Abstract
Chloride permeation through the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel is blocked by highly lyotropic permeant anions which bind tightly within the pore. Here we show that several different substitutions of a positively charged amino acid residue, arginine R334, in the putative outer mouth of the CFTR pore, greatly reduce the block caused by lyotropic Au(CN)2− ions applied to the intracellular side of the channel. Fixed positive charge at this site appears to play a role in Au(CN)2− binding, as judged by multiple substitutions of differently charged amino acid side chains and also by the pH dependence of block conferred by the R334H mutant. However, non-charge-dependent effects also appear to contribute to Au(CN)2− binding. Mutation of R334 also disrupts the apparent electrostatic interaction between intracellular Au(CN)2− ions and extracellular permeant anions, an interaction which normally acts to relieve channel block. All six mutations studied at R334 significantly weakened this interaction, suggesting that arginine possesses a unique ability to coordinate ion-ion interactions at this site in the pore. Our results suggest that lyotropic anions bind tightly to a site in the outer mouth of the CFTR pore that involves interaction with a fixed positive charge. Binding to this site is also involved in coordination of multiple permeant anions within the pore, suggesting that anion binding in the outer mouth of the pore is an important aspect in the normal anion permeation mechanism.
Ion channels are defined primarily by their ionic selectivity, the ability to allow certain ions to pass at a rate which approaches that of free diffusion while at the same time effectively excluding others. In highly selective voltage-gated cation channels, selectivity is determined by selective binding of the ion of physiological interest within a localized region of the pore known as the ‘selectivity filter’ (Doyle et al. 1998; McCleskey, 1999; Zhou et al. 2001). The apparent incongruity between tight binding and high conductance of the permeant ion is then resolved by electrostatic repulsion between ions bound simultaneously within the pore (Doyle et al. 1998; McCleskey, 1999; Miller, 2000; Morais-Cabral et al. 2001), such that a queue of permeant ions moves through the pore in a concerted fashion (Morais-Cabral et al. 2001; Bernèche & Roux, 2001).
While so much has recently been revealed about the mechanism of ion permeation in highly selective K+ channels, it is unknown how much of this mechanism may apply to anion channels. These channels are much less selective (reviewed in Dawson et al. 1999; Smith et al. 1999; Linsdell et al. 2000; Fahlke, 2001; Jentsch et al. 2002), having presumably not experienced the same kinds of evolutionary pressure to establish strong selectivity. Nevertheless, recent evidence suggests that Cl− channels may contain localized selectivity filters (Linsdell et al. 2000; Dutzler et al. 2002; Gong et al. 2002a) and that permeant anions bind to discrete sites within Cl− channel pores (Dawson et al. 1999; Linsdell, 2001; Fahlke 2001; Dutzler et al. 2002; Gong et al. 2002a). Furthermore, we have shown that different permeant anions move within the pore of the cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channel in a coupled manner (Gong & Linsdell, 2003), hinting at a possible role for ion-ion interactions in the normal Cl− permeation process.
We have used the Au(CN)2− ion, which shows both high permeability (Smith et al. 1999; Gong et al. 2002a) and tight binding within the CFTR channel pore (Smith et al. 1999; Gong et al. 2002a; Linsdell & Gong, 2002), as a probe of intrapore anion binding sites. Here we show that a positively charged arginine residue within the sixth transmembrane region (TM6) of CFTR makes a major contribution to anion binding within the pore, provide evidence that this residue contributes to a site important for interactions between permeant anions, and examine the molecular properties of this site which underlie its roles in anion conduction.
METHODS
Experiments were carried out on baby hamster kidney (BHK) cells transiently transfected with wild-type or mutated forms of human CFTR. Mutagenesis of CFTR was carried out as described previously (Gong et al. 2002a) and verified by DNA sequencing. Macroscopic CFTR currents were recorded from excised, inside-out membrane patches, as described in the accompanying paper (Gong & Linsdell, 2003). To isolate the pore-blocking effects of intracellular Au(CN)2−, channels were ‘locked’ in the open state by addition of 2 mm sodium pyrophosphate (PPi) to the intracellular solution following attainment of full PKA-stimulated current amplitude (Linsdell & Gong, 2002; Gong et al. 2002a; Gong & Linsdell, 2003). For high Cl− concentration experiments, both pipette (extracellular) and bath (intracellular) solutions contained (mm): 150 NaCl, 2 MgCl2, 10 N-tris[hydroxymethyl]methyl-2-aminoethanesulfonate (TES), pH 7.4. In some cases (Fig. 7), different extracellular pHs were studied; here, the pH buffer used in the extracellular solution was changed from TES to either 2-(N-morpholino)ethanesulfonic acid (MES) for solutions at pH 5.5, or [(2-hydroxy-1,1-bis[hydroxymethyl]ethyl)amino]-1-propanesulfonic acid (TAPS) at pH 9.0. As intracellular pH was not varied, all intracellular solutions contained TES. To examine the role of extracellular anions, NaCl in the pipette solution was completely replaced by sodium gluconate or NaSCN. Solutions were adjusted to the desired pH using NaOH. Given voltages have been corrected for liquid junction potentials existing between dissimilar pipette and bath solutions as described previously (Gong & Linsdell, 2003). Other methodological details, including the modified form of the Woodhull (1973) model used to fit the blocking effects of permeant Au(CN)2− ions, are as described in the accompanying paper (Gong & Linsdell, 2003).
Figure 7. Extracellular pH modifies Au(CN)2− block of R334H- but not wild-type CFTR.
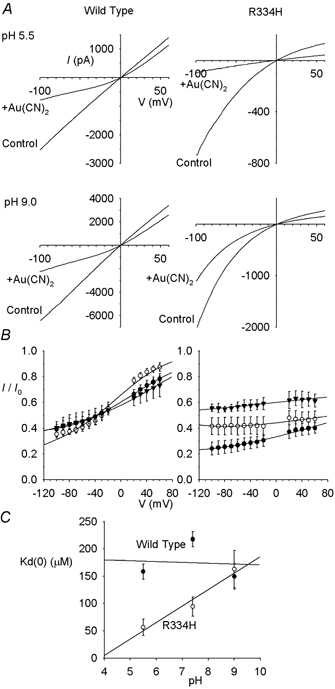
A, example I-V relationships for wild-type and R334H at extracellular pHs of 5.5 and 9.0, before (control) and after (+Au(CN)2) addition of 100 μm Au(CN)2− to the intracellular solution. In each case, the scale is current in pA on the ordinate and membrane potential in mV on the abscissa. B, mean I/I0 under these same conditions, at extracellular pH 5.5 (•), 7.4 (^) and 9.0 (▾). Mean of data from 3–5 patches, fitted as in Fig. 1B. C, effect of extracellular pH on Kd(0) in wild-type (•) and R334H (^).
In some cases (Figs 3D, 5, 6 and 8), the Kd(0) under different ionic conditions has been compared as the ratio between individual Kd measured with one anion (Kd(0)(X)) and the mean Kd measured with Cl− (Kd(0)(Cl)). Rectification of the macroscopic I-V relationship was quantified (Fig. 9A) by measuring the slope conductance at a voltage 50 mV more negative than the current reversal potential to that 50 mV more positive than the reversal potential, as described previously (Linsdell, 2001). The ratio of these two slope conductances was defined as the ‘rectification ratio’ (Fig. 9A); a rectification ratio greater than unity therefore indicates inward rectification of the I-V relationship. In some cases (Fig. 9B), the estimated current reversal potential has been used to estimate relative anion permeability as described in detail previously (Gong et al. 2002a). For graphical presentation of mean values, error bars represent ±s.e.m.
Figure 3. Au(CN)2− block of R334C is relatively independent of the extracellular anion.
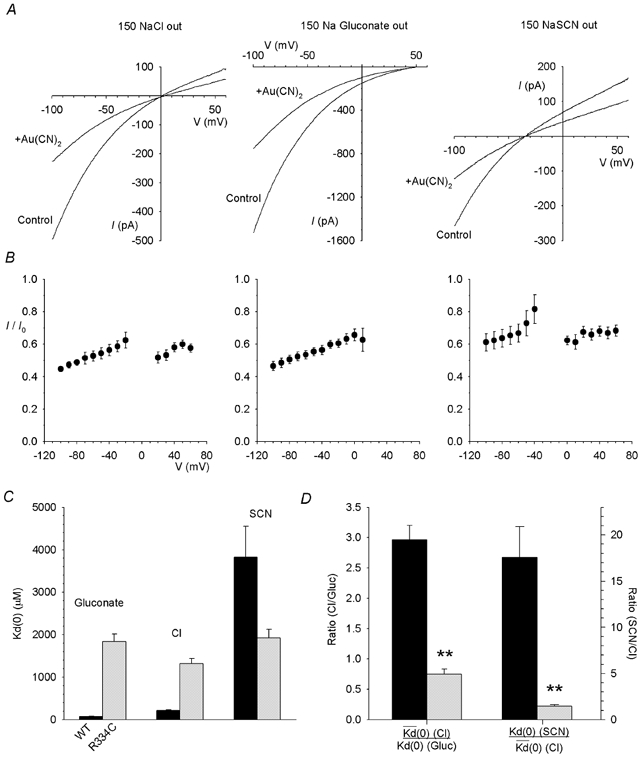
A, example R334C-CFTR I-V relationships recorded before (control) and after (+Au(CN)2) addition of 1 mm Au(CN)2− to the intracellular solution, with 150 mm NaCl, sodium gluconate or NaSCN present in the extracellular solution. B, mean fraction of control current remaining (I/I0) following addition of 1 mm Au(CN)2− for the same ionic conditions as shown in A. C, Kd(0) is strongly affected by the extracellular anion in wild-type (black bars) but not in R334C (grey bars). D, the effect of changing the extracellular anion (from Cl− to gluconate or from Cl− to SCN−) on the strength of Au(CN)2− block, as quantified by the ratio of Kd(0) values estimated under different ionic conditions as described in Methods, is significantly reduced in R334C (grey bars) relative to wild-type (black bars) (**P < 0.001). Mean of data from 5 patches in each case.
Figure 5. Mutagenesis of R334 alters the affinity, voltage dependence and extracellular anion dependence of Au(CN)2− block.
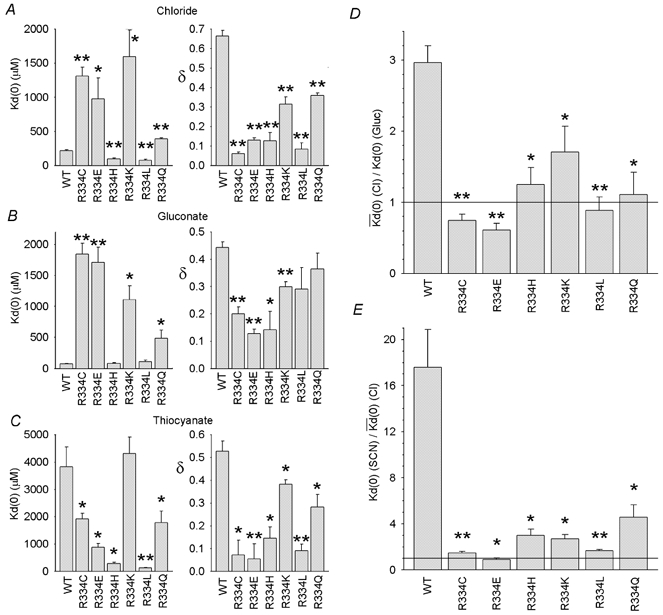
In A-C, Kd(0) and δ were calculated from data from individual patches with 150 mm NaCl (A), sodium gluconate (B) or NaSCN (C) in the extracellular solution. D and E, the effect of changing the extracellular anion (from Cl− to gluconate (D) or from Cl− to SCN− (E)), quantified by the Kd(0) ratio as in Fig. 3D, is significantly reduced in all R334 mutants relative to wild-type (*P < 0.05; **P < 0.001). Mean of data from 3–5 patches in each case.
Figure 6. Repulsive interactions with external Cl− ions contribute to the voltage dependence of Au(CN)2− block.
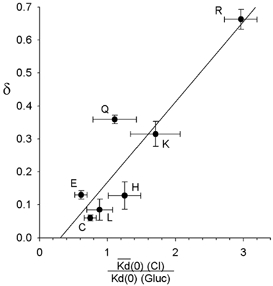
The observed fractional electrical distance (δ) for Au(CN)2− block in wild-type and each R334 mutant studied under high extracellular [Cl−] conditions appears well correlated with the effect of extracellular Cl− (relative to the impermeant gluconate) on the affinity of block. The fitted line is a linear regression with a correlation coefficient (r2) of 0.832. Parameters as described in Fig 5A and D. Different CFTR variants are identified on the plot by the amino acid present at position 334.
Figure 8. Extracellular pH does not affect extracellular anion dependence of Au(CN)2− block in R334H-CFTR.
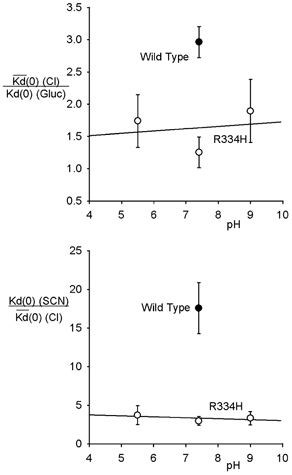
The effect of changing the extracellular anion (from Cl− to gluconate or from Cl− to SCN−), quantified by the Kd(0) ratio as in Fig. 3D, appears independent of pH in R334 and is significantly reduced relative to wild-type at pH 7.4. Mean of data from 3–5 patches in each case.
Figure 9. Mutation of R334 affects current rectification and anion selectivity.
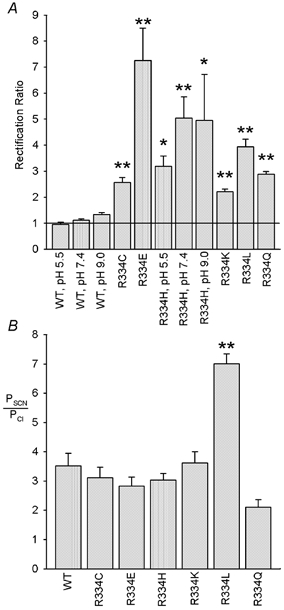
A, rectification of the macroscopic I-V relationship, quantified as the rectification ratio as described in Methods, is significantly increased following mutagenesis of R334 (*P < 0.05; **P < 0.001). Furthermore, this rectification acquires pH dependence in R334H not seen in wild-type. Mean of data from 3–5 patches. B, relative SCN− permeability, estimated from the reversal potential with 150 mm NaSCN in the extracellular solution, was unaltered in most mutants but significantly increased (**P < 0.001) in R334L. Mean of data from 3–5 patches.
RESULTS
Effect of CFTR pore mutations on anion interactions
Block of the wild-type CFTR Cl− channel pore by intracellular Au(CN)2− ions is strongly dependent on the extracellular Cl− concentration (Gong & Linsdell, 2003; Fig. 1), apparently due to repulsion between Au(CN)2− ions bound within the pore and Cl− ions bound to a site within the external entrance to the pore (Gong & Linsdell, 2003). Under conditions of high extracellular Cl− concentration, Au(CN)2− block is weakened in the CFTR pore mutants K335A, F337S and T338A (Gong et al. 2002a), suggesting that these pore residues may contribute to lyotropic anion binding site(s) within the pore. To investigate if weakened Au(CN)2− block in these mutants was associated with changes in the interaction between Au(CN)2− and Cl− ions within the pore, block by 100 μm intracellular Au(CN)2− ions was examined under conditions of high (154 mm) and low (4 mm; Cl− replaced by the impermeant gluconate) extracellular Cl− concentration (Fig. 1). In spite of the altered affinity and voltage dependence of block seen in these three mutants, in each case block was still significantly weakened by extracellular Cl− (Figs 1 and 2). In contrast, an additional mutation near the external end of TM6, R334C, not only caused a more dramatic weakening of Au(CN)2− block than previously studied mutants (Fig. 1), but also abolished or even reversed the Cl− dependence of both apparent affinity (as judged by the Kd(0); Fig. 2A) and voltage dependence (as judged by the fractional electrical distance, δ; Fig. 2B).
Figure 1. Extracellular Cl−-dependent Au(CN)2− block in wild-type and mutant forms of CFTR.
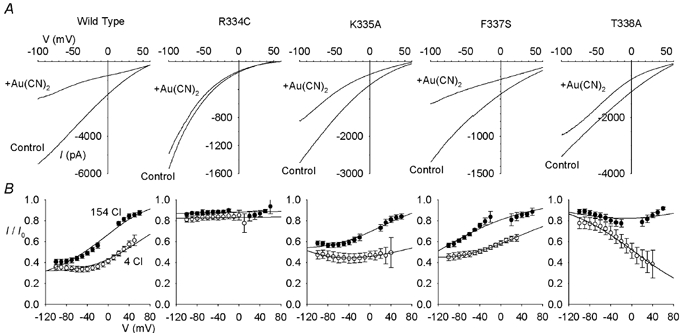
A, example leak subtracted CFTR macroscopic I-V relationships recorded with 4 mm Cl− and 150 mm gluconate in the extracellular solution. Currents were recorded following maximal CFTR channel activation with PKA and PPi, before (control) and after (+Au(CN)2) addition of 100 μm KAu(CN)2 to the intracellular solution. In each case, the scale is current in pA on the ordinate and membrane potential in mV on the abscissa. B, mean fraction of control current remaining (I/I0) at different voltages following addition of 100 μm Au(CN)2−, with 154 mm Cl− (•) or 4 mm Cl− plus 150 mm gluconate (^) in the extracellular solution. Mean of data from 4–5 patches in each case. These mean data have been fitted by a modified version of the Woodhull (1973) model as described in the accompanying paper (Gong & Linsdell, 2003).
Figure 2. Chloride-dependent Au(CN)2− block is differentially affected in different CFTR pore mutants.
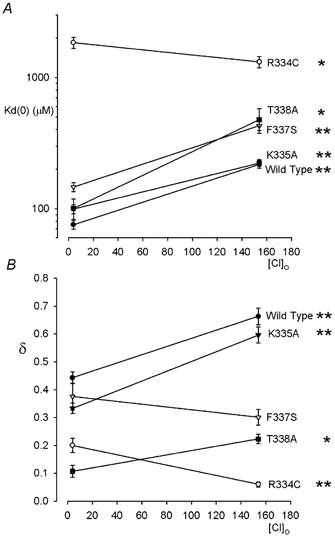
Mean Kd(0) (A) and δ (B) were estimated by analysis of data from individual patches such as those shown in Fig. 1, as described in the accompanying paper (Gong & Linsdell, 2003). In wild-type, K335A, F337S and T338A, high extracellular Cl− significantly weakens Au(CN)2− block and (except in F337S) increases the fraction of the transmembrane electric field apparently experienced by the blocker. In contrast, in R334C extracellular Cl− significantly weakens block and reduces its voltage dependence. Asterisks indicate significant difference from the data at 4 mm Cl− (*P < 0.05; **P < 0.001). Mean of data from 4–5 patches in each case.
Because the block of R334C-CFTR by Au(CN)2− was so weak compared to wild-type, we further characterized this mutant using 1 mm intracellular Au(CN)2− (Fig. 3A and B). Under these conditions, Au(CN)2− caused a weakly voltage-dependent block which, unlike the block of wild-type CFTR, was broadly similar with different extracellular anions (Cl−, gluconate, or SCN−; Fig. 3A and B). Whereas in wild-type, Kd(0) increases dramatically as the extracellular anion is made more lyotropic (gluconate → Cl−→ SCN−), this trend is apparently abolished in R334C (Fig. 3C). The effect of changing the extracellular anion on the apparent affinity of Au(CN)2− block in wild-type and R334C, quantified as the relative Kd(0) under different ionic conditions, is directly compared in Fig. 3D. The dramatic effects of changing the extracellular anion observed in wild-type were practically abolished in R334C.
Characterization of R334 as an anion binding site
The effects of the mutation R334C outlined above suggest that the positively charged arginine at this position normally contributes both to a lyotropic binding site and to a site which is crucial for electrostatic interactions between extracellular permeant ions and intracellular blocking ions. To investigate the structural determinants of this site, a number of different substitutions for arginine 334 were carried out.
Previously we reported that the mutant R334A could not be expressed in BHK cells (Gong et al. 2002a); in the course of the present study, we confirmed this previous finding, but did find that, in addition to R334C, five other mutants could be studied (Fig. 4). Each of these mutants was apparently effectively locked open by PPi (Fig. 4A). Block of wild-type, R334C-, R334E-, R334H-, R334K-, R334L- and R334Q-CFTR by 100 μm and 1 mm intracellular Au(CN)2− are compared in Fig. 4B. Block was affected in all mutants, depending on the ionic conditions used, but was particularly weakened in R334C, R334E and R334K (Fig. 5A–C). The voltage dependence of block was also reduced in all mutants studied (Fig. 5A–C).
Figure 4. Au(CN)2− block of different R334 mutants.
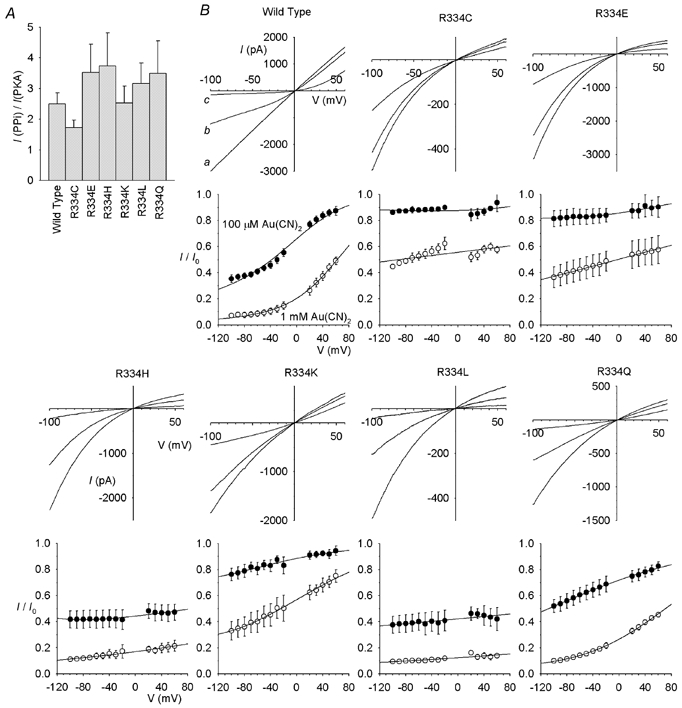
A, mean fractional increase in macroscopic current amplitude at −100 mV following addition of 2 mm PPi for wild-type and R334 mutant CFTR channels. Mean of data from 4–5 patches. B, block by Au(CN)2−. For each CFTR variant, example I-V relationships show the blocking effects of 100 μm and 1 mm Au(CN)2− with symmetrical Cl−-containing solutions. In each case, the scale is current in pA on the ordinate and membrane potential in mV on the abscissa. In each case the current amplitude was decreased in a concentration-dependent fashion by addition of Au(CN)2; for example, in wild-type, a represents control, b following addition of 100 μm Au(CN)2, and c following addition of 1 mm Au(CN)2. Beneath each I-V relationship, the corresponding mean I/I0 following addition of 100 μm (^) or 1 mm (•) Au(CN)2− is shown; this represents the mean of data from 4–5 patches, fitted as in Fig. 1B.
Figure 5 also compares the effects of changing the extracellular anion on the apparent affinity of block in different mutants. In wild-type, block was weakened on replacing the impermeant gluconate with the permeant Cl−, and further dramatically weakened on replacing Cl− with the more lyotropic SCN−. In every mutant studied, both of these effects of changing the extracellular permeant ion were greatly reduced, leading to significant decreases in the Kd(0) ratio in both cases (Fig. 5D and E).
Previously we suggested that part of the voltage dependence of Au(CN)2− block resulted from repulsive interactions with extracellular anions (Gong & Linsdell, 2003). The apparent effect of extracellular Cl− on the affinity of Au(CN)2− block in different mutants (as judged by the Kd(0) ratio for Cl−versus gluconate) was correlated with the voltage dependence of block observed with Cl− present in the extracellular solution (δ) (Fig. 6; r2= 0.832), consistent with the idea that the same repulsive Cl−-Au(CN)2− interactions that affect blocker affinity similarly affect blocker voltage dependence.
A direct investigation of the role of positive charge
A previous mutagenic investigation of arginine 334 emphasized the role played by the fixed positive charge at this position, and elegantly demonstrated this effect by titrating the side chain charge in R334H by changing the external pH (Smith et al. 2001). Although we have studied mutants with neutral, positively charged and negatively charged side chains, the pH dependence of R334H provides a unique opportunity to examine the effect of side chain charge independently of side chain shape. We therefore examined the effect of changing extracellular pH on Au(CN)2− block of wild-type and R334H-CFTR.
As shown in Fig. 7, Au(CN)2− blocked R334H more strongly at pH 5.5 than at pH 9.0, whereas block of wild-type was apparently pH independent. This suggests that the protonated histidine side chain at this position binds Au(CN)2− more strongly than the uncharged form. However, the effect of changing the extracellular anion on the apparent affinity of Au(CN)2− block was not strongly pH dependent in R334H (Fig. 8), and at all pHs studied the effect of changing from extracellular gluconate to Cl−, or from Cl− to SCN−, was small (as judged by the Kd(0) ratio) compared to the effect seen in wild-type at pH 7.4. These experiments on R334H at different pHs strongly suggest that Au(CN)2− blocking affinity and the interaction between intracellular Au(CN)2− ions and extracellular anions show different dependencies on side chain charge at position 334.
Other properties of R334 mutants
Our results suggest that R334 makes a strong contribution to anion binding in the CFTR pore, consistent with recent evidence that the positive charge at this position plays an important role in attracting anions to the extracellular mouth of the pore (Smith et al. 2001). Our experiments also revealed other properties of R334 mutants. As previously reported (Smith et al. 2001), all mutations at this position induced inward rectification of the I-V relationship (Fig. 4B); this effect is quantified in Fig. 9A. As noted by Smith et al. (2001), this effect was clearly charge dependent, being strongest in R334E and weak (but still significant) in R334K. Consistent with this notion, and again as noted by Smith et al. (2001), rectification was pH dependent in R334H but not in wild-type (Fig. 9A).
Our experiments on the effects of extracellular SCN− also allowed us to estimate the SCN− permeability of CFTR mutants (Fig. 9B). In contrast to mutations in the more central region of TM6, at F337 (Linsdell et al. 2000), T338 (Linsdell et al. 1998) and S341 (McCarty & Zhang, 2001), most substitutions for R334 had no significant effect on relative SCN− permeability. However, SCN− permeability was significantly increased in one mutant, R334L (Fig. 9B).
DISCUSSION
Arginine 334 and anion binding in the CFTR pore
The TM6 region plays a central role in forming the CFTR pore and determining its functional properties (Dawson et al. 1999; McCarty, 2000; Gupta et al. 2001; Gong et al. 2002a). Mutations within TM6 alter CFTR anion selectivity (Linsdell et al. 1998, 2000; McCarty & Zhang, 2001; Gong et al. 2002a) as well as the binding of permeant (Linsdell, 2001; Gong et al. 2002a) and blocking anions (McDonough et al. 1994; Gong et al. 2002b; Gupta & Linsdell, 2002). Weakened binding of the highly lyotropic Au(CN)2− ion was previously associated with mutagenesis of TM6 residues K335, F337, T338 and (to a lesser extent) I334 (Gong et al. 2002a). However, as shown in Figs 1 and 2, block by Au(CN)2− was more dramatically altered following mutagenesis of R334, implicating this residue as contributing to a high-affinity lyotropic anion binding site within the pore. Each of the mutations R334C, R334E and R334K greatly decreased Au(CN)2− affinity (Figs 4B and 5), increasing Kd(0) 4–7-fold under conditions of high extracellular Cl− (Fig. 5A), and 14–24-fold with the impermeant gluconate ion in the extracellular solution (Fig. 5B), conditions under which Au(CN)2− binding is studied in relative isolation from interactions with other anions. Thus perhaps somewhat surprisingly, combining these with our previous results (Gong et al. 2002a) suggests that the very lyotropic, highly permeant Au(CN)2− ion binds most strongly at the putative outer mouth of the pore.
Arginine 334 may also contribute to a binding site for larger CFTR open channel blockers. Previously we showed that the R334C mutation significantly weakened block by intracellular lonidamine (Gong et al. 2002b), consistent with the idea that permeant and blocking ions may share common binding sites within the pore. While the highly permeant Au(CN)2− is expected to have access to binding sites throughout the pore, it is not clear how a binding site near the extracellular mouth of the channel interacts with large intracellular blockers (as discussed in Gong et al. 2002b).
Assuming that Au(CN)2− interacts strongly with Cl− binding sites in CFTR (Linsdell & Gong, 2002), we speculate that R334 may contribute to a relatively high-affinity Cl− binding site within the CFTR pore. No positively charged amino acid side chains were identified in the primary Cl− binding site at the putative selectivity filter of the ClC crystal structure (Dutzler et al. 2002), although negatively charged side chains do bind Ca2+ ions in Ca2+ channel selectivity filters (Yang et al. 1993; Ellinor et al. 1995). Our results are consistent with R334 forming a high-affinity anion binding site in the external pore vestibule rather than the selectivity filter (see below); indeed, we find no evidence that R334 plays a major role in CFTR anion permeation selectivity (Fig. 9B; see below).
In addition to contributing to a high-affinity lyotropic anion binding site, R334 plays a crucial role in the interaction between intracellular Au(CN)2− and extracellular anions (Figs 3 and 5). In wild-type CFTR, changing the nature of the extracellular anion dramatically alters both the affinity and voltage dependence of block by intracellular Au(CN)2− (Fig. 5; Gong & Linsdell, 2003). We suggested that this reflects repulsion between bound Au(CN)2− ions and other anions bound to an extracellularly accessible lyotropic binding site (Gong & Linsdell, 2003). The effect of different extracellular anions on the affinity of Au(CN)2− block is greatly weakened or lost in all R334 mutants studied (Fig. 5D and E), implicating this residue as playing a crucial role in interactions between anions bound within the pore. Furthermore, correlated effects of R334 mutations on Cl−-Au(CN)2− interactions and on the voltage dependence of Au(CN)2− block (Fig. 6), support the view that such repulsive interactions normally contribute to blocker voltage dependence (Gong & Linsdell, 2003). In contrast, mutation of other nearby TM6 residues associated with weakened Au(CN)2− binding (K335A, F337S, T338A) showed similar sensitivity to extracellular Cl− concentration to that seen in wild-type (Figs 1 and 2). We propose that anion binding to a site involving R334 is crucial in coordinating anion-anion interactions within the pore. The apparent accessibility of this site to extracellular anions (Gong & Linsdell, 2003) is consistent with models which place R334 in the outer vestibule of the CFTR pore (Cheung & Akabas, 1996; Smith et al. 2001; Gong et al. 2002a).
The important role played by R334 in anion binding is consistent with previous findings that mutations at this site alter single channel conductance and the shape of the macroscopic I-V relationship (Smith et al. 2001). Indeed, we find a similar effect of mutations on I-V rectification in inside-out membrane patches (Fig. 9A) to those reported by Smith et al. (2001) in intact Xenopus oocytes. Our results in inside-out patches under symmetrical ionic conditions argue that this rectification is an intrinsic property of the open channel pore and does not reflect altered block by cytoplasmic constituents, one possibility raised by Smith et al. (2001).
In spite of its clear importance in anion binding, R334 does not appear to play a major role in lyotropic anion permeation selectivity (Fig. 9B). This is consistent with our previous suggestion that selectivity is determined predominantly over a short region of the pore located more cytoplasmically than R334 (Linsdell et al. 2000; Gong et al. 2002a) and that some anion binding sites within the pore are not intimately involved in the selectivity process (Gong et al. 2002a).
Separating the different effects of mutagenesis at arginine 334
The effects of mutagenesis of R334 on the apparent affinity of Au(CN)2− block and on its dependence on the extracellular anion are both consistent with disruption of an important anion binding site in the external mouth of the pore. Since there is broad agreement that the side chain at R334 lines the aqueous lumen of the CFTR pore (Cheung & Akabas, 1996; Smith et al. 2001), unlike the situation for another arginine residue in TM6, R347 (Cheung & Akabas, 1996; Cotten & Welsh, 1999; Smith et al. 2001), we assume that anion-amino acid side chain interactions dominate the permeation phenotype conferred by different R334 mutations, although as with all mutagenesis experiments, more indirect effects cannot be ruled out.
With either Cl− or gluconate in the extracellular solution, Au(CN)2− block was most dramatically weakened in the mutants R334C, R334E and R334K, which involve replacement of the positively charged arginine side chain with one neutral side chain (cysteine), one negatively charged side chain (glutamate) and one positively charged side chain (lysine). This suggests that charge does not play a dominant role in determining Au(CN)2− affinity at this site. In R334H, positive charge does appear to enhance Au(CN)2− binding, since low pH, which is expected to favour protonation of this side chain (see also Smith et al. 2001), increases the apparent affinity of block (Fig. 7).
All mutations at R334 interfered with the ability of different extracellular anions to affect the apparent affinity of Au(CN)2− block (Fig 5D and E), presumably due to a disruption of anion-anion interactions within the pore (see above). Thus, among the amino acids studied at position 334, the wild-type arginine appears unique in its ability to confer strong repulsive interactions between Au(CN)2− in the pore and extracellular anions.
The different sensitivity of different parameters of pore function on amino acid side chain charge at position 334 suggests that these aspects of the permeation mechanism may not be strongly interdependent or even controlled by similar structural features of the pore. These different effects of side chain charge are clearly demonstrated in R334H, which shows pH-dependent rectification (Fig. 9A) and Au(CN)2− affinity (Fig. 7) but pH-independent interactions between intracellular Au(CN)2− and extracellular anions (Fig. 8). The fact that tight Au(CN)2− binding and strong Au(CN)2−-anion interactions are separable by mutagenesis is also evident in R334H and R334L (Fig. 5).
The specific role of arginine
Of the six R334 mutants studied, none could reproduce the strong anion-anion interactions seen in wild-type (Fig. 5D and E) or the resulting voltage dependence of Au(CN)2− block (Fig. 6). This implies a specific role for arginine in coordinating interactions between anions bound simultaneously in the pore. Arginine has frequently been proposed as being particularly well suited to form anion binding sites in proteins (Riordan, 1979; Tabcharani et al. 1997; Dawson et al. 1999). We hypothesize that the large, highly hydrated surface area (Radzicka & Wolfenden, 1988) and diffuse positive charge of the guanidinium group of arginine gives it a unique ability to coordinate the binding of multiple anions within the pore. This could occur, for example, if the guanidinium group, which contains five hydrogen bond donors in a planar array (Dawson et al. 1999), was able to interact simultaneously with two different anions. However, we previously found that extracellular anions much larger than Cl−, such as ClO4−, were able to interact with intracellular Au(CN)2− (Gong & Linsdell, 2003). Alternatively, the long arginine side chain may allow it to bind a single anion in close proximity to a second anion bound to a distinct but nearby binding site; the nearby TM6 residues K335, F337 and T338 all appear to be involved in Au(CN)2− binding in the CFTR pore (Gong et al. 2002a).
Implications for the permeation mechanism in the CFTR pore
Our results suggest that R334, a pore-lining TM6 residue predicted to lie in the outer vestibule of the CFTR pore (Cheung & Akabas, 1996; Smith et al. 2001; Gong et al. 2002a), contributes a relatively high-affinity lyotropic anion binding site in the pore. Furthermore, this binding site appears to play a crucial role in the ability of extracellular anions to interact with blocking anions bound within the pore. We propose that R334 does, in fact, contribute to the extracellularly accessible lyotropic anion binding site predicted by the accompanying paper (Gong & Linsdell, 2003). Although other TM6 residues located more intracellularly than R334 also appear to contribute to lyotropic anion binding (e.g. K335, F337, T338; Fig. 1; Gong et al. 2002a), the effects of mutations studied to date suggest that the highly lyotropic Au(CN)2− anion binds most strongly at R334.
We proposed that electrostatic repulsion between anions bound simultaneously within the CFTR pore may help maximize the rate of Cl− permeation (Gong & Linsdell, 2003), a mechanism analogous to that which occurs in K+ channel (Doyle et al. 1998; Morais-Cabral et al. 2001) and Ca2+ channel pores (McCleskey, 1999), and which has been suggested on structural grounds in ClC-type Cl− channels (Dutzler et al. 2002). This mechanism appears to involve R334, but is not conferred simply by tight Au(CN)2− binding. Within K+ channel pores, K+ binding sites are spaced along the axis of the pore, both within and on either side of the selectivity filter, and ions move between these sites in a concerted fashion (Morais-Cabral et al. 2001; Zhou et al. 2001; Bernèche & Roux, 2001). In Ca2+ channel pores, a ring of negatively charged glutamate residues within the selectivity filter can coordinate one Ca2+ ion tightly or two Ca2+ ions weakly, leading to exit (permeation) of bound Ca2+ ions from doubly occupied pores (Yang et al. 1993; Ellinor et al. 1995; McCleskey, 1999). Calcium ions do not appear to bind tightly outside the selectivity filter region (Cibulsky & Sather, 2000). Although it is not at present clear which of these two mechanisms most closely resembles that of Cl− permeation in the CFTR pore, it is interesting to note that R334 does not appear to be intimately involved in the selectivity of anion permeation (Fig. 9B) and probably confers tight anion binding on the extracellular side of the anion selectivity filter (Gong et al. 2002a).
Acknowledgments
We thank Susan Burbridge and Angie Lewis for technical assistance. This work was supported by the Canadian Institutes of Health Research and the Canadian Cystic Fibrosis Foundation (CCFF). X.G. is a CCFF postdoctoral fellow. P.L. is a CCFF scholar.
REFERENCES
- Bernèche S, Roux B. Energetics of ion conduction through the K+ channel. Nature. 2001;414:73–77. doi: 10.1038/35102067. [DOI] [PubMed] [Google Scholar]
- Cheung M, Akabas MH. Identification of cystic fibrosis transmembrane conductance regulator channel-lining residues in and flanking the M6 membrane-spanning segment. Biophys J. 1996;70:2688–2695. doi: 10.1016/S0006-3495(96)79838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibulsky SM, Sather WA. The EEEE locus is the sole high-affinity Ca2+ binding structure in the pore of a voltage-gated Ca2+ channel. Block by Ca2+ entering from the intracellular pore entrance. J Gen Physiol. 2000;116:349–362. doi: 10.1085/jgp.116.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten JF, Welsh MJ. Cystic fibrosis-associated mutations at arginine 347 alter the pore architecture of CFTR. Evidence for disruption of a salt bridge. J Biol Chem. 1999;274:5429–5435. doi: 10.1074/jbc.274.9.5429. [DOI] [PubMed] [Google Scholar]
- Dawson DC, Smith SS, Mansoura MK. CFTR: mechanism of anion conduction. Physiol Rev. 1999;79:S47–75. doi: 10.1152/physrev.1999.79.1.S47. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3. 0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- Ellinor PT, Yang J, Sather WA, Zhang J-F, Tsien RW. Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron. 1995;15:1121–1132. doi: 10.1016/0896-6273(95)90100-0. [DOI] [PubMed] [Google Scholar]
- Fahlke C. Ion permeation and selectivity in ClC-type chloride channels. Am J Physiol Renal Physiol. 2001;280:F748–757. doi: 10.1152/ajprenal.2001.280.5.F748. [DOI] [PubMed] [Google Scholar]
- Gong X, Burbridge SM, Cowley EA, Linsdell P. Molecular determinants of Au(CN)2− binding and permeability within the cystic fibrosis transmembrane conductance regulator Cl− channel pore. J Physiol. 2002a;540:39–47. doi: 10.1113/jphysiol.2001.013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Burbridge SM, Lewis AC, Wong PYD, Linsdell P. Mechanism of lonidamine inhibition of the CFTR chloride channel. Br J Pharmacol. 2002b;137:928–936. doi: 10.1038/sj.bjp.0704932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X, Linsdell P. Coupled movement of permeant and blocking ions in the CFTR chloride channel pore. J Physiol. 2003;549:375–385. doi: 10.1113/jphysiol.2002.038216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta J, Evagelidis A, Hanrahan JW, Linsdell P. Asymmetric structure of the cystic fibrosis transmembrane conductance regulator chloride channel pore suggested by mutagenesis of the twelfth transmembrane region. Biochemistry. 2001;40:6620–6627. doi: 10.1021/bi002819v. [DOI] [PubMed] [Google Scholar]
- Gupta J, Linsdell P. Point mutations in the pore region directly or indirectly affect glibenclamide block of the CFTR chloride channel. Pflugers Arch. 2002;443:739–747. doi: 10.1007/s00424-001-0762-0. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Linsdell P. Relationship between anion binding and anion permeability revealed by mutagenesis within the cystic fibrosis transmembrane conductance regulator chloride channel pore. J Physiol. 2001;531:51–66. doi: 10.1111/j.1469-7793.2001.0051j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsdell P, Evagelidis A, Hanrahan JW. Molecular determinants of anion selectivity in the cystic fibrosis transmembrane conductance regulator chloride channel pore. Biophys J. 2000;78:2973–2982. doi: 10.1016/S0006-3495(00)76836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsdell P, Gong X. Multiple inhibitory effects of Au(CN)2− ions on cystic fibrosis transmembrane conductance regulator Cl− channel currents. J Physiol. 2002;540:29–38. doi: 10.1113/jphysiol.2001.013234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsdell P, Zheng S-X, Hanrahan JW. Non-pore lining amino acid side chains influence anion selectivity of the human CFTR Cl− channel expressed in mammalian cell lines. J Physiol. 1998;512:1–16. doi: 10.1111/j.1469-7793.1998.001bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty NA. Permeation through the CFTR chloride channel. J Exp Biol. 2000;203:1947–1962. doi: 10.1242/jeb.203.13.1947. [DOI] [PubMed] [Google Scholar]
- McCarty NA, Zhang Z-R. Identification of a region of strong discrimination in the pore of CFTR. Am J Physiol Lung Cell Mol Physiol. 2001;281:L852–867. doi: 10.1152/ajplung.2001.281.4.L852. [DOI] [PubMed] [Google Scholar]
- McCleskey EW. Calcium channel permeation: a field in flux. J Gen Physiol. 1999;113:765–772. doi: 10.1085/jgp.113.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S, Davidson N, Lester HA, McCarty NA. Novel pore-lining residues in CFTR that govern permeation and open-channel block. Neuron. 1994;13:623–634. doi: 10.1016/0896-6273(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Miller C. Ion channels: doing hard chemistry with hard ions. Curr Opin Chem Biol. 2000;4:148–151. doi: 10.1016/s1367-5931(99)00068-x. [DOI] [PubMed] [Google Scholar]
- Morais-Cabral JH, Zhou Y, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- Radzicka A, Wolfenden R. Comparing the polarities of the amino acids: side-chain distribution coefficients between the vapor phase, cyclohexane, 1-octanol, and neutral aqueous solution. Biochemistry. 1988;27:1664–1670. [Google Scholar]
- Riordan JF. Arginyl residues and anion binding sites in proteins. Mol Cell Biochem. 1979;26:71–92. doi: 10.1007/BF00232886. [DOI] [PubMed] [Google Scholar]
- Smith SS, Liu X, Zhang Z-R, Sun F, Kriewall TE, McCarty NA, Dawson DC. CFTR: covalent and noncovalent modification suggests a role for fixed charges in anion conduction. J Gen Physiol. 2001;118:407–431. doi: 10.1085/jgp.118.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Steinle ED, Meyerhoff ME, Dawson DC. Cystic fibrosis transmembrane conductance regulator. Physical basis for lyotropic anion selectivity patterns. J Gen Physiol. 1999;114:799–818. doi: 10.1085/jgp.114.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani JA, Linsdell P, Hanrahan JW. Halide permeation in wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels. J Gen Physiol. 1997;110:341–354. doi: 10.1085/jgp.110.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Ellinor PT, Sather WA, Zhang J-F, Tsien RW. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature. 1993;366:158–161. doi: 10.1038/366158a0. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Morais-Cabral JH, Kaufman A, Mackinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2. 0 Å resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]


