Abstract
Many important physiological processes involve changes in cell volume, e.g. the transport of salt and water in epithelial cells and the contraction of cardiomyocytes. In this study, we show that voltage-gated KCNQ1 channels, which are strongly expressed in epithelial cells or cardiomyocytes, and KCNQ4 channels, expressed in hair cells and the auditory tract, are tightly regulated by small cell volume changes when co-expressed with aquaporin 1 water-channels (AQP1) in Xenopus oocytes. The KCNQ1 and KCNQ4 current amplitudes precisely reflect the volume of the oocytes. By contrast, the related KCNQ2 and KCNQ3 channels, which are prominently expressed in neurons, are insensitive to cell volume changes. The sensitivity of the KCNQ1 and KCNQ4 channels to cell volume changes is independent of the presence of the auxiliary KCNE1–3 subunits, although modulated by KCNE1 in the case of KCNQ1. Incubation of the oocytes in cytochalasin D and experiments with truncated KCNQ1 channels suggest that KCNQ1 channels sense cell volume changes through interactions between the cytoskeleton and the N-terminus of the channel protein. From our results we propose that KCNQ1 and KCNQ4 channels play an important role in cell volume control, e.g. during transepithelial transport of salt and water.
KCNQ channels form a family of depolarization-activated K+ channels with five members. A number of mutations in the genes coding for channel α-subunits (KCNQ1–5) lead to human disease (see, e.g. Wang et al. 1996; Kubish et al. 1999; Jentsch, 2000). The voltage-regulated KCNQ channels have important functions in a number of excitable tissues. In the heart and in the inner ear, KCNQ1 associates with KCNE1 to form channels which are slowly activated by depolarization as compared to homomeric KCNQ1 channels. In cardiomyocytes the KCNQ1-KCNE1 channels conduct the slow delayed rectifier current, IKs (Wang et al. 1996; Sanguinetti et al. 1996; Barhanin et al. 1996), and in the central nervous system, KCNQ2-KCNQ3 channels mediate the so-called M-current (Wang et al. 1998).
KCNQ channels also have important functions in non-excitable tissues. In secretory epithelia, e.g. in the distal colon, KCNQ1 channels coassembled with auxiliary KCNE3 subunits may represent the major basolateral K+ conductance during cAMP-mediated secretion (Kunzelmann et al. 2001). KCNQ1-KCNE3 channels have a nearly linear voltage dependence and are therefore suitable for participation in secretion of salt and water in epithelial cells (Schroeder et al. 2000). KCNQ4 channels are expressed in the outer hair cells of the cochlea and in the type I hair cells in the vestibular organs (Kharkovets et al. 2000). Loss of KCNQ4 function is associated with a heritable form of deafness (Kubisch et al. 1999), most likely because KCNQ4 channels are responsible for extrusion of K+ taken up via mechanosensitive cation channels.
The transepithelial transport of salt and water demands a tight control of cell volume, and cell volume changes have been shown to modulate the activity of epithelial K+ channels (for reviews, see e.g. Lang et al. 1998; O'Neill, 1999), and in e.g. Ehrlich ascites tumour cells, K+ channels are involved in regulatory volume decrease (RVD) (see e.g. Niemeyer et al. 2001; Hoffmann & Hougaard, 2001). Several types of K+ channels may be regulated by cell volume changes, but although we have shown that certain Ca2+-activated K+ channels (SK and IK channels) may be regulated by cell volume through interactions with the cytoskeleton (Grunnet et al. 2002), the precise mechanism still seems obscure. It has earlier been suggested that the IKs current in cardiomyocytes is enhanced during cell swelling (Sasaki et al. 1994; Vandenberg et al. 1996), and in addition it has recently been shown that cloned KCNQ1 channels are activated by cell swelling after expression in mammalian cells (Kubota et al. 2002). Also, in airway epithelial cells, KCNQ1 channels seem to be involved in the RVD following cell swelling in hyposmolar medium (Lock & Valverde, 2000).
In the present paper we provide evidence that the KCNQ1 and the KCNQ4 channels, but not the KCNQ2-KCNQ3 channels, are indeed tightly regulated by small changes in cell volume. The activation of KCNQ1 and KCNQ4 channels by cell swelling and inhibition by cell shrinkage probably involves interaction between the cytoskeleton and the N-terminus of the channel proteins.
METHODS
Expression in Xenopus laevis oocytes
cDNAs coding for KCNQ1–4, sKCNQ1, KCNE1–3 and aquaporin 1 were subcloned into expression vectors and expressed in Xenopus laevis oocytes as described before (Schmitt et al. 2000; Grunnet et al. 2002). The oocytes were collected from Xenopus laevis frogs under anaesthesia (2 g l−1 Tricaine) according to national guidelines. For co-expression of KCNQ channels, KCNE β-subunits and aquaporin 1, the mRNAs were mixed in equal molar ratios, before a total amount of approx. 5 ng was injected. Oocytes were kept in Kulori medium (90 mm NaCl, 1 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 5 mm Hepes, pH 7.4) at 19 °C until electrophysiological measurements were performed.
Electrophysiological and cell volume measurements
All measurements were performed 3–5 days after RNA injection using a conventional two-electrode voltage-clamp set-up. For volume measurements the set-up was equipped with a CCD camera, and the volume was recorded online at a rate of 1 Hz with an accuracy of 3 in 10 000 (see Grunnet et al. 2002; Zeuthen et al. 2001). The measurements were done in medium that was isotonic (65 mm NaCl, 1 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 50 mm mannitol, 5 mm Hepes, pH 7.4 (188 mosmol kg−1)), hypotonic (65 mm NaCl, 1 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 5 mm Hepes, pH 7.4 (137 mosmol kg−1)) or hypertonic (65 mm NaCl, 1 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 100 mm mannitol, 5 mm Hepes, pH 7.4 (239 mosmol kg−1)) or as described in the figure legends. PKA was activated by raising intracellular cAMP by application of 1 mm 3-isobutyl-1-methylxanthine (IBMX) and 10 μm forskolin. Protein kinase C was activated by application of 10 nm phorbol 12-myristate 13-acetate (PMA). Data obtained from oocytes from at least three different frogs are given as means ±s.e.m.
RESULTS
Regulation of KCNQ channels by small changes in cell volume
To examine if KCNQ channels are sensitive to changes in cell volume, they were expressed in Xenopus oocytes together with or without AQP1 water-channels. KCNQ channel activity and oocyte volume were simultaneously recorded in a set-up which monitored both the current through the expressed KCNQ channels and the cell volume of the oocytes (Grunnet et al. 2002). The plasma membrane of endogenous oocytes is practically impermeable to water, and the presence of AQP1 water channels assured that the oocytes responded to changes in extracellular osmolarity with fast and significant changes in cell volume (Grunnet et al. 2002).
Figure 1A shows the results of an experiment where KCNQ1 channels were co-expressed with AQP1 channels in an oocyte. The expressed KCNQ1 channels were activated by depolarization using a pulsed protocol, 2 s at +20 mV separated by 4 s at −80 mV. In isotonic solutions, depolarizing pulses resulted in maximal currents of approx. 2 μA (measured at the end of the depolarization period). When the extracellular medium was made hypotonic, the oocyte immediately responded with a rapid increase in cell volume, saturating at a volume increase of about 8 %. The increase in oocyte volume was accompanied by a simultaneous increase in current through the expressed KCNQ1 channels to 172 % (s.e.m. = 12 %, n = 12) in comparison to controls. When the oocyte was exposed to a hypertonic extracellular medium it shrank by approx. 8 % and the shrinkage was accompanied by a decrease in the current through the expressed KCNQ1 channels to 55 % (s.e.m. = 9 %, n = 12) of control (see also Fig. 4A). When the oocyte was returned to isotonic medium, cell volume as well as current through the expressed KCNQ1 channels approached the initial levels (Fig. 1A). If KCNQ1 channels were expressed in the absence of AQP1 channels, neither the volume of the oocytes, nor the current through the expressed KCNQ1 channels changed significantly during hypo- or hypertonic challenges (Fig. 1B). This showed that KCNQ1 channels are not sensitive to changes in extracellular osmolarity per se. In another control experiment (Fig. 1C) oocytes expressing AQP1 channels in the absence of KCNQ1 channels were exposed to the same voltage protocol and the same osmotic challenges as applied in Fig. 1A. This result showed that endogeneous channels in the oocytes did not interfere with the measurements. The response of KCNQ1 channels to cell volume changes reflected the extent of swelling or shrinkage of the oocytes. The data in Fig. 1D show that KCNQ1 channels closely sense even very small changes in cell volume and that the relative response was linear for up to 4 % changes in cell volume.
Figure 1. Regulation of KCNQ1 channels by cell volume changes.
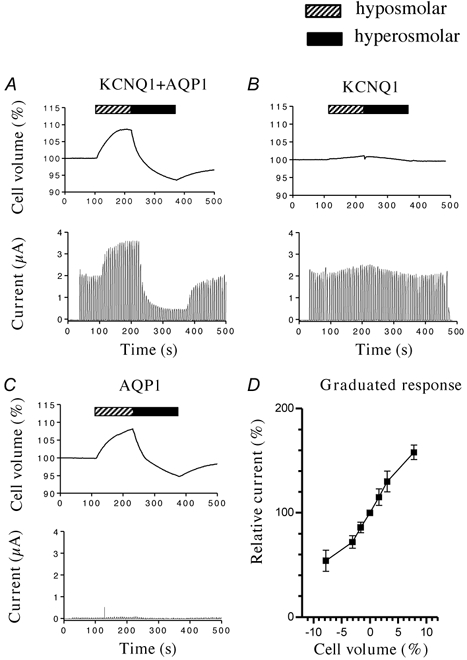
KCNQ1 channels and AQP1 (A), KCNQ1 channels (B) or AQP1 (C) were expressed in Xenopus laevis oocytes, and exposed to a pulsed voltage protocol: 4 s at − 80 mV and 2 s at +20 mV. When indicated, the oocytes were challenged with a hyposmolar (hatched bar, −50 mosmol l−1) or hyperosmolar (filled bar, +50 mosmol l−1) extracellular solution. The cell volume of the oocytes (upper traces) and the measured currents (lower traces) were simultaneously measured. In D, KCNQ1 channels co-expressed with AQP1 were activated as in A, before the oocytes were exposed to diferent extracellular osmolarities (−50, −20, −10, 0, +10, +20, +50 mosmol l−1). The figure shows the maximal current (measured at the end of the depolarization period) as a function of the measured oocyte volume. Data points shows the mean of 4 independent experiments ±s.e.m.
Figure 4. Mechanism for regulation of KCNQ1 channels by cell volume changes.
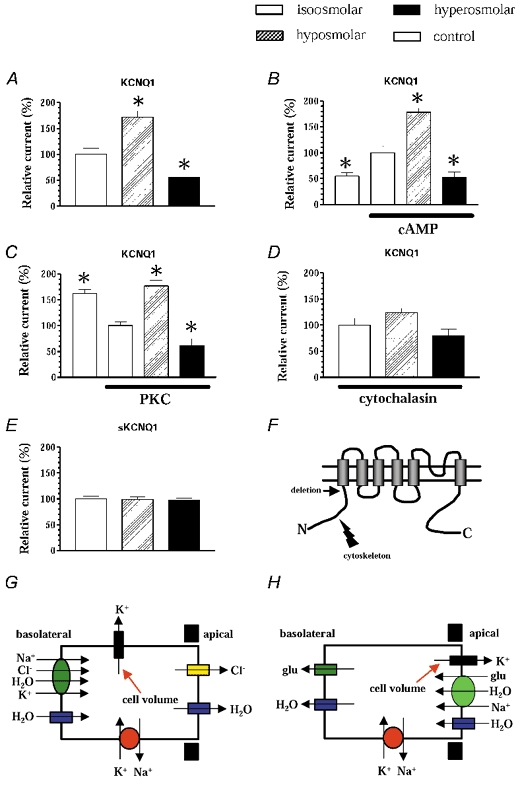
In all experiments KCNQ1 channels were co-expressed with AQP1 in Xenopus laevis oocytes, which were swollen in hyposmolar medium (−50 mosmol l−1, hatched bars) or shrunken in hyperosmolar medium (+50 mosmol l−1, filled bars). The columns show the maximal currents through the expressed channels at +60 mV relative to the currents measured in isotonic medium (open bars). A, the results for wild-type KCNQ1 channels. In B and C the expressed KCNQ1 channels were initially phosphorylated by protein kinase A (panel B) or protein kinase C (panel C), before the oocytes were subject to volume changes. The grey columns show the control currents before phosphorylation. D, the effect of incubation of the oocytes in 1 μm cytochalasin D for 30 min before measurements were made. In E sKCNQ1 channels were coexpressed with AQP1 and volume sensitivity measured as above. F, a model of KCNQ1; the arrow indicates the deletion of the N-terminal in sKCNQ1, which interacts with the cytoskeleton. The currents measured in the control situation ranged from 2.8 to 4.3 μA (for KCNQ1) or from 0.6 to 1.2 μA (for sKCNQ1). Columns show the means of 6–12 independent experiments ±s.e.m.; * Indicates significant differences (P < 0.01). G, model of a secretory epithelial cell, e.g. from distal colon. During cAMP-stimulated secretion Na+, Cl− and K+ are transported into the cell by the basolateral co-transport system (NKCC1), Cl− is secreted across the apical membrane through Cl− channels of the CFTR type and K+ is allowed to recycle through basolateral K+ channels of the KCNQ1 type. The basolateral co-transport system will directly transport or osmotically drive water into the cells. H, in absorptive cells, e.g. in the renal proximal tubule, water is coupled to the Na+-driven transport of solutes (e.g. glucose or amino acids) across the apical membrane (see Zeuthen et al. 2001). Recent studies have suggested that the KCNQ1 channels are expressed in the apical membrane in these cells (Vallon et al. 2001). In both cases a strict regulation of the involved transport systems is necessary during transepithelial transport of solutes and water, and the KCNQ1 channels are expected to play a crucial role for the overall transport. Modulation of the K+ channel activity by changes in cell volume is an obvious regulatory parameter.
Figure 2A shows that the I-V relationship for KCNQ1 channels is not changed during modulation by cell volume changes, indicating that the overall voltage sensitivity (and the selectivity) for KCNQ1 was not affected by cell volume changes. In the experiments shown in Fig. 2B, KCNQ1 co-expressed with AQP1 channels in Xenopus oocytes were activated at isotonic conditions and during swelling and shrinkage by depolarizations to +40 mV for 10 s to ensure full activation of the channels. A comparison of the normalized currents showed that the activation kinetics for the KCNQ1 channels apparently did not differ during cell volume changes. Thus, the effect of cell volume changes on KCNQ1 channels can be considered a mere change in the maximal current that can be conducted after voltage-dependent activation.
Figure 2. Kinetics for KCNQ1 channels during regulation by cell volume changes.
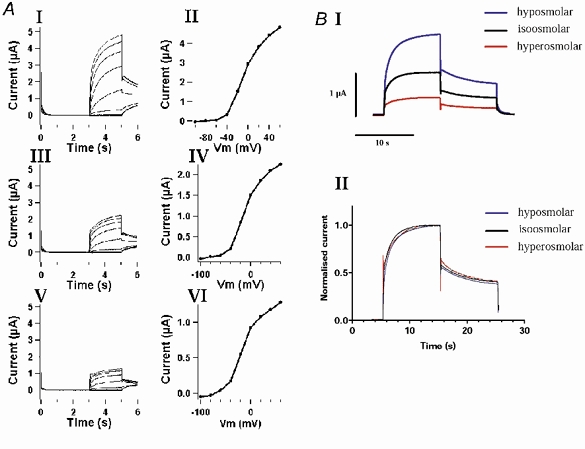
KCNQ1 channels were co-expressed with AQP1 in Xenopus laevis oocytes. A, the expressed KCNQ1 channels were activated by a step-protocol; from a holding potential of −80 mV (3 s) current was recorded at membrane potentials from −100 mV to +60 mV (20 mV steps) for 2 s, and finally the tail current was measured at −30 mV for 1 s. The figure shows the recorded currents and the corresponding I-V curves (plotted as the current measured at the end of the depolarization vs. voltage) in hyposmolar solution (−50 mosmol l−1; I and II) isosmolar solution (III and IV) hyperosmolar solution (+50 mosmol l−1; V and VI). B, the oocytes were kept at a holding potential of −80 mV, before the expressed KCNQ1 channels were activated by a depolarization to +40 mV (10 s). Tail currents were measured at −30 mV (10 s). I shows the recorded current traces in isosmolar, hyposmolar and hyperosmolar media and II shows the same currents normalized to the maximal measured current in each experiment (found after 10 s of depolarization).
Regulation of other KCNQ channels subtypes by changes in cell volume
Remarkably, KCNQ4 channels responded to cell swelling with larger changes in activity than KCNQ1 when co-expressed with AQP1 channels in oocytes (Fig. 3A). When the oocytes were exposed to osmotic challenges of ± 50 mosmol l−1 the current through the expressed KCNQ4 channels increased to 258 % (s.e.m. = 16 %, n = 8) of control in response to cell swelling and decreased to 30 % (s.e.m. = 3 %, n = 8) of control in response to cell shrinkage (Fig. 3A). In contrast to the KCNQ1 and KCNQ4 channels, the KCNQ2-KCNQ3 channels, which most likely mediate the neuronal M-current (Wang et al. 1998), were totally insensitive to changes in cell volume (Fig. 3A). In summary, the results showed that the KCNQ subtypes exhibited significant differences in their volume sensitivity; KCNQ4 channels responded to cell swelling with larger changes in activity than KCNQ1, whereas KCNQ2-KCNQ3 heteromers were insensitive
Figure 3. Sensitivity to cell volume changes for KCNQ subtypes and effect of β-subunits.

A, KCNQ1, KCNQ2/3 or KCNQ4 channels were co-expressed with AQP1 in Xenopus laevis oocytes. The KCNQ1 channels were activated by pulsed depolarizations to +60 mV for 2 s and the maximal currents (at the end of the depolarization period) were measured under isotonic conditions, and during cell swelling (−50 mosmol l−1) and cell shrinkage (+50 mosmol l−1). The columns show the relative changes in maximal current through the expressed KCNQ1 channels during swelling (hatched bars) and shrinkage (filled bars) as compared to the current under isotonic conditions. B, KCNQ1 channels were co-expressed with AQP1 in the presence or absence of the β-subunits KCNE1–3, activated by depolarizations to +60 mV for 2 s (20 s for KCNQ1-KCNE1) and their sensitivity to cell volume changes was determined as described above. The maximal currents in the control situation (isotonic medium) ranged from 1.2 to 4.8 μA depending on the expressed isoforms. Columns show the means of 4–12 independent experiments ±s.e.m. * Indicates significant differences (P < 0.01).
Effect of regulatory subunits of the KCNE type
The small subunits KCNE1–3 have significant effects on KCNQ-channel gating properties (Barhanin et al. 1996; Sanguinetti et al. 1996; Romey et al. 1997; Schroeder et al. 2000; Sanguinetti, 2000). We examined whether the co-expresssion of KCNEs could affect the responses to cell volume changes of the KCNQ1 channels. In the presence of KCNEs, KCNQ1 channels were still sensitive to cell volume changes (Fig. 3B). Co-expression of KCNE1 significantly attenuated the swelling-induced increase in KCNQ1 current, whereas the response to cell shrinking was unchanged. Co-expression of KCNE2 or KCNE3, on the other hand, did not alter the response of KCNQ1 to changes in cell volume.
Mechanism for regulation of KCNQ1 channels by cell volume changes
KCNQ1 channel proteins contain consensus sequences for protein kinase A and protein kinase C phosphorylation. It has been suggested that cell volume changes possibly could modulate membrane proteins through phosphorylation (Vandenberg, 1996; Lang et al. 1998; O'Neill, 1999). Therefore, we examined if KCNQ1 channels could still respond to cell volume changes after phosphorylation by protein kinase A and protein kinase C. Figure 4B and C shows that although phosphorylations have pronounced effects on the activity of KCNQ1 channels, the relative responses to cell volume changes are fully preserved after phosphorylation. This indicated that protein kinase A and C are not involved in the sensitivity of KCNQ1 channels to cell volume changes.
Next, we investigated how important an intact actin cytoskeleton was for the observed cell volume-dependent regulation of KCNQ1 channel activity. Oocytes co-expressing KCNQ1 and AQP1 were incubated with 1 μm cytochalasin D for 30 min before they were exposed to extracellular osmotic challenges. Incubation with cytochalasin D did not affect the current through the KCNQ1 channels at isotonic conditions, and did not have any effect on the ability of the oocytes to swell and shrink in response to osmotic challenges (data not shown). However, after treatment with cytochalasin, KCNQ1 channels no longer responded significantly to cell volume changes (Fig. 4D). The effect of cytochalasin D could be prevented if the oocytes were treated with 1 μm phalloidin, a compound antagonizing the destabilizing effect of cytochalasin D on actin filaments (data not shown). The experiments indicated that the sensitivity of KCNQ1 channels to changes in cell volume is critically dependent on an intact actin cytoskeleton.
The N-terminus of KCNQ1 channels is required for regulation by cell volume changes
Taking the cytochalasin experiments into account, it could be tempting to suggest that the cytoskeleton through actin or other cytoskeletal components interacts with parts of the channel protein. It has recently been shown that KCNQ1 protein, which has the 95 N-terminal amino acids deleted (short KCNQ1 or sKCNQ1), forms fully functional channels after expression in Xenopus oocytes (Schmitt et al. 2000). When these channels were co-expressed with AQP1 in oocytes, currents were totally insensitive to changes in cell volume (Fig. 4E and F). This experiment indicated that the N-terminus of KCNQ1 contains structural elements which render KCNQ1 channels sensitive to cell volume changes.
DISCUSSION
The present study examined the effect of cell volume changes on cloned voltage-regulated K+ channels of the KCNQ type. KCNQ1 and KCNQ4 channels, but not KCNQ2/3 channels, were readily stimulated by cell swelling and inhibited by cell shrinkage. In addition, it was shown that the KCNQ1 and KCNQ4 channels strictly reflected even very small changes in cell volume, and that the responses showed some saturation if the swelling or shrinkage exceeded 5 %. Sensitivity to such small changes in cell volume must be expected to be of physiological relevance, and we believe it is not an exaggeration to regard certain KCNQ channels as ‘sensors of cell volume’. In addition, this regulatory mechanism explains the observations that KCNQ1 channels in in vitro experiments seem to be activated by cell swelling in e.g. cardiomyocytes (Vandenberg et al. 1996) and airway epithelial cells (Lock & Valverde, 2000), and after expression in mammalian cells (Kubota et al. 2002). These observations suggest that the sensitivity of certain KCNQ channels to changes in cell volume is not a phenomenon restricted to expression systems, but most likely should be regarded as a general regulatory mechanism.
The strict regulation of certain KCNQ currents by cell volume could in principle be mediated through three mechanisms: changes in the number of active channels in the plasma membrane, modulation of the open probability of channels already in the plasma membrane or changes in the single channel conductances. At present we cannot distinguish between these possibilities. However, our data show that the sensitivity of KCNQ channels to cell volume changes is not critically dependent on regulatory subunits of the KCNE type although co-expressed KCNE1 to some extent modulates the volume sensitivity of KCNQ1, indicating that the volume sensitivity must be a property of the KCNQ1 and KCNQ4 α-subunits. These observations are consistent with Kubota et al. (2002), who showed that KCNQ1 channels, expressed in mammalian cells in the absence of KCNE1, are activated by cell swelling. However, in contrast, Lock & Valverde (2000) reported that in KCNE1 knock-out mice, the KCNQ1 channels failed to respond to cell swelling. The reason for this discrepancy is not clear, but may be due to the fact that the plasma membrane expression of KCNQ1 in the KCNE1 knock-out animals is low.
A large number of possible pathways could link cell volume and KCNQ channel activity (Lang et al. 1998; O'Neill, 1999). Apart from membrane depolarization, it has been suggested that KCNQ1 channels are regulated by Ca2+ and phosphorylation by protein kinase A and C (Schroeder et al. 2000; Boucherot et al. 2001; Kunzelmann et al. 2001), and we considered whether the volume sensitivity should be mediated through one of these mechanisms. In earlier studies it has not been possible by fluorescence methods to measure any significant changes in global intracellular Ca2+ in Xenopus oocytes during volume changes (Vandorpe et al. 1998). In addition, experiments employing the Ca2+-activated high-conductance K+ channel as a probe for Ca2+ have shown that changes in the oocyte volume do not result in changes in free Ca2+ in the close vicinity of the plasma membrane (Grunnet et al. 2002). Finally, in contrast to regulation by cell volume, regulation by intracellular Ca2+ seems to be dependent on co-expressed KCNE β-subunits (Boucherot et al. 2001). Taken together a Ca2+-mediated cell volume effect on KCNQ1 channels was not considered likely. Figure 4 shows that although KCNQ1 channels are clearly modulated by protein kinase A and C, phosphorylation apparently did not interact with the sensitivity to cell volume; the effects of phosphorylation and cell volume were additive.
Thus, considering the experiments show in Fig. 4D and E, our results suggest that KCNQ1 and KCNQ4 channels expressed in oocytes are strictly regulated by cell volume changes through interactions between the cytoskeleton and structural elements in the N-terminus of the channel protein. This regulatory mechanism could be expected to be ubiquitous for volume sensitivity in channels (see Lang et al. 1998; O'Neill 1999; Grunnet et al. 2002), but identification of possible N-terminal structures involved will require further experiments.
In particular, the sensitivity of certain KCNQ channels to cell volume changes could be an important regulatory parameter in epithelial cells and in cardiac myocytes. In secretory cells in the colonic crypts, KCNQ1-KCNE3 channels have been identified in the basolateral membrane of the crypt cells (Schroeder et al. 2000) and pharmacological studies have shown that recycling of K+ through the basolateral KCNQ1-KCNE3 channels is crucial for the transepithelial transport of Cl− (see Fig. 4G and Lohrman et al. 1995; Kunzelmann et al. 2001). Our results showed that KCNQ1 channels are not stimulated only by cAMP (Schroeder et al. 2000, Kunzelmann et al. 2001), but also by cell volume changes. During secretion massive amounts of water may be transported through the epithelial cells, and the regulation of the basolateral K+ channels must be strictly coordinated with that of the other transporters to avoid potentially fatal imbalances in intracellular ion and water content (see Fig. 4G). Indeed cell volume is an obvious regulatory parameter (see Weyand et al. 1998), and the fact that the effects of cAMP and cell volume changes on the KCNQ1 channels are additive (see Fig. 4) may explain the rather dramatic changes in basolateral K+ conductance seen e.g. during secretory diarrhoea.
In addition, the strict regulation of KCNQ1 channels by cell volume, especially by cell volume increases, makes these channels obvious modulators of epithelial re-absorption of solutes and water in other epithelia. Indeed, it has been shown that KCNE1−/− mice are volume depleted, hypokalaemic and suffer from an increased level of aldosterone (Arrighi et al. 2001; Warth & Barhanin, 2002; Attali, 2002). In proximal kidney tubules cells, where KCNQ1-KCNE1 channels most likely are located in the apical membrane (Vallon et al. 2001), volume is tightly regulated in response to the glucose and amino acid load. Solute take-up is associated with Na+ and water influx. The observed activation of epithelial KCNQ1-KCNE1 channels upon cell volume increase suggests an obvious cellular mechanism to exert tight cell-volume control. Volume-dependent activation of the KCNQ1 potassium channels most likely hyperpolarizes the cell membrane leading to an increase in the electrogenic Na+-dependent re-absorption of solutes as schematically diagrammed in Fig. 4H. A compromised reabsorption in the proximal tubule due to impaired function of the KCNQ1-KCNE1 channel will lead to volume depletion and in consequence hyperaldosteronaemia. In the distal tubule the aldosterone stimulation and increased tubular flow will stimulate K+ excretion and lead to hypokalaemia. It should be expected that these mechanisms play an important role in the pathophysiology of patients suffering from mutations in the KCNQ1 or KCNE1 genes.
Our results may provide an explanation for the observation that KCNQ1-KCNE1 channel activity, the molecular correlate of IKs, the slowly activating cardiac K+ current, is increased when cardiomyocytes swell during experimental procedures (Sasaki et al. 1994; Kubota et al. 2002) or during ischaemia (Vandenberg, 1996). In addition, our results emphasize that renal re-absorption problems, as seen in KCNE1−/− mice (see Attali, 2002), may add to the intrinsic cardiac problems that lead to episodic arrhythmic attacks in LQT1 patients.
Acknowledgments
Ms B. Lynderup and Ms T. Soland are thanked for expert technical assistance. This work was supported by grants from the Danish Medical Research Council, The Novo Nordisk Foundation, The Velux Foundation, The San Cataldo Foundation and the Le-Ducq Foundation.
REFERENCES
- Arrighi I, Bloch-Faure M, Grahammer F, Bleich M, Warth R, Mengual R, Drici MD, Barhanin J, Meneton P. Altered potassium balance and aldosterone secretion in a mouse model of human cognital long QT syndrome. Proc Natl Acad Sci U S A. 2001;98:8792–8792. doi: 10.1073/pnas.141233398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attali B. Human cognital QT syndrome: more than previously thought? Trends Pharmacol Sci. 2002;23:249–251. doi: 10.1016/s0165-6147(02)02029-1. [DOI] [PubMed] [Google Scholar]
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and IsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Boucherot A, Schreiber R, Kunzelmann K. Regulation and properties of KCNQ1 (KvLQT1) and impact of the cystic fibrosis transmembrane conductance regulator. J Membrane Biol. 2001;182:39–47. doi: 10.1007/s00232-001-0030-4. [DOI] [PubMed] [Google Scholar]
- Grunnet M, MacAulay N, Jørgensen NK, Jensen BS, Olesen SP, Klaerke DA. Regulation of cloned Ca2+-activated K+ channels by cell volume changes. Pflugers Arch. 2002;444:167–177. doi: 10.1007/s00424-002-0782-4. [DOI] [PubMed] [Google Scholar]
- Hoffmann EK, Hougaard C. Intracellular signalling involved in activation of the volume-sensitive K+ current in Ehrlich ascites tumour cells. Comp Biochem Physiol. 2001;130:355–366. doi: 10.1016/s1095-6433(01)00419-6. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nature Rev Neurosci. 2000;1:1–21. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin JP, Safieddine S, Schweitzer M, El-Amraoli A, Petit C, Jentsch TJ. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci U S A. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lütjohann B, Elamraoui A, Marlin S, Petit C, Jentsch TC. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–446. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- Kubota T, Horie M, Takano M, Yoshida H, Otani H, Sasayama S. Role of KCNQ1 in cell swelling-induced enhancement of the slowly activating delayed rectifier K+ current. Jpn J Physiol. 2002;52:31–39. doi: 10.2170/jjphysiol.52.31. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Hübner M, Schreiber R, Levy-Holzman R, Garty H, Bleich M, Warth R, Slavik M, Von Hahn T, Greger R. Cloning and function of the rat colonic epithelial K+ channel KvLQT1. J Membr Biol. 2001;179:155–164. doi: 10.1007/s002320010045. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Volkl H. The diversity of volume regulatory mechanisms. Cell Physiol Biochem. 1998;8:1–45. doi: 10.1159/000016269. [DOI] [PubMed] [Google Scholar]
- Lock H, Valverde MA. Contribution of the IsK (MinK) potassium channel subunit to regulatory volume decrease in murine tracheal epithelial cells. J Biol Chem. 2000;275:34849–34852. doi: 10.1074/jbc.C000633200. [DOI] [PubMed] [Google Scholar]
- Lohrmann E, Burhoff I, Nitschke RB, Lang HJ, Mania D, Englert HC, Hropot M, Warth R, Rohm M, Bleich M, Greger R. A new class of inhibitors of cAMP-mediated Cl− secretion in rabbit colon, acting by the reduction of cAMP-activated K+ conductance. Pflugers Arch. 1995;429:517–530. doi: 10.1007/BF00704157. [DOI] [PubMed] [Google Scholar]
- Niemeyer MI, Cid LP, Sepulveda FV. K+ conductance activated during regulatory volume decrease. The channels in Ehrlich cells and their possible molecular countarpart. Comp Biochem Physiol. 2001;130:565–575. doi: 10.1016/s1095-6433(01)00428-7. [DOI] [PubMed] [Google Scholar]
- O'Neill WC. Physiologial significance of volume-regulatory transporters. Am J Physiol. 1999;276:C995–C1011. doi: 10.1152/ajpcell.1999.276.5.C995. [DOI] [PubMed] [Google Scholar]
- Romey G, Attali B, Chouabe C, Abitbol I, Guillemare E, Barhanin J, Lazdunski M. Molecular mechanism and functional signigicance of the MinK control of the KvLQT1 channel activity. J Biol Chem. 1997;272:16713–16716. doi: 10.1074/jbc.272.27.16713. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC. Maximal function of minimal K+ channel subunits. Trends Pharmacol Sci. 2000;21:199–201. doi: 10.1016/s0165-6147(00)01475-9. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Assembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Mitsuiye T, Wang Z, Noma A. Increase of the delayed rectifier K+ and Na+-K+ pump currents by hypotonic solutions in gunea pig cardiac myocytes. Circ Res. 1994;75:887–895. doi: 10.1161/01.res.75.5.887. [DOI] [PubMed] [Google Scholar]
- Schmitt N, Schwarz M, Paretz A, Abitbol I, Attali B, Pongs O. A recessive C-terminal Jervell and Lange-Nielsen mutation of the KCNQ1 channel impairs subunit assembly. EMBO J. 2000;19:332–340. doi: 10.1093/emboj/19.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- Vallon V, Grahammer F, Richter K, Bleich M, Lang F, Barhanin J, Völkl H, Warth R. Role of KCNE1-dependent K+ fluxes in mouse proximal tubule. J Am Soc Nephrol. 2001;12:2003–2011. doi: 10.1681/ASN.V12102003. [DOI] [PubMed] [Google Scholar]
- Vandenberg JI, Rees SA, Wright AR, Powell TR. Cell swelling and ion transport pathways in cardiac cycle. Cardiovasc Res. 1996;32:85–97. [PubMed] [Google Scholar]
- Vandorpe DH, Shmukler BE, Jiang L, Lim B, Maylie J, Adelman JP, De Franceschi L, Cappellini MD, Brugnara C, Alper SL. cDNA cloning and functional characterization of the mouse Ca2+-gated K+ channel, mIK1. Roles in regulatory volume decrease and erythoid differentiation. J Biol Chem. 1998;273:21542–21553. doi: 10.1074/jbc.273.34.21542. [DOI] [PubMed] [Google Scholar]
- Wang Q. Positional cloning of a novel potassium channel gene: KVLQT mutations cause cardiac arrhythmias. Nature Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, Van Raay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating M. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nature Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: Molecular correlates of the M-current. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Warth R, Barhanin J. The multifaceted phenotype of the knockout mouse for the KCNE1 potassium. Am J Physiol Regul Integr Comp Physiol. 2002;282:R639–648. doi: 10.1152/ajpregu.00649.2001. [DOI] [PubMed] [Google Scholar]
- Weyand B, Warth R, Bleich M, Kerstan M, Kerstan D, Nitsche R, Greger R. Hypertonic cell shrinkage reduces the K+ conductance of rat colonic crypts. Pflugers Arch. 1998;436:227–232. doi: 10.1007/s004240050626. [DOI] [PubMed] [Google Scholar]
- Zeuthen T, Meinild AK, Loo DD, Wright EM, Klaerke DA. Isotonic transport by the Na+-glucose cotransporter SGLT1 from humans and rabbit. J Physiol. 2001;531:631–644. doi: 10.1111/j.1469-7793.2001.0631h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


