Abstract
Studies suggest that cytokines have a role in the biology of depression. In this study, we evaluated depression and cytokine levels in patients with and without chronic hepatitis C (HCV) to better assess how chronic infection alters cytokines levels and may contribute to depressive symptomotology. Twenty-three adults with (n = 16) and without (n = 7) HCV were recruited through the Portland VA Medical Center. Research participants were excluded for current substance abuse, psychotic disorder, liver cirrhosis, or interferon (IFN) therapy. Participants completed the Beck Depression Inventory-II (BDI-II) and a blood draw to evaluate plasma cytokine levels [i.e., interleukin (IL)-1β, IL-10 and tumor necrosis factor (TNF)- α]. T-tests were performed to compare cytokine levels in patients with versus those without HCV. HCV patients showed higher TNF-α values compared to patients without HCV (group means = 7.94 vs. 3.41 pg/mL, respecitively, p = 0.047). There were no significant differences between the groups for the other cytokines assessed. In patients with HCV, TNF-α and IL-1β levels (but not IL-10) were correlated with BDI-II scores [r = 0.594, p = 0.020 and r = 0.489, p = 0.055 (trend), respectively]. Taken together, these results show an association between severity of depressive symptoms and expression of proinflammatory cytokines in patients with HCV. Future studies should investigate how inflammatory mediators play a role in the expression of specific depressive symptoms in patients with chronic infection. Patients with HCV represent an interesting model to examine this relationship.
Keywords: cytokines, inflammation, chronic infection, Beck Depression Inventory, hypothalamic-pituitary-adrenal axis, serotonin
1. Introduction
A substantial body of evidence suggests a role for cytokines in the etiology of mood disorders, and in particular depression [14, 15, 20, 22]. Depressive symptoms often accompany diseases affecting the immune system including, but not limited to, HIV/AIDS [26], diabetes [3], multiple sclerosis [10] and cardiovascular disease [21]. Depressive symptoms are also common in patients with chronic hepatitis C viral infection (HCV) [1, 9, 12]. In a study of 293 patients with HCV, 35% had depression rating scale scores in the moderate-to-severe range prior to starting antiviral [interferon (IFN)-based] therapy [9]. The most recent findings in this area support and extend this observation by showing that when compared to healthy participants, patients with HCV have significantly greater scores for depression, as assessed using the Profile of Mood States [25]. However, the mechanisms contributing to depression in HCV patients, not undergoing antiviral therapy, have yet to be determined.
It is postulated that depression in HCV may be related to a dysregulation of the cytokine network. A number of studies provide evidence for the relationship between cytokine production and the development of depression. This hypothesis is consistent with the putative deficiencies in serotonergic transmission accompanying depression in patients with HCV [5, 7], as cytokines can reduce serotonin levels by decreasing the availability of tryptophan and altering the ratio of kynurenine/tryptophan [28].
Although an association between depressive symptoms and proinflammatory cytokines has been known for several decades, a mechanistic understanding of the specific contributions of immune mediators to the pathogenesis, pathophysiology and treatment of depression is only beginning to emerge. Thus, to better understand the contribution of immunological factors to the depressive symptomotology observed with HCV, we measured cytokine levels and depressive symptoms in patients with and without chronic HCV.
2. Materials and methods
2.1 Participants
The Institutional Review Board at the Portland Veteran’s Affairs Medical Center (VAMC) approved the study protocol and all participants provided written informed consent. Twenty-three adults with (n = 16) and without (n = 7) HCV were recruited through the Portland VAMC. Research participants were excluded for current severe mental illness, liver cirrhosis, any substance abuse within the past month, IFN therapy, history of traumatic brain injury or chemotherapy, or any medical conditions with cognitive effects.
2.2 Assessment of depression
All participants completed the 21-item Beck Depression Inventory-II (BDI-II) [4]. The BDI-II is a brief 21-item self-report instrument that has been shown to have good validity and reliability in various medical and psychiatric populations. We used this instrument in our previous studies that showed the efficacy of citalopram in attenuating depressive symptoms in HCV patients undergoing IFN therapy [11, 16].
In order to determine if specific depressive symptoms were associated with alterations in specific cytokines, items from the BDI-II were grouped into three symptom dimensions derived from the principle components analysis by Dunn et al. [8]. The three depressive symptom dimensions included: I) Negative Cognitions, II) Psychomotor Anhedonia and III) Vegetative Symptoms.
2.3 Blood sampling and cytokine measurements
Blood samples were obtained during study visits following the completion of the BDI-II. Whenever possible, study visits were scheduled to coincide with participants’ medical appointments at the Portland VAMC. Blood was drawn in the afternoons (mean time was 1:10pm, SD = 1.6 hours) by one-time venipuncture into cell preparation tubes (BD Vacutainer Systems, Franklin Lakes, NJ) containing 1 mL of 0.1M sodium citrate solution. The blood was then centrifugation at 1500 RCF for 20 minutes at room temperature (22–25° C). Plasma was separated, collected and immediately aliquoted in polypropylene tubes (Phenix Research Products, Hayward, CA) and frozen at −80 °C until assayed. Cytokines [IL-1β, IL-10, and tumor necrosis factor (TNF)-α] were measured using the Beadlyte Human Multi-Cytokine Beadmaster Kit (Upsate, Temecula, CA) and xMAP technology (Luminex, Austin, TX). All analyses were performed in duplicates. IL-1β and TNF-α were chosen for the T-helper 1 (Th1) proinflammatory cytokines to measure as several studies have found positive relationships between the expression of these cytokines and the etiology of sleep disturbances, fatigue, pain and depression [22, 29]. To investigate the relationships between Th1/Th2 cytokine balance in patients with and without HCV, IL-10 was selected for the Th2 anti-inflammatory cytokine, as this cytokine also shows changes that are correlated with the expression of depressive symptoms [19, 27].
2.4 Statistical analyses
Mann-Whitney U and t-tests were performed to compare cytokine levels in participants with versus those without HCV. Correlations between depression rating scale scores and cytokine levels were conducted using Pearson moment correlations or Spearman rho correlations for variables that did not have normal distributions. Statistical analyses were completed using SPSS for Windows, version 12.0 (Chicago, IL).
3. Results
3.1 Participants’ characteristics
Twenty-three adults with (n = 16) and without (n = 7) HCV participated in this study (age = 53.3 ± 5 years; 93% male; 91% Caucasian and 9% African-American). Patients without HCV had no history of substance use disorder (SUD), while 87.5% (14/16) of patients with HCV had a history of SUD (mean years of abuse = 19.5 ± 12.7; mean years in remission = 9.2 ± 9.07). Table 1 provides a summary of other demographic information, including group differences in age, sex, body mass index (BMI) and tobacco use.
Table 1.
Demographic characteristics of participants with (HCV+) and without (HCV−) chronic hepatitis C viral infection
| Group | Sex | Age1 | BMI1 | Tobacco use |
|---|---|---|---|---|
| HCV +
(n = 16) |
Males (n = 15)
Females (n = 1) |
53.25 ± 1.359 | 27.38 ± 1.002 | 11/16 currently using tobacco products |
| HCV −
(n = 7) |
Males (n = 5)
Females (n = 2) |
58.86 ± 2.415 | 36.60 ± 2.845 | 0/7 currently using tobacco products |
T-tests were used to compare age and BMI between the two groups. Participants without HCV did not report any current tobacco use, as opposed to 7 people with HCV; however, participants without HCV were significantly older (t = 2.162, p = 0.042) and had a significantly greater BMI (t = 3.83, p = 0.001) than individuals with HCV.
Patients with HCV had higher BDI-II scores as compared to patients without HCV (mean = 11.19, SD = 11.87 vs. mean = 5.59, SD = 0.207, respectively). This difference did not reach statistical significance (U = 28.000, exact p = 0.065) (Figure 1).
Figure 1.
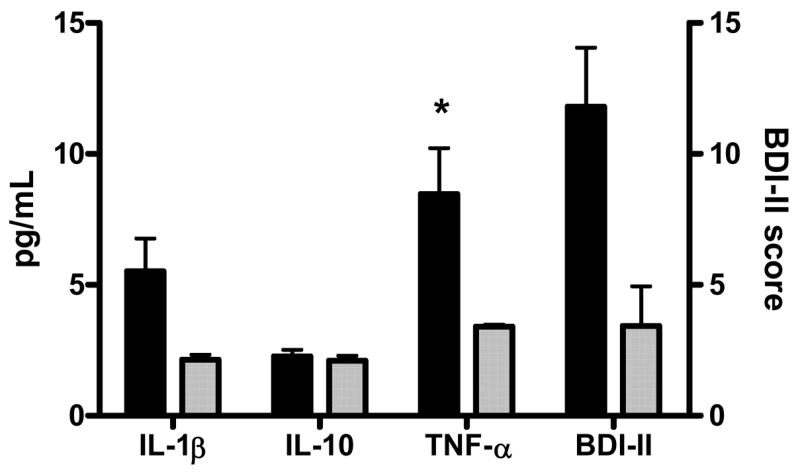
Mean cytokine levels and depression scores for patients with and without HCV. Error bars represent standard deviations. T-tests were performed to compare HCV+ and HCV− groups. Asterisk indicates p-value < 0.05. Black bars represent the HCV+ group and shaded bars represent the HCV− group.
3.2 Inflammatory mediators in participants with and without HCV
Figure 1 shows that patients with HCV had significantly higher TNF-α levels (U=24.000, exact p = 0.047) as compared to participants without HCV. There were no significant differences in plasma levels of IL-10 (t=1.929, p = 0.067) or IL-1β (U = 34.0, exact p = 0.154) between the two groups. However, it is likely that the small sample size hindered our ability to detect significant group differences between levels of IL-1β (HCV+ group = 5.47 ± 6.02 pg/mL vs. HCV− group = 2.14 ± 0.76 pg/mL) and possibly IL-10 (HCV+ group = 2.26 ± 0.20 pg/mL vs. HCV− group = 2.10 ± 0.09 pg/mL).
3.3 Correlations between depressive symptom severity and specific cytokine levels
In patients with HCV, TNF-α and IL-1β levels (but not IL-10) were positively correlated with BDI-II scores [r = 0.594, p = 0.020 and r = 0.489, p = 0.055 (trend), respectively]. No correlations approached significance (p > 0.10) for participants without HCV, though small sample size limited statistical power.
When cytokine levels for TNF-α, IL-1β and IL-10 were correlated with the BDI-II scores for all research participants collectively (i.e., HCV+ and HCV− groups), the strength of the relationships between depressive symptom severity and cytokine levels were attenuated (Table 2).
Table 2.
Correlations between BDI-II symptom dimensions and cytokine levels in patients with HCV (HCV+; n = 16) and in the entire study sample (all participants; N = 23).
| Depressive Symptom Dimension | IL-1β1 | TNF-α1 | IL-10 | |||
|---|---|---|---|---|---|---|
| HCV+ | All | HCV+ | All | HCV+ | All | |
| Negative Cognitions | r = 0.622,
p = 0.010* |
r = 0.542,
p = 0.009* |
r = 0.554,
p = 0.032* |
r = 0.542,
p = 0.009* |
r = −0.137,
p = 0.612 |
r = −0.12,
p = 0.956 |
| Psychomotor Anhedonia | r = 0.397,
p = 0.128 |
r = 0.338,
p = 0.114 |
r = 0.593,
p = 0.020* |
r = 0.489,
p = 0.021* |
r = −0.095,
p = 0.727 |
r = 0.039,
p = 0.860 |
| Vegetative Symptoms | r = 0.396,
p = 0.129 |
r = 0.372,
p = 0.081 |
r = 0.636,
p = 0.011* |
r = 0.544,
p = 0.009* |
r = −0.535,
p = 0.033* |
r = −0.183,
p = 0.403 |
| Total BDI-II Score | r = 0.489,
p = 0.055 |
r = 0.439,
p = 0.036* |
r = 0.594,
p = 0.020* |
r = 0.565,
p = 0.006* |
r = −0.113,
p = 0.678 |
r = 0.042,
p = 0.848 |
These variables did not have normal distributions; therefore, related analyses were conducted using Spearman rho correlations. Pearson product moment correlations were conducted for all other analyses.
p < 0.05
3.4 Association of depressive symptom dimensions with specific cytokine levels
In order to determine if specific depressive symptoms were associated with alterations in specific cytokines, items from the BDI-II were grouped into three symptom dimensions derived from the principle components analysis by Dunn et al. [8]. The three depressive symptom dimensions included: I) Negative Cognitions, II) Psychomotor Anhedonia and III) Vegetative Symptoms. In patients with HCV, IL-β and TNF-α levels were significantly correlated with Negative Cognitions; TNF-α levels were also significantly correlated with both Psychomotor Anhedonia and Vegetative Symptoms; and IL-10 negatively correlated with Vegetative Symptoms (Table 2).
4. Discussion
Chronic infection is hypothesized to elevate proinflammatory cytokines, which over time may cause- like chronic stress- depressive symptoms in the context of chronic HCV, leading to depression. To our knowledge, no other studies have directly examined the effects of chronic infection with HCV on cytokines and depressive symptoms, so clearly larger and more comprehensive studies in patients with HCV will be important to confirm such hypotheses.
Taken together, our data support an association between severity of depressive symptoms and increased expression of specific proinflammatory cytokines (i.e., IL-1β TNF-α) in patients with HCV. Given our small sample size, we cannot rule out the possibility that the association between HCV and increased cytokine levels may have occurred because HCV could be a marker for some other nonrandomly distributed factor, also contributing to elevated cytokines, such as history of substance abuse. However, it should be noted that patients in the HCV+ group had significantly lower BMIs and were significantly younger than HCV− participants (Table 1); both of these factors should reduce this possibility.
The potential mechanisms leading to cytokine-induced depression are numerous and were recently reviewed in Spalletta et al. [24]. Included among these mechanisms are several lines of evidence demonstrating how cytokines can contribute to HPA axis hyperactivity [17] as well as affect the serotonergic and dopaminergic systems [2, 5, 6] and subsequently lead to depressive symptomotology. There is, however, a need for a more integrated view of depression.
Elevated peripheral levels of proinflammatory cytokines, such as IL-1β and TNF-α are frequently observed in patients with major depression [13, 18] and mice lacking TNF-α receptors show antidepressant-like responses in behavioral measures of depression [23]. Wichers et al. recently found that in patients with HCV, baseline soluble IL-2 receptor (sIL-2r), IL-6, and IL-10 concentrations were significantly elevated in patients who developed depression during IFN therapy, as compared with those that did not, which suggests that increased immune activation may predict the development of depression during IFN therapy [27]. Our findings provide further support for hypotheses involving cytokine dysregulation and depression, specifically among patients with a chronic viral infection. Future studies should investigate how inflammatory mediators play a role in the expression of specific depressive symptoms, such as fatigue and anhedonia, in patients with chronic infection. Patients with HCV represent an interesting model to examine these relationships.
Acknowledgments
The authors would like to thank Tiffany Parcel, Jonathan Woodhouse and Adrianna Steele for their help with patient recruitment and data collection. The authors are additionally appreciative of Dr. Arthur Vandenbark for his help in reviewing and editing this manuscript. This research was supported in part by a grant from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer M. Loftis, Behavioral Health and Clinical Neurosciences Division, Portland VA Medical Center, Department of Psychiatry, Oregon Health & Science University, Portland, OR 97239 USA
Marilyn Huckans, Behavioral Health and Clinical Neurosciences Division, Portland VA Medical Center, Department of Psychiatry, Oregon Health & Science University, Portland, OR 97239 USA.
Samantha Ruimy, Behavioral Health and Clinical Neurosciences Division, Portland VA Medical Center, Portland, OR 97239 USA.
David J. Hinrichs, Research & Development, Portland VA Medical Center, Department of Molecular Microbiology and Immunology, Oregon Health & Science University, Portland, OR 97239 USA
Peter Hauser, Behavioral Health and Clinical Neurosciences Division, and the NW Hepatitis C Resource Center, Portland VA Medical Center, Departments of Psychiatry, Behavioral Neuroscience and Internal Medicine, Oregon Health & Science University, Portland, OR 97239 USA.
References
- 1.Angelino AF, Treisman GJ. Evidence-informed assessment and treatment of depression in HCV and interferon-treated patients. Int Rev Psychiatry. 2005;17:471–6. doi: 10.1080/02646830500381567. [DOI] [PubMed] [Google Scholar]
- 2.Anisman H, Kokkinidis L, Merali Z. Further evidence for the depressive effects of cytokines: anhedonia and neurochemical changes. Brain Behav Immun. 2002;16:544–56. doi: 10.1016/s0889-1591(02)00011-9. [DOI] [PubMed] [Google Scholar]
- 3.Astle F. Diabetes and depression: a review of the literature. Nurs Clin North Am. 2007;42:67–78. vii. doi: 10.1016/j.cnur.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Beck A, Steer R, Brown G. Beck Depression Inventory. 2. The Psychological Corporation; 1996. [Google Scholar]
- 5.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–73. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 7.Cozzi A, Zignego AL, Carpendo R, Biagiotti T, Aldinucci A, Monti M, Giannini C, Rosselli M, Laffi G, Moroni F. Low serum tryptophan levels, reduced macrophage IDO activity and high frequency of psychopathology in HCV patients. J Viral Hepat. 2006;13:402–8. doi: 10.1111/j.1365-2893.2005.00706.x. [DOI] [PubMed] [Google Scholar]
- 8.Dunn RT, Kimbrell TA, Ketter TA, Frye MA, Willis MW, Luckenbaugh DA, Post RM. Principal components of the Beck Depression Inventory and regional cerebral metabolism in unipolar and bipolar depression. Biol Psychiatry. 2002;51:387–99. doi: 10.1016/s0006-3223(01)01244-6. [DOI] [PubMed] [Google Scholar]
- 9.Fireman M, Indest DW, Blackwell A, Whitehead AJ, Hauser P. Addressing tri-morbidity (hepatitis C, psychiatric disorders, and substance use): the importance of routine mental health screening as a component of a comanagement model of care. Clin Infect Dis. 2005;40(Suppl 5):S286–91. doi: 10.1086/427442. [DOI] [PubMed] [Google Scholar]
- 10.Gold SM, Irwin MR. Depression and immunity: inflammation and depressive symptoms in multiple sclerosis. Neurol Clin. 2006;24:507–19. doi: 10.1016/j.ncl.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD, Meyers CA, Howell CD. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–7. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 12.Kraus MR, Schafer A, Csef H, Scheurlen M, Faller H. Emotional state, coping styles, and somatic variables in patients with chronic hepatitis C. Psychosomatics. 2000;41:377–84. doi: 10.1176/appi.psy.41.5.377. [DOI] [PubMed] [Google Scholar]
- 13.Leo R, Di Lorenzo G, Tesauro M, Razzini C, Forleo GB, Chiricolo G, Cola C, Zanasi M, Troisi A, Siracusano A, Lauro R, Romeo F. Association between enhanced soluble CD40 ligand and proinflammatory and prothrombotic states in major depressive disorder: pilot observations on the effects of selective serotonin reuptake inhibitor therapy. J Clin Psychiatry. 2006;67:1760–6. doi: 10.4088/jcp.v67n1114. [DOI] [PubMed] [Google Scholar]
- 14.Licinio J, Wong ML. The role of inflammatory mediators in the biology of major depression: central nervous system cytokines modulate the biological substrate of depressive symptoms, regulate stress-responsive systems, and contribute to neurotoxicity and neuroprotection. Mol Psychiatry. 1999;4:317–27. doi: 10.1038/sj.mp.4000586. [DOI] [PubMed] [Google Scholar]
- 15.Loftis JM, Hauser P. The phenomenology and treatment of interferon-induced depression. J Affect Disord. 2004;82:175–90. doi: 10.1016/j.jad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Loftis JM, Socherman RE, Howell CD, Whitehead AJ, Hill JA, Dominitz JA, Hauser P. Association of Interferon-α-Induced Depression and Improved Treatment Response in Patients with Hepatitis C. Neurosci Lett. 2004;365:87–91. doi: 10.1016/j.neulet.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 17.Maes M, Bosmans E, Meltzer HY, Scharpe S, Suy E. Interleukin-1 beta: a putative mediator of HPA axis hyperactivity in major depression? Am J Psychiatry. 1993;150:1189–93. doi: 10.1176/ajp.150.8.1189. [DOI] [PubMed] [Google Scholar]
- 18.Mikova O, Yakimova R, Bosmans E, Kenis G, Maes M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol. 2001;11:203–8. doi: 10.1016/s0924-977x(01)00081-5. [DOI] [PubMed] [Google Scholar]
- 19.O’Mahony SM, Myint AM, van den Hove D, Desbonnet L, Steinbusch H, Leonard BE. Gestational stress leads to depressive-like behavioural and immunological changes in the rat. Neuroimmunomodulation. 2006;13:82–8. doi: 10.1159/000096090. [DOI] [PubMed] [Google Scholar]
- 20.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, Ni H. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167:174–81. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 22.Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–17. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–85. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Spalletta G, Bossu P, Ciaramella A, Bria P, Caltagirone C, Robinson RG. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry. 2006;11:984–91. doi: 10.1038/sj.mp.4001879. [DOI] [PubMed] [Google Scholar]
- 25.von Wagner M, Lee JH, Kronenberger B, Friedl R, Sarrazin C, Teuber G, Herrmann E, Zeuzem S. Impaired health-related quality of life in patients with chronic hepatitis C and persistently normal aminotransferase levels. J Viral Hepat. 2006;13:828–34. doi: 10.1111/j.1365-2893.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 26.Voss JG, Dodd M, Portillo C, Holzemer W. Theories of fatigue: application in HIV/AIDS. J Assoc Nurses AIDS Care. 2006;17:37–50. doi: 10.1016/j.jana.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Wichers MC, Kenis G, Leue C, Koek G, Robaeys G, Maes M. Baseline immune activation as a risk factor for the onset of depression during interferon-alpha treatment. Biol Psychiatry. 2006;60:77–9. doi: 10.1016/j.biopsych.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 28.Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538–44. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 29.Wood LJ, Nail LM, Gilster A, Winters KA, Elsea CR. Cancer chemotherapy-related symptoms: evidence to suggest a role for proinflammatory cytokines. Oncol Nurs Forum. 2006;33:535–42. doi: 10.1188/06.ONF.535-542. [DOI] [PubMed] [Google Scholar]


