Abstract
Background:
Biventricular (BIV) pacing can improve cardiac function in heart failure (HF).
Objective:
To investigate the mechanisms of benefit of BIV pacing using a rabbit model of myocardial infarction (MI).
Methods:
New Zealand White rabbits were divided into 4 groups: sham-operated (C), MI with no pacing (MI), MI with right ventricular pacing (MI+RV), and MI with BIV pacing (MI+BIV), and underwent serial electrocardiograms and echocardiograms. At 4 weeks, hearts were excised and tissue was extracted from various areas of the left ventricle (LV).
Results:
Four weeks after coronary ligation, BIV pacing prevented systolic and diastolic dilation of the LV as well as the reduction in its fractional shortening, restored the QRS width and the rate-dependent QT intervals to their baseline values, and prevented the decline of the ether-a-go-go (erg) protein levels. This prevention of remodeling was not documented in the MI+RV groups.
Conclusions:
In this rabbit model of BIV pacing and MI, we demonstrate prevention of adverse mechanical and electrical remodeling of the heart. These changes may underlie some of the benefits seen with BIV pacing in HF patients with more severe LV dysfunction.
Keywords: Rabbit, Myocardial Infarction, Biventricular Pacing, Cardiac Remodeling
Heart Failure (HF) 1-5 is a disease of epidemic proportion in the United States, affecting over 5 million individuals. Every year, nearly 400,000 new cases are diagnosed, 1 million individuals are hospitalized and 300,000 die because of HF. Approximately half of the deaths are attributable to worsening pump function while the remainder are secondary to sudden cardiac death, predominantly from tachyarrhythmias.
Cardiac resynchronization therapy, also known as biventricular (BIV) pacing, has recently emerged as a modality for the treatment of advanced HF6-8. In patients with ventricular conduction abnormalities who continue to suffer from severe HF symptoms (New York Heart Association Class III-IV) despite optimal pharmacological therapy, BIV pacing can improve maximum oxygen consumption, exercise tolerance, and quality of life, as well as reduce the incidence of ventricular arrhythmias. More recent clinical trials 7, 8 also suggest a benefit from BIV pacing in improving the endpoints of death and hospitalization.
Despite this, little is known about the regional, cellular, and molecular mechanisms responsible for the benefits seen with BIV pacing. In this current study, we introduce a novel rabbit model of myocardial infarction (MI) and pacing, and analyze the effect of BIV pacing on the prevention of adverse electrical and mechanical remodeling following MI.
Materials and Methods
Study Design
New Zealand White rabbits (n=39; weight=3.4-5.2 kg) were divided into 4 groups: sham-operated controls (C group, n=9), where the rabbits underwent pericardial stripping only; MI with no pacing (MI group, n=11), where the rabbits underwent pericardial stripping and coronary ligation but no pacing; MI with right ventricular (RV) pacing (MI+RV group, n=9), where the rabbits underwent pericardial stripping, coronary ligation and RV pacing; and MI with BIV pacing (MI+BV group, n=10), where the rabbits underwent pericardial stripping, coronary ligation and BIV pacing. Rabbits in the pacing groups were continuously paced at a rate of 270 beats per minute, which is slightly higher than the maximum ambulatory heart rate in the rabbit, for 4-5 weeks after surgery until they were sacrificed, with a single interruption at 2 weeks after surgery for electrocardiographic recording. Rabbits had baseline electrocardiograms and echocardiograms on the day of surgery (before and immediately after the operation) and repeat electrocardiographic studies at 2 weeks and 4 weeks after surgery. At 4 weeks, all rabbits had a repeat echocardiogram done after the pacemaker was turned off for an empiric period of no less than 30 minutes. They were then sacrificed and their hearts were excised. The size of the infarct was measured from the epicardial surface. Cardiac tissue was collected from the MI, border zone, and the normal basal zone of the LV for molecular analyses.
Surgical preparation of rabbit
Rabbits were anesthetized using intramuscular injections of a mixture of ketamine (35 mg/kg) and xylazine (5 mg/kg). A 22 gauge intravenous catheter was inserted into the marginal ear vein for venous access. An arterial line was inserted into the middle ear artery for continuous hemodynamic monitoring. The rabbits were intubated with an endotracheal tube (3.0-mm ID) and mechanically ventilated (rate 40/min, tidal volume 15 ml) using room air enriched with oxygen. Isoflurane anesthesia (2.0-2.5%) was delivered to maintain general anesthesia during surgery. The rabbits were placed on a water blanket adjusted to 38 degrees Celsius. A pulse oxymeter was placed on the rabbit's tongue for continuous monitoring of oxygen saturation.
The chest was opened through the fourth left intercostal space. The heart was exposed through an incision of the pericardium and explored. The MI was induced by ligating the posterolateral division of the left coronary system using 5-0 Prolene suture at a level 50% between the atrioventricular groove and the apex. Successful ligation was confirmed by the presence of myocardial cyanosis with bulging and ST-segment changes in the amplified ECG signal. Lidocaine (1 to 4 mg/kg) was administered pre- and post-ligation as an antiarrhythmic agent, and a prophylactic antibiotic (Ancef 100mg IV) was administered before and after surgery.
Pacemaker Implantation
Pacemaker leads were sutured to the epicardial surface of both the RV and left ventricle (LV) or on the RV only, depending on the study group of the rabbit. The LV lead was placed on the LV free wall basally, away from the apical infarcted area. The leads (model 4965 and model 4968, Medtronic, St Paul, MN) were connected to a permanent pacemaker configured to pace the RV alone or both the RV and LV simultaneously. In case of BIV pacing, a Y-connector (model 2872, Medtronic, St Paul, MN) was used to pace the RV and LV simultaneously from a single-chamber (Kappa KSR403, Medtronic, St Paul, MN) pacemaker, programmed through research software to pace at a fast rate of 270 beats per minute, which is slightly higher than the maximum ambulatory heart rate of rabbits confined to a cage. The pacemaker and leads were placed in a subcutaneous pocket in the abdominal area of the rabbit. As a result of ventricular pacing at this rate, paced rabbits in the MI+RV and MI+BIV groups lacked atrioventricular synchrony post-operatively.
Echocardiography
Following anesthesia with ketamine and xylazine, rabbits were placed supine on a warming plate and the chest shaved. Needle electrocardiographic leads were attached to each limb for continuous ECG monitoring. Echocardiography was performed with the pacemaker turned off for no less than 30 minutes, with an Acuson Sequoia ultrasound machine using a dynamically focused 8 MHz transducer. Two-dimensional long and short axis images through the LV were obtained and the latter used to place the M-mode cursor perpendicularly through the LV septum and posterior wall, avoiding the papillary muscles and imaging at the level of maximum chamber dimension. Studies were recorded on 1/2″; S-VHS videotape and freeze frame images, including ECG, printed on a color printer.
Off-line data analysis was performed on the echocardiographic images. Left ventricular cross-sectional areas at end-diastole (CSAd) and at end-systole (CSAs) were measured from freeze frame 2-D mode in the short axis view by planimetry. End-diastole was taken to be at the point of maximal cavity dimension, and end-systole at the point of maximal anterior excursion of the posterior wall. Three or more beats were measured and averaged. The LV percent fractional area change (%FAC) was calculated as (CSAd−CSAs)/CSAd × 100. All echocardiograms were read in random order by an observer masked to the experimental group of the rabbits.
Cellular and Molecular Analysis of Cardiac Tissue
Hearts were removed from rabbits in each experimental group, and 200 mg samples from the various regions including 1) the apex, 2) the LV free wall at the base, 3) the RV free wall, and 4) the MI and 5) the peri-infarct area were excised. We performed immunoblots (Western blots) using antibodies to SERCA2A (Zymed Laboratories Inc., CA), GAPDH (Research Diagnostics Inc., NJ), the Na+-Ca2+ exchanger (Affinity BioReagents, Inc.) and ion channels including the ether-a-go-go related gene (erg) rabbit (anti-erg (Kv11.1) antibody (Cat# AB5908) from Upstate/Chemicon/Millipore Corp., Temecula, CA), Kv2.1, Kv4.2, and Kv4.3 (Alamone Laboratories, Israel). As previously described9, crude membrane preparations were isolated by differential centrifugation from the same regions of the hearts described above. Channels were solubilized with 1 % SDS, quantitated (BioRad), ∼100 μg of protein per lane was run on a 12% SDS-PAGE gel, transferred by semi-dry apparatus, blocked with PBS-5% milk, incubated overnight at 4°C with the primary antibody, washed with PBS-Tween, incubated with HRP-conjugated chicken anti-rabbit 2° antibody, and quantitated by chemiluminescence (NEN). Coomassie Blue staining of SDS-PAGE blots was performed to confirm equal loading. Custom-made polyclonal antibodies to rabbit SERCA2a directed against the peptide DPVPDPRAVNQDKK (AA 192-205) and to rabbit ERG directed against the peptide RQRKRKLSFRRRTD (AA 885-898) were generated in the chicken (affinity-purified IgY, Zymed Laboratories Inc., South San Francisco, CA). Confirmation of results was done by using anti-erg (Kv11.1) antibody (Cat# AB5908) from Upstate/Chemicon/Millipore Corp., Temecula, CA. Pre-immune IgY from egg yolk was used to determine the SERCA2a and ERG specific bands.
The autoradiographs were scanned using a Visioneer OneTouch 9220 scanner (Visioneer Hardware, Pleasanton, CA 94588) and Scanner and Camera Wizard computer program (Microsoft Corporation). The images were digitized for analysis using Quantity One quantitation software (BioRad Laboratories, Hercules, CA). Bands of interest were delineated and densitometries were calculated based on the number of pixels. We performed Westerns on tissue from 6 rabbits of each of the C, MI, MI+RV, and MI+BIV groups.
ERG gene expression of mRNA levels were determined by fluorescence-based kinetic real-time PCR (RT-PCR) using a Perkin-Elmer Applied Biosystems model 7000 sequence detection system. Total RNA was isolated using TRIzol reagent (Invitrogen Inc., Carlsbad, CA, USA) as described elsewhere10, 11. First strand cDNA synthesis was performed with 1 μg of RNA and SuperScript III RNase H− Reverse Transcriptase (200 u/μl) (Invitrogen Inc., Carlsbad, CA, USA) and the RT-PCR reaction was performed using 1 μg of cDNA, Absolute SYBR Green ROX Mix (ABgene, Foster City, CA), and rabbit specific ERG forward: 5'-CAGGCACCACGCATCCA-3' and reverse: 5'-CAGTCCCACACAGCCTTGAA-3' primers11. Quantification of Erg PCR product was performed with ABI PRISM 7000 SDS software Version 1.1 and expression of ERG was compared with the gene expression of β-actin using rabbit specific forward: 5'-CTGGCTGGCCGCGACCT-3' and reverse: 5'-GAACCGCTCATTGCCAATGGG-3' β-actin primer pairs with similar RT-PCR condition as that of ERG. The threshold cycle (CT) number at which detectable fluorescence for the ERG product exceeded background was compared to the β-actin product for each sample.
Data Analysis
Data are presented as mean ± standard error unless otherwise indicated. Continuous variables were compared by analysis of variance (ANOVA) with Scheffe's multiple range test. Data before and after an intervention were compared using ANOVA for repeated measurements. Graphs of QT versus RR intervals were constructed for all study groups. A p value less than or equal to 0.05 was considered statistically significant.
Results
Myocardial Infarction Size
The size of the MI was similar between the 3 infarcted (MI, MI+RV, and MI+BIV) rabbit groups. The size of the myocardial infarction as measured from the epicardial surface was 1.37±0.88 cm2, 0.93±0.75 cm2, and 1.13±1.22 cm2 for the MI, MI+RV, and MI+BIV groups respectively, p=0.893. Assuming a spherical LV shape, the MI size was approximately 10% of LV area in all groups.
Echocardiography
Echocardiograms were analyzed on 33 rabbits (9 Controls, 9 MI, 7 MI+RV, 8 MI+BIV) at baseline and 4 weeks post surgery and are shown in table 1. As expected, with coronary ligation, the diastolic (CSAd) and systolic (CSAs) LV cross-sectional areas on the short axis view increased in the MI and MI+RV pacing groups at 4 weeks post-MI compared to baseline values, whereas they remained unchanged in the sham-operated control rabbits. In the MI+BIV group, however, the increase in CSAd and CSAs was less pronounced and the LV dimensions remained significantly smaller than in the MI or MI+RV groups both in systole and diastole. The fractional area change (FAC) also exhibited a significant (p<0.01) decrease in the MI and MI+RV groups compared to the C and MI+BIV groups, except for the pair-wise comparison of the MI and MI+BIV groups, which exhibited a strong trend (p=0.052) toward significance.
Table 1.
Echocardiographic Data
| CSAd | CSAs | FAC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre Mean (SD) |
4w Mean (SD) |
Δ Mean (SD) |
Pre Mean (SD) |
4w Mean (SD) |
Δ Mean (SD) |
Pre Mean (SD) |
4w Mean (SD) |
Δ Mean (SD) |
|
|
C (n=9) |
196 (14) |
194 (10) |
0% (10) |
102 (17) |
97 (13) |
−4% (13) |
48 (9) |
50 (5) |
6% (11) |
|
MI (n=9) |
193 (15) |
243 *† (27) |
25% *† (18) |
93 (13) |
140*† (11) |
52% *† (20) |
52 (6) |
42 * (4) |
−19% * (13) |
|
MI+RV (n=7) |
195 (22) |
254 *† (27) |
33% *† (24) |
95 (12) |
157 *†‡ (25) |
72% *† (39) |
51 (5) |
38 (5) |
−26% *† (10) |
|
MI+BIV (n=8) |
190 (27) |
192 (20) |
3% (20) |
90 (16) |
103 (19) |
17% (29) |
53 (3) |
46 (6) |
−10% (12) |
All measurements represent mean±standard deviation
CSAd=cross-sectional area of LV in diastole (in mm2)
CSAs=cross-sectional area of LV in systole (in mm2)
FAC=fractional area change of LV (%)
Pre = Pre coronary ligation
4w = 4 weeks after surgery
Δ = percent change between pre-surgery and 4 weeks after surgery
p< 0.01 for unpaired t-test comparison of study groups with the control group
p< 0.01 for unpaired t-test comparison of study groups with the MI+BIV group
p< 0.05 for unpaired t-test comparison of MI+RV to MI group
Comparing the MI+RV group to the MI group reveals a significant increase in the CSAs (p=0.034) in the MI+RV group. The finding suggests a detrimental effect of RV but not BIV pacing on LV size and function after myocardial infarction.
Electrocardiography
Electrocardiograms (ECG) obtained on all 39 rabbits pre- and post-MI, as well as at 2 and 4 weeks after surgery were analyzed. All ECG data presented in table 2 were obtained while the pacemaker was turned off for no less than 30 minutes. Immediately post-MI, infarcted rabbits belonging to groups MI, MI+RV, and MI+BIV exhibited widening of the PR (post MI 84±9 ms versus pre-MI 77±11 ms, p=0.020), QRS (post-MI 46±10 ms versus pre-MI 38±6 ms, p<0.001), and QT (post-MI 184±16 ms versus pre-MI 164±16 ms, p<0.001) intervals compared to the immediate pre surgery parameters. There was no change in the RR interval acutely after MI (285±32 ms post-MI compared to 277±40 ms pre-MI, p=0.372).
Table 2.
Electrocardiographic Data
| RR (ms) Mean (SD) |
PR (ms) Mean (SD) |
QRS (ms) Mean (SD) |
QT (ms) Mean (SD) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | 2w | 4w | Pre | Post | 2w | 4w | Pre | Post | 2w | 4w | Pre | Post | 2w | 4w | |
|
C (n=9) |
316 (40) |
N/A | 294 (28) |
316 (31) |
78 (10) |
N/A | 75 (9) |
77 (10) |
36 (6) |
N/A | 35 (5) |
35 (9) |
180 (16) |
N/A | 171 (11) |
168 (17) |
|
MI (n=11) |
292 (45) |
294 (39) |
295 (41) |
285 (49) |
76 (15) |
84 (9) |
85 (4) |
78 (13) |
39 (7) |
46* (9) |
46*† (8) |
47*† (7) |
171 (18) |
180 (18) |
163 (18) |
177 (22) |
|
MI+RV (n=9) |
261 (40) |
288 (31) |
269 (19) |
291 (52) |
76 (12) |
86 (11) |
99*† (15) |
103*† (23) |
41 (7) |
52* (12) |
45† (4) |
47*† (9) |
157 (15) |
187* (16) |
155 (12) |
166 (18) |
|
MI+BIV (n=10) |
265 (34) |
273 (25) |
266 (31) |
300 (38) |
77 (6) |
82 (6) |
78 (9) |
84 (15) |
36 (5) |
41* (6) |
37 (2) |
39 (7) |
162 (11) |
185* (18) |
160 (34) |
148 (15) |
SD = standard deviation
N/A = not applicable
Pre = pre coronary ligation
Post = post coronary ligation (within 15 to 20 minutes)
2w = 2 weeks after surgery
4w = 4 weeks after surgery
p< 0.05 for paired t-test comparisons within each group compared to pre-MI value
p< 0.05 for unpaired t-test comparison of study groups with the MI+BIV group
Compared to rabbits in the control group (C group), the QRS complex was significantly wider in the MI and MI+RV, but not in the MI+BIV groups at 2 weeks and at 4 weeks post-MI, suggesting prevention of prolongation of depolarization time after myocardial infarction with BIV pacing. In fact, the QRS width in the MI+BIV group was significantly shorter than in the MI or in the MI+RV groups at 2 weeks (37±2 ms, 46±8 ms, and 45±4 ms, for the MI+BIV, MI, and MI+RV groups, respectively, p<0.02) and at 4 weeks (39±7 ms, 47±7 ms, 47±9 ms, for the MI+BIV, MI, and MI+RV groups, respectively, p<0.04).
Compared to rabbits in the C group, the PR interval was longer in the MI+RV, but not in the MI or MI+BIV groups at 2 weeks post-MI (78±9 ms, 85±4 ms, and 99±15 ms, for the MI+BIV, MI, and MI+RV groups, respectively, p<0.02), and 4 weeks (84±15 ms, 78±13 ms, and 103±33 ms, for the MI+BIV, MI, and MI+RV groups, respectively, p=0.05) post MI, suggesting a detrimental effect of right ventricular pacing that may slow down or prevent the recovery of atrioventricular conduction or conduction down the His-Purkinje system after myocardial infarction.
Graphs for the QT versus RR intervals were constructed for all 4 groups. At baseline, there were no significant differences between the groups in the slopes or intercepts of these graphs. At 4 weeks post surgery, the linear slopes were significantly higher in the C (s=0.48) and MI+BIV (s=0.39) groups compared to the MI (s=0.25) and MI+RV (s=0.17) groups, suggesting a direct effect of the mode of pacing on the QT interval modulation by heart rate (figure 1).
Figure 1.
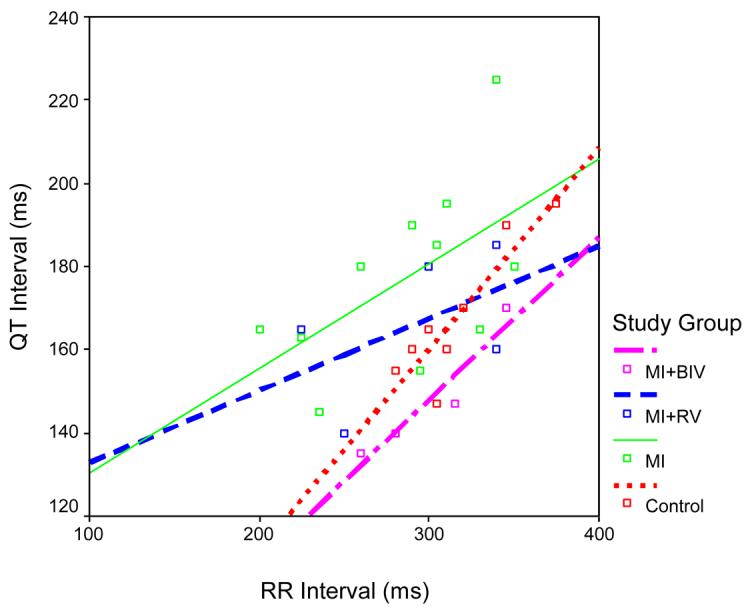
Scatter plots of QT versus RR and the best-fit linear equation for each of the 4 study groups at 4 weeks after surgery. Note the higher slope of the linear correlation between the QT and RR intervals in the C and BIV compared to the MI and MI+RV groups.
Tissue Analysis
Tissues were collected from rabbits in the C, MI, MI+RV, and MI+BIV groups from standardized areas of the LV and RV, with focus on the normal myocardial zone, the infarcted zone and the transitional zone bordering the scar. Testing the expression of K+ channels responsible for repolarization has shown no differences between these groups in the expression of Kv4.3, Kv2.1, or Kv4.2 (data not shown). In the areas far from the infarction zone, the expression of the Na+-Ca2+ exchanger and SERCA2a were also not different between these groups (data not shown).
Compared to the sham-operated control group, erg protein levels collected from LV areas far from the infarcted zone were significantly reduced in the MI group (p<0.0001) and the MI+RV group (p<0.0001), but not in the MI+BIV group (p=0.136). In fact, erg levels in the MI+BIV group were significantly higher than in the MI (p<0.0001) or MI+RV (p=0.0001) groups (figure 2). Levels of mRNA for the erg gene were not different among these 4 groups of rabbits (data not shown), suggesting a post-transcription mechanism for the reduction in the erg protein levels in the MI and MI+RV groups compared to the C and MI+BIV groups.
Figure 2.

A: Anti-ERG antibody and a shorter exposure of the blot to capture IgG band which is used as a reference to quantify rERG bands. B: Blot exposed to anti-ERG antibody + peptide (1:3 ratio). C: Graphic representation of rERG bands from three experiments in duplicate (n = 6 per group). Statistical analysis: *Cont. vs. MI p < 0.0001; **Cont. vs. RV p < 0.0001; Cont. vs. BiV p = 0.136; RV vs. MI p = 0.066; ***MI vs. BiV p < 0.0001; *****RV vs. BiV p < 0.0001.
Discussion
In this study, we present a novel rabbit model of myocardial infarction and pacing, in which we demonstrate prevention of adverse electrical and mechanical cardiac remodeling with BIV pacing compared to right ventricular pacing or no pacing after MI. Rabbits with myocardial infarction exhibit widening of the QRS complex after MI and dilation of systolic and diastolic left ventricular dimensions as well as reduction in the fractional area shortening. They also exhibit by Western blot analysis decrease in erg protein levels in normal areas of myocardium away from the infarcted LV zone. Our data show that biventricular pacing prevents, at least partially, some of these changes in cardiac electrical and mechanical properties, which may underlie some of the mechanisms of benefit of cardiac resynchronization therapy.
The sequence of events that lead to the mechanical and electrical remodeling following MI and the interplay between these two phenomena is still not fully elucidated. In the present rabbit model, coronary ligation leads not only to myocardial dysfunction as a result of the MI, but also to widening of the QRS complex. Given the apical location of the MI, it seems unlikely that the QRS widening is the result of a direct effect of the MI on the specialized cells of conduction, but may rather be secondary to other mechanisms, perhaps involving a slower velocity of electrical conduction in the MI border zone or even in areas distant from the actual scar. Be that as it may, the wider QRS complex may accentuate the injury in the MI group by creating dyssynchrony of mechanical activation of the LV, which is even more pronounced in the MI+RV group where RV pacing may result in more dyssynchrony. Mechanically, this translates into increased dilation and reduced function of the LV. BIV pacing restores synchrony to the LV activation, thereby resulting in less mechanical dilation and more preserved function. Evidence of reverse electrical remodeling with narrowing of the native QRS complex after cardiac resynchronization therapy has also been clinically documented12.These findings raise the question about the hypothetical value of BIV pacing in preventing adverse remodeling early after myocardial infarction.
Another aspect of electrical remodeling is the altered QT to RR interval relationship after MI or MI with RV pacing with significantly decreased slopes of the relationship compared to that of control rabbits. With BIV pacing, the slope of the QT to RR relationship remains identical to that of the sham-operated rabbits. This steeper relationship in the MI+BIV group, which translates into more QT shortening at faster heart rates, is probably the result of preserved erg protein levels, a major constituent of the IKr repolarization current. The preservation of erg and its effect on repolarization could explain the lower incidence of ventricular arrhythmias observed with BIV pacing in few clinical trials13 although the effect of cardiac resynchronization on arrhythmia burden remains highly controversial14.
The electrocardiographic findings may not be sufficient to explain the improved echocardiographic parameters documented in large multi-center clinical trials6-8 as well as in our current animal model. Our data have failed to demonstrate significant changes in cellular contractile markers such as the Na+-Ca2+ exchanger or SERCA2A. This is consistent with the lack of overt HF in our model. This does not rule out, however, the possibility of other markers being significantly altered by BIV pacing in the setting of a myocardial infarction, such as cytokines or matrix metaloproteinases that may contribute to adverse mechanical remodeling. It is possible that the normalized conduction may contribute to better synchrony between the atria and ventricles, therefore achieving a more optimal LV filling, as well as between the right and left ventricles, achieving improved myocardial efficiency. The relevance of these findings to patients with HF undergoing cardiac resynchronization remains to be elucidated.
Due to several inherent characteristics, the rabbit heart has been used for many years as a model for studying the pathophysiology of the human heart15-18. The rabbit heart has minimal collateral arteries and lacks a transmural gradient of the collateral blood flow19-21. Arrhythmias and death after coronary occlusion are less frequent in rabbits as compared with other species, and the distribution, size, and length of the coronary arteries are less variable in rabbits than in dogs22. For the above reasons, rabbits have served as a useful animal model for myocardial infarction by coronary artery ligation. Recently, a reliable MI model using coronary artery ligation in the rabbit on the basis of a bifurcation/trifurcation classification has been developed23, which was utilized in the current study in order to induce a MI while minimizing peri-operative death. The rabbit is also a good model system for electrophysiologic changes after MI. The rabbit cardiac action potential is similar to that of humans, and the underlying channels and currents are also homologous24-31. Moreover, the rabbit's size is large enough to accommodate the implantation of permanent pacemakers designed for humans32, 33 but modified to pace at fast rates. For all these reasons, we chose to use the rabbit in our present study.
Only a few animal studies have evaluated the mechanisms of response and benefit of BIV pacing. Liu et al.34 used a dog model of left bundle branch block to show the equivalence of LV and BIV pacing in correcting the paradoxical septal wall motion and improving cardiac function. Using tagged magnetic resonance imaging, Wyman et al.35 showed that BIV pacing had a greater impact on correcting spatial distribution of LV contraction than on improving the temporal synchronization of contraction. Similar findings showing that the mechanical synchrony achieved with LV or BIV pacing does not necessarily require electrical synchrony were documented by Leclercq et al.36 using tagged MRI, epicardial mapping, and hemodynamics in a dog model of HF and left bundle branch block. As clearly evident from these studies that have focused on the effect of biventricular pacing on the hemodynamic response of animals and on the regional characterization of the myocardium, there has been little insight into the cellular mechanisms responsible for the observed changes. Our present study makes use of immunoblotting to investigate the possible cellular and molecular mechanisms responsible for the benefits of cardiac resynchronization.
Human studies have focused on the effect of biventricular pacing in reducing left ventricular dyssynchrony37 and mitral regurgitation38, with limited insight into the possible cellular or molecular mechanisms involved in the effect of pacing on altering myocardial function. Many questions remain to be answered regarding the mechanisms of benefit from BIV pacing and the methods of optimization of these benefits. Questions as to optimal site or sites of LV pacing and their relative timing remain unsettled. Optimizing mechanical function in HF patients by altering parameters of pacing may prove essential in maximizing the functional cardiac benefit and minimizing the percentage of non-responders to this therapy. Other questions pertaining to the difference in myocardial substrate between ischemic and non-ischemic patients as well as to the effect of genetic polymorphisms on the response to BIV pacing are yet to be answered. Our results duplicate some of the echocardiographic data of large randomized prospective clinical trials that have demonstrated significant reduction with BIV pacing in left ventricular dimensions and improvement in myocardial function in HF patients6. This similarity underlines the relevance and validity of our rabbit model for the study of the effects of electrical stimulation on myocardial function, and allows the investigation of the possible cellular and molecular mechanisms responsible for these benefits since access to cardiac tissue is limited in clinical practice.
The present study has a number of limitations. First, contrary to current clinical practice, pacing in our rabbit model was initiated at the time of myocardial infarction before any cardiac remodeling has had a chance to occur. Therefore, our data demonstrate prevention of adverse cardiac remodeling with BIV pacing rather than reverse remodeling, which is observed clinically in HF patients undergoing resynchronization therapy. Second, in our model, pacing the right ventricle was done epicardially, which is different from the endocardial pacing typically used in clinical practice. It is unclear whether this difference affects the extrapolation of our results to humans. Although close to 100% ventricular pacing was achieved, device pacing with fusion or pseudofusion could not be ruled out. Also, a component of tachycardia-induced cardiomyopathy, although unlikely, could not be excluded with certainty. Third, in our model, the rabbits did not exhibit overt signs of HF and there was no invasive LV pressure monitoring over time. This is different from the current clinical practice where symptoms of advanced HF are necessary in order for a patient to qualify for the implantation of a BIV pacing device. The role of BIV pacing in patients with LV dysfunction but without overt HF symptoms is currently unknown. Prospective, randomized clinical trials addressing this question are currently enrolling patients. Finally, in our model, rabbits had significant QRS prolongation after coronary ligation, which is not a typical finding in patients who suffer from an acute MI. Also, since no echocardiographic measure of dyssynchrony was obtained in our study groups, it remains speculative whether RV pacing induced dyssynchrony in this model.
In summary, we present a rabbit model of myocardial infarction and pacing that constitutes a powerful tool for the study of the effect of BIV pacing on myocardial function. In this model, we demonstrate significant prevention of electrical and mechanical remodeling of the left ventricle with BIV pacing as demonstrated by ECG, echocardiography, and molecular tissue analysis.
Acknowledgement
This study was supported in part by grant H2004-031 from the National Heart Foundation (to SS) and grant K08 HL080106-01 A1 (to SS)
Abbreviations
- BIV
Biventricular pacing
- CSA
Cross sectional area
- ECG
Electrocardiogram
- FAC
Fractional area change
- HF
Heart failure
- LV
Left ventricle
- MI
Myocardial infarction
- RV
Right ventricle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The Framingham Study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 2.Bart BA, Shaw LK, McCants CB, Fortin DF, Lee KL, Califf RM, O'Connor CM. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol. 1997;30:1002–1008. doi: 10.1016/s0735-1097(97)00235-0. [DOI] [PubMed] [Google Scholar]
- 3.Ho KL, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: The Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 4.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 5.Zannad F, Briancon S, Juiiliere Y, Mertes PM, Villemot JP, Alla F, Virion JM, the EPICAL investigators Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: The EPICAL study. J Am Coll Cardiol. 1999;33:734–742. doi: 10.1016/s0735-1097(98)00634-2. [DOI] [PubMed] [Google Scholar]
- 6.Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, McKenna W, Fitzgerald M, Deharo JC, Alonso C, Walker S, Braunschweig F, Bailleul C, Daubert JC. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol. 2002;40:111–118. doi: 10.1016/s0735-1097(02)01932-0. [DOI] [PubMed] [Google Scholar]
- 7.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac resynchronization therapy with or without an Implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 8.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 9.Petkova-Kirova PS, Gursoy E, Mehdi H, McTiernan CF, London B, Salama G. Electrical remodeling of cardiac myocytes from mice with heart failure due to the overexpression of tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol. 2006;290:H2098–H2107. doi: 10.1152/ajpheart.00097.2005. [DOI] [PubMed] [Google Scholar]
- 10.Mehdi H, Manzi S, Desai P, Chen Q, Nestlerode C, Bontempo F, Strom SC, Zarnegar R, Kamboh MI. A functional polymorphism at the transcriptional initiation site in β2-glycoprotein I (apolipoprotein H) associated with reduced gene expression and lower plasma levels of β2-glycoprotein I. Eur J Biochem. 2003;270:230–238. doi: 10.1046/j.1432-1033.2003.03379.x. [DOI] [PubMed] [Google Scholar]
- 11.Rose J, Armoundas AA, Tian Y, DiSilvestre D, Burysek M, Halperin V, O'Rourke B, Kass DA, Marban E, Tomaselli GF. Molecular correlates of altered expression of potassium currents in failing rabbit myocardium. Am J Physiol Heart Circ Physiol. 2005;288:H2077–H2087. doi: 10.1152/ajpheart.00526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrickson CA, Spraqq DD, Cheng A, Capps M, Devaughn K, Marine JE, Calkins H, Tomaselli GF, Berger RD. Evidence for electrical remodeling of the native conduction system with cardiac resynchronization therapy. PACE. 2007;30:591–595. doi: 10.1111/j.1540-8159.2007.00717.x. [DOI] [PubMed] [Google Scholar]
- 13.Higgins SL, Yong P, Scheck D, McDaniel M, Bollinger F, Vadecha M, Desai S, Meyer DB, the Ventak CHF Investigators Biventricular pacing diminishes the need for implantable cardioverter defibrillator therapy. JACC. 2000;36:824–827. doi: 10.1016/s0735-1097(00)00795-6. [DOI] [PubMed] [Google Scholar]
- 14.Podesser B, Wollenek G, Seitelberger R, Siegel H, Wolner E, Firbas W, Tschabitscher M. Epicardial branches of coronary arteries and their distribution in the rabbit heart: the rabbit heart as a model of regional ischemia. Anat Rec. 1997;247:521–527. doi: 10.1002/(SICI)1097-0185(199704)247:4<521::AID-AR11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 15.Turitto G, El-Sherif N. Cardiac resynchronization therapy: a review of proarrhythmic and antiarrhythmic mechanisms. PACE. 2007;30:115–122. doi: 10.1111/j.1540-8159.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig G. Die terminale Strombahn des Kaninchenmyokards. Verh Anat Ges. 1971;65:479–493. [PubMed] [Google Scholar]
- 17.Langendorff O. Dntersuchungen am uberlebenden Saugetierherz. Arch Physiol. 1895;61:291–332. [Google Scholar]
- 18.Lancester ER. On the valves of the heart of “Ornithorhynchus paradcxus” compared to those of man. Proc Zool Soc Lond. 1882;52:42–64. [Google Scholar]
- 19.Miura T, Downey JM, Ooiwa H, Ogawa S, Adachi T, Noto T, Shizukuda Y, limura O. Progression of myocardial infarction in a collateral flow deficient species. Jpn Heart J. 1989;30:695–708. doi: 10.1536/ihj.30.695. [DOI] [PubMed] [Google Scholar]
- 20.Maxwell MP, Hearse DJ, Yellon DM. Species variation in the coronary collateral circulation during regional myocardial ischemia: a critical determinant of the rater of evolution and extent of myocardial infarction. Cardiovasc Res. 1987;21:737–746. doi: 10.1093/cvr/21.10.737. [DOI] [PubMed] [Google Scholar]
- 21.Harken AH, Simson MB, Haselgrove J, Wetstein L, Harden WR, Barlow CH. Early ischemia after complete coronary ligation in the rabbit, dog, pig, and monkey. Am J Physiol. 1981;241:H202. doi: 10.1152/ajpheart.1981.241.2.H202. [DOI] [PubMed] [Google Scholar]
- 22.Toyo-oka T, Kamishiro T, Fumino H, Masaki T, Hosoda S. Rabbit hearts for the critical evaluation of drugs to reduce the size of experimentally produced acute myocardial infarction. Jpn Heart J. 1984;25:623–632. doi: 10.1536/ihj.25.623. [DOI] [PubMed] [Google Scholar]
- 23.Lee BH, Kim WH, Choi MJ, Rho JR, Kim WG. Chronic Heart Failure Model in Rabbits Based on the Concept of the Bifurcation/Trifurcation Coronary Artery Branching Pattern. Art Org. 2002;26:360–365. doi: 10.1046/j.1525-1594.2002.06881.x. [DOI] [PubMed] [Google Scholar]
- 24.Pham T, Sosunov E, Gainullin R, Daniio P, Rosen M. Gender-related differences in susceptibility to lethal ventricular arrhythmias: roles of gonaldal steroids in normal and castrated rabbits. PACE. 2000;23:604. Abstract. [Google Scholar]
- 25.Pham T, Sosunov E, Gainullin R, Robinson R, Rosen M. Gender-related differences in transmurai dispersion of the L-type calcium current in rabbit ventricles. PACE. 2000;23:638. Abstract. [Google Scholar]
- 26.Cheng J, Kamiya K, Liu W, Tsuji Y, Toyama J, Kodama Heterogeneous distribution of the two components of delayed rectifier K+ current: a potential mechanism of the proarrhythmic effects of methanesulfonanilide class III agents. Cardiovasc Res. 1999;43:135–147. doi: 10.1016/s0008-6363(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 27.Choi B-R, Salama G. Simultaneous maps of action potentials (AP) and Ca2+ transients (Cal) reveal that Cai oscillations underlie cellular discordant altemans. Circulation. 1999;100:150–151. [Google Scholar]
- 28.Yehia AR, Shrier A, Lo KC, Guevara MR. Transient outward currents contributes to Wenckebach like rhythms in isolated rabbit ventricular cells. Am J Physiol. 1997;273:H1–H11. doi: 10.1152/ajpheart.1997.273.1.H1. [DOI] [PubMed] [Google Scholar]
- 29.Salata JJ, Jurkiewicz NK, Jow B, Folander K, Guinosso PJ, Jr, Raynor B, Swanson R, Fermimi B. IK of rabbit ventricle is composed of two currents: evidence for lKs. Am J Physiol. 1996;271:H2477–H2489. doi: 10.1152/ajpheart.1996.271.6.H2477. [DOI] [PubMed] [Google Scholar]
- 30.Veldkamp MW, van Ginneken AC, Bouman LN. Single delayed rectifier channels in the membrane of rabbit ventricular myocytes. Circ Res. 1993;72:865–878. doi: 10.1161/01.res.72.4.865. [DOI] [PubMed] [Google Scholar]
- 31.Fedida G, Giles WR. Regional variations in action potentials and transient outward currents in myocytes isolated from rabbit left ventricle. J Physiol. 1991;442:191–209. doi: 10.1113/jphysiol.1991.sp018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suto F, Cahill SA, Wilson GJ, Hamilton RM, Greenwald, Gross GJ. A novel rabbit model of variably compensated complete heart block. J Appl Physiol. 2001;92:1199–1204. doi: 10.1152/japplphysiol.00714.2001. [DOI] [PubMed] [Google Scholar]
- 33.Luchner A, Muders F, Dietl O, Friedrich E, Blumberg F, Protter AA, Riegger GAJ, Elsner D. Differential expression of cardiac ANP and BNP in a rabbit model of progressive left ventricular dysfunction. Cardiovasc Res. 2001;51:601–607. doi: 10.1016/s0008-6363(01)00316-9. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Tockman B, Girouard S, Pastore J, Walcott G, KenKnight B, Spinelli J. Left ventricular resynchronization therapy in a canine model of left bundle branch block. Am J Physiol Heart Circ Physiol. 2002;282:H2238–H2244. doi: 10.1152/ajpheart.00684.2001. [DOI] [PubMed] [Google Scholar]
- 35.Wyman BT, Hunter WC, Prinzen FW, Faris OP, McVeigh ER. Effects of single- and biventricular pacing on temporal and spatial dynamics of ventricular contraction. Am J Physiol Heart Circ Physiol. 2002;282:H372–H379. doi: 10.1152/ajpheart.2002.282.1.H372. [DOI] [PubMed] [Google Scholar]
- 36.Leclercq C, Faris O, Tunin R, Johnson J, Kato R, Evans F, Spinelli J, Halperin H, McVeigh E, Kass DA. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with left bundle-branch block. Circulation. 2002;106:1760–1763. doi: 10.1161/01.cir.0000035037.11968.5c. [DOI] [PubMed] [Google Scholar]
- 37.Dohi K, Suffoletto MS, Schwartzman D, Ganz LI, Pinsky MR, Gorcsan J., 3rd Utility of echocardiographic radial strain imaging to quantify left ventricular dyssynchrony and predict acute response to cardiac resynchronization therapy. Am J Cardiol. 2005;96:112–116. doi: 10.1016/j.amjcard.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 38.Kanzaki H, Bazaz R, Schwartzman D, Dohi K, Sade LE, Gorcsan J., 3rd A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy: insights from mechanical activation strain mapping. J Am Coll Cardiol. 2004;44:1619–1625. doi: 10.1016/j.jacc.2004.07.036. [DOI] [PubMed] [Google Scholar]


