection (Burger et al., 2004) and generates constitutive expression. Product was titered by Dr During’s lab and was approximately 5 × 1012 particles/ml.
Surgical procedures
To confirm that Narp is released into blood from the pituitary, we injected AAV expressing wild-type Narp into the paraventricular nucleus of the anterior hypothalamus of Narp KO mice and monitored Narp expression in the posterior pituitary and plasma. Animals were anesthetized with xylazine/ketamine and placed in a stereotaxic apparatus (David Kopf Instruments). Co-ordinates for unilateral injection (1.2uL injected over 8 minutes) into the paraventricular nucleus of the hypothalamus were: 0.85mm caudal to bregma, 0.15mm lateral and 5.5mm ventral from dura (Paxinos and Franklin, 2001). Animals were sacrificed 3 weeks after surgery. Brain and pituitary were processed for immunohistochemistry and plasma was collected for assaying Narp protein levels. Narp cell body staining was typically observed in a region around 500um in diameter centered on the paraventricular nucleus of the hypothalamus.
RESULTS
Narp immunostaining in the pituitary
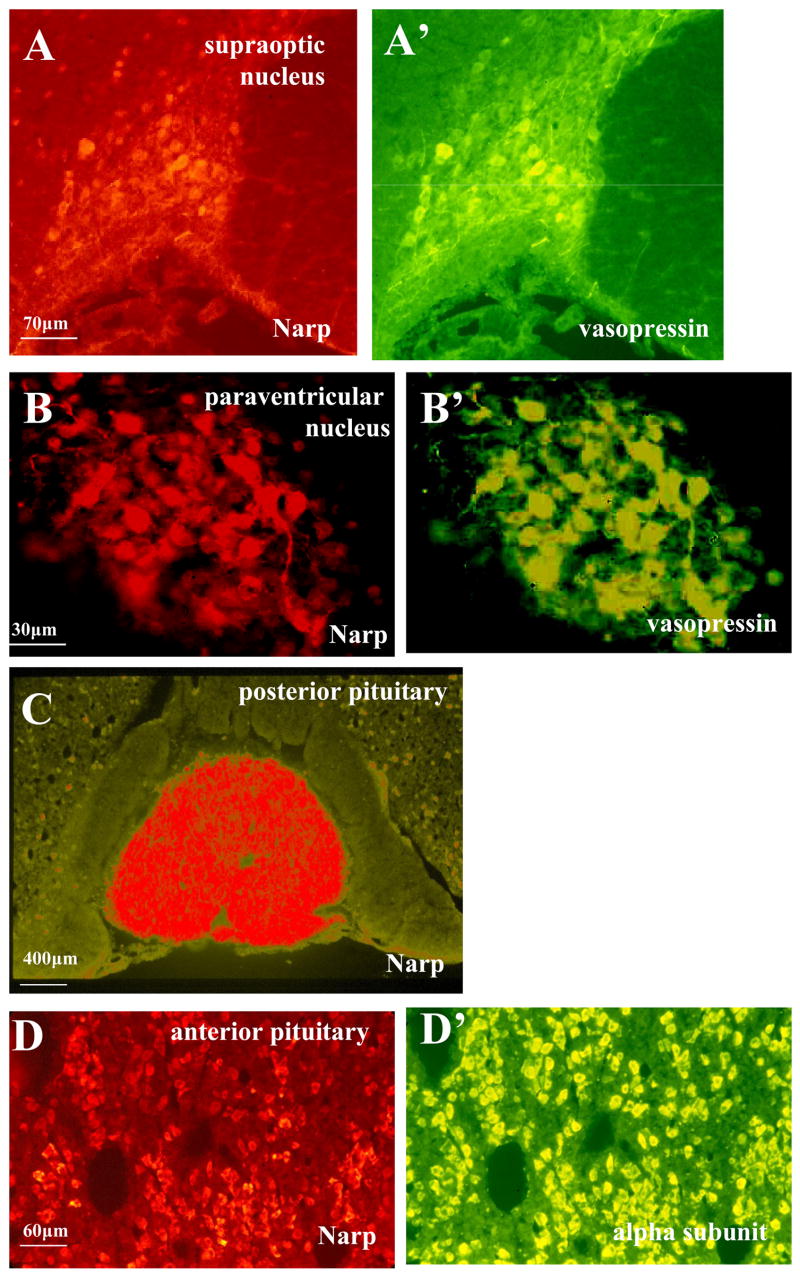
While conducting an immunohistochemical survey of Narp expression in rat brain, we found strong cell body staining in the supraoptic and paraventricular nuclei of the hypothalamus (figure 1A and B). To assess whether Narp is expressed by vasopressin neurons located in these nuclei, we performed double-labeling studies and found that nearly all of the Narp-positive neurons also stained for vasopressin and vice versa (figures 1A′ and 1B′). In contrast, oxytocin neurons were not Narp-positive (data not shown).
Figure 1.
Narp immunostaining in rat anterior hypothalamus and pituitary. Co-localization of Narp (red) and vasopressin (green) in the supraoptic (A,A′) and paraventricular nuclei (B,B′) of the rat anterior hypothalamus. (C) Intense staining of Narp terminals in the rat posterior pituitary. The intermediate lobe of the pituitary is essentially devoid of Narp. Scattered Narp cell body staining can be seen in the surrounding anterior pituitary. (D,D′) Narp (red) co-localizes with the alpha-subunit (green) in the anterior pituitary.
Previous immunohistochemical studies have shown that Narp is targeted to axons (Reti et al., 2002a). For example, in the hippocampus, prominent Narp staining is present in the mossy fiber pathway which contains axons of dentate granule cells. Accordingly, we checked whether Narp may also be targeted to the posterior pituitary which is innervated by the axons of vasopressin neurons. Staining of pituitary sections revealed robust Narp staining in the posterior pituitary (figure 1C). In contrast, the intermediate lobe was devoid of staining. Unexpectedly, we also found that a subset of anterior pituitary cells were also Narp-positive (figure 1D). To determine which subpopulation(s) of anterior pituitary cells stain for Narp, we performed double-staining with several hormone peptides secreted by these cells. These studies indicate that Narp staining is not limited to cells that express one of the major peptide hormones. Instead, Narp staining shows a high degree overlap with cells that express luteinizing hormone (LH), follicle-stimulating hormone (FSH) and thyroid-stimulating hormone (TSH), hormones which share a common alpha subunit (figure 1D′).
To confirm that Narp immunostaining detected in the pituitary reflects specific staining, we proceeded to check whether it is absent in mice with a targeted deletion of Narp. As observed in rat studies, we found strong Narp immunostaining in the posterior pituitary of wild type mice (figure 2A). In contrast, Narp staining was absent in the posterior pituitary of Narp knockout mice (figure 2A′). We also examined the pattern of Narp staining in the supraoptic and paraventricular nuclei of the mouse and found that it was much weaker than in the rat, suggesting that in mice Narp synthesized in the cell body may be more rapidly transported to the posterior pituitary than in the rat. Narp staining in mouse anterior pituitary was also relatively weak compared with that found in rats.
Figure 2.
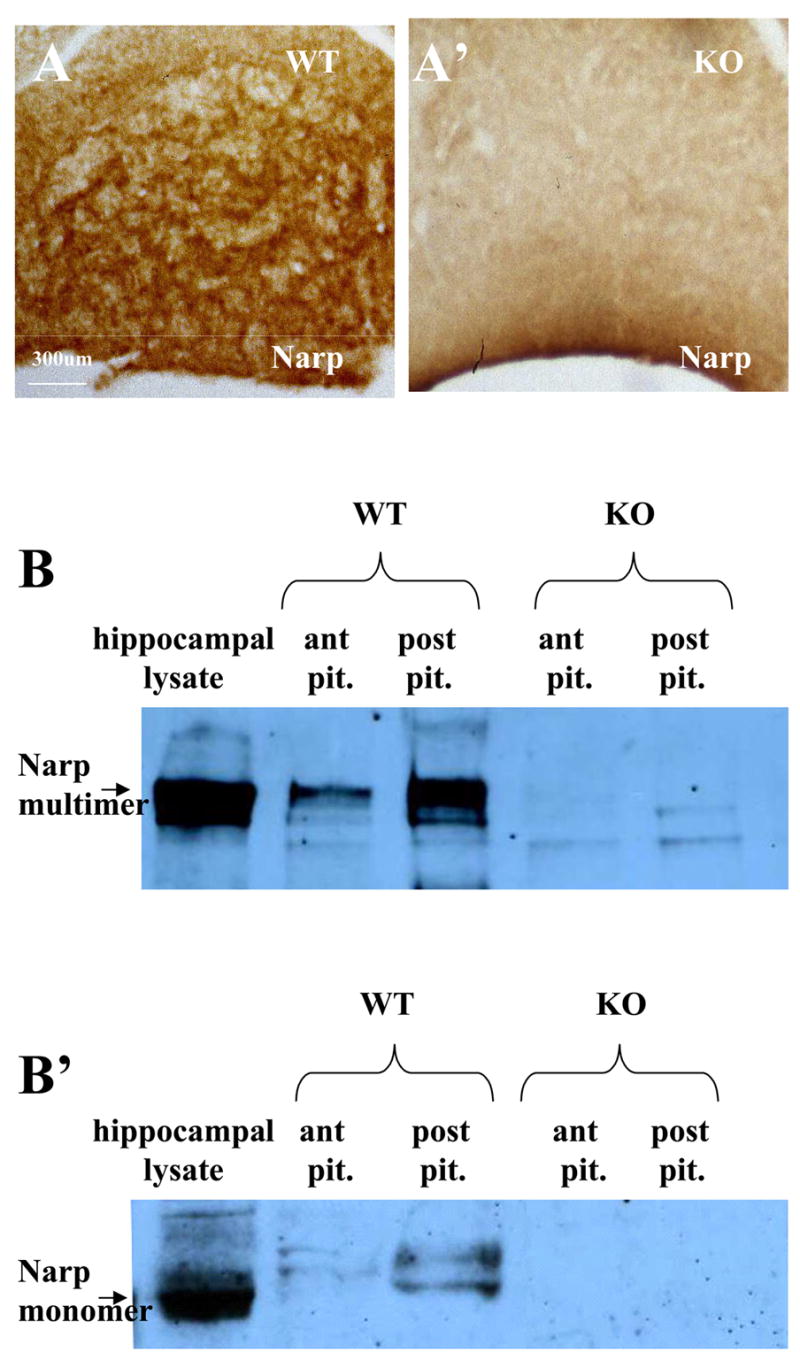
Specificity of Narp expression in pituitary. In mouse sections of the posterior pituitary processed for DAB immunohistochemistry, Narp staining is present in a wild-type mouse (A) but absent from a Narp KO (A′). (B) Western blot probed with Narp antibody showing hippocampal lysate and pituitary lysates from a wild type mouse and pituitary lysates from a Narp KO mouse. The native multimeric Narp complex is shown in (B). Addition of β-mercaptoethanol to the samples followed by boiling liberates Narp monomer (B′). Non-reduced bands in the anterior and posterior pituitary of wild-type mice co-migrate with the Narp band from hippocampal lysate. Note that equal amounts of protein were loaded into the anterior and posterior pituitary lanes and that the anterior pituitary Narp band is weaker than the posterior pituitary band indicating lower overall amounts of Narp in the anterior pituitary. In reduced lysates (B′), a duplex band is observed that migrates slightly slower than hippocampal Narp.
Immunoblot analysis of pituitary Narp
To provide additional confirmation that Narp is expressed in the pituitary, we also performed Narp immunoblot studies on mouse pituitary extracts. Previous studies characterizing Narp protein in hippocampus have demonstrated that it forms a large multimeric complex that is readily detected by immunoblot following SDS-PAGE performed under non-reducing conditions. However, under reducing conditions Narp protein migrates as a monomer with an estimated molecular weight of 46kD (Reti and Baraban, 2000). Analysis of anterior and posterior pituitary extracts under non-reducing conditions revealed a prominent band that co-migrates with the multimeric band detected in hippocampal lysates (figure 2B). Consistent with the immunostaining results, the intensity of the Narp band is much stronger in samples from posterior than anterior pituitary. Furthermore, these bands are absent in extracts prepared from Narp knockout mice (figure 2B). Although samples analyzed under reducing conditions displayed a similar intensity profile, the size of the bands detected in pituitary extracts differed from that in hippocampal samples (figure 2B′). Rather than a single major band, pituitary extracts contain two Narp bands that migrate more slowly than the hippocampal Narp band, suggesting that pituitary Narp may be subject to a different set of post-translational modifications.
EM localization of Narp in pituitary
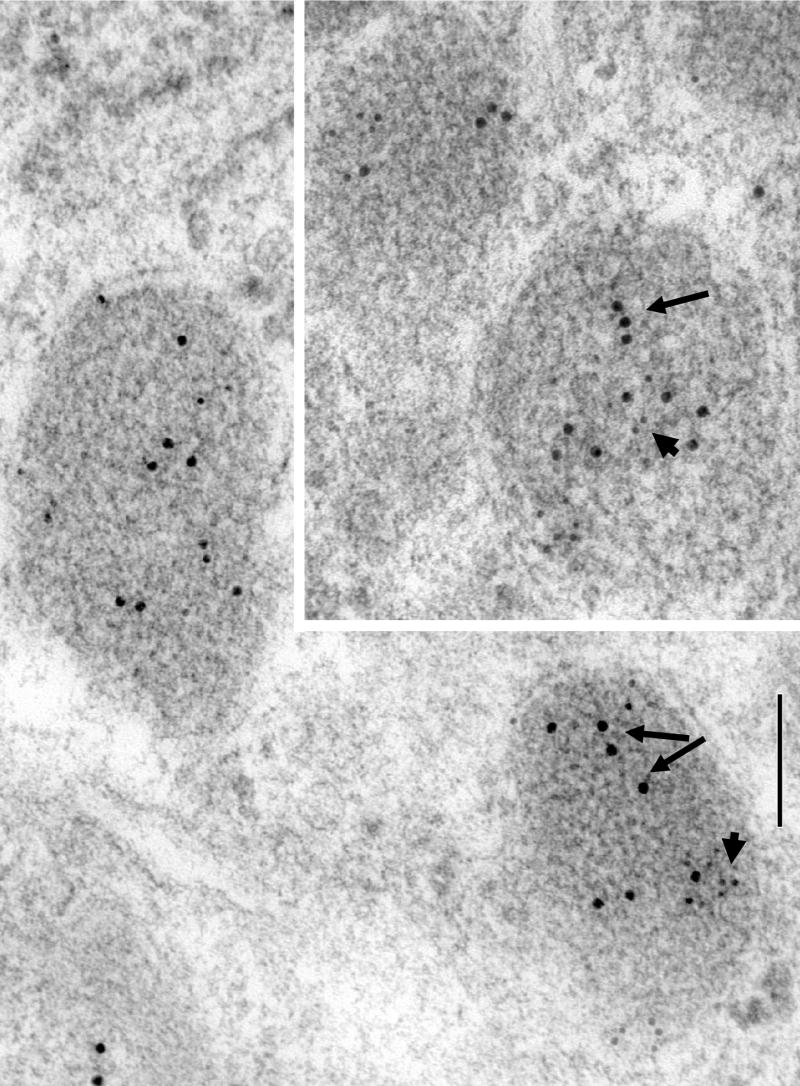
Since Narp is a secreted protein, we wanted to check if it is present in secretory vesicles in the pituitary. Accordingly, we conducted EM immunohistochemical studies on sections of anterior and posterior pituitary. Pilot studies on these sections indicated that Narp is selectively located in large dense core vesicles similar to those containing vasopressin and anterior pituitary hormones. Therefore, we proceeded to conduct double-labeling immunogold studies of Narp with vasopressin and alpha subunit in posterior and anterior pituitary sections, respectively, to assess whether they are co-localized in the same population of dense core vesicles. These studies clearly demonstrated that Narp and vasopressin are present in the same vesicles (figure 3). Similarly, numerous examples of double-labelling of Narp and alpha subunit were found in anterior pituitary sections (figure 4).
Figure 3.
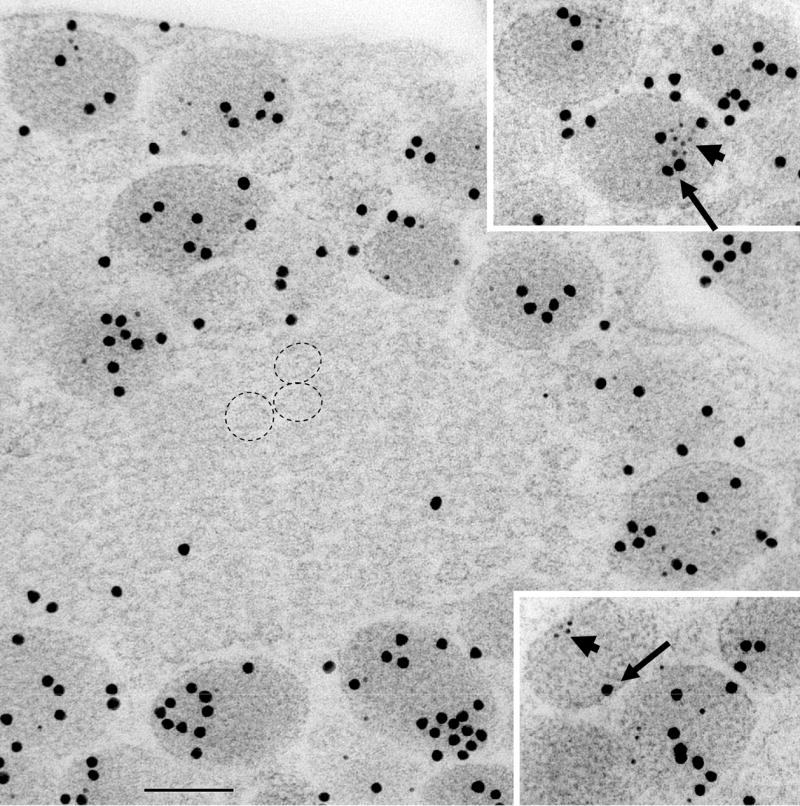
Composite of images showing cell processes in the posterior pituitary from a P35 rat double-labeled with 5 nm gold for Narp (short arrows) and 15 nm gold for vasopressin (long arrows). Note the concentration of Narp labeling in large, vasopressin-labeled secretory granules, while there is little or no labeling in small secretory vesicles (dashed circles). Scale bar is 100 nm.
Figure 4.
Composite of images showing cells in the anterior pituitary from a P35 rat double-labeled with 5 nm gold for Narp (short arrows) and 10 nm gold for alpha subunit (long arrows). Note the concentration of Narp labeling in large, alpha subunit-labeled secretory granules. Scale bar is 100 nm.
Detection of Narp in plasma
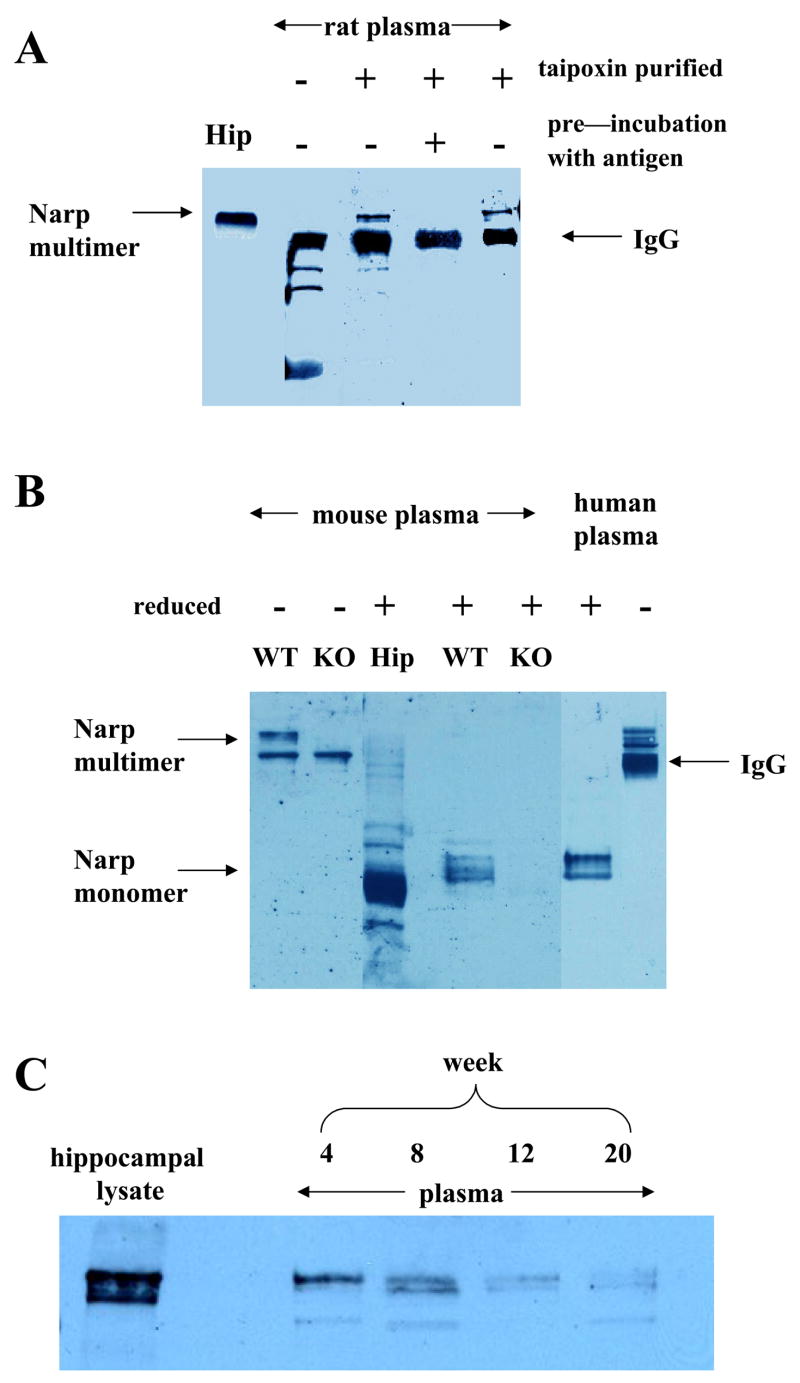
The localization of Narp to large, dense secretory vesicles in the pituitary raised the possibility that it may be released into the circulation just like other pituitary hormones. Although Narp forms large multimeric complexes estimated to be up to 12nm in cross-section (Xu et al., 2003), fenestrated capillaries have pores measuring 30 to 50 nm in diameter (Livingston, 1975; Seyama et al., 1980), suggesting that it could enter the systemic circulation. In initial studies, we were not able to detect Narp in rat plasma with our standard immunoblotting protocol. However, since Narp released from the pituitary would be diluted by the entire blood volume, we reasoned that this negative result may be due to insufficient sensitivity of this method. Accordingly, we employed taipoxin, a snake venom that binds with high affinity to Narp in the presence of calcium, as an affinity ligand to concentrate Narp from blood samples. Using this affinity column strategy, we detected a band that co-migrates with the multimeric Narp band detected in hippocampal lysates and is recognized by two Narp antibodies generated against distinct epitopes (figure 5A).
Figure 5.
Narp is present in plasma. In (A) Immunoblot of non-reduced lysates from rat plasma showing hippocampal lysate (Hip; 1st lane); native plasma (2nd lane); Narp isolated from plasma by taipoxin affinity chromatography (3rd lane) which co-migrates with Narp from hippocampal lysates. A non-specific band is present in the taipoxin eluate that is also visible in plasma (2nd lane) and is likely non-reduced IgG recognized by the secondary antibody. Preincubation of the antibody with the GST fusion protein blocks the appearance of the Narp band but not the non-specific band which migrates with a MW of about 220 (4th lane). A Narp C-terminal antibody also detects Narp in plasma (5th lane). (B) Detection of Narp in mouse plasma after isolation by taipoxin affinity chromatography. A slowly migrating, non-reduced Narp band is observed which is absent in the Narp KO. In reduced lysate from plasma, a cluster of bands is observed in samples from the wild-type mouse which migrates slightly slower than mouse hippocampal Narp and which is absent from the Narp KO. In the last two lanes we show Narp bands detected from reduced and non-reduced human plasma respectively which co-migrate with mouse Narp bands. The appearance and relative migration of the bands in the 4th and 6th lanes compared with the mouse hippocampal band is reminiscent of the Narp duplex band seen in reduced pituitary lysate adding further weight to the contention that Narp is being released into serum from pituitary. C. This panel shows non-reduced plasma lysate from mice aged 4, 8, 12 and 20 weeks processed in parallel.
To provide additional confirmation of the identity of this presumptive Narp band, we conducted similar studies on plasma obtained from wild type and Narp knockout mice (figure 5B). Although both samples contain non-specific, presumed IgG, bands of identical intensity, the Narp band is absent from the sample obtained from Narp knockout mice. Analysis of these samples under reducing conditions to release Narp monomers demonstrated a cluster of bands that migrate just above the hippocampal Narp monomer band, similar to the pattern observed in pituitary extracts. As expected, these bands are absent in samples obtained from Narp knockout mice. Human plasma displays the same pattern of Narp immunoreactive bands under both reducing and non-reducing conditions, indicating that the presence of Narp in plasma is not peculiar to rodents (figure 5B)
To assess whether Narp levels in plasma vary with age, we performed this affinity purification procedure on samples obtained from mice or rats ranging in age from 4 to 20 weeks. These results indicate that basal levels of Narp tend to be higher in younger animals within this age range (figure 5C).
Plasma Narp is released from pituitary
To check if Narp detected in plasma is released from pituitary, we evaluated Narp levels in hypophysectomized rats and compared them with age matched controls (figure 6A). We found that plasma Narp is markedly diminished by hypophysectomy strongly suggesting that Narp is released from pituitary into plasma. However, plasma Narp is not completely eliminated by hypophysectomy (figures 6A and 7B) suggesting there are other sources for it.
Figure 6.
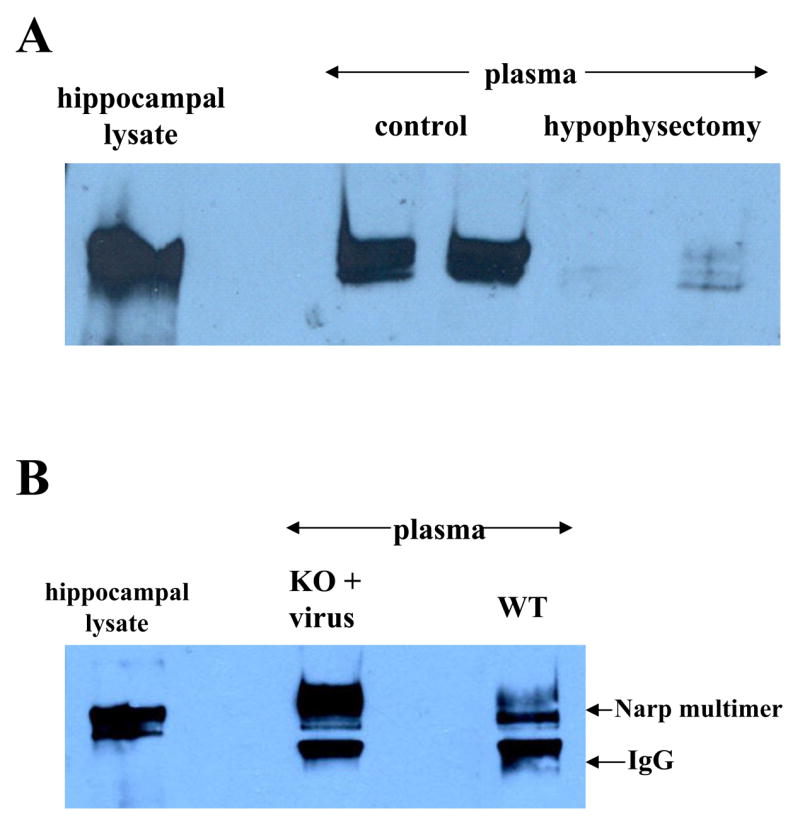
Plasma Narp is released from pituitary. (A) Non-reduced plasma Narp levels are markedly diminished by hypophysectomy in 5 week old rats compared with age matched controls. Samples were harvested 9 –10 days post-surgery. (B) Plasma Narp is detectable in a Narp KO mouse after it has been injected with AAV-wild type Narp into the paraventricular nucleus of the hypothalamus. The Narp band co-migrates with wild type plasma and hippocampal Narp. The intensity of the IgG band varied across batches of taipoxin beads.
Figure 7.
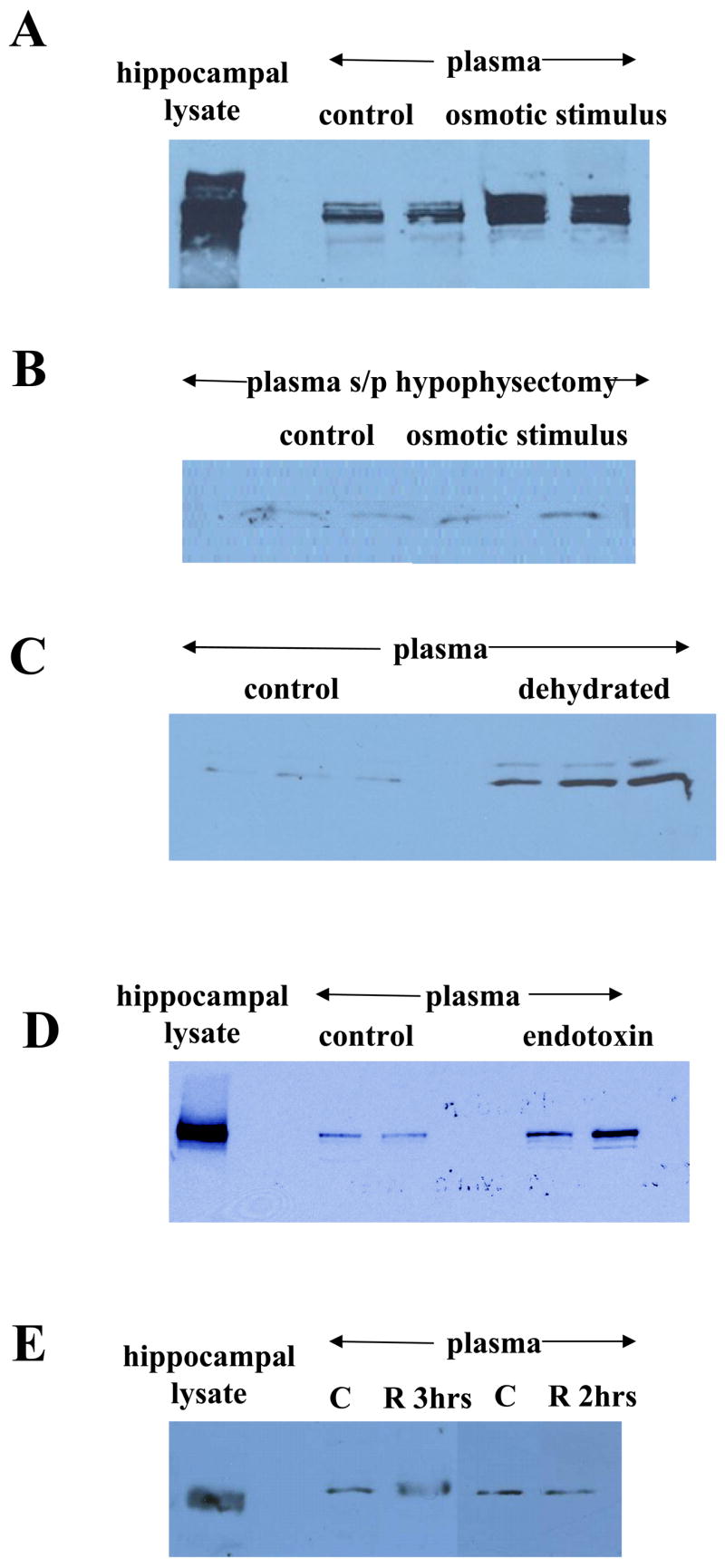
Regulation of plasma Narp. (A) Hypovolemic stress markedly increases levels of plasma Narp (non-reduced) in 5 week old rats compared with rats administered normal saline only. Increases are less robust in older rats (data not shown). (B) Five week old rats that were hypophysectomized do not show an increase in plasma Narp (non-reduced) levels after a hypovolemic stress compared with control hypophysectomized rats that were administered saline only. (C) Mice that have been dehydrated for 24 hours have increased levels of plasma Narp (reduced). (D) Mice treated with endotoxin also show increased levels of plasma Narp (non-reduced) (E) Restraint stress administered to 5 week old rats for either 2 or 3 hours failed to increase plasma Narp levels over control levels.
Although hypophysectomy markedly decreases plasma Narp, it is conceivable that the decrease could be due to an indirect effect of hypophysectomy on release of Narp into the circulation from another tissue in the hypophysectomized animals. Accordingly, to confirm that Narp can be released from the pituitary into plasma we injected an AAV vector containing a Narp insert into the paraventricular nucleus of the hypothalamus of a Narp KO mouse. This procedure resulted in expression of Narp in the posterior pituitary (data not shown) and plasma (figure 6B).
Regulation of plasma Narp
As Narp is located in secretory granules containing vasopressin, we hypothesized that stimulation triggering vasopressin release would also elicit release of Narp into plasma. Initially, we checked the effect of a hypovolemic stimulus, namely polyethylene glycol followed by hypertonic saline which is known to produce a robust rise in plasma vasopressin (Kondo et al., 2004). We observed that this osmotic stress produces a marked increase in plasma Narp levels in rats (figure 7A). To assess if the increase was due to release of Narp from pituitary we checked the effect of the same hypovolemic stimulus on rats that had undergone hypophysectomy (figure 7B). As observed previously, hypophysectomy decreased the basal level of plasma Narp in control rats. Furthermore, this surgical treatment blocked the rise in Narp observed in intact rats following this hypovolemic stress.
We also checked the effect of other stressors previously examined for their effect on vasopressin release into serum (Husain et al., 1979; Gibbs, 1984). We found that dehydration for 24 hours which causes an increase in plasma vasopressin also increases plasma Narp (figure 7C). Likewise endotoxin administration which increases vasopressin, also increases plasma Narp 12 hours later (figure 7D). Restraint stress is not known to increase vasopressin and we did not see a rise in plasma Narp when young rats were subjected to as much as 3 hours of restraint stress and sacrificed immediately afterwards (figure 7E).
DISCUSSION
To help understand how Narp secretion is regulated, we have studied its release from the posterior pituitary into the systemic circulation. These studies have revealed that Narp is co-localized with vasopressin in large secretory vesicles in the posterior pituitary. Furthermore, as predicted by this unexpected observation, we found that plasma Narp levels are elevated by a variety of stimuli that also drive vasopressin secretion from the pituitary. Thus, these findings indicate that Narp, like vasopressin, is secreted in an activity-dependent fashion.
We have focused our studies on the posterior pituitary. However, it is noteworthy that many of the pathways that display prominent Narp staining also contain neuropeptides. These include the hippocampal mossy fiber pathway, which contains dynorphin, the orexin neurons of the hypothalamus and the habenular-interpeduncular pathway, which contains substance P (Reti et al., 2002a,b). Accordingly, it may be interesting in future studies to assess whether Narp is also co-localized in neuropeptide containing vesicles in these pathways.
Prior to using this system to investigate Narp secretion, it was critical that we establish that the pituitary is the major source of Narp detected in plasma. This inference is supported by several lines of evidence. First, we have demonstrated using both immunohistochemical and biochemical approaches that Narp is present in both the anterior and posterior portions of the pituitary. Furthermore, the specificity of these findings has been corroborated by the absence of Narp staining or immunoreactive bands in sections or samples obtained from Narp knockout mice. Second, we have confirmed using affinity chromatography that both basal and stimulated levels of plasma Narp are markedly decreased by hypophysectomy. Third, we have demonstrated the plasma levels of Narp can be restored by injection of a viral construct expressing Narp into the hypothalamus of Narp knockout mice. Taken together, these findings provide compelling evidence that Narp is released from the pituitary into the systemic circulation.
Although these studies demonstrate that Narp in the systemic circulation comes from the pituitary, immunoblot analysis of plasma samples harvested from hypophysectomized rats did detect residual, low levels of Narp-positive bands. Although it is conceivable that these represent a low level of non-specific cross-reactivity with a contaminating protein, it is also possible that Narp may also be released into the circulation from other tissues. Unfortunately, the technical difficulty associated with performing hypophysectomy in mice prevented us from assessing whether this residual Narp-positive band is absent in Narp knockout mice.
We also noted Narp staining in cells containing LH, FSH, and TSH. To check whether Narp is produced in these cells or whether it is sequestered in them from elsewhere, we performed RT-PCR on anterior pituitary lysates (data not shown). Narp mRNA is readily detectable in these lysates suggesting Narp is produced by the subpopulation of anterior pituitary cells that express the alpha subunit. Additional studies are warranted to assess if Narp is released from these anterior pituitary cells.
We have also detected Narp reactivity in human plasma suggesting that monitoring this parameter may be useful clinically in assessing pituitary function. However to evaluate this possibility, additional studies are needed to assess whether it is expressed in the same subpopulation of cells as found in rodents, as well as the pattern of plasma Narp levels in a range of physiological and pathological states. Furthermore, determining Narp’s stability in blood would be useful in assessing whether it may be a more long-lived marker of pituitary hormone release than the classical pituitary hormones, which have relatively short half-lives (Robertson et al., 1973; Veldhuis et al., 2001).
Our observation that Narp is released into the bloodstream does not in itself imply that it acts as a hormone. It is well-established that there are proteins, such as neurophysins (Burton et al., 1971) and PAM (Wand et al., 1985), that are released from the pituitary vesicles, but are thought to play key roles in supporting normal processing of pituitary hormones (de Bree, 2000; Ferraro et al., 2005) and are not known to exert hormone-like actions on peripheral cells or tissues. Previous studies characterizing the role of Narp in brain have provided compelling evidence that it binds to and regulates trafficking of AMPA-type glutamate receptors (O’Brien et al., 1999; Xu et al., 2003). Accordingly, one possibility is that Narp acts in an autocrine fashion to regulate AMPA receptors locally on vasopressin neurons, conceivably modulating vasopressin release from the pituitary (Hiraswa et al., 2003). Another possibility is that Narp in blood may also affect trafficking of glutamate receptors in peripheral tissues. As there is evidence that glutamate receptors are located on endothelial and immune cells (Boldyrev et al., 2005), these represent potential targets. However, it is certainly possible that Narp may also affect other receptor types as well. In pilot studies, we have not detected a robust defect in renal function in Narp knockout mice indicating that Narp co-released from the pituitary with vasopressin is not critical for mediating its classical action on the kidney. However, since Narp is a member of the pentraxin family and peripheral pentraxins have been linked to mediating endothelial and immune cell function, further studies are warranted to check for a possible role of plasma Narp on these cell types. Precedent for this scenario is provided by endorphins, since they are released from the pituitary (Pettibone and Mueller, 1982) and are thought to regulate immune function by acting on opiate receptors expressed on immune cells (Sharp, 2006). Thus, it will be interesting in future studies to assess how Narp’s actions in the periphery compare and contrast with those of peripheral pentraxins.
Acknowledgments
We thank Dr. Ya-Xian Wang for help with the immunogold studies. We thank Drs Desheng Xu, Radhika Reddy and Paul Worley for providing Narp antibodies. This study was supported by RO1DA016303, 5P50DA000266-35 and by the NIDCD Intramural Program.
Abbreviations
- Narp
neuronal activity regulated pentraxin
- NP1
neuronal pentraxin 1
- NPR
neuronal pentraxin receptor
- KO
knockout
- PEG
polyethylene glycol
- ip
intraperitoneal
- NGS
normal goat serum
- TBS
tris-buffered saline
- MW
molecular weight
- PBS
phosphate buffered saline
- AAV
adeno-associated virus
- LH
luteinizing hormone
- FSH
follicle-stimulating hormone
- TSH
thyroid-stimulating hormone
- EM
electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bjartmar L, Huberman AD, Ullian EM, et al. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–81. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldyrev AA, Carpenter DO, Johnson P. Emerging evidence for a similar role of glutamate receptors in the nervous and immune systems. J Neurochem. 2005;95:913–8. doi: 10.1111/j.1471-4159.2005.03456.x. [DOI] [PubMed] [Google Scholar]
- de Bree FM. Trafficking of the vasopressin and oxytocin prohormone through the regulated secretory pathway. J Neuroendocrinol. 2000;12:589–94. doi: 10.1046/j.1365-2826.2000.00521.x. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–17. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Burton AM, Forsling ML, Martin MJ. Release of neurophysin, oxytocin and arginine vasopressin in the rat. J Physiol. 1971;217:23P–24. [PMC free article] [PubMed] [Google Scholar]
- Ferraro F, Eipper BA, Mains RE. Retrieval and reuse of pituitary secretory granule proteins. J Biol Chem. 2005;280:25424–35. doi: 10.1074/jbc.M414156200. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL. Calcium-Permeable AMPA Receptor Plasticity Is Mediated by Subunit-Specific Interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Gibbs DM. Dissociation of oxytocin, vasopressin and corticotropin secretion during different types of stress. Life Sci. 1984;35:487–91. doi: 10.1016/0024-3205(84)90241-8. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Mouginot D, Kozoriz MG, Kombian SB, Pittman QJ. Vasopressin differentially modulates non-NMDA receptors in vasopressin and oxytocin neurons in the supraoptic nucleus. J Neurosci. 2003;23:4270–7. doi: 10.1523/JNEUROSCI.23-10-04270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain MK, Manger WM, Rock TW, Weiss RJ, Frantz AG. Vasopressin release due to manual restraint in the rat: role of body compression and comparison with other stressful stimuli. Endocrinology. 1979;104:641–4. doi: 10.1210/endo-104-3-641. [DOI] [PubMed] [Google Scholar]
- Johnson AW, Crombag HS, Takamiya K, Baraban JM, Holland PC, Huganir RL, Reti IM. Selective role of Narp in processing sensory-specific incentive learning. 2007 doi: 10.1523/JNEUROSCI.4320-07.2007. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee HK, Takamiya K, Huganir RL. The role of synaptic GTPase activating protein in neuronal development and synaptic plasticity. J Neurosci. 2003;23:1119–24. doi: 10.1523/JNEUROSCI.23-04-01119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick LL, Matzuk MM, Dodds DC, Perin MS. Biochemical interactions of the neuronal pentraxins. Neuronal pentraxin (NP) receptor binds to taipoxin and taipoxin-associated calcium-binding protein 49 via NP1 and NP2. J Biol Chem. 2000;275:17786–92. doi: 10.1074/jbc.M002254200. [DOI] [PubMed] [Google Scholar]
- Kondo N, Arima H, Banno R, Kuwahara S, Sato I, Oiso Y. Osmoregulation of vasopressin release and gene transcription under acute and chronic hypovolemia in rats. Am J Physiol Endocrinol Metab. 2004;286:E337–46. doi: 10.1152/ajpendo.00328.2003. Epub 2003 Nov 12. [DOI] [PubMed] [Google Scholar]
- Livingston A. Morphology of the perivascular regions of the rat neural lobe in relation to hormone release. Cell Tissue Res. 1975;159:551–61. doi: 10.1007/BF00221710. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–23. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. Academic Press; New York: 2001. [Google Scholar]
- Petralia RS, Wenthold RJ. Immunocytochemistry of NMDA receptors. In: Li M, editor. Methods in Molecular Biology: NMDA Receptor Protocols. Humana Press; Totowa, NJ: 1999. pp. 73–92. [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Mueller GP. Adrenergic control of immunoreactive beta-endorphin release from the pituitary of the rat: in vitro and in vivo studies. J Pharmacol Exp Ther. 1982;222:103–8. [PubMed] [Google Scholar]
- Reti IM, Baraban JM. Sustained increase in Narp protein expression following repeated electroconvulsive seizure. Neuropsychopharmacology. 2000;23:439–43. doi: 10.1016/S0893-133X(00)00120-2. [DOI] [PubMed] [Google Scholar]
- Reti IM, Reddy R, Worley PF, Baraban JM. Prominent Narp expression in projection pathways and terminal fields. J Neurochem. 2002a;82:935–44. doi: 10.1046/j.1471-4159.2002.01051.x. [DOI] [PubMed] [Google Scholar]
- Reti IM, Reddy R, Worley PF, Baraban JM. Selective expression of Narp, a secreted neuronal pentraxin, in orexin neurons. J Neurochem. 2002b;82:1561–65. doi: 10.1046/j.1471-4159.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- Reti IM, Minor LB, Baraban JM. Prominent expression of Narp in central vestibular pathways: selective effect of labyrinth ablation. European Journal of Neuroscience. 2002c;16:1949–58. doi: 10.1046/j.1460-9568.2002.02284.x. [DOI] [PubMed] [Google Scholar]
- Reti IM, Baraban JM. Opiate withdrawal induces Narp in the extended amygdala. Neuropsychopharmacology. 2003;28:1606–13. doi: 10.1038/sj.npp.1300205. Epub, May 14. [DOI] [PubMed] [Google Scholar]
- Robertson GL, Mahr EA, Athar S, Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest. 1973;52:2340–2352. doi: 10.1172/JCI107423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlimgen AK, Helms JA, Vogel H, Perin MS. Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron. 1995;14:519–26. doi: 10.1016/0896-6273(95)90308-9. [DOI] [PubMed] [Google Scholar]
- Seyama S, Pearl GS, Takei Y. Ultrastructural study of the human neurohypophysis. III. Vascular and perivascular structures. Cell Tissue Res. 1980;206:291–302. doi: 10.1007/BF00232773. [DOI] [PubMed] [Google Scholar]
- Sharp BM. Multiple opioid receptors on immune cells modulate intracellular signaling. Brain Behav Immun. 2006;20:9–14. doi: 10.1016/j.bbi.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley P. Narp, a novel member of the pentraxin family, promotes neurie outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuis JD, Iranmanesh A, Naftolowitz D, Tatham N, Cassidy F, Carroll BJ. Corticotropin secretory dynamics in humans under low glucocorticoid feedback. J Clin Endocrinol Metab. 2001;86:5554–63. doi: 10.1210/jcem.86.11.8046. [DOI] [PubMed] [Google Scholar]
- Wand GS, Ney RL, Baylin S, Eipper B, Mains RE. Characterization of a peptide alpha-amidation activity in human plasma and tissues. Metabolism. 1985;34:1044–52. doi: 10.1016/0026-0495(85)90077-0. [DOI] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O’Brien RJ, Worley P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003;39:513–28. doi: 10.1016/s0896-6273(03)00463-x. [DOI] [PubMed] [Google Scholar]