Abstract
We investigated the effect of increased plasma adrenaline on hormone-sensitive lipase (HSL) activity and extracellular regulated kinase (ERK) 1/2 phosphorylation during exercise. Seven untrained men rested for 20 min and exercised for 10 min at 60 % peak pulmonary oxygen uptake on three occasions: with adrenaline infusion throughout rest and exercise (ADR), with no adrenaline infusion (CON) and with adrenaline infusion commencing after 3 min of exercise (EX+ADR). Muscle samples were obtained at rest before (Pre, −20 min) and after (0 min) infusion, and at 3 and 10 min of cycling. Exogenous adrenaline infusion increased (P < 0.05) plasma adrenaline at rest during ADR, which resulted in greater HSL activity (Pre, 2.14 ± 0.10 mmol min−1 (kg dry matter (dm))−1; 0 min, 2.74 ± 0.20 mmol min−1 (kg dm)−1). Subsequent exercise had no effect on HSL activity. During exercise in CON, HSL activity was increased (P < 0.05) above rest at 3 min but was not increased further by 10 min. The infusion of exogenous adrenaline at 3 min of exercise in EX+ADR resulted in a marked elevation in plasma adrenaline levels (3 min, 0.57 ± 0.12 nM; 10 min, 10.08 ± 0.84 nM) and increased HSL activity by 25 %. HSL activity at 10 min was greater (P < 0.05) in EX+ADR compared with CON. There were no changes between trials in the plasma concentrations of insulin and free fatty acids (FFA) and the muscle contents of free AMP, all putative regulators of HSL activity. ERK1/2 phosphorylation increased at 3 min in CON and EX+ADR. Because HSL activity did not increase during exercise when adrenaline was infused prior to exercise (ADR) and because HSL activity increased when adrenaline was infused during exercise (EX+ADR), we conclude that (1) high adrenaline levels can stimulate HSL activity regardless of the metabolic milieu and (2) large increases in adrenaline during exercise, independent of changes in other putative regulators, are able to further stimulate the contraction-induced increase in HSL activity. The results also demonstrate that increased ERK 1/2 phosphorylation coincides with elevated HSL activity, indicating that ERK 1/2 may mediate the contraction-induced increase in HSL activity early in exercise.
Hormone-sensitive lipase (HSL) is the rate-limiting enzyme of intramuscular triacylglycerol (IMTG) hydrolysis in skeletal muscle. In contrast to the study of adipose HSL, there exists little information pertaining to the regulation of skeletal muscle HSL. Studies in rat soleus have demonstrated persistent activation of HSL by pharmacological levels of adrenaline (4.4 μM) and transient increases in HSL during maximal tetanic contractions (Langfort 1999, 2000).
A stimulatory effect of adrenaline on HSL has also been proposed in humans. A study conducted in adrenaline-deficient adrenalectomised humans suggested that adrenaline was essential for HSL activation, as HSL activity was not increased during exercise in the absence of adrenaline. However, when adrenaline was infused to match concentrations measured in healthy control subjects, HSL activity increased (Kjaer et al. 2000). In contrast to this interpretation, HSL activity increased after 1 min of low intensity exercise (30 % V̇O2, peak) before plasma adrenaline was increased (Watt et al. 2003b) and HSL activity was decreased late in prolonged exercise (2 h) despite large increases in plasma adrenaline (Watt et al. 2003a). These data suggest that adrenaline may not be necessary to increase skeletal muscle HSL activity during exercise in healthy subjects as other intramuscular and/or hormonal factors may regulate HSL activity. In addition, HSL has five known phosphorylation sites (Holm et al. 2000; Greenberg et al. 2001) and the order of, and the degree of phosphorylation at these sites, will ultimately determine the net activation of HSL. In contrast to the known stimulatory effect of adrenaline on adipose HSL, the role of adrenaline on skeletal muscle HSL activity is poorly defined due to the use of pharmacological adrenaline concentrations, isolated animal muscles and inappropriate methodological designs. In this regard, the primary purpose of this study was to examine the effect of increased plasma adrenaline on HSL activity in two situations, (1) during rest and subsequent exercise and (2) once exercise had already commenced.
Aside from adrenergic regulation of HSL, we have previously suggested that an intramuscular factor regulates the early activation of HSL (Watt et al. 2003b). One such candidate is the mitogen-activated protein kinase (MAPK) cascades, which are an intracellular network of proteins that can convert mechanical and biochemical stimuli elicited by muscle contraction into intracellular responses by phosphorylating and activating various cytoplasmic proteins (Sakamoto & Goodyear, 2002). A recent study conducted in adipocytes has shown that HSL is a downstream target of extracellular signal-regulated kinase (ERK) 1/2 (also known as p42/44), and that increased ERK1/2 activity is involved in HSL activation (Greenberg et al. 2001). ERK1/2 may be involved in the rapid upregulation of HSL activity in skeletal muscle. Muscle contraction in rodent skeletal muscle increases ERK1/2 phosphorylation (Goodyear et al. 1996; Aronson et al. 1997a) and most studies have shown that ERK1/2 is phosphorylated during moderate intensity exercise (≈70 % V̇O2, max) in human skeletal muscle (Aronson et al. 1997b; Widegren et al. 1998; Yu et al. 2001). Given that the intracellular mechanisms responsible for skeletal muscle HSL activation are not known, and because ERK1/2 has been shown to increase adipose tissue HSL activity, our second aim was to examine the effects of exercise on ERK1/2 phosphorylation and HSL activity both with and without ADR infusion at rest and early in exercise.
We hypothesised that, (1) adrenaline infusion would increase resting HSL activity and that exercise would have no further effect on HSL activity, (2) adrenaline infusion during exercise would increase HSL activity above exercise alone, (3) ERK phosphorylation would occur early in exercise and coincide with increased HSL activity and (4) ERK phosphorylation would not be influenced by adrenaline infusion.
METHODS
Subjects
Seven healthy males (22 ± 2 years, 74 ± 3 kg, mean ±s.e.m.) volunteered to participate in the experiment. Subjects were recreationally active and peak pulmonary oxygen uptake (V̇O2, max) averaged 44.1 ± 1.9 ml min−1 (kg body mass)−1. The experimental procedures and possible risks were explained both verbally and in writing and subjects provided their written informed consent. The human ethics committees of the University of Guelph and McMaster University approved the study, which was carried out in accordance with the Declaration of Helsinki.
Experimental protocol
Subjects visited the laboratory on four occasions. On the first visit subjects completed an incremental cycling test (Quinton Excalibur, Quinton Instruments, Seattle, USA) to exhaustion to determine their V̇O2, peak. Expired gases were collected on-line (Quinton Q-plex 1, Quinton Instruments) to determine O2 uptake. Subjects visited the laboratory 3 h after eating on three subsequent occasions and cycled for 10 min at 60 % V̇O2, peak. Subjects were instructed to eat the same carbohydrate rich meal (70 %) on the morning of each trial and to refrain from alcohol, caffeine and exercise for the 24 h preceding each trial. Trials were randomised, counterbalanced and separated by at least 1 week.
Upon arrival at the laboratory subjects were fitted with a heart rate monitor (Polar Electro) and then rested quietly on a bed. A Teflon catheter was inserted into one forearm vein for blood sampling and was kept patent by a constant saline (0.9 %, no heparin) infusion. A second catheter was inserted in the contralateral arm for adrenaline or saline infusion. A resting blood sample was obtained following 30 min of rest (−20 min), after which the vastus lateralis was prepared for percutaneous needle biopsies. A small incision was made through the skin and deep fascia under local anaesthesia (2 % lidocaine (lignocaine), no adrenaline). An incision was made for each biopsy and incisions were separated by at least 3 cm.
Subjects then commenced one of three trials. On one occasion adrenaline was infused for 20 min at rest and during 10 min of exercise (ADR). Muscle samples were obtained before adrenaline infusion (−20 min), immediately prior to exercise (0 min) and following 3 and 10 min of cycle exercise. On another occasion subjects rested quietly during 20 min of saline infusion, and then moved to the cycle ergometer and exercised for 3 min, after which adrenaline infusion commenced until the cessation of exercise at 10 min (EXER + ADR). Muscles samples were obtained at rest, and 3 and 10 min of exercise. The final trial acted as a control trial (CON). Subjects rested quietly during 20 min of saline infusion then moved to the cycle ergometer and cycled for 10 min with muscle samples obtained at 3 and 10 min. A resting sample was not obtained during this trial to minimise the total number of biopsies and thereby minimise stress to the subjects. Also, we have previously shown that resting HSL activity is consistent between days (Watt et al. 2003b). All muscle samples were rapidly frozen in liquid nitrogen until analysis. During all trials, blood samples and heart rate were obtained prior to and after the 20 min infusion period (0 min) and at 3 and 10 min of exercise.
Analysis
One portion of heparinised whole blood was immediately deproteinised 1:5 with 0.6 % (w/v) perchloric acid (HClO4), and centrifuged. The HClO4 supernatant was stored at −80 °C and subsequently analysed for blood glucose and lactate (Bergmeyer, 1974). A second portion of whole blood was centrifuged and the plasma removed for the determination of FFA by an enzymatic colorimetric method (Wako NEFA C test kit, Wako Chemicals, VA, USA) and insulin by radioimmunoassay (Coat-a-Count insulin test kit, Diagnostics Products, CA, USA). A final portion of blood (1.5 ml) was added to 30 μl of EGTA/GSH, mixed thoroughly and centrifuged. The supernatant was stored at −80 °C and subsequently analysed for plasma adrenaline by radioimmunoassay (Adrenaline RIA, Labor Diagnostika Nord, Germany).
Skeletal muscle was freeze dried (> 8 h), carefully dissected free of non-muscle contaminants under magnification and powdered. One aliquot of powdered muscle was used for the determination of HSL activity (Langfort et al. 1999) as modified by Watt et al. (2003b). Briefly, the powdered muscle was homogenised on ice in 20 volumes of homogenising buffer using a rotating Teflon pestle on glass. After centrifugation the supernatant was removed and stored on ice for immediate analysis of HSLa. A substrate consisting of 5 mM triolein, 14 × 106 d.p.m. [9,10-3H]triolein, 0.6 mg phospholipid (phosphatidylcholine/phosphatidylinositol 3:1 w/w), 0.1 M potassium phosphate and 20 % BSA was emulsified by sonication (Fredrikson et al. 1981; Osterlund et al. 1996). The muscle homogenate supernatant (14 μl) was incubated at 37 °C with enzyme dilution buffer (86 μl) and 100 μl of triolein substrate (Langfort, 1999). The reaction was stopped after 20 min by the addition of 3.25 ml of a methanol-chloroform-heptane (10:9:7 v/v/v) solution and 1.1 ml of 0.1 M potassium carbonate-0.1 M boric acid were added to facilitate the separation of the organic and aqueous phases. The mixture was vortexed and then centrifuged at 1100 g for 20 min, and 1 ml of the upper phase containing the released fatty acids was removed for subsequent determination of radioactivity on a beta spectrometer (Beckman LS 5000TA). All measurements were made in triplicate and the mean of these values is reported. This assay measures the activity of HSL against a triolein substrate and not total HSL content.
A second aliquot of powdered muscle was extracted in 0.5 M HClO4 (1 mM EDTA) and neutralised with 2.2 M KHCO3. The supernatant was removed and used for the determination of ATP, PCr, creatine and lactate by spectrophotometric assays (Bergmeyer, 1974; Harris et al. 1974). All muscle metabolite and enzyme activity values are normalised to the highest total creatine content obtained from the nine biopsies within subjects.
A third aliquot of powered muscle was used to determine the phosphorylation of ERK 1/2. Muscle was homogenised (Polytron; Brinkman Instruments, Westbury, NY, USA) in ice-cold buffer containing 50 mM Hepes, 150 mM NaCl, 10 mM NaF, 1 mM Na3VO4, 5 mM EDTA, 0.5 % Triton X-100, 10 % glycerol (v/v), 2 μg ml−1 leupeptin, 100 μg ml−1 phenylmethylsulfonyl fluoride, and 2 μg ml−1 aprotinin. Homogenates were centrifuged and the supernatant was removed and rapidly frozen in liquid nitrogen. The protein concentration of the muscle lysates was subsequently determined according to the Bradford method. To determine ERK1/2 phosphorylation, muscle lysates were solubilised in Laemmli sample buffer (1 % SDS, 6 mg ml−1 EDTA, 0.06 M Tris(hydroxymethyl)aminomethane, 2 mg ml−1 bromophenol blue, 15 % glycerol and 5 % β-mercaptoethanol) and boiled for 5 min, resolved by SDS-PAGE on 12 % polyacrylamide gels, transferred to a nitrocellulose membrane, blocked with 3 % BSA and immunoblotted with the phospho-ERK1/2 map kinase antibody (1:1000; New England Biolabs, Beverley, MA, USA). This antibody detects endogenous levels of ERK1/2 only when dually phosphorylated at Thr202/Tyr204. After incubation with horseradish peroxidase-conjugated secondary antibody (1:2000; Amersham Biosciences, Castle Hill, NSW, Australia), the immunoreactive proteins were detected with enhanced chemiluminescence (Perkin Elmer, Rowville, VIC, Australia) and quantified by densitometry. The dual phosphorylation of ERK1 and ERK2, which reflects activity, was not different in muscle that was analysed wet or following freeze-drying and dissection (data not shown). Thus, freeze-dried muscle is a suitable preparation for measuring ERK1/2 phosphorylation.
Calculations and statistics
Free ADP and AMP concentrations were calculated as previously described (Watt et al. 2003b). Statistical analysis was performed by two-way analysis of variance with repeated measures (time × trial), and specific differences were located using a Student-Newman-Keuls post hoc test. Statistical significance was set at P ≤ 0.05. Data are expressed as the means ±s.e.m.
RESULTS
Plasma adrenaline concentrations at rest and during exercise
Plasma adrenaline at rest averaged 0.38 ± 0.06 nM and was not different between trials (Fig. 1). As expected, plasma adrenaline was increased (P < 0.05) during ADR at rest and was also increased (P < 0.05) from 0 min during exercise in ADR. Plasma adrenaline was unaffected during 20 min rest with saline infusion (CON and EX+ADR) and was not increased by 3 min of exercise. Plasma adrenaline remained at resting levels throughout exercise in CON and exogenous adrenaline infusion in EX+ADR resulted in marked increases in plasma adrenaline by 10 min (CON, 0.64 ± 0.08 nM; ADR, 4.96 ± 0.28 nM; EX+ADR, 10.08 ± 0.84 nM).
Figure 1. Plasma adrenaline before and during exercise at 60 % J, peak with adrenaline infusion commencing after −20 min (ADR) or 3 min (EX+ADR).
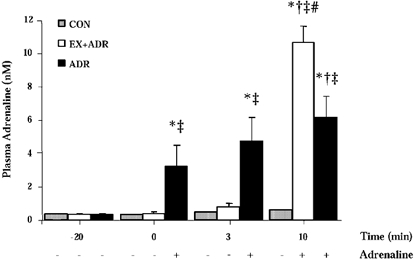
Values are means ±s.e.m., n = 7. * Significant difference from −20 min, † significant difference from 3 min of the same trial, ‡significant difference from CON at the same time point; # significant difference from ADR at the same time point, P < 0.05.
HSL activity at rest and during exercise
HSL activity at rest was not different between treatments (Fig. 2). HSL activity was increased (P < 0.05) by 28 % with adrenaline infusion at rest and no further increases were observed during exercise in ADR (Fig. 2). HSL activity was increased (P < 0.05) from rest by 3 min of exercise in CON and EX+ADR. No further increases were observed by 10 min in CON but adrenaline infusion in the EX+ADR trial increased (P < 0.05) HSL activity above CON (Fig. 2).
Figure 2. HSL activity before and during exercise at 60 % J, peak with adrenaline infusion commencing after −20 min (ADR) or 3 min (EX+ADR).
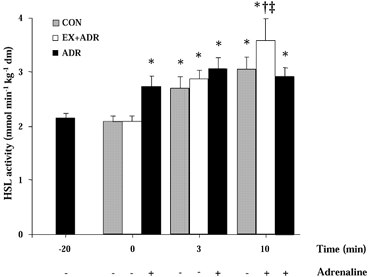
Values are means ±s.e.m., n = 7. * Significant difference from −20 min for ADR and 0 min for CON and EX+ADR, † significant difference from 3 min of the same trial, ‡significant difference from CON and ADR at the same time point, P < 0.05.
ERK 1/2 phosphorylation at rest and during exercise
Figure 3 shows a representative immunoblot from one subject's complete set of trials and the mean data plotted in figure form. Adrenaline infusion increased (P < 0.05) ERK1/2 phosphorylation 2-fold at rest in the ADR trial. ERK1/2 phosphorylation was further increased (P < 0.05) during exercise in ADR (Fig. 3). Exercise increased (P < 0.05) ERK1/2 phosphorylation at 3 min during CON and EX+ADR. ERK1/2 phosphorylation was further increased (P < 0.05) at 10 min in both trials, suggesting that increased adrenaline does not affect ERK1/2 phosphorylation after the commencement of exercise (Fig. 3). ERK1/2 phosphorylation was greater (P < 0.05) at 3 and 10 min in ADR compared with CON and EX+ADR.
Figure 3. ERK1/2 phosphorylation before and during exercise at 60 % V̇O2, peak with adrenaline infusion commencing after −20 min (ADR) or +3 min (EX+ADR).
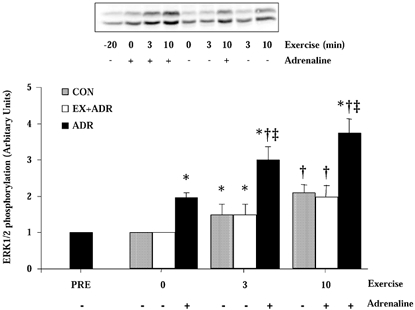
Top, representative immunoblot of phosphorylated ERK1 and ERK2 from human skeletal muscle biopsies at rest and during exercise, with (+) or without (−) exogenous adrenaline infusion. Below: ERK1/2 phosphorylation at rest and during 10 min exercise at 60 % V̇O2, peak without (CON) or with adrenaline infusion before and throughout exercise (ADR) or commencing after 3 min of exercise (EX+ADR). Values are means ±s.e.m., n = 6. * Significant difference from −20 min of same trial for ADR or 0 min for CON and EX+ADR; † significant difference from 0 and 3 min of the same trial; ‡ significant difference from corresponding time point for CON and EX +ADR, P < 0.05.
Muscle metabolite responses at rest and during exercise
Muscle contents of ATP were not different between trials at rest or during exercise (Table 1). Muscle phosphocreatine was decreased (P < 0.05) and lactate increased (P < 0.05) during exercise in all trials (Table 1). Accordingly, free AMP and free ADP contents were increased from rest during exercise (Table 1). There was no difference in muscle metabolite contents between CON and EX+ADR at all time points.
Table 1.
Muscle metabolite responses during 10 min excercise at 60% V̇O2, peak
| Trial | 0 min | 3 min | 10 min | |
|---|---|---|---|---|
| ATP | CON | — | 25.0 ± 0.6 | 24.7 ± 0.6 |
| EX + ADR | 25.1 ± 1.4 | 25.3 ± 1.4 | 25.0 ± 1.2 | |
| ADR | 24.8 ± 0.8 | 24.4 ± 1.3 | 25.5 ± 1.0 | |
| PCr | CON | — | 48.4 ± 6.1* | 48.7 ± 3.7* |
| EX + ADR | 85.7 ± 4.2 | 57.2 ± 3.6* | 58.2 ± 5.9* | |
| ADR | 82.3 ± 3.0 | 52.5 ± 3.7* | 48.3 ± 3.8* | |
| Lactate | CON | — | 24.2 ± 4.5* | 22.3 ± 2.7* |
| EX +ADR | 9.7 ± 0.9 | 22.5 ± 3.3* | 26.3 ± 3.0* | |
| ADR | 12.7 ± 1.1 | 23.3 ± 3.0* | 26.0 ± 3.5* | |
| Free AMp | CON | — | 1.57 ± 0.33* | 2.02 ± 0.41* |
| EX + ADR | 0.23 ± 0.04 | 1.40 ± 0.26* | 1.80 ± 0.31* | |
| ADR | 0.27 ± 0.04 | 2.06 ± 0.58* | 2.29 ± 0.27* | |
| Free ADP | CON | — | 245 ± 34* | 231 ± 29* |
| EX +ADR | 75 ± 5 | 174 ± 20* | 168 ± 22* | |
| ADR | 81 ± 7 | 202 ± 34* | 226 ± 16* |
Subjects performed trials without (CON) adrenaline infusion, with adrenaline infusion being initiated at 3 min of exercise (EX + ADR) or before and throughout (ADR) excerise. Values are means ±s.e.m., n = 7.
Significant difference from 0 min, P < 0.05.
Plasma substrate and hormone concentrations at rest and during exercise
Plasma glucose was not different between trials prior to the resting saline or ADR infusions (−20 min). Plasma glucose levels were increased (P < 0.05) by adrenaline infusion at rest in ADR and were further elevated after 10 min exercise (Table 2). Plasma glucose was unaltered by exercise in CON, but adrenaline infusion increased (P < 0.05) glucose at 10 min in EX+ADR. Plasma lactate was increased (P < 0.05) from rest during exercise and was not affected by adrenaline infusion (Table 2). Adrenaline was without effect on plasma insulin at rest (Table 2). Plasma insulin was decreased at 10 min in all trials and was unaffected by adrenaline infusion.
Table 2.
Plasma metabolite and hormone responses during 10 min exercise ar 60% V̇O2, peak
| Trial | −20 min | 0 min | 3 min | 10 min | |
|---|---|---|---|---|---|
| Glucose (mM) | CON | 4.1 ± 0.2 | 4.0 ± 0.3 | 4.1 ± 0.3 | 4.0 ± 0.3 |
| EX + ADR | 4.2 ± 0.3 | 4.4 ± 0.3 | 4.5 ± 0.3 | 4.6 ± 0.1*‡ | |
| ADR | 4.1 ± 0.2 | 4.6 ± 0.1* | 4.8 ± 0.3* | 5.1 ± 0.3*† | |
| Lactate (mM) | CON | 1.00 ± 0.21 | 1.03 ± 0.23 | 1.66 ± 0.18* | 3.50 ± 0.21*† |
| EX + ADR | 1.18 ± 0.18 | 0.91 ± 0.21 | 1.76 ± 0.29* | 4.22 ± 0.34*† | |
| ADR | 1.23 ± 0.16 | 1.36 ± 0.16 | 3.73 ± 0.41* | 5.54 ± 0.31*† | |
| Insulin (pM) | CON | 84.5 ± 32.9 | 75.0 ± 33.3 | 68.0 ± 31.5 | 35.1 ± 14.4* |
| EX + ADR | 69.8 ± 32.9 | 62.4 ± 28.5 | 41.8 ± 21.9 | 30.5 ± 9.2* | |
| ADR | 77.1 ± 27.5 | 60.1 ± 30.4 | 73.4 ± 27.3 | 54.8 ± 15.8* |
Subjects performed trials without (CON) adrenaline infusion, with adrenaline infusion being initiated at 3 min of exercise (EX + ADR) or before and throughout (ADR) excerise. Values are means ±s.e.m., n = 7.
Significant difference from −20 min
significant difference from 3 min
significant difference from corresponding time point in CON, p < 0.05.
DISCUSSION
Contraction and adrenaline have been shown to increase HSL activity in isolated rat skeletal muscle (Langfort et al. 1999, 2000); however, it is unknown whether these regulators act in synergy. In the present study we have demonstrated increased HSL activity with elevated adrenaline in resting human skeletal muscle, which was unaffected by the superimposition of muscle contraction. We have also demonstrated increased HSL activity in human skeletal muscle during moderate exercise, which was augmented by exogenous adrenaline infusion. These data suggest that skeletal muscle HSL is under dual control by contractions and adrenaline, and that the order of the stimuli reaching HSL may also important in determining enzymatic activity. In addition, we observed increased ERK1/2 phosphorylation at the onset of exercise that was associated with the rapid activation of HSL.
Consistent with previous work in rat skeletal muscle (Langfort et al. 1999), increasing adrenaline activated HSL at rest. From our understanding of adipose tissue lipolysis, it is likely that adrenaline increased cyclic AMP, resulting in activation of protein kinase A (PKA) and phosphorylation of HSL (for review see Langin et al. 1996). This mechanism has been demonstrated in rat skeletal muscle (Langfort et al. 1999). There is also the possibility that HSL activity at rest was increased by ERK activity. Activation of the ERK pathway in adipocytes phosphorylates HSL at Ser-600 and increases activity (Greenberg et al. 2001). ERK1/2 phosphorylation was increased concomitantly with HSL activity during adrenaline infusion at rest, indicating the possible involvement of ERK in β-adrenergic stimulation of HSL. Given that PKA and ERK phosphorylate HSL at Ser-563 and 600, respectively (Garton & Yeaman, 1990; Greenberg et al. 2001), the possibility arises that HSL is subject to multiple phosphorylation and parallel regulation during β-adrenergic stimulation at rest.
HSL activity was not augmented during exercise when plasma adrenaline concentrations were already high (i.e. ADR trial), which contrasts the finding of increased HSL activity during exercise when adrenaline infusion was commenced after the onset of exercise. The reason for the absence of change in HSL activity in the former condition is not readily apparent. Adrenaline infusion at rest may have activated HSL to near-maximal rates and further phosphorylation by other putative intramuscular and hormonal regulators associated with exercise may be without effect. In the case of the prior exercise followed by adrenaline infusion (i.e. EX+ADR trial), the exercise stimulus at 60 % V̇O2, peak may have been submaximal leaving room for adrenaline infusion to drive it higher. Alternatively, studies in adipose preparations suggest that phosphorylation of the serine sites on HSL are mutually exclusive such that phosphorylation of the ‘active’ Ser-563 site prevents phosphorylation of the ‘inactive’ Ser-565 site, and vice versa. (Garton & Yeaman, 1990). Although controversial, the present data support the suggestion that phosphorylation by PKA at Ser-563 may prevent subsequent phosphorylation of the other known sites (Ser-565, Ser-600), preventing changes in HSL activity (Anthonsen et al. 1998).
In a previous series of experiments conducted in the isolated rat soleus muscle, Langfort and co-workers (1999, 2000) demonstrated increased HSL activity during maximal tetanic contractions and pharmacological adrenaline administration. The authors reported HSL activation during contractions when β-adrenergic antagonists were added to the perfusion medium, suggesting parallel regulation by contraction-induced and adrenergic mechanisms. In contrast to these findings, a recent study conducted in adrenaline-deficient adrenalectomised patients demonstrated no change in HSL activity during moderate to heavy exercise. However, when adrenaline was infused during exercise, HSL activity increased to levels observed in aged-matched controls (Kjaer et al. 2000). These findings indicate that adrenaline was responsible for increased HSL activity during exercise in adrenalectomised patients but the study was not able to determine whether contractions or the increase in adrenaline was responsible for HSL activation during exercise in normal subjects. However, a recent study reported increased HSL activity in the absence of changes in plasma adrenaline during exercise at 30 % V̇O2, peak (Watt et al. 2003b). In addition, HSL increased within 1 min of exercise at power outputs of 30, 60 and 90 % V̇O2, peak, suggesting that activation occurred before adrenaline had time to accumulate in the blood. It is possible that a small increase in arterial plasma adrenaline, which may be observed in arterial blood after 3 min exercise at 60 % V̇O2, peak, may have exerted a permissive stimulatory effect on HSL activity. Evidently, such an effect would be missed when measuring venous adrenaline concentrations.
HSL activity increased at the onset of moderate exercise in the absence of increased plasma adrenaline during the CON and EX+ADR trials in the present study. The present data again suggests that effectors other than adrenaline are responsible for the increased HSL activity during exercise. However, when adrenaline was infused during exercise (EX+ADR), HSL was activated to levels above control, demonstrating that dual regulation of HSL by adrenaline and contraction is likely to occur, as previously suggested (Langfort et al. 2000). It is important to note that the plasma adrenaline levels observed at 10 min in EX+ADR are on the high end of the physiological spectrum and would not normally represent what occurs in a ‘normal’ exercise setting. The additive effect of exercise and adrenaline may also be explained by the presence of multiple HSL isoforms that are differentially regulated. If various isoforms existed, it would be expected that the effects of adrenaline and exercise would be additive, which evidently did not occur (Fig. 3). We have previously postulated that a contraction-related factor regulates the rapid activation of HSL at the onset of exercise (Watt et al. 2003a,b). In the present study we evaluated the possible role of ERK1/2 on HSL activity. ERK phosphorylates HSL in adipose tissue (Greenberg et al. 2001) and is elevated in skeletal muscle during muscle contraction in rodents (Goodyear et al. 1996; Aronson et al. 1997a; Ryder et al. 2000) and during exercise in man (Aronson et al. 1997b; Widegren et al. 1998; Yu et al. 2001). Although no study has previously investigated ERK1/2 phosphorylation in human skeletal muscle early in exercise, rodent studies reported increased ERK activity after 15 s of isometric contractions (Aronson et al. 1997a). We demonstrated concomitant increases in ERK1/2 phosphorylation and HSL activity at 3 min of exercise. Although temporal relationships do not prove causality, these are the first human data to indicate that ERK may be responsible, at least partly, for increased HSL activity at the onset of exercise. In this regard, HSL activity in contracting rat skeletal muscle was decreased when ERK activation was prevented by the blockade of MAPK kinase (MEK), an upstream activator of ERK (Donsmark et al. 2002).
Previous studies in skeletal muscle suggest that ERK activity is unaffected by β-adrenergic stimulation. Napoli et al. (1998) reported no change in ERK activity despite a 30-fold increase in serum adrenaline in resting rats. In human skeletal muscle, the activation of ERK1/2 occurs in only the contracting leg during single-legged exercise, suggesting that activation is related to local and not hormonal events (Aronson et al. 1997b; Widegren et al. 1998). However, adrenaline was not measured in these studies and plasma concentrations were likely to be low, which may preclude the observation of possible adrenergic effects. In the present study, we infused adrenaline to obtain high, yet physiological plasma concentrations (≈9 nM) and observed increased ERK1/2 phosphorylation in resting skeletal muscle (ADR), but no effect of adrenaline was demonstrated with exercise and adrenaline infusion (EX+ADR). The increased ERK1/2 at rest is consistent with a cyclic AMP-dependent mechanism observed in adipocytes (Shimizu et al. 1997; Lindquist et al. 2000), but is at odds with previous work in rat skeletal muscle (Napoli et al. 1998). These discrepant findings may be related to species and fibre type differences, or activation of ERK in control muscles during animal surgery. The absence of adrenergic effects on ERK during exercise in EX+ADR (Fig. 3) was unexpected in view of the ≈15-fold increase in adrenaline, and given that ERK1/2 phosphorylation increased during exercise in ADR despite modest increases in plasma adrenaline. Indeed, the effects of adrenaline and exercise on ERK1/2 phosphorylation during ADR appear to be additive, suggesting that cAMP-dependent and -independent regulators increase ERK in parallel. Further studies investigating the regulation of ERK during exercise are warranted.
β-Adrenergic stimulation has previously been shown to play an important role in skeletal muscle HSL activation. We have demonstrated a stimulatory effect of adrenaline on HSL activity when plasma concentrations were increased at rest and during exercise; however, adrenaline is without effect during exercise when elevated before the onset of exercise. These data support the possibility that HSL is controlled in parallel by β-adrenergic and contraction-related factors during exercise. The results also demonstrate that increased ERK 1/2 phosphorylation at the onset of exercise coincides with elevated HSL activity, indicating ERK 1/2 may mediate the contraction-induced increase in HSL activity early in exercise.
Acknowledgments
The authors would like to thank Marcus O'Neill for technical assistance and Drs Roger Fielding and Kirsten Howlett for assistance with the ERK1/2 analysis. This study was supported by grants from Natural Sciences and Engineering Research Council of Canada (L.L.S.) and the Canadian Institute for Health Research (G.J.F.H.).
REFERENCES
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- Aronson D, Dufresne SD, Goodyear LJ. Contractile activity stimulates the c-Jun NH2-terminal kinase pathway in rat skeletal muscle. J Biol Chem. 1997a;272:25636–25640. doi: 10.1074/jbc.272.41.25636. [DOI] [PubMed] [Google Scholar]
- Aronson D, Violan MA, Dufresne SD, Zangen D, Fielding RA, Goodyear LJ. Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. J Clin Invest. 1997b;99:1251–1257. doi: 10.1172/JCI119282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer HU. Methods in Enzymatic Analysis. New York: Academic Press; 1974. [Google Scholar]
- Donsmark M, Langfort J, Ploug T, Galbo H. Protein kinase C and a mitogen-activated kinase (MAPK) signalling pathway are involved in the contraction-induced activation of hormone-sensitive lipase in rat skeletal muscle. J Physiol. 2002;539.P:98P. [Google Scholar]
- Fredrikson G, Stralfors P, Nilsson NO, Belfrage P. Hormone-sensitive lipase from adipose tissue of rat. Methods Enzymol. 1981;71C:636–646. doi: 10.1016/0076-6879(81)71076-0. [DOI] [PubMed] [Google Scholar]
- Garton AJ, Yeaman SJ. Identification and role of the basal phosphorylation site on hormone-sensitive lipase. Eur J Biochem. 1990;191:245–250. doi: 10.1111/j.1432-1033.1990.tb19116.x. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Chang PY, Sherwood DJ, Dufresne SD, Moller DE. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol. 1996;271:E403–408. doi: 10.1152/ajpendo.1996.271.2.E403. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Shen WJ, Muliro K, Patel S, Souza SC, Roth RA, Kraemer FB. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J Biol Chem. 2001;276:45456–45461. doi: 10.1074/jbc.M104436200. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Holm C, Osterlund T, Laurell H, Contreras JA. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutr. 2000;20:365–393. doi: 10.1146/annurev.nutr.20.1.365. [DOI] [PubMed] [Google Scholar]
- Kjaer M, Howlett K, Langfort J, Zimmerman-Belsing T, Lorentsen J, Bulow J, Ihlemann J, Feldt-Rasmussen U, Galbo H. Adrenaline and glycogenolysis in skeletal muscle during exercise: a study in adrenalectomised humans. J Physiol. 2000;528:371–378. doi: 10.1111/j.1469-7793.2000.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Holm C, Galbo H. Stimulation of hormone-sensitive lipase activity by contractions in rat skeletal muscle. Biochem J. 2000;351:207–214. doi: 10.1042/0264-6021:3510207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Saldo M, Holm C, Galbo H. Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochem J. 1999;340:459–465. [PMC free article] [PubMed] [Google Scholar]
- Langin D, Holm C, Lafontan M. Adipocyte hormone-sensitive lipase: a major regulator of lipid metabolism. Proc Nutr Soc. 1996;55:93–109. doi: 10.1079/pns19960013. [DOI] [PubMed] [Google Scholar]
- Lindquist JM, Fredriksson JM, Rehnmark S, Cannon B, Nedergaard J. Beta 3- and alpha1-adrenergic Erk1/2 activation is Src- but not Gi-mediated in Brown adipocytes. J Biol Chem. 2000;275:22670–22677. doi: 10.1074/jbc.M909093199. [DOI] [PubMed] [Google Scholar]
- Napoli R, Gibson L, Hirshman MF, Boppart MD, Dufresne SD, Horton ES, Goodyear LJ. Epinephrine and insulin stimulate different mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Diabetes. 1998;47:1549–1554. doi: 10.2337/diabetes.47.10.1549. [DOI] [PubMed] [Google Scholar]
- Osterlund T, Danielsson B, Degerman E, Contreras JA, Edgren G, Davis RC, Schotz MC, Holm C. Domain-structure analysis of recombinant rat hormone-sensitive lipase. Biochem J. 1996;319:411–420. doi: 10.1042/bj3190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder JW, Fahlman R, Wallberg-Henriksson H, Alessi DR, Krook A, Zierath JR. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement of the mitogen- and stress-activated protein kinase 1. J Biol Chem. 2000;275:1457–1462. doi: 10.1074/jbc.275.2.1457. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Goodyear LJ. Invited review: intracellular signalling in contracting muscle. J Appl Physiol. 2002;93:369–383. doi: 10.1152/japplphysiol.00167.2002. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Tanishita T, Minokoshi Y, Shimazu T. Activation of mitogen-activated protein kinase by norepinephrine in brown adipocytes from rats. Endocrinology. 1997;138:248–253. doi: 10.1210/endo.138.1.4832. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Heigenhauser GJF, O'Neill M, Spriet LL. Hormone sensitive lipase activity and fatty acyl CoA content in human skeletal muscle during prolonged exercise. J Appl Physiol. 2003a doi: 10.1152/japplphysiol.01181.2002. DOI: 10.1152/japplphysiol.01181.2002. [DOI] [PubMed] [Google Scholar]
- Watt MJ, Heigenhauser GJF, Spriet LL. Effects of dynamic exercise intensity on the activation of hormone-sensitive lipase in human skeletal muscle. J Physiol. 2003b;547:301–308. doi: 10.1113/jphysiol.2002.034595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widegren U, Jiang XJ, Krook A, Chibalin AV, Bjornholm M, Tally M, Roth RA, Henriksson J, Wallberg-Henriksson H, Zierath JR. Divergent effects of exercise on metabolic and mitogenic signaling pathways in human skeletal muscle. FASEB J. 1998;13:1379–1389. doi: 10.1096/fasebj.12.13.1379. [DOI] [PubMed] [Google Scholar]
- Yu M, Blomstrand E, Chibalin AV, Krook A, Zierath JR. Marathon running increases ERK1/2 and p38 MAP kinase signalling to downstream targets in human skeletal muscle. J Physiol. 2001;536:273–282. doi: 10.1111/j.1469-7793.2001.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HH, Halbleib M, Ahmad F, Manganiello VC, Greenberg AS. Tumor necrosis factor-alpha stimulates lipolysis in differentiated human adipocytes through activation of extracellular signal-related kinase and elevation of intracellular cAMP. Diabetes. 2002;51:2929–2935. doi: 10.2337/diabetes.51.10.2929. [DOI] [PubMed] [Google Scholar]


