Abstract
The dentate gyrus (DG) of the adult hippocampus gives rise to progenitor cells, which have the potential to differentiate into neurons. To date it is not known whether sleep or sleep loss has any effect on proliferation of cells in the DG. Male rats were implanted for polysomnographic recording, and divided into treadmill sleep-deprived (SD), treadmill control (TC) and cage control (CC) groups. SD and TC rats were kept for 96 h on a treadmill that moved either for 3 s on/12 s off (SD group) or for 15 min on/60 min off (TC group) to equate total movement but permit sustained rest periods in TC animals. To label proliferating cells the thymidine analogue 5-bromo-2′-deoxyuridine (BrdU) was injected after the first 48 h of the experimental procedure in all groups (50 mg kg−1, i.p.). The percentage of time awake per day was 93.2 % in the SD group vs. 59.6 % in the TC group and 49.9 % in the CC group (P < 0.001). Stereological analysis showed that the number of BrdU-positive cells in the DG of the dorsal hippocampus was reduced by 54 % in the SD group in comparison with the TC and by 68 % in comparison with the CC group. These results suggest that sleep deprivation reduces proliferation of cells in the DG of the dorsal hippocampus.
It has been established that both neurons and glia continue to be generated in restricted adult brain structures including the olfactory bulb (Kaplan & Hinds, 1977), the subventricular zone lining the lateral ventricles (Vaysee & Goldman, 1990; Lois & Alvarez-Buylla, 1994) and the subgranular cell layer (SGZ) in the dentate gyrus (DG) of the hippocampus (Altman & Das, 1965; Kaplan & Hinds, 1977). Adult neurogenesis, the process whereby cells in these regions proliferate, survive and differentiate into neurons (Kuhn et al. 1996), has been shown to occur in several species including birds, rodents, non-human primates and humans (Altman & Das, 1965; Alvarez-Buylla & Nottebohm, 1988; Gould et al. 1997; Eriksson et al. 1998). Both positive and negative factors affect neurogenesis. Living in an enriched environment, exercise, acquisition of hippocampal-dependent learning tasks, and antidepressant drug administration increase neurogenesis (Kempermann et al. 1997; Van Praag et al. 1999b; Malberg et al. 2000; Shors et al. 2001), whereas ageing, environmental or psychosocial stress, and glucocorticoid exposure decrease neurogenesis (Kuhn et al. 1996; Gould et al. 1997; Cameron & McCay, 1999). In addition several growth factors including epidermal growth factor (EGF), insulin-like growth factor-I (IGF-I) and brain-derived neurotrophic factor (BDNF) have been shown to promote neurogenesis (Kuhn et al. 1997; Aberg et al. 2000; Pencea et al. 2001).
Many of these modulators of neurogenesis are also known to affect sleep, quantitatively and qualitatively. Exercise enhances sleep in rats (Gambelunghe et al. 2001) while ageing (Kirov & Moyanova, 2002) and chronic stress (Adrien et al. 1991) reduce sleep. Likewise, the intracerebroventricular infusion of EGF, IGF-I and BDNF enhances sleep (Kushikata et al. 1998, 1999; Obal et al. 1998). These facts suggest that sleep may play a role in the modulation of neurogenesis. However, to date it is unknown whether sleep or sleep loss have effects on the different stages of neurogenesis including cell proliferation.
The aim of the present study was to determine whether sleep loss affects cell proliferation in the DG in the dorsal hippocampus. To test this hypothesis we carried out the present study using the thymidine analogue 5-bromo-2′-deoxyuridine (BrdU) to label proliferating cells in the DG of sleep-deprived and control rats.
Part of this work has been presented previously in abstract form (Guzman-Marin et al. 2002).
METHODS
Animal preparation
All experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. All animal use protocols were reviewed and approved by the Internal Animal Care and Use Committee of the V.A. Greater Los Angeles Healthcare System. Male Sprague-Dawley rats (325.5 ± 1.16 g) were used for this experiment. Animals were housed individually in Plexiglas cages (27 cm × 29 cm × 30 cm) and kept under a 12 h:12 h light-dark cycle with access to water and food ad libitum. Under deep anaesthesia (ketamine 80 mg (kg body weight)−1i.p+ xylazine 10 mg (kg body weight)−1i.p.) and aseptic conditions, rats were surgically prepared for sleep-wake cycle monitoring. Five stainless steel screw electrodes were implanted in the skull (frontal and parietal bones) for electroencephalogram (EEG) recording, and four stainless steel wires were inserted into nuchal muscles for electromyogram (EMG) recording. All electrodes were connected with a plug and fixed to the skull with dental acrylic.
Experimental procedure and recording
After 1 week of recovery from surgery and 3 days of acclimatization to experimental conditions the procedures were started. Three groups of six rats each were studied: treadmill sleep-deprived (SD), treadmill control (TC) and cage control (CC). A pair of rats from the TC and SD groups was studied concurrently. All experimental procedures on the animals of the CC group were carried out separately after completion of experiments in the first two groups. TC and SD animals were studied in a Plexiglas enclosure (30 cm × 30 cm × 40 cm) with open top and bottom, which was positioned over a treadmill (90 cm × 58 cm × 14 cm). The treadmill speed was set at 10 cm s−1 and timed to move with either a 3 s on/12 s off (SD) or 15 min on/60 min off (TC) periodicity during the entire recording session (96 h). This schedule gave the TC animals sustained periods of rest but equated the total distance moved in SD and TC groups. Treadmill movement in 96 h was 6.91 km for the SD group and 6.84 km in the TC group (a 1 % difference). The direction of treadmill movement was switched every 15 min. For the CC group, rats were housed individually in Plexiglas cages. Animals in all groups were housed individually with continuous access to food and water and whenever possible treated identically. Lights were kept on a 12 h:12 h light-dark cycle and room temperature was kept at 23 °C.
For sleep-wake cycle recording EEG and EMG signals were filtered at 1.0 and 30 Hz and 10–250 Hz, respectively, and digitized at 256 Hz with the Pass Plus system (Delta Software, St Louis, MO, USA). Sleep-wake states were scored in 20 s epochs on the basis of the predominant state within the epoch. Wake was identified by low-voltage high-frequency EEG activity and sustained elevated neck muscle tone. A high-amplitude low-frequency EEG with decreased muscle activity was defined as non-rapid eye movement (NREM) sleep whereas rapid eye movement (REM) sleep was identified by moderate-amplitude EEG with dominant theta frequency (4–8 Hz), combined with low muscle tone. The percentages of each state were calculated for each 24 h period and the total 96 h period. One per cent of recordings could not be scored due to artifacts.
After the first 48 h of recording, animals from all groups were injected once with the thymidine analogue BrdU (50 mg kg−1i.p., dissolved in 0.9 % NaCl; Sigma), and returned to the treadmill or cage. For all groups BrdU was injected at approximately the same time (09:30–10:00 h). At the end of the 96 h, animals from all groups were deeply anaesthetized (nembutal, 100 mg kg−1i.p.), and perfused transcardially with ice-cold paraformaldehyde (4 %) followed by 10 and 30 % sucrose; brains were removed and processed for BrdU immunostaining.
Immunohistochemistry
Brains were sectioned at 40 μm in the frontal plane through the DG of the dorsal hippocampus. Immunohistochemistry for BrdU was performed on every sixth free-floating section. This ensured that the same cell would not be counted in two sections. Tissue was pretreated for BrdU immunostaining by DNA denaturation (2 m HCl at 37 °C for 30 min) followed by 10 min in borate buffer (pH 8.5). Sections were then incubated with a mouse anti-BrdU primary antibody (Roche, 1:400) for 48 h. Tissue from all groups was treated with aliquots from the same batch of antibodies. Sections were subsequently incubated with a biotinylated horse anti-mouse IgG (Vector Laboratories, 1:200), then reacted with avidin-biotin complex (Vector Elite 1:100) and developed with diaminobenzidine tetrahydrochloride (DAB, Sigma). Absence of the primary antibody resulted in an absence of specific nuclear staining.
Quantification of BrdU labelling
A modified version of the optical fractionator (Stereo Investigator, Microbrightfield) was used to obtain an unbiased estimate of total cell number (West et al. 1991). The sampling was performed according to unbiased stereological principles (West et al. 1991). The cell number (N) was estimated as:
where ΣQ− is the total number of cells actually counted in the dissectors; t is the mean thickness of the sections in an animal; h is the height of the dissector; asf, the area sampling fraction, is the area of the counting frame relative to the area associated with each x, y movement; and ssf, the section sampling fraction, is the fraction of the total sections sampled (West et al. 1991). In the present study the dissector height (h) was 15 μm; nuclei within the first 5 μm of the section were not counted. The section thickness was measured at roughly the middle of one of the two blades of the granule cell layer (∼25 μm) and the area sampling fraction was 150 μm × 130 μm.
The precision of estimates of the number of cells was expressed using the coefficient of error (CE). The stereological sampling scheme was considered adequate when CE was less than 0.10 (West & Gundersen, 1990).
In the present study our counting of BrdU-positive cells was carried out in the DG of the dorsal hippocampus. Our focus on the dorsal hippocampus stems from the following findings. A growing body of evidence indicates that dorsal (Moser & Moser, 1998) and ventral hippocampi (Kjesltrup et al. 2002) may play different functional roles and have different afferent and efferent cortical and subcortical connections (Moser & Moser, 1998). Based on lesion studies a septotemporal hippocampal functional differentiation has been suggested with a critical role of the dorsal hippocampus in several types of learning including spatial learning (Moser et al. 1993; Moser & Moser, 1998; Hock & Bunsey, 1998; Stoelzel et al. 2002). Spatial learning was shown to promote hippocampal neurogenesis, including cell proliferation (Lemaire et al. 2000) and, on the other hand, to be impaired by sleep deprivation (Youngblood et al. 1997, 1999; Smith & Rose, 1996, 1997; Smith et al. 1998).
The rostral boundary of the region analysed corresponds to the appearance of the granular cell layer of the DG whereas the caudal boundary, which corresponds to the level −4.5 mm from Bregma according to the Paxinos & Watson (1998)‘atlas’, was chosen where the ventral and dorsal parts of the CA2 and CA3 pyramidal cell layers coalesce forming continuous cell layers in frontal sections. The caudal boundary in our study was chosen in order to provide a clear anatomical landmark of the area of interest and to include the region destroyed in most dorsal hippocampus lesion studies. The dorsal hippocampus region we defined was not identical to that examined in all lesion studies. The area of the dorsal hippocampus affected was sometimes co-extensive with our area (Maren & Fanselow, 1997; Bonardi 2001), or was smaller (Hock & Bunsey, 1998) or extended slightly more caudally (Moser et al. 1993).
For analysis every sixth section was collected with random selection of the first section. The number of sections that fell within the predefined boundaries was identical in all brains; 11 sections per brain were analysed. This procedure ensured a systematic random sample of sections, in which all parts of the DG region analysed had the same opportunity of being represented (Gundersen, 1986). This selection process occasionally resulted in the selection of sections that had been cut from the leading and trailing surfaces of the DG of the dorsal hippocampus. These sections were equally weighted with complete sections during the selection process, in accordance with fractionator sampling rules (Gundersen, 1986). The final series of sections constituted a known fraction of the sections that passed through the DG of the dorsal hippocampus. Positive cells were counted in the entire granule cell layer (external and internal blade), subgranular region (proliferative zone located at the border of granule cell layer facing the hilus and about two granule cell diameters thick) and the hilus of the DG in the dorsal hippocampus by an experimenter blind to the experimental groups. BrdU-positive cells were counted in each section at ×400 under a light microscope (Nikon E600), omitting cells in the outermost focal plane. In order to rule out the possibility that the differences in the number of labelled cells among groups were due to differences in the volume of the regions analysed, the reference volume was calculated using Stereo Investigator by summing the traced areas for each section, multiplied by the distance between sampled sections.
Corticosterone analysis
In separate groups of animals (n = 6 each, 325.02 ± 1.56 g), blood samples were collected 48 h after initiating SD, TC or CC experimental manipulations, coinciding with the time of the BrdU injection (carried out at 09.30–10.00 h). Animals were rapidly killed by decapitation. The elapsed time from the time animals were picked up until blood collection was less than 1 min. Blood was collected from the trunk (a mixture of arterial and venous blood) into cooled polyethylene tubes with heparin as the anticoagulant, and centrifuged immediately (1500 g for 10 min) to separate plasma, which was stored at −20 °C until analysis was carried out. Plasma corticosterone was measured by radioimmuno assay (Essoterix, Agoura Hills CA, USA).
A one-way analysis of variance (ANOVA) followed by the Tukey post hoc test was used to determine statistical differences of the mean values of variables; results are expressed as means ±s.e.m.
RESULTS
None of the rats appeared debilitated during or after the 96 h of sleep deprivation and control procedures; animals appeared healthy, their coats were white and shiny, and no lesions on the tail or paws were evident as has been described for rats subjected to longer periods of sleep deprivation (Rechtschaffen et al. 1999).
Sleep-wake cycle parameters
The method of sleep deprivation used in this study had marked effects on the stages of the sleep-wake cycle (Fig. 1). The average percentage of time spent awake for 96 h was higher in the SD group (93.2 ± 0.5 %) in comparison with the TC (59.6 ± 1.4 %) and CC (49.9 ± 1.3 %) groups (F(2,15)= 422.2; P < 0.001).
Figure 1. Effects of sleep deprivation on the sleep-wake cycle.
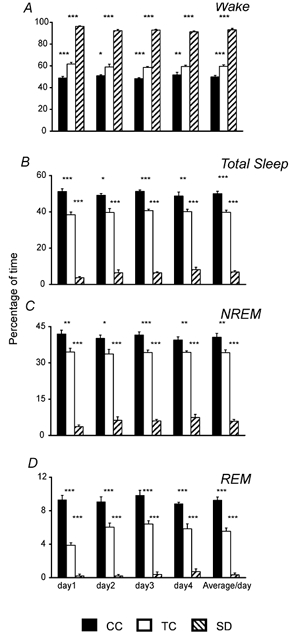
The graphs show the percentage of time spent in the different stages of the sleep-wake cycle (A, B, C and D represent waking, total sleep, NREM and REM sleep, respectively) in cage control (CC), treadmill control (TC) and sleep-deprived (SD) animals during each day of recording and the average for 96 h. Data are means ±s.e.m. for groups of 6 rats each. ***P < 0.001, **P < 0.01 and *P < 0.05. Comparisons between CC and SD were also significant (P < 0.001) in all cases. One-way ANOVA, followed by Tukey post hoc test.
Total sleep time was reciprocally reduced in the SD group with respect to the TC and CC groups (Fig. 1B). The mean percentages of total sleep time for 96 h were 50.07 ± 1.3 in the CC group, 39.7 ± 1.2 in the TC group and 6.8 ± 0.7 for the SD group (F(2,15)= 470.69; P < 0.001). Reductions were found in both NREM sleep (40.63 ± 1.6 % CC, 34.2 ± 1.2 % TC, vs. 5.8 + 0.7 % SD; F(2,15)= 281.9; P < 0.001; Fig. 1C) and REM sleep (9.3 ± 0.4 CC %, 5.5 ± 0.4 % TC vs. 0.3 ± 0.2 % SD; F(2,15)= 189.4; P < 0.001; Fig. 1D). Post hoc comparisons showed that differences in the percentages of each sleep-waking stage in 96 h were statistically significant between the SD group and both control groups, as well as between the TC and CC groups (P < 0.01, Fig. 1). These differences were consistent and significant on each day of the recording session (Fig. 1).
Cell proliferation
In all BrdU-injected rats, BrdU-positive nuclei were observed in the DG (Fig. 2), particularly in the SGZ, i.e. the border between the hilus and granule cell layer. In all groups BrdU-positive cells were darkly stained and irregularly shaped and appeared frequently in either singles or clusters of two or more. Among the clusters, it was possible to see pairs of closely apposed BrdU-positive cells in the SGZ (Fig. 2C). It is likely (Kornack & Rakic, 1999) that these doublets represent the daughters of a progenitor cell that had incorporated BrdU, suggesting that the BrdU labelling is due to its incorporation into DNA during the S phase of the cell cycle rather than other processes that could potentially result in BrdU labelling, such as DNA repair or apoptosis.
Figure 2. BrdU labelling in the DG of the hippocampus.
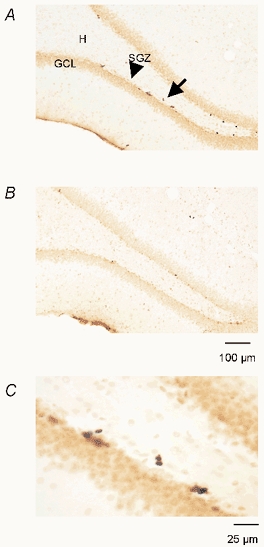
A and B, representative photomicrographs of sections from TC and SD animals, respectively. The majority of the BrdU-labelled cells were located in the subgranular cell layer (SGZ) indicated by an arrowhead in A. C represents a BrdU-labelled doublet (indicated by an arrow in A) in the SGZ at higher magnification. GCL, granule cell layer; H, hilus.
A one-way ANOVA showed statistically significant differences in the mean number of BrdU-labelled cells in the DG of the dorsal hippocampus among groups (F(2,15)= 25.9; P < 0.001). All pairwise multiple comparisons were also significant. SD rats exhibited 54 % fewer BrdU-positive cells compared to TC rats (P < 0.01) and 68 % (P < 0.01) fewer compared to CC rats (1452.5 ± 180.9 SD, 3153 ± 245.8 TC and 4520 ± 506.0 CC, Fig. 3A). TC rats showed a lower number of BrdU-positive cells in comparison with CC rats (P < 0.05). The same inter-group differences in the number of BrdU-positive cells were observed in most of the individual sections (Fig. 3B). The CEs of the estimated number of BrdU-positive cells between different groups were not different (0.07 ± 0.006 TC, 0.08 ± 0.006 SD and 0.07 ± 0.005 CC). The differences among groups in the DG reference volumes (2.6 ± 0.15 mm3 TC, 2.4 ± 0.07 mm3 SD and 2.3 ± 0.17 mm3 CC) were not statistically significant (F(2,15)= 0.7; P = 0.48).
Figure 3. Effects of sleep deprivation on proliferation of cells in the DG.
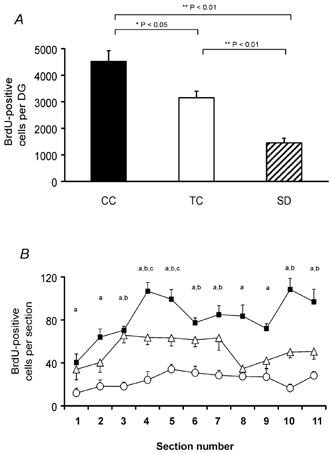
A, the number of BrdU-positive cells in the DG of the dorsal hippocampus was reduced in the SD group in comparison with the CT and CC groups. B, the reduction was significant in most of the sections analysed. Sections 1 and 11 correspond approximately to Bregma −1.8 and −4.5 mm, respectively. ▪, CC; △, TC; and ○, SD. a CC vs. SD, b TC vs. SD and c CC vs. TC (P < 0.05, one-way ANOVA, followed by Tukey post hoc test).
Plasma corticosterone
As indicated in Fig. 4, no significant differences were observed in serum corticosterone levels between SD, TC and CC groups (5.1 ± 1.2 μg dl−1 SD, 9.0 ± 1.6 TC and 7.1 ± 1.7 μg dl−1 CC; F(2,15)= 1.6; P = 0.227).
Figure 4. Comparison of serum corticosterone levels in SD and control groups.
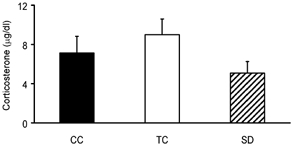
Corticosterone levels did not show significant differences among groups (F(2,15)= 1.6; P = 0.227 one-way ANOVA).
Discussion
This study used stereological sampling and counting methods to show a decrement in the number of BrdU-positive cells in the DG of the hippocampus in SD rats. We used a BrdU dose (50 mg (kg body weight)−1) thought to selectively label dividing cells (Rakic, 2002). Observed differences in cell proliferation between groups were independent of differences in reference volumes. The method of sleep deprivation used in this study caused reductions of both NREM and REM sleep by 83 and 95 %, respectively, compared with the TC group and by 86 and 97 %, respectively, compared with the CC group, but equated total locomotion in the SD and TC groups.
Since many negative regulators of cell proliferation have in common the influence of stress, and sleep deprivation is often perceived as a physiological stressor, it may be suggested that the effect of sleep deprivation on the proliferation of cells in the DG was caused by stress. However, our sleep deprivation procedure did not result in an increase in the corticosterone levels after 48 h of sleep deprivation, at the time when BrdU was administered. Corticosterone levels were comparable to basal glucocorticoid levels in the rat reported previously (Sapolsky et al. 1985). In addition, no visible changes were observed in the condition of rats after 96 h of SD, as was described after long-term deprivation periods using the disc-over water method (Rechtschaffen et al. 1999). These findings support the view that the reduction in the number of BrdU-positive cells was due to an effect of sleep loss rather than stress associated with experimental manipulation. Further, in previous studies the classical stress symptoms were very mild or absent in SD animals compared to control (Coenen & Van Luijtelaar, 1985; Rechtschaffen et al. 1989), and elevations of corticosterone levels during or after sleep deprivation induced by forced locomotion (as used in the present study) were also mild (Meerlo et al. 2002) or not statistically significant (Tobler et al. 1983). In our study the absence of stress responses as measured by corticosterone levels in the animals exposed to the treadmill could be related to the fact that rats voluntarily choose locomotor activity when given the opportunity to run (see below).
Increased physical activity is one of the factors known to promote proliferation of cells in the DG (Van Praag et al. 1999b). However the specific factors involved in this process are not well understood. It has been shown that exercise results in elevated trophic factor production (Gomez-Pinilla et al. 1998, 2002), angiogenesis (Black et al. 1990) or increased serotonin release (Chaouloff, 1997; Bequet et al. 2001; Cotman & Berchtold, 2002). Recently it was reported that exercise also enhances subsequent sleep (Gambelunghe et al. 2001), so it is possible that one of the factors contributing to the effect of exercise on cell proliferation is the amount of sleep following exercise. In the present study both SD and TC animals were exposed to the same housing conditions and equivalent total forced locomotion (both groups of animals spent 20 % of the total recording time in movement). The main difference between SD and TC animals in our experiment was the percentage of the total amount of sleep.
Although total distance was matched, locomotion was more sustained in the TC group compared to the SD group. It could be argued that this constituted greater physical activity. However, despite increased forced locomotion, the TC group did not show increased numbers of BrdU cells in comparison with the CC group. On the basis of these comparisons, we conclude that differences in cell proliferation between SD and TC rats are more likely to be related to differences in the amount of sleep rather than the pattern of physical activity. It should also be pointed out that, first, not all types of exercise increase cell proliferation. For example, forced exercise assessed by swimming, in contrast to running wheel exercise, did not increase cell proliferation in the DG (Van Praag et al. 1999b). Second, in an exercise study, mice ran an average of 4.8 km day−1 (Van Praag et al. 1999a) whereas in our study the total distance travelled for the 4 day period of recording was 6.8 km (1.7 km day−1). When provided with a running wheel, rats may voluntarily run as much as 48 km per 24 h for several days without apparent ill effects (Richter, 1967). Third, the slow treadmill speed used in the present study (10 cm s−1) was intended to keep animals awake rather than induce exercise.
Although the CC group had mildly increased sleep compared to the TC group, the increased cell proliferation in the CC group could also be due to differences in housing between groups. Thus, at this time, we cannot conclude that the differences in sleep amounts between the CC and TC groups affected cell proliferation.
Recent studies have shown that REM sleep deprivation impairs spatial learning as measured in the Morris water maze (Youngblood et al. 1997, 1999; Smith & Rose, 1996, 1997; Smith et al. 1998), but not cued (Smith et al. 1998) or non-spatial memory using the visible version of the maze (Smith & Rose, 1997). Since this type of spatial learning is known to promote cell proliferation (Lemaire et al. 2000), it is possible that impairment in spatial learning caused by sleep deprivation may contribute to the reduction in cell proliferation in the DG of the dorsal hippocampus.
The negative effects of sleep deprivation have been hypothesized to be related to the cellular consequences of prolonged waking including toxicity by excess glutamate release, free radicals, or elevated glucocorticoids, all of which may lead to cell damage (Inoue et al. 1995; Mamelak, 1997). However, it was shown recently that sleep deprivation from 5 to 14 days did not induce cell degeneration or death as determined by methods such as TUNEL and Fluoro-Jade labelling applied in different brain regions including the hippocampus (Cirelli et al. 1999). The present results suggest an alternative possibility that one of the consequences of sleep deprivation underlying its negative effects could be a reduction of cell proliferation.
Our observations suggest that mechanisms associated with sleep facilitate cell proliferation in the DG of adult rats. We note that sleep time is greatly elevated in fetal life and infancy at times of widespread neurogenesis. A hypothesis that sleep promotes brain development has received some support (Shaffery et al. 1998; Frank et al. 2001). Current approaches to sleep processes suggest possible underlying mechanisms. First, there is evidence that brain cellular energy storage in the form of glycogen is reduced after sleep deprivation and restored during sleep (Kong et al. 2002). In support of this view, recently it was reported that levels of mRNAs encoding glycogen synthase and glycogen phosphorylase were reduced following sleep deprivation (Petit et al. 2002). Since brain metabolism depends primarily on glucose supply, a reduction in its availability would reduce the rate of synthesis of high-energy compounds (ATP) and limit anabolic processes including protein synthesis (Rolfe & Brown,1997; Martin & MacNeill, 2002). This interpretation is consistent with the finding that cerebral protein synthesis is lower during waking than during sleep (Maquet, 1995; Nakanishi et al. 1997). One signal of reduced energy stores is an increase in adenosine levels (Bennington & Heller, 1995). During sustained waking, adenosine release is increased in the basal forebrain and neocortex (Porkka-Heiskanen et al. 1997, 2000) and during the nocturnal active phase, in the hippocampus (Huston et al. 1996). Adenosine inhibits neurite growth in vitro (Thevananther et al. 2001). Sleep deprivation may also affect cell proliferation through changes in neurotrophic factors levels. Recently it was reported that even short-term REM sleep deprivation causes a decrease in BDNF levels in the brainstem and cerebellum, and a decrease of NGF in the hippocampus (Sei et al. 2000).
Another approach stems from recent work showing that homeostatically regulated sleep-like states having a circadian distribution are conserved across species, at least among insects and most cordates (e.g. Shaw et al. 2000; Hendricks et al. 2000). Moreover, in vivo observations indicate that the circadian clock may play a role in growth control. For example, cell proliferation and apoptosis in rapidly renewing tissues exhibit circadian synchronization (Bjarnason et al. 1999; Ruifrok et al. 1998) and some circadian genes (Per 2) affect the temporal expression of genes involved in cell cycle regulation (Fu et al. 2002). Thus, the circadian pattern of sleep may have a basic role in the biology of cell proliferation. We can speculate that in the nervous system, sleep supports an essential stage within the approximately 24 h cell-division cycle (Cameron & McKay, 2001).
The reduction of cell proliferation in the DG resulting from sleep deprivation may have clinical significance. Reductions in hippocampal volume have been reported in depression (Sheline et al. 1996, 1999; Shah et al. 1998; Bremner et al. 2000). Previous investigators have suggested that suppression of neurogenesis could contribute to the neuropathology of depression, but have linked this suppression to increased glucocorticoid release (Duman et al. 1999; Jacobs et al. 2000). Sleep fragmentation, a consistent feature of depression, could be an additional factor contributing to the suppression of neurogenesis.
It remains to be determined how sleep deprivation affects the survival and differentiation of these cells into neurons. Also unknown is whether or not an increase in sleep duration above baseline during recovery from sleep deprivation affects neurogenesis. The present study does not differentiate between the effects of NREM and REM sleep. It remains to be determined whether these sleep stages contribute differentially to the proliferation of cells in the DG.
Acknowledgments
We gratefully acknowledge Dr Lutz Slomianka for valuable comments on the manuscript. The authors wish to thank Anna L. Stone, Keng-Tee Chew, Melinda Wu and Feng Xu for their excellent technical assistance. This research was supported by the US Department of Veterans Affairs Medical Research service and US National Institutes of Health grants MH 47480, HL 60296.
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;15:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrien J, Dugovic C, Martin P. Sleep-wakefulness patterns in the helpless rat. Physiol Behav. 1991;49:257–262. doi: 10.1016/0031-9384(91)90041-l. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Bequet F, Gomez-Merino D, Berthelot M, Guezennec CY. Exercise-induced changes in brain glucose and serotonin revealed by microdialysis in rat hippocampus: effect of glucose supplementation. Acta Physiol Scand. 2001;173:223–230. doi: 10.1046/j.1365-201X.2001.00859.x. [DOI] [PubMed] [Google Scholar]
- Bjarnason GA, Jordan RC, Sothern RB. Circadian variation in the expression of cell-cycle proteins in human oral epithelium. Am J Pathol. 1999;154:613–622. doi: 10.1016/S0002-9440(10)65306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonardi C. Dorsal hippocampal lesions impair appetitive classical conditioning to localized cues. Eur J Neurosci. 2001;13:1435–1443. doi: 10.1046/j.1460-9568.2001.01504.x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psych. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RDG. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Chaouloff F. Effects of acute physical exercise on central serotonergic systems. Med Sci Sports Exerc. 1997;29:58–62. doi: 10.1097/00005768-199701000-00009. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Shaw PJ, Rechtschaffen A, Tononi G. No evidence of brain cell degeneration after long-term sleep deprivation in rats. Brain Res. 1999;840:184–193. doi: 10.1016/s0006-8993(99)01768-0. [DOI] [PubMed] [Google Scholar]
- Coenen AML, Van Luijtelaar ELJM. Stress induced by three procedures of deprivation of paradoxical sleep. Physiol Behav. 1985;35:501–504. doi: 10.1016/0031-9384(85)90130-1. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psych. 1999;46:1181–1191. doi: 10.1016/s0006-3223(99)00177-8. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- Gambelunghe C, Rossi R, Mariucci G, Tantucci M, Ambrosini MV. Effects of light physical exercise on sleep regulation in rats. Med Sci Sports Exerc. 2001;33:57–60. doi: 10.1097/00005768-200101000-00010. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, So V, Kesslak JP. Spatial learning and physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise. Neurosci. 1998;85:53–61. doi: 10.1016/s0306-4522(97)00576-9. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJG. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones. J Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- Guzman-Marin R, Stewart D, Stone A, Suntsova N, Gong H, Szymusiak R, McGinty D. Miami FL USA: Society for Neuroscience; Sleep deprivation reduces proliferation of new cells in the subgranular zone of hippocampal dentate gyrus in the rat. Program no.577.8. [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Hock BJ, Bunsey MD. Differential effects of dorsal and ventral hippocampal lesions. J Neurosci. 1998;18:7027–7032. doi: 10.1523/JNEUROSCI.18-17-07027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston JP, Haas HL, Boix F, Pfister M, Decking U, Schrader J, Schwarting RKW. Extracellular adenosine levels in neostratum and hippocampus during rest and activity periods of rats. Neurosci. 1996;73:99–107. doi: 10.1016/0306-4522(96)00021-8. [DOI] [PubMed] [Google Scholar]
- Inoue S, Honda K, Komoda Y. Sleep as neuronal detoxification and restitution. Behav Brain Res. 1995;69:91–96. doi: 10.1016/0166-4328(95)00014-k. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psych. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn GH, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kirov R, Moyanova S. Distinct sleep-wake stages in rats depend differentially on age. Neurosci Lett. 2002;322:134–136. doi: 10.1016/s0304-3940(02)00096-4. [DOI] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99:10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Shepel N, Holden C, Mackiewicz M, Pack A, Geiger J. Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci. 2002;22:5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornack D, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn GH, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn GH, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushikata T, Fang J, Chen Z, Wang Y, Krueger JM. Epidermal growth factor enhances spontaneous sleep in rabbits. Am J Physiol. 1998;275:R509–514. doi: 10.1152/ajpregu.1998.275.2.R509. [DOI] [PubMed] [Google Scholar]
- Kushikata T, Fang J, Krueger JM. Brain-derived neurotrophic factor enhances spontaneous sleep in rats and rabbits. Am J Physiol. 1999;276:R1334–1338. doi: 10.1152/ajpregu.1999.276.5.R1334. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamelak M. Neurodegeneration, sleep, and cerebral energy metabolism: a testable hypothesis. J Geriatr Psychiatry Neurol. 1997;10:29–32. doi: 10.1177/089198879701000106. [DOI] [PubMed] [Google Scholar]
- Maquet P. Sleep function(s) and cerebral metabolism. Behav Brain Res. 1995;69:75–83. doi: 10.1016/0166-4328(95)00017-n. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiol Learn Mem. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Martin IV, MacNeill SA. ATP-dependent DNA ligases. Genome Biol. 2002;3:3005.1–3005.7. doi: 10.1186/gb-2002-3-4-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P, Koehl M, Van Der Borgh T, Turek FW. Sleep restriction alters the hypothalamic-pituitary-adrenal response to stress. J Neuroendocrinol. 2002;14:397–402. doi: 10.1046/j.0007-1331.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Sun Y, Nakamura RK, Mori K, Ito M, Suda S, Namba H, Storch FI, Dang TP, Mendelson W, Mishkin M, Kennedy C, Gillin JC, Smith CB, Sokoloff L. Positive correlations between cerebral protein synthesis rates and deep sleep in Macaca mulatta. Eur J Neurosci. 1997;9:271–279. doi: 10.1111/j.1460-9568.1997.tb01397.x. [DOI] [PubMed] [Google Scholar]
- Obal F, Kapas L, Bodosi B, Krueger JM. Changes in sleep in response to intracerebral injection of insulin-like growth factor-1 in the rat. Sleep Res Online. 1998;1:87–91. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit JM, Tobler I, Allaman I, Borbely AA, Magistretti PJ. Sleep deprivation modulates brain mRNAs encoding genes of glycogen metabolism. Eur J Neurosci. 2002;16:1163–1167. doi: 10.1046/j.1460-9568.2002.02145.x. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neurosci. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci. 2002;3:65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: X. Integration and discussion of the findings. Sleep. 1989;12:68–87. [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM, Gilliland MA, Bauer K. Effects of method, duration, and sleep stage on rebounds from sleep deprivation in the rat. Sleep. 1999;22:11–31. doi: 10.1093/sleep/22.1.11. [DOI] [PubMed] [Google Scholar]
- Richter CP. Sleep and activity: their relation to the 24-hour clock. In: Kety SS, Evart EV, Williams HL, editors. Sleep and Altered States of Consciousness. Baltimore: Williams and Wilkins; 1967. pp. 8–29. [PubMed] [Google Scholar]
- Rolfe D, Brown G. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- Ruifrok A, Weil M, Thames H, Mason K. Diurnal variations in the expression of radiation-induced apoptosis. Radiat Res. 1998;149:360–365. [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoids exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sei H, Saitoh D, Yamamoto K, Morita K, Morita Y. Differential effect of short-term REM sleep deprivation on NGF and BDNF protein levels in the rat brain. Brain Res. 2000;877:387–390. doi: 10.1016/s0006-8993(00)02708-6. [DOI] [PubMed] [Google Scholar]
- Shaffery JP, Oksenberg A, Marks GA, Speciale SG, Mihailoff G, Roffwarg HP. REM sleep deprivation in monocularly occluded kittens reduces the size of cells in LGN monocular segment. Sleep. 1998;21:837–845. doi: 10.1093/sleep/21.8.837. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM. Cortical gray matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry. 1998;172:527–532. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci U S A. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–375. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Smith CT, Conway JM, Rose GM. Brief paradoxical sleep deprivation impairs reference, but not working memory in the radial arm maze task. Neurobiol Learn Mem. 1998;69:211–217. doi: 10.1006/nlme.1997.3809. [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol Behav. 1996;59:93–97. doi: 10.1016/0031-9384(95)02054-3. [DOI] [PubMed] [Google Scholar]
- Smith C, Rose GM. Posttraining paradoxical sleep in rats is increased after spatial learning in the Morris water maze. Behav Neurosci. 1997;111:1197–1204. doi: 10.1037//0735-7044.111.6.1197. [DOI] [PubMed] [Google Scholar]
- Stoelzel CR, Stavnezer AJ, Denenberg VH, Ward M, Markus EJ. The effects of aging and dorsal hippocampal lesions: performance on spatial and nonspatial comparable versions of the water maze. Neurobiol Learn Mem. 2002;78:217–233. doi: 10.1006/nlme.2001.4054. [DOI] [PubMed] [Google Scholar]
- Thevananther S, Rivera A, Rivkees SA. A1 adenosine receptor activation inhibits neurite process formation by rho kinase-mediated pathways. Neuroreport. 2001;12:3057–3063. doi: 10.1097/00001756-200110080-00015. [DOI] [PubMed] [Google Scholar]
- Tobler I, Murison R, Ursin R, Ursin H, Borbely AA. The effects of sleep deprivation and recovery sleep on plasma corticosterone in the rat. Neurosci Lett. 1983;35:297–300. doi: 10.1016/0304-3940(83)90333-6. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999a;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999b;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vaysse PJ, Goldman JE. A clonal analysis of glial lineages in neonatal forebrain development in vitro. Neuron. 1990;5:227–235. doi: 10.1016/0896-6273(90)90160-h. [DOI] [PubMed] [Google Scholar]
- West MJ, Gundersen H. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Youngblood BD, Smagin GN, Elkins PD, Ryan DH, Harris RB. The effects of paradoxical sleep deprivation and valine on spatial learning and brain 5-HT metabolism. Physiol Behav. 1999;67:643–649. doi: 10.1016/s0031-9384(99)00120-1. [DOI] [PubMed] [Google Scholar]
- Youngblood BD, Zhou J, Smagin GN, Ryan DH, Harris RB. Sleep deprivation by the flower pot technique and spatial reference memory. Physiol Behav. 1997;61:249–256. doi: 10.1016/s0031-9384(96)00363-0. [DOI] [PubMed] [Google Scholar]


