Abstract
At certain synapses in the brain, Ca2+-permeable AMPA receptor (AMPAR) channels represent an important pathway for synaptically controlled Ca2+ entry. However, the molecular determinants of this Ca2+ influx are poorly defined. In NMDA receptor (NMDAR) channels, where the influx is much greater, the extracellular vestibule, specifically the M3 segment and regions C-terminal to it in the NR1 subunit, contains elements critical to their high Ca2+ influx under physiological conditions. We therefore investigated the contribution of homologous positions in AMPAR as well as kainate receptor (KAR) subunits to the process of Ca2+ influx. Substitutions of a conserved asparagine (N) in M3 of AMPAR GluR-B(Q) channels strongly attenuated Ca2+ permeability measured using reversal potentials under biionic conditions and fractional Ca2+ currents recorded under physiological conditions. Hence, as in NMDAR channels, the conserved N makes a significant contribution to Ca2+ influx in AMPAR channels. In addition, C-terminal to M3, substitutions of negatively (glutamate, E) or positively (arginine, R) charged residues also altered Ca2+ influx. However, in contrast to charged residues occupying homologous positions in NMDAR channels, these effects were about equal and opposite suggesting that this ER in AMPARs does not contribute significantly to the mechanism of Ca2+ influx. Opposite charge substitutions of two negative residues C-terminal to M3 in KAR GluR-6(Q) subunits had no effect on Ca2+ permeability. We conclude that the different contribution of residues C-terminal to M3 to Ca2+ permeation in NMDAR and non-NMDAR channels reflects a different positioning of these residues relative to the tip of the M2 loop.
Glutamate receptor (GluR) channels, when activated by glutamate, represent a pathway for Ca2+ entry into a cell (Dingledine et al. 1999). At most synapses, the major mechanism mediating this Ca2+ entry is the N-methyl-d-aspartate receptor (NMDAR) subtype. However, at certain synapses, typically those associated with interneurons, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptor (AMPAR) channels can also be Ca2+ permeable (Burnashev, 1996). Indeed, the Ca2+ influx mediated by Ca2+-permeable AMPAR channels has physiological significance, underlying synaptic modulation (Gu et al. 1996; Mahanty & Sah, 1998; Liu & Cull-Candy, 2000) as well as functional relationships between glial cells and synapses (Iino et al. 2001). Despite this importance, determinants of Ca2+ influx in Ca2+-permeable AMPAR and kainate receptor (KAR) channels remain poorly defined.
In GluR channels, Ca2+ permeability as well as channel block and single channel conductance are strongly influenced by the amino acid occupying a functionally critical position in the M2 loop, the Q/R site in non-NMDARs and the N site (or N + 1 site) in NMDARs (Dingledine et al. 1999). RNA editing of the Q/R site in the AMPAR GluR-B (or GluR2) or the KAR GluR-5 and -6 subunits results in a polar glutamine (Q) being replaced by a positively charged arginine (R) in the mature protein (Seeburg et al. 1998). Channels containing the edited or R-form of these subunits are essentially Ca2+-impermeable (Burnashev et al. 1995) with homomeric R-forms of KAR channels even permeable to Cl− (Burnashev et al. 1996). Hence, the Q/R site, specifically in its edited form, is critical to defining Ca2+ permeability. Still, the positively charged arginine can have indirect actions on permeating ions. Accordingly, its effect on Ca2+ influx does not indicate that glutamine occupying the Q/R site defines the Ca2+ permeability properties of channels containing only non- or unedited subunits. Consistent with this idea, the Q/R site is positioned external to the channel's narrow constriction (Kuner et al. 2001), suggesting that the action of the arginine is mainly electrostatic. Hence, other elements in the pore may also contribute to the process of Ca2+ influx in Ca2+-permeable AMPAR channels. Identifying such elements will help to clarify the mechanism of Ca2+ influx in AMPAR channels and will be useful in the development of mutant mice to study its functional significance.
In NMDAR channels, the extracellular vestibule contains key determinants of their high Ca2+ influx (Watanabe et al. 2002). These determinants, associated with the NR1 subunit, include DRPEER, a highly charged motif located C-terminal to the M3 segment as well as a highly conserved asparagine (N632) within the M3 SYTANLAAF motif (see Fig. 1). Some of these domains, such as the conserved N, are present in non-NMDAR subunits. On the other hand, most of DRPEER is unique to NR1. DR, however, is conserved in terms of charge, being represented by a negatively charged glutamate (E) and an arginine (R) in non-NMDAR subunits. Further, within regions homologous to DRPEER, KAR subunits possess not only ER but also an additional negative charge at position 634 (E634). It is unclear how these externally located domains contribute to the process of Ca2+ influx in non-NMDAR, especially given that the magnitude of this influx is much lower than that in NMDAR channels.
Figure 1. Sequence alignment of GluR subtypes.
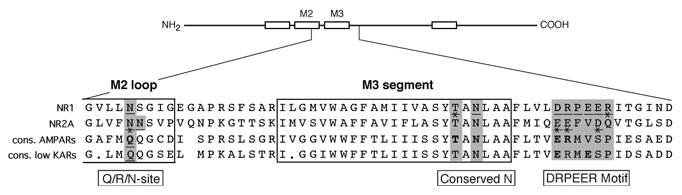
Top, schematic drawing of a GluR subunit with the four hydrophobic domains (M1-M4) shown as open boxes. Bottom, enlarged region (thin lines) comparing the amino acid residues of NR1, NR2A and the consensus sequences of AMPARs (GluR-A, -B, -C, -D) and low-affinity KARs (GluR-5, -6, -7). The dots in the KAR consensus sequence indicate positions that are occupied by non-identical amino acid residues. Open positions represent gaps within the alignment. For NMDAR subunits, residues that have been mutated and tested for effects on Ca2+ influx are underlined (Watanabe et al. 2002). Mutant channels containing these substitutions either significantly altered Ca2+ influx relative to wild type or if not, the underlined marker is crossed out. The shaded areas reflect the homologous positions to them in all subunits. Bold amino acid residues depict positions where site-directed mutagenesis was used to introduce opposite charged or neutral (alanine, A, cysteine, C) residues in non-NMDAR subunits. For the Q/R site, the glutamine Q, of the unedited form of GluR-B was substituted by an asparagine (N). The numbering for the conserved N in the different subunits is: NR1, 632; NR2A, 629; GluR-A, 615; GluR-B, 619; GluR-6, 623.
In the present study, we investigated the contribution of these homologous elements in Ca2+-permeable non-NMDAR channels to Ca2+ influx (Fig. 1). We find that the conserved N in M3 in AMPAR channels makes a significant contribution to this process. On the other hand, charged residues located C-terminal to M3 make either minor (AMPAR) or no (KAR) contribution in contrast to those in NMDAR. We conclude that this difference between the GluR subtypes reflects a structural difference between them, possibly a much more external location of these positions in non-NMDAR subunits relative to the tip of the M2 loop.
METHODS
Molecular biology
All experiments were performed with previously described expression constructs for wild type NMDAR, AMPAR and KAR subunits (see Jatzke et al. 2002 for references). The KAR subunit, GluR-6, was fully edited within the M1 segment (V,C) (Köhler et al. 1993). AMPAR subunits were identified following the nomenclature of Seeburg (1993), with the amino acid occupying the Q/R site indicated in parentheses. Point mutations in GluR-A(Q), GluR-B(Q), GluR-B(N) and GluR-6(Q) were generated either by the QuikChange site-directed mutagenesis kit (Stratagene, LaJolla, CA, USA) or by other PCR-based methods using Platinum Pfx DNA polymerase (Invitrogen, Carlsbad, CA, USA) or Pfu poly DNA polymerase (Stratagene). Positive clones were subcloned back into the respective wild type clone present in the eukaryotic expression vector pRK. All constructs were sequenced over the entire length of the replaced fragment. All AMPAR subunits were of the flip-splice variant form. Channels were expressed transiently in human embryonic kidney 293 (HEK 293) cells using FuGene 6 (Roche, Indianapolis, IN, USA). A vector for enhanced green fluorescent protein (pEGFP-C1, Clontech, Palo Alto, CA, USA) was co-transfected at a ratio of 1:9. Cells were recorded from 1to 3 days after transfection.
Electrophysiology
Currents were recorded at room temperature (20–23 °C) using an EPC-9 amplifier with PULSE software (HEKA Elektronik, Lambrecht, Germany), low-pass filtered at 300–500 Hz and digitized at 2 kHz. Pipettes had resistances of 2–4 MΩ when filled with the pipette solution and measured in the Na+ reference solution. External solutions were applied using a piezo-driven double-barrel application system. The open tip response (10–90 % rise time) of the application system was < 500 μs. For NMDARs, one barrel contained the external solution plus glycine (20 μm), while the other barrel contained the same solution with added glutamate (100 μm). For non-NMDARs, the glutamate concentration was 1 or 3 mm. To minimize desensitization, we included in all external solutions for AMPARs 15 μm cyclothiazide (CTZ; stock solution was 10 mm CTZ in 100 mm NaOH). For KARs, we initially incubated cells for 2–3 min in concanavalin A (0.3 mg ml−1) and included concanavalin A in all reference solutions. Unless otherwise stated, all chemicals were obtained from Sigma (St Louis, MO, USA) or J. T. Baker (Phillipsburg, NJ, USA).
Experimental protocols
Fractional Ca2+ currents
Fura-2 (1 mm) was loaded into cells via the patch pipette to measure the fraction of the total current carried by Ca2+ (see Neher, 1995). Briefly, cells were illuminated alternatively at 360 and 380 nm (2–10 Hz) by a polychromatic illumination system, with fluorescence signals measured using a photodiode (T.I.L.L. Photonics, München, Germany). Fractional Ca2+ currents (Pf) were quantified using the relationship, Pf (%) = 100QCa/QT, where QCa and QT are the charge carried by Ca2+ and the total charge, respectively, during a defined time interval. QT was derived from the current integral. QCa was derived from the relationship, QCa=ΔF380/fmax, where ΔF380 is the change in the fluorescence signal with 380 nm excitation and fmax is the proportionality constant between the charge carried by inward Ca2+ and ΔF380. ΔF380 was normalized to the fluorescence of beads (4.5 μm diameter fluoresbrite BB beads, Polysciences, Inc., Warrington, PA, USA; lot no. 481613) and expressed in ‘bead units’ (BU).
In measuring fractional Ca2+ currents, our intracellular solution consisted of (mm): 140 KCl, 10 Hepes, 1 K5 fura-2, pH 7.2 (KOH). The external solution consisted of (mm): 140 NaCl and 10 Hepes, pH 7.2 (NaOH) and added CaCl2 (1.8 or 20 mm). Fura-2 was obtained from Molecular Probes (Eugene, OR, USA).
Ca2+ permeability
We used two different approaches to quantify Ca2+ permeability relative to Na+ (PCa/PNa). The first approach was based on measuring changes in the reversal potential, ΔErev, for glutamate-activated currents on replacing Na+ in a Na+-based reference solution with Ca2+ (e.g. Jatzke et al. 2002). The reference solution consisted of (mm): 140 NaCl and 10 Hepes, pH 7.2 (NaOH). The high Ca2+-containing solution consisted of (mm): 110 Ca2+ and 10 trizma base, pH 7.3 (HCl). For 0.5, 1.8 or 10 mm, Ca2+ was added to a NMDG-based solution (mm): 140 NMDG and 10 Hepes, pH 7.2 (HCl). The pipette solution consisted of (mm): 140 KCl, 10 BAPTA, 10 Hepes, pH 7.2 (KOH). NMDG shows a weak permeability in non-NMDAR channels. We assumed that this NMDG permeability (PNMDG/PNa) was approximately 0.02 in GluR-A(Q) and GluR-B(Q), 0.003 in GluR-B(N) and 0.01 in GluR-6(Q) channels (see Jatzke et al. 2002 and references therein). We also assume that the mutations in the extracellular vestibule do not alter PNMDG/PNa. In measuring reversal potentials, all potentials have been corrected for junction potentials and are indicated as E. ΔErev values were converted to PCa/PNa using the Lewis equation (see eqn (7) in Wollmuth & Sakmann, 1998).
The other approach we used to quantify PCa/PNa is based on Pf measurements and relates Ca2+ permeability to Pf values using Goldman-Hodgkin-Katz (GHK) assumptions. We followed the approach of Jatzke et al. 2002 (eqn (1)) where we explicitly defined the intracellular and extracellular monovalent composition and their relative permeabilities. All Pf measurements were quantified relative to the reversal potential.
Block by internal polyamines
We used the rectification in the current-voltage relation for Ca2+-permeable AMPAR channels as an index of the block by intracellular polyamines. To quantify this rectification, we generated current-voltage relations from −80 to 40 mV in 10 mV increments (see Fig. 2E). A fourth-order polynomial was then fitted to the current-voltage relation to verify that the reversal potential was within several millivolts of zero. To derive the rectification ratio, we divided the absolute current at −50 mV by the average current from 10 to 40 mV (|I−50 mV|/avg(I10–40 mV)).
Figure 2. Effect of substitutions of the conserved N in GluR-B(Q) channels on Ca2+ permeability.
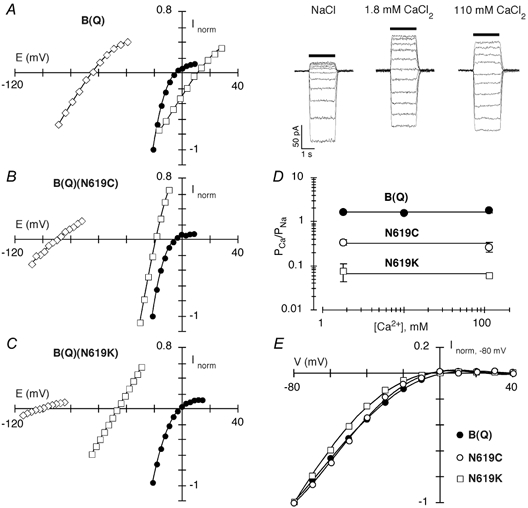
A–C, average glutamate-activated currents at different membrane potentials, in 2–5 mV increments, in cells expressing GluR-B(Q) (A), GluR-B(Q)(N619C) (B) or GluR-B(Q)(N619K) (C) subunits. The cells were bathed in either 140 mm NaCl (•), 1.8 mm CaCl2, 140 mm NMDG (⋄) or 110 mm CaCl2 (□). The NaCl recording is an average of the currents before and after each CaCl2 recording. For comparison, current amplitudes are normalized (Inorm) to the amplitude at −20 mV in the NaCl solution. A, right panel, corresponding whole-cell glutamate-activated currents (glutamate application, horizontal bar, 200 ms) for the GluR-B(Q) current-voltage plot shown in the left panel. All external solutions contained cyclothiazide (CTZ; 15 μm). D, average PCa/PNa (±2 s.e.m.), derived from changes in reversal potentials, such as those shown in A–C, for various Ca2+ concentrations. The continuous lines are the average PCa/PNa for all concentrations tested. These average PCa/PNa values are: 1.72, GluR-B(Q); 0.31, B(Q)(N619C); and 0.07, B(Q)(N619K). A minimum of 4 cells was recorded at each concentration. E, current-voltage relationships for cells expressing GluR-B(Q), B(Q)(N619C) or B(N)(N619K) subunits. For comparison, current amplitudes are normalized to that at −80 mV. Cells were bathed in 5 mm CaCl2 and140 mm NaCl.
Gating kinetics
To determine basic gating characteristics, we recorded currents at −60 mV in outside-out patches isolated from HEK 293 cells in the absence of CTZ. To derive the time constant of desensitization (τdes), we fitted a single exponential to the current decay during a 100 ms glutamate application. The extent of desensitization, represented as a percentage (% des), was derived from the ratio of steady-state (Is) to peak (IP) current amplitudes during the 100 ms application (% des = 100(1 - IS/IP)). To derive the time constant of deactivation (τdeact), we fitted a single exponential to the current decay following a 1 ms glutamate application.
Glutamate concentration dependence
The functional concentration- response curve was measured in the whole-cell mode and in the presence of CTZ. Cells were held at −60 mV and solutions containing various concentrations of glutamate (0.01–5 mm) were applied with current amplitudes normalized to that in 5 mm glutamate, plotted as a function of concentration and fitted with the Hill equation 1/(1 + (EC50/[Glu])nH) where EC50 the concentration to achieve half-maximal response and nH the Hill coefficient.
Data analysis
All curve fitting was done using Igor Pro (WaveMetrics, Inc., Lake Oswego, OR, USA). Results are reported as means ±s.e.m. and shown graphically as means ± 2 s.e.m. An analysis of variance was used to test for statistical differences with the Tukey test used for multiple comparisons. Significance was assumed if P < 0.05.
RESULTS
The mechanism of Ca2+ influx appears to be comparable across all Ca2+-permeable AMPAR channels (e.g. Jatzke et al. 2002). As a background for many of our experiments, we therefore typically used the unedited form of GluR-B, where a glutamine occupies the Q/R site (GluR-B(Q)) since other substitutions of the Q/R site already exist for this subunit (e.g. GluR-B(N)). We initially examined the contribution of the conserved asparagine to the process of Ca2+ influx in AMPAR channels.
GluR-B(Q) channels containing substitutions of the conserved N in the M3 segment alter Ca2+ permeability
Figure 2A–C shows current-voltage relations for cells expressing wild type (B(Q)) or mutant GluR-B(Q) channels containing either cysteine (B(Q)(N619C)) or the positively charged lysine (B(Q)(N619K)) substituted for the conserved asparagine in the M3 segment. (Cells transfected with the alanine substitution of this position did not yield detectable glutamate-activated currents.) Cells were bathed either in the Na+ reference solution (filled circles) or in solutions where Na+ was replaced by different concentrations of Ca2+, 1.8 mm (open diamonds) or 110 mm (open squares). For all channels, currents reversed near −5 mV in the Na+ solution. For wild type when Na+ was replaced by Ca2+, the reversal potential was shifted along the axis by 19.2 ± 0.7 mV (mean ±s.e.m.) (n = 6) in 110 mm Ca2+ and −59.0 ± 0.6 mV (n = 7) in 1.8 mm Ca2+. For the mutant channels, the reversal potential shifts differed significantly from those in wild type. Indeed, for B(Q)(N619C) and B(Q)(N619K) channels, the shifts were −15.3 ± 2.6 mV (n = 4) and −45.5 ± 1.0 mV (n = 4), respectively, in 110 mm Ca2+ and −84.5 ± 0.8 mV (n = 3) and −95.9 ± 0.9 mV (n = 5), respectively, in 1.8 mm Ca2+.
We used these shifts in reversal potentials to derive average Ca2+ permeability ratios (PCa/PNa; Fig. 2D). For wild type, PCa/PNa was essentially concentration independent with an average PCa/PNa of around 1.72. For both mutant channels, PCa/PNa was again essentially concentration independent, but was greatly reduced in magnitude to around 0.31 for B(Q)(N619C) and 0.07 for B(Q)(N619K). Hence, substitutions of the conserved asparagine strongly attenuate Ca2+ influx as assayed by reversal potentials.
Substitutions of the conserved N could indirectly affect Ca2+ permeation by disrupting pore structure, including that of the M2 loop where the Q/R site is located. To test this idea, we characterized the rectifying current-voltage relation in GluR-B(Q), B(Q)(N619C) and B(Q)(N619K) channels as an index of the block by intracellular polyamines (Bowie & Mayer, 1995; Koh et al. 1995). Figure 2E shows that the normalized current-voltage relations in these channels are highly comparable. For GluR-B(Q), the rectification ratio (see Methods) was 50 ± 10 (n = 5). For B(Q)(N619C) and B(N)(N619K), the ratios were 39 ± 7 (n = 4) and 62 ± 11 (n = 3), values not significantly different from those in wild type. Since the cysteine substitution (B(Q)(N619C)) is critical to our conclusion, we also compared its gating properties with those of wild type to verify that its effects on Ca2+ permeation are not due to changes in gating. As summarized in Table 1, none of the measured parameters including the rate (τdes) and extent (% des) of desensitization, the rate of deactivation (τdeact) and the glutamate concentration for half-maximal activation (EC50) were significantly different for B(Q) and B(Q)(N619C) channels. Hence, although we cannot completely rule out alternative functions, this lack of an effect on other channel properties suggests that substitutions of the conserved N are directly affecting Ca2+ influx.
Table 1.
Comparison of gating properties of wild type and mutant GluR-B (Q) channels
| Subunit | τdes | % des | τdeact | EC50 |
|---|---|---|---|---|
| combination | (ms) | (ms) | (μm) | |
| GluR-B(Q) | 5.8 ± 0.5 (6) | 96 ± 0.8 (7) | 0.6 ± 0.1 (3) | 110 ± 10 |
| B(Q)(N619C) | 5.8 ± 0.6 (3) | 97 ± 0.5 (3) | 0.7 ± 0.1 (3) | 120 ± 20 |
Values shown as means ±s.e.m. (number of recordings). For the EC50 measurements, at least three cells were recorded at each concentration. The external solution contained 1.8 mm CaCl2 and 140 mm NaCl. τdes, time constant of desensitization; % des, extent (% des) of desensitization; τdeact, time constant of deactivation.
A polar threonine (T) residue is present in the highly conserved SYTANLAAF motif and is exposed to the water interface in the NMDAR NR1 subunit (Beck et al. 1999). GluR-B(Q) channels containing an alanine (A) but not a lysine (K) substitution of this position were functional (data not shown). Still, in these channels, B(Q)(T617A), Ca2+ permeability measured using reversal potentials in 1.8 mm Ca2+ (1.5 ± 0.1, n = 4) was comparable to that in wild type (∼1.70) (for B(Q)(N619C) the comparable value was ∼0.35). We did not explore this position or other polar residues in M3 further, but this result suggests that the contribution of the conserved N is specific, possibly because of its structural location relative to the central axis of the pore.
Substitutions of the conserved N in the M3 segment alter Ca2+ influx under physiological conditions
An alternative means to quantify Ca2+ influx in channels with both a monovalent and Ca2+ permeability is the use of dye overload to measure the fraction of the total current carried by Ca2+ (Neher, 1995). Relative to measuring Ca2+ permeability using reversal potentials, measuring fractional Ca2+ currents (Pf) is advantageous, since it directly quantifies Ca2+ influx under physiological conditions, is model independent and can be used to characterize Ca2+ influx over a wide voltage range.
Figure 3A illustrates the dye overload approach to measuring the fraction of the total current carried by Ca2+. The upper panel shows whole-cell glutamate activated currents in HEK 293 cells expressing, from left to right, GluR-B(Q), B(Q)(N619C) or B(Q)(N619K). In all examples, the external solution contained 1.8 mm Ca2+ in 140 mm NaCl. The application of glutamate (horizontal bar) generates an inward current. It also generates a decrement in the fluorescence signal at 380 nm excitation (F380). With dye overload, changes in the fluorescence signal (ΔF380) are proportional to the total Ca2+ influx (QCa), with the proportionality constant defined by fmax according to the relationship QCa=ICadt =ΔF380/fmax. Fractional Ca2+ currents were derived from the relationship Pf (%) =QCa/QT× 100. QT, the total charge during the defined time interval, was derived from the current integral (shaded area in the current plot) and is about of equal magnitude for all three records allowing one to directly compare the Ca2+ (F380) signal. The magnitude of ΔF380 is greatly attenuated in the mutant channels compared with wild type. As summarized in Fig. 3B, mean Pf measurements in B(Q)(N619C) (1.3 ± 0.1 %, n = 7) and B(Q)(N619K) (0.3 ± 0.1 %, n = 5) were significantly less than those in wild type (4.0 ± 0.3 %, n = 6) channels. A similar attenuation in Pf measurements occurs in 20 mm Ca2+ (See Fig. 4A). Thus, the conserved N, like that in NMDAR channels, contributes to the process of Ca2+ influx under physiological conditions.
Figure 3. Effect of substitutions of the conserved N in GluR-B(Q) channels on fractional Ca2+ currents.
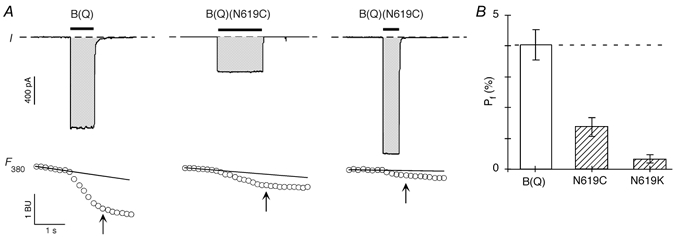
A, simultaneous measurement of whole-cell currents (I; upper panels) and fluorescence intensity with 380 nm excitation (F380; lower panels) evoked by glutamate applications (horizontal bars) in HEK 293 cells expressing wild type GluR-B(Q) (left panel), GluR-B(Q)(N619C) (middle panel) or GluR-B(Q)(N619K) (right panel) subunits. The potential (V) was −60 mV to the reversal potential (see Methods). In the current records (upper panels), the dashed lines represent ‘0′ current, and the shaded regions correspond to the current integral (QT), which was approximately the same for each of the example records. The F380 plot is expressed in bead units (BU). We derived ΔF380 as the difference between the F380 amplitude at the indicated time (arrow) and the baseline F380 signal (horizontal line), extrapolated from a linear fit to the F380 amplitudes prior to the glutamate application. Cells were bathed in 1.8 mm CaCl2 and 140 mm NaCl. B, mean Pf values measured at −60 mV relative to the reversal potential. The dashed line represents the average Pf value for GluR-B(Q) channels. It also approximately represents the predicted Pf for a channel that is cation non-selective, that is PNa/PK= 1 =PCa/PNa (see Jatzke et al. 2002). From left to right n = 6, 6 and 5.
Figure 4. Voltage and concentration dependence of Ca2+ permeability in GluR-B(Q) and GluR-B(Q)(N619C) channels.
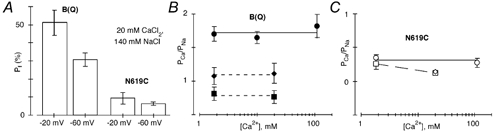
A, mean Pf values measured at −20 mV (left bars) or −60 mV (right bars) in cells expressing GluR-B(Q) or B(Q)(N619C) subunits. Cells were bathed in 20 mm CaCl2 and 140 mm NaCl. From left to right n = 7, 5, 6 and 7. B, comparison of PCa/PNa derived either from changes in reversal potential (•) or from Pf measurements at −20 mm (♦) or −60 mV (▪). Recordings made from GluR-B(Q) channels. The continuous line represents the average PCa/PNa (1.72) derived from changes in the reversal potential (Fig. 2D). The dashed lines show the average PCa/PNa derived from Pf measurements in 1.8 and 20 mm Ca2+ at −20 mV (1.1) or −60 mV (0.76). C, recordings made from B(Q)(N619C) channels. The continuous line shows the average PCa/PNa (0.31) derived from changes in the reversal potential (Fig. 2D). The dashed line has no theoretical meaning. Due to the reduced Ca2+ influx and small current amplitudes, we could not reliably measure Pf values at −20 mV in 1.8 mm Ca2+ in these channels.
In summary, in mutant channels containing substitutions of the conserved N in M3, Ca2+ permeability derived from reversal potentials is considerably less than unity and Pf values are much less than those expected for a channel that is non-selective for Ca2+ (∼4 %). These results, therefore, demonstrate that the conserved N in M3 represents a critical determinant of Ca2+ influx in Ca2+-permeable AMPAR channels.
The conserved N contributes to the deviation from GHK in AMPAR channels
Changes in reversal potentials and Pf measurements both assay the magnitude of Ca2+ influx. One approach to compare these values directly is to convert Pf measurements to Ca2+ permeability using GHK assumptions (e.g. Schneggenburger et al. 1993; Burnashev et al. 1995; Jatzke et al. 2002). We use GHK here not to test its validity for GluR channels, but rather as a reference point to compare across different experimental approaches and conditions. To convert Pf values to Ca2+ permeability, we follow the approach of Jatzke et al. (2002), yielding, in our ionic conditions, a Ca2+ permeability ratio of PCa/PNa.
Based on a comparison of the voltage and concentration dependence of PCa/PNa derived from changes in reversal potentials or Pf measurements, AMPAR channels including GluR-B(Q) follow and deviate from GHK (see Fig. 8B in Jatzke et al. 2002). Figure 4B summarizes these characteristics for wild type GluR-B(Q) channels. PCa/PNa derived from changes in reversal potentials (filled circles) is concentration independent as are those derived from Pf measurements at −20 mV (filled diamonds) or −60 mV (filled squares). Such concentration-independent PCa/PNa values are expected from GHK. On the other hand, while Pf measurements are intrinsically voltage dependent, when this voltage dependency follows GHK, a single PCa/PNa will describe the values over the entire voltage range. Pf measurements in AMPAR channels show much stronger voltage dependence than expected relative to GHK (Burnashev et al. 1995), with PCa/PNa derived from Pf measurements getting greater in magnitude as one approaches the reversal potential (Jatzke et al. 2002). Accordingly, PCa/PNa values derived from Pf values are around 0.76 at −60 mV and 1.1 at −20 mV (Fig. 4B). Finally, because of this voltage dependence, there is a quantitative difference between PCa/PNa derived from changes in reversal potentials, which is around 1.72, and that derived from Pf measurements.
Figure 4C summarizes the voltage and concentration dependence of PCa/PNa in B(Q)(N619C) channels. As in wild type, PCa/PNa derived from reversal potentials (open circles) was concentration independent, though PCa/PNa derived from Pf measurements at −60 mV (open squares) did show a weak concentration dependence. More distinctive relative to wild type was that PCa/PNa derived from Pf measurements at −60 and in 1.8 mm Ca2+ (0.26 ± 0.03) was comparable to PCa/PNa derived from changes in reversal potentials (∼0.31). In addition, PCa/PNa derived from Pf measurements in 20 mm Ca2+ at - 20 mV (0.14 ± 0.02) was not significantly different from PCa/PNa derived at −60 mV (0.12 ± 0.02). Thus, the conserved N in M3 contributes to the pore properties that underlie the deviation from GHK in AMPAR channels, including the strong voltage dependence of PCa/PNa derived from Pf measurements.
The ER element does not contribute strongly to the mechanism of Ca2+ influx in AMPAR channels
In NMDAR channels, the highly charged DRPEER motif in NR1 strongly influences the magnitude of Ca2+ influx under physiological conditions (Watanabe et al. 2002). In terms of charge, DR in DRPEER is conserved in AMPAR subunits in the form of a negatively charged glutamate (E) and a positively charged arginine (R) (see Fig. 1). To study the contribution of ER to Ca2+ influx as well as its relationship to DR in DRPEER, we generated mutant channels in which these residues were either neutralized (Fig. 5) or replaced with oppositely charged residues (Figs 5 and 6).
Figure 5. The effect of substitutions of two charged residues C-terminal to M3 on Ca2+ influx in AMPAR channels.
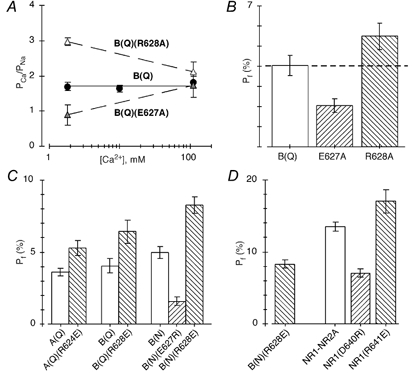
A, average PCa/PNa derived from changes in reversal potentials for various Ca2+ concentrations in cells expressing GluR-B(Q), B(Q)(E627A) or B(Q)(R628A) subunits. The dashed lines have no theoretical significance. B(Q) values are the same as those shown in Fig. 2B. B, mean Pf values measured at −60 mV relative to the reversal potential. Cells were bathed in 1.8 mm CaCl2 and 140 mm NaCl. The dashed line represents the average Pf value for GluR-B(Q) channels. C and D, mean Pf values measured at −60 mV relative to the reversal potential. Cells were bathed in 1.8 mm CaCl2 and 140 mm NaCl. Note the scale change in D with the value for B(N)(R628E) (8.3 ± 0.3 %) shown in both panels. A minimum of 4 recordings was made for each mean value.
Figure 6. Concentration and voltage dependence of Ca2+ permeability in channels containing opposite charge substitutions.
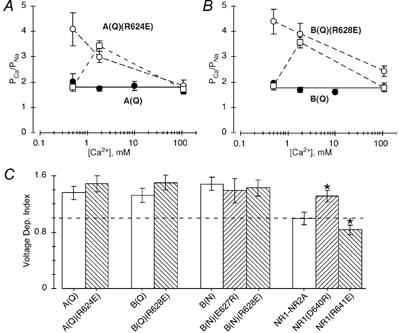
A, average PCa/PNa derived from changes in the reversal potential for various Ca2+ concentrations in cells expressing GluR-A(Q) (•) or A(Q)(R624E) (○) subunits. The continuous line reflects the average PCa/PNa (1.6) in wild type GluR-A(Q) over a concentration range of 0.5 to 110 mm Ca2+. The dashed lines have no theoretical meaning. PCa/PNa for NMDAR NR1-NR2A channels normalized to the value at 110 mm Ca2+ in wild type GluR-A(Q) channels are shown by □. The NMDAR values are from Jatzke et al. (2002) with the actual PCa/PNa values being about 3.8, 7.2 and 3.6, when measured in 0.5, 1.8 or 110 mm Ca2+, respectively. A minimum of four recordings was made at each concentration. B, as in A except subunits are GluR-B(Q) (•) and -B(Q)(R628E) (○). C, voltage dependence of Ca2+ permeability, (PCa/PNa)−20 mV/(PCa/PNa)−60 mV, for various wild type and mutant GluR subunit combinations. PCa/PNa was derived from Pf values measured either at −20 or −60 mV (Table 2). The dashed line (= 1) corresponds to a channel where PCa/PNa shows no voltage dependence. * Values that are significantly different from those for their respective wild type channel.
Figure 5A and B shows that neutralization of either E or R alters Ca2+ influx and in a manner expected from charge neutralization. In particular, PCa/PNa derived from reversal potentials in 1.8 mm Ca2+ (Fig. 5A) was, relative to wild type (∼1.72), either significantly decreased (0.9 ± 0.1, n = 5) in channels where the negative charge was neutralized, B(Q)(E627A), or significantly increased (3.0 ± 0.1, n = 5) in channels where the positive charge was neutralized, B(Q)(R628A). PCa/PNa was not significantly different from that in wild type in 110 mm Ca2+, leading to PCa/PNa being strongly concentration dependent. Paralleling the PCa/PNa values in 1.8 mm Ca2+, Pf measurements at −60 mV and in physiological conditions were significantly decreased in B(Q)(E627A) to 2.1 ± 0.2 % (n = 9) or significantly increased in B(Q)(R628A) to 5.5 ± 0.3 % (n = 8) compared with about 4 % in wild type channels (Fig. 5B).
The effect of charge neutralization of ER on Ca2+ influx is consistent with the idea that these positions, similar to DR in the NR1 NMDAR subunit (Watanabe et al. 2002), are positioned in the ion conduction pathway and exposed to the water interface. However, the significance of ER to the overall mechanism of Ca2+ entry in wild type channels does not seem great, since neutralization of either charge produces about equal and opposite effects. Hence, the E-to-A mutation reduces Pf values by about 1.9 % whereas the R-to-A mutation increases them by about 1.5 %, suggesting that in wild type channels these charges may simply cancel each other out. Consistent with this idea, in a double-mutant channel where both charged residues were inverted (GluR-B(Q)(E627R/R628E)), Ca2+ permeability measured in 1.8 mm Ca2+ was indistinguishable (PCa/PNa= 1.8 ± 0.1, n = 3) from that in wild type (1.7 ± 0.1, n = 7) (data not shown). We will return to this point later, but note here that individual charge substitutions of ER are consistent with this idea that these charges simply cancel each other out (Figs 5C and D and 6).
Opposite charge substitutions of ER in AMPAR channels
To further compare the function of ER in AMPARs to DR and DRPEER in general in NMDARs, we introduced opposite charge substitutions of E and R. The E-to-R substitution in all backgrounds except for GluR-B(N) did not yield glutamate-activated currents. The basis for this lack of functionality is unknown. Nevertheless, in terms of comparing ER to DR, a key experiment is the R-to-E substitution, which produced functional channels in all backgrounds.
Figure 5C summarizes Pf measurements at −60 mV and in 1.8 mm Ca2+ for mutant channels containing opposite charge substitutions of E or R in three different AMPAR backgrounds, GluR-A(Q), GluR-B(Q) and GluR-B(N). In all channels containing the R-to-E substitution, Pf measurements were significantly greater than those in their respective background. In A(Q)(R624E), Pf measurements were 5.3 ± 0.3 % (n = 7) compared with 3.6 ± 0.1 % (n = 9) in GluR-A(Q), whereas in B(Q)(R628E), they were 6.4 ± 0.4 % (n = 5) compared with about 4 % in GluR-B(Q). In GluR-B channels where the glutamine at the Q/R site is replaced by asparagine (N) GluR-B(N), Pf measurements are around 5 % (5.0 ± 0.2, n = 11). In the double-mutant channels, B(N)(R628E), Pf measurements were significantly increased to 8.3 ± 0.3 % (n = 4). The E-to-R substitution produced functional channels only in GluR-B(N), and in these mutant channels, B(N)(E627R), Pf values were significantly reduced to 1.6 ± 0.2 % (n = 4).
In GluR-B(N) channels, the E-to-R substitution decreased Pf measurements by about 3.4 % whereas the R-to-E substitution increased them by 3.3 %. This equal and opposite effect of these charge reversals is similar to that observed for charge neutralization in GluR-B(Q) (see Fig. 2D and Fig. 3B), and supports the idea that in AMPAR channels these charges make little contribution to Ca2+ influx under physiological conditions. To test the comparable functional significance of DR in DRPEER, we measured Pf values for wild type and mutant NMDAR channels containing opposite charge substitutions of DR in the NR1 subunit (Fig. 5D). For wild type channels, Pf values were around 13.5 % (13.5 ± 0.3 %, n = 13). Consistent with previous results (Watanabe et al. 2002), Pf measurements were significantly reduced in NR1(D640R) channels to 7.0 ± 0.3 % (n = 12) and significantly increased in NR1(R641E) to 17.1 ± 0.8 % (n = 12). Nevertheless, D640R reduced Pf values by about 6.4 % whereas R641E increased them by only 3.6 %, suggesting that these charges are not equivalent in NMDAR channels. Thus, DR yields a net negativity that is of importance to Ca2+ influx in contrast to ER in AMPAR channels.
The R-to-E substitution in AMPAR channels increases Ca2+ influx via a different mechanism than DRPEER in NMDAR channels
In AMPAR channels, the R-to-E substitution increases Pf values for all three backgrounds tested (Fig. 5C). Presumably this effect arises because this substitution generates a high degree of negativity, C-terminal to M3, comparable with DRPEER in NMDARs. However, the results shown in Fig. 6 indicate that, while this R-to-E substitution does increase Ca2+ influx, it does so by a mechanism distinct from that produced by DRPEER.
Figure 6A and B shows PCa/PNa measured using changes in reversal potentials over a wide concentration range in AMPAR channels containing the R-to-E substitution. In both A(Q)(R624E) (Fig. 6A) and B(Q)(R628E) (Fig. 6B) channels (open circles) and in contrast to their respective wild type backgrounds (filled circles), PCa/PNa was strongly concentration dependent, getting significantly greater in magnitude at lower Ca2+ concentrations. Such a pattern of PCa/PNa is indicative of surface charges suggesting that the effect of the R-to-E substitution is to increase the concentration of Ca2+ at the surface of the channel relative to the bulk concentration. In contrast, surface charges do not contribute significantly to ion permeation in NMDAR channels (Zarei & Dani, 1994) as is illustrated by the concentration dependence of PCa/PNa in these channels (Wollmuth & Sakmann, 1998). To compare the overall concentration dependence of PCa/PNa in GluR subtypes, we normalized the PCa/PNa values in NMDAR channels (Fig. 6A and B, open squares) to that measured in 110 mm Ca2+ in AMPAR channels. Like mutant AMPAR channels containing the R-to-E substitution, PCa/PNa increases in 110 to 1.8 mm Ca2+. However, they diverge at 0.5 mm Ca2+ where PCa/PNa continues to increase in mutant AMPAR channels but declines in NMDAR channels. Although the basis for this concentration dependence of PCa/PNa at low concentrations in NMDAR channels is unknown, it is associated with DRPEER (data not shown; Watanabe et al. 2002) and is incompatible with surface charges.
PCa/PNa derived from Pf measurements is strongly voltage dependent in wild type AMPAR channels (e.g. Fig. 4B). To characterize this voltage dependence, we divided the average PCa/PNa derived from the Pf at −20 mV by that derived at −60 mV ((PCa/PNa)−20 mV/(PCa/PNa)−60 mV). As shown in Fig. 6C, this voltage-dependent index was around 1.4 for all wild type AMPAR channels, and was not significantly changed in any of the mutant channels containing charge substitutions of ER as well as GluR-B(N) channels. On the other hand, PCa/PNa derived from Pf measurements are voltage independent in wild type NMDAR channels, as shown by the voltage-dependent index of 1 (Fig. 6C). In addition, opposite charge substitutions of DR in NR1 significantly alters this voltage dependence, either increasing it (NR1(D640R)) or decreasing it (NR1(R641E)). Hence, the R-to-E substitution in AMPAR subunits, while increasing Ca2+ influx, does not shift the voltage dependence of Pf measurements towards that found in NMDAR channels. In addition, DR in NMDAR NR1 but not ER in AMPARs is positioned in the pore such that it can influence the voltage dependence of Pf values. As proposed in Discussion, this difference, as well as the surface charge effect in mutant AMPAR channels, may reflect that ER in AMPARs is positioned more externally relative to the tip of the M2 loop than DR in DRPEER in NMDAR channels.
Negative charges C-terminal to M3 in the KAR GluR-6 subunit do not contribute to Ca2+ permeability
C-terminal to M3 in KAR subunits, ER is present as it is in AMPAR subunits but there is an additional negative charge, specifically a glutamate (E) at position 634 (Fig. 1). Hence, KAR subunits have a potential high degree of negativity C-terminal to M3, similar to NMDAR channels, yet in contrast are poorly Ca2+ permeable (Burnashev et al. 1995; Jatzke et al. 2002). To test the functional significance of these negative charges to Ca2+ influx in KAR subunits, we substituted them with the oppositely charged arginine (R) (Fig. 7).
Figure 7. The effect of opposite charge substitutions of negative charges located C-terminal to M3 in KAR subunits on Ca2+ permeability.
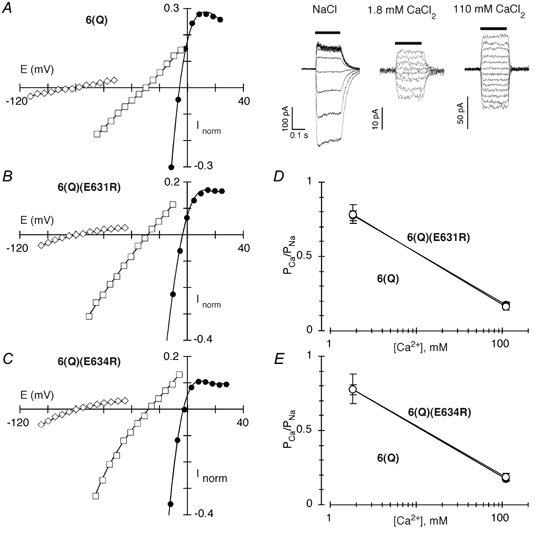
A–C, peak glutamate-activated currents at different membrane potentials, in 2–5 mV increments, in cells expressing GluR-6(Q) (A), GluR-6(Q)(E631R) (B) or GluR-6(Q)(E634R) (C) subunits. Recordings were made and displayed as in Fig. 2, but because of the smaller relative current amplitudes in the Ca2+-containing solutions, a more restricted range of the display is shown. A, right, corresponding whole-cell glutamate-activated currents (glutamate application, horizontal bar, 200 ms) for the GluR-6(Q) current-voltage plot shown to the left. Concanavalin A (0.3 mg ml−1) was included in the reference solution. D and E, average PCa/PNa derived from changes in the reversal potential for various Ca2+ concentrations in cells expressing wild type GluR-6(Q) (filled symbols) or 6(Q)(E631R) (D) or 6(Q)(E634R) (E) (open symbols). The lines have no theoretical meaning. A minimum of 4 recordings was made at each concentration.
Figure 7A–C shows current-voltage relations for cells expressing wild type (6(Q)) or mutant GluR-6(Q) channels containing opposite charge substitutions of either position 631 (6(Q)(E631R)) or 634 (6(Q)(E634R)). In the Na+ reference solution (filled circles) currents reversed in all channels near −5 mV. For wild type when Na+ was replaced by Ca2+, the reversal potential was shifted along the axis by −22.7 ± 1.0 mV (n = 5) in 110 mm Ca2+ and −75.4 ± 0.4 mV (n = 6) in 1.8 mm Ca2+. The reversal potential shifts for the mutant channels did not differ significantly from those in wild type. For 6(Q)(E631R) and 6(Q)(E634R), the shifts were −25.4 ± 1.1 mV (n = 5) and −23.9 ± 1.7 mV (n = 4), respectively, in 110 mm Ca2+, and −76.5 ± 0.7 mV (n = 3) and −76.8 ± 1.3 mV (n = 3) in 1.8 mm Ca2+. Correspondingly, Ca2+ permeability in channels containing these opposite charge substitutions (open circles in Fig. 7D and E), was indistinguishable from that in wild type (filled circles) at both concentrations tested. This lack of an effect of the opposite charge substitution on PCa/PNa is in direct contrast to what is observed for AMPAR channels even for the more subtle charge neutralization (Fig. 5A). Thus, these negative charges in KAR subunits do not contribute to Ca2+ influx, presumably because they are either not exposed to the water interface or they are positioned so externally that they have no effect on ion permeation. In either case, this region in KAR channels shows a distinct structural difference from that in NMDAR and AMPAR channels.
DISCUSSION
Extracellular vestibule determinants of Ca2+ influx in AMPAR channels
In GluRs, the M2 loop covers the approximate internal half of the ion pore (Kuner et al. 1999). The Q/R site, located at the tip of the M2 loop (Kuner et al. 2001), represents a key determinant of the Ca2+ permeability properties of AMPAR channels. Although structural determinants of the extracellular vestibule in non-NMDAR channels are unknown, it is presumably formed, in homology to NMDARs, by multiple transmembrane segments including M1, M3 and M4 (Beck et al. 1999). Still, in NMDAR channels, the M3 segment forms the central part of the channel leading to the tip of the M2 loop (Sobolevsky et al. 2002a), and only substitutions of polar or charged residues within M3 and regions C-terminal to it alter Ca2+ influx (Watanabe et al. 2002). Therefore, to identify possible determinants of Ca2+ influx in Ca2+-permeable AMPAR channels, we focused on M3 and regions C-terminal to it (Fig. 1). Several candidate positions were identified including polar residues in M3 and charged ones C-terminal to M3.
Our results demonstrate that a conserved asparagine (N) in M3 and specifically within the highly conserved SYTANLAAF motif contributes to the mechanism of Ca2+ influx in Ca2+-permeable AMPAR channels (Figs 2–4). Indeed, substitutions of this position strongly attenuated Ca2+ permeability measured using reversal potentials and fractional Ca2+ currents (Pf) measured under physiological conditions. This contribution appears specific since these same mutations do not alter polyamine block (Fig. 2E), suggesting that the general structure of the M2 loop including that of the Q/R site is undisturbed. Thus, in Ca2+-permeable AMPAR channels, the process of Ca2+ influx involves multiple sites, including (at minimum) the Q/R site and the conserved N in M3.
The M3 segment in GluRs is an important determinant of channel gating (Kohda et al. 2000; Sobolevsky et al. 2002a; Jones et al. 2002). AMPAR channels also show subconductance states (e.g. Rosenmund et al. 1998), some of which might have different permeation properties. Substitutions of the conserved N could therefore disrupt permeation processes indirectly, by altering the distribution of AMPAR subconductance states and/or by introducing subconductance states with different permeation properties (Schneggenburger & Ascher, 1997). Although we cannot completely rule out effects on channel gating, we believe that substitutions of the conserved N are, at minimum, directly affecting Ca2+ permeation. First, the cysteine substitution had no apparent affect on channel gating (Table 1). Second, the magnitude of the effect was consistent with the substitution and both indices of Ca2+ influx (Ca2+ permeability measured using reversal potentials and Pf measurements) gave the same general answer. Finally, current amplitudes were uniform and showed a single reversal potential for all mutant channels measured in the present study, in contrast to what is observed for mutant NMDAR channels which show subconductance states with different permeation properties (Schneggenburger & Ascher, 1997).
Other channels that have mixed monovalent/Ca2+ permeability, such as NMDAR (Watanabe et al. 2002) and neuronal nicotinic AChR (Bertrand et al. 1993) channels, also contain multiple structural elements involved in Ca2+ permeability that are distributed throughout the pore. This contrasts to voltage-gated Ca2+ channels where the high Ca2+ selectivity - Ca2+ is the only permeant ion in these channels under physiological conditions - is restricted to a single locus (Ellinor et al. 1995). It is unknown how these different structural elements contribute to the overall process of Ca2+ influx in channels with mixed monovalent/Ca2+ permeability. Nevertheless, in GluR-B(Q) channels containing substitutions of the conserved N, Ca2+ permeability derived from either reversal potentials (Fig. 2) or Pf measurements (Figs 3 and 4) is significantly less than unity. Hence, these channels now select against Ca2+ relative to monovalent ions, suggesting that simple models where a single permeation barrier defines all features of monovalent and Ca2+ selectivity may be inappropriate.
Deviation of Ca2+ permeability from GHK in AMPAR channels is mediated by the extracellular vestibule
Pf values are inherently voltage dependent. However, if this voltage dependence follows GHK, then a single PCa/PNa will describe the Pf values over the entire voltage range. Pf values in AMPAR channels show a much stronger voltage dependence than expected relative to GHK (Burnashev et al. 1995), with PCa/PNa derived from Pf values getting greater in magnitude as one approaches the reversal potential (Jatzke et al. 2002) (see Fig. 4B, left panel). Due to this voltage dependence, PCa/PNa derived from reversal potentials and Pf values in AMPAR channels show a strong quantitative difference. Many of these deviations from GHK are greatly attenuated in channels containing substitutions of the conserved N (Fig. 4C). On the other hand, the asparagine substitution of the Q/R site does not alter the deviation from GHK (Jatzke et al. 2002) nor do ER substitutions (Fig. 6C; Table 2). Hence, the deviations from GHK found in AMPAR channels appear to be due primarily to energetic features of the extracellular vestibule as defined by the M3 segment.
Table 2.
Fractional Ca2+ currents (Pf) and PCa/PNa derived from Pf measurements in wild type and mutant GluR channels
| Subunit combination | V | Pf | PCa/PNa | n | ΔPf |
|---|---|---|---|---|---|
| (mV) | (%) | ||||
| GluR-A(Q) | −20 | 6.2 ± 0.5 | 0.95 ± 0.06 | 8 | |
| −60 | 3.6 ± 0.1 | 0.70 ± 0.03 | 9 | ||
| A(Q)(R624E) | −20 | 10.2 ± 0.8 | 1.62 ± 0.10 | 5 | — |
| −60 | 5.3 ± 0.3 | 1.09 ± 0.05 | 7 | — | |
| GluR-B(Q) | −20 | 6.9 ± 0.4 | 1.07 ± 0.06 | 6 | |
| −60 | 4.0 ± 0.3 | 0.81 ± 0.05 | 6 | ||
| B(Q)(R628E) | −20 | 12.2 ± 1.2 | 2.00 ± 0.07 | 5 | — |
| −60 | 6.4 ± 0.4 | 1.33 ± 0.06 | 5 | — | |
| GluR-B(N) | −20 | 9.5 ± 0.4 | 1.50 ± 0.07 | 11 | 0 |
| −60 | 5.0 ± 0.2 | 1.01 ± 0.04 | 11 | 0 | |
| B(N)(E627R) | −20 | 2.9 ± 0.3 | 0.43 ± 0.05 | 4 | −6.6 |
| −60 | 1.6 ± 0.2 | 0.31 ± 0.04 | 4 | −3.4 | |
| B(N)(R628E) | −20 | 15.1 ± 1.5 | 2.50 ± 0.13 | 4 | 5.6 |
| −60 | 8.3 ± 0.3 | 1.75 ± 0.06 | 4 | 3.3 | |
| NR1-NR2A | −20 | 17.3 ± 0.7 | 3.81 ± 0.12 | 13 | 0 |
| −60 | 13.5 ± 0.3 | 3.84 ± 0.09 | 13 | 0 | |
| NR1(D64OR)-NR2A | − 20 | 11.8 ± 0.6 | 2.47 ± 0.08 | 5 | −5.5 |
| −60 | 7.0 ± 0.3 | 1.88 ± 0.05 | 12 | −6.5 | |
| NR1(R641E)-NR2A | −20 | 18.9 ± 1.2 | 4.20 ± 0.13 | 6 | 1.6 |
| −60 | 17.1 ± 0.8 | 5.06 ± 0.10 | 12 | 3.6 |
Values are shown as means ±s.e.m. The external solution contained 1.8 mM CaCl2 and 140 mM NaCl. Pf measurements were converted to PCa/PNa using GHK (see Jatzke et al. 2002). ΔPf is the difference of the mean Pf values between the mutant channel and its respective background and are shown only for those channels where both E (or D) and R substitutions formed functional channels.
Subtype-specific contribution of charged residues C-terminal to M3 to Ca2+ influx
The DRPEER motif, C-terminal to M3 in the NMDAR NR1 subunit, is a key determinant of the high Ca2+ influx mediated by these channels (Watanabe et al. 2002). Our results clarify how this motif contributes to the much greater Ca2+ influx in NMDAR channels relative to non-NMDAR subtypes. In particular, of all negative charges located C-terminal to M3, only DRPEER generates a large negativity that can influence Ca2+ influx under physiological conditions. In part, this net negativity arises because of the excess of exposed negatively charged residues. All three negative charges (D, E and E) are exposed whereas only the first positively charged arginine is exposed (Watanabe et al. 2002). KAR subunits also have a net negativity in this region (Fig. 1), but none of these negatively charged residues contribute to Ca2+ influx (Fig. 7). This could reflect a local structural difference - these positions, in contrast to homologous ones in NR1, may not be exposed to the water interface - or, alternatively, there may be a more global structural difference between subunits (see below).
Although the additional negative charges in DRPEER are important to its function, even the negativity provided by D relative to R is significant. In particular, the D-to-R substitution reduces Pf values by about 6.5 %, considerably more than the 3.6 % increase produced by the R-to-E substitution (Fig. 5D). (An analysis of the alanine substitutions of these positions (Watanabe et al. 2002) yields a comparable disparity.) Further, because these substitutions alter the voltage dependence of Pf values, this asymmetry is even more dramatic at −20 mV (Table 2). On the other hand, in AMPAR channels containing substitutions of residues in ER, either neutralizing them or reversing the charge (Fig. 5), Ca2+ influx was also altered relative to wild type. Nevertheless, these substitutions have about equal and opposite effects on Ca2+ influx measured using reversal potentials and Pf measurements. In GluR-B(Q) channels, for example, the E-to-A substitution reduces Pf values by about 1.9 %, whereas the R-to-A substitution increases them by about 1.5 % (Fig. 3B). Similarly, in GluR-B(N) channels, the E-to-R substitution decreases Pf values by about 3.4 %, whereas the R-to-E substitution increases them by 3.3 % (Fig. 5C). In addition, because these substitutions do not alter the voltage dependence of Pf values, this equal and opposite effect occurs over the entire voltage range (Table 2). Thus, ER in AMPARs, in contrast to DR in NR1, makes little or no contribution to the mechanism of Ca2+ influx.
Structural asymmetry between GluR subunits
The mechanism of Ca2+ influx in Ca2+-permeable GluR channels, including NMDAR channels, remains unknown. Clearly, the negativity provided by DRPEER is critical to the overall process as well as to the distinction between subtypes (Ca2+-permeable AMPAR vs. NMDAR). However, our results suggest that an additional difference between subtypes must also exist. Consider mutant AMPAR channels containing the R-to-E substitution, including the double-mutant channels GluR-B(N)(R624E) (Fig. 5). In these channels, there is an increase in negativity C-terminal to M3, similar to DRPEER in NR1, leading to an increase in Ca2+ influx. However, the mechanism by which this increase occurs appears to be fundamentally different from that produced by DRPEER. Specifically, in AMPAR R-to-E mutant channels, this additional negativity acts as a surface charge (Fig. 6A and B). In contrast, surface charges do not make a significant contribution to ion permeation in NMDAR channels (Zarei & Dani, 1994), as illustrated by the concentration dependence of Ca2+ permeability in wild type NMDAR channels (Fig. 6A and B). Further, in R-to-E mutant channels, PCa/PNa derived from Pf values remains voltage dependent rather than becoming voltage independent as in NMDAR channels (Fig. 6C). Finally, substitutions of DR in NMDAR but not ER in AMPAR channels alter the voltage dependence of Pf values (Fig. 6C).
What is the structural basis for this difference between subtypes? In part it could reflect a local structural difference, that is, negative charges may be exposed in some instances (e.g. DRPEER in NR1) whereas in other subunits (e.g. KAR and NR2) they are not. However, this explanation cannot account for the results delineated above for the R-to-E mutant channels. An alternative explanation and one consistent with the above results (though not proved by them) is that there is a global structural difference between subunits. In a comparison of NMDAR subunits, we found that relative to the tip of the M2 loop the M3 segments are staggered relative to each other, with positions in the NR2C M3 located about four amino acids more externally than homologous ones in NR1 (Sobolevsky et al. 2002b). This asymmetry may account for the fact that a cluster of three negative charges C-terminal to M3 in NR2A - occupying positions homologous to DRPEER (see Fig. 1) - makes no contribution to Ca2+ influx (Watanabe et al. 2002). Hence, although DRPEER is located externally, it is positioned closer to the tip of the M2 loop than homologous residues in NR2. The more external positioning of charged residues C-terminal to M3 in NR2 subunits - and presumably also in AMPAR and KAR subunits - is sufficient to remove them from significantly influencing Ca2+ influx. Nevertheless, the overall positioning of subunits in NMDAR, AMPAR and KAR channels is unknown. Clarifying this issue will help define basic channel properties such as ion permeation and block.
Acknowledgments
We thank Drs A. Sobolevsky, M. Yelshanksy and J. Watanabe for their comments on the manuscript, and L. Rooney, M. Yelshanksy, W. Raab and S. Masterson for technical assistance. M. Hernandez was a Howard Hughes Medical Institute (HHMI Grant no. 52003052) Undergraduate Summer Research Scholar. This work was supported by NIH RO1 grant NS39102 and a Sinsheimer Scholars Award (L.P.W.).
REFERENCES
- Beck C, Wollmuth LP, Seeburg PH, Sakmann B, Kuner T. NMDAR channel segments forming the extracellular vestibule inferred from the accessibility of substituted cysteines. Neuron. 1999;22:559–570. doi: 10.1016/s0896-6273(00)80710-2. [DOI] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiery A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc Natl Acad Sci U S A. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Burnashev N. Calcium permeability of glutamate-gated channels in the central nervous system. Curr Opin Neurobiol. 1996;6:311–317. doi: 10.1016/s0959-4388(96)80113-9. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Villarroel A, Sakmann B. Dimensions and ion selectivity of recombinant AMPA and kainate receptor channels and their dependence on Q/R site residues. J Physiol. 1996;496:165–173. doi: 10.1113/jphysiol.1996.sp021674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol. 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Ellinor PT, Yang J, Sather WA, Zhang JF, Tsien RW. Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron. 1995;15:1121–1132. doi: 10.1016/0896-6273(95)90100-0. [DOI] [PubMed] [Google Scholar]
- Gu JG, Albuquerque C, Lee CJ, MacDermott AB. Synaptic strengthening through activation of Ca2+-permeable AMPA receptors. Nature. 1996;381:793–796. doi: 10.1038/381793a0. [DOI] [PubMed] [Google Scholar]
- Iino M, Goto K, Kakegawa W, Okado H, Sudo M, Ishiuchi S, Miwa A, Takayasu Y, Saito I, Tsuzuki K, Ozawa S. Glia-synapse interaction through Ca2+-permeable AMPA receptors in Bergmann glia. Science. 2001;292:926–929. doi: 10.1126/science.1058827. [DOI] [PubMed] [Google Scholar]
- Jatzke C, Watanabe J, Wollmuth LP. Voltage and concentration dependence of Ca2+ permeability in recombinant glutamate receptor subtypes. J Physiol. 2002;538:25–39. doi: 10.1113/jphysiol.2001.012897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KS, Van Dongen HM, Van Dongen AM. The NMDA receptor M3 segment is a conserved transduction element coupling ligand binding to channel opening. J Neurosci. 2002;22:2044–2053. doi: 10.1523/JNEUROSCI.22-06-02044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D-S, Burnashev N, Jonas P. Block of native Ca2+-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol. 1995;486:305–312. doi: 10.1113/jphysiol.1995.sp020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohda K, Wang Y, Yuzaki M. Mutation of a glutamate receptor motif reveals its role in gating and d2 receptor channel properties. Nat Neurosci. 2000;3:315–322. doi: 10.1038/73877. [DOI] [PubMed] [Google Scholar]
- Köhler M, Burnashev N, Sakmann B, Seeburg PH. Determinants of Ca2+permeability in both TM1 and TM2 of high-affinity kainate receptor channels: diversity by RNA editing. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- Kuner T, Beck C, Sakmann B, Seeburg PH. Channel-lining residues of the AMPA receptor M2 segment: structural environment of the Q/R site and identification of the selectivity filter. J Neurosci. 2001;21:4162–4172. doi: 10.1523/JNEUROSCI.21-12-04162.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner T, Wollmuth LP, Sakmann B. The ion-conducting pore of glutamate receptor channels. In: Jonas P, Monyer H, editors. Ionotropic Glutamate Receptors in the CNS. Berlin: Springer-Verlag; 1999. pp. 219–249. [Google Scholar]
- Liu SQ, Cull-Candy SG. Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature. 2000;405:454–458. doi: 10.1038/35013064. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Neher E. The use of fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology. 1995;34:1423–1442. doi: 10.1016/0028-3908(95)00144-u. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Ascher P. Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron. 1997;18:167–177. doi: 10.1016/s0896-6273(01)80055-6. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Zhou Z, Konnerth A, Neher E. Fractional contribution of calcium to the cation current through glutamate receptor channels. Neuron. 1993;11:133–143. doi: 10.1016/0896-6273(93)90277-x. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Seeburg PH, Higuchi M, Sprengel R. RNA editing of brain glutamate receptor channels: mechanism and physiology. Brain Res Brain Res Rev. 1998;26:217–229. doi: 10.1016/s0165-0173(97)00062-3. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Beck C, Wollmuth LP. Molecular rearrangements of the extracellular vestibule in NMDAR channels during gating. Neuron. 2002a;33:75–85. doi: 10.1016/s0896-6273(01)00560-8. [DOI] [PubMed] [Google Scholar]
- Sobolevsky AI, Rooney L, Wollmuth LP. Staggering of Subunits in NMDAR Channels. Biophys J. 2002b;83:3304–3314. doi: 10.1016/S0006-3495(02)75331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J, Beck C, Kuner T, Premkumar L, Wollmuth LP. DRPEER: A motif in the extracellular vestibule conferring high Ca2+ flux rates in NMDA receptor channels. J Neurosci. 2002;22:10209–10216. doi: 10.1523/JNEUROSCI.22-23-10209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmuth LP, Sakmann B. Different mechanisms of Ca2+ transport in NMDA and Ca2+-permeable AMPA glutamate receptor channels. J Gen Physiol. 1998;112:623–636. doi: 10.1085/jgp.112.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarei MM, Dani JA. Ionic permeability characteristics of the N-methyl-d-aspartate receptor channel. J Gen Physiol. 1994;103:231–248. doi: 10.1085/jgp.103.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]


