Abstract
Studies in isolated mouse stomach showed that bombesin releases somatostatin. We characterized the effects of exogenous bombesin on gastric acid secretion in mice and determined the involvement of somatostatin and somatostatin receptor type 2 (SSTR2) by using somatostatin immunoneutralization, the SSTR2 antagonist, PRL-2903, and SSTR2 knockout mice. Gastric acid secretion was monitored under basal and pentagastrin-, histamine- or bethanechol-stimulated conditions in urethane-anaesthetized mice. Bombesin (10–40 μg kg−1 h−1) and somatostatin-14 (20 μg kg−1 h−1) were infused i.v. 10 and 30 min after PRL-2903 or somatostatin antibody pretreatment, respectively. Urethane-anaesthetized wild-type mice had low basal acid secretion (0.12 ± 0.01 μmol (10 min)−1) compared with SSTR2 knockout mice (1.43 ± 0.10 μmol (10 min)−1). Somatostatin antibody and PRL-2903 increased basal secretion in wild-type mice but not in SSTR2 knockout animals. In wild-type mice, bombesin inhibited secretagogue-stimulated acid secretion in a dose-dependent manner, and somatostatin-14 inhibited pentagastrin-stimulated secretion. In wild-type mice pretreated with somatostatin antibody or PRL-2903 and in SSTR2 knockout mice, bombesin and somatostatin-14 i.v. infusion did not alter the increased gastric acid secretion. These results indicate that, in mice, bombesin inhibits gastric acid secretion through the release of somatostatin and the activation of SSTR2. These observations strengthen the important role of SSTR2 in mediating somatostatin inhibitory actions on gastric acid secretion.
Bombesin and bombesin-like peptides administered centrally and peripherally influence gastric acid secretion (Martínez & Taché, 2000). Consistent reports showed that bombesin acts in the brain to inhibit gastric acid secretion in several mammalian species (Martínez & Taché, 2000). By contrast, either stimulation or inhibition of gastric acid secretion has been reported in response to peripheral administration of bombesin and bombesin-like peptides (Helman & Hirschowitz, 1987). In cats, dogs and humans the intravenous infusion of bombesin increases gastric acid secretion through gastrin-dependent mechanisms (Helman & Hirschowitz, 1987; Bado et al. 1989; Kovacs et al. 1995; Hildebrand et al. 2001). However, the pattern of the acid response was found to be influenced by the dose since, in some cases, intravenous injection of bombesin at high doses resulted in the inhibition of gastric secretion in dogs and humans (Helman & Hirschowitz, 1987; Walsh et al. 1988). In rats, peripheral bombesin either inhibits or stimulates gastric acid secretion (Bertaccini et al. 1973; Rossowski et al. 1989; Sandvik et al. 1989; Schubert et al. 1991a; Martínez et al. 1995). Earlier reports showed that bombesin stimulates gastrin release both in vivo and in isolated G cells in culture (Sugano et al. 1987; Kovacs et al. 1995; Squires et al. 1999). Other studies performed in vitro in isolated perfused rat stomach also showed that peripheral bombesin stimulates the secretion of somatostatin (Sandvik et al. 1989, 1997; Schubert & Hightower, 1989). Therefore the differential gastric acid secretory responses may be dependent upon the balance between the release and action of gastric stimulant (gastrin) and inhibitor (somatostatin) of acid secretion (Sandvik et al. 1989). However, in vivo studies in conscious or urethane-anaesthetized rats showed that immunoneutralization of somatostatin failed to influence bombesin antisecretory action (Martínez et al. 1995). These controversial results suggested that, under in vivo conditions, bombesin-induced inhibition of gastric acid secretion does not depend on the release of somatostatin in rats.
Previous studies in vitro, using an isolated mouse stomach preparation, showed that bombesin/gastrin releasing peptide (GRP) neurons of the gastric fundus inhibited neurally stimulated acid secretion by inducing the release of somatostatin (Schubert et al. 1991a, b). In addition, a direct inhibitory effect of bombesin-GRP on parietal cells was also suggested (Sandvik et al. 1989; Schubert et al. 1991a, b). However, neither the effects of bombesin on gastric acid secretion, nor the functional significance of somatostatin changes induced by bombesin in the regulation of gastric acid secretion observed in vitro have been characterized under in vivo conditions in mice.
Somatostatin actions are mediated through the activation of five different receptor subtypes (SSTR1 to SSTR5; Patel, 1997). The cloning and characterization of the five receptor subtypes have allowed the development of selective agonists and antagonists (Liapakis et al. 1996; Patel, 1999). In addition, genetically modified animals with deletion of a specific receptor subtype have been used to study the biological actions of somatostatin and the role of the different receptor subtypes (Martínez, 2002). Although the five somatostatin receptor subtypes are localized in the stomach (Prinz et al. 1994; Le Romancer et al. 1996; Krempels et al. 1997; Sternini et al. 1997), functional in vivo studies in rats, dogs and mice, as well as in vitro studies in human, rat and dog antral tissue, suggest that somatostatin effects on gastric acid secretion are mediated through the activation of SSTR2 (Rossowski et al. 1994; Lloyd et al. 1995; Zaki et al. 1996; Aurang et al. 1997; Fung & Greenberg, 1997; Patel, 1997; Martínez et al. 1998).
The objectives of the present study were first to characterize the action of peripheral infusion of bombesin on gastric acid secretion in mice by assessing changes in gastric secretory response to various secretagogues. Second, we examined whether the bombesin effect is meditated by somatostatin release using in vivo immunoneutralization of somatostatin. Lastly, the role of SSTR2 was investigated using wild-type mice and mice with specific deletion of the SSTR2 receptor gene (Zheng et al. 1997), and the selective SSTR2 antagonist, PRL-2903 (Rossowski et al. 1998; Kawakubo et al. 1999).
METHODS
Animals
Adult male mice (20–30 g, 3–6 months of age) were used. Mice deficient for SSTR2 were generated by gene targeting in mouse embryonic stem cells using a neomycin cassette with the entire SSTR2 gene on a 129Sv/C57B16 hybrid background (Zheng et al. 1997). The original knockout mice were genotyped to be homozygous −/− SSTR2 mutant or +/+ wild-type mice by Southern blot analysis (Zheng et al. 1997) and then maintained from the F1 as inbred colonies. Mice used in the present study were born from different litters; all descendants were born from genotyped littermates obtained through inbreeding. The appearance, behaviour, and gastrointestinal and brain morphology of knockout mice appeared indistinguishable from those of wild-type mice (Zheng et al. 1997; Martínez et al. 1998). Mice were maintained under controlled conditions of temperature (22 °C) and humidity (60 %), with food (Harlan Ibérica S.A., Spain) and tap water ad libitum. All experiments were performed in mice fasted for 18–20 h but with free access to water up to the beginning of the experiments. Animal care and handling were carried out in accordance with the regulations of the American Physiological Society.
Treatments
Pentagastrin (Peptavlon; Ayerst Laboratories, New York, NY, USA), histamine (Sigma, St Louis, MO, USA), bethanechol (Sigma), somatostatin-14 (Peptides International, Louisville, KY, USA) and bombesin (Sigma) were dissolved in 0.9 % saline. The preferential SSTR2 agonist DC 32-87 (also known as NC 8–12; Dr D. H. Coy, Tulane University, New Orleans, LA, USA; Table 1) (Patel & Srikant, 1994; Patel, 1997; Rossowski et al. 1998) was dissolved in 0.01 % acetic acid to a concentration of 1 μg μl−1 and subsequent dilutions were made in 0.9 % saline. The selective SSTR2 antagonist, PRL-2903 (also known as DC 41–33; Dr D. H. Coy; Table 1) (Rossowski et al. 1998) was dissolved in 0.9 % saline. All solutions were prepared immediately before each experiment. Purified monoclonal somatostatin antibody (CURE S.6, CURE: Digestive Diseases Research Center, UCLA, Los Angeles, CA, USA) was used for in vivo immunoneutralization (Wong et al. 1990). Purified monoclonal antibody to keyhole limpet haemocyanin (KLH) was used as a control. Production, characterization and purification of the monoclonal antibodies have been described in detail previously (Kovacs et al. 1989; Wong et al. 1990). Doses of compounds were selected according to previous studies in mice (Martínez et al. 1998; Piqueras et al. 2003).
Table 1.
Structure and relative receptor affinity of somatostatin-14 and its analogues
| Peptide | Structure | Relative receptor affinity* | Activity |
|---|---|---|---|
| Somatostatin-14 | Ala–Gly-c[Cys-Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr-Ser-Cys] | SSTR1 ≈ SSTR2 ≈ SSTR3 ≈ SSTR4 ≈ SSTR5 | Agonist (endogenous ligand) |
| DC 32-87 (NC 8-12) | d-Phe-c[Cys-Tyr-d-Trp-Lys-Abu-Cys]-Nal-NH2 | SSTR2 > SSTR5 >> SSTR3 >> SSTR1 ≈ SSTR4 | Agonist |
| PRL-2903 (DC 41-33) | Fpa-c[d-Cys-Pal -d-Trp-Lys-Tle-Cys]-Nal-NH2 | SSTR2 >> SSTR3 >> SSTR5 >> SSTR1 ≈ SSTR4 | Antagonist |
Based on binding affinity for cloned human receptors (Rossowski et al. 1998; Patel, 1999). Abu, aminobutanoic acid (dl-α-aminobutyric acid); Fpa, 4-fluorophenylalanine; Pal, 3-pyridylalanine; Tle, tertleucine; Nal, 3-(2-naphthyl)alanine.
Gastric acid secretion measurements
Gastric acid secretion was monitored in urethane-anaesthetized mice as previously described, with minor modifications (Martínez et al. 1998; Piqueras et al. 2003). Fasted wild-type (+/+) and SSTR2 knockout (−/−) mice were anaesthetized with urethane (1.25 g kg−1, about 0.2 ml, i.p.). The trachea was cannulated to ensure a clear airway and the oesophagus was ligated. The abdomen was then opened and the pylorus was ligated. An incision was made in the non-glandular portion of the stomach, the gastric lumen was rinsed until clean with warm 0.9 % saline, and a double-lumen gastric cannula was inserted through the forestomach incision. A catheter (30 gauge needle inserted into polyethylene E-10 tubing; Baxter, Irvine, CA, USA) was placed into the ileal vein for constant intravenous infusion of saline (0.1 ml h−1) and administration of substances. Gastric acid secretion was determined by continuous intragastric perfusion with warm saline (pH 7.0, 0.3 ml min−1). After the surgery, a 30–45 min period was allowed for stabilization and thereafter the effluents were collected at 10 min intervals and back-titrated to pH 7.0 (0.001 N NaOH) with an automatic titrator (Radiometer Copenhagen).
Experimental protocols
Gastric acid secretion was monitored every 10 min for 30–60 min before treatments and for an additional 30 min after ending peptide infusion in urethane-anaesthetized mice. Vehicle or peptide infusion was performed using an i.v. infusion rate of 0.1 ml h−1.
Effects of i.v. infusion of bombesin on basal and/or secretagogue-stimulated gastric acid secretion in wild-type and SSTR2 knockout mice
In wild-type mice, after a 30 min basal period, either saline or bombesin (10, 15, 20 or 40 μg kg−1) was infused for 1 h. In other studies, after a 30 min basal gastric secretion, pentagastrin (16 μg kg −1 h−1), histamine (5 mg kg−1 h−1) or bethanechol (0.6 mg kg−1 h−1) was infused i.v. and 30 min later, either saline or bombesin (10, 15, 20 or 40 mg kg−1) was infused i.v. for 1 h. In SSTR2 knockout mice, after a 1 h basal period, either saline or bombesin (10, 15, 20 or 40 μg kg−1) was infused for a 1 h period.
Effects of i.v. infusion of bombesin on gastric acid secretion in wild-type and SSTR2 mice pretreated with somatostatin monoclonal antibody
In wild-type mice, after a 30 min basal period, CURE S.6 purified somatostatin monoclonal antibody (150 μg mouse−1, 0.1 ml) was injected i.v. Thirty minutes later saline, somatostatin-14 (20 μg kg−1 h−1) or bombesin (20 μg kg−1 h−1) was infused i.v. for a 1 h period. In SSTR2 knockout mice, after a 1 h basal period, either somatostatin monoclonal antibody (150 μg mouse−1) or control antibody (150 μg mouse−1) was administered i.v. (0.1 ml).
Effects of i.v. infusion of somatostatin-14 and the preferential SSTR2 agonist DC 32-87 on gastric acid secretion in wild-type and SSTR2 knockout mice
In wild-type mice, after a 30 min basal period, pentagastrin (16 μg kg−1 h−1) or histamine (5 mg kg−1 h−1) was infused i.v. and 30 min later, either somatostatin-14 (20 μg kg−1 h−1) or DC 32-87 (20 μg kg−1 h−1), or the appropriate vehicle was infused i.v. for 1 h. In SSTR2 knockout mice, after a 30 min basal period, either somatostatin-14 (20 μg kg−1 h−1) or DC 32-87 (20 μg kg−1 h−1), or their respective vehicle was infused i.v. for 1 h.
Effects of the selective SSTR2 antagonist PRL-2903 on basal secretion in wild-type and SSTR2 knockout mice
In wild-type animals, after a 30 min basal period, PRL-2903 was administered as a bolus (1.5 mg kg−1, 0.1 ml, i.v.) followed by a continuous i.v. infusion (1.5 mg kg−1 h−1) during a 2 h period (total dose administered: 4.5 mg kg−1). In other studies, 10 min after starting PRL-2903 infusion, either somatostatin-14 (20 μg kg−1 h−1) or bombesin (20 μg kg−1 h−1), or the appropriate vehicle was infused i.v. for 1 h. In SSTR2 knockout mice, after a 1 h basal period, PRL-2903 was administered as a bolus (1.5 mg kg−1, 0.1 ml, i.v.) followed by a 2 h infusion (1.5 mg kg−1 h−1).
All animals were humanely killed, following current regulations, at the end of the experiments.
Statistical analysis
Gastric acid secretion (μmol per time) is expressed as mean ± s.e.m. Net secretion was calculated by subtracting the mean basal secretion for 1 h from the secretion during the period of interest. Differences between two groups were determined by Student's paired or unpaired t test, as appropriate. Differences between multiple groups were determined by analysis of variance (ANOVA) followed, when necessary, by a Student-Newman-Keuls multiple comparisons test. Within-group differences in acid secretion over time were assessed by repeated measures ANOVA followed, when necessary, by a Student-Newman-Keuls multiple comparisons test. Data were considered statistically significant when P was ≤ 0.05.
RESULTS
Effects of bombesin on basal and secretagogue-stimulated gastric acid secretion in wild-type mice and on basal secretion in SSTR2 knockout mice
Wild-type urethane-anaesthetized mice had a low basal gastric acid secretion (0.61 ± 0.03 μmol h−1), which was not modified by the i.v. infusion of vehicle or bombesin (10–40 μg kg−1 h−1) (Table 2). However, bombesin inhibited the acid secretory response to pentagastrin, histamine and bethanechol (Fig. 1). Pentagastrin infusion increased gastric acid secretion reaching a plateau 2.3-fold (mean plateau value: 0.30 ± 0.01 μmol (10 min)−1) over basal values (0.13 ± 0.03 μmol (10 min)−1) within 20 and 30 min. The infusion of bombesin (10, 15 or 20 μg kg−1 h−1, 1 h) dose-dependently inhibited pentagastrin-stimulated secretion with a net reduction of 27.7 ± 8.9 % (n = 6; P > 0.05vs. pentagastrin + vehicle), 45.9 ± 12.8 % (0.60 ± 0.16 μmol h−1, n = 6; P < 0.05vs. pentagastrin + vehicle: 1.03 ± 0.30 μmol h−1) and 85.4 ± 8.7 % (0.06 ± 0.14 μmol h−1, n = 6; P < 0.05vs. pentagastrin + vehicle; F4,23 = 5.234, P = 0.004), respectively (Fig. 1A). Histamine (5 mg kg−1 h−1) stimulated gastric acid secretion and the plateau response reached values 15- to 20-fold (mean plateau value: 2.18 ± 0.10 μmol (10 min)−1) over basal (0.11 ± 0.01 μmol (10 min)−1) after 60 min. The infusion of bombesin (20 or 40 μg kg−1 h−1) inhibited the net histamine response by 30.8 ± 16.4 % (8.61 ± 2.04 μmol h−1, n = 6; P > 0.05vs. vehicle: 12.43 ± 2.41 μmol h−1, n = 7) and 64.2 ± 4.4 % (4.44 ± 0.54 μmol h−1, n = 6; P < 0.05vs. vehicle; F3,19 = 7.702, P = 0.001), respectively (Fig. 1B). Bethanechol (0.6 mg kg−1 h−1) stimulated basal secretion by 12-fold (mean plateau value: 1.10 ± 0.09 μmol (10 min)−1) over basal values (0.09 ± 0.02 μmol (10 min)−1), reaching a secretory plateau within 40 min after starting the infusion. Bombesin (20 μg kg−1 h−1) inhibited the net secretory response to bethanechol by 48.8 ± 13.9 % (3.16 ± 0.86 μmol h−1, n = 4; P < 0.05vs. vehicle: 6.51 ± 1.17 μmol h−1; F2,10 = 13.25, P = 0.002; Fig. 1C).
Table 2.
Effects of bombesin on basal gastric acid secretion in urethane-anaesthetized wild-type and SSTR2 knockout mice *
| Bombesin (μg kg−1 h−1, i.v.) | ||||||
|---|---|---|---|---|---|---|
| Vehicle | 10 | 15 | 20 | 40 | ||
| Wild-type mice | Basal | 0.61 ± 0.03 | 0.65 ± 0.06 | 0.45 ± 0.07 | 0.58 ± 0.12 | 0.51 ± 0.06 |
| 0–1 h | 0.57 ± 0.05 | 0.51 ± 0.07 | 0.35 ± 0.05 | 0.41 ± 0.05 | 0.51 ± 0.16 | |
| n | 3 | 4 | 4 | 4 | 4 | |
| SSTR2 knockout mice | Basal | 12.61 ± 3.68 | 12.04 ± 0.47 | 11.54 ± 3.06 | 14.01 ± 3.76 | 11.84 ± 0.33 |
| 0–1 h | 9.21 ± 1.77 | 9.47 ± 1.50 | 10.54 ± 2.27 | 15.97 ± 4.20 | 9.67 ± 0.33 | |
| n | 6 | 4 | 6 | 6 | 5 | |
After a basal period, either vehicle (saline, 0.1 ml h−1) or bombesin (10−40 μg kg−1 h−1) was infused i.v. for 1 h (0–1 h period); acid secretion was monitored at 10 min intervals throughout the experimental time. Data represent accumulated gastric acid output in μmol h−1 and are expressed as means ± s.e.m. for the number of animals indicated for each treatment (n).
Figure 1. Effects of bombesin on secretagogue-stimulated gastric acid secretion in wild-type mice.
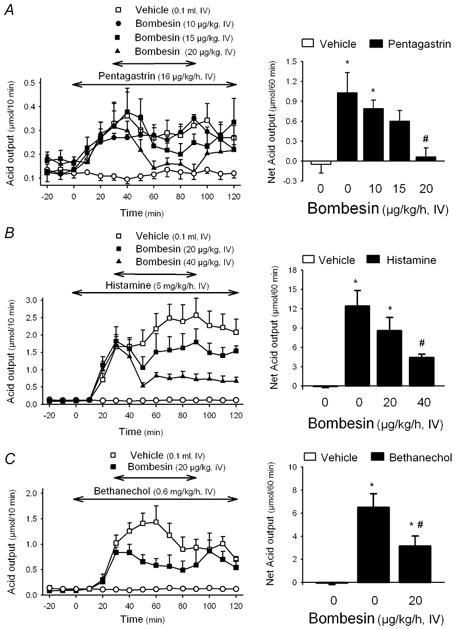
In urethane-anaesthetized wild-type mice, after a 30 min basal period, gastric acid secretion was stimulated with pentagastrin (A), histamine (B) or bethanechol (C). Thirty minutes after starting secretagogue administration, bombesin was infused i.v. for 1 h. Acid secretion was monitored at 10 min intervals throughout the experiment. Left panels represent time course changes in gastric acid output in 10 min intervals. The open circles in the three panels represent time course changes in acid secretion in animals receiving only an i.v. infusion of vehicle saline throughout the experimental time. Right panels show the net change in cumulative acid output for the 1 h period of bombesin or vehicle infusion. * P < 0.05vs. non-stimulated secretion; #P < 0.05vs. respective secretagogue + vehicle group (ANOVA). Pentagastrin stimulation: F4,23 = 5.234, P = 0.004. Histamine stimulation: F3,19 = 7.702, P = 0.001. Bethanechol stimulation: F2,10 = 13.25, P = 0.002.
In urethane-anaesthetized SSTR2 knockout mice, basal secretion was between 10- and 12-times higher than that observed in wild-type animals (SSTR2 knockout: 1.43 ± 0.1 μmol (10 min)−1, n = 11; wild-type: 0.12 ± 0.01 μmol (10 min)−1, n = 4; mean values from basal secretion monitored during a 2 h period; P < 0.05). Bombesin infusion (10, 15, 20 or 40 μg kg−1 h−1 for 1 h) failed to modify the high basal acid secretion in SSTR2 knockout mice (Table 2).
Effects of somatostatin-14 and the preferential SSTR2 agonist DC 32-87 on acid secretion in wild-type and SSTR2 knockout mice
In wild-type animals, pentagastrin infusion (16 μg kg−1 h−1) stimulated gastric acid secretion, reaching a plateau response within 30 min (1.25 ± 0.05 μmol h−1, n = 9, pooled data from groups treated with vehicle for somatostatin-14 or vehicle for DC 32-87). This represents a 2.5- and a 2.2-fold increase when compared with basal values (0.49 ± 0.07 μmol h−1; P < 0.05) or vehicle infusion (0.57 ± 0.05 μmol h−1, n = 3; P < 0.05), respectively. Somatostatin-14 (20 μg kg−1 h−1) reduced the secretory response to pentagastrin by 47.2 ± 8.9 % (0.67 ± 0.12 μmol h−1, n = 7; P < 0.05vs. pentagastrin + vehicle: 1.25 ± 0.04 μmol h−1, n = 5; Fig. 2A). Likewise, DC 32-87 (20 μg kg−1 h−1) inhibited the secretory response to pentagastrin by 47.1 ± 6.3 % (0.67 ± 0.07 μmol h−1, n = 6; P < 0.05vs. pentagastrin + vehicle: 1.26 ± 0.12 μmol h−1, n = 4; Fig. 3A). Acid secretion stimulated by an i.v. infusion of histamine (5 mg kg−1 h−1) resulted in a 12-fold increase above basal secretion from the 40–50 min period (1.56 ± 0.13 μmol (10 min)−1, P < 0.05vs. basal secretion: 0.13 ± 0.02 μmol (10 min)−1). Somatostatin-14 infusion (20 μg kg−1 h−1) reduced the plateau secretory response to histamine by 41.0 ± 12.7 % (5.50 ± 1.18 μmol h−1; P = 0.074vs. vehicle: 9.35 ± 1.81 μmol h−1; n = 4 for each group; Fig. 4).
Figure 2. Effects of somatostatin-14 on gastric acid secretion in wild-type and SSTR2 knockout mice.
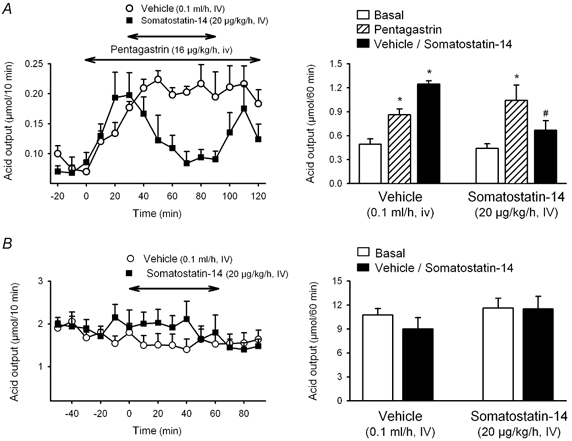
In urethane-anaesthetized wild-type mice (A) after a 30 min basal period, acid secretion was stimulated with pentagastrin and 30 min later either somatostatin or vehicle was infused i.v. for 1 h. Acid secretion was monitored at 10 min intervals throughout the experimental time. In SSTR2 knockout mice (B), due to the high basal secretion under urethane anaesthesia, the effects of somatostatin were determined under basal conditions. After a 60 min basal period either somatostatin or vehicle was infused i.v. for 1 h. Acid secretion was monitored at 10 min intervals throughout the experimental time. Left panels represent time course changes in gastric acid output at 10 min intervals. Right panels show the cumulative acid response. * P < 0.05vs. respective basal. #P < 0.05vs. pentagastrin. Wild-type mice: F5,30 = 6.891, P < 0.001. SSTR2 knockout mice: F3,20 = 1.285, P = 0.307).
Figure 3. Effects of the SSTR2 agonist DC 32-87 on gastric acid secretion in wild-type and SSTR2 knockout mice.
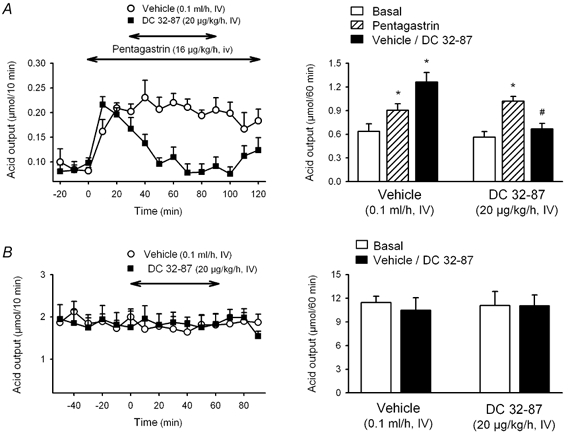
In urethane-anaesthetized wild type mice (A) after a 30 min basal period, acid secretion was stimulated with pentagastrin, and 30 min later either DC 32-87 or vehicle was infused i.v. for 1 h. Acid secretion was monitored at 10 min intervals throughout the experimental time. In SSTR2 knockout mice (B), due to the high basal secretion under urethane anaesthesia, the effects of DC 32-87 were determined under basal conditions. After a 60 min basal period either DC 32-87 or vehicle was infused i.v. for 1 h. Acid secretion was monitored at 10 min intervals throughout the experimental time. Left panels represent time course changes in gastric acid output in 10 min intervals. Right panels show the cumulative acid response. * P < 0.05vs. respective basal. #P < 0.05vs. pentagastrin. Wild-type mice: F5,24 = 9.205, P < 0.001. Knockout mice: F3,14 = 0.0374, P = 0.99).
Figure 4. Effects of somatostatin-14 on histamine-stimulated gastric acid secretion in wild-type mice.
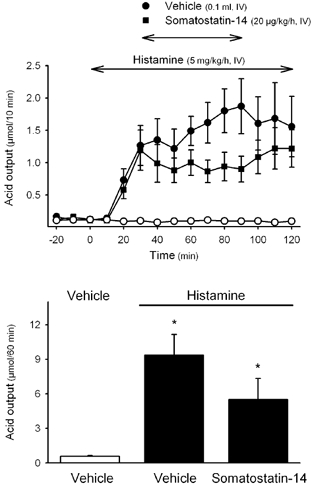
In urethane-anaesthetized wild type mice, after a 30 min basal period, acid secretion was stimulated with histamine, and 30 min later either somatostatin-14 or vehicle was infused i.v. for 1 h. In one group of animals, only vehicles were infused throughout the experiment (open circles in the upper panel). Acid secretion was monitored at 10 min intervals throughout the experimental time. The upper panel shows time course changes in gastric acid secretion and the lower panel the cumulative acid response for the 1 h infusion period of somatostatin-14 or vehicle. * P < 0.05vs. non-stimulated secretion. F2,8 = 9.409, P = 0.008.
Neither somatostatin-14 (20 μg kg−1 h−1, n = 6) nor DC 32-87 (20 μg kg−1 h−1, n = 6) modified the high basal acid secretion in urethane-anaesthetized SSTR2 knockout mice (Figs 2B and 3B).
Effects of bombesin and somatostatin-14 on gastric acid secretion in wild-type and SSTR2 knockout mice pretreated with somatostatin monoclonal antibody
In wild-type mice, the somatostatin monoclonal antibody CURE S.6 (150 μg mouse−1, i.v.) increased basal gastric acid secretion 20 min after administration, reaching a 4- to 5-fold plateau increase over basal secretory rates, that lasted for the 80 min experimental period. Infusion of bombesin (20 μg kg−1 h−1, i.v.) during the plateau phase, 30 min after monoclonal antibody administration, did not affect acid secretion (1.90 ± 0.08 μmol h−1, n = 5) when compared with vehicle-treated animals (2.01 ± 0.14 μmol h−1, n = 5; P > 0.05; Fig. 5). In the same experimental conditions, the i.v. infusion of somatostatin-14 (20 μg kg−1 h−1) 30 min after the antibody administration failed also to modify gastric acid secretion (2.29 ± 0.08 μmol h−1, n = 3; P > 0.05vs. vehicle; F2,10 = 2.693, P = 0.116; Fig. 5).
Figure 5. Effects of somatostatin immunoneutralization on somatostatin-14 and bombesin effects on gastric acid secretion in wild-type mice.
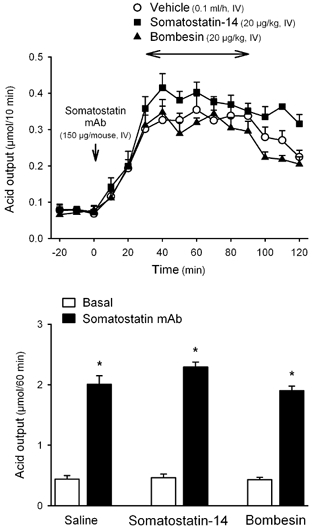
In urethane-anaesthetized wild-type mice, after a 30 min basal period, somatostatin (CURE S.6) monoclonal antibody (mAb; 150 μg mouse−1) was administered i.v. Thirty minutes later somatostatin-14, bombesin or vehicle was infused i.v. during a 1 h period. Acid secretion was monitored at 10 min intervals throughout the experimental time. The upper panel shows time course changes in acid secretion at 10 min intervals. The lower panel shows the cumulative acid response for the different treatments. * P < 0.05vs. respective basal secretion.
The somatostatin monoclonal antibody (150 μg mouse−1, i.v.) did not modify basal gastric acid output in SSTR2 knockout mice when compared with non-treated or control antibody (KLH)-treated animals (Table 3).
Table 3.
Effect of somatostatin monoclonal antibody (mAb) on basal gastric acid secretion in SSTR2 knockout mice *
| No treatment | Control Ab (KLH) | Somatostatin mAb | |
|---|---|---|---|
| Basal | 8.04 ± 1.01 | 7.17 ± 1.68 | 9.25 ± 3.02 |
| 0–1 h post-treatment | 9.75 ± 1.18 | 8.35 ± 2.59 | 8.82 ± 2.97 |
| 1–2 h post-treatment | 9.91 ± 1.27 | 10.71 ± 4.14 | 13.43 ± 4.34 |
| n | 4 | 4 | 4 |
After a basal period either the somatostatin mAb CURE S.6 (150 μg mouse−1) or control Ab (keyhole limpet haemocyanin, KLH; 150 μg mouse−1) was administered i.v. and acid secretion was monitored for the next 2 h. Data represent accumulated gastric acid output in μmol h−1 and are expressed as means ± s.e.m. for the number of animals indicated for each treatment (n).
Effect of the SSTR2 antagonist PRL-2903 on basal secretion in wild-type and SSTR2 knockout mice, and on bombesin and somatostatin-14 action in wild-type mice
In wild-type mice, PRL-2903 administered as a bolus (1.5 mg kg−1) followed by a continuous 2 h i.v. infusion (1.5 mg kg−1 h−1; n = 5), stimulated acid secretion reaching a stable secretory plateau within 20–30 min, that was maintained throughout the following 90 min infusion period (Fig. 6A). The plateau acid response reached mean values (1.04 ± 0.05 μmol (10 min)−1, n = 5) that represented an 11- and 7-fold increase over basal secretion (0.09 ± 0.01 μmol (10 min)−1, n = 5; P < 0.05) and vehicle-treated group, respectively (0.15 ± 0.01 μmol (10 min)−1; n = 4 P < 0.05). By contrast, in SSTR2 knockout mice, similar PRL-2903 administration did not modify basal gastric acid secretion. During a 1 h infusion time of PRL-2903, cumulative acid secretion was 13.59 ± 1.57 μmol (n = 4), which was not different from basal (12.87 ± 2.30 μmol h−1) or secretion in vehicle-treated animals (12.00 ± 5.67 μmol h−1, n = 4; Fig. 6B).
Figure 6. Effects of the SSTR2 antagonist PRL-2903 on gastric acid secretion in wild-type (A) and SSTR2 knockout mice (B).
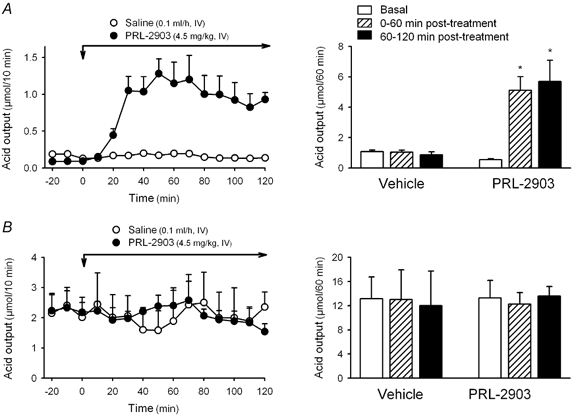
In urethane-anaesthetized mice, after a 30 min basal period, PRL-2903 was administered as a bolus (1.5 mg kg−1, i.v.) followed by a continuous i.v. infusion (1.5 mg kg−1 h−1) during a 2 h period (total dose administered: 4.5 mg kg−1). Control groups received vehicle saline (bolus of 0.1 ml and 2 h infusion of 0.1 ml h−1). Acid secretion was monitored at 10 min intervals throughout the experimental time. Left panels represent time course changes in acid secretion. Right panels represent the cumulative response for the different treatments. * P < 0.05vs. basal or secretion in vehicle-treated animals.
In wild-type mice, when somatostatin-14 (20 μg kg−1 h−1) was infused in the presence of PRL-2903 the secretory response was not changed indicating that the inhibitory effect of the peptide was completely blocked (PRL-2903 + somatostatin: 5.45 ± 0.48 μmol h−1, n = 4; P > 0.05vs. PRL-2093 + vehicle: 6.19 ± 1.17 μmol h−1, n = 3; Fig. 7A). Similarly, the inhibitory effect of bombesin (20 μg kg−1 h−1, i.v.) was also no longer observed when the peptide was perfused in the presence of PRL-2903 (6.17 ± 0.80 μmol h−1, n = 4; P > 0.05vs. PRL-2903 + vehicle: 6.16 ± 1.21 μmol h−1, n = 5; F5,21 = 9.516, P < 0.001; Fig. 7B).
Figure 7. Effects of somatostatin-14 and bombesin on PRL-2903-stimulated gastric acid secretion in wild-type mice.
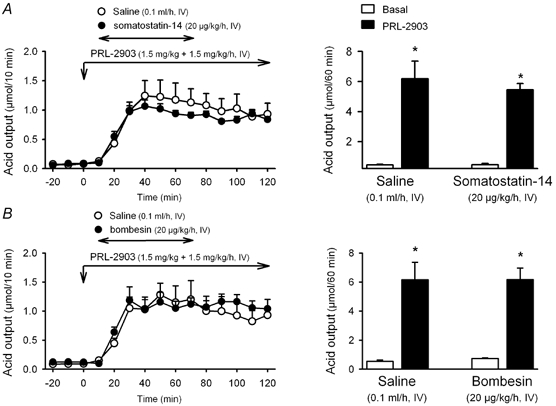
In urethane-anaesthetized wild-type mice, gastric acid secretion was stimulated with PRL-2903 (bolus of 1.5 mg kg−1 and infusion of 1.5 mg kg−1 h−1 for 2 h). Ten minutes after starting the i.v. infusion of PRL-2903 a 1 h infusion of somatostatin-14 (A), bombesin (B) or vehicle was started. Acid secretion was monitored at 10 min intervals throughout the experimental time. Left panels show time course changes in acid secretion. Right panels show cumulative acid output for the different treatments. * P < 0.05vs. respective basal secretion. Somatostatin-14: F3,10 = 31.999, P < 0.001. Bombesin: F3,14 = 17.379, P < 0.001.
DISCUSSION
The present study established that peripheral infusion of bombesin dose-dependently inhibits the gastric acid response to pentagastrin, bethanechol and histamine while not influencing the low basal acid secretion (about 1.0 μmol h−1) in urethane-anaesthetized mice. Likewise in an isolated luminally perfused mouse stomach preparation, bombesin, added to the serosal medium, resulted in an inhibition of histamine-stimulated gastric acid secretion (Schubert & Hightower, 1989). However, bombesin under these in vitro conditions, associated with a basal secretion of 5.4 μmol h−1, induced a dose-related and receptor-specific inhibition of basal gastric acid secretion (Schubert & Hightower, 1989). Therefore, it is likely that the lack of bombesin influence on basal secretion in the present study results from the low basal gastric acid secretion in urethane-anaesthetized mice. The inhibition of secretagogue-stimulated gastric acid secretion induced by exogenous bombesin is consistent with previous studies showing that bombesin/GRP-containing neurons activated by electrical field stimulation reduce the neurally mediated acid response in isolated mouse stomach preparations (Schubert et al. 1991a).
Convergent evidence supports a role of somatostatin in mediating bombesin antisecretory action in mice. We showed that intravenous infusion of somatostatin has similar effects to intravenous infusion of bombesin and significantly inhibited pentagastrin- and reduced histamine-stimulated gastric acid secretion in wild-type mice. In addition, immunoneutralization of somatostatin with the monoclonal somatostatin antibody CURE S.6 (Wong et al. 1990) prevented the bombesin antisecretory effect. Efficacy of the antibody treatment is demonstrated by the increase in basal gastric secretion observed in wild-type mice, associated with the immunoneutralization of endogenously released somatostatin in urethane-anaesthetized rodents (Yang et al. 1990; Martínez et al. 1995) as well as by the blockade of intravenous somatostatin-14-induced inhibition of gastric acid secretion. Lastly, although somatostatin release was not directly monitored in the present conditions, previous studies in isolated mouse stomach demonstrated that exogenous bombesin induces a dose-related stimulation of gastric somatostatin release (Schubert & Hightower, 1989) and intramural stimulation of bombesin-containing neurons also results in GRP receptor-dependent release of somatostatin (Schubert et al. 1991a). Further reports in rats have established that bombesin stimulates somatostatin release from mucosal segments of both fundus and antrum by distinct mechanisms (Schubert et al. 1991b). In the rat fundus, bombesin induces somatostatin secretion by direct interaction with specific bombesin/GRP receptors (Schubert et al. 1991b), which have been located on D cells by autoradiography (Nakamura et al. 1988) as well as on enriched primary fundic D cell cultures by immunohistochemistry (Schaffer et al. 1997). However, in the antrum, bombesin indirectly modulates somatostatin secretion through gastrin release (Schubert et al. 1991b).
Somatostatin exerts its biological actions through the activation of five different receptor subtypes (SSTR1 to SSTR5) (Martínez, 2002). Consistent with our previous report (Martínez et al. 1998), we showed that SSTR2 mediates the somatostatin antisecretory effect in urethane-anaesthetized mice. First, the preferential SSTR2 agonist, DC 32-87 (Rossowski et al. 1994) inhibited pentagastrin-stimulated gastric acid secretion in wild-type mice. Second, blockade of somatostatin/STTR pathways by peripheral administration of the SSTR2 antagonist, PRL-2903 (Rossowski et al. 1994), or somatostatin antibody pretreatment (Wong et al. 1990) in wild-type mice and by SSTR2 gene deletion in SSTR2 knockout mice (Zheng et al. 1997) resulted in a rise in basal gastric acid secretion. Interestingly, in SSTR2 knockout mice, the levels of basal acid secretion equal the maximal acid secretion elicited by histamine, suggesting that sustained inhibitory action of somatostatin through SSTR2 receptors maintained the stomach in a low basal secretory state. Lastly, exogenous somatostatin-14 no longer inhibited gastric acid secretion in wild-type mice pretreated with the SSTR2 antagonist or basal acid secretion in SSTR2 knockout mice.
In the present study, convergent findings demonstrate that the somatostatin-dependent inhibition of gastric acid secretion induced by bombesin is mediated by the activation of SSTR2. The infusion of bombesin, at doses effective in inhibiting acid secretion in wild-type animals, did not influence the high basal acid secretion characteristic of SSTR2 knockout mice under urethane anaesthesia. Bombesin antisecretory effects were also abolished by the pharmacological blockade of SSTR2 with the somatostatin analogue PRL-2903 in wild-type animals. The efficacy of PRL-2903 at the SSTR2 was demonstrated by the increase in basal secretion associated with the antagonism of endogenously released somatostatin action (Yang et al. 1990). Moreover, the antisecretory effect of exogenous somatostatin-14 was also blocked in the presence of the SSTR2 antagonist. These observations establish that functional SSTR2 are necessary for peripheral bombesin-induced somatostatin-dependent inhibitory effects on gastric acid secretion in mice.
Recent studies using SSTR2 knockout/lacZ knockin mice revealed that the vast majority of epithelial cells in the middle portion of the corpus expressed SSTR2. Most of these cells were also H+-K+-ATPase-positive being, therefore, identified as parietal cells (Allen et al. 2002). In addition, many of the enterochromaffin-like (ECL) cells in the corpus and pylorus of the mouse were also shown to express SSTR2 (Allen et al. 2002). Therefore, it is likely that the SSTR2-mediated antisecretory effect of peripheral infusion of bombesin in mice results from both a direct effect on the parietal cells as well as from inhibition of histamine release from ECL cells (Prinz et al. 1994). This is supported by the demonstration that intravenous infusion of bombesin inhibits gastric acid secretion stimulated by various secretagogues including pentagastrin, bethanechol and histamine, which act either directly on ECL (gastrin) or on parietal cells (bethanechol and histamine) (Pfeiffer et al. 1990; Prinz et al. 1999; Lindstrom et al. 2001). Studies in the isolated mouse stomach have shown that somatostatin induces a prompt decrease in histamine secretion and that neutralization of endogenous somatostatin results in a rise in histamine release (Vuyyuru & Schubert, 1997). Moreover, in the same in vitro preparation, somatostatin was effective in inhibiting pentagastrin-stimulated acid secretion, while only partially effective in inhibiting direct stimulation of parietal cells by bethanechol, and ineffective against histamine-stimulated secretion (Komasaka et al. 2002). In our study, somatostatin infused at doses effective in inhibiting by 50 % the plateau response to pentagastrin infusion, prevented the further rise in acid secretion elicited by histamine infusion for 1 h, while not influencing the acid response reached by histamine prior to starting the infusion of somatostatin. This may support a dual action of somatostatin inhibiting histamine release from ECL cells as well as a direct action on parietal cells. However, the difference in the antisecretory effect of somatostatin between these two secretagogues may also be related to the 9-fold higher acid response induced by histamine compared with pentagastrin.
The present results contrast with previous observations in which the immunoneutralization of somatostatin, using the same monoclonal antibody, failed to modify bombesin-induced inhibition of basal secretion in conscious rats or pentagastrin-stimulated secretion in urethane-anaesthetized rats (Martínez et al. 1995). These differences are not related to an inefficacy of the antibody to immunoneutralize somatostatin in the target tissues in rats. In urethane-anaesthetized rats, the antibody treatment significantly increased basal acid secretion and blocked the effects of exogenous somatostatin, indicating an effective immunoneutralization of the peptide both in the target tissue (stomach) and in blood (Martínez et al. 1995). The present and previous data may suggest a species-specific difference between rats and mice in the mechanisms of action of bombesin to suppress acid secretion. However, in the present study, it is of note that the somatostatin immunoneutralization resulted in a 4- to 5-fold lower increase in basal gastric acid secretion compared with the pharmacological blockade of SSTR2 receptors or the deletion of the SSTR2 gene. Therefore, it cannot be ruled out that bombesin-induced somatostatin release may not be as efficiently blocked by immunoneutralization in rats as in mice. Based on the present studies, it will be important to assess whether blocking SSTR2 receptors may be more efficient to unmask the role of this pathways in bombesin antisecretory effect in rats.
Previous reports showed that bombesin also stimulates gastric acid secretion depending upon the regimen of administration and the species considered. In rats, stimulation of acid secretion by bombesin has also been reported with different protocols of administration (Rossowski et al. 1989; Sandvik et al. 1989; Weigert et al. 1996). Such differential acid secretory responses to peripheral bombesin have been attributed to the interplay between the release and actions of gastrin and somatostatin (Sandvik et al. 1989). In the present study, bombesin administered intravenously as an infusion or as a bolus (data not shown) did not increase basal gastric acid secretion in wild-type mice. Moreover, when acid secretion was stimulated with secretagogues only inhibitory responses were observed, suggesting an action of somatostatin-dependent inhibitory mechanisms over the stimulatory gastrin-dependent actions.
Therefore, it should be expected that elimination of the somatostatin-dependent mechanisms may reveal a stimulatory response associated to gastrin release. The present results showed that bombesin did not alter the high basal gastric acid secretion induced by the blockade of SSTR2 receptors, by deletion of the gene (SSTR2 knockout mice) or by the use of SSTR2 antagonist, PRL-2903, in wild-type mice. It is worth mentioning that under these conditions, gastric acid secretion reached levels similar to those induced by a maximal dose of histamine, making it unlikely that any change in gastrin release induced by bombesin, when the somatostatin signalling pathways are blocked, could be manifested by a further increase in acid secretion. However, during somatostatin antibody treatment that resulted in a lower rise in basal acid secretion compared with the blockade of SSTR2 receptors, gastric acid values were not modified by bombesin, arguing against a stimulatory action of bombesin on gastric acid secretion in mice. Moreover, the lack of stimulatory effect of bombesin in SSTR2 knockout mice does not seem to be related to a maximal secretory state in these animals due to a sustained lack of somatostatin inhibitory action, since secretory responses to exogenously administered secretagogues (gastrin and histamine) can still be observed in these animal (data not shown).
In summary, results obtained using immunoneutralization of somatostatin by monoclonal antibody, pharmacological blockade of SSTR2 receptor and SSTR2 knockout mice showed that intravenous infusion of bombesin inhibits gastric acid secretion in mice and that this effect is mediated through the release of somatostatin and the activation of SSTR2, probably localized on ECL and parietal cells (Fig. 8). Although no direct measurements of somatostatin release from the stomach were possible in vivo, the results obtained are consistent with earlier in vitro studies in mouse and other species that demonstrated the release of somatostatin by bombesin and identified the pathways mediating this release. Moreover, we strengthen previous observations showing that SSTR2 is the main receptor subtype whereby somatostatin suppresses gastric acid secretion. Several studies have shown that other neuropeptides that inhibit gastric acid secretion also release somatostatin or have a somatostatin-dependent mechanism of action. For instance, it has been shown that glucose-dependent insulinotropic polypeptide (GIP)- and glucagon-like polypeptide (GLP)-induced inhibition of acid secretion in rats are mediated through somatostatin release; while the effects of amylin and adrenomedullin were only partially mediated by somatostatin (Briard et al. 1997). Some of these observations have been further confirmed in vitro by showing that amylin increased somatostatin release and decreased histamine release in an isolated mouse stomach preparation (Zaki et al. 2002). Studies in vitro using somatostatin immunoneutralization also suggested that the effects of pituitary adenylate cyclase-activating polypeptide (PACAP) are related to the release of somatostatin (Li et al. 2000). These observations, together with the present results suggest that gastric D cells may function as a common target for a variety of gut peptides, in which input is translated into the release of somatostatin and, in turn, the activation of SSTR2 on ECL and parietal cells, leading to an inhibition of acid output. Whether other neuropeptides or inhibitory mechanisms also depend on somatostatin to act as an integrative factor requires further studies using similar functional approaches to those used in this work (pharmacological blockade, immunoneutralization and/or use of genetically modified animal models).
Figure 8. Schematic representation of the local mechanisms controlling gastric acid secretion in the mouse gastric mucosa in vivo as it relates to bombesin/GRP actions.
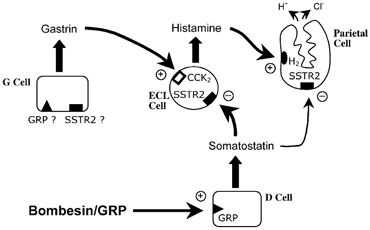
Bombesin/GRP will act primarily on D cells, through GRP receptors, stimulating the release of somatostatin. In turn, somatostatin will activate SSTR2 receptors located primarily on ECL cells, inhibiting the release of histamine, and also on parietal cells. Therefore, acid secretion will be inhibited via a double mechanism: inhibition of histamine release; and, to a minor degree, direct inhibition of parietal cell activity. So far, there is no evidence for the presence of somatostatin receptors in G cells which could mediate a direct action of somatostatin on G cells inhibiting gastrin release. In addition, the results obtained argue against the existence, in mice, of a direct stimulatory action of bombesin in G cells, and therefore the presence of GRP receptors in these cells is questionable.
Acknowledgments
This work was supported by the Conselleria de Cultura Educació i Ciència-Generalitat Valenciana, grant GV99-23-1-4 and the National Institute of Diabetes and Digestive and Kidney Diseases grants DK-30110 and DK-41301 (Antibody-RIA Core). L. Piqueras is supported by a fellowship from the San Pablo-CEU Foundation. The authors thank Dr D. H. Coy (Tulane University Medical Center, New Orleans, LA, USA) for the generous donation of DC 32-87 and PRL-2903; and Dr J. H. Schaeffer (Merck Research Laboratories) for the generous donation of the SSTR2 knockout breeders.
References
- Allen JP, Canty AJ, Schulz S, Humphrey PP, Emson PC, Young HM. Identification of cells expressing somatostatin receptor 2 in the gastrointestinal tract of Sstr2 knockout/lacZ knockin mice. J Comp Neurol. 2002;454:329–340. doi: 10.1002/cne.10466. [DOI] [PubMed] [Google Scholar]
- Aurang K, Wang J, Lloyd KC. Somatostatin inhibition of acid and histamine release by activation of somatostatin receptor subtype 2 receptors in rats. J Pharmacol Exp Ther. 1997;281:245–252. [PubMed] [Google Scholar]
- Bado A, Lewin JM, Dubrasquet M. Effects of bombesin on food intake and gastric acid secretion in cats. Am J Physiol. 1989;256:R181–186. doi: 10.1152/ajpregu.1989.256.1.R181. [DOI] [PubMed] [Google Scholar]
- Bertaccini G, Erspamer V, Impicciatore M. The actions of bombesin on gastric secretion of the dog and the rat. Br J Pharmacol. 1973;49:437–444. doi: 10.1111/j.1476-5381.1973.tb17254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briard N, Dutour A, Epelbaum J, Sauze N, Slama A, Oliver C. Species differences between male rat and ram pituitary somatostatin receptors involved in the inhibition of growth hormone secretion. Eur J Endocrinol. 1997;137:545–555. doi: 10.1530/eje.0.1370545. [DOI] [PubMed] [Google Scholar]
- Fung LC, Greenberg GR. Characterization of somatostatin receptor subtypes mediating inhibition of nutrient-stimulated gastric acid and gastrin in dogs. Regul Pept. 1997;68:197–203. doi: 10.1016/s0167-0115(96)02122-2. [DOI] [PubMed] [Google Scholar]
- Helman CA, Hirschowitz BI. Divergent effects of bombesin and bethanechol on stimulated gastric secretion in duodenal ulcer and in normal men. Gastroenterology. 1987;92:1926–1933. doi: 10.1016/0016-5085(87)90626-3. [DOI] [PubMed] [Google Scholar]
- Hildebrand P, Lehmann FS, Ketterer S, Christ AD, Stingelin T, Beltinger J, Gibbons AH, Coy DH, Calam J, Larsen F, Beglinger C. Regulation of gastric function by endogenous gastrin releasing peptide in humans: studies with a specific gastrin releasing peptide receptor antagonist. Gut. 2001;49:23–28. doi: 10.1136/gut.49.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo K, Coy DH, Walsh JH, Taché Y. Urethane-induced somatostatin mediated inhibition of gastric acid: reversal by the somatostatin 2 receptor antagonist, PRL-2903. Life Sci. 1999;65:L115–120. doi: 10.1016/s0024-3205(99)00340-9. [DOI] [PubMed] [Google Scholar]
- Komasaka M, Horie S, Watanabe K, Murayama T. Antisecretory effect of somatostatin on gastric acid via inhibition of histamine release in isolated mouse stomach. Eur J Pharmacol. 2002;452:235–243. doi: 10.1016/s0014-2999(02)02309-9. [DOI] [PubMed] [Google Scholar]
- Kovacs TO, Lloyd KC, Wong H, Walsh JH. Inhibition of bombesin-stimulated acid secretion by immunoneutralization of gastrin in dogs. Am J Physiol. 1995;268:G54–58. doi: 10.1152/ajpgi.1995.268.1.G54. [DOI] [PubMed] [Google Scholar]
- Kovacs TOG, Walsh JH, Maxwell V, Wong HC, Azuma T, Katt E. Gastrin is a major mediator of the gastric phase of acid secretion in dogs: proof by monoclonal antibody neutralization. Gastroenterology. 1989;97:1406–1413. doi: 10.1016/0016-5085(89)90383-1. [DOI] [PubMed] [Google Scholar]
- Krempels K, Hunyady B, O'Carroll AM, Mezey E. Distribution of somatostatin receptor messenger RNAs in the rat gastrointestinal tract. Gastroenterology. 1997;112:1948–1960. doi: 10.1053/gast.1997.v112.pm9178687. [DOI] [PubMed] [Google Scholar]
- Le Romancer M, Cherifi Y, Levasseur S, Laigneau JP, Peranzi G, Jais P, Lewin MJ, Reyl-Desmars F. Messenger RNA expression of somatostatin receptor subtypes in human and rat gastric mucosae. Life Sci. 1996;58:1091–1098. doi: 10.1016/0024-3205(96)00063-x. [DOI] [PubMed] [Google Scholar]
- Li P, Chang TM, Coy D, Chey WY. Inhibition of gastric acid secretion in rat stomach by PACAP is mediated by secretin, somatostatin, and PGE(2) Am J Physiol Gastrointest Liver Physiol. 2000;278:G121–127. doi: 10.1152/ajpgi.2000.278.1.G121. [DOI] [PubMed] [Google Scholar]
- Liapakis G, Hoeger C, Rivier J, Reisine T. Development of a selective agonist at the somatostatin receptor subtype sstr1. J Pharmacol Exp Ther. 1996;276:1089–1094. [PubMed] [Google Scholar]
- Lindstrom E, Chen D, Norlen P, Andersson K, Hakanson R. Control of gastric acid secretion:the gastrin-ECL cell-parietal cell axis. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:505–514. doi: 10.1016/s1095-6433(00)00331-7. [DOI] [PubMed] [Google Scholar]
- Lloyd KCK, Wang J, Aurang K, Grönhed P, Coy DH, Walsh JH. Activation of somatostatin receptor subtype 2 inhibits acid secretion in rats. Am J Physiol. 1995;268:G102–106. doi: 10.1152/ajpgi.1995.268.1.G102. [DOI] [PubMed] [Google Scholar]
- Martínez V. Somatostatin receptors and the regulation of gastric function. In: Taché Y, Goto Y, Ohning G, Yamada T, editors. Gut-Brain Peptides in the New Millenium. Los Angeles: CURE Foundation; 2002. pp. 167–178. [Google Scholar]
- Martínez V, Curi AP, Torkian B, Schaeffer JM, Wilkinson HA, Walsh JH, Taché Y. High basal gastric acid secretion in somatostatin receptor subtype 2 knockout mice. Gastroenterology. 1998;114:1125–1132. doi: 10.1016/s0016-5085(98)70417-2. [DOI] [PubMed] [Google Scholar]
- Martínez V, Taché Y. Bombesin and the brain-gut axis. Peptides. 2000;21:1617–1625. doi: 10.1016/s0196-9781(00)00293-x. [DOI] [PubMed] [Google Scholar]
- Martínez V, Yang H, Wong HC, Walsh JH, Taché Y. Somatostatin antibody does not influence bombesin-induced inhibition of gastric acid secretion in rats. Peptides. 1995;15:1–6. doi: 10.1016/0196-9781(94)00152-v. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Oda M, Kaneko K, Akaiwa Y, Tsukada N, Komatsu H, Tsuchiya M. Autoradiographic demonstration of gastrin-releasing peptide-binding sites in the rat gastric mucosa. Gastroenterology. 1988;94:968–976. doi: 10.1016/0016-5085(88)90555-0. [DOI] [PubMed] [Google Scholar]
- Patel YC. Molecular pharmacology of somatostatin receptor subtypes. J Endocrinol Invest. 1997;20:348–367. doi: 10.1007/BF03350317. [DOI] [PubMed] [Google Scholar]
- Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- Patel YC, Srikant CB. Subtype selectivity of peptide analogs for all five cloned human somatostatin receptors (hsstr 1–5) Endocrinology. 1994;135:2814–2817. doi: 10.1210/endo.135.6.7988476. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Rochlitz H, Noelke B, Tacke R, Moser U, Mutschler E, Lambrecht G. Muscarinic receptors mediating acid secretion in isolated rat gastric parietal cells are of M3 type. Gastroenterology. 1990;98:218–222. doi: 10.1016/0016-5085(90)91314-v. [DOI] [PubMed] [Google Scholar]
- Piqueras L, Corpa JM, Martinez J, Martínez V. Gastric hypersecretion associated to iodoacetamide-induced mild gastritis in mice. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:140–150. doi: 10.1007/s00210-002-0670-7. [DOI] [PubMed] [Google Scholar]
- Prinz C, Sachs G, Walsh JH, Coy DH, Wu SV. The somatostatin receptor subtype on rat enterochromaffinlike cells. Gastroenterology. 1994;107:1067–1074. doi: 10.1016/0016-5085(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Prinz C, Zanner R, Gerhard M, Mahr S, Neumayer N, Hohne-Zell B, Gratzl M. The mechanism of histamine secretion from gastric enterochromaffin-like cells. Am J Physiol. 1999;277:C845–855. doi: 10.1152/ajpcell.1999.277.5.C845. [DOI] [PubMed] [Google Scholar]
- Rossowski WJ, Cheng BL, Jiang NY, Coy DH. Examination of somatostatin involvement in the inhibitory action of GIP, GLP-1, amylin and adrenomedullin on gastric acid release using a new SRIF antagonist analogue. Br J Pharmacol. 1998;125:1081–1087. doi: 10.1038/sj.bjp.0702160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossowski WJ, Gu Z-F, Akarca US, Jensen RT, Coy DH. Characterization of somatostatin receptor subtypes controlling rat gastric acid and pancreatic amylase release. Peptides. 1994;15:1421–1424. doi: 10.1016/0196-9781(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Rossowski WJ, Murphy WA, Jiang NY, Yeginsu O, Ertan A, Coy DH. Effects of a novel bombesin antagonist analogue on bombesin-stimulated gastric acid secretion and growth hormone release in the pentobarbital-anesthetized rat. Scand J Gastroenterol. 1989;24:121–128. doi: 10.3109/00365528909092249. [DOI] [PubMed] [Google Scholar]
- Sandvik AK, Brenna E, Sundan A, Holst JJ, Waldum HI. Bombesin inhibits histamine release from the rat oxyntic mucosa by a somatostatin-dependent mechanism. Scand J Gastroenterol. 1997;32:427–432. doi: 10.3109/00365529709025076. [DOI] [PubMed] [Google Scholar]
- Sandvik AK, Holst JJ, Waldum HL. The effect of gastrin-releasing peptide on acid secretion and the release of gastrin, somatostatin, and histamine in the totally isolated, vascularly perfused rat stomach. Scand J Gastroenterol. 1989;24:9–15. doi: 10.3109/00365528909092232. [DOI] [PubMed] [Google Scholar]
- Schaffer K, Herrmuth H, Mueller J, Coy DH, Wong HC, Walsh JH, Classen M, Schusdziarra V, Schepp W. Bombesin-like peptides stimulate somatostatin release from rat fundic D cells in primary culture. Am J Physiol. 1997;273:G686–695. doi: 10.1152/ajpgi.1997.273.3.G686. [DOI] [PubMed] [Google Scholar]
- Schubert ML, Hightower J. Inhibition of acid secretion by bombesin is partly mediated by release of fundic somatostatin. Gastroenterology. 1989;97:561–567. doi: 10.1016/0016-5085(89)90625-2. [DOI] [PubMed] [Google Scholar]
- Schubert ML, Hightower J, Coy DH, Makhlouf GM. Regulation of acid secretion by bombesin/GRP neurons of the gastric fundus. Am J Physiol. 1991a;260:G156–160. doi: 10.1152/ajpgi.1991.260.1.G156. [DOI] [PubMed] [Google Scholar]
- Schubert ML, Jong MJ, Makhlouf GM. Bombesin/GRP-stimulated somatostatin secretion is mediated by gastrin in the antrum and intrinsic neurons in the fundus. Am J Physiol. 1991b;261:G885–889. doi: 10.1152/ajpgi.1991.261.5.G885. [DOI] [PubMed] [Google Scholar]
- Vuyyuru L, Schubert ML. Histamine, acting via H3 receptors, inhibits somatostatin and stimulates acid secretion in isolated mouse stomach. Gastroenterology. 1997;113:1545–1552. doi: 10.1053/gast.1997.v113.pm9352856. [DOI] [PubMed] [Google Scholar]
- Squires PE, Meloche RM, Buchan AM. Bombesin-evoked gastrin release and calcium signaling in human antral G cells in culture. Am J Physiol. 1999;276:G227–237. doi: 10.1152/ajpgi.1999.276.1.G227. [DOI] [PubMed] [Google Scholar]
- Sternini C, Wong H, Wu SV, De GR, Yang M, Reeve JJ, Brecha NC, Walsh JH. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J Comp Neurol. 1997;386:396–408. [PubMed] [Google Scholar]
- Sugano K, Park J, Soll AH, Yamada T. Stimulation of gastrin release by bombesin and canine gastrin-releasing peptides. Studies with isolated canine G cells in primary culture. J Clin Invest. 1987;79:935–942. doi: 10.1172/JCI112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JH, Kovacs TOG, Maxwell V, Cuttitta F. Bombesin-like peptides as regulators of gastric function. Ann N Y Acad Sci. 1988;547:217–224. doi: 10.1111/j.1749-6632.1988.tb23890.x. [DOI] [PubMed] [Google Scholar]
- Weigert N, Li YY, Lippl F, Coy DH, Classen M, Schusdziarri V. Role of endogenous bombesin-peptides during vagal stimulation of gastric acid secretion in the rat. Neuropeptides. 1996;30:521–527. doi: 10.1016/s0143-4179(96)90033-5. [DOI] [PubMed] [Google Scholar]
- Wong HC, Walsh JH, Yang H, Taché Y, Buchan AM. A monoclonal antibody to somatostatin with potent in vivo immunoneutralizing activity. Peptides. 1990;11:707–712. doi: 10.1016/0196-9781(90)90185-8. [DOI] [PubMed] [Google Scholar]
- Yang H, Wong H, Wu V, Walsh JH, Taché Y. Somatostatin monoclonal antibody immunoneutralization increases gastrin and gastric acid secretion in urethane-anesthetized rats. Gastroenterology. 1990;99:659–665. doi: 10.1016/0016-5085(90)90952-w. [DOI] [PubMed] [Google Scholar]
- Zaki M, Harrington L, McCuen R, Coy DH, Arimura A, Schubert ML. Somatostatin receptor subtype 2 mediates inhibition of gastrin and histamine secretion from human, dog, and rat antrum. Gastroenterology. 1996;111:919–924. doi: 10.1016/s0016-5085(96)70059-8. [DOI] [PubMed] [Google Scholar]
- Zaki M, Koduru S, McCuen R, Vuyyuru L, Schubert ML. Amylin, released from the gastric fundus, stimulates somatostatin and thus inhibits histamine and acid secretion in mice. Gastroenterology. 2002;123:247–255. doi: 10.1053/gast.2002.34176. [DOI] [PubMed] [Google Scholar]
- Zheng H, Bailey A, Jiang MH, Honda K, Chen HY, Trumbauer ME, Van Der Ploeg LH, Schaeffer JM, Leng G, Smith RG. Somatostatin receptor subtype 2 knockout mice are refractory to growth hormone-negative feedback on arcuate neurons. Mol Endocrinol. 1997;11:1709–1717. doi: 10.1210/mend.11.11.0016. [DOI] [PubMed] [Google Scholar]


