Abstract
The acute hypercapnic ventilatory response (AHCVR) arises from both peripheral and central chemoreflexes. In humans, one technique for identifying the separate contributions of these chemoreflexes to AHCVR has been to associate the rapid component of AHCVR with the peripheral chemoreflex and the slow component with the central chemoreflex. Our first aim was to validate this technique further by determining whether a single slow component was sufficient to describe AHCVR in patients with bilateral carotid body resections (BR) for glomus cell tumours. Our second aim was to determine whether the slow component of AHCVR was diminished following carotid body resection as has been suggested by studies in experimental animals. Seven BR subjects were studied together with seven subjects with unilateral resections (UR) and seven healthy controls. A multifrequency binary sequence in end-tidal PCO2 was employed to stimulate ventilation dynamically under conditions of both euoxia and mild hypoxia. Both two- and one-compartment models of AHCVR were fitted to the data. For BR subjects, the two-compartment model fitted significantly better on 1 out of 13 occasions compared with 22 out of 28 occasions for the other subjects. Average values for the chemoreflex sensitivity of the slow component of AHCVR differed significantly (P < 0.05) between the groups and were 0.95, 1.38 and 1.50 l min−1 Torr−1 for BR, UR and control subjects, respectively. We conclude that, without the peripheral chemoreflex, AHCVR is adequately described by a single slow component and that BR subjects have sensitivities for the slow component that are lower than those of control subjects.
A recurring problem when investigating the chemoreflex responses to CO2 in human studies has been our relative inability to separate the level of stimulation at the peripheral chemoreceptors from the level of stimulation at the central chemoreceptors. This has made it difficult to ascribe with any certainty a given response in expired minute ventilation (V̇E) to either the central chemoreflex or the peripheral chemoreflex. However, the central and peripheral chemoreflexes do differ in relation to their speed of response by about one order of magnitude, and consequently one approach to separating their contributions to V̇E has been to ascribe the fast component of the response to the peripheral chemoreflex and the slow component to the central chemoreflex (Swanson & Bellville, 1975; DeGoede et al. 1985). Early experiments using this approach tended to focus on modelling the ventilatory response to step changes in end-tidal partial pressure of CO2 (PET,CO2; Swanson & Bellville, 1975; Bellville et al. 1979; Gardner, 1980; Dahan et al. 1990). More recently, other patterns have been employed to produce dynamic stimulation in PET,CO2 that have been devised to optimise the separation of the peripheral and central chemoreflex components (Pedersen et al. 1999a, b; Fatemian & Robbins, 2001; Nieuwenhuijs et al. 2001; Fatemian et al. 2003). Pedersen et al. (1999b) demonstrated that a two-compartment model (i.e. with fast and slow components) was required to model the data, but a remaining concern has to be whether the fast and slow components can be accurately ascribed to the peripheral and central chemoreflexes, respectively. The first aim of this study was to assess whether a two-compartment model is still required to describe the responses of patients who have undergone bilateral carotid body resections and therefore lack a peripheral (carotid body) chemoreflex response to CO2.
One fundamental question concerning the chemoreflex responses to CO2 is whether the peripheral and central components of the response are independent and additive with respect to their effects on V̇E. In relation to acute changes in the level of stimulation at the chemoreceptors, the majority of the data suggest that this is the case (van Beek et al. 1983; Clement et al. 1995). In relation to longer-term alterations, the picture is less clear-cut. For example, in the goat, the data of Pan et al. (1998) suggest that the reduction in ventilatory sensitivity to CO2 following bilateral carotid body denervation may become more marked in the first few days following surgery than it is immediately following denervation, although after day 4 the sensitivity increases again. The second aim of this study was to assess whether humans who have undergone bilateral carotid body resection exhibit a reduction in central chemosensitivity to CO2.
METHODS
Patients and volunteers
Fourteen patients and seven volunteers were recruited. Seven of the patients had previously undergone bilateral resection of the carotid bodies and the other seven had undergone unilateral resection of the carotid bodies. The operations had been carried out between 1 and 26 years before the current experiments. All subjects were otherwise healthy. The control subjects were reasonably matched for age and sex with the patients.
Patients had undergone carotid body resection because they had developed tumours of one or both carotid bodies (glomus tumour or chemodectoma). These tumours were due to a mutation of the gene encoding for succinate-ubiquinone oxireductase subunit D (SDHD) on chromosome 11q23, as part of the syndrome of head and neck hereditary paraganglioma. SDHD is a small part of cytochrome b588 of the mitochondrial respiratory chain complex II. In the majority of Dutch patients (all patients arising from the Dutch founder families) a missense mutation of Asp92→ Tyr has been reported (van der Mey, 1992; Baysal et al. 2000; Jansen, 2001).
The study was approved by Leiden University Medical Centre ethics committee. All subjects gave their informed, written consent, and the experiments conformed with the guidelines laid down in the Declaration of Helsinki.
Study design
Each subject was studied in the laboratory on one occasion. After a 30 min period of rest, a 10 min measurement was made of the subject's natural PET,CO2 under air-breathing conditions. Following this, the subject's ventilatory response to CO2 against a background of euoxia (end-tidal partial pressure of O2, PET,O2 = 100 Torr) was measured. Finally, the subject's ventilatory response to CO2 against a background of mild hypoxia (PET,O2 = 75 Torr) was measured.
The ventilatory responses to CO2 were assessed by dynamic variation of PET,CO2. The core stimulus was a multifrequency binary sequence (MFBS) in which PET,CO2 varied between +2 and +10 Torr above the subject's natural air-breathing value. The sequence lasted 1408 s, and included a total of 26 transitions between the two levels of PET,CO2. The particular sequence employed had been optimised to separate the contributions of peripheral and central chemoreflex stimulation to overall V̇E (Pedersen et al. 1999b). The MFBS was imposed twice under conditions of euoxia and twice under conditions of hypoxia. For measurements under both euoxic and hypoxic conditions, the first MFBS was separated from the second MFBS by a 5 min period during which PET,CO2 was held at +2 Torr above the subject's natural air-breathing value. For the measurements made under hypoxic conditions, hypoxia was induced 20 min prior to the start of the first MFBS to allow time for hypoxic ventilatory depression to develop before the MFBS began and so provide a more stable background against which to perform the MFBS. Isocapnia was maintained during the induction of hypoxia, and this allowed a measurement to be made of the ventilatory response to hypoxia.
Apparatus and technique
The subjects were seated comfortably in a hospital bed and breathed through a face mask (Vital Signs, Totowa, NJ, USA). Respired gases were sampled and analysed for PCO2 and PO2 using a Datex Multicap gas monitor (Datex-Engstrom, Helsinki, Finland). Arterial O2 saturation (SO2) was monitored by pulse oximetry. Respired flows were measured with a pneumotachograph connected to a pressure transducer. The pressure signal was integrated to yield a volume signal. Corrections were made for the changes in gas viscosity due to changes in the oxygen content of the inhaled gas mixture. All data were logged and stored to a personal computer for further analysis.
Gas mixtures were supplied to the subject via a T-piece connected to the pneumotachograph. One arm of the T-piece received a gas mixture with a total flow of 50 l min−1 from three mass flow controllers (Bronkhorst High Tech BV-F202, The Netherlands). These three mass flow controllers set the individual flows for O2, CO2 and N2 in response to signals received from the computer. The PET,CO2 and PET,O2 of the subject were regulated using the technique of end-tidal forcing (Robbins et al. 1982). The data acquisition and control software were custom built (RESREG(c), E. Kruyt and E. Olofsen, Leiden University Medical Center).
Data analysis
Modelling
The purpose of fitting models to the data was twofold. First, it was to assess whether a separate peripheral chemoreflex component could be identified from the three subject groups, and the second was to compare parameter values for the ventilatory response to CO2 between the three subject groups.
The two models fitted to the data were those of Pedersen et al. (1999b). These were closely related to the earlier models of Bellville et al. (1979) and Dahan et al. (1990). The first of these was a one-compartment model representing just the central chemoreflex response to CO2, and the second was a two-compartment model representing both the central and the peripheral chemoreflex responses to CO2.
The equations describing the two-compartment model are as follows:
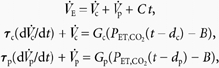 |
where V̇c and V̇p are the slow (central) and fast (peripheral) components of the ventilatory response, respectively, and C is a trend term. In this model, V̇c and V̇p are determined by first-order differential equations in which PET,CO2 is the input at time t delayed by either dc or dp for the central and peripheral chemoreflex components, respectively. Gc and Gp are the sensitivities for the central and peripheral chemoreflex loops, and τc and τp their time constants. Parameter B is the extrapolated value for PET,CO2 for which steady-state V̇E is zero. The one-compartment model is obtained from this model by removing the fast component (i.e. Gp, and hence V̇p, are zero). Assuming that PET,CO2 remains constant during a single breath, these differential equations can be solved to form a set of difference equations that provide V̇E for breath n+ 1 as a function of PET,CO2 at breath n+ 1 and V̇E at breath n.
One complication associated with modelling respiratory data is that successive breaths are not independent observations, but show a degree of correlation with previous breaths. To address this issue, a parallel noise model that describes the correlation that exists between successive breaths (Liang et al. 1996) was fitted to the data simultaneously with the deterministic models described above.
The models were fitted to the data using a standard subroutine for the minimisation of a sum of squared residuals (Numerical Algorithms Group, Oxford, UK).
Model comparison
F-ratio tests were used to compare the goodness of fit between the two models for each group. The statistic is given by (Armitage & Berry, 1987):
where RSS1 and df1 are the residual sum of squares and the remaining degrees of freedom for the larger (two-compartment) model and RSS2 and df2 are the residual sum of squares and the remaining degrees of freedom for the smaller (one-compartment) model, respectively. This test indicates whether, after allowing for the different number of parameters, the two-compartment model is a significantly better fit to the data set than the one-compartment model. An important assumption of this test is that the residuals are uncorrelated. This assumption is provided for by including the noise model, which ensures that the residuals are white (Pedersen et al. 1999b).
Comparison of model parameters between protocols and groups
Analysis of variance (ANOVA, SPSS statistical package) was used to compare model parameters. In the case of comparisons between protocols within a group, protocol was employed as a fixed factor and subjects as a random factor. In the case of comparisons between groups, group and protocol were employed as fixed factors. Statistical significance was assumed at P < 0.05.
RESULTS
Subjects
The physical characteristics of the subjects are given in Table 1. Of note, air-breathing PET,CO2 in the bilaterally resected group was significantly higher (by 4.6 ± 1.3 Torr, mean ± s.d.) than for either the unilaterally resected group (P < 0.005) or the control group (P < 0.05). The unilaterally resected group and control group did not differ in this respect.
Table 1.
Physical characteristics of bilaterally carotid body-resected (BR), unilaterally carotid body-resected (UR) and control (C) subjects
| Subject no. | Sex | Weight (kg) | Height (m) | Age (years) | Pet.cO2 (Torr) | Date of surgery | |
|---|---|---|---|---|---|---|---|
| 002 | M | 80 | 1.96 | 48 | 45.8 | 06.06.96 | |
| 004 | M | 82 | 1.93 | 28 | 45.0 | 15.06.90 | |
| 005 | M | 87 | 1.78 | 49 | 42.0 | 21.07.90 | |
| BR | 006 | F | 62 | 1.60 | 45 | 46.5 | 20.09.00 |
| 111 | F | 68 | 1.62 | 51 | 42.8 | 19.04.00 | |
| 168 | F | 62 | 1.54 | 51 | 50.3 | 01.09.02 | |
| 953 | M | 69 | 1.73 | 49 | 43.5 | 21.02.90 | |
| Mean ± S.D. | 73 ± 10 | 1.74 ± 0.16 | 46 ± 8 | 45.1 ± 2.8 | |||
| 001 | M | 65 | 1.70 | 35 | 45.8 | 19.01.90 | |
| 003 | M | 94 | 1.93 | 42 | 39.8 | 07.03.90 | |
| 004b | M | 84 | 1.83 | 56 | 36.0 | 01.01.76 | |
| UR | 007 | F | 62 | 1.54 | 50 | 36.0 | 01.11.00 |
| 104 | F | 56 | 1.72 | 32 | 41.3 | 14.10.93 | |
| 105 | M | 82 | 1.87 | 30 | 44.3 | 01.01.00 | |
| 110 | F | 105 | 1.78 | 45 | 36.8 | 01.01.83 | |
| Mean ± S.D. | 78 ± 18 | 1.77 ± 0.13 | 41 ± 10 | 40.0 ± 4.0 | |||
| 103 | M | 77 | 1.78 | 31 | 39.8 | — | |
| 217 | M | 72 | 1.74 | 43 | 42.8 | — | |
| 222 | F | 77 | 1.78 | 39 | 40.5 | — | |
| C | 225 | F | 55 | 1.57 | 57 | 42.8 | — |
| 227 | M | 75 | 1.82 | 59 | 39.0 | — | |
| 243 | F | 65 | 1.7 | 55 | 41.3 | — | |
| 244 | M | 89 | 1.84 | 55 | 41.3 | — | |
| Mean ± S.D. | 73 ± 11 | 1.75 ± 0.09 | 48 ± 11 | 41.0 ± 1.4 | |||
For each subject, the ventilatory response to hypoxia was calculated as the difference between V̇E during the second and third minutes of hypoxia and V̇E during the 2 min period immediately prior to the onset of hypoxia. The ventilatory response to hypoxia averaged 1.5 ± 1.7, 3.8 ± 3.0 and 7.3 ± 2.7 l min−1 (mean ± s.d.) for the bilaterally resected subjects, unilaterally resected subjects and controls, respectively. The value for bilaterally resected subjects did not differ significantly from zero. The apparent slight increase in V̇E in the bilaterally resected subjects may have been due to a small but significant increase in PET,CO2 of 0.9 ± 0.3 Torr between euoxia and hypoxia.
General observations and model fitting
An example of the ventilatory response to the MFBS in PET,CO2 together with an example of the fit of the two-compartment model to the data is shown in Fig. 1. The changes in ventilation clearly followed the variations in PET,CO2. The response to the second MFBS was very similar to the first one. The fit of the model to data is illustrated both including and excluding the stochastic component of the model that was obtained as part of the overall fitting process. The residuals calculated without the stochastic component clearly show the presence of longer-term trends. These trends in the residuals are absent when the stochastic component is included in the calculation.
Figure 1. Example of data and model fits for a bilaterally carotid body-resected subject under conditions of euoxia (left) and hypoxia (right).
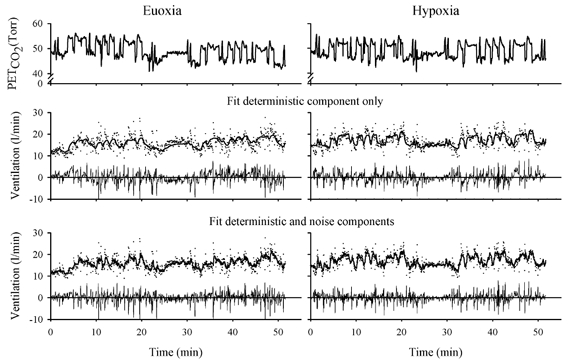
The top panel shows the multifrequency binary sequence (MFBS) in PET,CO2. The two other panels show the ventilation data and model fits. Dots represent ventilation data and lines represent the model output; residuals are shown below each fit. The middle panel shows just the deterministic component of the model fit, i.e. the component that arises directly from the input stimulus. The model follows the pattern of variations in ventilation but the residuals remain correlated with many successive residuals being either all positive or all negative. The bottom panel shows the entire model fit. This includes the noise model, which models the intrinsic correlation between breaths. In this case the residuals are much less correlated, with no long runs of successive residuals being either all positive or all negative.
Model comparison
The residual sums of squares and F-ratio test results for both model fits for all subjects are shown in Table 2. There was a clear distinction between the results for the bilaterally resected subjects compared with the unilaterally resected subjects and healthy controls. For the unilaterally resected subjects and the healthy controls, the two-compartment model fitted the data significantly better than the one-compartment model on 22 out of 28 occasions, with no distinction between data gathered against a background of euoxia and data gathered against a background of hypoxia. An F-ratio test conducted on the overall data from the unilaterally resected and control subjects indicated that the two-compartment model fitted the data significantly better than the one-compartment model against both background levels of oxygen tension.
Table 2.
Residual sum of squares (RSS) for both model fits for BR, UR and C groups
| Euoxia | Hypoxia | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Subject no. | N | RSS1/df1 (1 min−1)2 | RSS2/df2 (1 min−1)2 | F | n | RSS1/df1 (1 min−1)2 | RSS2/df2 (1 min−1)2 | F | |
| 002 | 709 | 6.71 | 6.70 | n.s. | 695 | 8.05 | 8.13 | n.s. | |
| 004 | 862 | 6.42 | 6.39 | n.s. | 444 | 6.44 | 6.54 | n.s. | |
| 005 | 828 | 4.92 | 4.93 | n.s. | 885 | 3.91 | 3.87 | P < 0.005 | |
| BR | 006 | 828 | 3.69 | 3.68 | n.s. | 903 | 2.94 | 2.97 | n.s. |
| 111 | 722 | 1.37 | 1.37 | n.s. | 745 | 1.60 | 1.62 | n.s. | |
| 168 | 764 | 2.14 | 2.19 | n.s. | — | — | — | — | |
| 953 | 419 | 2.17 | 2.17 | n.s. | 438 | 2.70 | 2.74 | n.s | |
| Overall | 4.08 | 4.08 | n.s. | 4.11 | 4.11 | n.s. | |||
| 001 | 907 | 5.48 | 5.49 | n.s. | 929 | 7.90 | 7.91 | n.s. | |
| 003 | 863 | 18.99 | 18.71 | P < 0.005 | 1004 | 11.58 | 11.32 | P < 0.005 | |
| 004b | 713 | 16.02 | 15.65 | P < 0.005 | 321 | 19.47 | 19.28 | P < 0.05 | |
| UR | 007 | 843 | 2.40 | 2.04 | P < 0.005 | 949 | 2.27 | 1.97 | P < 0.005 |
| 104 | 599 | 3.29 | 3.19 | P < 0.005 | 759 | 0.81 | 0.81 | P < 0.05 | |
| 105 | 736 | 1.67 | 1.65 | P < 0.05 | 760 | 1.92 | 1.90 | P < 0.005 | |
| 110 | 641 | 8.32 | 8.10 | P < 0.005 | 750 | 5.01 | 4.98 | P < 0.005 | |
| Overall | 8.18 | 7.99 | P < 0.005 | 6.03 | 5.87 | P < 0.005 | |||
| 103 | 804 | 3.93 | 3.81 | P < 0.005 | 935 | 5.12 | 5.03 | P < 0.005 | |
| 217 | 714 | 8.45 | 8.21 | P < 0.005 | 655 | 9.74 | 9.29 | P < 0.005 | |
| 222 | 866 | 7.58 | 7.50 | P < 0.01 | 958 | 10.85 | 10.37 | P < 0.005 | |
| C | 225 | 977 | 2.40 | 2.38 | P < 0.005 | 1146 | 2.77 | 2.78 | n.s. |
| 227 | 780 | 2.59 | 2.60 | n.s. | 802 | 2.11 | 2.13 | n.s. | |
| 243 | 737 | 5.05 | 5.05 | n.s. | 848 | 3.99 | 3.98 | P < 0.05 | |
| 244 | 779 | 5.52 | 5.42 | P < 0.005 | 832 | 5.67 | 5.53 | P < 0.005 | |
| Overall | 4.98 | 4.90 | P < 0.005 | 5.57 | 5.39 | P < 0.005 | |||
N is the number of fitted data points, RSS1 and RSS2 are the residual sums of squares and df1 and df2 are the degrees of freedom (n minus number of model parameters) for the smaller and the larger models, respectively. Overall values are calculated as ΣRSS/Σdf.
The results from the bilaterally resected subjects contrasted markedly with those from the unilaterally resected subjects and healthy controls. For the bilaterally resected subjects, the two-compartment model fitted significantly better than the one-compartment model for only one out of the 13 data sets. For the bilaterally resected subjects overall, the two-compartment model did not fit the data significantly better than the one-compartment model for either the data gathered against a euoxic background or for the data gathered against a hypoxic background.
Model parameters
The values of the parameters obtained by fitting the two-compartment model to the data are presented in Tables 3–5. Within each group of subjects, comparisons were drawn between the parameter values for the two protocols (euoxia vs. hypoxia). For the control subjects, there was a significant increase in Gp with hypoxia (P < 0.05, ANOVA). There was a similar-sized increase in Gp for the unilaterally resected subjects, but in this case the observation did not reach significance. For the bilaterally resected subjects, there was no increase in the magnitude of Gp with hypoxia.
Table 3.
Model parameters for control subjects
| Subject no. | Gc (1 min−1 Torr−1) | τc (s) | dc (s) | Gp (1 min−1 Torr−1) | τp (s) | dp (s) | B (Torr) | C (1 min−1 min−1) | F | Rv/Rw | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 103 | 1.78 | 132.9 | 11.0 | 0.28 | 3.3 | 4.8 | 36.9 | 0.108 | 0.97 | 0.05 | |
| 217 | 1.78 | 223.2 | 19.9 | 0.64 | 9.9 | 4.9 | 38.8 | 0.010 | 0.84 | 0.22 | |
| 222 | 1.88 | 224.6 | 7.4 | 0.69 | 8.5 | 8.5 | 39.5 | 0.032 | 0.89 | 0.22 | |
| Euoxia | 225 | 0.89 | 55.6 | 4.8 | 0.14 | 0.04 | 4.7 | 36.4 | 0.124 | 0.98 | 0.01 |
| 227 | 1.80 | 100.5 | 14.8 | 0.16 | 2.4 | 6.5 | 35.7 | −0.005 | 0.90 | 0.09 | |
| 243 | 0.80 | 97.1 | 11.2 | 0.15 | 0.7 | 6.0 | 35.3 | 0.131 | 0.97 | 0.01 | |
| 244 | 0.64 | 204.9 | 11.7 | 0.82 | 19.8 | 5.0 | 37.4 | 0.025 | 0.91 | 0.02 | |
| Mean | 1.37 | 148.4 | 11.5 | 0.41 | 6.4 | 5.8 | 37.1 | 0.061 | 0.92 | 0.09 | |
| ± S.D. | 0.558 | 68.8 | 4.89 | 0.294 | 7.01 | 1.39 | 1.55 | 0.0580 | 0.052 | 0.094 | |
| 103 | 2.03 | 104.7 | 14.6 | 0.62 | 5.6 | 4.2 | 37.5 | 0.117 | 0.92 | 0.30 | |
| 217 | 2.09 | 245.8 | 19.3 | 0.71 | 6.7 | 7.0 | 38.9 | 0.027 | 0.85 | 0.17 | |
| 222 | 2.04 | 133.1 | 13.8 | 1.13 | 7.5 | 6.3 | 39.5 | 0.062 | 0.78 | 0.50 | |
| Hypoxia | 225 | 1.13 | 54.5 | 10.4 | 0.20 | 1.5 | 5.5 | 34.8 | 0.132 | 0.97 | 0.04 |
| 227 | 2.05 | 98.6 | 19.5 | 0.35 | 4.6 | 6.3 | 37.0 | 0.124 | 0.99 | 0.03 | |
| 243 | 0.99 | 105.9 | 8.0 | 0.43 | 10.9 | 3.7 | 36.0 | 0.092 | 0.84 | 0.27 | |
| 244 | 1.09 | 165.9 | 4.7 | 0.85 | 12.7 | 5.2 | 37.0 | 0.055 | 0.85 | 0.07 | |
| Mean | 1.63 | 129.8 | 12.9 | 0.61 | 7.1 | 5.4 | 37.2 | 0.087 | 0.88 | 0.20 | |
| ± S.D. | 0.527 | 61.39 | 5.56 | 0.318 | 3.78 | 1.19 | 1.61 | 0.0399 | 0.076 | 0.172 | |
Gc, central chemoreflex sensitivity; Gp, peripheral chemoreflex sensitivity; τc, central chemoreflex time constant; τp, peripheral chemoreflex time constant; dc, central chemoreflex delay; dp, peripheral chemoreflex delay; B, extrapolated value for PET,CO2 at which V̇E = 0; C, trend term; F, system gain for noise component; Rv/Rw, variance ratio for process and measurement noise.
Table 5.
Model parameters for bilaterally carotid body-resected subjects
| Subject no. | Gc (1 min−1 Torr−1) | τc (s) | dc (s) | Gp (1 min−1 Torr−1) | τp (s) | dp (s) | B (Torr) | C (1 min−1 min−1) | F | Rv/Rw | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 002 | 0.93 | 123.0 | 19.9 | 0.43 | 15.6 | 5.4 | 34.4 | 0.092 | 0.63 | 0.49 | |
| 004 | 2.34 | 207.0 | 19.5 | 0.41 | 21.3 | 8.4 | 38.5 | 0.058 | 0.83 | 0.12 | |
| 005 | 0.60 | 52.4 | 3.9 | 0.26 | 26.4 | 12.2 | 34.5 | 0.128 | 0.85 | 0.17 | |
| Euoxia | 006 | 0.55 | 23.4 | 17.7 | 0.18 | 0.9 | 6.0 | 31.3 | 0.033 | 0.84 | 0.90 |
| 111 | 0.28 | 209.3 | 19.6 | 0.09 | 0.1 | 12.3 | 33.5 | 0.069 | 0.81 | 0.34 | |
| 168 | 0.62 | 101.7 | 4.7 | 0.04 | 7.2 | 4.8 | 26.9 | 0.066 | 0.94 | 0.01 | |
| 953 | 1.32 | 98.3 | 4.1 | 0.08 | 2.6 | 16.8 | 35.5 | 0.145 | 0.90 | 0.40 | |
| Mean | 0.95 | 116.4 | 12.8 | 0.21 | 10.6 | 9.4 | 33.5 | 0.084 | 0.83 | 0.35 | |
| ± S.D. | 0.696 | 70.91 | 8.02 | 0.159 | 10.56 | 4.48 | 3.63 | 0.0399 | 0.098 | 0.296 | |
| 002 | 0.80 | 73.9 | 18.5 | 0.33 | 13.6 | 4.8 | 33.2 | 0.087 | 0.92 | 0.03 | |
| 004 | 1.29 | 47.7 | 9.2 | 0.07 | 3.9 | 4.2 | 29.4 | 0.098 | 0.68 | 0.59 | |
| 005 | 1.05 | 190.0 | 16.1 | 0.64 | 13.4 | 14.5 | 39.2 | 0.024 | 0.80 | 0.23 | |
| Hypoxia | 006 | 0.76 | 79.3 | 16.9 | 0.09 | 3.1 | 8.5 | 33.4 | 0.008 | 0.73 | 5.44 |
| 111 | 0.50 | 282.8 | 4.7 | 0.03 | 16.0 | 4.8 | 37.0 | −0.028 | 0.80 | 0.23 | |
| 168 | — | — | — | — | — | — | — | — | — | — | |
| 953 | 1.31 | 75.0 | 8.2 | 0.11 | 29.8 | 16.8 | 35.0 | 0.147 | 0.79 | 0.80 | |
| Mean | 0.95 | 124.8 | 12.3 | 0.21 | 13.30 | 8.9 | 34.5 | 0.056 | 0.79 | 1.22 | |
| ± S.D. | 0.321 | 91.98 | 5.62 | 0.235 | 9.72 | 5.47 | 3.39 | 0.0654 | 0.081 | 2.086 | |
Symbols as in Table 3.
Table 4.
Model parameters for unilaterally carotid body-resected subjects
| Subject no. | Gc (1 min−1 Torr−1) | τc (s) | dc (s) | Gp (1 min−1 Torr−1) | τp (s) | dp (s) | B (Torr) | C (1 min−1 min−1) | F | Rv/Rw | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 001 | 1.48 | 55.4 | 6.7 | 0.04 | 1.1 | 11.2 | 39.0 | 0.146 | 0.89 | 0.27 | |
| 003 | 1.58 | 46.9 | 16.5 | 0.56 | 2.0 | 6.4 | 33.1 | 0.239 | 0.95 | 0.03 | |
| 004b | 2.04 | 61.9 | 6.3 | 0.38 | 3.4 | 6.1 | 32.1 | 0.201 | 0.97 | 0.02 | |
| Euoxia | 007 | 1.27 | 186.3 | 6.3 | 0.29 | 0.8 | 6.3 | 30.9 | 0.078 | 0.92 | 0.03 |
| 104 | 0.71 | 264.7 | 6.9 | 0.21 | 15.5 | 6.1 | 35.1 | 0.017 | 0.93 | 0.02 | |
| 105 | 0.96 | 43.5 | 4.5 | 0.11 | 0.2 | 4.0 | 36.2 | 0.160 | 0.85 | 0.42 | |
| 110 | 1.61 | 62.8 | 16.1 | 0.48 | 0.1 | 5.3 | 34.1 | 0.030 | 0.97 | 0.04 | |
| Mean | 1.38 | 103.1 | 9.0 | 0.29 | 3.3 | 6.5 | 34.3 | 0.124 | 0.92 | 0.12 | |
| ± S.D. | 0.443 | 86.93 | 5.02 | 0.191 | 5.50 | 2.24 | 2.71 | 0.0850 | 0.044 | 0.161 | |
| 001 | 2.04 | 86.3 | 9.9 | 0.34 | 6.7 | 5.8 | 40.9 | 0.013 | 0.88 | 0.17 | |
| 003 | 2.01 | 164.3 | 5.5 | 1.12 | 16.3 | 3.7 | 34.8 | 0.092 | 0.83 | 0.13 | |
| 004b | 2.20 | 72.7 | 12.4 | 0.54 | 1.5 | 8.7 | 32.9 | 0.261 | 0.97 | 0.01 | |
| Hypoxia | 007 | 1.06 | 189.5 | 13.3 | 0.36 | 1.4 | 5.6 | 29.2 | 0.022 | 0.89 | 0.10 |
| 104 | 0.49 | 94.6 | 18.4 | 0.13 | 5.2 | 3.3 | 30.4 | 0.060 | 0.98 | 0.02 | |
| 105 | 0.73 | 138.5 | 1.6 | 0.62 | 18.5 | 3.5 | 37.6 | 0.17 | 0.90 | 0.18 | |
| 110 | 1.13 | 48.7 | 10.4 | 0.29 | 0.15 | 5.1 | 30.9 | 0.107 | 0.98 | 0.01 | |
| Mean | 1.38 | 113.5 | 10.2 | 0.48 | 7.11 | 5.1 | 33.8 | 0.104 | 0.92 | 0.09 | |
| ± S.D. | 0.693 | 51.55 | 5.44 | 0.323 | 7.42 | 1.89 | 4.23 | 0.0877 | 0.059 | 0.075 | |
Symbols as in Table 3.
There were no significant changes in any of the other parameters with hypoxia for any of the subject groups with one exception. The exception was that there was a significant increase in Gc with hypoxia in the control subjects (P < 0.001).
In order to determine the effect of carotid body resection on the parameters, comparisons were drawn across the three groups. There were significant differences in the values for Gp between subject groups (P < 0.05). Post hoc testing revealed that Gp was significantly lower in the bilaterally resected subjects than in the controls (P < 0.005). Apart from the differences in Gp, there were also differences in the values for Gc (P < 0.05). Post hoc testing revealed that Gc was significantly lower in the bilaterally resected subjects than in the controls (P < 0.05).
Other differences included the observation that parameter B was significantly lower for both bilaterally and unilaterally resected subjects compared with the control group (P < 0.05). Finally, for the stochastic component of the model, the value for parameter F was significantly lower for the bilaterally resected subjects compared with the other two subject groups (P < 0.001) which suggests that there may be less correlation between successive breaths in the absence of peripheral chemoreceptors.
DISCUSSION
The major results from this study were, first, in the bilaterally carotid body-resected subjects, a two-compartment model of the ventilatory response to CO2 failed to fit the data significantly better than a one-compartment model. Second, in the bilaterally resected subjects, the slow component of the ventilatory sensitivity to CO2 was smaller than in either the control subjects or the unilaterally resected subjects.
Subjects
There have been a number of studies of the control of breathing in humans who have undergone bilateral carotid body resection (for review see Honda, 1985). These resections were performed in an attempt to relieve symptoms of breathlessness associated with asthma. Thus, the results from these studies are likely to be complicated by the presence of some degree of pulmonary pathology. A second group of subjects that have been studied are those who have had bilateral carotid endarterectomy and this has resulted in bilateral denervation of the carotid bodies (Wade et al. 1970). These subjects lack the pulmonary pathology of the first group but have the disadvantage of having established cerebrovascular disease. The subjects of the present study now form a third group. They are relatively young and do not have established cardiorespiratory disease.
The PET,CO2 of the bilaterally resected subjects was significantly greater than that of the control subjects by 4.6 Torr. This is close to the value of 5.8 Torr reported by Wade et al. (1970) for the rise in arterial PCO2 for the subjects who had undergone (staged) bilateral carotid endarterectomy and had lost their ventilatory response to hypoxia. These results show that the carotid bodies play a significant role in setting normal levels of V̇E and blood gas tensions in humans.
Dynamic separation of peripheral and central contributions to V̇E
A number of studies in experimental animals have suggested that the fast component of the ventilatory response to CO2 may be linked with the peripheral chemoreflex and the slow component of the ventilatory response to CO2 may be linked with the central chemoreflex. Daubenspeck (1973) demonstrated that the rapid component of the ventilatory response to sinusoidal variations in PCO2 could be abolished by denervation of the carotid bodies. In anaesthetised cats, DeGoede et al. (1985) compared the gains of the peripheral and central chemoreflexes obtained from the dynamics of the ventilatory response to CO2 with the gains obtained by directly varying the PCO2 at either chemoreceptor using their technique of artificial brainstem perfusion. They found that the gains obtained by the two techniques were similar. Berkenbosch et al. (1989) reported that a step change in the arterial PCO2 of the blood perfusing just the brainstem resulted in a single slow exponential change in V̇E.
Bellville et al. (1979) studied the ventilatory response to step changes in PET,CO2 in carotid body-resected humans. Although they did not compare different models for the ventilatory response to CO2 in these subjects, they did fit a two-compartment model to their data. With this model, they reported the fast (peripheral) sensitivity to average 0.16 l min−1 Torr−1 in euoxia and 0.20 l min−1 Torr−1 in hypoxia. These values are very similar to those obtained in the present study (0.21 l min−1 Torr−1 in both euoxia and hypoxia). In the present study, we have shown that the inclusion of this fast component in the overall model does not lead to a significant improvement in the fit of the model to the data. We suggest that the relatively small values for Gp in the bilaterally resected subjects arise through a combination of imperfections in the model of the dynamics of the central chemoreflex and noise.
Central chemoreflex sensitivity in bilaterally carotid body-resected subjects
Although not clearly significant, Bellville et al. (1979) reported that central chemosensitivity appeared to be lower, by about half, in the patients who had undergone carotid body resection than in the normal controls. The present study confirms that the magnitude of the slow component is smaller in the bilaterally resected subjects than it is in the other two groups. There are at least two possible interpretations of this result. The first is that there is a component of the peripheral chemoreflex response to CO2 that is slow. The second is that there is interaction between the peripheral and central chemoreflexes such that the presence of peripheral input augments the sensitivity of the central chemoreflex.
One theoretical reason in favour of the first hypothesis is that, following a change in PET,CO2, there should be a component of the change in pH of the arterial blood that is slow. For example, in the case of a step increase in PET,CO2, there will be a rise in bicarbonate in the arterial blood which will then partially redistribute itself into the peripheral tissues during circulation of the blood through the systemic capillaries. If PET,CO2 remains constant, this redistribution will result in a progressive acidification of the arterial blood. Consequently, if the arterial chemoreceptors primarily respond to pH (Hornbein & Roos, 1963; Donnelly et al. 1982), then in vivo there should be a slow component within their response to changes in PET,CO2. However, in anaesthetised cats in which the PET,CO2 of the central chemoreflex was maintained constant by artificial perfusion of the brainstem, a single fast time constant was found to be sufficient to describe the ventilatory response to a step change in PET,CO2 (Berkenbosch et al. 1988). This result tends to suggest that the magnitude of any slow component within the peripheral chemoreflex response is small.
If there were a slow component within the peripheral chemoreflex response to CO2, then it might reasonably be predicted that there would be an immediate reduction in the slow component of ventilatory response to CO2 following carotid body denervation. However, experimental studies in animals have found that it takes two or more days for the decline in the ventilatory sensitivity to develop fully (Pan et al. 1998; Rodman et al. 2001). This would seem to be more compatible with a slow regulation of central chemoreflex sensitivity by peripheral chemoreflex activity – i.e. a slowly developing central–peripheral chemoreflex interaction.
If part of the slow component of the response does arise at the carotid bodies, then it would be reasonable to predict that the magnitude of the slow component of the chemoreflex response to CO2 would be increased in hypoxia. Although a degree of interaction between the central and peripheral chemoreflexes could also give rise to this result, the absence of any increase in the magnitude of the slow component with hypoxia would make a peripheral chemoreflex origin for any of the slow component unlikely. Unfortunately, the data in relation to this are contradictory. Using step changes in PET,CO2, Bellville et al. (1979) report a non-significant increase in central chemoreflex sensitivity of 0.31 l min−1 Torr−1 (compared with a non-significant increase of 0.09 l min−1 Torr−1 for the carotid body-resected subjects) and Dahan et al. (1990) report almost no change (fall of ∼0.02 l min−1 Torr−1). Using MFBS, Pedersen et al. (1999b) report a non-significant fall of 0.31 l min−1 Torr−1. However, Fatemian et al. (2003) report a significant rise in Gc with hypoxia of 0.6 l min−1 Torr−1, and in the present study there was a significant increase of 0.26 l min−1 Torr−1 (compared with no change for the carotid body-resected subjects).
If part of the slow component of the response does arise at the carotid bodies and is incorrectly attributed to the central chemoreflex in the modelling process, then a further prediction that can be made is that the pure delay of the central chemoreflex in normal subjects will be intermediate between that measured for the bilaterally carotid body-resected subjects and the pure delay for the peripheral chemoreflex. Our data lend no support to this prediction.
In summary, it is clear from the present study that, in humans, there is a reduction in the magnitude of the slow chemoreflex response to CO2 following carotid body resection. However, there must remain a degree of uncertainty as to whether this is caused by the loss of some tonic influence by the peripheral chemoreceptors on central chemoreflex sensitivity or whether it simply results from the loss of a slow component that exists within the peripheral chemoreflex response to CO2.
Acknowledgments
This study was supported by the Wellcome Trust.
References
- Armitage P, Berry G. Statistical Methods in Medical Research. Oxford: Blackwell Scientific Publications; 1987. [Google Scholar]
- Baysal BE, Ferrell RE, Willett Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW, III, Cornelisse CJ, Devilee P, Devlin B. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- Bellville JW, Whipp BJ, Kaufman RD, Swanson GD, Aqleh KA, Wiberg DM. Central and peripheral chemoreflex loop gain in normal and carotid body-resected subjects. J Appl Physiol. 1979;46:843–853. doi: 10.1152/jappl.1979.46.4.843. [DOI] [PubMed] [Google Scholar]
- Berkenbosch A, De Goede J, Ward DS, Olievier CN, Vanhartevelt J. Dynamic response of peripheral chemoreflex loop to changes in end-tidal CO2. J Appl Physiol. 1988;64:1779–1785. doi: 10.1152/jappl.1988.64.5.1779. [DOI] [PubMed] [Google Scholar]
- Berkenbosch A, Ward DS, Olievier CN, De Goede J, Vanhartevelt J. Dynamics of ventilatory response to step changes in PCO2 of blood perfusing the brain stem. J Appl Physiol. 1989;66:2168–2173. doi: 10.1152/jappl.1989.66.5.2168. [DOI] [PubMed] [Google Scholar]
- Clement ID, Pandit JJ, Bascom DA, Dorrington KL, O'Connor DF, Robbins PA. An assessment of central-peripheral ventilatory chemoreflex interaction using acid and bicarbonate infusions in humans. J Physiol. 1995;485:561–570. doi: 10.1113/jphysiol.1995.sp020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, De Goede J, Berkenbosch A, Oliver ICW. The influence of oxygen on the ventilatory response to carbon dioxide in man. J Physiol. 1990;428:485–499. doi: 10.1113/jphysiol.1990.sp018223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenspeck JA. Frequency analysis of CO2 regulation: afferent influences on tidal volume control. J Appl Physiol. 1973;35:662–672. doi: 10.1152/jappl.1973.35.5.662. [DOI] [PubMed] [Google Scholar]
- DeGoede J, Berkenbosch A, Ward DS, Bellville JW, Olievier CN. Comparison of chemoreflex gains obtained with two different methods in cats. J Appl Physiol. 1985;59:170–179. doi: 10.1152/jappl.1985.59.1.170. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Smith E, Dutton RE. Carbon dioxide versus H ion as a chemoreceptor stimulus. Brain Res. 1982;245:136–138. doi: 10.1016/0006-8993(82)90347-x. [DOI] [PubMed] [Google Scholar]
- Fatemian M, Gamboa A, Leon-Velarde F, Rivera-Ch M, Palacios JA, Robbins PA. Plasticity in respiratory motor control: Selected contribution: Ventilatory response to CO2 in high altitude natives and patients with chronic mountain sickness. J Appl Physiol. 2003;94:1279–1287. doi: 10.1152/japplphysiol.00859.2002. [DOI] [PubMed] [Google Scholar]
- Fatemian M, Robbins PA. Selected contribution: chemoreflex responses to CO2 before and after an 8 h exposure to hypoxia in humans. J Appl Physiol. 2001;90:1607–1614. doi: 10.1152/jappl.2001.90.4.1607. [DOI] [PubMed] [Google Scholar]
- Gardner WN. The pattern of breathing following step changes of alveolar partial pressures of carbon dioxide and oxygen in man. J Physiol. 1980;300:55–73. doi: 10.1113/jphysiol.1980.sp013151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y. Role of carotid chemoreceptors in control of breathing at rest and in exercise: studies on human subjects with bilateral carotid body resection. Jpn J Physiol. 1985;35:535–544. doi: 10.2170/jjphysiol.35.535. [DOI] [PubMed] [Google Scholar]
- Hornbein TF, Roos A. Specificity of H ion concentration as a carotid chemoreceptor stimulus. J Appl Physiol. 1963;18:580–584. doi: 10.1152/jappl.1963.18.3.580. [DOI] [PubMed] [Google Scholar]
- Jansen JC. Paragangliomas of the head and neck: clinical implications of growth rate and genetics. 2001. PhD Thesis, Leiden University, Leiden.
- Liang P-J, Pandit JJ, Robbins PA. Statistical properties of breath-to-breath variations in ventilation at constant end-tidal PCO2 and PO2 in humans. J Appl Physiol. 1996;81:2274–2286. doi: 10.1152/jappl.1996.81.5.2274. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijs D, Sarton E, Teppema LJ, Kruyt E, Olievier I, van Kleef J, Dahan A. Respiratory sites of action of propofol: absence of depression of peripheral chemoreflex loop by low-dose propofol. Anesthesiology. 2001;95:889–895. doi: 10.1097/00000542-200110000-00017. [DOI] [PubMed] [Google Scholar]
- Pan LG, Forster HV, Martino P, Strecker PJ, Beales J, Serra A, Lowry TF, Forster MM, Forster AL. Important role of carotid afferents in control of breathing. J Appl Physiol. 1998;85:1299–1306. doi: 10.1152/jappl.1998.85.4.1299. [DOI] [PubMed] [Google Scholar]
- Pedersen MEF, Dorrington KL, Robbins PA. Effects of dopamine and domperidone on ventilatory sensitivity to hypoxia after 8 h of isocapnic hypoxia. J Appl Physiol. 1999a;86:222–229. doi: 10.1152/jappl.1999.86.1.222. [DOI] [PubMed] [Google Scholar]
- Pedersen MEF, Fatemian M, Robbins PA. Identification of fast and slow ventilatory responses to carbon dioxide under hypoxic and hyperoxic conditions in humans. J Physiol. 1999b;521:273–287. doi: 10.1111/j.1469-7793.1999.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PA, Swanson GD, Howson MG. A prediction-correction scheme for forcing alveolar gases along certain time courses. J Appl Physiol. 1982;52:1353–1357. doi: 10.1152/jappl.1982.52.5.1353. [DOI] [PubMed] [Google Scholar]
- Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol. 2001;91:328–335. doi: 10.1152/jappl.2001.91.1.328. [DOI] [PubMed] [Google Scholar]
- Swanson GD, Bellville JW. Step changes in end-tidal CO2: methods and implications. J Appl Physiol. 1975;39:377–385. doi: 10.1152/jappl.1975.39.3.377. [DOI] [PubMed] [Google Scholar]
- van Beek JHGM, Berkenbosch A, de Goede J, Olievier CN. Influence of peripheral O2 tension on the ventilatory response to CO2 in cats. Respir Physiol. 1983;51:379–390. doi: 10.1016/0034-5687(83)90030-0. [DOI] [PubMed] [Google Scholar]
- van der Mey AGL. Head and neck paragangliomas: A clinical, genetic and pathological study of glomus tumors. 1992. PhD Thesis, Leiden University, Leiden.
- Wade JG, Larson CP, Hickey RF, Ehrenfeld WK, Severinghaus JW. Effect of endarterectomy on carotid chemoreceptor and baroreceptor function in man. N Engl J Med. 1970;282:823–829. doi: 10.1056/NEJM197004092821501. [DOI] [PubMed] [Google Scholar]


