Abstract
The role of endogenous GABA and ATP in regulating transmitter release from primary afferent terminals in the superficial dorsal horn of the spinal cord is still controversial. ATP is co-released with GABA from some inhibitory dorsal horn neurons raising the possibility that ATP could act in concert with GABA to regulate transmitter release from primary afferent terminals if receptors to both transmitters are expressed there. Using electrophysiology together with immunocytochemistry, we have investigated the expression of ATP-gated P2X and GABAA receptors by identified subpopulations of dorsal root ganglion (DRG) neurons known to project primarily to the superficial dorsal horn. Expression of the heat-sensitive vanilloid receptor 1 (VR1) and sensitivity to capsaicin were used to characterize DRG neurons sensitive to noxious heat. Both P2X and GABAA receptors were expressed on the majority of DRG neurons examined. Recording compound action potentials (CAPs) from dorsal roots in the presence of muscimol, α,β-methylene-ATP (α,β-meATP) or capsaicin resulted in depression of CAP in the slow and medium conducting fibres, indicating cognate receptor expression on the small diameter axons. Dorsal root-evoked dorsal root potentials (DR-DRPs), reflecting depolarization of primary afferent terminals by endogenously released substances, were depressed by the GABAA receptor antagonist SR95531 and α,β-meATP. These results suggest that GABAA and P2X receptors are expressed on DRG cell bodies and slow fibre axons, many of which are heat-nociceptive. These fibres project to the superficial lamina of the dorsal horn where the receptors may function to modulate transmitter release near their central terminals.
Regulation of transmitter release from central terminals of nociceptive sensory fibres is an important step in processing nociceptive signalling. These terminals are subject to synaptic regulation mediated by a wide array of metabotropic and ionotropic receptors. Two neurotransmitters shown to be co-released from dorsal horn neurons, ATP and GABA (Jo & Schlichter, 1999), have both been proposed to modulate synaptic release from terminals of primary afferents, raising the possibility that these two neurotransmitters may act in concert.
GABA is the best-known endogenous mediator of presynaptic inhibition on primary afferent terminals in the spinal cord (Rudomin & Schmidt, 1999). GABAergic modulation of sensory input to the dorsal horn has been demonstrated in several physiological studies (Carstens et al. 1987; Willis, 1999). Due to a high intracellular chloride concentration in dorsal root ganglion (DRG) neurons (Alvarez-Leefmans et al. 1988; Sung et al. 2000), GABA has a depolarizing effect on primary afferent terminals (primary afferent depolarization or PAD) in the spinal cord and causes a reduction of neurotransmitter release (Rudomin & Schmidt, 1999; Willis, 1999). GABA is released from inhibitory dorsal horn neurons at both dendritic and axonal release sites but the proximity of GABA release sites to most afferent terminals is unclear. Axo-axonic and dendro-axonic GABAergic synapses onto glomerular primary afferent terminals in the dorsal horn provide one example of a morphological substrate for GABA-mediated presynaptic inhibition (Ribeiro-da-Silva et al. 1985; Carlton & Hayes, 1991).
Evidence for the importance of extracellular ATP in sensory nerve function is provided by the high expression of most cloned P2X receptor subunits by DRG neurons (Dunn et al. 2001). The P2X3 subunit in particular is almost exclusively expressed by nociceptive neurons (Chen et al. 1995). A role for ATP and the ATP-gated P2X receptors in modulation of transmitter release from the central terminals of sensory neurons has been suggested because activation of presynaptically localized P2X receptors increases spontaneous (Gu & MacDermott, 1997) and evoked (Nakatsuka & Gu, 2001) glutamate release from DRG neurons. This is further supported by immunocytochemical evidence that P2X3 receptors are expressed at primary afferent terminals in lamina II of the spinal cord (Vulchanova et al. 1998) and that they are localized presynaptically (Llewellyn-Smith & Burnstock, 1998).
To investigate whether GABA and ATP could act as modulators of transmitter release from nociceptors terminating within the complex circuitry of the dorsal horn, we have used surface marker antibodies to identify DRG neuron subpopulations and electrophysiology to define ATP and GABAA receptor expression by those subpopulations. We have used capsaicin and antibodies to vanilloid receptor 1 (VR1) to identify neurons that are noxious heat-sensitive neurons. Finally, we have investigated the expression of ATP and GABAA receptors on central axons and nerve terminals of sensory neurons in spinal cord and dorsal root preparations, employing functional approaches to localize presynaptic GABAA and P2X receptors.
Methods
Dissociation of DRG neurons
Postnatal day 16 (P16) to P26 rats were anaesthetized with isoflurane and decapitated, in accordance with the Institutional Animal Care and Use Committee of Columbia University. The cervical, thoracic and lumbar DRGs were isolated, incubated with 1 mg ml−1 Type III trypsin and 250 u ml−1 Type IV collagenase from Clostridium histolyticum (both Sigma, Saint Louis, MO, USA) for 1 h in minimal essential medium (MEM), then washed and mechanically dissociated with glass pipettes. Isolated cells were plated on glass coverslips coated with poly-d-lysine and laminin (Sigma) and incubated at 37 °C for 2–24 h in MEM + 10 % heat-inactivated horse serum (HS) (Gibco, Grant Island, NY, USA) before use. Data were from dissociations using 28 different animals. Functional and immunocytochemical data were usually collected in parallel from cells of the same dissociation.
Immunocytochemistry of dissociated DRG neurons
For labelling of surface antigens of live DRG neurons, dissociated DRG neurons plated on glass coverslips were washed in regular bath solution (see Electrophysiology) and then incubated with primary antibody diluted in bath solution. Mouse anti-SSEA4 IgG monoclonal antibody was used at a dilution of 1:3; the FE-A5 (A5) antibody (mouse IgM supernatant) was used at 1:5 and A5 ascites at 1:100 (anti-SSEA4 and FE-A5 antibodies were from Developmental Studies Hybridoma Bank, University of Iowa, IA, USA). The LA4 antibody (mouse IgM ascites) was used at 1:100 and LD2 (mouse IgM supernatant) at 1:3 (both LA4 and LD2 were a gift from Dr J. Dodd, Columbia University). Neurons were incubated with primary antibodies for 1 h at room temperature. After washing with bath solution, the cells were incubated (30 min) with the secondary antibody, either Cy3-conjugated goat anti-mouse IgM or Cy3-conjugated goat anti-mouse IgG (Cy3-conjugated secondaries were from Jackson Immunoresearch Laboratories, West Grove, PA, USA), both diluted 1:500 in bath solution. Neurons were then washed and placed in the recording chamber on a microscope stage where they were continuously perfused with bath solution for electrophysiological experiments. DRG neurons were observed using a × 40 water immersion objective and staining was identified under fluorescence.
For surface marker/VR1 double labelling, cells were fixed and permeabilized (0.1 % saponin) before staining was performed. Affinity-purified rabbit anti-VR1 (gift from Dr D. Julius, University of California, San Francisco, 1:5000) or rabbit anti-VR1 N-terminus (1:1000, Neuromics, Northfield, MN, USA) was used simultaneously with the SSEA4, LA4, LD or A5 antibodies. Secondary antibodies were Alexa 488-conjugated goat anti-rabbit IgG.
Fixed cells were mounted in Vectashield (Vector Laboratories, Inc., Burlingame, CA, USA). Images were acquired using a Dage RC300 CCD camera, a Scion LG-3 frame grabber and ScionImage software. Stainings were compared with controls that did not include the primary antibody. Positive staining was defined by a threshold pixel value five times brighter than controls.
Electrophysiology
DRG neurons stained for surface markers were identified under fluorescence and marker-positive cells were patch clamped in whole-cell mode. Staining was localized on the membrane surface only. When adjusting the focal plane on the centre of the cells, a dotted or continuous fluorescent ring was observed at the perimeter of the cell. When the focal plane was adjusted to the top of the cell, a punctuate staining of the cell surface was observed. Patch pipettes had resistances ranging from 3 to 5 MΩ. Internal solution contained (mm): 130 Cs2MeSO4, 10 NaCl, 0.5 CaCl2, 10 Hepes and 5 EGTA. pH was adjusted to 7.3 with CsOH and osmolarity to 308 mosmol l−1 with sucrose. The external bath solution contained (mm): 145 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 5.5 glucose and 10 Hepes. Under the above conditions the calculated ECl was −67 mV. Membrane currents recorded using the Axopatch 200B (Axon Instruments, Union City, CA, USA), were filtered at 2 kHz and digitized at 5 kHz. Junction potential was corrected for all recordings. The telegraphed values of manual capacitance compensation were taken for the cell capacitance measurements. Drug solutions were applied to cells by local perfusion through capillary tubes (1.1 mm inner diameter) positioned near the cell of interest. Solution flow was driven by gravity (flow rate 1–2 ml min−1, solution exchange: 60–80 ms).
Dorsal root compound action potential and dorsal root-dorsal root potential recordings
Rat pups from P19-P2 were used for dorsal root compound action potential (CAP) studies. Rats were anaesthetized and decapitated. Spinal cords with roots and DRGs attached were removed and placed in ice-cold oxygenated (95 % O2 and 5 % CO2) Krebs solution containing (mm): 125 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4, 25 glucose, 1 MgCl2, 2 CaCl2, pH 7.4, 320 mosmol l−1. After removal of the dura mater, all ventral roots were cut near the root entry zone. Lower lumbar and sacral dorsal roots with their ganglia attached were removed from the spinal cord and transferred to an incubation chamber superfused with Krebs solution at room temperature for recovery (1 h). The isolated roots were then placed in a submersion-type recording chamber and superfused continuously with Krebs solution at 23–25 °C. The ganglion was separated from the rest of the root by a Vaseline barrier, to isolate it from drug perfusion. Two suction electrodes, one for stimulation and the other for recording, were placed at a distance of 0.8–2 mm apart.
Three components of the CAP could be distinguished, referred to as fast, medium and slow conducting groups. Their conduction velocities were 1.8 ± 0.2 m s−1 (n = 13), 0.47 ± 0.02 m s−1 (n = 15) and 0.21 ± 0.01 m s−1 (n = 15), respectively. Similar conduction velocities have been observed on excised dorsal root preparations at room temperature (Agrawal & Evans, 1986). However, the conduction velocity for the fast component recorded under these conditions is considerably slower than that recorded from a more intact preparation (Fitzgerald, 1985). Sometimes small intermediate components were present among these three groups but they were too irregular to analyse systematically. In 2 out of 15 dorsal root CAP recordings, faster CAPs (5.6 ± 0.9 m s−1) were observed while in another recording, a slower component was recorded (0.12 m s−1). The fast conducting CAPs were capsaicin insensitive while the slow conducting CAPs were capsaicin sensitive. The medium CAPs showed a moderate degree of sensitivity to capsaicin (see Results).
For dorsal root evoked dorsal root potential (DR-DRP) recordings, sections from lumbar to sacral spinal cords with dorsal roots and their ganglia attached were isolated from P19-P23 rats as described above. Cuts of the spinal cord were made with a pair of sharp scissors so that several spinal cord pieces with at least two dorsal roots at each side were obtained. These spinal cord portions were sagittally hemisected using a razor blade and then transferred to an incubation chamber. A suction electrode was placed on one of the two roots approximately 1.5–3 mm from the entry zone and was used for stimulation. A second suction electrode was placed on the adjacent dorsal root close to the entry zone and used as a recording electrode. For some experiments, DRGs were cut from the dorsal roots for CAP or DR-DRP recording. No significant difference was found between the data obtained from those with or without attached DRGs.
Dorsal roots were stimulated antidromically at 0.05–0.2 Hz for CAPs or orthodromically at 0.017–0.05 Hz for DR-DRP using an ISO-Flex Stimulus Isolator (AMPI, Jerusalem, Israel). Stimulus duration was 100 μs and stimulation intensity was adjusted to be high enough to elicit a slow fibre CAP or DR-DRP without damaging the preparations. Only data collected from roots with stable CAPs or DR-DRPs were included for analysis. Extracellular voltage signals were recorded by a Cygnus ER1 differential amplifier (Cygnus Technologyor, Delaware Water Gap, PA, USA) or Dagan EX1 differential amplifier (Dagan Corporation, Minneapolis, MI, USA) with a 4001 headstage. CAPs were sampled at 20 kHz and filtered at 10 kHz while the DR-DRPs were either sampled at 10 kHz for the entire record or for just the first 120 ms and with the rest of the record sampled at 833 Hz. All physiological data were acquired with pCLAMP 6 (Axon Instruments). In the DR-DRP experiments, when dual electrode recording was performed, the acquisition rate was 10 kHz for 800 ms. To analyse DR-DRPs, control Tpeak (time for DR-DRP to reach the maximal amplitude) was first obtained then subsequent DR-DRP amplitudes were measured at that Tpeak. For CAP analysis, we measured the relative amplitudes of each component of the CAP from the positive local maximal potential to the negative voltage peak (Fig. 5).
Figure 5. Dorsal root recordings show P2X and GABAA receptor-mediated depression of slow and medium conducting fibres and their capsaicin sensitivities.
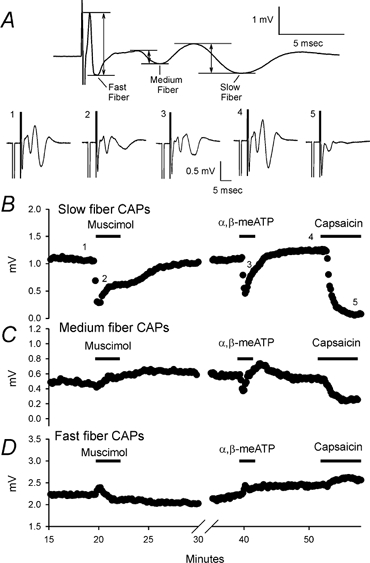
A, top panel illustrates how the amplitudes of fast, medium and slow fibre compound action potentials (CAPs) were measured. The amplitude of a specific CAP was measured from the local maximal positive potential before the negative potential to the local minimal potential corresponding to the CAP. This trace is the same as trace 1 in the bottom panel. Bottom panel shows CAPs recorded in normal Krebs buffer and in the presence of different drugs. Numbers 1–5 indicate the traces taken at the time indicated by the numbers in B. Traces 1 and 4 were in control conditions before each drug application. Traces 2, 3 and 5 were recorded at the time of the strongest responses to drug. Each trace represents an average of 5 records. B–D, time course of the drug effects on slow, medium and fast CAP amplitudes. B, typical effects of muscimol (50 μm), α,β-meATP (100 μm) and capsaicin (1 μm) on slow CAP. All of the drugs depressed slow fibre CAPs indicating that P2X, GABAA and VR1 receptors are expressed on slow conducting fibres. C, medium fibre CAPs were depressed by α,β-meATP and capsaicin but not muscimol. D, neither α,β-meATP nor capsaicin had depressing effects on fast CAPs. Muscimol slightly depressed the fast CAP. The small enhancing effects of the drugs on fast CAPs were presumably due to a decrease of overlapping components of the medium CAPs.
α,β-Methylene-ATP (α,β-meATP), pyridoxal phosphate 6-azophenyl-2′,4′-disulfonic acid (PPADS), muscimol and capsaicin were from Sigma. SR95531 and NF023 were from Tocris (Ellisville, MO, USA).
Statistical analysis
Statistical analysis was carried out using Student's paired t test, ANOVA or the Kruskal-Wallis test depending on normality of the data. Data were expressed as the mean ± s.e.m. or as the mean and range.
Results
Subpopulations of acutely dissociated DRG neurons
Immunocytochemical markers LA4, LD2, A5 and SSEA4 were used to pre-identify subpopulations of nociceptive DRG neurons. LA4 marks a population of primary afferents that project to inner lamina II of the dorsal horn (Dodd & Jessell, 1985). This population includes nearly all of the fluoride-resistant acid phosphatase (FRAP) neurons and all of the somatostatin-positive neurons as well as other unidentified neuronal types. LA4-positive neurons make up the same population of DRG neurons marked by the lectin IB4 (Lee et al. 2001) and have been identified as giving rise to C-fibres (Fang et al. 2000). LD2 marks all somatostatin-expressing neurons and a few of the FRAP-positive neurons, making the LD2-positive DRG subpopulation partially overlap that marked by LA4 (Dodd & Jessell, 1985). However, there are LD2-positive neurons that do not overlap with LA4 (Lee et al. 2001) as originally suggested by the observation that central terminals marked by LD2 stain most strongly in outer lamina II of the dorsal horn (Dodd & Jessell, 1985). SSEA4 marks a population of medium diameter DRG neurons that terminate in laminae I, III and IV. SSEA4-positive DRG neurons are not labelled by antibodies to LA4 and LD2 and do not include the substance P (SP)-positive DRG neurons. Neurons expressing A5 terminate most strongly in lamina I, they overlap somewhat with LA4 and LD2 but also include 50 % of the SP-expressing neurons. Because all four probes, LA4, LD2, SSEA4 and A5, are surface markers, we used them to identify cell populations prior to performing physiological and pharmacological tests.
We determined the percentage of DRG neurons from P16 to P26 rat pups that were labelled by each of the four markers. Examples of staining with each antibody are shown in Fig. 1. SSEA4 stained 9 % (n = 1495; examples in Fig. 1A) and LD2 stained 28 % of the neurons counted (n = 572; examples in Fig. 1B). LA4 stained 65 % (n = 644; examples in Fig. 1C) whereas A5 stained 23 % (n = 525; examples in Fig. 1D) of the neurons counted. The proportions of these populations in dissociated DRG neurons were roughly comparable to those counted by Dodd & Jessell (1985; 11 %, 20–25 %, 45–50 % and 40–45 %, respectively) in whole ganglia from adult rats except for the A5-positive neurons that appeared to be underrepresented in our dissociated cell preparation.
Figure 1. Subpopulations of DRG neurons are identified by antibodies to carbohydrate markers.
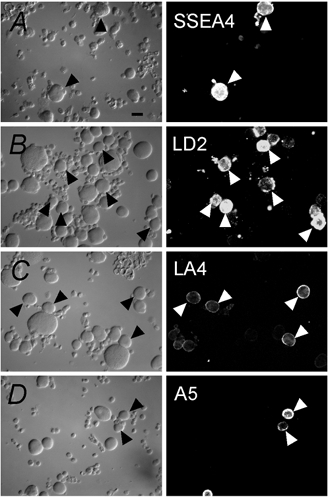
Acutely dissociated DRG neurons express the surface markers SSEA4 (A), LD2 (B), LA4 (C) and A5 (D). On the left, panels are differential interference contrast (DIC) images. On the right, corresponding fluorescence images are displayed. Arrowheads point to examples of positively stained cells. Scale bar: 20 μm.
Expression of VR1
Expression of VR1 and sensitivity to capsaicin are properties of most noxious heat-sensing nociceptors (Caterina et al. 2000). We used electrophysiology and immunocytochemistry to identify these nociceptors within our four subpopulations. Electrophysiological recordings from DRG neurons pre-identified with one of the four surface markers were carried out to assess expression of functional VR1 by activation with capsaicin. Capsaicin (1 μm) applied for 2.5 s produced an inward current in a portion of cells tested (Fig. 2A and B). In SSEA4-positive DRG neurons, the lowest percentage of neurons responded to capsaicin, whereas LA4-positive neurons had the highest percentage of responders (Fig. 2B). More than half of the LD2- and A5-positive neurons tested were also responsive to capsaicin. There were no significant differences (one-way ANOVA, P > 0.05) in the amplitudes of the capsaicin responses in LA4 (6.2 ± 2.7 pA pF−1, 17 ± 3 pF, n = 8), LD2 (5.4 ± 1.6 pA pF−1, 21 ± 3 pF, n = 6) and A5 (4.9 ± 1.2 pA pF−1, 22 ± 2 pF, n = 7) subpopulations of DRG neurons. In the two SSEA4 cells that responded to capsaicin the amplitude was 1.93 ± 0.73 pA pF−1 (46 ± 3 pF).
Figure 2. Capsaicin currents and VR1 protein expression in DRG neuron subpopulations are compared.
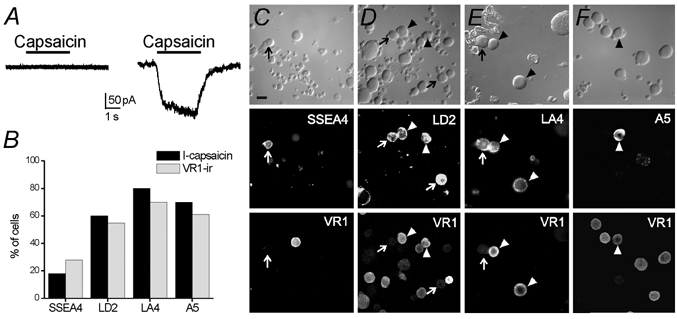
A, sample current responses to capsaicin (1 μm) were recorded from pre-labelled acutely dissociated DRG neurons. The current trace on the left was recorded from an SSEA4-positive neuron that was capsaicin insensitive. The record on the right was obtained from an A5-positive DRG neuron that responded to capsaicin with an inward current. B, summary histogram shows the percentage of capsaicin-sensitive (I-capsaicin) neurons in SSEA4- (n = 11), LA4- (n = 10), LD2- (n = 10) and A5- (n = 10) positive neurons and VR1-immunopositive (VR1-ir) cells in the SSEA4 (n = 54), LA4 (n = 144), LD2 (n = 217) and A5 (n = 146) DRG neuron subpopulations. C–F, double-immunocytochemistry is shown for the capsaicin receptor, VR1, and the surface markers in acutely dissociated DRG neurons. Arrowheads show examples of co-localization of VR1 and surface marker. Arrows show examples of surface marker-positive neurons that do not express VR1. Scale bar: 20 μm.
Recent experiments have indicated that some heat-insensitive sensory fibres are responsive to capsaicin, raising the possibility that not all capsaicin-sensitive DRG neurons express VR1 (Ringkamp et al. 2001). As an independent test of VR1 expression in DRG neuron subpopulations, immunocytochemistry was performed for each cell surface marker and for VR1 (Fig. 2C-F). The combined functional and immunocytochemical evidence (Fig. 2B) indicates that comparable percentages of each of the four groups of pre-identified sensory neurons express VR1 and capsaicin sensitivity. This suggests that in most neurons, VR1 mediates capsaicin sensitivity, consistent with the VR1 knockout studies of Caterina et al. (2000). Furthermore, this heat transduction protein does not appear to be exclusively expressed by, or excluded from, any of the subpopulations identified by the surface markers LA4, LD2, A5 and SSEA4, although it is less likely to be expressed by SSEA4-positive sensory neurons.
Functional P2X receptor expression in DRG neuron subpopulations
The percentage of ATP-responsive neurons in each of the pre-identified four cell groups was determined by electrophysiology. Figure 3A and B shows data from one of these experiments. Two out of four neurons in the field stained positive with antibody to LA4 (Fig. 3A). A patch electrode was applied to the lower LA4-positive neuron (Fig. 3A) and was voltage clamped at −60 mV. α,β-meATP, a potent agonist for ATP receptors including rapidly desensitizing subunits (Lewis et al. 1995) and ATP, a pan-agonist for all ATP-gated P2X receptors, were used to activate the receptors. Both agonists were applied for 2.5 s at 30 μm and both activated this LA4-positive neuron with a rapidly desensitizing response (Fig. 3B). A 5 min wash was performed between agonist applications to allow at least partial recovery from desensitization in this and all other experiments when multiple agonist applications were made.
Figure 3. Functional P2X receptors are identified and characterized in pre-identified DRG neurons.
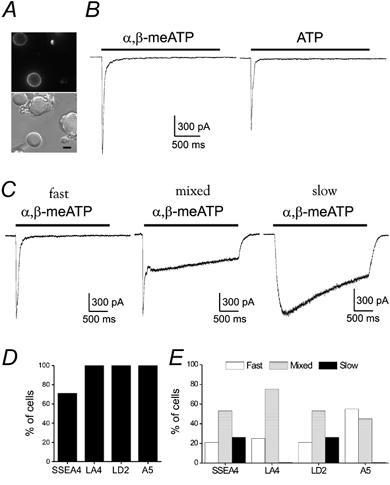
A, top, acutely dissociated DRG neurons stained with the LA4 antibody (live staining). Bottom, positively stained neuron (lower left) is patched in whole-cell mode. Scale bar: 20 μm. B, ATP (30 μm) and α,β-meATP (30 μm) activated inward currents in the LA4-positive DRG neuron in A. C, α,β-meATP currents show three desensitization phenotypes: left, a rapidly desensitizing (fast) current recorded from an A5-positive DRG neuron; middle, a mixed response with a rapidly and slowly desensitizing component from an LD2 pre-labelled neuron; right, a slowly desensitizing current from a SSEA4-positive neuron. D, histogram showing the percentage of neurons in each subpopulation responding to either α,β-meATP or ATP (n = 25, n = 16; n = 20; n = 28, respectively). E, distribution of fast, mixed and slowly desensitizing responses to α,β-meATP for each DRG neuron subpopulation is shown (n = 20 SSEA4-, 16 LA4-, 19 LD2-, 14 A5-positive cells).
Sensitivity to ATP agonists was tested in a similar manner on neurons in each of the four pre-identified neuronal populations. Within the LA4-, LD2- and A5-positive DRG neurons tested, all neurons responded to α,β-meATP and/or ATP (Fig. 3D), indicating widespread expression of P2X receptors. In SSEA4-positive DRG neurons, however, 72 % of the cells tested were sensitive to ATP or α,β-meATP (Fig. 3D). Thus while some SSEA4-positive neurons do not express detectable levels of functional ATP-gated P2X receptors, the majority of all neurons tested do.
When sensitivity to ATP and α,β-meATP was compared within each group of neurons, most neurons were sensitive to both agonists. All LA4- and A5-positive DRG neurons tested (n = 14 and 27) responded to both α,β-meATP and ATP, indicating the presence of rapidly desensitizing subunits (Lewis et al. 1995). Eighteen of 19 LD2-positive neurons and 16 of 19 SSEA4-positive neurons responded to both agonists while one LD2-positive neuron and three SSEA4-positive neurons responded only to ATP. The absence of α,β-meATP sensitivity indicates the lack of rapidly desensitizing subunits in receptors expressed by these four neurons, although this was a rare occurrence.
Response kinetics of different P2X receptors strongly affect the physiological consequences of receptor activation (Labrakakis et al. 2000). We used α,β-meATP to categorize the P2X receptor-mediated currents evoked within each group of pre-identified DRG neurons. This agonist, similar to ATP, induced three types of inward currents in DRG neurons, determined by their desensitization time course as previously described (Burgard et al. 1999; Grubb & Evans, 1999; Labrakakis et al. 2000). Figure 3C shows examples of each response type recorded from three different neurons, including a fast response with a transient, rapidly desensitizing inward current, a mixed response with a combination of fast and slow responses and a slow response with only a slowly desensitizing inward current. We separated responses into the three types illustrated in Fig. 3C for each of the four marked groups of DRG neurons (Fig. 3E). The fast response was most prominent in the A5 population.
To assess the amount of fast component in response to α,β-meATP in different cell populations, we normalized the peak amplitudes of the rapidly desensitizing component of the α,β-meATP-activated currents with respect to cell capacitance (Table 1). LA4-positive neurons had the largest mean amplitude current in response to α,β-meATP while SSEA4-positive neurons had the smallest mean amplitude current. To assess the slow component of the α,β-meATP-activated responses in the mixed and slow current types, we measured the current amplitude at the end of the 2.5 s drug application and normalized it to the cell capacitance (Table 1). These data show that although P2X receptors are highly expressed in all subpopulations of DRG neurons tested, their density varies within each subpopulation.
Table 1.
α,β-meATP-evoked current amplitudes
| SSEA4 | LA4 | LD2 | A5 | |
|---|---|---|---|---|
| Normalized mean peak current amplitude* (pApF−1) | −17 | −62 | −31 | −35 |
| Range | −2 to −100 | −16 to −174 | −10 to −75 | −13 to −181 |
| Cell capacitance (pF) | 43 ± 3 | 19 ± 2 | 19 ± 2 | 26 ± 2 |
| n | 12 | 14 | 17 | 26 |
| Normalized mean amplitude of the desensitizing component† (pA pF−1) | −6 | −13 | −16 | −7 |
| Range | −0.6 to −54 | −6 to −36 | −0.7 to −31 | −0.3 to −60 |
| Cell capacitance (pF) | 38 ± 4 | 20 ± 2 | 19 ± 1 | 26 ± 4 |
| n | 12 | 10 | 14 | 11 |
Peak and desensitizing component amplitudes of the four populations were compared.
Among the four subpopulations, peak current amplitudes were significantly different (P < 0.05) using the Kruskal-Wallis test. The SSEA4/LA4 and SSEA4/LD2 pairs were significantly different (P < 0.05) using Dunn's post test.
Among the four subpopulations, amplitudes of the desensitizing components were significantly different (P < 0.05) using the Kruskal-Wallis test.
The SSEA4/LA4, SSEA4/LD2 and LD2/A5 pairs were significantly different (P < 0.05) using Dunn's post test.
Differential expression of GABAA receptor by subpopulations of DRG neurons
To detect expression of functional GABAA receptors in DRG neuron subpopulations, we used the whole-cell patch clamp method on pre-identified DRG neurons as described above for functional P2X receptors. The chloride concentration in the pipette solution was low with a calculated reversal potential for Cl− of −67 mV. The holding potential, maintained under voltage clamp, was moved to −10 mV several minutes before drug application. The GABAA-selective agonist, muscimol, was applied for 2.5 s at 100 μm. Muscimol induced outward currents (Fig. 4A), as expected for the activation of GABAA receptors and the opening of Cl− channels. All SSEA4-positive neurons responded to muscimol (Fig. 4B) with relatively high current amplitudes (Fig. 4C) while the A5-positive population was also strongly muscimol sensitive. The LD2-positive neurons had the lowest percentage of responsive neurons and the lowest mean amplitude currents. These data indicate that while GABAA receptor expression is widespread among DRG neurons, it is not uniform among subpopulations.
Figure 4. Functional GABAA receptor expression was tested in DRG neuron subpopulations.
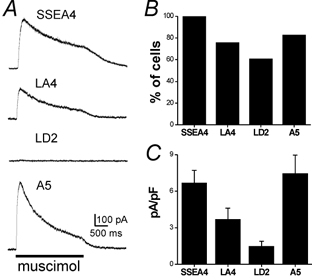
A, representative responses to 100 μm muscimol at a holding potential of −10 mV were recorded from acutely dissociated DRG neurons pre-identified as SSEA4-, LA4-, LD2- and A5-positive. B, histogram showing the percentage of cells for each subpopulation marker that responded to muscimol (total SSEA4 n = 15, LA4 n = 15, LD2 n = 18, A5 n = 17). C, mean muscimol-evoked peak current normalized to cell capacitance is plotted for each DRG neuron subpopulation (SSEA4: 6.3 ± 1 pA pF−1, 39 ± 4 pF, n = 10; LA4: 3.7 ± 0.9 pA pF−1, 19 ± 2 pF, n = 13; LD2: 1.5 ± 0.4 pA pF−1, 21 ± 2 pF, n = 11; A5: 7.1 ± 1.4 pA pF−1, 30 ± 4 pF, n = 12). Error bars show s.e.m. Statistical analysis of the peak currents show significant differences among the four subpopulations (one-way ANOVA, P < 0.05). Newman-Keuls post test showed significant differences (P < 0.05) between SSEA4/LD2, LA4/A5 and LD2/A5 pairs.
ATP-P2X and GABAA receptor expression on sensory fibre axons
Experiments on acutely dissociated DRG neurons provide useful information on the expression of receptors by sensory neurons. This information, however, is restricted to the cell soma and might not reflect receptor expression on axons or at central endings of primary afferents as shown recently for AMPA receptors (Lee et al. 2002). Therefore, we extended our experiments to test for functional expression of ATP-gated P2X and GABAA receptors on dorsal root axons. We tested this by recording compound action potentials (CAPs) elicited from lower lumbar and sacral dorsal roots in the absence and presence of agonists for P2X and GABAA receptors. We compared these drug effects with those of capsaicin, which was used to identify the large functionally defined class of nociceptors sensitive to noxious heat.
We monitored changes of the slowest inward deflection of the CAP waveform (Fig. 5), representing the slow conducting sensory fibres. Capsaicin (1 μm), applied at the end of each experiment, strongly depressed the slow CAP (84 ± 4 %; n = 15; P < 0.01 paired t test). These data confirm that the majority of slow conducting fibres in our preparations (13/14 roots tested were sacral 1–3) are capsaicin sensitive. α,β-meATP (100 μm) inhibited the slow CAP (Fig. 5), with a mean inhibition of 43 ± 4 % (n = 10; P < 0.01). α,β-meATP produced no change in the slow CAP in the presence of pyridoxal phosphate 6-azophenyl-2′,4′-disulfonic acid (PPADS) (1 ± 4 % enhancement; 75 μm; n = 3; P > 0.05; data not shown), a P2 receptor antagonist. In addition, PPADS alone did not affect the CAP (n = 12; data not shown). Similarly, muscimol (50 μm) had a strong depressive effect on the slow component of the CAP (Fig. 5B) producing a 63 ± 4 % inhibition (n = 15; P < 0.01). Muscimol, capsaicin and α,β-meATP did not produce significant changes on the fast conducting portion of the CAP (P > 0.05; Fig. 5D). For the medium conducting CAP, capsaicin and α,β-meATP depressed CAP by 34 ± 8 % (n = 14; P < 0.05) and 35 ± 4 % (n = 9; P < 0.05), respectively, while muscimol depressed CAP by 13 ± 8 %, n = 13; P < 0.01; Fig. 5C). These data indicate expression of P2X and GABAA receptors on medium and slow conducting primary afferent fibres and are consistent with the expression of these same receptors on the subpopulation of small and medium diameter DRG cell bodies.
ATP-P2X and GABAA receptor-mediated modulation of DR-DRPs
The dorsal root-dorsal root potential (DR-DRP) was recorded from one dorsal root following stimulation of the neighbouring dorsal root. The DR-DRP is mediated, at least in part, by polysynaptic activation of the central terminals of the dorsal root from which the recording is made. If GABAA and P2X receptors are expressed by sensory afferents near the terminals and are activated by endogenous release of transmitters during the DR-DRP, applications of receptor antagonists should modulate the recorded DRP. The GABAA receptor antagonist SR95531 (3 μm) was applied to the spinal cord preparation while recording DR-DRPs and it produced a strong depression (80 ± 6 %; n = 9; P < 0.01). Further addition of 75 μm PPADS produce no further reduction. Figure 6A shows an example. Simultaneous recordings of CAPs from the dorsal roots, by a third suction electrode placed on the dorsal root between the stimulating electrode and the spinal cord, showed no sensitivity of the CAPs to SR95531 or PPADS (Fig. 6B). This indicates that the inhibitory effect of GABAA receptor antagonists on the DR-DRP is not due to alteration of the action potential in the dorsal roots. The observed depression of the DR-DRP is consistent with the idea that activation of GABAA receptors on primary afferents is due to GABA released from interneurons (Eccles et al. 1963). α,β-meATP (100 μm) caused depression of the DR-DRP by 19 ± 4 % (n = 9, P < 0.01) and depression of the CAP by 28 ± 3 % (n = 9, P < 0.01) in the same preparations. An example of α,β-meATP action is shown in Fig. 7. Application of PPADS (75 μm), a generic P2 receptor antagonist, depressed the DR-DRP by 12 ± 3 % (n = 9, P < 0.01 for paired t test, data not shown). However, NF023 (150 μm), a subunit-dependent P2X receptor antagonist (Soto et al. 1999) did not change the DR-DRP (n = 5, P > 0.05, data not shown). These data demonstrate that endogenous activation of GABAA receptors contributes strongly to the DR-DRP. P2X receptor activation is able to modulate this response although we were unable to confirm endogenous activation of these receptors with two different antagonists within this recording protocol.
Figure 6. PPADS does not affect the residual dorsal root-dorsal root potential (DR-DRP) after GABAA receptor blockade.
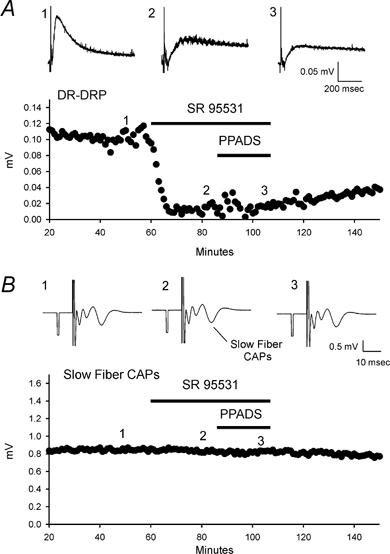
A, inhibition of GABAA receptors by 3 μm SR95531 depressed DR-DRP. Application of 75 μm PPADS to block P2X receptors did not produce further effects. Top panel, records of DR-DRP before, during SR95531 and during SR95531 and PPADS. Bottom panel, time course of DR-DRP amplitudes and the drug application time. B, SR95531 and PPADS had no effect on CAP. CAP is recorded from the root of the same preparation as A. Traces 1–3 in A and B correspond to the same recording time. All of the traces are the averages of five individual records.
Figure 7. Activation of P2X receptor depresses DR-DRP amplitude.
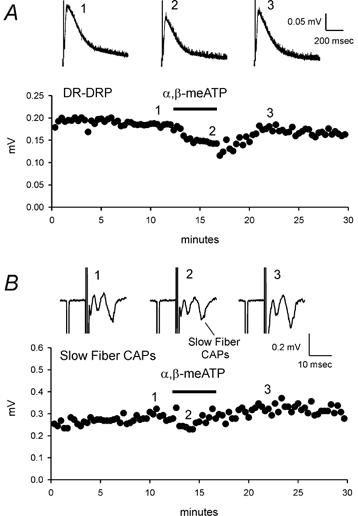
A, α,β-meATP (100 μm) inhibited DR-DRP. Top panel, DR-DRP records before, during and after α,β-meATP application. Bottom panel, time course of the DR-DRP amplitudes before and after α,β-meATP treatment. B, α,β-meATP depressed slow CAP. Top panel, CAP records before and after α,β-meATP application. Bottom panel, time course of the slow fibre CAP amplitudes. All of the traces are the averages of 5 individual records.
Discussion
Subpopulation distribution of VR1, P2X receptors and GABAA receptors
The surface markers LA4, LD2, SSEA4 and A5, were previously characterized as marking subpopulations of DRG neurons terminating in specific lamina of the superficial dorsal horn (Dodd & Jessell, 1985). The distributions of the DRG neuron subpopulations in our dissociated preparations were fairly comparable to those found in whole ganglia (Dodd & Jessell, 1985). We have examined receptor expression by these subpopulations of sensory neurons using expression of VR1 and capsaicin responsiveness to show nociceptive properties of the identified subpopulations. This is possible because VR1, the receptor for the vanilloid capsaicin, has been shown to be a key transducer of noxious heat stimuli (Caterina et al. 2000).
In the SSEA4-positive subpopulation, VR1 expression and capsaicin sensitivity occurred with low frequency. This population consists of DRG neurons with a wide range of cell diameters that include both muscle and somatic afferents (Perry & Lawson, 1998). These afferents terminate in laminae I, III and IV and have been suggested to be low threshold fibres such as Aδ- and Aβ-fibres (Dodd & Jessell, 1985; Perry & Lawson, 1998). The majority of neurons in the LA4, A5 and LD2 subpopulations are sensitive to capsaicin and express VR1. This is in agreement with previous results showing high expression of VR1 mRNA (Michael & Priestley, 1999) or protein (Tominaga et al. 1998; Guo et al. 1999) in subpopulations of small DRG neurons. The percentage of IB4-positive neurons expressing VR1 changes over the first few postnatal weeks with no corresponding change in the percentage of substance P-positive neurons expressing VR1 (Guo et al. 2001). Our experiments were carried out over postnatal ages at which the expression of VR1 has still not stabilized in the IB4 population, and thus we may be underestimating the level of VR1 expression in that group in fully mature animals. Nevertheless, it is these neurons with fibres projecting to laminae I and II (LA4/IB4, LD2, and A5) that make up, at least in part, the high threshold, slow conducting fibres in the dorsal roots (C- and some Aδ-fibres) that are sensitive to capsaicin.
Functional P2X receptors were highly expressed in our labelled subpopulations of DRG neurons known to terminate in laminae I-III of the superficial dorsal horn. This is consistent with the observation that most P2X receptor subunits (P2X1-6) are found in DRG neurons. All neurons in the A5, LD2 and LA4 subpopulations and the majority of the SSEA4 subpopulation responded to activation of P2X receptors. Interestingly, a high proportion of neurons responded to the subunit-specific agonist α,β-meATP, an agonist that activates receptors containing rapidly desensitizing subunits (Lewis et al. 1995; Grubb & Evans 1999; Dunn et al. 2001).
In contrast to neurons in the adult central nervous system, activation of GABA receptors on DRG neurons in the peripheral nervous system causes a depolarization due to Cl− efflux. This in turn is due to active intracellular Cl− accumulation in DRG neurons (Sung et al. 2000). In fact, GABAA receptor-induced depolarization of the primary afferent terminals has been proposed to be a key element controlling presynaptic inhibition of transmitter release from these neurons (Rudomin & Schmidt, 1999). We observed differences in the expression of GABAA receptors by the various subpopulations of sensory neurons that terminate in laminae I–III. The SSEA4 subpopulation showed the highest percentage and largest amplitude of muscimol responses compared to the other subpopulations we tested. The SSEA4-positive DRG neurons are mostly capsaicin-insensitive (Fig. 2), Aδ/Aβ-fibre neurons (Perry & Lawson, 1998) that respond more strongly to GABA than do capsaicin-sensitive, C-fibre neurons. In contrast, LD2 and LA4 subpopulations had the lowest percentage of muscimol-responding neurons with the smallest current amplitudes. A lower GABA sensitivity for C-fibre neurons with some of the neurons showing no response to GABA was previously demonstrated for DRG neurons associated with slow conducting fibres (Desarmenien et al. 1984). Our percentage of non-responding neurons was somewhat higher than in the study of Desamenien et al. (1984), which may be due to differences in the method of tissue preparation, age of the animals, cell selection criteria and the agonists used. Nevertheless, our data show that the majority of small diameter DRG neurons express GABAA receptors on their cell bodies and central fibres. This supports the suggestion that GABAA receptors may be expressed on the central terminals of these fibres and could contribute to presynaptic inhibition there (Willis, 1999).
Functional P2X and GABAA receptors on axons and near primary afferent terminals
α,β-meATP depressed the amplitude of the slow and medium CAPs recorded from dorsal roots. The capsaicin sensitivity and slower conduction velocity of these CAPs imply a nociceptor-fibre origin (Caterina et al. 2000). Thus our data suggest expression of P2X receptors on C- and Aδ-fibres in dorsal roots. Previous reports have demonstrated P2X receptor action on vagus nerve C-fibres (Trezise et al. 1993; 1994; Irnich et al. 2001) and on terminals of capsaicin-insensitive Aδ-fibres (Nakatsuka et al. 2002). Muscimol also affected slow and medium conducting CAPs, consistent with expression of GABAA receptors by those fibre types. Absence of a muscimol and α,β-meATP effect on faster conducting fibres might indicate the absence of GABAA and P2X receptors on myelinated A-fibre axons along the root without precluding the expression of these receptors by other parts of the same primary afferent neurons (i.e soma and central terminals). Although receptor trafficking in the DRG neurons is not well studied, differential targeting of P2X receptor subunits has been previously proposed (Vulchanova et al. 1997). Poor access to the axon surface by muscimol and α,β-meATP due to heavy myelination could be another reason for the absence of responses in our experiments.
Expression of neurotransmitter receptors along axons has been described for other receptors such as the kainate receptors (Agrawal & Evans, 1986; Schmitz et al. 2001). The physiological relevance of axonal neurotransmitter receptors is not yet clear. Their activation can modulate action potential propagation and firing. For kainate receptors a concentration-dependent dual role has been observed; low kainate concentrations enhance hippocampal mossy fibre CAPs while higher concentrations decrease it (see review by Schmitz et al. 2001). In the case of P2X receptors, release of ATP from injured nerves or glia could cause action potential firing (Cook et al. 1997; Gu & MacDermott, 1997) or conduction block on the axons serving as a signal of nerve injury. Moreover, extracellular ATP acting on axonal P2X receptors might play a role in neuron-glia interactions.
Dorsal root depolarization recorded after stimulation of a neighbouring dorsal root is due in part to release of GABA from dorsal horn neurons that activates GABAA receptors near the primary afferent terminals (Willis, 1999). Our experiments showing SR95531 inhibition of the DR-DRP support this hypothesis. In addition we show the DR-DRP is depressed by α,β-meATP. This effect may be caused by the depressive effect of α,β-meATP on the CAP. For example, modulation of the incoming action potential by P2X receptors could lead to a reduction of neurotransmitter release at the primary afferent terminals.
Physiological implications
Our results show that the majority of DRG neurons terminating in laminae I–III express both P2X and GABAA receptors on their cell bodies and centrally directed axons. It is possible that these receptors are expressed on or near their primary afferent terminals. In this case, endogenous GABA and ATP are expected to activate afferent terminals following stimulation of an independent set of afferents, consistent with our observations for GABA antagonists on DR-DRPs. We were unable to obtain clear evidence for a contribution from endogenous ATP to the DR-DRP. Nevertheless, there is evidence for endogenously released ATP modulating glutamate release from primary afferents terminating in laminae I-III and V (Li et al. 1998; Nakatsuka & Gu, 2001). In addition, Jo & Schlichter (1999) showed co-release of ATP and GABA from dorsal horn neurons in culture. Thus, it is possible that ATP and GABA synergistically depolarize primary afferent terminals. Because Ca2+ entry through P2X receptors enhances synaptic transmission, the initial effect could enhance release of transmitter from the primary afferent terminals. With stronger input possibly in conjunction with ATP receptor desensitization, the depolarization could cause presynaptic inhibition. Both GABAA and P2X receptor families have a variety of desensitization kinetics that are subunit dependent. The consequences of presynaptic receptor activation are expected to be dependent on the kinetics of the particular receptors involved at each terminal (e.g. Labrakakis et al. 2000).
This variety of properties suggests that there is likely to be a complex interaction between ATP and GABA in regulating transmitter release. In addition, both transmitters may co-operate in their depolarizing actions to drive antidromic action potentials to the peripheral terminals of these afferents. This has been proposed as a key mechanism inducing neurogenic inflammation (Willis, 1999). Besides the activation of P2X and GABAA receptors, ATP and GABA also interact with their metabotropic receptors, P2Y and GABAB, respectively. Additionally, adenosine, the degradation product of ATP, activates adenosine receptors to inhibit transmitter release. As both ionotropic and metabotropic receptors can be found on the presynaptic primary afferent terminals, activation of any set of receptors will have complex effects on transmitter release.
Acknowledgments
This work was supported by NIH/NS 37549 and NS 40428. We thank Drs David Julius, Jane Dodd and Susan Morton for graciously providing antibodies. We also thank Aiming Shang for excellent technical assistance.
References
- Agrawal SG, Evans RH. The primary afferent depolarizing action of kainate in the rat. Br J Pharmacol. 1986;87:345–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ, Gamino SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurones studied with ion-selective microelectrodes. J Physiol. 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard EC, Niforatos W, Van Biesen T, Lynch KJ, Touma E, Metzger RE, Kowaluk EA, Jarvis MF. P2X receptor-mediated ionic currents in dorsal root ganglion neurons. J Neurophys. 1999;82:1590–1598. doi: 10.1152/jn.1999.82.3.1590. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hayes ES. GABAergic vesicle-containing dendrites and spines: a critical element in processing sensory input in the monkey dorsal horn. Neurosci Lett. 1991;121:40–42. doi: 10.1016/0304-3940(91)90644-9. [DOI] [PubMed] [Google Scholar]
- Carstens E, Gilly H, Schreiber H, Zimmermann M. Effects of midbrain stimulation and iontophoretic application of serotonin, noradrenaline, morphine and GABA on electrical thresholds of afferent C- and A-fibre terminals in cat spinal cord. Neuroscience. 1987;21:395–406. doi: 10.1016/0306-4522(87)90130-8. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- Desarmenien M, Santangelo F, Loeffler JP, Feltz P. Comparative study of GABA-mediated depolarizations of lumbar A delta and C primary afferent neurones of the rat. Exp Brain Res. 1984;54:521–528. doi: 10.1007/BF00235477. [DOI] [PubMed] [Google Scholar]
- Dodd J, Jessell TM. Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn of rat spinal cord. J Neurosci. 1985;5:3278–3294. doi: 10.1523/JNEUROSCI.05-12-03278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Schmidt RF, Willis WD. Pharmacological studies on presynaptic inhibition. J Physiol. 1963;168:500–530. doi: 10.1113/jphysiol.1963.sp007205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, Lawson S. Sensory receptor properties of isolectin B4-positive (IB4+) and negative (IB4-) dorsal root ganglion (DRG) neurons in vivo in the rat. Soc Neurosci Abstr. 2000;26:354. [Google Scholar]
- Fitzgerald M. The post-natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. J Physiol. 1985;364:1–18. doi: 10.1113/jphysiol.1985.sp015725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur J Neurosci. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- Guo A, Simone DA, Stone LS, Fairbanks CA, Wang J, Elde R. Developmental shift of vanilloid receptor 1 (VR1) terminals into deeper regions of the superficial dorsal horn: correlation with a shift from TrkA to Ret expression by dorsal root ganglion neurons. Eur J Neurosci. 2001;14:293–304. doi: 10.1046/j.0953-816x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Irnich D, Burgstahler R, Bostock H, Grafe P. ATP affects both axons and Schwann cells of unmyelinated C fibres. Pain. 2001;92:343–50. doi: 10.1016/S0304-3959(01)00277-9. [DOI] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Labrakakis C, Gerstner E, MacDermott AB. Adenosine triphosphate-evoked currents in cultured dorsal root ganglion neurons obtained from rat embryos: desensitization kinetics and modulation of glutamate release. Neuroscience. 2000;101:1117–1126. doi: 10.1016/s0306-4522(00)00373-0. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Bardoni R, Tong CK, Engelman HS, Joseph D, Magherini PC, MacDermott AB. Functional expression of AMPA receptors on central terminals of rat dorsal root ganglion neurons and presynaptic inhibition of glutamate release. Neuron. 2002;35:135–146. doi: 10.1016/s0896-6273(02)00729-8. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Kong H, Manzini MC, Albuquerque C, Chao MV, MacDermott AB. Kainate receptors expressed by a subpopulation of developing nociceptors rapidly switch from high to low Ca2+ permeability. J Neurosci. 2001;21:4572–45781. doi: 10.1523/JNEUROSCI.21-13-04572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Li P, Calejesan AA, Zhuo M. ATP P2x receptors and sensory synaptic transmission between primary afferent fibers and spinal dorsal horn neurons in rats. J Neurophysiol. 1998;80:3356–3360. doi: 10.1152/jn.1998.80.6.3356. [DOI] [PubMed] [Google Scholar]
- Llewellyn-Smith IJ, Burnstock G. Ultrastructural localization of P2X3 receptors in rat sensory neurons. Neuroreport. 1998;9:2545–2550. doi: 10.1097/00001756-199808030-00022. [DOI] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka T, Furue H, Yoshimura M, Gu JG. Activation of central terminal vanilloid receptor-1 receptors and alpha beta-methylene-ATP-sensitive P2X receptors reveals a converged synaptic activity onto the deep dorsal horn neurons of the spinal cord. J Neurosci. 2002;22:1228–1237. doi: 10.1523/JNEUROSCI.22-04-01228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuka T, Gu JG. ATP P2X receptor-mediated enhancement of glutamate release and evoked EPSCs in dorsal horn neurons of the rat spinal cord. J Neurosci. 2001;21:6522–6531. doi: 10.1523/JNEUROSCI.21-17-06522.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry MJ, Lawson SN. Differences in expression of oligosaccharides, neuropeptides, carbonic anhydrase and neurofilament in rat primary afferent neurons retrogradely labelled via skin, muscle or visceral nerves. Neuroscience. 1998;85:293–310. doi: 10.1016/s0306-4522(97)00629-5. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A, Pignatelli D, Coimbra A. Synaptic architecture of glomeruli in superficial dorsal horn of rat spinal cord, as shown in serial reconstructions. J Neurocytol. 1985;14:203–220. doi: 10.1007/BF01258448. [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Peng YB, Wu G, Hartke TV, Campbell JN, Meyer RA. Capsaicin responses in heat-sensitive and heat-insensitive A-fiber nociceptors. J Neurosci. 2001;21:4460–4468. doi: 10.1523/JNEUROSCI.21-12-04460.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Frerking M, Nicoll RA. Presynaptic kainate receptors at hippocampal mossy fiber synapses. Proc Natl Acad Sci U S A. 2001;98:11003–11008. doi: 10.1073/pnas.191351498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Lambrecht G, Nickel P, Stuhmer W, Busch AE. Antagonistic properties of suramin analogue NF023 at heterologously expressed P2X receptors. Neuropharmacology. 1999;38:141–149. doi: 10.1016/s0028-3908(98)00158-0. [DOI] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Trezise DJ, Kennedy I, Humphrey PP. Characterization of purinoceptors mediating depolarization of rat isolated vagus nerve. Br J Pharmacol. 1993;110:1055–1060. doi: 10.1111/j.1476-5381.1993.tb13920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezise DJ, Kennedy I, Humphrey PP. The use of antagonists to characterize the receptors mediating depolarization of the rat isolated vagus nerve by alpha, beta-methylene adenosine 5′-triphosphate. Br J Pharmacol. 1994;112:282–288. doi: 10.1111/j.1476-5381.1994.tb13065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptors subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]


