Abstract
We tested the hypothesis that endogenous interleukin (IL)-10 limits the fever induced by a Gram-negative bacterial toxin (Escherichia coli lipopolysaccharide, LPS) and a Gram-positive bacterial toxin (Staphylococcus aureus), when these toxins are injected into a subcutaneous air pouch (i.po.) in rats. Injection of LPS or S. aureus caused fevers that were reduced in amplitude and duration by simultaneous administration of rat recombinant IL-10. The inhibition of fever by IL-10 was accompanied by a significant reduction in the toxin-evoked increases in concentrations of immunoreactive IL-6 at the site of inflammation and of IL-6 and IL-1 receptor antagonist in the circulation. Conversely, neutralisation of endogenous IL-10 in the pouch increased the amplitude and dramatically increased the duration of toxin-evoked fever, and augmented toxin-induced increases in pouch tumour necrosis factor-α, IL-1β, and especially IL-6. Our data support a crucial regulatory role for endogenous IL-10 in limiting the fever responses during both Gram-negative and Gram-positive infections.
The pro-inflammatory cytokines tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6 are believed to be key mediators underlying the febrile response (see for reviews, Kluger, 1991; Zeisberger, 1999). Although much is known about their role in the mechanisms initiating fever (for reviews, see Kluger, 1991; Elmquist et al. 1997; Dinarello et al. 1999; Zeisberger, 1999; Roth & de Souza, 2001), the mechanisms by which the fevers are maintained, limited and reduced (defervescence) have been little studied, although centrally acting endogenous antipyretic peptides (e.g. arginine vasopressin, α-melanocyte stimulating hormone and adrenocorticotrophic hormone) have been shown to limit febrile responses (see Tatro, 2000, for review).
The internalisation of lipopolysaccharide (LPS)-‘LPS receptor’ complexes, and (pyrogenic) cytokine-cytokine receptor complexes (Vuk-Pavlovic & Kovach, 1989; Sacht et al. 1999), and the downregulation of receptors for pro-inflammatory, pyrogenic cytokines (Neta et al. 1992; Tsujimoto & Oku, 1992; van der Poll et al. 1997a; Barrera et al. 2001) are possible ways in which fever responses are limited. Other means include the toxin-stimulated release of a number of endogenous ligands, including IL-1 receptor antagonist (IL-1ra), the endogenous antagonist of IL-1 (Arend 1990; Eisenberg et al. 1990; Hannum et al. 1990), soluble receptors for IL-1 and TNF (Ulich et al. 1994; Fernandez-Botran, 2000) and the so-called anti-inflammatory cytokines. An increasing number of cytokines have been shown to have activities that oppose or downregulate inflammatory processes. Among these are IL-4, IL-10, IL-13 and transforming growth factor-β. These so-called ‘anti-inflammatory cytokines’ are believed to modulate immune and inflammatory events, allowing resolution of the inflammation and a return of the damaged tissue to its normal state (Feghali & Wright, 1997; Opal & DePalo, 2000). Indeed, IL-6, although generally regarded as a ‘pro-inflammatory’ cytokine, has both pro-inflammatory and anti-inflammatory actions (see Dinarello, 1997).
IL-10 is an anti-inflammatory cytokine produced by Th2 lymphocytes and monocytes (Fiorentino et al. 1989) that potently inhibits the production of the pro-inflammatory cytokines TNF, IL-1, IL-6 and IL-8, while upregulating the expression of IL-1ra (Howard & O'Garra, 1992, Jenkins et al. 1994). Elevated plasma IL-10 concentrations have been reported in patients with sepsis (Marchant et al. 1994b; van der Poll et al. 1997b) and after the injection of LPS into experimental animals (Durez et al. 1993; Wang et al. 2001). In addition, exogenous administration of recombinant IL-10 protects mice from lethal endotoxaemia by reducing TNF release (Gérard et al. 1993; Howard et al. 1993; Marchant et al. 1994a), and neutralisation of endogenously produced IL-10 in endotoxaemic mice results in an increased production of several pro-inflammatory cytokines, and enhanced mortality (Bermudez & Champsi, 1993; Standiford et al. 1995). In addition, it was shown that IL-10-knockout mice have an increased likelihood of inflammatory bowel disease (Rennick et al. 1997), higher mortality rates after experimentally induced sepsis (Berg et al. 1995), and develop an exacerbated and prolonged fever in response to i.p. injection of LPS, but not to localised turpentine injection (Leon et al. 1999). Collectively, these data imply that IL-10 may function as an endogenous antipyretic in response to systemic LPS, and may have therapeutic potential in acute and chronic inflammatory diseases.
Toxins from both Gram-negative (Escherichia coli LPS) and Gram-positive (Staphylococcus aureus) bacteria initiate the release of a cascade of pro-inflammatory cytokines following their administration to experimental animals (see Kluger, 1991; Plata-Salamán et al. 1998; Turrin et al. 2001). Although Gram-negative and Gram-positive pyrogens elicit similar fevers, similar acute-phase reactions and apparently similar sickness behaviours, differences in the processes underlying the responses to the two types of toxin were suggested some years ago (Goelst & Laburn, 1991; Mitchell & Laburn, 1997). It was reported that S. aureus may be capable of causing fever independently of the cytokine cascade (Goelst & Laburn, 1991). Consequently, responses to S. aureus might be expected to be less susceptible to IL-10-induced inhibition of the production of endogenous pyrogenic cytokines, if that indeed is the mechanism of action of IL-10. More recently, evidence has accumulated to indicate that LPS and S. aureus toxin activate different cell-surface Toll-like receptors (TLR), TLR4 and TLR2, respectively (see Akira, 2003), and that the stimulation of these different receptors can lead to different profiles of cytokine production, and potentially, different sensitivities to the actions of anti-inflammatory cytokines such as IL-10 (see O'Neill, 2002).
Using a model of localised inflammation in rats, we have examined the potential antipyretic role of IL-10 in fevers evoked by Gram-negative and Gram-positive toxins. In this particular model, in which an exogenous pyrogen, such as LPS, is injected into a subcutaneous air pouch (Miller et al. 1997), the exogenous pyrogen remains at the site of its administration (Cartmell et al. 2001) and the fever appears to be dependent upon LPS-induced IL-6, which finds its way into the blood from the pouch, in contrast to LPS and LPS-induced TNF and IL-1, which are produced but remain in the pouch (Cartmell et al. 2000). The sequence of events in response to intrapouch (i.po.) injection of S. aureus has yet to be elucidated. Given the capacity of IL-10 to inhibit the production of the pro-inflammatory cytokines TNF, IL-1 and IL-6, and to increase IL-1ra production, our experiments were designed to investigate the role of exogenous and endogenous IL-10 on experimentally induced fever and cytokine production, both at the site of inflammation and in the circulation.
METHODS
Male and female Sprague-Dawley rats (250–300 g) were used in all experiments. Animals were housed in a controlled environment at an ambient temperature of 24 ± 2 °C (mean ±s.e.m.) on a 12:12 h light:dark cycle (lights on from 07.30 to 19.30 h). Food (pelleted rat chow, Epol, Johannesburg, South Africa) and water were provided ad libitum. Unless specified otherwise, at the end of the experimental period, animals were killed by an overdose of a rising concentration of carbon dioxide. All procedures were approved by the Animal Ethics Committee of the University of the Witwatersrand (South Africa) under clearance 2001/73/4.
Measurement of body temperature
The core body temperature of unrestrained rats was measured by remote biotelemetry, using temperature-sensitive radiotelemeters (TA10TA-F40, Data Sciences) implanted intraperitoneally whilst animals were under ketamine (80 mg kg−1i.m.) and xylazine (4 mg kg−1i.m.) anaesthesia. Animals were housed individually after surgery and allowed at least 1 week to recover from surgery before experimentation. Transmitter output frequency (Hz) was monitored at 10 min intervals by an antenna mounted in a receiver board, which was situated beneath the cage of each animal, and the data was logged into a peripheral processor (VitalView, Minimitter, Sunriver, OR, USA) connected to a personal computer. The telemeters and receiver were calibrated against a quartz thermometer (Quat, Heraeus) to an accuracy of 0.1 °C.
Air pouch
A subcutaneous air pouch was formed, as described by Edwards et al. (1981), immediately after implantation of the radiotelemeter while animals were still under ketamine and xylazine anaesthesia (see above). Briefly, 20 ml of sterile air (filtered through 0.2 μm Acrodisc, Gelman Sciences, USA) was injected into the subcutaneous tissue of the dorsal midline, caudal to the scapulae. Three days after the initial pouch formation, animals were briefly re-anaesthetised (3 % halothane (Fluothane, Zeneca) in oxygen) and the air pouches were reinflated with a further 10 ml of sterile air, to maintain open cavities. On day 6, rat recombinant (rr) IL-10, sheep anti-rat IL-10 serum (anti-IL-10), pre-immune sheep serum (PIS), pyrogen, or vehicle (saline) was injected directly into the air pouches of lightly restrained (hand held), conscious animals.
Materials
The following reagents were employed: rrIL-10, in the form of recDNA IL-10tyr149, a stable analogue with full biological activity (Ball et al. 2001), E. coli-derived (NIBSC, UK) with an endotoxin content < 4 ng (40 IU) mg−1 of protein, as measured in a Limulus Amoebocyte Lysate test (European Pharmacopoeia, 1999); sheep anti-rat IL-10 serum (raised against recDNA rat IL-10tyr149, NIBSC) and PIS (NIBSC), both with endotoxin content < 0.24 ng (2.4 IU) ml−1 of protein; purified LPS from E. coli (serotype, 0111:B4, Sigma, UK) and killed organisms (cell walls) of S. aureus (Sigma). LPS was reconstituted with saline (sterile, pyrogen-free 0.9 % (w/v) saline, Sabax, Johannesburg, South Africa) and injected at a dose of 100 μg kg−1. The cell walls of S. aureus, heat-killed and fixed in formalin, were supplied as a suspension in phosphate-buffered saline, and injected at a final dose of 3 × 1011 cell walls kg−1.
Experimental protocol
Experiment 1
Rats were injected i.po. with rrIL-10 (200 μg kg−1) or saline, immediately followed by an injection (i.po.) of LPS (100 μg kg−1, n = 5), S. aureus (3 × 1011 cell walls kg−1, n = 5) or saline (1 ml kg−1, n = 5). Core body temperature was monitored for 24 h after injection. A minimum of 1 week was allowed to elapse between injections in the same animal. Blood and pouch fluid samples (for assay of cytokines) were collected from separate groups of animals (under terminal anaesthesia with halothane), 4 h after injection of LPS or saline (n = 5 per treatment group). Sampling of the inflammatory exudates within the pouch was achieved by lavaging the pouch with 1 ml of sterile saline. The lavage fluid was quickly aspirated, centrifuged (3000 g, 4 °C, 10 min), with the resulting supernatant collected and stored at −70 °C until assayed. Blood was collected by cardiac puncture into sterile tubes containing pyrogen-free heparin (10 IU ml−1) and centrifuged (5300 g, 4 °C, 10 min). Plasma was stored at −70 °C until assayed.
Experiments 2 and 3
Anti-rat IL-10 serum or PIS was injected (1 ml, i.po.) into rats at the same time as the injection (i.po.) of LPS (100 μg kg−1, n = 5 per treatment), S. aureus (3 × 1011 cell walls kg−1, n = 5 per treatment), or saline (1 ml kg−1, n = 5). Body temperature was monitored continuously for 72 h. Blood and pouch fluid samples (for assay of cytokines) were collected from separate groups of antiserum-treated animals (under terminal anaesthesia with halothane), 5 and 25 h after injection of LPS, S. aureus or saline (n = 5 per treatment group).
Enzyme-linked immunosorbent assay (ELISA)
Concentrations of TNF-α, IL-1β, IL-6, IL-1ra and IL-10 in the plasma and pouch fluid were measured using rat-specific sandwich ELISAs (NIBSC), as described previously (Safieh-Garabedian et al. 1995; Rees et al. 1999a,b; Cunha et al. 2000; Ball et al. 2001). The ELISA for rat IL-1β detects both precursor and mature IL-1β but not TNF-α, IL-1α, IL-6, IL-1ra or IL-10. Similarly, the ELISAs for rat TNF-α, IL-6, IL-1ra and IL-10 do not cross-react with rat IL-1β, rat IL-1α or each other (NIBSC). The sensitivities of the assays were: IL-1β and IL-6, 1.9 pg ml−1; TNF, IL-1ra and IL-10, 3.9 pg ml−1. Given the high concentrations of cytokines at the site of inflammation compared to those in the circulation, some samples required large dilutions. The assay detection limit, which allows for the sample dilution factor, therefore differs between different biological fluids and treatment groups.
Statistical analysis
All data are expressed as means ±s.e.m. for five animals. Temperature responses were plotted as body temperature-time curves. Peak change in body temperature and fever indices (FIs, °C h, calculated as the time integral of the body temperature elevation above the temperature prevailing at the time of injection) were determined. Data were analysed using either ANOVA, followed by a Tukey-Kramer Multiple Comparisons post hoc test for differences between more than two groups, or Student's t test for differences, at the same time point, between two groups. Cytokine concentrations, at the various time points and in the various biological fluids, were compared with the corresponding concentrations measured in control animals, at the same time and in the same biological fluid using either ANOVA, followed by a Tukey-Kramer Multiple Comparisons post hoc test for differences between more than two groups, or Student's t test for differences at the same time point, between two groups. Comparisons between different cytokines and/or time points were not analysed. Where cytokine concentrations were undetectable, samples were assigned a value equivalent to the detection limit of the assay. A two-tailed probability P < 0.05 was considered statistically significant.
RESULTS
Effect of exogenous IL-10 on LPS-evoked fever and cytokine production
LPS (100 μg kg−1, i.po.) evoked fever that began approximately 2 h after its injection and reached a maximum value of 39.0 ± 0.1 °C at 3 h, significantly higher than the 37.0 ± 0.2 °C in vehicle-injected animals (Fig. 1, upper panel, P < 0.001, ANOVA, Tukey post hoc test). Exogenous IL-10 (200 μg kg−1, i.po.) significantly reduced both the amplitude and duration of the LPS-induced fever (P < 0.001, ANOVA, Tukey post hoc test): the maximum body temperature in the presence of IL-10 was 37.8 ± 0.1 °C (Fig. 1A) and the FI (the area under the fever curve) was reduced by 67 %. Exogenous IL-10 alone had no effect on body temperature: body temperature following IL-10 injection was not different from that of saline-injected animals (P > 0.05, ANOVA, Tukey post hoc test). IL-10 attenuated the amplitude of the S. aureus-evoked fever, on days 2 and 3, but had no effect on the duration of the fever (Fig. 2).
Figure 1. Effect of exogenous IL-10 on LPS-evoked fever and cytokine production.
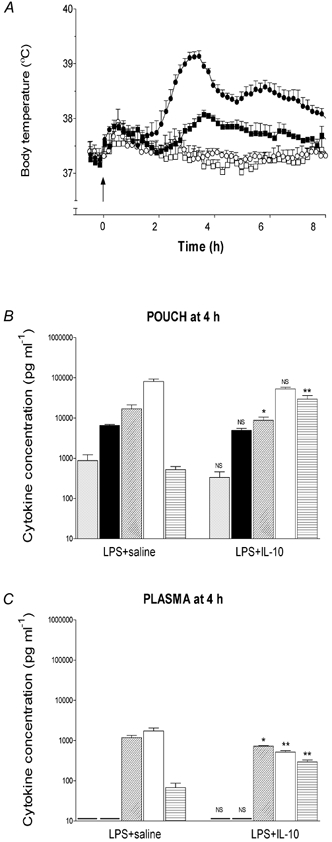
A, fever response of rats injected intrapouch with LPS (100 μg kg−1, filled symbols, n = 5) or saline (open symbols, n = 5) and IL-10 (200 μg kg−1, squares) or saline (1 ml kg−1, circles) at 10.00 h (arrow). B and C, concentrations of immunoreactive TNF-α (grey columns), IL-1β (black columns), IL-6 (diagonal hatched columns), IL-1ra (open columns) and IL-10 (horizontal hatched columns) at the site of LPS injection (pouch) and in the plasma, respectively, 4 h after treatment with IL-10. Data are expressed as means ±s.e.m., n = 5. - indicates value below the detection limit of the assay. NS, not significant, *P < 0.05, **P < 0.01vs. LPS + saline, Student's t test.
Figure 2. Effect of exogenous IL-10 on S. aureus-evoked fever.
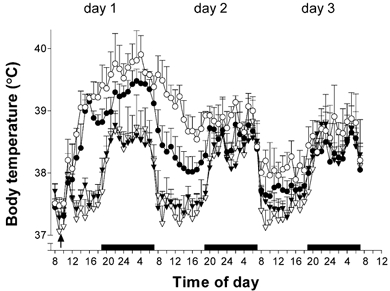
The fever response of rats injected i.po. with S. aureus (3 × 1011 cell walls kg−1, circles, n = 10) or saline (triangles, n = 10), and IL-10 (200 μg kg−1, filled symbols) or saline (1 ml kg−1, open symbols). The results are presented as means ±s.e.m., n = 5. The arrow indicates the time of injection; the black bars indicate periods of darkness (lights out).
TNF-α, IL-1β, IL-6, IL-1ra and IL-10 were detected in the pouch (site of injection) 4 h after injection of LPS (Fig. 1B). In contrast, only IL-6, IL-1ra and IL-10 were detected in the plasma (Fig. 1C). Both local (pouch) and circulating IL-6, and circulating IL-1ra concentrations evoked by LPS were reduced by 48, 39 and 70 %, respectively, 4 h after injection of exogenous IL-10 (Fig. 1B and C).
Effect of neutralising endogenous IL-10 within the pouch on LPS-induced fever and cytokine concentrations
The temperature responses of control animals (PIS + saline; anti-IL-10 serum + saline) were similar for the duration of the experiment (3 days; P > 0.05, ANOVA, Tukey post hoc test; Fig. 3A). LPS (+ PIS), injected i.po., evoked fever that began 2–3 h after LPS injection and reached a maximum value of 38.2 ± 0.2 °C, significantly higher than that of 37.2 ± 0.1 °C in controls (P < 0.001, Fig. 3A). Injection (i.po.) of sheep anti-IL-10 serum significantly increased the peak fevers evoked by LPS to 38.8 ± 0.2 °C, and fevers were maintained during the day at some 0.5–0.8 °C higher than in animals treated with LPS + PIS for the duration of the experiment (3 days).
Figure 3. Treatment with anti-IL-10 serum prolongs the duration of LPS-evoked fever.
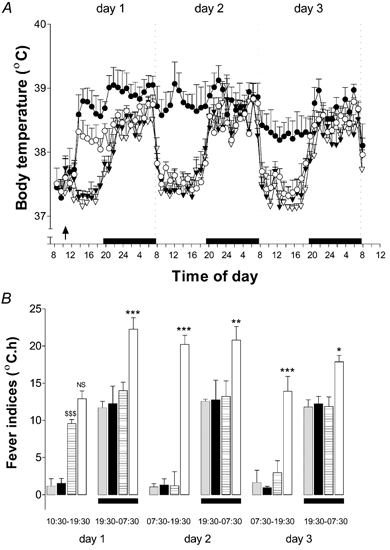
A, i.po. injection of sheep anti-IL-10 serum (1 ml, filled symbols) or sheep pre-immune serum (PIS, 1 ml, open symbols) followed by injection (i.po.) of LPS (100 μg kg−1, circles) or saline (1 ml kg−1, triangles). The results are presented as means +s.e.m., n = 5. The arrow indicates the time of injection; the black bars indicate periods of darkness (lights out). B, fever indices (FIs, °C h, means ±s.e.m., n = 5) calculated from the time of injection for rats receiving: PIS + saline (grey columns); anti-IL-10 serum + saline (black columns); PIS + LPS (horizontal hatched columns); anti-IL-10 serum + LPS (open columns). FIs are time integrals of the change in body temperature from the pre-injection body temperature prevailing on the day of injection. Black bars indicate periods of darkness (lights out). NS, not significant; ***P < 0.001 PIS + LPS vs. anti-IL-10 + LPS; ANOVA (Tukey post hoc test) for the same time frame. $$$P < 0.001 PIS + saline vs. PIS + LPS; ANOVA (Tukey post hoc test) for the same time frame.
Figure 3B shows the FIs calculated for 12 h time periods for a total of 72 h after injection. The FI takes into account both the amplitude and the duration of the fever. The 9 h FI (i.e. from injection until lights out, 10.30–19.30 h on day 1) evoked in response to injection of LPS was eightfold higher than in controls (PIS + saline; anti-IL-10 + saline; P < 0.001, ANOVA, Tukey post hoc test, Fig. 3), but was similar in animals treated with PIS (horizontal hatched columns) or anti-IL-10 (open columns, P > 0.05, ANOVA, Tukey post hoc test). Treatment with anti-IL-10 potentiated the 12 h night FI on day 1 (twofold), and both the day and night FIs evoked by LPS on days 2 and 3 after injection (approximately twofold).
Treatment with sheep anti-rat IL-10 serum (i.po.), at the same time as injection of LPS, also affected concentrations of cytokines (Fig. 4) at the site of injection (pouch) and in the plasma. Treatment with anti-rat IL-10 serum abolished the increase in pouch IL-10 concentrations and augmented the increases in local (pouch) IL-6 (twofold; Fig. 4A), but had no effect on local and circulating concentrations of TNF-α, IL-1β and IL-1ra, and circulating IL-6, evoked by LPS 5 h after injection (Fig. 4). Pouch fluid concentrations of IL-1β, IL-6, IL-1ra and IL-10, evoked by LPS, remained elevated 25 h after injection of LPS and, except for IL-1ra and IL-10, were even higher in animals in which endogenous IL-10 was neutralised (Fig. 4B). In the circulation, only IL-1ra (Fig. 4, lower panel) concentrations were potentiated by treatment with anti-IL-10, 25 h after injection of LPS.
Figure 4. Effect of treatment with anti-IL-10 serum on LPS-induced cytokine production.
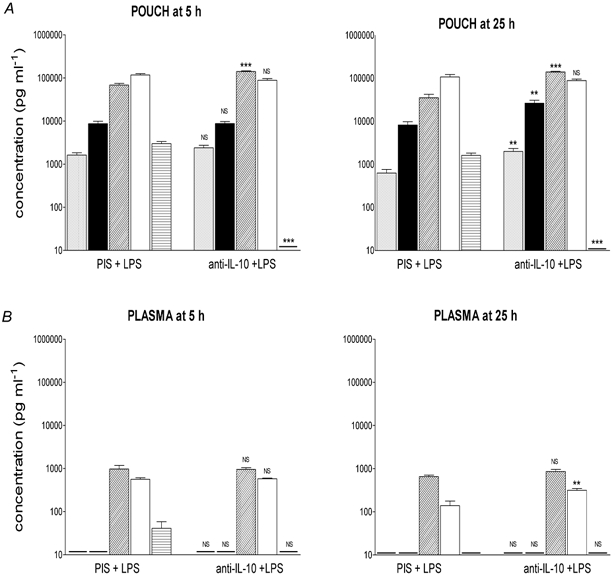
Concentrations of immunoreactive TNF-α (grey columns), IL-1β (black columns), IL-6 (diagonal hatched columns), IL-1ra (open columns) and IL-10 (horizontal hatched columns) at the site of injection (pouch, A) and in the plasma (B), 5 and 25 h after injection (i.po.) of PIS + LPS (100 μg kg−1) or anti-IL-10 + LPS. Data are expressed as means ±s.e.m., n = 5. - indicates value below the detection limit of the assay. NS, not significant; **P < 0.01, ***P < 0.001; PIS + LPS vs. anti-IL-10 + LPS, ANOVA (Tukey post hoc test).
Effect of neutralising endogenous IL-10 on S. aureus-induced fever and local and circulating cytokine concentrations
i.po. injection of S. aureus (3 × 1011 cell walls kg−1, i.po.) evoked fever approximately 2 h after injection, with an initial peak of 38.9 ± 0.2 °C at 6 h (Fig. 5, upper panel). Treatment with anti-rat IL-10 serum significantly potentiated the fever peak to 39.3 ± 0.3 °C, and fevers following S. aureus + anti-IL-10 were maintained some 0.5 °C higher than in animals treated with S. aureus+ PIS (Fig. 5, upper panel). Treatment with anti-IL-10 serum also significantly potentiated the mean night-time body temperatures evoked by S. aureus from 38.8 ± 0.1 to 39.2 ± 0.1 °C, and body temperatures were higher than in appropriate controls (i.e. PIS + saline: mean, 38.2 ± 0.1 °C and anti-IL-10+saline: mean, 38.3 ± 0.1 °C). The sharp fall in body temperature observed with the onset of daytime (lights on, day 2) was abolished in animals treated with S. aureus+ anti-IL-10, and temperatures evoked by S. aureus+ anti-IL-10 were sustained about 1 °C higher than in animals treated with S. aureus+ PIS (38.4 ± 0.1 °C) and 2.5 °C higher than in control animals (PIS + saline, 36.9 ± 0.1 °C; anti-IL-10 + saline, 36.9 ± 0.1 °C). A similar pattern was observed on day 3.
Figure 5. Treatment with anti-IL-10 serum exacerbates the magnitude of S. aureus-evoked fever.
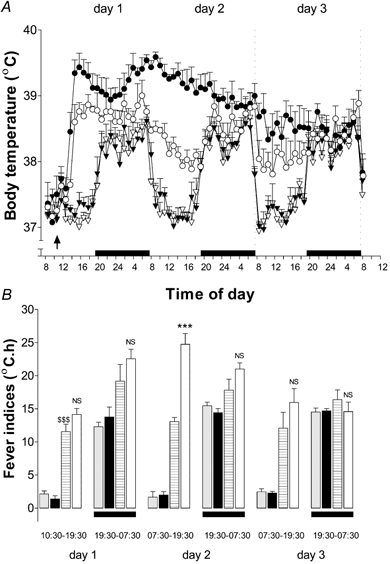
A, i.po. injection of sheep anti-IL-10 serum (1 ml, filled symbols) or PIS (1 ml, open symbols) immediately followed by injection (i.po.) of S. aureus (3 × 1011 cell walls kg−1, circles) or saline (1 ml kg−1, triangles). The results are presented as means ±s.e.m., n = 5. The arrow indicates time of injection; bars indicate periods of darkness (lights out). B, FIs (°C h, means ±s.e.m., n = 5) calculated from the time of injection for rats receiving: PIS + saline (grey columns); anti-IL-10 serum + saline (black columns); PIS +S. aureus (horizontal hatched columns); anti-IL-10 serum +S. aureus (open columns). FIs were calculated as in Fig. 2. Bars indicate periods of darkness (lights out). Data are expressed as means ±s.e.m. NS, not significant; ***P < 0.001 PIS +S. aureus vs. anti-IL-10 +S. aureus; ANOVA (Tukey post hoc test) for the same time frame. $$$P < 0.001 PIS + saline vs. PIS +S. aureus; ANOVA (Tukey post hoc test) for the same time frame.
The lower panel on Fig. 5 shows the 12 h FIs calculated for the 3 day period following injection of S. aureus (or saline) in animals treated with PIS or anti-IL-10. The FI (10.30–19.30 h) for the (daytime) period of 9 h immediately subsequent to injection of S. aureus was eightfold higher than in controls (PIS + saline; anti-IL-10 + saline; Fig. 5B) and remained so for the entire 3 days following injection. Treatment with anti-IL-10 had no effect on the FI evoked by S. aureus for the first 20 h after injection (Fig. 5B), but potentiated the 12 h (daytime) FI (by 75 %) on day 2 after injection. By day 3, there was no difference between the two groups. The 12 h (night-time FI) evoked by S. aureus was 1.6-fold higher than in controls on day 1 after injection (Fig. 5B), but was similar in animals injected with anti-IL-10 or PIS. By the evening of day 2, there was no difference between control animals and animals injected with S. aureus (P > 0.05, ANOVA, Tukey post hoc).
Treatment with sheep anti-rat IL-10 serum, at the same time as injection of S. aureus, also had effects upon concentrations of cytokines (Fig. 6) at the site of injection (pouch) and in the plasma. Treatment with anti-IL-10 abolished the increase in pouch IL-10 concentrations evoked by S. aureus (as expected) and potentiated the increases in TNF-α (4-fold), IL-1β (2.5-fold), and IL-6 (4.8-fold) at the site of injection (Fig. 6A; P < 0.01, ANOVA, Tukey post hoc test); and increased circulating concentrations (approximately 4-fold) of IL-6 (P < 0.01, ANOVA, Tukey post hoc test) and IL-1ra (P < 0.01, ANOVA, Tukey post hoc test; Fig. 6B) 5 h after injection. Pouch concentrations of TNF-α, IL-1β, IL-6, IL-1ra and IL-10 remained elevated 25 h after injection of S. aureus. However, only pouch IL-6 concentrations were increased significantly (5-fold) at this time point in animals treated with anti-IL-10 (P < 0.001, ANOVA, Tukey post hoc test; Fig. 6A), and pouch IL-10 concentrations still were undetectable in this same group of animals. Treatment with anti-IL-10 had no effect on circulating cytokine concentrations 25 h after injection of S. aureus (Fig. 6B).
Figure 6. Effect of treatment with anti-IL-10 serum on S. aureus-induced cytokine production.
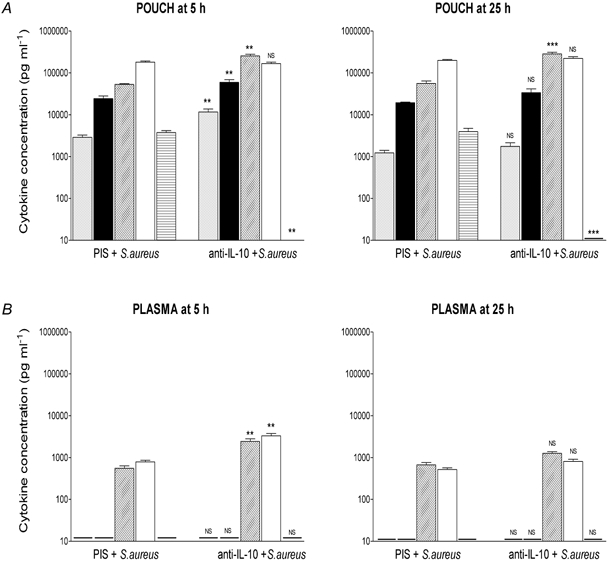
Concentrations of immunoreactive TNF-α (grey columns), IL-1β (black columns), IL-6 (diagonal hatched columns), IL-1ra (open columns) and IL-10 (horizontal hatched columns) at the site of injection (pouch, A) and in the plasma (B) 5 and 25 h after injection (i.po.) of PIS +S. aureus (3 × 1011 cell walls kg−1) or anti-IL-10 +S. aureus. Data are expressed as means ±s.e.m., n = 5. - indicates value below the detection limit of the assay. NS, not significant; **P < 0.01, ***P < 0.001; PIS +S. aureus vs. anti-IL-10 +S. aureus, ANOVA (Tukey post hoc test).
Discussion
The cloning of recDNA rat IL-10tyr149, a stable IL-10 analogue with full biological activity (Ball et al. 2001), and the generation of a sheep antiserum neutralising this molecule have permitted the study of the role of endogenous IL-10 in limiting the fevers caused by local injections of E. coli LPS and S. aureus toxin. We report that exogenous rat IL-10 is a potent inhibitor of the fevers caused by local injections of LPS and S. aureus, which is consistent with previous studies using only LPS as the inflammatory stimulus (Nava et al. 1997; Pajkrt et al. 1997; Leon et al. 1999: Ledeboer et al. 2002) and raises the possibility that IL-10 may be useful in treating inflammation and fever. The marked inhibitory effect of IL-10 on the amplitude of fever and, in the case of LPS, on the duration of fever, contrasted with its much less dramatic effects on the concentrations of pyrogenic cytokines in the pouch and of IL-6 in the blood. It is possible that IL-10 had a more marked effect on concentrations of pyrogenic cytokines in the pouch at times before we made our first measurements. In addition, actions by blood-borne IL-10, concentrations of which were elevated following its administration into the pouch, may have contributed to its antipyretic effect. Since the ELISA for rat IL-10 does not discriminate between endogenous and injected (i.e. tyr149) IL-10, it is not possible to say with certainty that the IL-10 immunoreactivity detected in the plasma was derived from the pouch. The effect of IL-10 was unlikely to have involved the induction of IL-1ra by IL-10 (Cassatella et al. 1994; Jenkins et al. 1994). Indeed, IL-10 actually reduced the concentrations of IL-1ra in the plasma.
The profiles of the fever responses to LPS and S. aureus (in the absence of anti-IL-10) were different. The response to S. aureus was of much longer duration, > 48 h, compared with about 10 h for the response to LPS. In the case of the S. aureus-induced fever, which lasted into the night-time of day 2, the nychthemeral rhythm (increased body temperature during the hours of darkness; see Luker et al. 2000) obscured the night-time fever response. In spite of this, neutralisation of endogenous IL-10 in the pouch had profound effects upon the fever responses to both LPS and S. aureus. Anti-IL-10 increased the amplitude and greatly increased, from about 10 h to > 72 h, the duration of LPS-evoked fever, whereas anti-IL-10 increased only the amplitude, but markedly so, of the already long-lasting S. aureus-induced fever. Comparison of pouch and plasma IL-10 concentrations at 5 and 25 h after injection of LPS or S. aureus alone, revealed no significant differences between the two time points, despite the dramatic increase in body temperature in response to toxin + anti-IL-10 at 25 h. Nevertheless, our data suggest strongly that locally produced IL-10 has a role as an endogenous antipyretic. In contrast, in another widely used model of local inflammation, in which turpentine was injected subcutaneously, endogenous IL-10 did not appear to have a role in the fever. IL-10-knockout mice (which lack the functional IL-10 gene in all tissues in the body) developed the identical, typical 4–8 h fever observed in wild-type mice (Leon et al. 1999), whereas i.p. (systemic) injection of LPS in IL-10-knockout mice evoked fever that was observed on both the 1st and 2nd day after injection (Leon et al. 1999). It is plausible, however, that redundancies of cytokine actions in vivo have developed in the mediation of fever to turpentine in knockout mice (Paul, 1989; see Kluger, 1991).
Despite the profound effects on fever of neutralisation of IL-10 observed in the present study, the effects of neutralisation of IL-10 on concentrations of pyrogenic cytokines were less dramatic. Enhanced plasma levels of IL-6 in IL-10-knockout mice at 4 h (corresponding with maximum fever) but not the following day (also a time point of maximum fever) have been reported by Leon et al. (1999) and led the authors to hypothesise that IL-10 has an endogenous antipyretic action during LPS-induced fever due to its capacity to inhibit the production of endogenous IL-6. Our data in part support such a role for endogenous IL-6. Exogenous IL-10 administration significantly decreased both pouch and plasma IL-6 concentrations 4 h after injection of LPS and, despite the absence of a significant increase in plasma IL-6 concentrations in animals treated with LPS + anti-IL-10 compared to animals injected with LPS alone, local IL-6 concentrations (that is, in the pouch) were increased at both 5 and 25 h after injection, time points at which exacerbated fever occurred. i.po. administration of S. aureus+ anti-IL-10 similarly resulted in significant increases in pouch IL-6 concentrations at both 5 and 25 h after injection, and in plasma IL-6 concentrations at 5 h, lending further support to the hypothesis proposed by Leon and colleagues. Of particular note was the finding that following injection of LPS + anti-IL-10, body temperature remained elevated in the absence of increases in plasma concentrations of IL-6 over and above those obtained with LPS given alone, suggesting that plasma IL-6 was not solely responsible for the prolonged fevers evoked by LPS in the presence of anti-IL-10. This result was unexpected since there is evidence to suggest that IL-6 is the final circulating mediator of fever in this model of localised LPS-induced infection/ inflammation (Cartmell et al. 2000). Interestingly, IL-6-knockout mice failed to develop fever in response to i.p. injection of LPS (Chai et al. 1996), although at high doses of LPS fever was evoked, probably through the production of additional endogenous mediators that compensated for the lack of IL-6 (Kozak et al. 1998). Also worthy of note is that in the present study IL-6 concentrations were significantly elevated in the absence of fever (Fig. 4, LPS + PIS, 25 h). Either this observation calls into question the role of IL-6 in fever or it implies that IL-6 in the periphery needs to act in concert with another mediator to be pyrogenic.
In the present study, anti-IL-10 had no effect on the concentrations of TNF-α and IL-1β in the pouch at 5 h after LPS injection, by which time the fever had reached its peak, but did increase concentrations of TNF-α and IL-1β in the pouch at 25 h after LPS injection. The opposite was true for S. aureus: anti-IL-10 increased concentrations of TNF-α and IL-1β in the pouch at 5 h after S. aureus injection, again by which time the fever had reached its peak, but did not increase concentrations of TNF-α and IL-1β in the pouch at 25 h after S. aureus injection. Similarly, anti-IL-10 treatment had no significant effect on circulating concentrations of TNF-α or IL-1β, at both 5 and 25 h. In fact, the only cytokine that was increased significantly in the plasma in response to treatment with either LPS or S. aureus and anti-IL-10 was IL-1ra. This finding was surprising since IL-10 has been shown to induce the expression of IL-1ra (Cassatella et al. 1994; Jenkins et al. 1994). The mechanism underlying the prolonged fever response to LPS in animals in which IL-10 had been neutralised remains to be resolved. It is plausible that another cytokine or chemokine might be produced in the pouch in response to exogenous pyrogens, and find its way into the blood. Our preliminary data suggest that cytokine-induced neutrophil chemoattractant-1, which is structurally similar to the pyrogenic human chemokine IL-8 (Zagorski & DeLarco, 1993), might be produced in the pouch in response to exogenous pyrogens. It has been proposed that both IL-6 and IL-8 have important roles in transforming acute into chronic inflammation (Marin et al. 2001).
Alternatively, vagal afferents may convey communication between pyrogen-sensitive cells in the periphery and the brain (Dantzer, 1994; Watkins et al. 1995), especially if pyrogens are present at low doses in the abdomen rather than in the blood (Bluthéet al. 1996; Romanovsky et al. 1997). The evidence for such a pathway has burgeoned over the last 5 years (see Zeisberger, 1999; Romanovsky, 2000). However, the importance of this route, and the question of whether or not it has a special role in abdominal pyrogen assault, remains controversial (see Romanovsky, 2000). This pathway of communication certainly cannot be advanced as a general mechanism by which pro-inflammatory cytokines trigger the cascade of thermoregulatory events taking place in response to systemic pyrogenic challenge (Rivest et al. 2000). In a view similar to that advanced for vagal signalling, modest evidence has been provided recently for the participation of cutaneous afferents in the transport of immune information from the skin to the brain, in the genesis of fever (Ross et al. 2000).
For the present, we can report that locally produced IL-10 has an important role as an endogenous antipyretic, and that it is unlikely to achieve its antipyretic effect primarily by inhibition of the pro-inflammatory cytokines TNF, IL-6 and IL-1. It is likely that other endogenous pyrogens, and or chemokines, contribute to the generation of fever to localised LPS or S. aureus administration.
Acknowledgments
We thank Dr Deryn Petty (University of the Witwatersrand, South Africa) for undertaking the surgical procedures and the staff of the Central Animal Service, University of the Witwatersrand, Medical School, South Africa, for the provision and care of the animals. The equipment and funds for the in vivo studies were provided by the Research Committee of the University of the Witwatersrand, South Africa. Neutralising IL-10 antiserum, recombinant cytokines and cytokine ELISAs were provided by the centralised facility (Division of Endocrinology, NIBSC, UK) of the European Community Concerted Action Program BIOMED I ‘Cytokines in the Brain’ (PL931450) and BIOMED 2 Anti-inflammatory cytokines (CT 97–2492).
References
- Akira S. Mammalian toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- Arend WP. Interleukin-1 receptor antagonist: discovery, structure and properties. Prog Growth Factor Res. 1990;2:193–205. doi: 10.1016/0955-2235(90)90018-f. [DOI] [PubMed] [Google Scholar]
- Ball C, Vigues S, Gee CK, Poole S, Bristow AF. Rat interleukin-10: production and characterisation of biologically active protein in a recombinant bacterial expression system. Eur Cytokine Netw. 2001;12:187–193. [PubMed] [Google Scholar]
- Barrera P, Joosten LA, den Broeder AA, Van De Putte LB, Van Riel PL, Van Den Berg WB. Effects of treatment with a fully human anti-tumour necrosis factor alpha monoclonal antibody on the local and systemic homeostasis of interleukin 1 and TNFalpha in patients with rheumatoid arthritis. Ann Rheum Dis. 2001;60:660–669. doi: 10.1136/ard.60.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg DJ, Kuhn R, Rajewsky K, Muller W, Menon S, Davidson N, Grunig G, Rennick D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J Clin Invest. 1995;96:2339–2347. doi: 10.1172/JCI118290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez LE, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–3097. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé RM, Michaud B, Kelley KW, Dantzer R. Vagotomy blocks behavioural effects of interleukin-1 injected via the intraperitoneal route but not via other systemic routes. Neuroreport. 1996;7:2823–2827. doi: 10.1097/00001756-199611040-00083. [DOI] [PubMed] [Google Scholar]
- Cartmell T, Luheshi GN, Hopkins SJ, Rothwell NJ, Poole S. Role of endogenous interleukin-1 receptor antagonist in regulating fever induced by localised inflammation in the rat. J Physiol. 2001;531:171–180. doi: 10.1111/j.1469-7793.2001.0171j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmell T, Poole S, Turnbull AV, Rothwell NJ, Luheshi GN. Circulating interleukin-6 mediates the febrile response to localised inflammation in rats. J Physiol. 2000;526:653–661. doi: 10.1111/j.1469-7793.2000.00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassatella MA, Meda L, Gasperini S, Calzetti F, Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994;179:1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Z, Gatti S, Toniatti C, Poli V, Bartfai T. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever responses to lipopolysaccharide or IL-1β: a study on IL-6-deficient mice. J Exp Med. 1996;183:311–316. doi: 10.1084/jem.183.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol. 2000;130:1418–1424. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. How do cytokines say hello to the brain? Neural versus humoral mediation. Eur Cytokine Netw. 1994;5:271–273. [PubMed] [Google Scholar]
- Dinarello CA. Role of pro- and anti-inflammatory cytokines during inflammation: experimental and clinical findings. J Biol Regul Homeost Agents. 1997;11:91–103. [PubMed] [Google Scholar]
- Dinarello CA, Gatti S, Bartfai T. Fever: links with an ancient receptor. Curr Biol. 1999;9:R147–150. doi: 10.1016/s0960-9822(99)80085-2. [DOI] [PubMed] [Google Scholar]
- Durez P, Abramowicz D, Gerard C, Van Mechelen M, Amraoui Z, Dubois C, Leo O, Velu T, Goldman M. In vivo induction of interleukin 10 by anti-CD3 monoclonal antibody or bacterial lipopolysaccharide: differential modulation by cyclosporin A. J Exp Med. 1993;177:551–555. doi: 10.1084/jem.177.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JCW, Sedgwick AD, Willoughby DA. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- Eisenberg SP, Evans RJ, Arend WP, Verderber E, Brewer MT, Hannum CH, Thompson RC. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990;343:341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends Neurosci. 1997;20:565–570. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- European Pharmacopoeia. European Pharmacopoeia. 3. Strasbourg: European Pharmacopoeia; 1999. pp. 41–46. suppl,. 2.6.14. Bacterial endotoxins, pp. [Google Scholar]
- Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997;2:12–26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R. Soluble cytokine receptors: novel immunotherapeutic agents. Expert Opin Investig Drugs. 2000;9:497–514. doi: 10.1517/13543784.9.3.497. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse helper T cell IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gérard C, Bruyns C, Marchant A, Abramowicz D, Vandenabeele P, Delvaux A, Fiers W, Goldman M, Velu T. Interleukin-10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxaemia. J Exp Med. 1993;177:547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelst K, Laburn HP. Response of body temperature and serum iron concentration to repeated pyrogen injection in rabbits. Pflugers Arch. 1991;417:558–561. doi: 10.1007/BF00372951. [DOI] [PubMed] [Google Scholar]
- Hannum CH, Wilcox CJ, Arend WP, Joslin FG, Dripps DJ, Heimdal PL, Armes LG, Sommer A, Eisenberg SP, Thompson RC. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- Howard M, Muchamuel T, Andrade S, Menon S. Interleukin-10 protects mice from lethal endotoxaemia. J Exp Med. 1993;177:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M, O'Garra A. Biological properties of IL-10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- Jenkins JK, Malyak M, Arend WP. The effects of interleukin-10 on interleukin-1 receptor and antagonist and interleukin-1 production in human monocytes and neutrophils. Lymphokine Cytokine Res. 1994;13:47–54. [PubMed] [Google Scholar]
- Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak W, Kluger MJ, Soszynski D, Conn CA, Rudolph K, Leon LR, Zheng H. IL-6 and IL-1β in fever. Studies using cytokine-deficient (knockout) mice. Ann N Y Acad Sci. 1998;856:33–47. doi: 10.1111/j.1749-6632.1998.tb08310.x. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Binnekade R, Breve JJ, Bol JG, Tilders FJ, Van Dam AM. Site-specific modulation of LPS-induced fever and interleukin-1 beta expression in rats by interleukin-10. Am J Physiol. 2002;282:R1762–1772. doi: 10.1152/ajpregu.00766.2001. [DOI] [PubMed] [Google Scholar]
- Leon LR, Kozak W, Rudolph K, Kluger MJ. An antipyretic role for interleukin-10 in LPS fever in mice. Am J Physiol. 1999;276:R81–89. doi: 10.1152/ajpregu.1999.276.1.R81. [DOI] [PubMed] [Google Scholar]
- Luker F, Mitchell D, Laburn HP. Fever and motor activity in rats following day and night injections of Staphylococcus aureus cell walls. Am J Physiol. 2000;279:R610–616. doi: 10.1152/ajpregu.2000.279.2.R610. [DOI] [PubMed] [Google Scholar]
- Marchant A, Bruyns C, Vandenabeele P, Ducarme M, Gérard C, Delvaux A, De Groote D, Abramowicz D, Velu T, Goldman M. Interleukin-10 controls interferon-γ and tumor necrosis factor production during experimental endotoxaemia. Eur J Immunol. 1994a;24:1167–1171. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- Marchant A, Deviere J, Byl B, De Groote D, Vincent JL, Goldman M. Interleukin-10 production during septicaemia. Lancet. 1994b;343:707–708. doi: 10.1016/s0140-6736(94)91584-9. [DOI] [PubMed] [Google Scholar]
- Marin V, Montero-Julian FA, Gres S, Boulay V, Bongrand P, Farnarier C, Kaplanski G. The IL-6-soluble IL-6Ralpha autocrine loop of endothelial activation as an intermediate between acute and chronic inflammation: an experimental model involving thrombin. J Immunol. 2001;167:3435–3442. doi: 10.4049/jimmunol.167.6.3435. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Luheshi GN, Rothwell NJ, Hopkins SJ. Local cytokine induction by lipopolysaccharide in the rat air pouch and its relationship to the febrile response. Am J Physiol. 1997;272:R857–861. doi: 10.1152/ajpregu.1997.272.3.R857. [DOI] [PubMed] [Google Scholar]
- Mitchell D, Laburn HP. Macrophysiology of fever. In: Nielsen, Johannsen B, Nielsen R, editors. Thermal Physiology. Copenhagen: August krogh Institute; 1997. pp. 249–263. [Google Scholar]
- Nava F, Calapai G, Facciolá G, Cuzzocrea S, Marciano MC, De Sarro A, Caputi AP. Effects of interleukin-10 on water intake, locomotory activity, and rectal temperature in rat treated with endotoxin. Int J Immunopharmacol. 1997;19:31–38. doi: 10.1016/s0192-0561(97)00006-4. [DOI] [PubMed] [Google Scholar]
- Neta R, Sayers TJ, Oppenheim JJ. Relationship of TNF to interleukins. Immunol Ser. 1992;56:499–566. [PubMed] [Google Scholar]
- O'Neill LA. Toll-like receptor signal transduction and the tailoring of innate immunity: a role for Mal? Trends Immunol. 2002;23:296–300. doi: 10.1016/s1471-4906(02)02222-6. [DOI] [PubMed] [Google Scholar]
- Pajkrt D, Camoglio L, Tiel-Van Buul MCM, De Bruin K, Cutler DL, Affrime MB, Rikken G, Van Der Poll T, Ten Cate JW, Van Deventer SJH. Attenuation of proinflammatory response by recombinant human IL-10 in human endotoxaemia. J Immunol. 1997;158:3971–3977. [PubMed] [Google Scholar]
- Paul WE. Pleitropy and redundancy: T-cell derived lymphokines in the immune response. Cell. 1989;57:521–524. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Plata-Salamán CR, Ilyin SE, Gayle D, Flynn MC. Gram-negative and Gram-positive bacterial products induce differential cytokine profiles in the brain: analysis using an integrative molecular-behavioral in vivo model. Int J Mol Med. 1998;1:387–397. doi: 10.3892/ijmm.1.2.387. [DOI] [PubMed] [Google Scholar]
- Rees G, Ball C, Ward HL, Gee CK, Tarrant G, Mistry Y, Poole S, Bristow AF. Rat interleukin 6: expression in recombinant Escherichia coli. Cytokine. 1999a;11:95–103. doi: 10.1006/cyto.1998.0408. [DOI] [PubMed] [Google Scholar]
- Rees GS, Gee CK, Ward HL, Ball C, Tarrant GM, Poole S, Bristow AF. Rat tumor necosis factor-α: expression in recombinant Pichia pastoris, purification, characterization and development of a novel ELISA. Eur Cytokine Netw. 1999b;10:383–392. [PubMed] [Google Scholar]
- Rennick DM, Fort MM, Davidson NJ. Studies with IL-10-/- mice: an overview. J Leukoc Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- Rivest S, Lacroix S, Valières L, Nadeau S, Zhang J, Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med. 2000;223:22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA. Fever: the role of the vagus nerve. Auton Neurosci. 2000;85:1–55. doi: 10.1016/S1566-0702(00)00232-0. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol. 1997;273:R407–413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- Ross G, Roth J, Störr B, Voigt K, Zeisberger E. Afferent nerves are involved in the febrile response to injection of LPS into artificial subcutaneous chambers in guinea pigs. Physiol Behav. 2000;71:305–313. doi: 10.1016/s0031-9384(00)00358-9. [DOI] [PubMed] [Google Scholar]
- Roth J, De Souza GP. Fever induction pathways: evidence from responses to systemic or local cytokine formation. Braz J Med Biol Res. 2001;34:301–314. doi: 10.1590/s0100-879x2001000300003. [DOI] [PubMed] [Google Scholar]
- Sacht G, Brigelius-Flohe R, Kiess M, Sztajer H, Flohe L. ATP-sensitive association of mortalin with the Il-1 receptor type I. Biofactors. 1999;9:49–60. doi: 10.1002/biof.5520090107. [DOI] [PubMed] [Google Scholar]
- Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Interleukin-1 beta contributes to the inflammation-induced increase in nerve-growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford TJ, Strieter RM, Lukacs NW, Kunkel SL. Neutralization of IL-10 increases lethality in endotoxemia. J Immunol. 1995;155:2222–2229. [PubMed] [Google Scholar]
- Tatro JB. Endogenous antipyretics. Clin Infect Dis. 2000;31:S190–201. doi: 10.1086/317519. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M, Oku N. Regulation of TNF receptors. Immunol Ser. 1992;56:149–160. [PubMed] [Google Scholar]
- Turrin NP, Gayle D, Ilyin SE, Flynn MC, Langhans W, Schwartz GJ, Plata-Salamán CR. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res Bull. 2001;54:443–453. doi: 10.1016/s0361-9230(01)00445-2. [DOI] [PubMed] [Google Scholar]
- Ulich TR, Yi ES, Yin S, Smith C, Remick D. Intratracheal administration of endotoxin and cytokines. VII. The soluble interleukin-1 receptor and the soluble tumor necrosis factor receptor II (p80) inhibit acute inflammation. Clin Immunol Immunopathol. 1994;72:137–140. doi: 10.1006/clin.1994.1117. [DOI] [PubMed] [Google Scholar]
- Van Der Poll T, Coyle SM, Kumar A, Barbosa K, Agosti JM, Lowry SF. Down-regulation of surface receptors for TNF and IL-1 on circulating monocytes and granulocytes during human endotoxemia: effect of neutralization of endotoxin-induced TNF activity by infusion of a recombinant dimeric TNF receptor. J Immunol. 1997a;158:1490–1497. [PubMed] [Google Scholar]
- Van Der Poll T, De Waal Malefyt R, Coyle SM, Lowry SF. Antiinflammatory cytokine responses during clinical sepsis and experimental endotoxaemia: sequential measurements of plasma soluble interleukin (IL)-1 receptor type II, IL-10, and IL-13. J Infect Dis. 1997b;175:118–122. doi: 10.1093/infdis/175.1.118. [DOI] [PubMed] [Google Scholar]
- Vuk-Pavlovic S, Kovach JS. Recycling of tumor necrosis factor-alpha receptor in MCF-7 cells. FASEB J. 1989;3:2633–2640. doi: 10.1096/fasebj.3.14.2556313. [DOI] [PubMed] [Google Scholar]
- Wang Q, Fang CH, Hasslegren P-O. Intestinal permeability is reduced and IL-10 levels are increased in septic IL-6 knockout mice. Am J Physiol. 2001;281:R1013–1023. doi: 10.1152/ajpregu.2001.281.3.R1013. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Cytokine to brain communication: a review and analysis of alternative mechanisms. Life Sci. 1995;57:1011–1026. doi: 10.1016/0024-3205(95)02047-m. [DOI] [PubMed] [Google Scholar]
- Zagorski J, Delarco JE. Rat CINC (cytokine-induced neutrophil chemoattractant) is the homolog of the human GRO proteins but is encoded by a single gene. Biochem Biophys Res Commun. 1993;190:104–110. doi: 10.1006/bbrc.1993.1017. [DOI] [PubMed] [Google Scholar]
- Zeisberger E. From humoral fever to neuroimmunological control of fever. J Therm Biol. 1999;24:287–326. [Google Scholar]


