Abstract
The circadian pacemaker is differentially sensitive to the resetting effects of retinal light exposure, depending upon the circadian phase at which the light exposure occurs. Previously reported human phase response curves (PRCs) to single bright light exposures have employed small sample sizes, and were often based on relatively imprecise estimates of circadian phase and phase resetting. In the present study, 21 healthy, entrained subjects underwent pre- and post-stimulus constant routines (CRs) in dim light (∼2–7 lx) with maintained wakefulness in a semi-recumbent posture. The 6.7 h bright light exposure stimulus consisted of alternating 6 min fixed gaze (∼10 000 lx) and free gaze (∼5000–9000 lx) exposures. Light exposures were scheduled across the circadian cycle in different subjects so as to derive a PRC. Plasma melatonin was used to determine the phase of the onset, offset, and midpoint of the melatonin profiles during the CRs. Phase shifts were calculated as the difference in phase between the pre- and post-stimulus CRs. The resultant PRC of the midpoint of the melatonin rhythm revealed a characteristic type 1 PRC with a significant peak-to-trough amplitude of 5.02 h. Phase delays occurred when the light stimulus was centred prior to the critical phase at the core body temperature minimum, phase advances occurred when the light stimulus was centred after the critical phase, and no phase shift occurred at the critical phase. During the subjective day, no prolonged ‘dead zone’ of photic insensitivity was apparent. Phase shifts derived using the melatonin onsets showed larger magnitudes than those derived from the melatonin offsets. These data provide a comprehensive characterization of the human PRC under highly controlled laboratory conditions.
Early human entrainment studies led to the belief that the primary entraining agent for humans was not light, but rather social interaction (Wever, 1979; Aschoff & Wever, 1981). However, due to concerns about the design of these experiments (primarily the use of self-selected lighting schedules) and the subsequent demonstration that light cycles indeed can entrain human circadian rhythms (Czeisler et al. 1981; Wever et al. 1983; Honma et al. 1987a), this belief is now considered unwarranted (Czeisler, 1995). Numerous studies have reported that single pulses of bright light are capable of generating modest phase delay and phase advance shifts of the human circadian pacemaker (Honma et al. 1987b; Kennaway et al. 1987; Buresová et al. 1991; Dawson & Campbell, 1991; Laakso et al. 1993; Van Cauter et al. 1993; Samková et al. 1997; Honma et al. 1997; Parry et al. 1997b; Shanahan et al. 1999; Zeitzer et al. 2000). Several studies have determined the phase-dependent phase shifting effects of bright light by applying single pulses at two different phases (Honma et al. 1987b; Dawson et al. 1993; Van Cauter et al. 1993).
The best quantification of the phase dependence of light-induced phase shifts is obtained by deriving a phase response curve (PRC), in which the response to a specific light stimulus is evaluated thoroughly and systematically over the entire circadian cycle. The exact shape and timing of the resulting PRC provides precise information concerning the overall relationship between phase shift magnitude and circadian phase of the stimulus over the entire circadian cycle.
There have been five previous reports of PRCs to light in humans. A comprehensive study using three consecutive days containing 5 h exposures of bright light revealed a type 0 PRC (Czeisler et al. 1989) in which phase shifts as large 12 h were observed when the stimulus was centred near the core body temperature minimum. The four other PRC studies employed single light pulses but presented insufficient data for adequate resolution of phase dependence. The earliest study (Honma & Honma, 1988) reveals a PRC without a significant phase-delay region, which is clearly inconsistent with the reports of many studies from other laboratories that have routinely demonstrated phase delays to single light pulses. Furthermore, the PRCs derived, one for 3 h and one for 6 h light pulses, consisted of only nine and eight data points, respectively, distributed unevenly over the circadian cycle. As a result there were significant gaps in the PRC, with one of them over 7 h long (almost one-third of the entire cycle) (Honma & Honma, 1988). A subsequent study by Minors et al. (1991) shows PRCs with a similar paucity of data. Using one experimental protocol, 10 data points are presented, whereas in another only six are presented, and gaps in the PRC appear for periods as long as 11 h, or almost half of the circadian cycle. The phase-delay region of the curve was especially poorly defined. Interpretation of these data is further complicated by the use of two different light intensities (5000 and 9000 lx) for the light stimulus and the use of mathematical methods, rather than a constant routine (CR) protocol to eliminate masking effects on the phase measurement variable (core body temperature) – a procedure that can lead to inaccurate phase estimates (Klerman et al. 1999). Another study, which was a preliminary retrospective analysis, also suffers from a lack of data (Jewett et al. 1994). Only 11 data points were acquired, resulting in poor characterization of the early portion of the phase-delay region, and the region of peak amplitude of the advance region of the PRC. Furthermore, the light pulse duration was inconsistent, varying in duration from 4.0 to 6.2 h. Finally, the most comprehensive single pulse PRC study by Van Cauter et al. (1994) employed well-controlled conditions and methodology including a CR to assess the circadian phase. However, the experimental protocol in this study incorporated the pre-stimulus circadian phase assessment, the light exposure stimulus, and the post-stimulus circadian phase assessment in a single fixed-duration CR. Consequently, they were only able to report phase shifts for a region spanning less than half of the circadian cycle; in order to span the entire circadian cycle, CR durations would have had to be extended substantially. Therefore, in order to construct a more comprehensive PRC to single light pulses we have administered bright light over the entire range of circadian phases and have used a precise method (the entire plasma melatonin profile assessed in dim light with controlled posture) to assess circadian phase resetting.
METHODS
Prior to admission to the laboratory all subjects were screened for any current or previous medical and psychiatric disorders or abnormalities as revealed by urine and blood testing, electrocardiographic recording, physical history and examination, psychometric testing, and psychological interview. Subjects with a history of any regular night work within the past 3 years or travel across more than two time zones within the past 3 months were excluded. Subjects maintained a regular 8 h sleep schedule based upon their habitual sleep and wake times for at least 2 weeks prior the start of the study. Compliance was verified using a time-stamped voicemail phone answering system that subjects called at each sleep and wake time. For at least the last week prior to starting the study, compliance was further verified by actigraphy (Ambulatory Monitoring Inc., Ardsley, NY, USA). Subjects were required to abstain from caffeine, nicotine, alcohol and all medications or supplements prior to the start of the study; compliance was evaluated by a urinary toxicology screen upon admission to the laboratory. Subjects who were successfully screened and began the study were also required to exhibit a normal circadian rhythm in core body temperature with a minimum fitted amplitude of 0.14 °C during the first CR (see below) before they were qualified to continue the study (Czeisler et al. 1989; Duffy et al. 1996; Jewett et al. 1997; Shanahan et al. 1999; Zeitzer et al. 2000). Subjects were then randomly assigned to a circadian phase for receiving their bright light exposure. All subjects were informed of study procedures and their status as volunteers prior to participation, gave their informed consent, and were paid for their participation. The experimental protocol was approved by the institutional review board of Brigham and Women's Hospital and all procedures used conformed with the Declaration of Helsinki.
Subjects remained in a private laboratory suite to which only technicians and investigators had access for the duration of the study. An environment free of usual time cues was maintained such that subjects had no access to information concerning the time of day or day of the week. The 9 or 10 day in-laboratory segment of the study protocol (Fig. 1) began with three baseline days with 8 h sleep episodes in darkness scheduled at their habitual times, and 16 h of ambulatory wakefulness in < 150 lx. Upon waking from the third sleep episode, subjects began a pre-stimulus CR, which lasted from 27.1 to 49.7 h on days 4–5 of the protocol. The duration of the pre-stimulus CR, and therefore, the timing of the following light exposure day, was varied systematically across the 24 h day at 2 h intervals in different subjects. This timing was calculated relative to either the phase of the fitted core body temperature minimum during the pre-stimulus CR (n = 16), or to the timing of the subject's habitual wake time (n = 7). Following the pre-stimulus CR, subjects underwent a light exposure day on day 6 consisting of an 8 h sleep episode, an ambulatory 16 h wake episode with a seated 6.7 h bright light exposure (see below for details) centred in the middle of the wake episode, and another 8 h sleep episode. Subjects then underwent a post-stimulus CR of 30.9 to 65.2 h duration that was followed by a final sleep episode. The 10 day protocol was identical to the 9 day protocol, except that the post-stimulus CR was 15 h longer and the final sleep episode was 14 h long.
Figure 1. A raster plot of the experimental protocol for subject 1843.

Consecutive days are plotted from top to bottom. Clock time is indicated on the horizontal axis. The protocol began with three 24 h baseline days on the subject's habitual sleep–wake schedule with 16 h wake episodes in < 150 lx illumination (□) and 8 h sleep opportunities in darkness (▪). A pre-stimulus CR in dim light (< 15 lx, ▪) began on day 4 and continued until the 8 h sleep episode on day 5. The light exposure day consisted of 16 h of ambulatory wakefulness in dim light, interrupted by a seated 6.7 h bright light exposure (∼10 000 lx fixed gaze, □) centred in the middle of the wake episode. A subsequent 8 h scheduled sleep episode was followed by a post-stimulus CR and a recovery sleep episode. The timing of the melatonin midpoints for evaluation of the phase shift is indicated by ▵. For this subject, the light exposure generated a phase delay of −3.60 h in the melatonin midpoint..
The CR protocol (Duffy & Dijk, 2002) consisted of continuous enforced wakefulness in a semi-recumbent posture. Subjects were restricted to the bed for the entire CR. Hourly snacks and fluids were provided in order to maintain evenly distributed caloric and fluid intake throughout the CR. Technicians were present at all times to assist the subjects in maintaining wakefulness and to ensure protocol compliance.
All lighting was delivered from fluorescent bulbs mounted throughout the ceiling behind lexan panels, which blocked ultraviolet light. During wake-time on the baseline days, subjects were exposed to < 150 lx in the maximum reasonable angle of gaze (typical illumination in the angle of gaze ranged from ∼60 to ∼130 lx). During all sleep episodes subjects were in darkness and did not have access to any light sources. From the beginning of the first CR until discharge at the end of the study, illumination was maintained at < 15 lx in any angle gaze (typical horizontal angle of gaze ranged from ∼2 to ∼7 lx). For the bright-light exposure, subjects were seated throughout and were accompanied by a technician. They were instructed to fix their gaze at a target on the wall near to the ceiling. For 3 min at the start of the bright-light exposure, the illumination was gradually increased to a final intensity of ∼10 000 lx in this fixed angle of gaze at this target, followed by 3 min of maintained fixed gaze at the ∼10 000 lx intensity. This was followed by alternating 6 min episodes of free gaze about the room and 6 min episodes of fixed gaze at the target. During the free-gaze episodes, subjects experienced illumination ranging from ∼5000 to ∼9000 lx depending upon their angle of gaze during this interval. The last 6 min fixed-gaze episode consisted of 3 min at the ∼10 000 lx intensity, followed by 3 min during which the illumination was gradually reduced to the pre-stimulus dim intensity level of < 15 lx. The total light exposure duration was 6.7 h. This stimulus duration was selected to ensure that the phase shifts would be of sufficient magnitude to yield a statistically significant PRC amplitude, and because this protocol was part of a series of comparison studies including PRCs to two and three consecutive stimuli, which required stimuli of sufficient duration to generate amplitude suppression and type 0 resetting, respectively. Technicians monitored subjects throughout the entire light exposure to implement the timing of the fixed and free-gaze episodes, record the illumination received by the subjects in their angle of gaze for each 6 min episode, and ensure that subjects kept their eyes open and received the light exposure at the scheduled times.
Core body temperature was recorded throughout the study at 1 min intervals with a rectal temperature thermistor (Yellow Springs Instrument Company, Yellow Springs, OH, USA). The amplitude of the circadian rhythm of core body temperature during the CR was evaluated from a dual-harmonic least-squares fit to the data with correlated noise (Brown & Czeisler, 1992). Beginning on day 2 of the protocol, blood samples were collected at 30 min intervals from an indwelling intravenous catheter throughout the entire study protocol. Hourly saliva samples were routinely collected during CRs in the event that blood samples could not be collected. Plasma or salivary melatonin concentration was determined by radioimmunoassay (DiagnosTech, Osceola, WI, USA). Three different evaluations of phase from the individual daily plasma melatonin profiles were performed. For the evaluation of melatonin onset, offset, and midpoint, the peak amplitude of a 3-harmonic fit to the raw data from the melatonin profile in the pre-stimulus CR was first calculated. Melatonin onset (DLMOn) for both the pre- and post-stimulus melatonin profiles was then defined as the time at which the rising phase of the melatonin profile (using linear interpolation between relevant data points) crossed a threshold value of 25 % of the fitted 3-harmonic peak amplitude. Melatonin offset (DLMOff) was defined as time at which the falling phase of the melatonin profile crossed the 25 % threshold. Melatonin midpoint was defined as the average time between DLMOn and DLMOff for each melatonin profile. Melatonin phase shifts were evaluated as the difference in clock time between the melatonin phase evaluated during the pre-stimulus CR, and the melatonin phase evaluated 3 days later during the post-stimulus CR. As per normal convention, phase delays are plotted in all figures as negative values and phase advances as positive values.
RESULTS
Forty-three subjects were successfully screened, impanelled, and randomized into the study. Of these, one subject withdrew from the study, two were disimpanelled for failing to meet the core body temperature amplitude criteria on the pre-stimulus CR, three were disimpanelled due to onset of illness during the study, and two were disimpanelled due to errors in execution of the protocol. Of the remainder, 23 male (n = 16) and female (n = 7) subjects aged 19–44 years (average = 27.16 ± 7.46 years (s.d.)) were randomized to a 9 (n = 17) or 10 (n = 6) day bright light study protocol. Plasma melatonin data sufficient to determine phase shifts were acquired from 21 of these subjects. Sufficient blood and saliva samples were unavailable from one subject. Blood sampling was unavailable in another subject (18K8) throughout the study; melatonin phase for this subject was determined from salivary melatonin collected every 30 min during both CRs. The midpoint of the melatonin profile on the pre-stimulus CR (n = 23, including the salivary assay) occurred at an average ± s.d. of 4.05 ± 0.78 h (range: 2.69–5.79 h) before the subjects' habitual wake times, which is considered a normal phase relationship in entrained subjects (Duffy et al. 2002).
An example of the melatonin data from a subject experiencing a phase delay to a light exposure is shown in Fig. 2. Using the midpoint of the melatonin profile on the pre- and post-stimulus CRs as a phase marker, phase-delay shifts of up to −3.60 h and phase-advance shifts of up to +2.01 h were observed in response to the single 6.7 h bright-light exposure. The full PRC to this stimulus is shown in Fig. 3 and is plotted relative to the phase of the melatonin midpoint (22 h) determined on the pre-stimulus CR, which occurs approximately 2 h before the fitted core body temperature minimum (circadian phase 0 h). The data from circadian phases 6–18 have been double plotted to better demonstrate the PRC shape. A dual harmonic sinusoidal regression of the raw data reveals a peak-to-trough amplitude of the PRC of 5.02 h, with a 95 % confidence interval that does not include zero. Because there were 3 intervening days between the pre- and post-stimulus phase assessments and the endogenous circadian period for each subject was unknown, it was not possible to separately determine the relative amplitude of the phase-delay and phase-advance regions of the PRC with any certainty. Therefore, the horizontal baseline in Fig. 3 (dashed line) is plotted based upon an anticipated average delay drift of the pacemaker of 0.18 h per day (i.e. an average circadian period of 24.18 h) (Czeisler et al. 1999). Using this baseline and the sinusoidal regression, the baseline-to-peak amplitude of the phase-advance region at circadian phase 4.05 h is 2.32 h, and that of the phase-delay region at circadian phase 19.38 h is 2.70 h. A relatively rapid transition from phase delays to phase advances occurs near circadian phase 0 h and a somewhat more gradual transition from phase advances to phase delays occurs in the subjective day approximately 12 h later.
Figure 2. The melatonin profile from the pre-stimulus to the post-stimulus CRs.
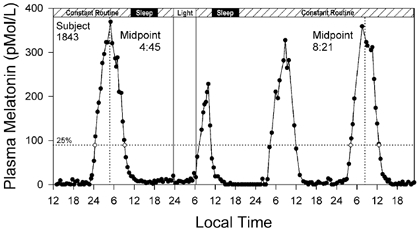
Plasma melatonin on the vertical axis is plotted against local time on the horizontal axis for subject 1843 in Fig. 1. The protocol procedure is indicated on the bar at the top of the figure. The 25 % threshold (89.5 pmol l−1, dotted horizontal line) of the peak amplitude of the fitted 3-harmonic curve from the pre-stimulus CR was used to calculate the pre- and post-stimulus DLMOn and DLMOff values (⋄). The midpoints between DLMOn and DLMOff used to calculate the light-induced phase delay for this subject (04:45 h to 08:21 h = −3.60 h) are indicated by the vertical dotted lines. Note suppression of the second melatonin peak in the figure during the light exposure.
Figure 3. The PRC to the bright light stimulus using melatonin midpoints as the circadian phase marker.
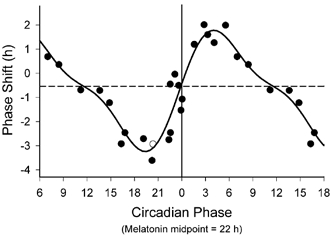
Phase advances (positive values) and delays (negative values) are plotted against the timing of the centre of the light exposure relative to the melatonin midpoint on the pre-stimulus CR (defined to be 22 h), with the core body temperature minimum assumed to occur 2 h later at 0 h. Data points from circadian phases 6–18 are double plotted. The filled circles represent data from plasma melatonin, and the open circle represents data from salivary melatonin in subject 18K8 from whom blood samples were not acquired. The solid curve is a dual harmonic function fitted through all of the data points. The horizontal dashed line represents the anticipated 0.54 h average delay drift of the pacemaker between the pre- and post-stimulus phase assessments.
The PRCs derived using DLMOn (defined to occur at circadian phase 17 h) and DLMOff (defined to occur at circadian phase 3 h) are shown in Fig. 4A and B, respectively. Again, the circadian phases are plotted so that the core body temperature minimum is estimated to occur at approximately circadian phase 0 h. The general waveform characteristics of these PRCs are similar to those for the melatonin midpoint PRC. Dual harmonic sinusoidal regression fits through the data reveal a fitted peak-to-trough amplitude of 5.41 h for the DLMOn data and of 4.60 h for the DLMOff data, with 95 % confidence intervals that do not include zero. The relationship between phase shifts calculated using DLMOff and those calculated using DLMOn is plotted in Fig. 4C, together with a linear regression through these data points (solid line). This regression is highly significant (r2 = 0.92) with a slope of 0.84 (95 % confidence interval: 0.73 to 0.96). This suggests that phase shifts measured by DLMOff are smaller than those measured by DLMOn, since the slope of the correlation is significantly lower than unity. Furthermore, a paired t test analysis reveals that phase shifts evaluated with the DLMOn marker are significantly different from those evaluated with the DLMOff marker (P < 0.05). This holds true even when the phase shifts are corrected by the average anticipated overall drift in phase between the pre- and post-stimulus phase assessments of 0.54 h (0.18 h day−1 × 3 days).
Figure 4. Comparison of the PRCs using melatonin onset (DLMOn) and offset (DLMOff).

A and B, PRCs plotted as in Fig. 3, except that all values are single plotted. The horizontal dashed line represents the anticipated 0.54 h average delay drift of the pacemaker between the pre- and post-stimulus phase assessments. The fitted peak-to-trough amplitude of the DLMOn PRC (5.41 h) appears slightly larger than that of the DLMOff PRC (4.60 h). C, correlation between the DLMOn and DLMOff phase shifts. The continuous line with a slope of unity represents identical phase shifts between the two phase markers. The dotted line is the linear regression through the data points with a slope (0.84) significantly less than unity (P < 0.05). This indicates that both phase advances and phase delays determined using DLMOff tend to be smaller than those determined using DLMOn.
DISCUSSION
The PRC to the single bright light stimulus in this study is consistent with type 1 resetting. Generally, phase delays are generated for pulses centred before circadian phase 0 h, and phase advances are generated for pulses centred after circadian phase 0 h. Unlike the previously reported type 0 PRC, in which light pulses centred very near the critical phase at the core body temperature minimum produce phase shifts as large as 12 h in magnitude (Czeisler et al. 1989), phase shifts in the type 1 PRC presented here are near zero when the light stimulus is centred close to circadian phase 0 h. The transition from delays to advances in this critical region of the PRC is rapid, while the transition from phase advances to phase delays during the subjective day is more gradual. These results support our contention that humans are capable of both type 1 and type 0 resetting depending upon the strength of the resetting stimulus applied (Kronauer et al. 1993). These results also support our position that three iterations of this single pulse PRC cannot explain the type 0 resetting observed in response to three consecutive pulses of bright light (Kronauer et al. 1993; Beersma & Daan, 1993).
The phase shift magnitudes reported in this study are similar to those reported in other studies of light-induced phase shifts in humans. The phase delays in this study are also consistent in magnitude with those reported to single light pulses in an intensity response curve recently reported by Zeitzer et al. (2000), in which phase delay magnitudes of about −3 h were observed for light intensities from 1000 to 10 000 lx in a very similar experimental protocol. Because the free-running periods of the subjects are not known, it is not possible to be certain of the degree to which circadian phase drifted between the pre- and post-stimulus phase estimates. This precludes any definitive analysis of the relative magnitude of the phase-delay and phase-advance regions of the PRC. However, assuming an average free-running period of 24.18 h, the phase-delay and phase-advance regions appear roughly equivalent in magnitude, consistent with predictions of the mathematical model of Kronauer et al. (1999). On the other hand, the marginally larger size of the phase delay relative to the phase advance (2.70 h vs. 2.32 h), if real, would be consistent with data from other PRC studies also showing slightly larger phase delays than advances (Minors et al. 1991; Dawson et al. 1993; Jewett et al. 1994; Van Cauter et al. 1994). The peak-to-trough amplitude of the PRC (from the maximum phase delay to the maximum phase advance) is about 5 h. This agrees well with the peak-to-trough amplitude in two PRC studies using bright light exposures of about 6 h in duration (Jewett et al. 1994; Honma & Honma, 1988). PRCs using 3–4 h duration bright light pulses have reported peak-to-trough amplitudes of about 3–4 h (Minors et al. 1991; Dawson et al. 1993; Van Cauter et al. 1994). Given the significant protocol differences between this study and previous PRC studies in terms of light stimulus characteristics, background lighting conditions, recording conditions, and phase markers used, this degree of consistency in results suggests that the PRC to single bright light pulses is a robust and replicable phenomenon.
The subjective day region of the PRC in many organisms is characterized by a region of relative insensitivity to light-induced resetting, and has been described as a ‘dead zone’ of the PRC. However, there is no evidence of a significant dead zone in this study (see Figs 3 and 4). Phase advances diminish gradually during the course of the early subjective day, ultimately yielding to gradually increasing phase delays later in the subjective day. This suggests that the human circadian pacemaker is sensitive to light-induced resetting throughout the subjective day. This absence of a dead zone is consistent with that typically observed in diurnal, but not nocturnal, rodents (Pohl, 1982), for the type 0 PRC in humans (Jewett et al. 1997), and possibly with PRCs observed in primates (Rauth-Widmann et al. 1991; Hoban & Sulzman, 1985). However, it is also possible that the long duration of the light stimulus in this study could be obscuring the presence of a dead zone, which might be revealed in a PRC to a shorter duration stimulus.
Studies examining the profile of melatonin secretion in rodents following phase shifts to light stimuli have indicated that the onset and offset of melatonin secretion do not always phase shift in a parallel manner. Accordingly, the hypothesis has been suggested that there may be two coupled oscillators, an evening or E oscillator associated with melatonin onset, and a morning or M oscillator associated with melatonin offset (Pittendrigh & Daan, 1976; Illnerová & Vanecek, 1982; Elliott & Tamarkin, 1994; Illnerová & Sumová, 1997). Illnerová and coworkers have observed that following phase-delaying light stimuli in rats, the shift in pineal N-acetyl-transferase (NAT, the biochemical precursor to melatonin secretion) onset is immediate. The phase delay in NAT offset is generally slightly smaller than that of NAT onset and increases rapidly in magnitude over subsequent days. Following phase-advancing light stimuli, it is the shift in NAT offset that is immediate, whereas the shift in NAT onset is notably smaller and may be negligible at first (reviewed in Illnerová & Sumová, 1997). In contrast to the pattern of light-induced phase delays noted in rats above, Elliott & Tamarkin (1994) have reported a tendency for the melatonin offset in hamsters to shift before that of the melatonin onset following phase-delaying light pulses. However, their results following phase-advancing light stimuli concurred with those of Illnerova & Sumová (1997), with the shift in melatonin offset occurring immediately, whereas the shift in melatonin onset advances only after several days of transient adjustment.
It is difficult to make a thorough comparison of the relative phase shifting tendency of melatonin onset and offset between this study and those of the rodent studies. Unlike the rodent studies, this study has not examined the full time course of phase-shift magnitude on subsequent days following the bright light stimulus. However, it is interesting that the tendency in this study is for phase shifts in melatonin onset to be larger than those in melatonin offset throughout the PRC, which is in sharp contrast to the observations in rodents for phase-advance shifts, in which the onset of NAT or melatonin undergoes marked transient behaviour before the phase advances are equivalent in magnitude to those observed in the offset of NAT or melatonin.
Other human studies reporting on the light-induced phase shifts of melatonin onset and offset also provide mixed results as to which phase marker shows the larger phase-shifting response. Although results from some laboratories have suggested a tendency for the phase shift in melatonin onset to be greater than the phase shift in melatonin offset (Lewy et al. 1985; Foret et al. 1993; Laakso et al. 1993; Deacon & Arendt, 1994; Hashimoto et al. 1997; Honma et al. 1997; Van Cauter et al. 1998), others have suggested the opposite relationship (Cagnacci et al. 1997; Parry et al. 1997a, b), and others has reported mixed results in separate studies (Buresová et al. 1991; Samková et al. 1997). The most important factor that may explain these contradictory findings in the relationship between the phase shift observed in melatonin onset and offset in response to a light regimen is that these studies have employed a wide variety of light interventions and experimental protocols with different light intensities and durations, making comparisons across studies problematic. Therefore, a definitive evaluation of the relative behaviour of phase shifts in melatonin onset and offset must await controlled in-laboratory studies, ideally with multiple phase assessments following the light exposure to evaluate any transient behaviour of the phase markers.
Although light is considered to be the dominant synchronizing agent for circadian rhythms, a recent entrainment study in a blind individual (Klerman et al. 1998), and studies evaluating the phase-shifting effects of exercise (Van Reeth et al. 1994) suggest that non-photic agents may have effects on the human circadian pacemaker. In this study protocol, the sleep–wake, activity and social interaction schedule have been shifted concurrently with the light stimulus. It is therefore possible that the phase shifts observed in this study include a contribution from non-photic cues. The appropriate control experiment required to definitively quantify this contribution would be to shift the sleep–wake schedule in darkness, a protocol that is technically very difficult to conduct in sighted human subjects. However, previous studies in our laboratory employing protocols very similar to that of this study have shown that the contribution of non-photic agents must be minimal, if present at all. Duffy et al. (1996) demonstrated that shifting the sleep–wake and activity schedule for 3 consecutive days with a bright light stimulus targeted to generate either phase advances or phase delays, yielded robust phase shifts. However, identical control protocols for both the phase-advancing and phase-delaying schedules in the absence of bright light yielded only small phase delays consistent with the anticipated drift of the pacemaker due to the longer-than-24 h average period expected for human subjects (Czeisler et al. 1999). Zeitzer et al. (2000) have shown that reduction of the light intensity in a phase-delaying light stimulus reduces the phase-shift amplitude in a predictable manner, and that despite a shift in the sleep–wake schedule, the stimuli with the lowest light intensities yield minimal phase delays, again consistent with the drift due to circadian period. Similarly, a recent study in our laboratory evaluating shifts of the sleep–wake and activity schedule in dim light at a variety of circadian phases has revealed very small phase shifts which are similar in form to the PRC in this study, suggesting that dim light exhibits small phase-shifting effects, and reaffirming that the contribution of non-photic cues, if any, is minimal (unpublished observations).
The data in this study have provided for a comprehensive characterization of the human PRC under highly controlled laboratory conditions. The PRC data from this study, together with the data from the intensity response curve of Zeitzer et al. (2000), provide information necessary for accurately predicting the response of the human circadian pacemaker to a specific light stimulus at a specific circadian phase. This has practical relevance for the design of light exposure interventions for resetting the circadian pacemaker in conditions such as shift work, sleep disorders with a circadian component, and jet lag. Furthermore, these data also suggest that the pattern of daily light exposure to both bright outdoor illumination during the day-time as well as indoor artificial illumination during both day-time and night-time significantly contributes to the subsequent timing and entrainment of the circadian pacemaker.
Acknowledgments
This work was supported by NIMH R01-MH45130 and NASA Cooperative Agreement NCC 9–58 with the NSBRI to C.A.C., NHLBI Senior NRSA Fellowship F33-HL09588 to S.B.S.K., ARO 1999-1-0241 and NASA Cooperative Agreement NCC 9–58 with the NSBRI to M.E.J., and Swiss National Science Foundation no. 823A-046640 to C.C., and was performed in a GCRC supported by NCRR MO1-RR02635. We also acknowledge the invaluable contributions of the research assistants in the Division of Sleep Medicine for the extensive subject recruitment, screening and data management; the technical and nursing staff of the GCRC of Brigham and Women's Hospital for executing the demanding experimental protocols; and the research subjects for persevering through the intensive experimental procedures.
References
- Aschoff J, Wever R. The circadian system of man. In: Aschoff J, editor. Biological Rhythms: Handbook of Behavioral Neurobiology. New York: Plenum Press; 1981. pp. 311–331. [Google Scholar]
- Beersma DG, Daan S. Strong or weak phase resetting by light pulses in humans? J Biol Rhythms. 1993;8:340–347. doi: 10.1177/074873049300800407. [DOI] [PubMed] [Google Scholar]
- Buresová M, Dvoráková M, Zvolsky P, Illnerová H. Early morning bright light phase advances the human circadian pacemaker within one day. Neurosci Lett. 1991;121:47–50. doi: 10.1016/0304-3940(91)90646-b. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Soldani R, Yen SSC. Contemporaneous melatonin administration modifies the circadian response to nocturnal bright light stimuli. Am J Physiol. 1997;272:R482–486. doi: 10.1152/ajpregu.1997.272.2.R482. [DOI] [PubMed] [Google Scholar]
- Czeisler CA. The effect of light on the human circadian pacemaker. In: Waterhouse JM, editor. Circadian Clocks and their Adjustment. Chichester: John Wiley and Sons, Inc.; 1995. pp. 254–302. Ciba Foundation Symposium no. 183. [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk D-J, Kronauer RE. Stability, precision, and near-24-h period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Richardson GS, Zimmerman JC, Moore-Ede MC, Weitzman ED. Entrainment of human circadian rhythms by light-dark cycles: a reassessment. Photochem Photobiol. 1981;34:239–247. [PubMed] [Google Scholar]
- Dawson D, Campbell SS. Timed exposure to bright light improves sleep and alertness during simulated night shifts. Sleep. 1991;14:511–516. doi: 10.1093/sleep/14.6.511. [DOI] [PubMed] [Google Scholar]
- Dawson D, Lack L, Morris M. Phase resetting of the human circadian pacemaker with use of a single pulse of bright light. Chronobiol Int. 1993;10:94–102. doi: 10.3109/07420529309059697. [DOI] [PubMed] [Google Scholar]
- Deacon SJ, Arendt J. Phase-shifts in melatonin, 6-sulphatoxymelatonin and alertness rhythms after treatment with moderately bright light at night. Clin Endocrinol. 1994;40:413–420. doi: 10.1111/j.1365-2265.1994.tb03940.x. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Dijk D-J. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk D-J, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab. 2002;282:E297–303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- Elliott JA, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. J Comp Physiol [A] 1994;174:469–484. doi: 10.1007/BF00191713. [DOI] [PubMed] [Google Scholar]
- Foret J, Touitou Y, Aguirre A, Benoit O. Early bright light exposure: effect on plasma melatonin patterns measured in subjects held in 24-h constant conditions. In: Touitou Y, Arendt J, Pévet P, editors. Melatonin and the Pineal Gland: from Basic Science to Clinical Application. Amsterdam: Elsevier Science Publishers; 1993. pp. 215–218. [Google Scholar]
- Hashimoto S, Kohsaka M, Nakamura K, Honma H, Honma S, Honma K-I. Midday exposure to bright light changes the circadian organization of plasma melatonin rhythm in humans. Neurosci Lett. 1997;221:89–92. doi: 10.1016/s0304-3940(96)13291-2. [DOI] [PubMed] [Google Scholar]
- Hoban TM, Sulzman FM. Light effects on circadian timing system of a diurnal primate, the squirrel monkey. Am J Physiol. 1985;249:R274–280. doi: 10.1152/ajpregu.1985.249.2.R274. [DOI] [PubMed] [Google Scholar]
- Honma K, Hashimoto S, Endo T, Honma S. Light and plasma melatonin rhythm in humans. Biol Signals. 1997;6:307–312. doi: 10.1159/000109142. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S. A human phase response curve for bright light pulses. Jpn J Psychiat Neurol. 1988;42:167–168. [Google Scholar]
- Honma K, Honma S, Wada T. Entrainment of human circadian rhythms by artificial bright light cycles. Experientia. 1987a;43:572–574. doi: 10.1007/BF02143589. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S, Wada T. Phase-dependent shift of free-running human circadian rhythms in response to a single bright light pulse. Experientia. 1987b;43:1205–1207. doi: 10.1007/BF01945525. [DOI] [PubMed] [Google Scholar]
- Illnerová H, Sumová A. Photic entrainment of the mammalian rhythm in melatonin production. J Biol Rhythms. 1997;12:547–555. doi: 10.1177/074873049701200609. [DOI] [PubMed] [Google Scholar]
- Illnerová H, Vanecek J. Two-oscillator structure of the pacemaker controlling the circadian rhythm of N-acetyltransferase in the rat pineal gland. J Comp Physiol [A] 1982;145:539–548. [Google Scholar]
- Jewett ME, Kronauer RE, Czeisler CA. Phase/amplitude resetting of the human circadian pacemaker via bright light: a further analysis. J Biol Rhythms. 1994;9:295–314. doi: 10.1177/074873049400900310. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Rimmer DW, Duffy JF, Klerman EB, Kronauer RE, Czeisler CA. Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am J Physiol. 1997;273:R1800–1809. doi: 10.1152/ajpregu.1997.273.5.r1800. [DOI] [PubMed] [Google Scholar]
- Kennaway DJ, Earl CR, Shaw PF, Royles P, Carbone F, Webb H. Phase delay of the rhythm of 6-sulphatoxy melatonin excretion by artificial light. J Pineal Res. 1987;4:315–320. doi: 10.1111/j.1600-079x.1987.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Lee Y, Czeisler CA, Kronauer RE. Linear demasking techniques are unreliable for estimating the circadian phase of ambulatory temperature data. J Biol Rhythms. 1999;14:260–274. doi: 10.1177/074873099129000678. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Rimmer DW, Dijk DJ, Kronauer RE, Rizzo JF, 3rd, Czeisler CA. Nonphotic entrainment of the human circadian pacemaker. Am J Physiol. 1998;274:R991–996. doi: 10.1152/ajpregu.1998.274.4.r991. [DOI] [PubMed] [Google Scholar]
- Kronauer RE, Forger D, Jewett ME. Quantifying human circadian pacemaker response to brief, extended, and repeated light stimuli over the photopic range. J Biol Rhythms. 1999;14:500–515. doi: 10.1177/074873099129001073. [DOI] [PubMed] [Google Scholar]
- Kronauer RE, Jewett ME, Czeisler CA. Commentary: the human circadian response to light - strong and weak resetting. J Biol Rhythms. 1993;8:351–360. doi: 10.1177/074873049300800409. [DOI] [PubMed] [Google Scholar]
- Laakso M-L, Hätönen T, Stenberg D, Alila A, Smith S. One-hour exposure to moderate illuminance (500 lux) shifts the human melatonin rhythm. J Pineal Res. 1993;15:21–26. doi: 10.1111/j.1600-079x.1993.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL, Singer CM. Immediate and delayed effects of bright light on human melatonin production: Shifting ‘dawn’ and ‘dusk’ shifts the dim light melatonin onset (DLMO) Ann NY Acad Sci. 1985;453:253–259. doi: 10.1111/j.1749-6632.1985.tb11815.x. [DOI] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- Parry BL, Berga SL, Mostofi N, Klauber MR, Resnick A. Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. J Biol Rhythms. 1997a;12:47–64. doi: 10.1177/074873049701200107. [DOI] [PubMed] [Google Scholar]
- Parry BL, Udell C, Elliott JA, Berga SL, Klauber MR, Mostofi N, Leveau B, Gillin JC. Blunted phase-shift responses to morning bright light in premenstrual dysphoric disorder. J Biol Rhythms. 1997b;12:443–456. doi: 10.1177/074873049701200506. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents V. Pacemaker structure: a clock for all seasons. J Comp Physiol [A] 1976;106:333–355. [Google Scholar]
- Pohl H. Characteristics and variability in entrainment of circadian rhythms to light in diurnal rodents. In: Aschoff J, Daan S, Groos GA, editors. Vertebrate Circadian Systems: Structure and Physiology. Berlin: Springer-Verlag; 1982. pp. 339–346. [Google Scholar]
- Rauth-Widmann B, Thiemann-Jägar A, Erkert HG. Significance of nonparametric light effects in entrainment of circadian rhythms in owl monkeys (Aotus lemurinus griseimembra) by light-dark cycles. Chronobiol Int. 1991;8:251–266. doi: 10.3109/07420529109063930. [DOI] [PubMed] [Google Scholar]
- Samková L, Vondrasová D, Hájek I, Illnerová H. A fixed morning awakening coupled with a low intensity light maintains a phase advance of the human circadian system. Neurosci Lett. 1997;224:21–24. doi: 10.1016/s0304-3940(97)13460-7. [DOI] [PubMed] [Google Scholar]
- Shanahan TL, Kronauer RE, Duffy JF, Williams GH, Czeisler CA. Melatonin rhythm observed throughout a three-cycle bright-light stimulus designed to reset the human circadian pacemaker. J Biol Rhythms. 1999;14:237–253. doi: 10.1177/074873099129000560. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Moreno-Reyes R, Akseki E, L'Hermite-Balériaux M, Hirschfeld U, Leproult R, Copinschi G. Rapid phase advance of the 24-h melatonin profile in response to afternoon dark exposure. Am J Physiol. 1998;275:E48–54. doi: 10.1152/ajpendo.1998.275.1.E48. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne MM, Blackman JD, Leproult R, Ofek G, L'Hermite-Balériaux M, Refetoff S, Turek FW, Van Reeth O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol. 1994;266:E953–963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne MM, Blackman JD, Scherberg NH, Leproult R, Refetoff S, Van Reeth O. Preliminary studies on the immediate phase-shifting effects of light and exercise on the human circadian clock. J Biol Rhythms. 1993;8:S99–S108. [PubMed] [Google Scholar]
- Van Reeth O, Sturis J, Byrne MM, Blackman JD, L'Hermite-Baleriaux M, Leproult R, Oliner C, Refetoff S, Turek FW, Van Cauter E. Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin secretion in normal men. Am J Physiol. 1994;266:E964–974. doi: 10.1152/ajpendo.1994.266.6.E964. [DOI] [PubMed] [Google Scholar]
- Wever RA. The Circadian System of Man: Results of Experiments under Temporal Isolation. New York: Springer-Verlag; 1979. pp. 1–276. [Google Scholar]
- Wever RA, Polasek J, Wildgruber CM. Bright light affects human circadian rhythms. Pflugers Arch. 1983;396:85–87. doi: 10.1007/BF00584704. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk D-J, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


