Abstract
Previous work in this laboratory has demonstrated that cerebral hypoperfusion increases the expression of prostaglandin endoperoxide synthase-2 (PGHS-2) in ovine fetal brain regions. Endogenously produced prostaglandins, in turn, partially mediate the fetal hypothalamus- pituitary-adrenal (HPA) axis response to arterial hypotension. In separate experiments, we have found that oestradiol stimulates fetal HPA axis activity. The present experiments were designed to test the hypothesis that oestradiol increases the expression of PGHS isoforms, and that oestradiol augments the PGHS response to cerebral hypoperfusion. Sixteen fetal sheep of known gestational ages (124–128 days' gestation at the time of study) were subjected to chronic catheterization and implantation of extravascular occluder around the brachiocephalic artery. Eight fetuses were subjected to subcutaneous implantation of a pellet containing 17β-oestradiol (release rate 5 mg (21 days)−1). Brachiocephalic occlusion (BCO) stimulated adrenocorticotropin (ACTH), cortisol and arginine vasopressin (AVP) secretion, responses that were augmented by oestradiol. One hour after the beginning of a 10 min period of BCO, PGHS-1 mRNA was increased in fetal brainstem and hypothalamus, and PGHS-2 mRNA was increased in fetal brainstem. Oestradiol, by itself, increased the abundance of PGHS-2 mRNA in brainstem and cerebellum, and augmented the PGHS-2 mRNA response to BCO in brainstem. In contrast, oestradiol had no significant effect on the abundance of PGHS-1 mRNA in any brain region. PGHS-1 and PGHS-2 protein levels did not reflect the changes in the respective mRNAs. The abundance of both proteins was increased in cerebral cortex in response to BCO, and the abundance of PGHS-2 protein was increased by both oestradiol and BCO in the hippocampus. The results of this study confirm and extend the results of our previous studies, demonstrating an effect of cerebral hypoperfusion on the expression of both isoforms of PGHS. We conclude that oestradiol increases the expression of PGHS-2 in specific fetal brain regions, and that there is an interaction between oestradiol and BCO in the control of PGHS-2 expression in the fetal brainstem. We expect that at later time-points, the changes in mRNA would be followed by similar changes in enzyme abundance at the protein level. We speculate that at least a part of the effect of oestradiol on fetal HPA axis function is mediated by an interaction between oestradiol and prostaglandin biosynthesis in the fetal brain.
The activity of the fetal hypothalamus-pituitary-adrenal axis (HPA) and the responsiveness of this endocrine axis to acute stress plays an important role in the maintenance of fetal homeostasis, the co-ordination of fetal maturation, and the timing of birth. The control of this endocrine axis is complex, involving brain maturation, development and modulation of corticosteroid negative feedback, and activity of visceral afferent nerves, including but not restricted to the arterial baroreceptors and chemoreceptors.
Previous work in this laboratory has demonstrated that oestradiol has a potent stimulatory effect on the fetal HPA axis (Wood & Saoud, 1997; Saoud & Wood, 1997; Wood et al. 2001). In the fetal sheep, as in other mammalian species, the activity of the fetal adrenal is involved in the control of oestrogen biosynthesis. In the sheep fetus, increases in plasma cortisol concentration at the end of gestation induce the activity of 17α-hydroxylase (cytochrome P450c17) in the placenta which, in turn, synthesizes oestrogen at the expense of progesterone. It is the increase in oestrogen-to-progesterone ratio that ultimately stimulates labour and delivery of the lamb. We have speculated that the stimulation of ACTH by oestradiol represents one limb of a positive feedback loop. This positive feedback loop accelerates ACTH and oestrogen biosynthesis until the process is terminated by delivery. We have also proposed that the increasing concentrations of oestradiol in late gestation augment fetal HPA responses to stress. This augmented HPA activity therefore occurs at a time when fetal stress resulting from hypoxia, hypercapnia and hypotension are more common.
There is a rich literature demonstrating that prostaglandin E2 (PGE2) stimulates fetal ACTH secretion. Thorburn and colleagues have reported that intravenous infusions of PGE2 stimulate HPA function, and that if infused long enough and in high enough amounts, PGE2 initiates parturition (Thorburn, 1991; Young & Thorburn, 1994). We have demonstrated that ACTH responses to hypotension are in part mediated by the generation of prostanoids. We have proposed that the relevant prostanoid generation that mediates ACTH responses to hypotension is within the fetal brain, specifically in brain regions containing baroreceptor and chemoreceptor pathways (Tong et al. 1999; Wood & Tong, 1999). In support of this hypothesis, we have found that cerebral hypoperfusion upregulates the expression of PGHS-2 mRNA in fetal brainstem and hypothalamus (Tong et al. 2000)
The present study was designed to test the hypothesis that oestradiol augments the expression of prostaglandin biosynthetic enzymes in the fetal brain. Specifically, we propose that oestradiol increases the expression of prostaglandin endoperoxide synthase-1 and/or −2 (PGHS-1 and PGHS-2, or COX-1 and COX-2) in fetal brainstem and other regions important for baroreceptor and chemoreceptor signalling. We propose that oestradiol will augment PGHS expression in the normotensive fetus, and that it will amplify the effect of cerebral hypoperfusion on PGHS expression.
METHODS
The experiments were approved by the University of Florida Animal Care and Use Committee and were performed in accordance with the Guiding Principles for Use of Animals of the American Physiological Society. We studied 16 fetal sheep of known gestational ages. Barbados strain pregnant ewes all had singleton (n = 8) and twin (n = 4) pregnancies of 124–128 days gestation at the time of experimentation.
Fetal surgery
Fetal surgery was performed as previously described (Tong & Wood, 1999). Food was withheld from the pregnant ewe for 24 h before surgery. Before and during surgery, the ewe was anaesthetized with halothane (0.5–2.0 %) in oxygen. The uterus was exposed using a midline incision and the fetal hindlimbs were located. Through small incision in the uterus, both hindlimbs were delivered and a polyvinylchloride catheter (0.030 in i.d., 0.050 in o.d.) was inserted into each femoral artery and the tip advanced to the subdiaphramatic aorta. Before closing the incision, oestradiol (E2; 5 mg (21 days)−1) or placebo implants (Innovative Research of America, Sarasota, FL, USA) were placed into the fetus subcutaneously. After closing the incision, an amniotic fluid catheter was sewn to the hindlimb before it was returned to the amniotic cavity. After catheterization of the fetal hindlimbs, the uterus was incised near the fetal head. The head was delivered through the incision, and a single midline incision was made over the trachea at the level of the angle of the jaw. Through this incision the lingual arteries were exposed and catheterized. The tips of the lingual catheters were advanced retrograde into the common carotid arteries. After closing the incision, the catheters were secured by suturing them to the skin rostral to the incision. Next, the left forelimb was delivered through the incision, and the chest was exposed to the level of the third intercostal space. The chest was incised and the ribs were spread, exposing the brachiocephalic artery, around which a brachiocephalic occluder (In Vivo Metric, 8 mm diameter, Healdsburg, CA, USA) was placed. The chest was closed, the fetus returned to the amniotic space, and the second uterine incision closed. All catheters were routed to the flank of the ewe, where they exited the skin and were held in place within the cloth pocket. The maternal abdominal incisions were closed in layers. Free ends of the catheters were held in place with a cloth pocket held to the skin with a commercial bandage (Spandage, Medi-Tech International, Brooklyn, NY, USA). The placement of all the catheters was later confirmed at necropsy, which was performed at the end of the experimental period.
Summary of experimental procedures
All ewes were allowed 5 days recovery after surgery. Each fetus was subjected to one experiment. One-half of the fetuses were subjected to a 10 min period of cerebral hypoperfusion, caused by brachiocephalic occlusion (BCO). The other half of the fetuses were subjected to a 10 min period of sham-BCO, in which the occluder catheter was manipulated but the occluder was not inflated. In each experiment, fetal blood samples (5 ml) were drawn immediately before, at the end, and 10 min after the end of BCO or sham-BCO.
Lingual arterial pressure was monitored for 35 min, starting 5 min before the period of BCO or sham-BCO, in order to verify a proper occlusion. One hour after beginning the BCO (or sham-BCO), the fetus and ewe were killed by an overdose of pentobarbital. Brains were rapidly removed and dissected to isolate brain regions of interest. Tissues were snap-frozen in an acetone-dry ice solution and stored at −80 °C until mRNA was extracted or Western blot analyses were performed.
Endocrine data
Blood samples for analysis of plasma hormone concentrations were placed into chilled tubes containing Na4EDTA. Plasma was separated from red blood cells centrifugally (3000 g; Sorvall Instruments), then aliquoted and stored at −20 °C in a manual defrost freezer until assay. Plasma adrenocorticotropin (ACTH), cortisol and arginine vasopressin (AVP) concentrations were measured using radioimmunoassay as described previously (Bell et al. 1991; Raff et al. 1991; Wood et al. 1993). Plasma oestradiol concentrations were measured using an EIA kit purchased from Oxford Biomedical (catalogue number EA70; Oxford, MI, USA) as described previously (Wood et al. 2003).
PGHS expression data
At sacrifice, the following regions were dissected: (1) hypothalamus, (2) hippocampus, (3) brainstem, (4) pituitary, (5) cerebral cortex and (6) cerebellum. All tissue samples were placed in RNAse-free polypropylene tubes, snap-frozen in liquid nitrogen or in an acetone-dry ice bath, and stored at −80 °C until used for mRNA or protein extraction.
Messenger RNA was extracted from frozen tissues using Trizol (Life Technologies) according to the manufacturer's instructions. After extraction of mRNA, all samples were treated with RNase-free DNase (Promega Corp., Madison, WI, USA) to remove any residual genomic DNA. Samples were incubated with DNase 37 °C for 30 min, followed by DNase stop solution, followed by incubation at 65 °C for 10 min to inactivate the DNase.
PGHS-1, PGHS-2 and 18S rRNA abundance were measured using real-time RT-PCR using pre-mixed reagents (One-Step RT-PCR master mix kit, Applied Biosystems, Foster City, CA, USA). This methodology has been reported previously (Tong et al. 2002). Each reaction was run in a total volume of 25 μl, and contained 10 ng (PGHS-1 and PGHS-2) or 0.1 ng (18S rRNA) RNA. The reaction times and temperatures were 48 °C for 30 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. Primer and Taqman probe sets were designed from known sequences for ovine PGHS-1 and PGHS-2 using Primer Express version 1.0 software (Applied Biosystems). Primer/probe sets for 18S rRNA were purchased from Applied Biosystems. The sequences of the individual primers and probes are reported in Table 1. Forward and reverse primer concentrations for all three reactions were 100 nm. Probe concentrations for PGHS-1 and PGHS-2 were 200 nm, and for 18S rRNA was 50 nm. Each plate was set up to include sample unknowns from all animal groups for a single gene, plus no-template controls from each primer/probe set, and a no-RT control group (to assess genomic DNA contamination) for each animal using the 18S ribosomal control primer/probes. For all three real-time RT-PCR reactions, dilution of mRNA yielded linear and proportional decreases in measured mRNA abundance, validated by quantifying changes in cycle threshold for dilution of mRNA.
Table 1.
PGHS primer and probe sequences used in real-time RT-PCR
| Forward primer sequence (5′-3′) | Reverse primer sequence (5′-3′) | Probe sequence (5′-3′) | |
|---|---|---|---|
| PGHS-1 | GGCACCAACCTCATGTTTGC | TCTTGCCGAAGTTTTGAAGA | TTCTTTGCCCAACACTTCACCCATCA |
| PGHS-2 | GCACAAATCTGATGTTTGCATTCT | CTGGTCCTCGTTCATATCTGCTT | TGCCCAGCACTTCACCCATCAATTTT |
For Western blot analysis of PGHS-1 and PGHS-2 protein, we used methods similar to those that we have previously reported (Tong et al. 2002). Homogenization of tissue was carried out using boiling lysis buffer containing 1 % SDS, 1.0 mm sodium orthovanadate, 10 mm Tris 7.4 (Sigma Chemical Co., St Louis, MO, USA). Homogenized samples were boiled for 5 min and centrifuged at 11 000 g at 4 °C for 20 min to remove particulate matter. Supernatants were aliquoted into microcentrifuge tubes, and stored at −80 °C until Western blot analysis. Samples (15 μg protein per lane), assayed using the Bradford method, were loaded onto 7.5 % Criterion Tris-HCl gel (BioRad Co., San Rafael, CA, USA) for SDS-PAGE. Each gel accommodates 16 lanes of unknowns, one lane of molecular weight markers (Rainbow markers, Amersham Co.) and one lane of a positive control. After electrophoresis the proteins were transferred onto nitrocellulose membranes overnight at 22 V, blocked, and probed with primary antibody. PGHS-1 and PGHS-2 were immunostained using polyclonal anti-PGHS-1 and anti-PGHS-2 antisera PG21 (1:500) and PG27b (1:1000), respectively, from Oxford Biomedical (Oxford, MI, USA). Staining was visualized using goat peroxidase-conjugated anti-rabbit IgG (catalogue no. A0545, concentration 1:5000, Sigma Chemical Co.), and ECL reagent (Amersham). Blots were analysed using Quantity One software (BioRad Co.). The results of the densitometry were expressed as relative optical density (OD) units.
Calculations and statistical analyses
In each assay, the cycle threshold (CT) value for each sample, for each gene, in all groups, was determined in triplicate. The mean value for these was used to determine ΔCT by subtracting the mean values from triplicates of the 18S ribosomal RNA values from each sample. Values of ΔCT were analysed statistically without further transformation, since this variable was normally distributed. These data were analysed by two-way ANOVA. Relative differences among groups were calculated as the fold change relative to the untreated (no oestradiol, no BCO) group. The ΔΔCT values were calculated as the difference between the individual ΔCT value and the mean ΔCT of the untreated group. Fold changes were calculated using the following equation:
Values of plasma ACTH, AVP and cortisol concentrations were analysed using a three-way ANOVA test corrected for repeated measures in one dimension (time). If relevant interaction effects were significant, we performed pairwise comparison of individual means using Tukey's multiple range test. Fetal plasma oestradiol concentrations were analysed using Student's unpaired t test. Fetal arterial blood pressure, corrected by subtraction of amniotic fluid pressure, was analysed by a three-way ANOVA test corrected for repeated measures in one dimension (time), and if appropriate, by Tukey's test. The tissue mRNA and protein data were analysed by two-way ANOVA. If the interaction term was significant, a pairwise comparison of means using Tukey's multiple range test was used. All statistical tests were performed using SPSS version 11 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Fetal arterial blood gases are reported in Table 2. There was no statistically significant difference in blood gas or pH values among the four experimental groups. Fetal arterial blood pressure was significantly elevated in the oestradiol-treated fetuses (P < 0.05, significant main effect of oestradiol treatment in three-way ANOVA; Fig. 1). Lingual arterial blood pressure was decreased by BCO during the period of occlusion, and was unchanged in the fetuses not subjected to BCO (Fig. 1). Fetal plasma oestradiol concentrations were higher in the oestradiol-treated fetuses compared with the placebo-treated fetuses (62 ± 6 pg ml−1vs. 103 ± 12 pg ml−1, respectively; P < 0.001).
Table 2.
Fetal arterial blood gases and pH values in experimental groups
| Variable | Control | BCO | Oestradiol | Oestradiol + BCO |
|---|---|---|---|---|
| Pa,O2 | 19.1±0.8 | 18.6 ± 1.1 | 19.33 ± 1.4 | 16.5 ± 0.7 |
| Pa,CO2 | 48.4 ± 2.1 | 53.0 ± 2.0 | 50.4 ± 0.7 | 50.9 ± 1.3 |
| pHa | 7.388 ± 0.005 | 7.377 ± 0.009 | 7.392 ± 0.009 | 7.391 ± 0.009 |
BCO, brachiocephalic occlusion. Means ±s.e.m., n = 4 per group.
Figure 1. Fetal lingual arterial blood pressure.
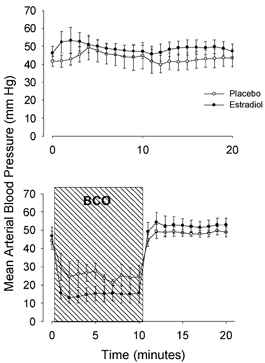
The fetal mean lingual arterial blood pressure in fetal sheep that were (bottom) and were not (top) subjected to a 10 min period of brachiocephalic occlusion (BCO, represented by hatched area). Oestradiol-treated groups are represented by filled symbols and placebo-treated groups are represented by open symbols. Data are presented as means ±s.e.m.
Plasma ACTH concentration was increased by BCO, and the ACTH response to BCO was augmented by oestradiol (Fig. 2, top panel). Analysis of the data by three-way ANOVA revealed statistically significant main effects of oestradiol (P < 0.001), BCO (P < 0.001), oestradiol × time interaction (P < 0.01), BCO × time interaction (P < 0.001) and oestradiol × BCO × time three-way interaction (P < 0.01). Post-hoc analyses of individual group means using Tukey's test revealed that plasma ACTH concentrations were significantly elevated at 10 min with oestradiol plus BCO (P < 0.001) compared with mean values from other groups. Plasma cortisol concentrations increased in response to BCO, but not in response to oestradiol alone (Fig. 2, middle panel). Analysis of the cortisol data by three-way ANOVA revealed main effects of oestradiol (P < 0.01), BCO (P < 0.001) and time (P < 0.05), as well as significant oestradiol × BCO interaction (P < 0.01). Tukey's test revealed that cortisol was significantly elevated only with both oestradiol treatment and BCO (P < 0.001) compared with mean values from other groups. Fetal plasma AVP concentration increased in response to BCO and oestradiol (Fig. 2, bottom panel). Results of the three-way ANOVA revealed significant main effects of oestradiol (P < 0.05), BCO (P < 0.05) and time (P < 0.01), as well as a significant oestradiol × BCO interaction (P < 0.05).
Figure 2. Fetal plasma concentrations of adrenocorticotropin, cortisol and arginine vasopressin.
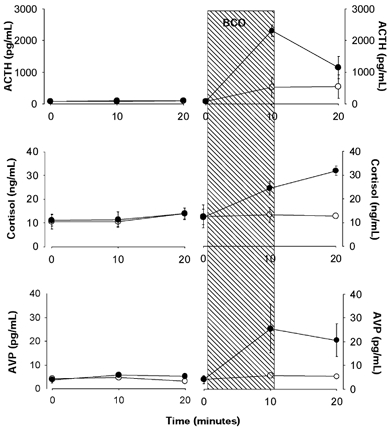
Top panels, adrenocorticotropin (ACTH); middle panels, cortisol; and bottom panels, arginine vasopressin (AVP). Oestradiol-treated groups are represented by filled symbols and placebo-treated groups are represented by open symbols. The 10 min period of brachiocephalic occlusion (BCO) is represented by the hatched area. Data are presented as means ±s.e.m.
PGHS-1 mRNA was significantly increased by BCO in brainstem and hypothalamus (Fig. 3). Analysis of the data by ANOVA revealed significant main effects of BCO in brainstem and hypothalamus (both P < 0.05). Oestradiol did not alter the expression of PGHS-1 in any brain region. PGHS-2 mRNA was increased significantly by oestradiol in brainstem and in cerebellum, but not in the other brain regions or in the pituitary (Fig. 4). In brainstem, there were statistically significant main effects of BCO and oestradiol treatment on PGHS-2 mRNA abundance in the two-way ANOVA (both P < 0.01), as well as significant oestradiol × BCO interaction (P < 0.05). In cerebellum, there was a significant main effect of oestradiol (P < 0.05).
Figure 3. Changes in PGHS-1 mRNA abundance in five brain regions and pituitary.
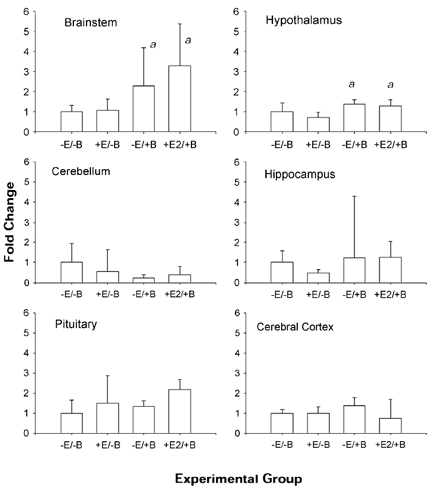
Data are presented as means ±s.e.m. (n = 4 per group). Statistically significant main effect of BCO is represented by a. B is brachiocephalic occlusion; E and E2 are oestradiol.
Figure 4. Changes in PGHS-2 mRNA abundance in five brain regions and pituitary.
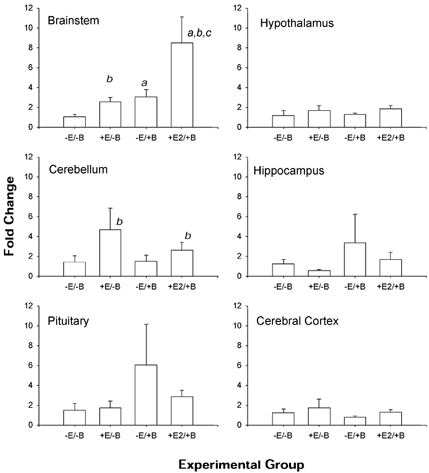
Data are presented as means ±s.e.m. (n = 4 per group). Statistically significant main effect of BCO is represented by a and statistically significant main effect of oestradiol is represented by b. Statistically significant BCO-oestradiol interaction is represented by c.
PGHS-1 and PGHS-2 protein levels did not reflect the changes in mRNA abundance (Fig. 5 and Fig. 6, respectively). The abundance of both proteins was increased in cerebral cortex in response to BCO (significant main effect of BCO for both proteins; P < 0.01 for both), but there was no effect of oestradiol on the protein expression levels in this brain region. PGHS-2 protein was significantly increased by both oestradiol (P < 0.05) and BCO (P < 0.005) in the hippocampus. In this region, there was also a significant interaction between BCO and oestradiol (P < 0.05).
Figure 5. Changes in PGHS-1 protein abundance in five brain regions.
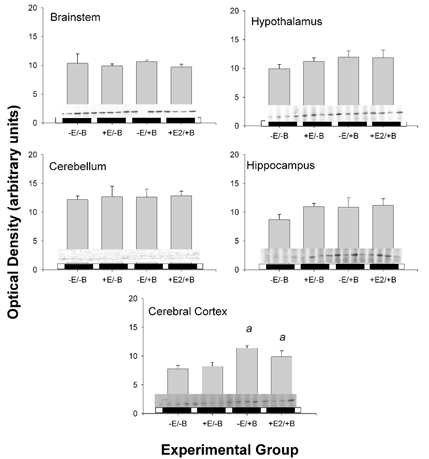
Data are presented as means ±s.e.m. Western blots are reproduced above horizontal bars delimiting the experimental groups, in the order represented on the x- axis (n = 4 per group). Statistically significant main effect of BCO is represented by a.
Figure 6. Changes in PGHS-2 protein abundance in five brain regions.
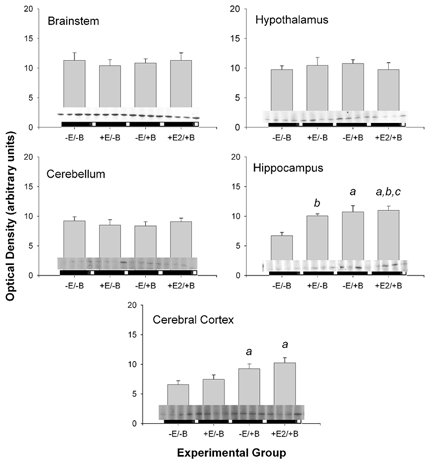
Data are presented as means ±s.e.m. Western blots are reproduced above horizontal bars delimiting the experimental groups, in the order represented on the x-axis (n = 4 per group). Statistically significant main effect of BCO is represented by a and statistically significant main effect of oestradiol is represented by b. Statistically significant BCO-oestradiol interaction is represented by c.
Discussion
The results of the present study demonstrate that oestradiol alters the expression of PGHS-2 in fetal brainstem and cerebellum, and that oestradiol augments the PGHS-2 response to hypotension in the brainstem. The fetal brainstem is particularly important in cardiovascular regulation because of the integration of baroreceptor and chemoreceptor afferent activity that occurs in this brain region.
We propose that at least a portion of the effect of oestradiol on central cardiovascular reflex pathways and, in turn, on fetal HPA function, might be mediated by local generation of prostaglandins in the fetal brainstem. We have previously demonstrated that indomethacin, a non-selective PGHS-1/PGHS-2 inhibitor, partially blocks the reflex ACTH and AVP responses to hypotension in the fetus. This effect of indomethacin is dependent upon the presence of intact baroreceptor/chemoreceptor afferent pathways (Tong et al. 1998). We have also reported that cerebral hypoperfusion results in generation of PGE2 in brainstem interstitial fluid (Tong et al. 1999). In addition to the immediate increase in PGE2 biosynthesis locally, cerebral hypoperfusion also results in a decrease in PGHS activity, presumably due to the mechanism-based inactivation of the enzyme in the brainstem (Tong et al. 1999). Twenty minutes after the end of a 10 min period of cerebral hypoperfusion, there is a dramatic upregulation of PGHS-2 mRNA in the fetal brainstem (Tong et al. 2000), an effect that persists until 1 h, the time-point chosen for the present study. We interpret these mRNA results to reflect replacement of the enzyme after mechanism-based inactivation in situ. In the present study, oestradiol alone increases PGHS-2 abundance in the brainstem. The present design does not allow assessment of whether there is a primary stimulatory effect of oestradiol on PGHS-2 transcription or whether the rate of transcription is upregulated in response to an unknown downstream effect of mechanism-based inactivation of the enzyme.
The PGHS-2 response to cerebral hypoperfusion measured in the present study is similar to that measured in previous studies with the following exceptions. In a previous study focusing on mRNA responses (20 min after the end of a 10 min BCO), we found highly significant upregulation of PGHS-2 mRNA in cerebellum and hippocampus, in addition to brainstem (Tong et al. 2000). In the present study (60 min after the onset of a 10 min BCO), we found PGHS-2 mRNA upregulated in cerebellum and brainstem but not in hippocampus. Rather, we found significant increases in the PGHS-2 protein in hippocampus. The differences between the two studies might possibly reflect a more rapid and shorter-lasting response for transcriptional regulation of PGHS-2 in the hippocampus than in brainstem or cerebellum. Differences in PGHS-2 protein are also noticeable between 30 and 60 min (after the onset of a 10 min period of BCO). Between 0 and 30 min, we reported a statistically significant increase in the abundance of PGHS-2 protein at 140 kDa, a form that might represent dimerized enzyme. At 30 min, we found only small and insignificant changes in abundance at the 72 kDa (monomeric) molecular weight. In the present study at 60 min, the 140 kDa form was not quantifiable, suggesting a change in the status of PGHS-2 integrity or turnover at that time-point.
PGHS-1 was affected by BCO, but not by oestradiol. Comparisons of the results of this study with previous studies might reveal underlying physiological processes. We have previously reported that complete baroreceptor and chemoreceptor denervation dramatically reduced PGHS-1 mRNA in fetal hypothalamus, suggesting that the expression of this enzyme in hypothalamus might be dependent upon baroreceptor or chemoreceptor activity. In sinoaortic-denervated fetuses, a 10 min BCO did not significantly alter PGHS-1 mRNA or protein abundance in any of the tested regions of the fetal brain (Tong et al. 1999, 2000). In the present study, PGHS-1 mRNA was increased 60 min after the onset of a 10 min BCO in hypothalamus and brainstem. Whether this upregulation was identifiable because of the later time-point (perhaps the PGHS-1 response is slower than the PGHS-2 response) or because of the presence of intact baroreceptor and chemoreceptor afferent fibres is unknown. It is possible that the difference is at least partially explained by time course, because the protein was not yet increased at 60 min.
Our results are generally in agreement with published studies from other groups, although relatively little is known about regulation of these enzymes in ovine fetal brain. Degi and colleagues reported that PGHS-2 was increased at both the mRNA and protein levels in cerebral cortex and hippocampus after cerebral ischaemia (produced by experimentally increasing intracranial pressure; Degi et al. 1998b). Interestingly, in their experimental paradigm, the time course of increase in mRNA (2–4 h) and protein (8 h) suggested a relatively slow response to the insult. In other experiments, the same group reported a similar time course of PGHS-2 response in choroid plexus (Thrikawala et al. 1998) and in visual cortex (Degi et al. 2001). Interestingly, the piglet expresses very low levels of PGHS-1 mRNA and protein and does not appear to increase PGHS-1 expression in response to ischaemia (Degi et al. 1998a, 1998b, 2001; Thrikawala et al. 1998). This might be the result of species differences, since we (Tong et al. 2000) and others (Norton et al. 1996) have found PGHS-1 expression in the ovine fetal brain. However, the differences could also be related to methodologic differences, including different methods for inducing cerebral ischaemia.
The ACTH, cortisol and AVP responses to oestradiol and to reduced cerebral perfusion pressure were generally similar to previous results from this laboratory (Wood & Saoud, 1997; Saoud & Wood, 1997; Purinton & Wood, 2002) as well as other laboratories (Rose et al. 1981; Ross et al. 1986). We were surprised by the lack of stimulation of ACTH and cortisol by oestradiol alone. We have consistently found that oestradiol augments fetal ACTH secretion, although in previous studies we used repeated sampling protocols to demonstrate the augmentation of the fetal HPA axis (Saoud & Wood, 1997; Wood et al. 2003). Fetal HPA axis activity has a marked ultradian rhythm which could, in part, be related to uterine activity (Challis et al. 1981; Lye et al. 1985). It is possible that in the present study the sampling frequency was too low reliably to detect the increased levels of ACTH and cortisol. It is perhaps more likely that the effect of oestradiol on fetal ACTH secretion might be dependent upon fetal gestational age. The fetuses used in this study are, on average, younger than in previous studies (Wood & Saoud, 1997; Saoud & Wood, 1997). Nevertheless, we did see a significant augmentation of the ACTH and AVP responses to BCO. Cortisol responses were possibly not increased by oestradiol because of the limitation of the response by the maximum adrenal secretory rate.
In late gestation, the ovine placenta secretes increasing amounts of oestradiol (Challis, 1971; Challis & Patrick, 1981). We have proposed that the rising concentrations of oestradiol in fetal plasma act to increase fetal ACTH as a part of a positive feedback loop which ultimately results in the onset of labour (Saoud & Wood, 1997). Present and past results from this laboratory (Purinton & Wood, 2002) demonstrate that the action of oestradiol on the fetal brain includes augmentation of AVP. Results of several studies have established that PGE2 injected into the brain stimulates the secretion of both ACTH and AVP (Hedge & Hanson, 1972; Brooks, 1992; Currie et al. 1994). Although we have established that intact peripheral baroreceptors and chemoreceptors are not required for oestradiol augmentation of ACTH and AVP secretion, we propose that it is possible that oestradiol stimulates both endocrine systems by augmenting PGE2 synthesis and action along the brainstem pathways that are involved in visceral afferent control of ACTH and AVP secretion (Purinton & Wood, 2002). The results of the present study do not prove, but are consistent with, this possibility.
We conclude that oestradiol augments the transcription of PGHS-2 in ovine fetal brainstem and cerebellum, and that it increases the magnitude of the brainstem PGHS-2 response to cerebral hypoperfusion. The stimulus of cerebral hypoperfusion, itself, increased transcription of PGHS-1 (independent of PGHS-2), in brainstem and hypothalamus. We speculate that the upregulation of prostaglandin biosynthetic enzymes is a reflection of the upregulation of prostaglandin biosynthesis in these brain regions, and we suggest the possibility that at least a part of the oestradiol effect on fetal ACTH and AVP secretion is the result of oestrogen-stimulated brain prostaglandin biosynthesis. The present results are consistent with this hypothesis, but more specific experiments will be needed to directly establish a cascade involving oestradiol, prostaglandins and neuroendocrine responses.
Acknowledgments
This work was supported by a Grant-in-Aid (to C.E.W.) and a Predoctoral Fellowship Award (to D.G.) from the Florida/Puerto Rico Affiliate of the American Heart Association, and by HD33053 from the NIH (to C.E.W.). The authors would like to thank Dr Colin Sumners for helpful suggestions along the way.
References
- Bell ME, Wood CE, Keller-Wood M. Influence of reproductive state on pituitary-adrenal activity in the ewe. Domest Anim Endocrinol. 1991;8:245–254. doi: 10.1016/0739-7240(91)90060-w. [DOI] [PubMed] [Google Scholar]
- Brooks AN. Prostaglandin E2 stimulates adrenocorticotrophin and cortisol secretion via a hypothalamic site of action in fetal sheep. J Dev Physiol. 1992;18:173–177. [PubMed] [Google Scholar]
- Challis G. Sharp increase in free circulating oestrogen immediately before parturition in sheep. Nature. 1971;229:208. doi: 10.1038/229208a0. [DOI] [PubMed] [Google Scholar]
- Challis G, Patrick JE. Fetal and maternal estrogen concentrations throughout pregnancy in the sheep. Can J Physiol Pharmacol. 1981;59:970–978. doi: 10.1139/y81-148. [DOI] [PubMed] [Google Scholar]
- Challis G, Patrick JE, Cross J, Workewych J, Manchester E, Power S. Short-term fluctuations in the concentration of cortisol and progesterone in fetal plasma, maternal plasma and amniotic fluids from fetal sheep during late pregnancy. Can J Physiol Pharmacol. 1981;59:261–267. doi: 10.1139/y81-041. [DOI] [PubMed] [Google Scholar]
- Currie IS, Gillies G, Brooks AN. Modulation of arginine vasopressin secretion from cultured ovine hypothalamic cells by glucocorticoids and opioid peptides. Neuroendocrinology. 1994;60:360–367. doi: 10.1159/000126770. [DOI] [PubMed] [Google Scholar]
- Degi R, Bari F, Beasley TC, Thrikawala N, Thore C, Louis TM, Busija DW. Regional distribution of prostaglandin H synthase-2 and neuronal nitric oxide synthase in piglet brain. Pediat Res. 1998a;43:683–689. doi: 10.1203/00006450-199805000-00018. [DOI] [PubMed] [Google Scholar]
- Degi R, Bari F, Thrikawala N, Beasley TC, Thore C, Louis TM, Busija DW. Effects of anoxic stress on prostaglandin H synthase isoforms in piglet brain. Brain Res Dev Brain Res. 1998b;107:265–276. doi: 10.1016/s0165-3806(98)00022-4. [DOI] [PubMed] [Google Scholar]
- Degi R, Thore C, Bari F, Thrikawala N, Nogradi A, Robins G, Domoki F, Beasley TC, Busija DW. Ischemia increases prostaglandin H synthase-2 levels in retina and visual cortex in piglets. Graefes Arch Clin Exp Ophthalmol. 2001;239:59–65. doi: 10.1007/pl00007899. [DOI] [PubMed] [Google Scholar]
- Hedge GA, Hanson SD. The effects of prostaglandins on ACTH secretion. Endocrinology. 1972;91:925–933. doi: 10.1210/endo-91-4-925. [DOI] [PubMed] [Google Scholar]
- Lye SJ, Wlodek ME, Challis JRG. Possible role of uterine contractions in the short-term fluctuations of plasma ACTH concentration in fetal sheep. J Endocrinol. 1985;106:R9–11. doi: 10.1677/joe.0.106r009. [DOI] [PubMed] [Google Scholar]
- Norton JL, Adamson SL, Bocking AD, Han VK. Prostaglandin-H synthase-1 (PGHS-1) gene is expressed in specific neurons of the brain of the late gestation ovine fetus. Brain Res Dev Brain Res. 1996;95:79–96. doi: 10.1016/0165-3806(96)00065-x. [DOI] [PubMed] [Google Scholar]
- Purinton SC, Wood CE. Oestrogen augments the fetal ovine hypothalamus-pituitary-adrenal axis in response to hypotension. J Physiol. 2002;544:919–929. doi: 10.1113/jphysiol.2002.025635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff H, Kane C, Wood CE. Vasopressin responses to hypoxia and hypercapnia in late-gestation fetal sheep. Am J Physiol. 1991;260:R1077–1081. doi: 10.1152/ajpregu.1991.260.6.R1077. [DOI] [PubMed] [Google Scholar]
- Rose JC, Meis P, Morris M. Ontogeny of endocrine (ACTH, vasopressin, cortisol) responses to hypotension in lamb fetuses. Am J Physiol. 1981;240:E656–661. doi: 10.1152/ajpendo.1981.240.6.E656. [DOI] [PubMed] [Google Scholar]
- Ross MG, Ervin MG, Leake RD, Habeeb O, Fisher DA. Isovolemic hypotension in ovine fetus: plasma arginine vasopressin response and urinary effects. Am J Physiol. 1986;250:E564–569. doi: 10.1152/ajpendo.1986.250.5.E564. [DOI] [PubMed] [Google Scholar]
- Saoud CJ, Wood CE. Modulation of ovine fetal adrenocorticotropin secretion by androstenedione and 17beta-estradiol. Am J Physiol. 1997;272:R1128–1134. doi: 10.1152/ajpregu.1997.272.4.R1128. [DOI] [PubMed] [Google Scholar]
- Thorburn GD. The placenta, prostaglandins, and parturition: a review. Reprod Fertil Dev. 1991;3:277–294. doi: 10.1071/rd9910277. [DOI] [PubMed] [Google Scholar]
- Thrikawala N, Bari F, Beasley TC, Thore C, Busija DW. Effects of ischemia on prostaglandin H synthase-2 expression in piglet choroid plexus. Prostaglandins Other Lipid Mediat. 1998;56:77–87. doi: 10.1016/s0090-6980(98)00044-6. [DOI] [PubMed] [Google Scholar]
- Tong H, Dhillon H, Wood CE. Induction of PGHS-2 mRNA in response to cerebral hypoperfusion in late-gestation fetal sheep. Prostaglandins Other Lipid Mediat. 2000;62:165–172. doi: 10.1016/s0090-6980(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Tong H, Gridley KE, Wood CE. Induction of immunoreactive prostaglandin H synthases 1 and 2 and fos in response to cerebral hypoperfusion in late-gestation fetal sheep. J Soc Gynecol Investig. 2002;9:342–350. [PubMed] [Google Scholar]
- Tong H, Lakhdir F, Wood CE. Endogenous prostanoids modulate the ACTH and AVP responses to hypotension in late-gestation fetal sheep. Am J Physiol. 1998;275:R735–741. doi: 10.1152/ajpregu.1998.275.3.R735. [DOI] [PubMed] [Google Scholar]
- Tong H, Richards E, Wood CE. Prostaglandin endoperoxide synthase-2 abundance is increased in brain tissues of late-gestation fetal sheep in response to cerebral hypoperfusion. J Soc Gynecol Investig. 1999;6:127–135. doi: 10.1016/s1071-5576(99)00012-x. [DOI] [PubMed] [Google Scholar]
- Tong H, Wood CE. Indomethacin attenuates the cerebral blood flow response to hypotension in late-gestation fetal sheep. Am J Physiol. 1999;277:R1268–1273. doi: 10.1152/ajpregu.1999.277.5.R1268. [DOI] [PubMed] [Google Scholar]
- Wood CE, Cudd TA, Kane C, Engelke K. Fetal ACTH and blood pressure responses to thromboxane mimetic U46619. Am J Physiol. 1993;265:R858–862. doi: 10.1152/ajpregu.1993.265.4.R858. [DOI] [PubMed] [Google Scholar]
- Wood CE, Gridley KE, Keller-Wood M. Biological activity of 17beta-estradiol-3-sulfate in ovine fetal plasma and uptake in fetal brain. Endocrinology. 2003;144:599–604. doi: 10.1210/en.2002-220764. [DOI] [PubMed] [Google Scholar]
- Wood CE, Saoud CJ. Influence of estradiol and androstenedione on ACTH and cortisol secretion in the ovine fetus. J Soc Gynecol Investig. 1997;4:279–283. [PubMed] [Google Scholar]
- Wood CE, Saoud CJ, Stoner TA, Keller-Wood M. Estrogen and androgen influence hypothalamic AVP and CRF concentrations in fetal and adult sheep. Regul Pept. 2001;98:63–68. doi: 10.1016/s0167-0115(00)00231-7. [DOI] [PubMed] [Google Scholar]
- Wood CE, Tong H. Central nervous system regulation of reflex responses to hypotension during fetal life. Am J Physiol. 1999;277:R1541–1552. doi: 10.1152/ajpregu.1999.277.6.R1541. [DOI] [PubMed] [Google Scholar]
- Young IR, Thorburn GD. Prostaglandin E2, fetal maturation and ovine parturition. N Z J Obstet Gynaecol. 1994;34:342–346. doi: 10.1111/j.1479-828x.1994.tb01086.x. [DOI] [PubMed] [Google Scholar]


