Abstract
Eukaryotic cells have multiple forms of endocytosis which maintain cell surface homeostasis. One explanation for this apparent redundancy is to allow independent retrieval of surface membranes derived from different types of vesicles. Consistent with this hypothesis we find that sea urchin eggs have at least two types of compensatory endocytosis. One is associated with retrieving cortical vesicle membranes, and formed large endosomes by a mechanism that was inhibited by agatoxin, cadmium, staurosporine and FK506. The second type is thought to compensate for constitutive exocytosis, and formed small endosomes using a mechanism that was insensitive to the above mentioned reagents, but was inhibited by phenylarsine oxide (PAO), and by microinjection of mRNA encoding Src kinase. Both mechanisms could act concurrently, and account for all of the endocytosis occurring during early development. Inhibition of either form did not trigger compensation by the other form, and phorbol ester treatment rescued the endocytotic activity blocked by agatoxin, but not the retrieval blocked by PAO.
Keywords: Sea Urchin, FM 1-43, endocytosis, PAO, agatoxin, Src kinase, FK506, staurosporine, PMA
INTRODUCTION
Compensatory endocytosis retrieves excess surface membrane into endosomes following exocytotic activity (Gundelfinger et al., 2003). It is one of the primary means by which cells maintain their surface area. Some cell types are known to have multiple forms of compensatory endocytosis (Engisch and Nowycky, 1998; Smith and Neher, 1997), but the purpose for having more than one retrieval mechanism is not apparent. One explanation is to have a backup mechanism for this important function. A second reason is that different retrieval mechanisms might have distinctive kinetic constraints (Artalejo et al., 2002). For example, “kiss and run” exocytosis might require a rapid retrieval mechanism that allows endosome fission before the exocytotic vesicle membrane fully collapses into the cell surface (Fulop et al., 2005; Fulop and Smith, 2006). In contrast, retrieval following full vesicle collapse might be mediated by a slower mechanism. A third explanation is to provide the flexibility to independently regulate the retrieval and processing of membranes derived from different types of vesicles following exocytosis.
Fertilization activates several membrane trafficking events involved in preventing polyspermy, protecting the developing embryo and establishing cell polarity (Jaffe and Cross, 1986; Miyake and McNeil, 1998). In sea urchin fertilization results in the release of calcium from intracellular stores (Shen, 1995; Steinhardt et al., 1977). Elevation of intracellular calcium triggers a massive exocytotic event called the cortical reaction. Approximately 15,000 pre-docked cortical vesicles fuse with the plasma membrane, secrete their contents, double the egg surface area, and thus construct the fertilization envelope, a protective barrier around the egg that helps prevent polyspermy (Jaffe et al., 1978; McCulloh, 1985). Within minutes of the cortical reaction, calcium influx through agatoxin-sensitive calcium channels initiates a form of compensatory endocytosis that retrieves the newly inserted cortical vesicle membranes and thus compensates for the increase in cell surface area (Smith et al., 2000; Vogel et al., 1999). Little is known about how calcium influx activates this compensatory mechanism. In addition to cortical vesicle exocytosis, constitutive exocytosis, the seemingly unregulated ongoing exocytotic delivery of membrane to the cell surface has been observed as soon as 30 minutes after fertilization (Frejtag et al., 2003). Little is known about the mechanism of constitutive exocytosis in developing eggs. Because a net increase in membrane surface area was not observed during the first 5 hours after fertilization, it was postulated that membranes inserted into the cell surface by constitutive exocytosis, is compensated for by a form of compensatory endocytosis (Frejtag et al., 2003).
Cortical vesicle exocytosis occurs in sea urchin eggs within three minutes of fertilization (Eddy and Shapiro, 1976; Shafi et al., 1994). Compensatory retrieval of the cortical vesicle membranes occurs within the first 15 minutes following fertilization (Whalley et al., 1995), and the first cell division typically occurs 1.5 hours post fertilization (at 15°C). It is not known if other forms of exocytosis and endocytosis occur during early development. We were particularly interested in determining if other forms of compensatory endocytosis were occurring, and in learning how the embryo integrated these membrane trafficking events.
Our strategy was to use the fluorescent styryl dye FM1-43 to monitor exocytotic and endocytotic activity from 30 minutes post fertilization, a time where cortical vesicle membrane retrieval has reached completion but before the first cell division, to the 8-cell stage (approximately 4 hours later). FM1-43 partitions into both aqueous and lipidic environments (Betz et al., 1996), and has been used to study exocytosis (Terasaki, 1995), endocytosis (Whalley et al., 1995), and cell surface homeostasis in urchins (Frejtag et al., 2003). FM1-43 is only fluorescent when in a lipidic environment. Furthermore, FM1-43 leaves a membrane from the same leaflet that it entered. Thus, the outer leaflet of the plasma membrane of eggs placed in artificial sea water (ASW) containing FM1-43 will initially fluoresce, and the extent of this initial fluorescence should be proportional to the cell surface area. With time, and prolonged incubation in FM1-43, an increase in fluorescence should be observed if a cell has exocytotic activity (see supplemental figure 1). Assuming FM1-43 has free access to the cell surface, the fluorescent signal from a cell should be proportional to the FM1-43 labeled cell surface area, plus the labeled luminal surface area of endosomes which have pinched off from the plasma membrane (FM1-43 should be trapped in the lumen of these endosomes). Complicating interpretation of fluorescent FM1-43 signals from cells is the possibility that endosomes that have already taken up FM1-43, may subsequently fuse with the plasma membrane. If this happens in the presence of extracellular FM1-43, we expect there to be no net change in fluorescence. Further complicating the interpretation of these signals is low pH quenching of FM1-43 fluorescence (Betz et al., 1996). Thus, if endosomes acidify, their FM1-43 signal might be attenuated. Finally, it is important to verify that FM1-43 itself is not perturbing the phenomenon under investigation. In this study we use FM1-43 to investigate the differences between these two compensatory retrieval mechanisms.
MATERIALS AND METHODS
Obtaining and handling gametes
Sea urchins were obtained from: Marinus (Long Beach, CA; Strongylocentrotus purpuratus, Lytechinus pictus), Charles Hollahan (Santa Barbara, CA; Strongylocentrotus purpuratus, Lytechinus pictus), and Jennifer M. Keller (Beaufort, NC; Lytechinus variagatus) and maintained in artificial sea water (ASW) in marine aquaria. As specific species of sea urchins only have eggs for a limited season, we performed experiments on three different species to allow us to conduct experiments throughout the year. ASW is routinely prepared by dissolving “Instant Ocean” (Aquarium Systems, Mentor, OH) per the manufacturer’s directions and adjusting the osmolarity to 1000 ± 10 mOsm and pH to 8.0. For experiments ASW was prepared using the formula of the Marine Biological Laboratory (Woods Hole, MA): 425 mM NaCl, 9 mM KCl, 9.3 mM CaCl2, 19.9 mM MgCl2, 25.5 mM MgSO4, 2.1 mM NaHCO3. Eggs and sperm were obtained as previously described (Vogel, et al., 1999).
Multiphoton and Confocal Microscopy
A Zeiss 510 NLO laser scanning microscope coupled to a Mira or Chameleon Ti:sapphire laser (Coherent, Santa Clara, CA) was used for fluorescent imaging as well as laser scanning differential interference contrast (DIC) imaging. FM1-43 fluorescence was excited at 780 nm and emission was collected through a 565–615 nm band pass filter. Tetramethylrhodamine fluorescence was excited at 850 nm and emission was collected through a 535–590 nm band pass filter. Hoechst 33342 was excited at 780 nm and its emission was collected through a 390–465 nm band pass filter. Cascade blue dextran was excited at 820 nm and its emission was collected through a 535–590 nm band pass filter. Laser scanning confocal microscopy of FM1-43 stained eggs was performed using 514nm excitation and fluorescence was collected through a 535–590 nm band pass filter.
Assays for measuring instantaneous and cumulative FM1-43 fluorescence
Sea urchin eggs were fertilized with sperm (0.5 μl/ml) in a single pool and aliquots of the fertilized eggs were added to wells of a low fluorescence microtiter dish in triplicate for each time point. FM1-43 (final concentration of 4 μM) was added to the wells at set intervals to measure changes in the embryo surface area and membrane flux. Fluorescence (excitation: 480, emission 580) was determined immediately (‘Instantaneous’) and over time (‘Cumulative’) on a Molecular Devices (Sunnyvale, CA) Spectramax Gemini XS fluorometer. Cell-stage number was monitored by light microscopy.
Preparation of sea urchin egg membranes
Sea urchin eggs were dejellied and washed as previously described (Vogel et al., 1999) and then washed twice in PKME (425 mM KCl, 10 mM MgCl2, 5 mM EGTA, 50 mM PIPES) buffer, pH 6.7, with 1 mM benzamidine. The eggs were resuspended in 4 volumes of PKME with 1 mM benzamidine, 1 mM BAPTA and 0.2 mM PMSF and homogenized by hand at 4°C. The homogenate was diluted 10 fold in homogenizing buffer and centrifuged at 250 xg 5 min. The supernatant was aspirated and the whitish layer on top of the yellow pellet of unbroken eggs was collected in homogenizing buffer. This layer contained plasma membrane and docked cortical granules (cell surface complex). It was resuspended to its original volume in homogenizing buffer and centrifuged at 300 xg 10 min. The pellet was resuspended in KEA (450 mM KCl, 50 mM NH4Cl, 25 mM EGTA) buffer, pH 9.1, with 1 mM benzamidine, 1 mM BAPTA and 0.2 mM PMSF at room temperature and homogenized by hand. The suspension was centrifuged at 500 xg 10 min and the crude membrane fraction in the pellet was resuspended in ASW for use in determining the pH dependence of styryl dye fluorescence.
Fluid phase membrane retrieval assay
A fluid phase uptake assay that measures the amount of tetramethylrhodamine-dextran (mol. wt. 3000) was used to determine the effect of endocytotic inhibitors on constitutive and compensatory endocytosis as previously described (Vogel et al., 1999).
Cloning and mRNA synthesis of Src and SrcK277M
Restriction enzymes were obtained from New England Bio Labs (Ipswich, MA). Src cDNA clone from Strongylocentrotus purpuratus (Gen Bank Accession # AY063749) was a generous gift from Dr. Kathy Foltz. The cDNA sequence coding for the open reading frame of Src was PCR amplified using a sense primer that contains a T7 promoter site (bold) 5′-GTA CTA ATA CGA CTC ACT ATA GGA TGG GAT GTG TTA ATA GT-3′ and an anti-sense oligo 5′-GTA CTT AAT CGG CCT CCT TGT A -3′ using PFU ultra (Stratgene, La Jolla, San Diego). The cDNA was cloned into Zero BLUNT II TOPO PCR cloning kit (Invitrogen, Carlsbad, CA) and sequenced. This construct was used to make mRNA for microinjections and also to generate the mutant Src K277M using site directed PCR mutagenesis. The sense 5′ CCT GTC GCT GTC AAG ACT CTG ATG GAA GGG ACC ATG TCA CCG GCA 3′ and the antisense primer 5′ TGC CGG TGA CAT GGT CCC TTC CAT CAG AGT CTT GAC AGC GAC AGG 3′ (mutation in bold) were combined with the TOPO Src clone from above and amplified. The PCR product was treated with Dpn1, and transformed into TOP 10 cells (Invitrogen). Individual clones were grown and sequenced to confirm the mutation. Messenger RNA was synthesized in vitro from linearized template cDNA with a mMESSAGE mMACHINE T7 ultra kit (Ambion Inc.). The mRNA was purified using MEGAclear RNA (Ambion) purification kit.
Microinjection of mRNA
Sea urchin eggs (Lytechinus variegatus) were attached to glass bottomed chambers and fertilized in ASW. A single blastomere of a two-cell stage embryos was co-injected with mRNA (0.51 mg/ml) encoding SpSK1 (Strongylocentrotus purpuratus Src kinase) and with Cascade blue dextran (0.1 mg/ml, Molecular Probes) used as a fluorescent injection marker. An Eppendorf FemtoJet microinjector coupled to an InjectMan NI 2 was used for all sea urchin embryo microinjections. Control embryos were injected with either fluorescent dextran alone, or co-injected with mRNA encoding a dominant negative Src mutant (K277M). After injection cells were incubated at 22°C for 1 hour, and then incubated in ASW containing FM1-43 (4 μM) for 30 min. Finally, cells were washed 3 times with ASW to remove extracellular FM 1-43 and imaged by two photon microscopy.
Evaluating the effect of PAO on cell division
Sea urchin eggs (Strongylocentrotus purpuratus) were fertilized in ASW. Fifteen minutes after fertilization the embryos were incubated in freshly made ASW containing 10 μM PAO, (made from a 100 mM PAO stock solution in DMSO) for 10 min. Control embryos were incubated in ASW containing a 1:10,000 dilution of DMSO. Control and treated cells were washed three times with ASW to remove PAO, and incubated with FM 1-43 for an additional 30 minutes. Finally, embryos were washed with ASW to remove non-internalized FM 1-43 and imaged at 60 and 130 minutes after fertilization by two photon microscopy. FM1-43 labeled embryos were stained with Hoechst 33342 (20μg/ml; Molecular Probes) in ASW for 15 minutes, washed 3 times with ASW, and cell nuclei visualized with 2-photon microscopy.
RESULTS
Embryo surface area does not change during early development
To determine if there is any net change in embryo surface area during development, we measured the instantaneous FM1-43 fluorescence of developing sea urchin embryos using a 96-well fluorimeter. By measuring FM1-43 fluorescence within seconds of its addition to embryos we obviate many of the interpretation difficulties caused by internalization of dye. We fertilized batches of eggs and added FM1-43 to aliquots at various times during development. Fluorescence was measured within 30 seconds of FM1-43 addition (Figure 1A). The instantaneous FM1-43 fluorescence did not change over a 4.5-hour period spanning the 1 to 8-cell stages of development. This suggests that the surface area of the developing embryo does not change during this period. A static instantaneous FM1-43 signal could indicate the absence of any exocytotic and endocytotic activity in the embryo. Alternatively, exocytosis might occur, but membrane insertion is compensated for by endocytosis. To discriminate between these possibilities we monitored the cumulative FM1-43 fluorescence for each of the individual points used to measure the instantaneous FM1-43 signal (Figure 1A). In these experiments the cumulative FM1-43 fluorescence was measured every 15 minutes. Regardless of which stage embryos were analyzed, the FM1-43 signal always doubled over the first 30 minutes following the addition of the dye. The highest rates of increase were always observed in the first measured time segment. This can be more clearly seen in figure 1B where we have superimposed all of the individual traces. The fluorescent signal reached a quasi-plateau value approximately 30 minutes after FM1-43 addition. Typically, a small continuous rise in fluorescence persisted from 60 minutes after FM1-43 addition. As mentioned above the interpretation of cumulative FM1-43 fluorescence is complex, nonetheless, it is possible to reach some simple conclusions from this experiment. An increase in FM1-43 fluorescence is indicative of exocytotic activity in the developing embryo. Because the kinetics of these traces were identical despite being initiated over a period spanning the 1 to 8-cell stages, the rate of exocytosis was constant over this period. This would be expected for constitutive exocytosis. Furthermore, identical kinetic traces suggest that it is unlikely that barriers form during this period that hinder FM1-43’s access to portions of individual blastomeres. In support of this contention, it is known that tight junctions form much later than the 8-cell stage (Andreuccetti et al., 1987) and electron microscopy of 2-cell through 8-cell stage embryos revealed ample space for FM1-43 access between adjacent blastomeres (Frejtag et al., 2003). Thus, if the embryonic surface area remains constant through the 8-cell stage, and there is continuous constitutive exocytotic activity, we are forced to conclude that membrane added by exocytosis is compensated for by endocytosis.
Figure 1.
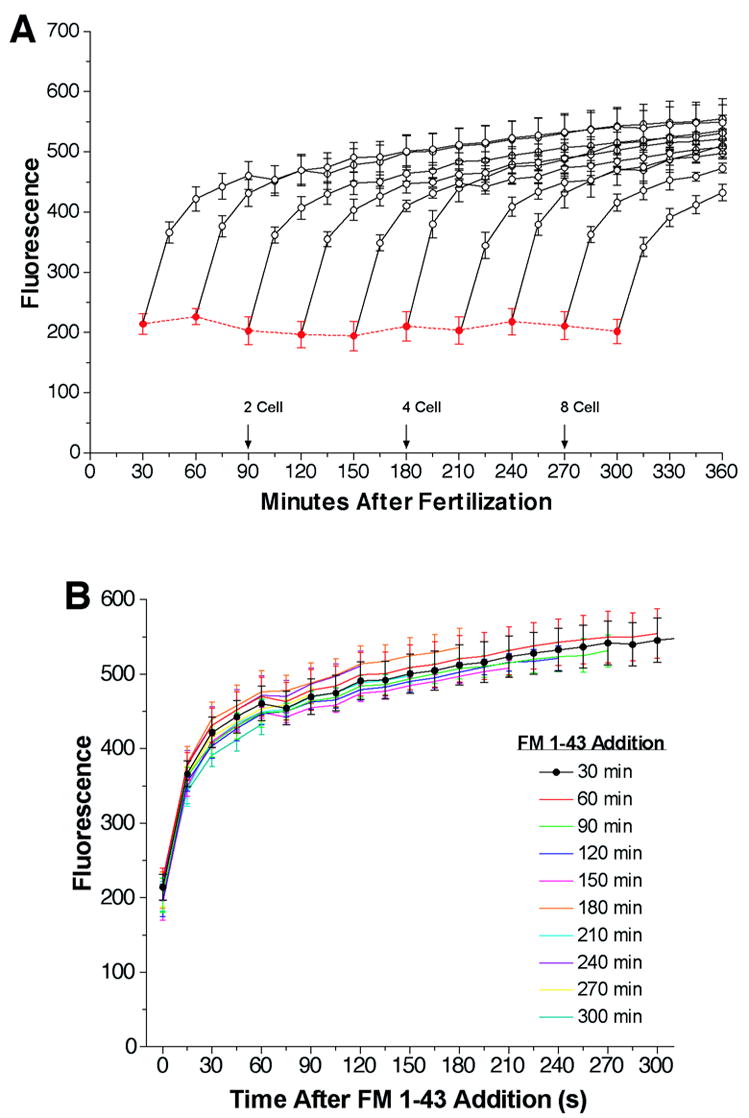
The instantaneous and cumulative FM1-43 fluorescence observed during early development. Panel A: A 5% suspension of sea urchin eggs (Lytechinus pictus) was fertilized with sperm (0.5 μl/ml) in a single pool and aliquots of the fertilized eggs were added to wells of a low fluorescence microtiter dish in triplicate for each time point and kept at 16°C. At 30-minute intervals after fertilization FM1-43 was added to the wells at a final concentration of 4 μM to measure the embryo surface area and membrane flux. Fluorescence (Ex 480/Em 580) was determined immediately (‘Instantaneous’ in RED) and at 15 min intervals (‘Cumulative’ in BLACK). Between readings, eggs were kept at 16°C. Indications of cell-stage number are shown when ~90% of the eggs were at that stage. All points are mean ± SEM of three experiments. Panel B: Traces in panel A are overlaid by plotting them as a function of time after FM1-43 addition.
FM1-43 trapped in the lumen of endosomes is released by exocytosis
We were interested in resolving an apparent paradox observed in figure 1. Addition of FM1-43 at any time during early development resulted in an initial increase in fluorescence indicating a steady ongoing exocytotic activity during this period. In contrast, all of these individual cumulative traces reached a quasi-plateau value within an hour of FM1-43 addition. If the rate of constitutive exocytosis is constant during this period why do individual FM1-43 traces flatten out? Several explanations are possible including pH quenching of FM1-43 trapped in endosomes, direct inhibition of membrane trafficking by FM1-43, and the recycling of FM1-43 labeled endosomes back to the cell surface. Control experiments ruled out pH effects and direct inhibition by FM1-43 (see Supplemental figures 2 & 3). The third explanation is that FM1-43 labeled endosomes might recycle back to the cell surface. FM1-43 uptake and re-release was first observed with synaptic vesicle recylcling at the neuromuscular junction (Betz and Bewick, 1992). Normally in the presence of extracellular FM1-43 there should be an increase in cell fluorescence proportional to the surface area of vesicle membrane inserted by exocytotic activity. If, however, the lumen of secretory vesicles have been pre-loaded during previous rounds of endocytosis, a net change in FM1-43 fluorescence may not be observed. Thus, if membrane recycling to the cell surface is occurring, the initial rise in fluorescence of a cumulative FM1-43 trace should still be proportional to the rate of exocytosis because few, if any labeled vesicles exist. With time, however, the proportion of endosomes with FM1-43 trapped in their lumens will increase. Therefore, the probability that pre-labeled vesicles may fuse with the cell surface will also increase. Eventually, the cumulative FM1-43 trace should approach a plateau value. To test if membrane recycling could explain the plateau during early development an experiment was devised to test if pre-labeled endosomes release FM1-43 during this period. If extracellular FM1-43 is removed at any point during a cumulative trace, there should be a decrease in cell fluorescence. Because embryo surface area does not change during this period, we reasoned that a constant drop in FM1-43 fluorescence, proportional to the surface area of the embryo, should always be observed upon perfusion. This decrease in FM1-43 fluorescence is expected to be rapid because it should only be limited by the rate of FM1-43 disassociation from the outer leaflet of the plasma membrane. After FM1-43 loading, if endosomes harboring FM1-43 do fuse with the cell surface, a further decrease in cell fluorescence should be observed in the absence of extracellular FM1-43. Endosome-plasma membrane fusion should liberate FM1-43 trapped in the lumen of these vesicles. A decrease in cell fluorescence associated with the fusion of FM1-43 labeled vesicles might be slower than FM1-43 washout from the cell surface because it will be limited by both the FM1-43 membrane disassociation rate and the exocytotic rate of FM1-43 labeled endosomes. Finally, this slower phase in cell FM1-43 fluorescence decrease should not be observed if extracellular FM1-43 is removed prior to any significant endocytotic dye uptake. The black filled circles in figure 2 depict a cumulative FM1-43 trace when dye was added to eggs 30 minutes after fertilization. Upon addition of FM1-43 there was an instantaneous rise in fluorescence from 0 to approximately 200 fluorescence units (fu). A rapid drop of 200 fu, to almost 0 fluorescence units was observed if extracellular FM1-43 was immediately removed by perfusion into ASW (see dashed line connecting to open black circles). The slight discrepancy from 0 is caused by irreversible FM1-43 staining of components of the egg fertilization envelope. With prolonged incubation (1 hr) in FM1-43, the cumulative fluorescence grew to a value of almost 500 fu. When FM1-43 was removed after 10, 20, 30, 40, and 50 minutes of prolonged incubation in FM1-43 we always observed a rapid 200 fu drop in fluorescence (dashed lines) indicating that the surface area of the embryo was constant over this period. In contrast to the decrease in fluorescence observed when extracellular FM1-43 was immediately removed, FM1-43 removal after an incubation period (even as short as 10 minutes) resulted in a second slower phase of fluorescence decrease. These traces decayed to plateau values over a 10 minute period that was always significantly greater than 0 fu. This suggests that internalized FM1-43 can be released from the embryo by a mechanism with a half life of ~ 3–5 minutes. A sizable fraction of internalized FM1-43 apparently is never released from the embryo.
Figure 2.
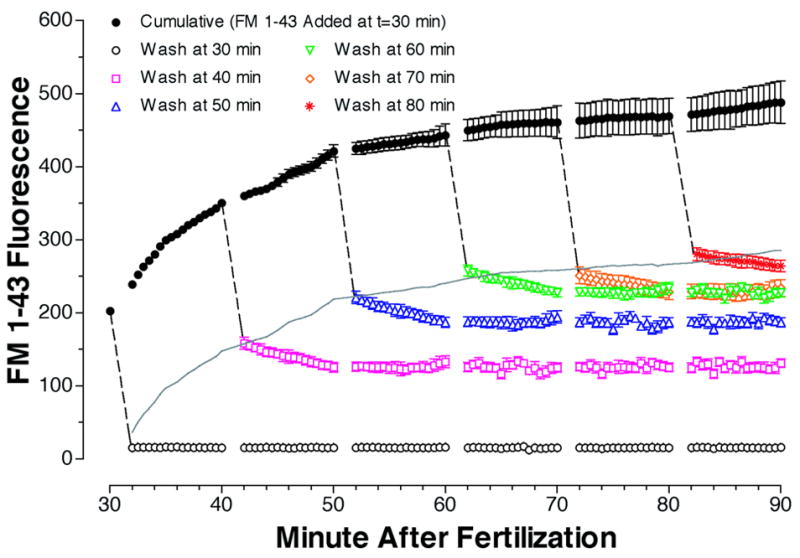
Recycling of internalized FM1-43. Sea urchin eggs (Lytechinus variegatus) were fertilized with sperm in a single pool and kept at room temperature. 30 min after fertilization FM1-43 was added to a final concentration of 4 μM. Samples were immediately removed to determine the time course of cumulative FM1-43 fluorescence (BLACK filled circles). Another set of samples was diluted 7 fold with artificial seawater, microfuged for 4 seconds, resuspended in ASW (final dilution of FM1-43 = 1400 fold) and placed in microtiter dish wells to determine non-dissociated fluorescence (BLACK open circles). Readings were taken at 30-second intervals beginning 2 minutes after washing. Other samples were washed and fluorescence determined in the same manner at 10 min intervals after adding FM1-43 (i.e. RED squares, BLUE triangles, etc.). The rapid decrease in fluorescence upon dilution of FM1-43 (dashed black lines) should be proportional to embryo surface area. Grey line estimates the fraction of the cumulative FM1-43 fluorescence originating from internalized FM1-43 (cumulative FM1-43 fluorescence minus embryo surface fluorescence). All points are mean ± SEM, n=3.
Two unique biochemical mechanisms mediate compensatory endocytosis during early development
Because cortical vesicle exocytosis is triggered by calcium released from intracellular stores (Shen and Buck, 1993), it does not require extracellular calcium. In contrast, the endocytotic retrieval of cortical vesicle membranes is dependent on extracellular calcium (Vogel et al., 1999). In this instance, local calcium influx through P-type calcium channels triggers compensatory endocytosis (Smith et al., 2000). To determine if the compensatory endocytosis observed following constitutive exocytosis is also dependent on extracellular calcium, FM1-43 fluorescence was measured with (figure 3 black circles) or without (red circles) extracellular calcium. Calcium did not alter either the instantaneous (dashed lines) or cumulative (solid lines) FM1-43 signals, indicating that both constitutive exocytosis and its compensatory endocytosis do not require extracellular calcium. This supports the hypothesis that sea urchin embryos utilize two different forms of compensatory endocytosis to maintain cell surface homeostasis: a calcium-dependent form to retrieve cortical vesicle membranes following triggered exocytosis, and a calcium-independent form to retrieve membrane inserted into the cell surface by constitutive exocytosis.
Figure 3.
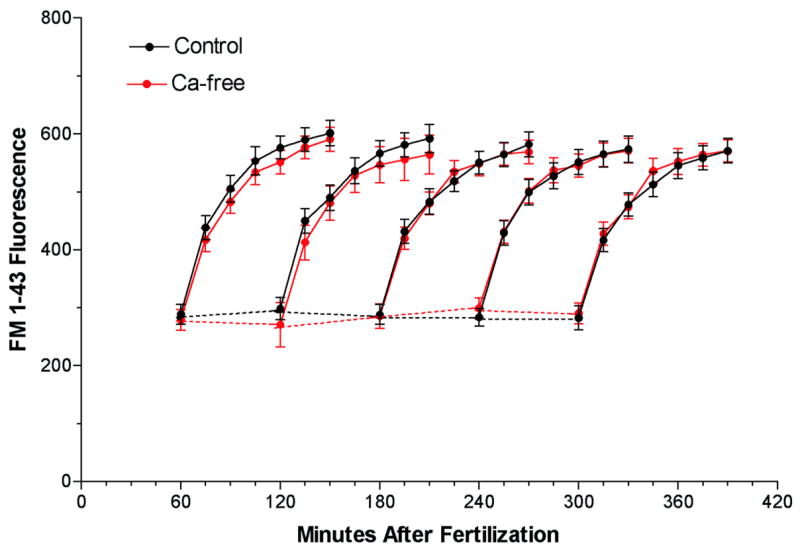
Extracellular calcium is not required for constitutive exocytosis. A suspension of sea urchin eggs (Strongylocentrotus purpuratus) were fertilized in a single pool. Embryos were placed in calcium-free seawater (RED) or normal seawater (BLACK) at 60 minutes post fertilization. At 60-minute intervals after fertilization, FM1-43 was added to aliquots at a final concentration of 4 μM to measure the instantaneous (dashed lines) and cumulative (solid lines) fluorescence. All points are mean ± SEM, n=6.
The signaling pathway activated by calcium influx, which triggers membrane retrieval in sea urchin eggs, is not known but calcineurin (Liu et al., 1994; Lawson and Maxfield, 1995; Bauerfeind et al., 1997; Engisch and Nowycky, 1998; (Bauerfeind et al., 1997; Chan and Smith, 2001; Engisch and Nowycky, 1998; Lawson and Maxfield, 1995; Liu et al., 1994) and protein kinase C (Aballay et al., 1999; Chan and Smith, 2003; Findlay et al., 1989; Robinson et al., 1993) have both been implicated to play a role in other cell types. Furthermore, it is possible that both the calcium-dependent and –independent forms of compensatory endocytosis activate the same endocytotic mechanism, but use either calcium or a different second messenger to signal retrieval. Alternatively, these two forms of compensatory endocytosis might involve fundamentally different intracellular components to signal retrieval. To expose what cellular components might be involved in activating these two retrieval mechanisms, several inhibitors of cellular signaling components were screened for their ability to block fluid-phase marker uptake by either the compensatory endocytosis of cortical vesicle membranes (figure 4A, ‘compensatory endocytosis’ dark grey bars), or the endocytosis following constitutive exocytosis (‘constitutive endocytosis’ light grey bars). As expected from their dependence on extracellular calcium (figure 3), compensatory endocytosis was blocked by the P/Q-channel specific inhibitor agatoxin, as well as by the more non-specific calcium channel blocker cadmium (see also (Vogel et al., 1999)). In contrast, constitutive endocytosis was not inhibited by these channel blockers. Compensatory endocytosis was also inhibited by staurosporine, a non-specific kinase inhibitor, and by FK 506, an inhibitor of the calcium activated serine/threonine phosphatase calcineurin. Compensatory endocytosis was not inhibited by phenylarsine oxide (PAO), a tyrosine phosphatase inhibitor. In contrast, constitutive endocytosis was not inhibited by staurosporine or FK 506, but was inhibited by PAO. Receptor mediated endocytosis in adrenal glomerulosa cells was completely blocked by PAO treatment, but did not require extracellular calcium (Hunyady et al., 1991). To further characterize the involvement of phosphatases in the mechanism of constitutive endocytosis, we measured dose-response relationships for six tyrosine specific phosphatase inhibitors, as well as for okadaic acid, another serine/threonine specific phosphatase inhibitor (figure 4B). All tyrosine specific phosphatase inhibitors blocked constitutive endocytosis, but okadaic acid did not.
Figure 4.
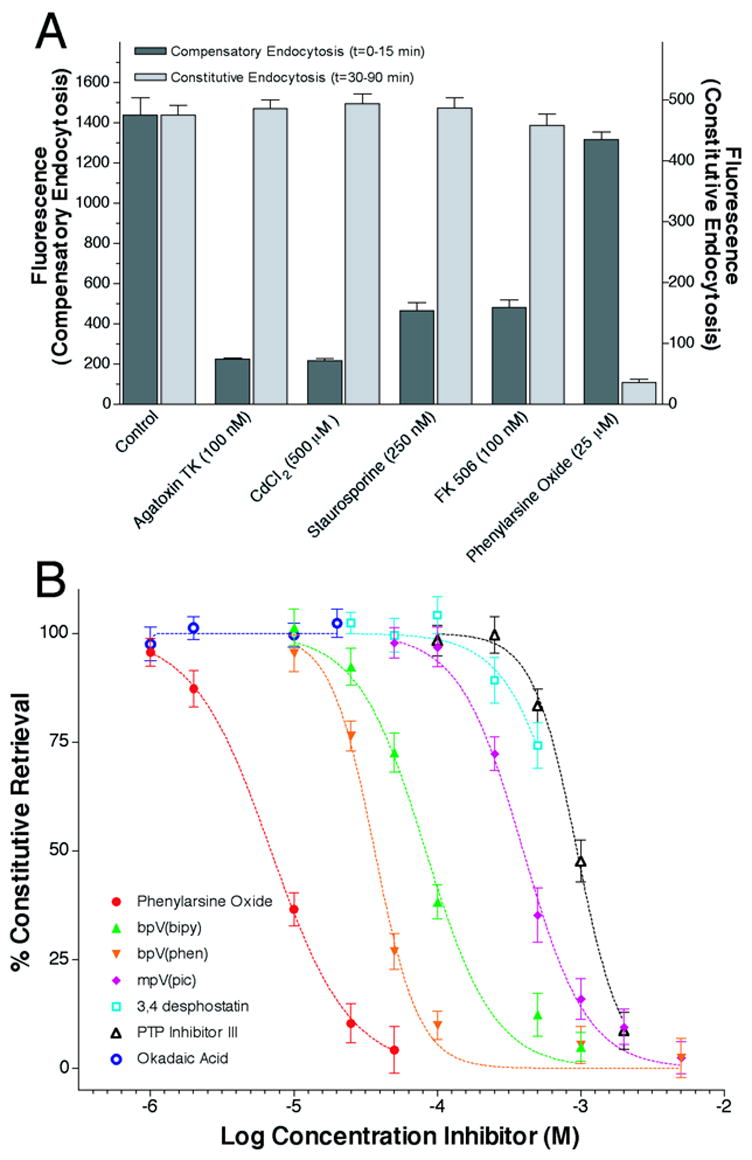
A tyrosine phosphatase regulates constitutive endocytosis. Panel A: Sea urchin embryos (Strongylocentrotus purpuratus) were placed in seawater containing the fluid phase marker tetramethylrhodamine dextran (3,000 mol.wt.) from 0–15 minutes post fertilization to monitor the compensatory endocytosis of cortical vesicle membranes (dark gray bars), or from 30–90 minutes post fertilization to monitor constitutive endocytosis (light gray bars). Endocytotic inhibitors were added prior to fertilization at the indicated concentrations. All points are mean ± SEM, n=5. Panel B: Extracellular tetramethylrhodamine dextran uptake by constitutive endocytosis between 30–90 minutes post fertilization was monitored as a function of phosphatase inhibitor concentration. Points are mean ± SEM, n=6.
It is known that calcium dependent compensatory endocytosis begins at fertilization following cortical vesicle exocytosis and is typically finished by 15 minutes after fertilization (Whalley et al., 1995). We have also shown that constitutive exocytosis and its PAO sensitive form of compensatory endocytosis is fully operant by 30 minutes after fertilization and continues for at least the first 5 hours of development (Figure 1, (Frejtag et al., 2003)). To determine if the PAO sensitive form occurs during the first 30 minutes of development, as well as to determine if other forms of endocytosis in addition to the agatoxin and PAO sensitive forms might operate during this period we investigated whether these two inhibitors could account for all of the FM1-43 uptake over the first 30 minutes of development. Fertilized eggs were treated with either PAO, agatoxin, or a mixture of both inhibitors, and FM1-43 uptake was compared with uptake of control eggs over the first 30 minutes of development (Figure 5A). Neither one of these two endocytotic inhibitors could fully block FM1-43 uptake. Agatoxin blocked approximately 63% of uptake while PAO blocked 36%. Together, they account for almost all of the FM1-43 uptake occurring during this period. As expected, eggs treated with both agatoxin and PAO blocked virtually all FM 1-43 uptake. Similarly, two-photon imaging of eggs treated with both cadmium and PAO revealed a complete block of fluid-phase uptake during the first 30 minutes of development (Figure 5B). In control eggs both large (blue arrow heads) and small (yellow arrow heads) endosomes containing the fluorescent fluid phase marker were observed. Interestingly, treatment with cadmium alone blocked the formation of the large endosomes while PAO treatment blocked the formation of the small endosomes. Thus, evidence in support of two forms of retrieval, a calcium dependent form into large endosomes formed from cortical vesicle membranes (Smith et al., 2000) and a PAO sensitive form which might represent uptake into small coated pits (Fisher and Rebhun, 1983) were obtained when retrieval was monitored using either the membrane probe FM1-43 or the fluid phase marker tetramethylrhodamine dextran.
Figure 5.
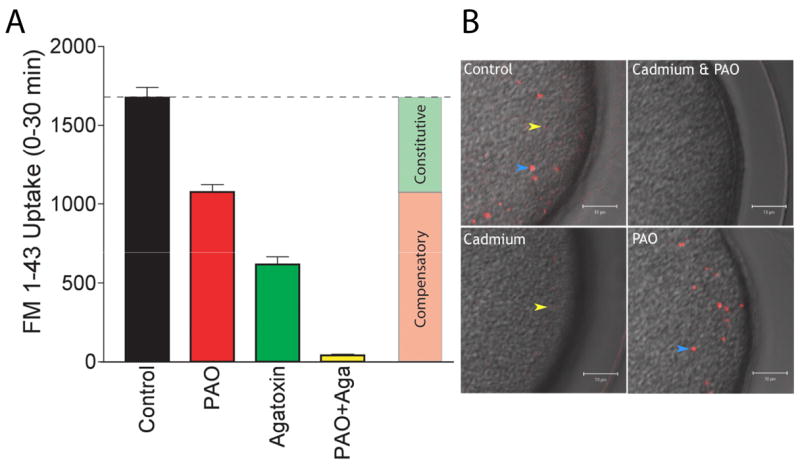
Two forms of endocytosis account for virtually all of the uptake observed during early development. Panel A: A suspension of sea urchin eggs (Strongylocentrotus purpuratus) were treated with either 10 μM PAO (red bar), 100 nM agatoxin-TK (green bar), or a combination of both inhibitors (yellow bar) and FM1-43 uptake during a 30 minute period following fertilization was measured and compared with control eggs (black bar). PAO was added 2 minutes after the addition of sperm, along with 4 μM FM1-43. Agatoxin was added at the same time as sperm and FM1-43. When both PAO and agatoxin were used the agatoxin and sperm first added, followed 2 minutes later by the addition of PAO and FM1-43. Control eggs were treated with sperm and FM1-43 at the same time. 30 minute after the addition of FM1-43 eggs were washed in ASW containing 1 mg/ml trypan blue (to quench any tightly bound extracellular FM1-43 fluorescence), resuspended in ASW, and aliquots transferred to a microtiter plate for fluorescence determination. Each point represents data in triplicate from three separate animals and is the mean ± SEM, n=3. Panel B: Two-photon microscopy was used to image the uptake of a fluorescent fluid phase marker (100 μM tetramethylrhodamine-dextran mol. wt. 3000, TMR-dex) in fertilized eggs (Strongylocentrotus purpuratus), or eggs treated with cadmium (500 μM), PAO (10 μM), or a mixture of cadmium and PAO. Eggs were fertilized with sperm, and at 2 minutes after fertilization inhibitors were added along with the TMR-dex. Eggs were washed with ASW 30 minutes later (to remove extracellular TMR-dex) and imaged. Images are an overlay of the two-photon TMR-dex fluorescence image and the laser-scanning DIC image. Yellow arrow head indicates the small endosomes formed by the PAO sensitive form of endocytosis and the blue arrow heads indicates the large endosomes formed by the cadmium sensitive form of endocytosis. Size bars are 10 μm.
Phenylarsine oxide inhibits constitutive endocytosis and cell division
When exocytotic reactions are coupled to compensatory endocytotic reactions, it is often difficult to determine if inhibitors act directly on endocytosis, or indirectly by inhibiting exocytosis. To determine if PAO was acting directly on constitutive endocytosis, we measured the instantaneous (figure 6, solid symbols) and cumulative (open symbols) FM1-43 fluorescence from developing embryos in the presence (red and grey boxes) or absence (blue circles) of PAO over a 2 hour period starting 30 minutes after fertilization (i.e. after cortical vesicle retrieval has finished). We reasoned that if PAO completely inhibits endocytosis, the instantaneous and cumulative FM1-43 traces following PAO treatment should be identical. Furthermore, if constitutive exocytosis is still occurring after PAO treatment, both the instantaneous and cumulative FM1-43 traces should increase with time, at a rate equivalent to the rate of constitutive exocytosis. This is what was observed. In untreated embryos the instantaneous FM1-43 signal did not change with time while the cumulative FM1-43 signal initially increased to a quasi-plateau level (compare solid and open blue circles in figure 6). After PAO treatment, both the instantaneous and cumulative FM1-43 signal increased at a steady rate and these traces were indistinguishable (compare red solid squares and grey open squares in figure 6). This suggests that constitutive endocytosis was completely inhibited by PAO but constitutive exocytosis occurred at a steady rate throughout this period.
Figure 6.
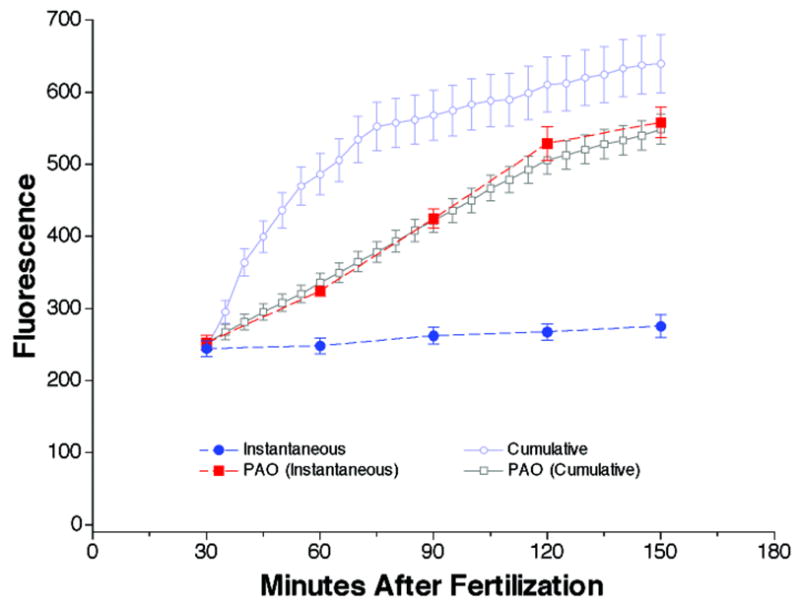
PAO inhibits constitutive endocytosis. Comparison of the instantaneous (solid symbols with dashed lines) and cumulative (open symbols with solid lines) FM1-43 fluorescence from developing sea urchin embryos (Strongylocentrotus purpuratus) plotted as a function of time after fertilization either with (RED and GREY squares) or without (light and dark BLUE circles) PAO pre-treatment (25 μM). All points are mean ± SEM, n= 3.
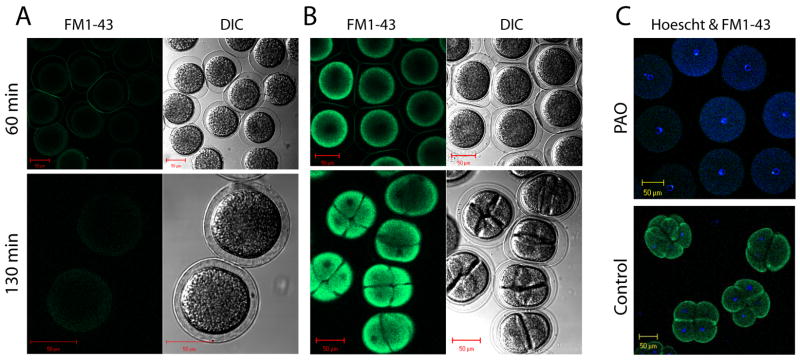
PAO treatment is known to inhibit cytokinesis in yeast (Oustrin et al., 1995). Differential interference contrast microscopy and two-photon fluorescent microscopy was used to evaluate the affects of PAO on cell division in eggs. Eggs were fertilized, and following envelope elevation treated with PAO for 10 min. At 20 min post-fertilization the eggs were incubated in ASW containing FM1-43 for 30 min. Embryos were washed with ASW to remove external FM1-43 and imaged at 60 and 130 minutes post-fertilization (figure 7). At 130 minutes post-fertilization mock treated embryos had gone through at least two rounds of cell division, while cell division in PAO treated embryos appeared arrested. As expected, PAO treated embryos had no observable FM1-43 fluorescence while uptake was abundant in controls. Subsequent staining of PAO treated embryos with Hoechst dye revealed a single nucleus per embryo while each blastomere of mock treated embryos contained a single Hoechst stained nucleus.
Figure 7.
PAO inhibits cell division. Fertilized eggs (Strongylocentrotus purpuratus) were either incubated for 10 minutes with 10 μM PAO (panel A), or mock treated (panel B), and then incubated in ASW containing FM1-43 (4 μM) for 30 minutes. Eggs were next washed with ASW to remove extracellular FM1-43 and imaged with two-photon microscopy at 60 and 130 minutes post fertilization to image both cell division (by DIC) and FM1-43 uptake (green fluorescence). Next, both control embryos and those treated with PAO were stained with Hoechst stain (20 μg/ml) for 15 minutes, washed with ASW and cell nuclei were visualized (see blue fluorescence in panel C) with two-photon microscopy. Size bars are 50 μm.
Microinjection of mRNA encoding Src mimics the effects of PAO
The PAO inhibition of both the calcium independent form of FM1-43 uptake, and of cell division suggests that PAO might be acting as a non-specific inhibitor. Nonetheless, PAO failed to inhibit the calcium dependent form of endocytosis (Figure 4 & 5), and it only partially blocked constitutive exocytosis (Figure 6). Thus, an alternative explanation for the PAO inhibition observed is that tyrosine phosphorylation signaling is utilized by the calcium independent form of FM1-43 uptake as well as by the cell division mechanism. If true, we reasoned that over-expression of a tyrosine kinase might have the same effect as PAO treatment (inhibition of a tyrosine phosphatase might be equivalent to overexpression of a tyrosine kinase). To test this eggs were fertilized, and after the first cell division one of two blastomeres were injected with cascade blue dextran (CB-dex) alone (Figure 8A), or co-injected with mRNA encoding either sea urchin Src-kinase (Giusti et al., 2003)(panel C) or a mutant SrcK277M (panel B) which corresponds to a dominant negative mutation in mammalian cSrcK298M (Imamura et al., 2001; Mukhopadhyay et al., 1995). After a 1 hour incubation period embryos were incubated for an additional 30 minutes in ASW containing FM1-43 to allow for endocytotic dye internalization. Embryos were then washed with ASW to remove FM1-43 from the embryo surface and imaged by two-photon microscopy. Cells of embryos injected with CB-dex developed from the injected blastomere as indicated by the presence of the blue fluorescence. All of the embryo’s cells, both those containing cascade blue dextran and those without the marker were evenly labeled with internalized FM1-43 (panel A) indicating that CB-dex did not interfere with normal development or with FM1-43 uptake. This pattern was always observed in embryos injected with CB-dex alone (n = 25 embryos). A similar pattern was observed for embryos injected with RNA encoding the dominant negative Src kinase (n = 25, panel B). In contrast, both cell division and FM 1-43 uptake was inhibited in cells derived from the blastomere injected with RNA encoding Src kinase (panel C). Typically the blastomere injected with Src RNA would undergo the next round of cytokinesis and then stop dividing. In contrast, the blastomere that was not injected would continue to divide. This pattern was observed in 21 out of 25 injected embryos. Presumably, over-expression of functional Src is responsible for inhibiting FM1-43 uptake and cell division.
Figure 8.
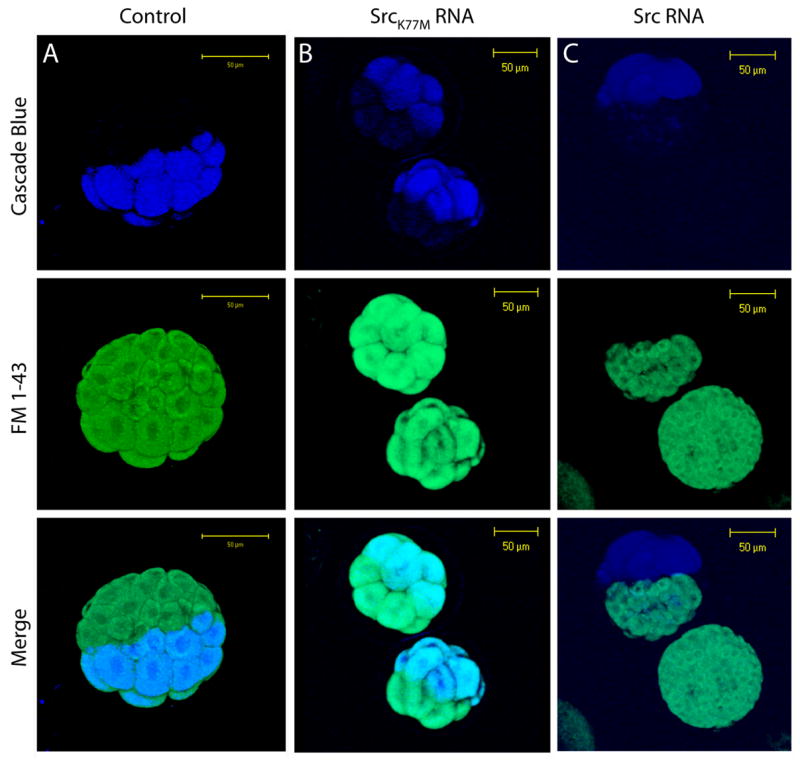
Src over-expression mimics the effect of PAO on FM1-43 uptake and cell division. Sea urchin eggs (Lytechinus variegatus) were attached to glass-bottomed chambers and fertilized. At the two-cell stage one of the two blastomeres was injected with cascade-blue dextran (CB-dex, 0.1 mg/ml) alone (panel A), or co-injected with mRNA (0.51 mg/ml) encoding either a constitutive inactive mutant of Src (SrcK277M) or encoding active sea urchin Src (SpSFK1). Next, embryos were incubated for 1 hour at 22°C, and then incubated for an additional 30 minutes in ASW containing 4 μM FM1-43. Embryoes were then washed and imaged by two-photon microscopy. Size bars are 50 μm.
The phorbol ester PMA rescues the agatoxin but not the PAO block of compensatory endocytosis
While the PAO sensitive form of compensatory endocytosis appears to be inhibited by tyrosine kinase activity, and presumably promoted by tyrosine phosphatase activity, the selective inhibition of the agatoxin sensitive form of compensatory endocytosis with both staurosporine and the calcineurin inhibitor FK506 (Figure 4A) suggests that this form of compensatory endocytosis is inhibited by both serine-threonine phosphorylation and a calcium-dependent de-phosphorylation events. Because staurosporine is a non-specific inhibitor of serine-threonine kinases we speculated that some of staurosporine’s more susceptible targets might inhibit this form of endocytosis and thus mask the existence of another calcium-dependent kinase activity which would promote this form of endocytosis. Accordingly we tested whether PMA, a phorbol ester known to activate protein kinase-C, could rescue the agatoxin sensitive form of compensatory endocytosis. PMA treatment of unfertilized eggs failed to stimulate endocytotic activity (Figure 9) as previously reported (Whalley et al., 1995). In contrast, a dose-dependent tetramethylrhodamine-dextran uptake was observed with PMA treatment of fertilized eggs if their calcium-dependent form of endocytosis had been previously blocked with agatoxin.
Figure 9.
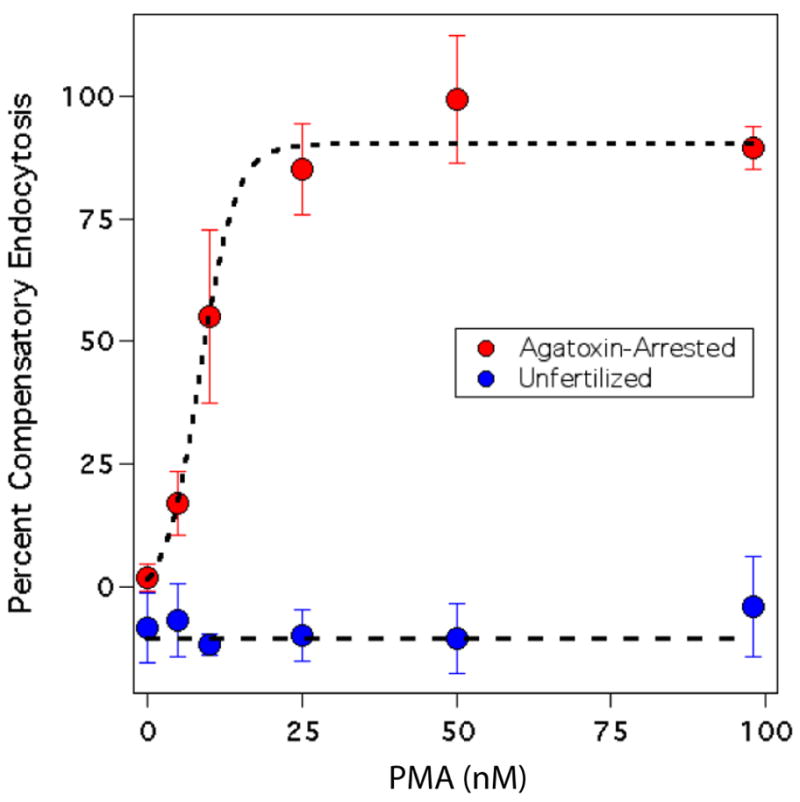
Phorbol ester rescues the agatoxin sensitive form of endocytosis. Either unfertilized eggs (Strongylocentrotus purpuratus, Blue circles) or eggs fertilized in the presence of agatoxin-TK (100 nM, red circles), were incubated for 15 minutes in ASW containing 100 μM tetramethylrhodamine-dextran (to monitor fluid phase uptake) and varying concentrations of PMA. The amount of uptake was normalized to the amount of uptake detected for control eggs (100%) and to fertilized eggs in calcium free ASW (0%). All points are mean ± SD, n=6. Note that the unfertilized eggs had a small negative value. Presumably this is due to a small amount of PAO sensitive uptake in calcium free ASW.
The ability to specifically block the calcium dependent form of compensatory endocytosis with agatoxin and to then subsequently rescue that activity with phorbol ester treatment affords the opportunity to directly test if phorbol ester treatment can rescue endocytosis blocked by PAO under conditions where prior to phorbol ester treatment both cortical granule membranes as well as the membranes inserted by constitutive exocytosis should be trapped on the cell surface. Fertilized eggs were treated with either agatoxin, PAO, or a mixture of both inhibitors, and FM1-43 uptake was assayed between 30–60 minutes post fertilization and compared with uptake in control cells (Figure 10, blue bars). Significant FM1-43 uptake was observed in both control eggs and those treated with agatoxin (co-applied with sperm), and the amount of uptake in these samples was indistinguishable. In contrast, virtually no FM1-43 uptake was observed in eggs treated with PAO (applied immediately after fertilization) or eggs treated with both agatoxin and PAO. Similar results were obtained when PAO was applied for two minutes prior to fertilization (data not shown). When the same experiment was performed but with the addition of PMA at the same time as FM1-43 (between 30–60 minutes post fertilization) a significant increase in FM 1-43 uptake was observed in the eggs treated with either agatoxin or the mixture of agatoxin and PAO (Figure 10, red bars). While the absolute magnitude of the FM 1-43 uptake observed in the agatoxin treated cells was larger than in the cells treated with both agatoxin and PAO, the magnitude of the augmentation observed in these cells was similar. This PMA induced augmentation was not observed in control eggs, or eggs treated with PAO alone. These results indicate that eggs treated with both agatoxin and PAO have membranes trapped in their cell surface associated with both cortical granule exocytosis and constitutive exocytosis. PMA treatment of these cells appeared to stimulate the retrieval of membranes trapped on the surface as a result of agatoxin treatment, but did not rescue membranes trapped on the cell surface due to PAO treatment.
Figure 10.
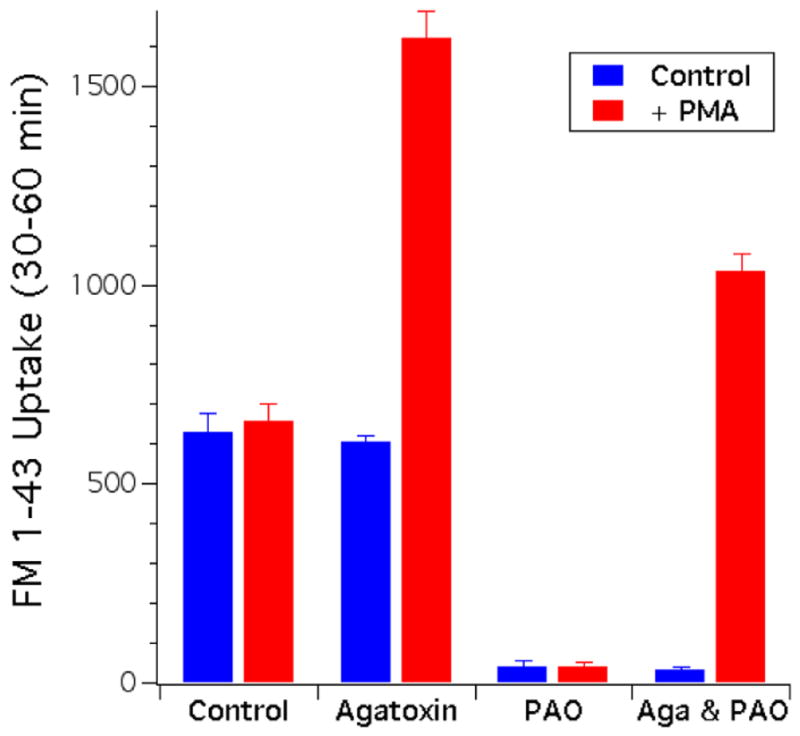
Phorbol ester does not rescue the PAO block. Blue bars depict the FM1-43 uptake of fertilized eggs (Strongylocentrotus purpuratus) treated with agatoxin-TK (100 nM), PAO (10 μM), or a combination of both inhibitors, and compares uptake with the uptake observed in control cells. FM1-43 (4 μM) was added at 30 minutes post-fertilization and eggs were incubated in the presence of FM1-43 and inhibitors for an additional 30 minutes. Eggs were then washed with ASW containing trypan blue (1 mg/ml) to quench any residual extracellular FM1-43 fluorescence and uptake was measured. Agatoxin was added with sperm, while PAO was added 2 minutes after the addition of sperm. Red Bars depict the same experiment outlined above but in the presence of 100 nM PMA added 5 minutes before fertilization and present up to the removal of FM1-43 at 60 minutes post-fertilization. All point represent mean±SEM, n=3 using eggs from three different animals each assayed in triplicate. Note the dramatic increase in FM1-43 uptake observed in eggs treated with agatoxin and PMA, and the lack of an increase in eggs treated with PAO and PMA.
DISCUSSION
Two experiments indicate that the net surface area of the sea urchin embryo remains constant during the first three cell divisions. Figure 1 shows that the instantaneous FM1-43 signal did not change, and in figure 2 a constant drop in FM1-43 fluorescence, proportional to the surface area of the embryo, was always observed immediately upon removal of extracellular FM1-43. It is clear that changes in embryonic surface area can be detected with FM1-43 based assays because an increase in the instantaneous FM1-43 signal was observed when endocytosis was inhibited with a tyrosine phosphatase inhibitor (see figure 6). Thus, it is unlikely that the sensitivity of the FM1-43 based assays used in this study limits our ability to detect changes in exocytosis and endocytosis. It is also unlikely that these FM1-43 based assays are limited by changes in dye access to the cell surface because the kinetics of dye labeling were identical for embryos labeled at the 1, 2, 4, and 8-cell stages (figure 1). Furthermore, there was no evidence for any inhibitory activity of FM1-43 on membrane trafficking (supplemental figure 3), nor did the pH sensitivity of styryl dyes significantly alter the fluorescent signal of developing sea urchin embryos incubated in ASW containing FM1-43 (supplemental figure 2). Thus, the simplest interpretation for the observed static instantaneous FM1-43 signals in figure 1 is that the net surface area of the developing embryo did not change between the one and eight-cell stages.
Sea urchin eggs use a simple mechanism to couple calcium triggered exocytosis to compensatory endocytosis. When cortical vesicles fuse with the plasma membrane following fertilization they insert vesicular P-type calcium channels into the cell surface (Smith et al., 2000). Local calcium influx through these channels activates compensatory endocytosis. In the present study, we found that this form of membrane retrieval is blocked by the non-specific kinase inhibitor staurosporine, as well as by the serine/threonine phosphatase inhibitor FK506 (figure 4). PMA, an activator of protein kinase-C, was found to rescue this form of endocytosis when calcium influx was blocked with agatoxin (figure 9). PMA did not trigger endocytosis in unfertilized eggs (figure 9), nor did it trigger FM1-43 uptake when applied 30 minutes after fertilization (figure 10), indicating that it can only trigger uptake when the membranes of cortical vesicles are trapped on the cell surface. Together these experiments suggest that local calcium influx through P-channels activates both protein kinase-C, as well as the calcium-activated phosphatase calcineurin to trigger this form of membrane retrieval. Inhibition by staurosporine, however, indicates that other kinase signaling cascades might be able to negatively regulate this mechanism.
Cells that have considerable triggered exocytotic activity often have constitutive exocytotic activity as a housekeeping function. Thus, it may be difficult to decipher the regulation of compensatory endocytosis because multiple forms of vesicle fusion and endocytosis are potentially operant. We used the styryl dye FM1-43 to confirm that developing sea urchin embryos do have constitutive exocytotic activity. When FM1-43 was added to developing sea urchin embryos at any point between 30 and 300 minutes post fertilization, a large and rapid increase in FM1-43 fluorescence was always observed (figure 1A). Because FM1-43 fluorescence is proportional to the cumulative surface area of dye-accessible membrane, an increase in FM1-43 fluorescence with time is indicative of exocytotic activity. The kinetics of FM1-43 staining was identical at these different time points (figure 1B) suggesting that the rate of exocytosis was constant over this period. A 74.0 ± 5.3 % increase in FM1-43 fluorescence (mean ± SD, n=10 traces in triplicate) was observed over a 15 minute incubation period initiated by the addition of FM1-43. Without knowledge of the size of the vesicles involved in constitutive exocytosis, or knowing what proportion of FM1-43 fluorescence is derived from plasma membrane resident dye (as compared to fluorescence caused by dye binding to other membrane components) it is not possible to derive accurate rates of exocytosis from this FM1-43 data. Still, because embryos did show a robust and constant increase in FM1-43 fluorescence, we conclude that between 30–300 minutes post fertilization constitutive exocytotic activity is present.
Instantaneous FM1-43 measurements indicated that the net surface area of the developing sea urchin embryo remains constant during the first 3 cell divisions (see red filled circles in figure 1A). Cumulative FM1-43 measurements during this same period, however, indicated that constitutive exocytosis is occurring throughout this period (see black open circles in figure 1A). If these interpretations are correct, we are forced to conclude that a process, such as compensatory endocytosis, must be removing the same amount of membrane that is delivered to the embryo surface by constitutive exocytosis. Three additional experiments support this interpretation. First, multiphoton microscopy revealed that a fluorescent fluid phase marker is localized inside small cytoplasmic puncta when eggs are incubated in seawater containing tetramethylrhodamine dextran and agatoxin from 0 to 30 minutes post fertilization. Because this fluorescence could not be washed away by extracellular perfusion this experiment indicates that the structures labeled with tetramethylrhodamine dextran by this protocol have detached from the cell surface by endocytosis to form endosomes. Second, only when endosomes had been previously loaded with FM1-43 did we see a slow release of FM1-43 fluorescence (−3.2 ± 0.8 fluorescent-units/min) upon removal of extracellular FM1-43 (compare open black circles and open red squares in figure 2). This can be interpreted as exocytotic release of previously internalized endosomes, and is consistent with our previous finding that sea urchin endosomes are fusion competent (Ikebuchi et al., 2001). Finally, when endocytosis was blocked with PAO, the net surface area of the embryo increases at a rate of 2.8 ± 0.2 fluorescent-units/min (figure 6). Because this rate and the rate of FM1-43 release from endosomes (−3.2 ± 0.8 fluorescent-units/min) are so similar (other than sign) it suggests that the release of FM1-43 from endosomes, and the increase in surface membrane, are mediated by the same cellular reaction: constitutive exocytosis. Thus, in sea urchin there is ample evidence for surface area homeostasis, mediated by constitutive exocytosis, and compensatory endocytosis.
The progressive increase in fluorescence observed when FM1-43 was added to developing embryos (figure 1) proceeded at an initial rate of 10.3 ± 0.6 fluorescent-units/min. This rate was greater than FM1-43 release from endosomes (figure 2a, −3.2 ± 0.8 fluorescent-units/min), and for the increase in surface membrane following phenylarsine oxide treatment (figure 6, 2.8 ± 0.2 fluorescent-units/min). This is surprising because all three rates should approximate the rate of constitutive exocytosis. One explanation for this discrepancy would be that constitutive exocytosis is composed of two forms of exocytosis; one which apparently uses vesicles recycled from the plasma membrane, and a second form which does not. This data can be explained if the second form of exocytosis was: 1) sensitive to phenylarsine oxide, 2) use vesicles whose membranes are also retrieved by the PAO sensitive form of retrieval, and 3) uses vesicles synthesized de novo. This hypothesis is supported by the observation that cumulative FM1-43 traces do not reach a fixed asymptote (see figure 1), even after 2 hours of FM1-43 incubation. It is interesting to note that while some vesicles that internalize FM1-43 can be rereleased, the bulk of internalized FM1-43 is never released from the embryo (figure 2).
The calcium requirement for compensatory endocytosis is controversial. Evidence in support of a direct calcium dependence for compensatory endocytosis has been established for the retrieval of sea urchin egg cortical vesicle membranes (Smith et al., 2000; Vogel et al., 1999). It was enlightening to discover that sea urchin embryos also have another form of compensatory endocytosis that does not require extracellular calcium (figure 3). While we cannot rule out the possibility that this form of endocytosis simply uses intracellular stores of calcium instead of extracellular calcium to trigger retrieval, this seems unlikely because in addition to its insensitivity to agatoxin, cadmium, and the extracellular calcium concentration, it was also insensitive to FK506 (see figure 4) an inhibitor of a calcium sensitive phosphatase, and to PMA, an activator of protein kinase-C. Furthermore, this form of endocytosis was inhibited by PAO (figure 4A and B) while the calcium-dependent form of compensatory endocytosis was not (figure 4A). Thus, it is likely that these two compensatory retrieval mechanisms use different signaling cascades and components. We suggest that the calcium dependent form of compensatory endocytosis utilizes calcium based signaling to activate the serine-threonine specific phosphatase, calcineurin, and perhaps protein kinase-C. Further, we propose that the form of endocytosis that compensates for constitutive exocytosis, uses an intracellular signaling cascade that either activates a tyrosine phosphatase or inhibits the activity of a tyrosine kinase, perhaps Src, to enable retrieval.
One unexpected finding of this study was that in addition to blocking FM1-43 uptake (figure 4), PAO treatment also blocked some constitutive exocytotic activity (figure 6) and most strikingly it blocked cell division (figure 7). Inhibition of cell division was also observed with the 5 other tyrosine phosphatase inhibitors tested in figure 4B (data not shown). One trivial explanation for these multiple effects is that these compounds are simply killing the developing embryo. This seems unlikely because 1. PAO pre-treatment of unfertilized eggs did not block fertilization as measured by the rising of the fertilization envelope, 2. PAO treatment did not completely block the calcium dependent form of compensatory endocytosis (figure 5), and 3. PAO treatment did not block the ability of PMA to rescue agatoxin sensitive retrieval. A more biologically relevant explanation for the multiple effects of PAO is that these cellular functions are either regulated by tyrosine phosphorylation cascades, or tyrosine phosphorylation plays an integral role in their mechanisms. Consistent with this explanation is the finding that micro-injection of mRNA encoding sea urchin Src kinase also blocked FM1-43 uptake as well as cell division (figure 8). Inhibition of uptake and cell division was not observed when CB-dex was injected into blastomeres, or when RNA encoding SrcK277M, a constitutively inactive mutant of Src was injected. Furthermore, injection of Src RNA did not immediately block cell division, injected blastomeres typically went through one or two rounds of cytokenesis before cell division was arrested. This is consistent with control experiments where RNA encoding Venus, a rapidly folding yellow spectral variant of GFP (Nagai et al., 2002), was injected into a blastomere. The expression of its faint yellow fluorescence could be observed within 30 min. of RNA injection, and was readily detected by the blastula stage (See supplemental figure 4). Presumably, Src expression subsequent to mRNA injection follows a similar time course. Regardless, the trivial explanation of damage as a result of Src RNA injection seems unlikely. Because Src has been found in membrane rafts thought to be required for cytokinesis (Ng et al., 2005), it is interesting to speculate on whether the multiple effects of PAO and Src RNA injection result from parallel sites of action, or from a common site shared by these susceptible mechanisms. Blocking endocytosis might reduce the rate of constitutive exocytosis by reducing the number of endosomes available to subsequently fuse with the plasma membrane as observed in figure 2. Similarly, a specific form of exocytosis (Conner and Wessel, 1999; O’Halloran, 2000; Shuster and Burgess, 2002; Straight and Field, 2000) or endocytosis (Dhonukshe et al., 2006; Konopka et al., 2006; Shen and Temple, 2002) might be required for cell division. Further experimentation is required to investigate these possibilities.
We have previously shown that cortical vesicle proteins inserted into the cell surface by exocytosis are specifically retrieved by a calcium dependent form of compensatory endocytosis mediated by agatoxin sensitive calcium channels (Smith et al., 2000). Proteins associated with the plasma membrane prior to cortical vesicle exocytosis were found to be occluded by this retrieval process. Here we have extended this observation to show that treatment with PMA can trigger the retrieval of cortical vesicle membranes between 30–60 minutes after fertilization, even after agatoxin treatment (figure 10). Because the effects of agatoxin on P-type voltage-gated calcium channels are thought to be irreversible over a time scale of minutes (Smith et al., 2000), these experiments suggest that calcium is required to activate protein kinase C for retrieval and is not required for subsequent steps. Furthermore, the observation that the membranes of both cortical vesicles and those inserted by constitutive exocytosis were both trapped on the cell surface by treatment with both agatoxin and PAO, yet only retrieval correlated with the cortical vesicles was observed after PMA treatment, again suggesting that the calcium dependent form of compensatory endocytosis is specific to the retrieval of cortical vesicle proteins, and extends this observation by arguing that this form of compensatory endocytosis can not retrieve the membranes associated with constitutive exocytosis. The cellular picture that emerges is akin to the behavior of FedEx and UPS trucks. While there is no apparent reason why a truck from one delivery company could not pick up a package from another, this behavior is rarely observed. Similarly, we observe that the endocytotic activity triggered by PMA treatment does not retrieve membranes trapped by PAO treatment, but does retrieve membranes trapped by agatoxin treatment (figure 10). Likewise, membranes trapped by agatoxin treatment were not retrieved by the PAO sensitive retrieval mechanism (when left operational).
In conclusion, during early development there is extensive membrane trafficking to and from sea urchin embryo surface membranes. There is evidence for a least three forms of exocytosis; calcium triggered cortical vesicle exocytosis (during the first three minutes following fertilization), constitutive exocytosis (from 30 to 300 minutes post fertilization) of de novo vesicles, and the constitutive fusion of recycled endosomes with the plasma membrane. None of these forms of exocytosis requires extracellular calcium. Despite this extensive exocytotic activity, excess membrane is not generated by these processes during early development. The membranes delivered to the cell surface by these three forms of exocytosis are all compensated for by endocytosis. Two forms of compensatory endocytosis were found to be responsible for maintaining this embryonic cell surface homeostasis. A calcium dependent form was found to be sensitive to FK506 and triggered by PMA, and is involved in the retrieval of the membranes of cortical vesicles from the cell surface during a 15-minute period following fertilization. An apparently calcium independent form of compensatory endocytosis was found to be inhibited by six different tyrosine phosphatase inhibitors as well as by over-expression of Src kinase. This form of endocytosis is responsible for compensating for the membrane added to the embryo surface by both de novo vesicle fusion (constitutive exocytosis), and the fusion of endosomes formed by previous retrieval. Under physiological conditions the calcium dependent form of endocytosis occurs during the first 15 minutes of development while the second, PAO sensitive form occurs over at least the following 5 hours. Our experiments indicate that both mechanism can occur concomitantly, and still maintains a membrane specificity for retrieval with no observable overlap. While the mechanism of this remarkable specificity requires further study, we can conclude that the two different compensatory retrieval mechanisms present in sea urchin eggs are not redundant, rather they are used to retrieve specific subsets of membrane from the cell surface.
Supplementary Material
Acknowledgments
We thank C. Thaler, H. Puhl and S. Koushik for their advice and expertise, and Dr. Kathy Foltz for providing the sea urchin Src cDNA clone SpSFK1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aballay A, Stahl PD, Mayorga LS. Phorbol ester promotes endocytosis by activating a factor involved in endosome fusion. J Cell Sci. 1999;112:2549–57. doi: 10.1242/jcs.112.15.2549. [DOI] [PubMed] [Google Scholar]
- Andreuccetti P, Barone Lumaga MR, Cafiero G, Filosa S, Parisi E. Cell junctions during the early development of the sea urchin embryo (Paracentrotus lividus) Cell Differ. 1987;20:137–46. doi: 10.1016/0045-6039(87)90427-1. [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Elhamdani A, Palfrey HC. Sustained stimulation shifts the mechanism of endocytosis from dynamin-1-dependent rapid endocytosis to clathrin- and dynamin-2-mediated slow endocytosis in chromaffin cells. Proc Natl Acad Sci U S A. 2002;99:6358–63. doi: 10.1073/pnas.082658499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R, Takei K, De Camilli P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem. 1997;272:30984–92. doi: 10.1074/jbc.272.49.30984. [DOI] [PubMed] [Google Scholar]
- Betz WJ, Bewick GS. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–3. doi: 10.1126/science.1553547. [DOI] [PubMed] [Google Scholar]
- Betz WJ, Mao F, Smith CB. Imaging exocytosis and endocytosis. Curr Opin Neurobiol. 1996;6:365–71. doi: 10.1016/s0959-4388(96)80121-8. [DOI] [PubMed] [Google Scholar]
- Chan SA, Smith C. Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells. J Physiol. 2001;537:871–85. doi: 10.1111/j.1469-7793.2001.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SA, Smith C. Low frequency stimulation of mouse adrenal slices reveals a clathrin-independent, protein kinase C-mediated endocytic mechanism. J Physiol. 2003;553:707–17. doi: 10.1113/jphysiol.2003.053918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SD, Wessel GM. Syntaxin is required for cell division. Mol Biol Cell. 1999;10:2735–43. doi: 10.1091/mbc.10.8.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Baluska F, Schlicht M, Hlavacka A, Samaj J, Friml J, Gadella TW., Jr Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev Cell. 2006;10:137–50. doi: 10.1016/j.devcel.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Eddy EM, Shapiro BM. Changes in the topography of the sea urchin egg after fertilization. J Cell Biol. 1976;71:35–48. doi: 10.1083/jcb.71.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engisch KL, Nowycky MC. Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. J Physiol (Lond) 1998;506:591–608. doi: 10.1111/j.1469-7793.1998.591bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay DM, Michelangeli VP, Robinson PJ. Protein kinase-C-induced down-regulation of calcitonin receptors and calcitonin-activated adenylate cyclase in T47D and BEN cells. Endocrinology. 1989;125:2656–63. doi: 10.1210/endo-125-5-2656. [DOI] [PubMed] [Google Scholar]
- Fisher GW, Rebhun LI. Sea urchin egg cortical granule exocytosis is followed by a burst of membrane retrieval via uptake into coated vesicles. Dev Biol. 1983;99:456–72. doi: 10.1016/0012-1606(83)90295-6. [DOI] [PubMed] [Google Scholar]
- Frejtag W, Burnette J, Kang B, Smith RM, Vogel SS. An increase in surface area is not required for cell division in early sea urchin development. Dev Biol. 2003;259:62–70. doi: 10.1016/s0012-1606(03)00184-2. [DOI] [PubMed] [Google Scholar]
- Fulop T, Radabaugh S, Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J Neurosci. 2005;25:7324–32. doi: 10.1523/JNEUROSCI.2042-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Smith C. Physiological stimulation regulates the exocytic mode through calcium activation of protein kinase C in mouse chromaffin cells. Biochem J. 2006;399:111–9. doi: 10.1042/BJ20060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti AF, O’Neill FJ, Yamasu K, Foltz KR, Jaffe LA. Function of a sea urchin egg Src family kinase in initiating Ca2+ release at fertilization. Dev Biol. 2003;256:367–78. doi: 10.1016/s0012-1606(03)00043-5. [DOI] [PubMed] [Google Scholar]
- Gundelfinger ED, Kessels MM, Qualmann B. Temporal and spatial coordination of exocytosis and endocytosis. Nat Rev Mol Cell Biol. 2003;4:127–39. doi: 10.1038/nrm1016. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Merelli F, Baukal AJ, Balla T, Catt KJ. Agonist-induced endocytosis and signal generation in adrenal glomerulosa cells. A potential mechanism for receptor-operated calcium entry. J Biol Chem. 1991;266:2783–8. [PubMed] [Google Scholar]
- Ikebuchi Y, Baibakov B, Smith RM, Vogel SS. Plasma Membrane Resident ‘Fusion Complexes’ Mediate Reconstituted Exocytosis. Traffic. 2001;2:654–667. doi: 10.1034/j.1600-0854.2001.20908.x. [DOI] [PubMed] [Google Scholar]
- Imamura T, Huang J, Dalle S, Ugi S, Usui I, Luttrell LM, Miller WE, Lefkowitz RJ, Olefsky JM. beta -Arrestin-mediated recruitment of the Src family kinase Yes mediates endothelin-1-stimulated glucose transport. J Biol Chem. 2001;276:43663–7. doi: 10.1074/jbc.M105364200. [DOI] [PubMed] [Google Scholar]
- Jaffe LA, Cross NL. Electrical regulation of sperm-egg fusion. Annu Rev Physiol. 1986;48:191–200. doi: 10.1146/annurev.ph.48.030186.001203. [DOI] [PubMed] [Google Scholar]
- Jaffe LA, Hagiwara S, Kado RT. The time course of cortical vesicle fusion in sea urchin eggs observed as membrane capacitance changes. Dev Biol. 1978;67:243–8. doi: 10.1016/0012-1606(78)90314-7. [DOI] [PubMed] [Google Scholar]
- Konopka CA, Schleede JB, Skop AR, Bednarek SY. Dynamin and cytokinesis. Traffic. 2006;7:239–47. doi: 10.1111/j.1600-0854.2006.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Maxfield FR. Ca(2+)- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature. 1995;377:75–9. doi: 10.1038/377075a0. [DOI] [PubMed] [Google Scholar]
- Liu JP, Sim AT, Robinson PJ. Calcineurin inhibition of dynamin I GTPase activity coupled to nerve terminal depolarization. Science. 1994;265:970–3. doi: 10.1126/science.8052858. [DOI] [PubMed] [Google Scholar]
- McCulloh DH. Cortical reaction of sea urchin eggs: rate of propagation and extent of exocytosis revealed by membrane capacitance. Development. 1985;27:178. [Google Scholar]
- Miyake K, McNeil PL. A little shell to live in: Evidence that the fertilization envelope can prevent mechanically induced damage of the developing sea urchin embryo. Biol Bull. 1998;195:214–5. doi: 10.2307/1542846. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature. 1995;375:577–81. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol. 2002;20:87–90. doi: 10.1038/nbt0102-87. [DOI] [PubMed] [Google Scholar]
- Ng MM, Chang F, Burgess DR. Movement of membrane domains and requirement of membrane signaling molecules for cytokinesis. Dev Cell. 2005;9:781–90. doi: 10.1016/j.devcel.2005.11.002. [DOI] [PubMed] [Google Scholar]
- O’Halloran TJ. Membrane traffic and cytokinesis. Traffic. 2000;1:921–6. [PubMed] [Google Scholar]
- Oustrin ML, Belenguer P, Leroy D, Hoffmann I, Ducommun B. Effect of phenylarsine oxide on the fission yeast Schizosaccharomyces pombe cell cycle. Biochimie. 1995;77:279–87. doi: 10.1016/0300-9084(96)88137-5. [DOI] [PubMed] [Google Scholar]
- Robinson PJ, Sontag JM, Liu JP, Fykse EM, Slaughter C, McMahon H, Sudhof TC. Dynamin GTPase regulated by protein kinase C phosphorylation in nerve terminals. Nature. 1993;365:163–6. doi: 10.1038/365163a0. [DOI] [PubMed] [Google Scholar]
- Shafi NI, Vogel SS, Zimmerberg J. Using caged calcium to study sea urchin egg cortical granule exocytosis in vitro. Methods: A Companion to Methods in Enzymology. 1994;6:82–92. [Google Scholar]
- Shen Q, Temple S. Creating asymmetric cell divisions by skewing endocytosis. Sci STKE. 2002;2002:PE52. doi: 10.1126/stke.2002.162.pe52. [DOI] [PubMed] [Google Scholar]
- Shen SS. Mechanisms of calcium regulation in sea urchin eggs and their activities during fertilization. Curr Top Dev Biol. 1995;30:63–101. doi: 10.1016/s0070-2153(08)60564-5. [DOI] [PubMed] [Google Scholar]
- Shen SS, Buck WR. Sources of calcium in sea urchin eggs during the fertilization response. Dev Biol. 1993;157:157–69. doi: 10.1006/dbio.1993.1120. [DOI] [PubMed] [Google Scholar]
- Shuster CB, Burgess DR. Targeted new membrane addition in the cleavage furrow is a late, separate event in cytokinesis. Proc Natl Acad Sci U S A. 2002;99:3633–8. doi: 10.1073/pnas.052342699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Neher E. Multiple forms of endocytosis in bovine adrenal chromaffin cells. J Cell Biol. 1997;139:885–94. doi: 10.1083/jcb.139.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RM, Baibakov B, Ikebuchi Y, White BH, Lambert NA, Kaczmarek LK, Vogel SS. Exocytotic Insertion of Calcium Channels Constrains Compensatory Endocytosis to Sites of Exocytosis. J Cell Biol. 2000;148:755–768. doi: 10.1083/jcb.148.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt R, Zucker R, Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977;58:185–96. doi: 10.1016/0012-1606(77)90084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight AF, Field CM. Microtubules, membranes and cytokinesis. Curr Biol. 2000;10:R760–70. doi: 10.1016/s0960-9822(00)00746-6. [DOI] [PubMed] [Google Scholar]
- Terasaki M. Visualization of exocytosis during sea urchin egg fertilization using confocal microscopy. J Cell Sci. 1995;108:2293–300. doi: 10.1242/jcs.108.6.2293. [DOI] [PubMed] [Google Scholar]
- Vogel SS, Smith RM, Baibakov B, Ikebuchi Y, Lambert NA. Calcium influx is required for endocytotic membrane retrieval. Proc Natl Acad Sci U S A. 1999;96:5019–24. doi: 10.1073/pnas.96.9.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley T, Terasaki M, Cho MS, Vogel SS. Direct membrane retrieval into large vesicles after exocytosis in sea urchin eggs. J Cell Biol. 1995;131:1183–92. doi: 10.1083/jcb.131.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.