Abstract
There is some evidence, mainly from rodent studies, that any factor which alters the final total number of nephrons formed, during nephrogenesis, will result in hypertension in adult life. Sheep, programmed to become hypertensive by exposure to synthetic glucocorticoid (dexamethasone, 0.48 mg h−1, for 48 h) early in development (∼27 days of gestation), were killed at 7 years of age, and had nephron counting performed by unbiased stereology. Mean arterial pressure was 83 ± 4 mmHg in the dexamethasone (DEX) group (n = 5), and 73 ± 5 in the control (CON; n = 7; P < 0.05). The total nephron number, in the right kidney (249 070 ± 14 331; n = 5) was significantly lower (P < 0.01) than that of controls (402 787 ± 30 458; n = 7). Mean glomerular volume was larger in the DEX than the CON group (P < 0.01), but there was no significant difference in the sclerosis index between the two groups. Low nephron number was associated with grossly enlarged and dilated proximal tubules and greater accumulation of collagen type I and type III in the tubular interstitium and periadventitia of the renal cortical vessels. These data suggest that the hypertensive programming effect of glucocorticoid treatment, early in kidney development, results, at least in part, from impaired nephrogenesis.
Some time ago we discovered, serendipitously, that if pregnant ewes were treated with dexamethasone, 0.48 mg h−1, for 48 h between days 26 and 28 of gestation, the offspring developed hypertension (Dodic et al. 1998). The hypertension was evident by 5 months after birth, increased with age, and was accompanied by reset baroreflexes, increased cardiac output, and eventually with left ventricular hypertrophy and decreased cardiac functional reserve (Dodic et al. 1998, 1999, 2001). Importantly, the phenomenon was reproducible, in both males and females (Dodic et al. 2002a) and could be produced by the use of high physiological doses of cortisol (5 mg h−1) over the same time period (Dodic et al. 2002b). The time at which the steroid treatment was given coincided with the very early stages of nephrogenesis in the permanent metanephric kidney (Wintour et al. 1996) when the ureteric bud had invaded the metanephric mesenchyme and started branching. In sheep the permanent metanephric kidney completes nephrogenesis by ∼130 days of gestation (Moritz & Wintour, 1999) which is well before birth (term is 145–150 days). In a similar study in rats, it was shown that treatment of the pregnant rat, at days 15–16, with dexamethasone, resulted in adult offspring with 30 % decrease in total nephron number, and hypertension (Ortiz et al. 2001). The rat kidney, at this time, is also preglomerular (Bertram et al. 2000), as the rat has only 20 % of the adult nephron number at birth, and does not complete nephrogenesis until 8–10 days after birth. The times of glucocorticoid treatment, relative to metanephric development were thus comparable in the rat and sheep studies. It was hypothesized that early glucocorticoid treatment would have decreased nephron number in the adult ovine offspring. When the hypertensive adult sheep were approximately 7 years of age they were killed and their kidneys collected, appropriately, for nephron counting. The recommended ‘gold standard’ method for assessment of nephron number is unbiased stereology (Nyengaard & Bendtsen, 1990; Bertram, 2001; Douglas-Denton et al. 2002) and this was used for the current experiment.
METHODS
Animals
All experiments were approved by the Animal Ethics Committee of the Howard Florey Institute, and conducted according to the guidelines of the National Health and Medical Research Council of Australia. Data from animals used in this study have been reported previously (Dodic et al. 1998, 1999, 2001). Ewes were infused intravenously with dexamethasone (11.5 mg day−1 (DEX)) for 48 h at a mean gestational age of 27 days. Five female offspring were studied until 7 years of age along with seven female control animals (CON). All animals were born at term. At birth these animals were of similar body weights (4.7 ± 0.3 kg in the DEX group; n = 5) compared with the CON group (birth weight 4.6 ± 0.2 kg; n = 7). At 7 years of age, mean arterial blood pressure was significantly higher in the DEX group (83 ± 4 mmHg vs.73 ± 5 mmHg in the CON group; P < 0.05) (Dodic et al. 2001).
Kidney sampling
At 7 years of age animals were killed with an overdose of pentobarbital sodium (Lethabarb, Arnolds, Boronia, Australia: 100 mg (kg body weight)−1). Right kidneys were perfusion-fixed with 4 % paraformaldehyde, weighed (fixed weight), and immersion-fixed in 10 % formalin. Kidneys were cut in the transverse plane into halves. One half was taken ‘randomly’ for further sampling. The sampled half was cut into two sections and these were cut into approximately 5 mm slices. Every third slice was taken for further sampling, with the first slice chosen at random from the first three. The medulla was trimmed but a thin ‘rim’ adjacent to the cortex was left intact to ensure no glomeruli were excised or damaged. Sampled slices were cut into strips approximately 5 mm in width, and then cut into smaller blocks suitable for processing. Every 15th block of tissue was sampled, with the first block chosen at random from the first 15. Tissue blocks were processed by embedding in glycolmethacrylate (Technovit 7100, Haraeus Kulzer Gmbh, Germany). Blocks were exhaustively sectioned at a nominal thickness of 15 μm with every 10th and 11th section sampled, the first of which was chosen at random from the first 10 sections. Sections were routinely stained with periodic acid-Schiff reagent (PAS).
Estimating total glomerular number (Nglom, kid)
Total glomerular number was estimated using physical dissectors as described in detail previously (Douglas-Denton et al. 2002). In brief, total glomerular number was estimated using:
where 2 was the inverse of the first sampling fraction (1/2 of the whole kidney), 3 was the inverse of the second sampling fraction (1/3 of the slices), 15 was the inverse of the third sampling fraction (1/15 of the blocks), 10 was the inverse of the section sampling fraction (1/10 of the sections), PS/2PF was the fraction of the section area used for counting glomeruli, and Q− was the actual number of glomeruli counted. Approximately, 100–150 glomeruli were counted for each kidney.
Estimating glomerular tuft and renal corpuscle volumes
Mean glomerular tuft (Vglom) and renal corpuscle (Vcorp) volumes were estimated using:
and
whereVV(glom,kid) and NV(glom,kid) are volume density and numerical density, respectively, of glomeruli in kidney and VV(corp,kid) and NV(corp,kid) are volume density and numerical density, respectively, of renal corpuscles in kidney.
Total glomerular tuft (Vglom(tot)) and renal corpuscle (Vcorp(tot)) volumes were also estimated using:
and
Sclerotic index
Four blocks of tissue from each kidney were processed to paraffin, sectioned at 5 μm and stained with PAS. A semiquantitative method was used to evaluate the degree of glomerulosclerosis. Simultaneously, the stereological method of Weibel & Gomez (1962) was used to estimate mean glomerular volume (Vglom) using:
where K is 1.01 which is the size distribution coefficient that assumes a coefficient of variation (CV) for glomerular size of 10 %, and β is 1.38, which is the shape coefficient of a sphere.
As shrinkage is known to occur during paraffin processing, glomerular volume was estimated again so that correlations with sclerosis could be investigated. During counting, sampled glomeruli were given a semiquantitative sclerosis score between 0 and 4. Normal (no sclerosis) glomeruli were representative of 0. Glomeruli with 1–25 % sclerosis were graded as 1, glomeruli with 26–50 % as 2, glomeruli with 51–75 % as 3, and glomeruli with 76–100 % as 4. Exactly 100 glomeruli per kidney were evaluated.
Kidney morphology
Sections of the perfused kidney not taken for nephron counting and embedded in paraffin were cut into approximately 4 μm slices (DEX, n = 4 and CON, n = 2). Slides were then stained with haematoxylin and eosin or Masson trichrome (as an indicator of collagen deposition in the renal cortex). Briefly, sections were stained with celestin blue, rinsed and stained with Mayer's haematoxylin. Following a further rinse in water, Biebrich scarlet-acid fuchsin was added. Sections were again washed and underwent differentiation with 5 % tungstophophoric acid and were then stained with aniline blue. Sections were placed in acetic acid, rehydrated and coverslipped.
Determination of collagen content
The total collagen content of the kidney from the CON (n = 7) and DEX (n = 5) group of animals was determined as previously described (Samuel et al. 1996). Triplicate 10 μl aliquots from each sample were analysed for hydroxyproline content using a scaled-down procedure (Bergman & Loxley, 1963). Hydroxyproline values were then converted to collagen content by multiplying by a factor of 7.46 (Caspari et al. 1977).
Determination of collagen types
Collagen was extracted from 2 mg of the dry weight tissue (from each of the control and dexamethasone-treated animals) as described (Samuel et al. 1996). The maturely cross-linked collagen was extracted by limited pepsin digestion (enzyme to substrate ratio, 1:10; 24 h) before the aliquots of each sample were analysed by SDS-PAGE on 5 % (w/v) acrylamide gels containing 3.5 % (w/v) acrylamide stacking gels. Interrupted electrophoresis with delayed reduction of the disulfide bonds of type III collagen was used to separate the α1(I) chains from the α1(III) collagen chains (Sykes et al. 1976). The gels were stained overnight with 0.1 % (w/v) Commassie blue (R-250) containing 45 % (v/v) methanol and 7 % (v/v) acetic acid. Densitometry of the individual collagen bands was performed using a Bio-Rad GS-710 calibrated imaging densitometer.
Statistics
All data are expressed as means ± s.e.m. Glomerulosclerosis data were tested using a Mann–Whitney rank sum test as the data failed normality tests. Other parameters were tested using a Student's t test.
RESULTS
Nephron number
Body and kidney weights were similar in the two groups of animals (see Table 1). Kidney weight as a proportion of body weight was also similar. Total glomerular number ranged between 208 278 in a DEX animal and 464 508 in a CON animal. Values for all parameters are shown in Table 1. The mean number of glomeruli was significantly higher in the CON group (P < 0.01). Mean glomerular tuft and renal corpuscle volumes were significantly (P < 0.01) higher in DEX animals compared to the CON group. Total glomerular tuft and renal corpuscle volumes were not significantly different.
Table 1.
Body weight and parameters in the right kidney of control (CON; n = 7) and prenatally dexamethasone-treated (DEX; n = 5) animals
| CON | DEX | |
|---|---|---|
| Body weight (kg) | 55.4 ± 2.4 | 56.0 ± 1.9 |
| Fixed kidney weight (g) | 67.8 ± 3.6 | 68.8 ± 1.4 |
| Kidney/body weight (g kg−1) | 1.22 ± 0.04 | 1.23 ± 0.03 |
| Total glomerular number | 402787 ± 30458 | 249070 ± 14331** |
| Mean tuft volume (mm3× 10−3) | 4.3 ± 0.2 | 5.6 ± 0.3** |
| Total glomerular tuft volume (cm3) | 1.7 ± 0.2 | 1.4 ± 0.1 |
| Mean renal corpuscle volume (mm3× 10−3) | 6.2 ± 0.3 | 8.1 ± 0.5** |
| Total renal corpuscle volume (cm3) | 2.5 ± 0.2 | 2.0 ± 0.1 |
P < 0.01.
The degree of glomerulosclerosis was not significantly different between the two groups. Both DEX and CON groups consisted of glomerular profiles with approximately 15 % sclerosed glomeruli. The percentage of glomeruli in each grade is shown in Fig. 1. Mean glomerular volume did not change with the degree of glomerulosclerosis. Renal corpuscle volume showed a slight increase from grade 0 to 1, and a further increase from grade 1 to 2. The volume decreased in grade 3 profiles. Two grade 4 profiles were observed in the DEX animals, and none in the CON animals.
Figure 1. Glomerulosclerosis index in adult control and prenatally dexamethasone-treated animals.
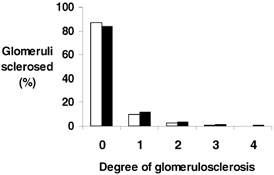
Degree of glomerulosclerosis (graded 0 (no sclerosis) to 4 (> 75 % sclerosis); see text for details) in control (CON; □) and prenatally dexamethasone-treated animals (DEX; ▪) at 7 years of age.
Kidney morphology
As shown in Fig. 2, in three out of four DEX animals (C, D and F) the proximal tubules (large arrows) were markedly dilated and enlarged compared to two animals from the CON group (A and B). In one animal from the DEX group (E), morphology of the proximal tubules resembled the two animals from the CON group (A and B). As Fig. 2 demonstrates, there was no noticeable collagen accumulation in the glomeruli of any animal from either CON or DEX group (A–F). However, excess collagen can be seen in the tubular interstitium (C, D and F) and the periadventitia of cortical vessels (D) in animals from the DEX group whose sections were stained with Masson trichrome – an indicator of collagen deposition in the renal cortex (C, D and F; small arrows).
Figure 2. Renal morphology in adult control and prenatally dexamethasone-treated animals.
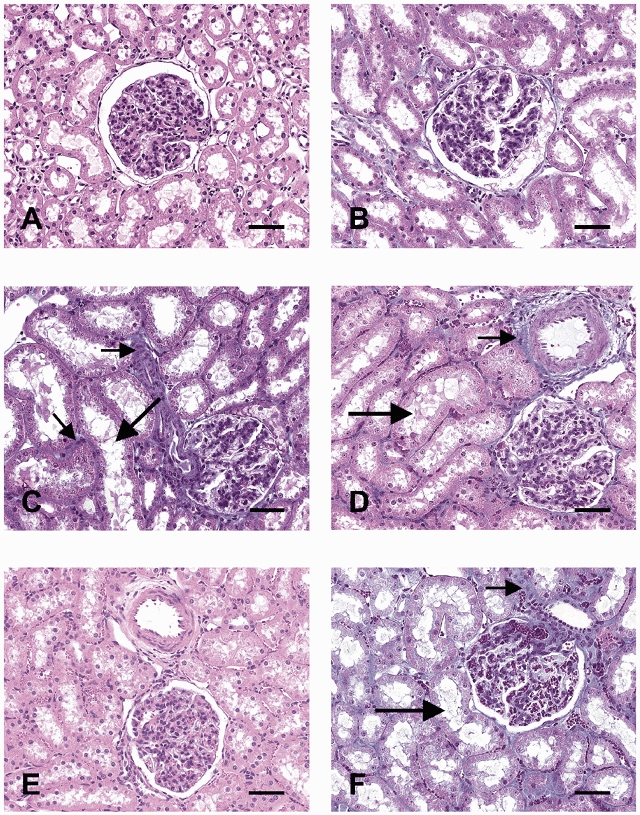
Representative photomicrographs of the renal cortex from two animals from the control group (CON; A and B) and four animals prenatally treated with dexamethasone (DEX; C–F); Tissues shown in A and E were stained with haematoxylin and eosin and those in B, C, D and F were stained with Masson trichrome. In three out of four DEX animals (C, D and F) the proximal tubules (large arrows) were markedly dilated and enlarged. There was no noticeable collagen accumulation in the glomeruli of any animal, but excess collagen can be seen in the tubular interstitium and the periadventitia of cortical vessels (small arrows). Bar represents 50 μm.
Collagen: content and types
While the overall wet weights of the kidneys from the CON and the DEX group of animals were similar, there was a small, but non-significant increase (∼10 %) in the total dry weight of the kidneys from the DEX group of animals (Fig. 3A). This was reflected in a tendency towards higher total kidney collagen content (∼22 %) found in the DEX group (421 ± 60 mg) compared to the collagen content in the CON group of animals (357 ± 38 mg) (P = 0.08) (Fig. 3A).
Figure 3. Kidney dry weight, total collagen content and collagen types (I and III) in adult control and prenatally dexamethasone-treated animals.
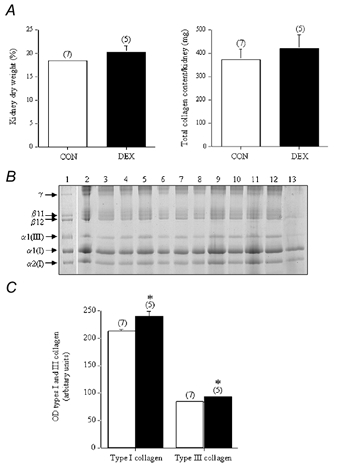
A, the total kidney dry weight, expressed as a percentage of the wet weight of tissue and total kidney collagen content (converted from the hydroxyproline values) from the control group (CON; □ n = 7) and dexamethasone-treated group of animals (DEX; ▪; n = 5). B, the pepsin-digested collagen (which represents the maturely cross-linked collagen) was analysed by SDS-PAGE, using delayed reduction of the disulphide bonds with 10% β-mercaptoethanol. The samples consist of a type I and type III collagen standard (from human dermis) (lane 1) and the pepsin digests of renal tissues from the CON (lanes 2–8) and the DEX group of animals (lanes 9–13). Type I collagen is normally composed of two α1(I) chains and one α2(I) chain, while type III collagen is composed of three α1(III) chains. β11 and β12 represent dimers of two μ1(I) chains and an α1(I)/α2(I) chain, respectively, while γ represents collagen trimers. C, the optical density (OD) of the type I and type III collagen chains (from the individual samples of kidneys taken from the CON (□;) and DEX (▪;) groups of animals was determined using a Bio-Rad GS-710 Calibrated Imaging Densitometer. The results from the individual samples were then grouped. *P < 0.05, when compared to OD values from the CON group.
The ovine kidney was predominantly composed of type I (α1(I) and α2(I) subunits) and type III collagen, while type V collagen was not detected (Fig. 3B). As determined by densitometry measurements of the collagen bands, the small increase in total renal collagen content in the DEX group (Fig. 3A) was reflected by significant increases in both type I collagen (240 ± 10 vs. 213 ± 4 in the CON group; P < 0.05) and type III collagen (93 ± 3 vs. 84 ± 1 in the CON group; P < 0.05) (Fig. 3C).
DISCUSSION
The finding that, in a long-gestation species, nephron number can be decreased by glucocorticoid treatment, at such an early stage of kidney development, suggests that there are critical periods for programming hypertension at much earlier stages than would have been predicted. It is worth pointing out that at the time of glucocorticoid treatment the future metanephric kidney contains no glomeruli at all. The human metanephros is in a similar stage of development towards the end of the second month of pregnancy (Moritz & Wintour, 1999). It is consistent with data showing that in the fetuses of protein-deprived pregnant rats an increased rate of apoptosis of metanephric mesenchyme cells can be detected by day 15 (Welham et al. 2002). It is also consistent with a number of other studies in rats and sheep in which decreased nephron number and subsequent adult hypertension have resulted from different perturbations of the maternal-fetal environment (Langley-Evans et al. 1999; Gilbert & Merlet-Benichou, 2000; Woods, 2000; Manning & Vehaskari, 2001; Ortiz et al. 2001; Moritz et al. 2002b). However, mere reduction in nephron number in the adult does not necessarily lead to hypertension (Brenner, 1987; Narkun-Burgess et al. 1993). Substantial changes were observed in late-gestation kidneys from early-dexamethasone-treated fetuses – increased maturity, and upregulation of angiotensinogen, and functional angiotensin II receptors (Moritz et al. 2002a). Compensatory increases in sodium channel expression, which can contribute to the final hypertension, were seen in rats (Bertram et al. 2001; Manning et al. 2002).
The important fact seems to be that there is a different outcome when reduction in nephron number occurs during nephrogenesis, rather than after nephrogenesis is complete. This suggests that it is not nephron number, per se, that is the critical factor in the development of hypertension, but the compensations made in the remaining kidney, if the reduction occurs during nephrogenesis.
Numerous studies have shown that donation of a kidney, by a healthy adult, does not necessarily result in deterioration of renal function and hypertension (Narkun-Burgess et al. 1993; Fehrman-Ekholm et al. 2001). Unilateral nephrectomy of the adult animal also does not necessarily result in hypertension (Chevalier, 1982). Unilateral nephrectomy during the period of active nephrogenesis, in both rats and sheep, results in the later development of hypertension (Woods, 2000; Moritz et al. 2002b). In a relatively recent study it was shown that children with congenital unilateral renal agenesis had elevated 24 h blood pressures compared with others who had had unilateral renal nephrectomy, after birth, for Wilm's tumour (Mei-Zahav et al. 2001).
Our second major finding was that low nephron number was associated with grossly enlarged and dilated proximal tubules and greater accumulation of collagen type I and type III in the tubular interstitium and periadventitia of renal cortical vessels. In these very same animals we have already demonstrated a higher collagen type I content in the heart (Dodic et al. 2001). Taken together, higher accumulation of collagen in both kidney and heart may have resulted from the structural changes in these two organs that have developed as a result of chronic non-treated hypertension.
The enlargement of the proximal tubules probably accounts, at least in part, for the observation that, in the hypertensive animals, the total kidney weights were the same although the nephron number was significantly reduced. It is also possible that medullary tubules were enlarged, but this was not investigated.
In summary, we have found a reduced nephron number in adult sheep, programmed to become hypertensive by exposure to excess glucocorticoid early in gestation. There was no difference in the sclerosis index, indicating that this probably resulted from a defect occurring during the period of nephrogenesis. It is worth emphasising that these animals were not growth-retarded at birth. Growth retardation in sheep has been reported to be accompanied by a reduced nephron number (Bains et al. 1996). However, higher accumulation of renal collagen (outside glomeruli) might be the result of long-standing hypertension. When taken in context with other data from humans and animals, in which hypertension resulted when nephrogenesis was impaired, it is highly suggestive that the kidney abnormality is an essential part of the aetiology of the subsequent hypertension. The most remarkable fact is that the timing of the glucocorticoid treatment is so early in development. It also explains, in part, why glucocorticoid treatment, in late gestation, when nephrogenesis is nearing completion, in sheep, does not result in subsequent hypertension (Moss et al. 2001).
More generally it is reasonable to suggest that any situation in which nephron number is reduced, during nephrogenesis, such as that occurring in the offspring of mothers with diabetes (Amri et al. 2001), or mild vitamin A deficiency (Lelievre-Pegorier et al. 1998), may also lead to ‘programmed’ hypertension. Thus the implications of the findings are not necessarily restricted to the effects of early prenatal stress.
The incidence of adult essential hypertension is 25 % of those aged 45 years and older, and in at least half of these people there are no well-established genetic or environmental risk factors (McKenzie et al. 1996; Smithies et al. 2000). Maybe some subtle influence on renal development, occurring in the second month of gestation, has this long-term result.
Acknowledgments
This work was supported by a Block Grant (No. 983001) from the National Health and Medical Research Council of Australia.
References
- Amri K, Freund N, Van Huyen JP, Merlet-Benichou C, Lelievre-Pegorier M. Altered nephrogenesis due to maternal diabetes is associated with increased expression of IGF-II mannose-6-phosphate receptor in the fetal kidney. Diabetes. 2001;50:1069–1075. doi: 10.2337/diabetes.50.5.1069. [DOI] [PubMed] [Google Scholar]
- Bains RK, Sibbons PD, Murray RD, Howard CV, Van Velzen D. Stereological estimation of the absolute number of glomeruli in the kidneys of lambs. Res Vet Sci. 1996;60:122–125. doi: 10.1016/s0034-5288(96)90005-3. [DOI] [PubMed] [Google Scholar]
- Bergman I, Loxley R. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal Chem. 1963;35:1961–1965. [Google Scholar]
- Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet, during pregnancy programmes altered expression of the GR and 11 β-HSD2: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142:2841–2853. doi: 10.1210/endo.142.7.8238. [DOI] [PubMed] [Google Scholar]
- Bertram JF. Counting in the kidney. Kidney Int. 2001;59:792–796. doi: 10.1046/j.1523-1755.2001.059002792.x. [DOI] [PubMed] [Google Scholar]
- Bertram JF, Young RJ, Spencer K, Gordon I. Quantitative analysis of the developing rat kidney: absolute and relative volumes and growth curves. Anat Rec. 2000;258:128–135. doi: 10.1002/(SICI)1097-0185(20000201)258:2<128::AID-AR2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Brenner BM. Nephron adaptation to renal injury or ablation. Am J Physiol. 1987;249:F324–337. doi: 10.1152/ajprenal.1985.249.3.F324. [DOI] [PubMed] [Google Scholar]
- Caspari PG, Newcomb M, Gibson K, Harris P. Collagen in the normal and hypertrophied human ventricle. Cardiovasc Res. 1977;11:554–558. doi: 10.1093/cvr/11.6.554. [DOI] [PubMed] [Google Scholar]
- Chevalier RL. Glomerular number and perfusion during normal and compensatory renal growth in the guinea pig. Pediatr Res. 1982;16:436–440. doi: 10.1203/00006450-198206000-00007. [DOI] [PubMed] [Google Scholar]
- Dodic M, Abouantoun T, O'Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension. 2002a;45:729–734. doi: 10.1161/01.hyp.0000036455.62159.7e. [DOI] [PubMed] [Google Scholar]
- Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour EM, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB J. 2002b;16:1017–1026. doi: 10.1096/fj.01-1045com. [DOI] [PubMed] [Google Scholar]
- Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci. 1998;94:149–155. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- Dodic M, Peers A, Coghlan JP, May CN, Lumbers ER, Yu Z-Y, Wintour EM. Altered cardiovascular hemodynamics and baroreceptor-heart rate reflex in adult sheep after prenatal exposure to dexamethasone. Clin Sci. 1999;97:103–109. [PubMed] [Google Scholar]
- Dodic M, Samuel C, Moritz K, Wintour EM, Morgan J, Grigg L, Wong J. Impaired cardiac functional reserve and left ventricular hypertrophy in adult sheep after prenatal dexamethasone exposure. Circ Res. 2001;89:623–629. doi: 10.1161/hh1901.097086. [DOI] [PubMed] [Google Scholar]
- Douglas-Denton R, Moritz KM, Bertram JF, Wintour EM. Compensatory renal growth after unilateral nephrectomy in the ovine fetus. J Am Soc Nephrol. 2002;13:406–410. doi: 10.1681/ASN.V132406. [DOI] [PubMed] [Google Scholar]
- Fehrman-Ekholm I, Duner F, Brink B, Tyden G, Elinder CG. No evidence of accelerated loss of kidney function in living kidney donors: results from a cross-sectional follow-up. Transplantation. 2001;72:444–449. doi: 10.1097/00007890-200108150-00015. [DOI] [PubMed] [Google Scholar]
- Gilbert T, Merlet-Benichou C. Retinoids and nephron mass control. Pediatr Nephrol. 2000;15:1137–1144. doi: 10.1007/s004670000385. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci. 1999;64:965–974. doi: 10.1016/s0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- Lelievre-Pegorier M, Vilar J, Ferrier ML, Moreau E, Freund N, Gilbert T, Merlet-Benichou C. Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int. 1998;54:1455–1462. doi: 10.1046/j.1523-1755.1998.00151.x. [DOI] [PubMed] [Google Scholar]
- McKenzie HS, Lawler EV, Brenner BM. Congenital oligonephropathy; the flaw in essential hypertension? Kidney Int. 1996;100:S30–34. [PubMed] [Google Scholar]
- Manning J, Vehaskari VM. Low birth weight associated adult hypertension in the rat. Pediatr Nephrol. 2001;16:417–422. doi: 10.1007/s004670000560. [DOI] [PubMed] [Google Scholar]
- Mei-Zahav M, Korzets Z, Cohen I, Kessler O, Rathaus V, Wolach B, Pomeranz A. Ambulatory blood pressure monitoring in children with a solitary kidney-comparison between unilateral renal agenesis and uninephrectomy. Blood Press Monit. 2001;6:263–267. doi: 10.1097/00126097-200110000-00007. [DOI] [PubMed] [Google Scholar]
- Moritz KM, Johnson K, Douglas-Denton R, Wintour EM, Dodic M. Maternal glucocorticoid treatment programs alterations in the renin-angiotensin system of the ovine fetal kidney. Endocrinology. 2002a;143:4455–4463. doi: 10.1210/en.2002-220534. [DOI] [PubMed] [Google Scholar]
- Moritz KM, Wintour EM. Functional development of the meso-and metanephros. Pediatr Nephrol. 1999;13:171–178. doi: 10.1007/s004670050587. [DOI] [PubMed] [Google Scholar]
- Moritz KM, Wintour EM, Dodic M. Fetal uninephrectomy leads to postnatal hypertension and compromised renal function. Hypertension. 2002 b;39:1071–1076. doi: 10.1161/01.hyp.0000019131.77075.54. [DOI] [PubMed] [Google Scholar]
- Moss TJ, Sloboda DM, Gurrin LC, Harding R, Challis JR, Newnham JP. Programming effects in sheep of prenatal growth restriction and glucocorticoid exposure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R960–970. doi: 10.1152/ajpregu.2001.281.3.R960. [DOI] [PubMed] [Google Scholar]
- Narkun-Burgess DM, Nolan CR, Norman JE, Page WF, Miler PL, Meyer TW. Forty-five year follow-up after uninephrectomy. Kidney Int. 1993;43:1110–1115. doi: 10.1038/ki.1993.156. [DOI] [PubMed] [Google Scholar]
- Nyengaard JR, Bendtsen TF. A practical method to count the number of glomeruli in the kidney as exemplified in various animal species. Acta Stereol. 1990;9:243–258. [Google Scholar]
- Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001;59:1663–1669. doi: 10.1046/j.1523-1755.2001.0590051663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel CS, Butkus A, Coghlan JP, Bateman JF. The effect of relaxin on collagen metabolism in the non-pregnant rat pubic symphysis: the influence of estrogen and progesterone in regulating relaxin activity. Endocrinology. 1996;137:3884–3890. doi: 10.1210/endo.137.9.8756561. [DOI] [PubMed] [Google Scholar]
- Smithies O, Kim H-S, Takahashi T, Edgell MH. Importance of quantitative genetic variations in the etiology of hypertension. Kidney Int. 2000;58:2265–2280. doi: 10.1046/j.1523-1755.2000.00411.x. [DOI] [PubMed] [Google Scholar]
- Sykes B, Puddle B, Francis M, Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976;72:1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Gomez DM. A principle for counting tissue structures on random structures. J Appl Physiol. 1962;17:343–348. doi: 10.1152/jappl.1962.17.2.343. [DOI] [PubMed] [Google Scholar]
- Welham SJM, Wade A, Woolf AS. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the star of rat metanephrogenesis. Kidney Int. 2002;61:1231–1242. doi: 10.1046/j.1523-1755.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- Wintour EM, Alcorn DA, Butkus A, Congiu M, Ernest L, Pompolo S, Potocnik S. Ontogeny of hormonal and excretory functon of the meso-and meta-nephros in the ovine fetus. Kidney Int. 1996;50:1624–1633. doi: 10.1038/ki.1996.478. [DOI] [PubMed] [Google Scholar]
- Woods LL. Fetal origins of adult hypertension; a renal mechanism? Curr Opin Nephrol Hypertens. 2000;9:419–425. doi: 10.1097/00041552-200007000-00014. [DOI] [PubMed] [Google Scholar]


