Abstract
Ghrelin is a newly discovered orexigenic peptide originating from the stomach. However, its action in regulating the fed and fasted motor activity of the digestive tract is not fully understood. In the present study, we examined the effects of intracerebroventricular (i.c.v.) and intravenous (i.v.) injection of ghrelin on the physiological fed and fasted motor activities in the stomach and duodenum of freely moving conscious rats. i.c.v. and i.v. injection of ghrelin induced fasted motor activity in the duodenum in normal fed rats, while i.v. injection of ghrelin induced fasted motor activity in both the stomach and duodenum in vagotomized rats. The effects of i.c.v. and i.v. injected ghrelin were blocked by growth hormone secretagogue receptor (GHS-R) antagonist given by the same route and also blocked by immunoneutralization of neuropeptide Y (NPY) in the brain. The effects of i.v. injected ghrelin were not altered by i.c.v. injection of GHS-R antagonist in vagotomized rats. Injection of GHS-R antagonist blocked the fasted motor activity in both the stomach and duodenum in vagotomized rats but did not affect the fasted motor activity in normal rats. Low intragastric pH inhibited the effect of ghrelin. The present results indicate that ghrelin is involved in regulation of fasted motor activity in the stomach and duodenum. Peripheral ghrelin may induce the fasted motor activity by activating the NPY neurons in the brain, probably through ghrelin receptors on vagal afferent neurons. Once the brain mechanism is eliminated by truncal vagotomy, ghrelin might be primarily involved in the regulation of fasted motor activity through ghrelin receptors on the stomach and duodenum. The action of ghrelin to induce fasted motor activity is strongly affected by intragastric pH; low pH inhibits the action.
Ghrelin is an endogenous ligand for the growth hormone secretagogue receptor (GHS-R) and a novel orexigenic peptide isolated from the stomach (Kojima et al. 1999). The feeding stimulatory action of ghrelin induced by central or peripheral administration has been well documented previously (Asakawa et al. 2000; Wren et al. 2000; Inui 2001; Nakazato et al. 2001; Shintani et al. 2001). The plasma level of ghrelin is elevated before meals (Cummings et al. 2001) and suppressed by glucose ingestion (Djurhuus et al. 2002). The release of ghrelin from the stomach is stimulated by such peptides as gastrin and cholecystokinin (Murakami et al. 2002). In addition to the feeding stimulatory action, ghrelin exerts peripheral actions such as stimulating gastric acid secretion (Date et al. 2000a; Masuda et al. 2000) and increasing gastric motility (Masuda et al. 2000). However its action in the regulation of fasted motor activities of the gastrointestinal tract is not fully understood. Previous studies have shown that feeding behaviour and the physiological fed (postprandial) and fasted (interdigestive) motor activities in the digestive tracts are tightly related. For example neuropeptide Y (NPY), a powerful orexigenic peptide in the brain, induces fasted motor activity when it is given intracerebroventricularly (I.C.V.) (Fujimiya et al. 2000). On the other hand, feeding inhibitory peptides such as corticotrophin releasing factor (CRF)/urocortin, cholecystokinin (CCK) and bombesin cause the disruption of fasted motor activity when they are given I.C.V. or intravenously (I.V.) (Rodriguez-Membrilla et al. 1996; Hashmonai & Szurszewski, 1998; Kihara et al. 2001). The effect of NPY on gastrointestinal motility is attributed to the NPY neurons in the brain but not to the peripheral NPY neuron (Fujimiya et al. 2000). Ghrelin primarily originates from the endocrine cells in the stomach (Date et al. 2000b; Tomasetto et al. 2000) and a small amount exists in the arcuate nucleus of the hypothalamus (Kojima et al. 1999). The localization of ghrelin receptors (GHS-R) has also been investigated previously. An in situ hybridization study showes that GHS-R and NPY were colocalized in the arcuate nucleus of the hypothalamus (Willesen et al. 1999), and ghrelin stimulated the food intake through Y1 receptors in the brain (Asakawa et al. 2000). A recent study has shown that peripheral ghrelin does not enter the brain in rodents, while human ghrelin enters the brain across the blood- brain barrier (Banks et al. 2002). Involvement of vagal afferent nerves in mediating the action of peripheral ghrelin on the brain NPY neurons seems highly possible, because a recent study has shown the localization of ghrelin receptor mRNA on vagal afferent neurons by in situ hybridization (Date et al. 2002).
Ghrelin has a structural resemblance to motilin, and the ghrelin receptor exhibits a 50 % identity with the motilin receptor (Asakawa et al. 2000). Motilin originates from the endocrine cells in the duodenum and induces the fasted motor pattern in the gastrointestinal tract (Itoh, 1997). However motilin induces the fasted motility when it is given peripherally but not when it is given centrally (Hashmonai et al. 1987), despite the fact that motilin given centrally stimulates food intake (Asakawa et al. 1998). Although both NPY and motilin stimulate food intake, their sites of action in inducing fasted motor activity in the digestive tracts seem to differ; NPY acts centrally but motilin acts peripherally.
In the present study we investigated the effect of I.C.V. and I.V. injection of ghrelin on the physiological fed and fasted motor activity of the gastrointestinal tract by performing experiments on conscious rats (Fujimiya et al. 2000; Kihara et al. 2001). To examine the site of action of ghrelin in affecting the motor activity of the digestive tract, GHS-R antagonist with or without truncal vagotomy or surgical sympathectomy was applied combined with ghrelin.
METHODS
Animal experiments
Male Wistar rats weighing 200–250 g at the initial period of the experiment were used. Care of animals was conducted in accordance with the Guide for Use of Experimental Animals of Shiga University of Medical Science, which was standardized to Japanese national guidelines. Rats were housed individually under controlled temperature (21–24 °C) and light (lights on 8:00–20:00) conditions with free access to water and laboratory chow pellets (CE-2; Clea, Japan).
Animal preparation
The rats were anaesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg (kg body wt)−1 Nembutal; Abbott Laboratories, North Chicago, IL, USA), placed in a stereotaxic apparatus, and implanted with a guide cannula (25-gauge; Eicom, Kyoto, Japan), which reached the right lateral ventricle. Stereotaxic co-ordinates were 0.8 mm posterior to bregma, 2.0 mm right lateral to the midline, and 4.5 mm below the outer surface of the skull using a Kopf stereotaxic frame (Tujunga, CA, USA) with the incisor bar set at the horizontal plane passing through bregma and lambda. The guide cannula was secured with dental cement anchored by two stainless steel screws fixed on the dorsal surface of the skull. After surgery, a dummy cannula (Eicom) was inserted into each guide cannula, and a screw cap (Eicom) was put on the guide cannula to prevent blockade. The animals were allowed to recover for at least 4 days after this operation. On the day of the experiment, the dummy cannula was replaced by a microinjection cannula (AMI-5; Eicom) connected to a polyethylene tube (PE-50, Clay Adams, Parsippany, USA). The placement of the cannula was verified at the end of the experiment by injection of 10 μl dye (0.05 % cresyl violet) and examination of the brain slice.
At 4–6 days after the brain operation, rats were deprived of food and given free access to water for 18 h before an abdominal operation. They were anaesthetized with pentobarbital sodium (I.P. 50 mg kg−1), and a motility recording device was implanted as follows. Two manometric catheters (3-Fr, 1 mm diameter, ATOM, Tokyo, Japan) with side holes were inserted through the fistula in the gastric body and the tips placed at the gastric antrum and 3 cm distal to the pylorus. Catheters were fixed to the gastric wall by purse-string sutures, which ran subcutaneously to emerge at the top of the neck and were secured at the animal's skin. In some animals, a catheter (3-Fr, 1 mm diameter) was placed in the right jugular vein. This ran subcutaneously to emerge at the top of the neck beside the manometric catheter, and used for I.V. administration of peptides. The catheter was filled with heparinized saline to prevent obstruction. During the first postoperative day, the animals were allowed water but no food, and allowed to recover for 1 week before the experiment measuring the gut motility. Animals were fasted for 18 h before the start of the experiment.
I.C.V. and I.V. injection of peptides
Ghrelin (Rat, Peptide Institute, Osaka, Japan) doses of 0.01 μg (0.003 nmol), 0.1 μg (0.03 nmol) and 1 μg (0.3 nmol) were dissolved in saline in a 10 μl volume for I.C.V. injection and doses of 0.1, 1 and 10 μg (3 nmol) were dissolved in saline in a 0.3 ml volume for I.V. injection. The doses of ghrelin were determined according to the effective doses to stimulate food intake (Wren et al. 2000; Masuda et al. 2000; Shintani et al. 2001). A dose of 1 nmol of GHS-R antagonist, (D-Lys3)GHRP-6 (Bachem California, Inc., USA) was injected I.C.V. with or without injection of ghrelin and 100 nmol of (D-Lys3)GHRP-6 was injected I.V. with or without injection of ghrelin. Vehicle control was made by I.C.V. or I.V. injection of saline. For immunoneutralization of brain NPY, 5 μl of anti-NPY antiserum (Inui et al. 1990) or normal rabbit serum plus 5 μl of saline was injected I.C.V. 5 min before ghrelin.
Measurement of gastroduodenal motility
Gastroduodenal motility was measured in conscious, freely moving rats by a manometric method. On the day of the experiment, the manometric catheter was connected to a pressure transducer (TP-400T; Nihon Koden Kogyo, Tokyo, Japan); the catheter was protected from being bitten by a flexible metal sheath and connected to an infusion swivel (dual type, 20-gauge; Instech Laboratories, Plymouth Meeting, PA, USA) to allow free movement. The catheter was continuously infused with bubble-free 0.9 % saline at a rate of 1.5 ml h−1 by a low-compliance capillary infusion system using a heavy-duty pump (CVF-3100; Nihon Koden). The data were recorded on a polygraph (RM-6100; Nihon Koden) and stored in a MacLab system (MacLab/8e, AD Instruments Ply, Power Book 1400cs; Apple Computer). Results are expressed as means ± S.D. At the end of the experiments measuring gut motility, animals were killed by intraperitoneal injection of excess doses of pentobarbital.
Experimental design
The experiments were performed on animals in the fasted or fed state, the latter being achieved by giving one piece of laboratory chow (4 g) to the fasted animals, and checking that it was eaten completely. After the gastroduodenal motility was ascertained to have changed to the fed motor pattern for 30 min in normal rats or 40 min (in vagotomized rats), I.C.V. or I.V. injection of peptides was performed. GHS-R antagonist or anti-NPY antiserum, was injected 5 min before ghrelin.
Truncal vagotomy and surgical sympathectomy
Involvement of autonomic nerves with the effects of ghrelin was examined, because fasted motor activity in the gastrointestinal tracts is under the regulation of the autonomic nervous system (Marik et al. 1975, Marlett & Code 1979). Four to six days after the brain operation, truncal vagotomy or surgical sympathectomy was performed as follows. The rats were anaesthetized with sodium pentobarbital (50 mg (kg body wt)−1). After a midline incision of the abdominal wall, the lower part of the oesophagus was exposed and anterior and posterior branches of the vagal nerve were incised above the hepatic and coeliac branches. In sham-operated rats, the vagal trunks were similarly exposed but not cut. For surgical sympathectomy, the roots of the coeliac and superior mesenteric arteries were exposed and the prevertebral ganglia between these arteries were completely removed. In sham-operated rats, the superior and mesenteric ganglia were similarly exposed but not removed. Two manometric catheters and an I.V. injection catheter were placed as mentioned above. Vagotomized and sham-operated rats were fed liquid diets for 4 days after the operation; they were then given laboratory chow; sympathectomized rats were always given normal laboratory chow. Measurement of gastroduodenal motility was performed 1 week after these operations. The completeness of vagotomy and sympathectomy was confirmed by morphological studies for cholinergic and noradrenergic nerve fibres in the gastrointestinal tract. For cholinergic nerve fibres, vagotomized animals were fixed for enzyme histochemistry and loss of acetylcholine esterase-positive fibres in the gastrointestinal tract was determined by light microscopic observation.
Measurement of intragastric pH
Rats were deprived of food but given free access to water for 18 h before the experiment. The rats were anaesthetized with sodium pentobarbital (I.P.; 50 mg (kg body wt)−1) and midline incisions were made in their abdominal walls. The lower parts of the oesophagus and pylorus were exposed and quickly ligated, then a catheter was inserted through the gastric fistula with the tip placed at the gastric antrum. The ligature around the oesophagus and pylorus was then removed. The intragastric fluid contents were collected through the catheter and the pH was measured by pH test paper (Tokyo, Japan). Measurement of pH was performed in the fasting state after 16 h starvation and at 30 and 60 min after the start of feeding of one piece of laboratory chow (4 g). The pH values of these three different points were analysed in rats with and without I.P. administration of H2 blocker, famotidine (Gaster, Yamanouchi Pharmaceutical Co., Tokyo, Japan) at a dose of 2 mg (kg body wt)−1, 2 h before the start of the experiment.
Statistical analysis
As the amplitude of pressure waves varied considerably between animals, the drug effects were evaluated by changes in the frequency of the phase III-like contractions in the fasted motor activated rats and the percentage motor index (% MI) was measured in the fed motor activated rats both with drug-injected and saline-injected controls. Phase III-like contractions were defined as clusters of strong contractions with an amplitude of more than 10 cmH2O (Fujimiya et al. 2000). The frequency of the fasted pattern was obtained from the average of the onset of the phase III-like contractions per hour as shown by arrowheads in the figures. MI was defined as the summation of the amplitude of contractions per minute. Values of % MI were calculated as 100 × (mean MI for 20 min after drug infusion)/(mean MI for 20 min after vehicle infusion) (Yamamoto et al. 1999). In the experiments of single or combined use of I.C.V. injection of ghrelin, a 20 min period was chosen immediately after ghrelin injection, while in the experiments of I.V. injection of ghrelin, a 20 min period was chosen after the time interval shown in Table 2. Results in Tables are expressed as means ± S.D., however, results in Figures are expressed as means ±s.e.m. Statistical analyses of the data were performed by Student's paired t test. One-way analysis of variance (ANOVA) and Dunn's post hoc test were used to test the significance of differences among groups. Values of P < 0.05 were considered statistically significant.
Table 2.
Time intervals between injection and initiation of phase III-like contractions in the duodenum (min)
| i.v. Ghrelin (Fig. 1B) | 29.6 ± 4.9a | (n = 3) |
| i.c.v. GHS-R antagonist +i.v. ghrelin (Fig. 3B) | 28.3 ± 4.6 | (n = 3) |
| i.v. Ghrelin in vagotomized rats (Fig. 4B) | 9.0 ± 1.6** | (n = 3) |
| i.v. Gherlin in sympathectomized rats (Fig. 7A) | 33.1 ± 7.3 | (n = 3) |
| i.v. Gherlin in famotidine-treated rats (Fig. 10) | 1.9 ± 0.2** | (n = 3) |
Values are means ±s.d.
P < 0.01 compared witha. Figure number shows a representative trace.
RESULTS
Effects of I.C.V. and I.V. inj ection of ghrelin on gastroduodenal motility
In animals in the fasted state, cyclic changes of pressure waves were detected in both the antrum and duodenum by the manometric method (Fujimiya et al. 2000), including a quiescence period (phase I-like contractions) followed by a grouping of strong contractions (phase III-like contractions; Fig. 1A and B). The frequency of the phase III-like activity in the antrum was 5.3 ± 0.5 h−1 (n = 6) and that in the duodenum was 5.6 ± 0.8 h−1 (n = 6) (Table 1). After food intake, the fasted motor pattern was immediately disrupted and replaced by the fed motor pattern, which consisted of irregular contractions of high frequency: 3.8 ± 0.6 min−1 (n = 10) in the antrum and 1.8 ± 0.6 min−1 (n = 10) in the duodenum (Fig. 1A and B).
Figure 1.
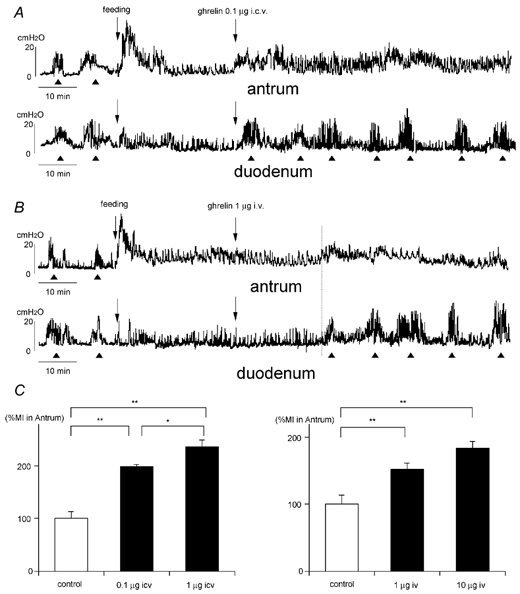
A and B, effects of intracerebroventricular (i.c.v., A) and intravenous (i.v., B) injection of ghrelin on the fed motor activity of the antrum and duodenum. The fasted motor patterns in the antrum and duodenum are disrupted by food intake and replaced by the fed motor pattern. The phase III-like contractions of fasted motility are indicated by arrowheads. i.c.v. injection of ghrelin induces the fasted pattern in the duodenum and increases the motor activity in the antrum (A). i.v. In B, injection of ghrelin causes the same effects, but they appear in about 30 min after i.v. injection. C, comparison of %MI in the antrum induced by i.c.v. and i.v. injection of ghrelin. *P < 0.05; ** P < 0.01.
Table 1.
Frequency of phase III-like contractions per hour
| Antrum | Duodenum | |
|---|---|---|
| Fasted pattern in normal rats | 5.3 ± 0.5 (n = 6) | 5.6 ± 0.8 (n = 6) |
| i.c.v. Ghrelin given in fed state (Fig. 1A) | Fed pattern remains | 6.7 ± 1.6 (n = 6) |
| i.v. Ghrelin given in fed state (Fig. 1B) | Fed pattern remains | 6.8 ± 1.3 (n = 6) |
| i.c.v. Gherlin given fastes state (Fig. 2A) | 12.7 ± 1.2**(n = 3) | 11.7 ± 0.6**(n = 3) |
| i.v. Gherlin given fastes state (Fig. 2B) | 10.3 ± 0.6**(n = 3) | 9.3 ± 1.2 (n = 3) |
| i.c.v. GHS-R antagoinst +i.v. ghrelin (Fig. 3B) | Fed pattern remains | 7.7 ± 0.6 (n = 3) |
| Fasted pattern after vagotomy (Fig. 4A and B) | 15.3 ± 2.0* (n = 6) | 9.7 ± 1.9**(n = 6) |
| i.v. Gherlin in vagotomized rats (Fig. 4B) | 15.0 ± 1.7**(n = 3) | 11.3 ± 1.2**(n = 3) |
| i.c.v. GHS antagonist in fasted state (Fig. 5A) | 5.3 ± 0.6 (n = 3) | 6.3 ± 0.6 (n = 3) |
| i.c.v. GHS antagonist in vagotomized state (Fig. 5B) | 11.7 ± 2.1**(n = 3) | 9.7 ± 2.9**(n = 3) |
| i.v. GHS antagonist in fasted state (Fig. 6A) | 5.3 ± 1.5 (n = 3) | 5.3 ± 0.5 (n = 3) |
| Fasted pattern after sympathectomy (Fig. 7A) | 5.7 ± 1.2 (n = 3) | 6.0 ± 1.2 (n = 3) |
| i.v. Gherlin in sympathectomized rats (Fig. 7A) | Fed pattern remains | 7.3 ± 1.2 (n = 3) |
| i.v. Gherlin in famotidine-treated rats (Fig. 10) | Fed pattern remains | 6.7 ± 1.5 (n = 3) |
Values are means ±s.d.
P < 0.01 compared with values in normal rats. Figure number shows a representative trace.
When I.C.V. injection of ghrelin (0.1 and 1 μg) was given to animals in the fed state, the fed motor pattern remained in the antrum but the % MI was significantly increased compared with I.C.V. injection of saline (Fig. 1A and C), while the fed motor pattern in the duodenum disappeared and the fasted motor pattern appeared immediately after I.C.V. injection of ghrelin (Fig. 1A); this pattern consisted of phase III-like contractions with a frequency of 6.7 ± 1.6 h−1 (n = 6; Table 1). When I.V. injection of ghrelin (1 μg and 10 μg) was given to animals in the fed state, the fed motor pattern remained in the antrum followed by an increase of % MI (Fig. 1B and C) within 29.6 ± 4.9 min (n = 3) after the I.V. injection, while the fed motor pattern in the duodenum was replaced by the fasted pattern within 29.6 ± 4.9 min (n = 3) after I.V. injection (Fig. 1B, Table 2); this consisted of phase III-like contractions with a frequency of 6.8 ± 1.3 h−1 (n = 6) (Table 1).
With I.C.V. injection of ghrelin (0.1 and 1 μg) to animals in the fasted state, the fasted motor pattern remained, however the frequency of phase III-like contractions was significantly increased in both the antrum (12.7 ± 1.2 h−1, n = 3) and duodenum (11.7 ± 0.6 h−1, n = 3) for 26.3 ± 2.3 min (n = 3) compared to those in normal animals (Fig. 2A; Table 1). The same phenomenon was observed when ghrelin (1 and 10 μg) was injected I.V. to animals in the fasted state (Fig. 2B). Here the frequency of phase III-like contractions was changed to 10.3 ± 0.6 h−1 (n = 3) in the antrum and 9.3 ± 1.2 h−1 (n = 3) in the duodenum for 14.6 ± 2.3 min (n = 3; Table 1).
Figure 2. Effects of I.C.V. (A) and i.v. (B) injection of ghrelin on the fasted motor activities of the antrum and duodenum.
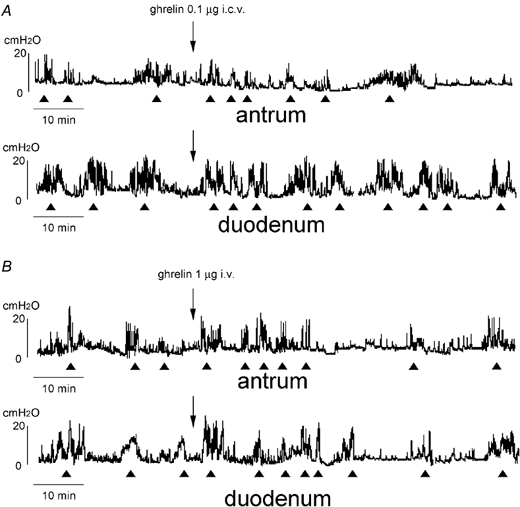
The fasted motor patterns remain but the frequency of phase III-like contractions increases for about 15–25 min after injection of i.c.v. (A) and i.v. (B) injection of ghrelin.
To examine whether the effect of I.V.injection of ghrelin on motor activity was mediated by GHS-Rs located in the brain or periphery, GHS-R antagonist was injected either I.C.V. or I.V. before ghrelin. Changes in the motor pattern induced by I.V. injection of ghrelin seen in Fig 1B and C were blocked by the I.V. injection of GHS-R antagonist (Fig. 3A and C). However changes in the motor pattern induced by I.V. injection of ghrelin were not altered by I.C.V. injection of GHS-R antagonist (Fig. 3B and C). On the contrary, changes in the motor pattern induced by I.C.V. injection of ghrelin seen in Fig 1A and C were blocked by I.C.V. injection of GHS-R antagonist, but not altered by I.V. injection of GHS-R antagonist (data not shown).
Figure 3.
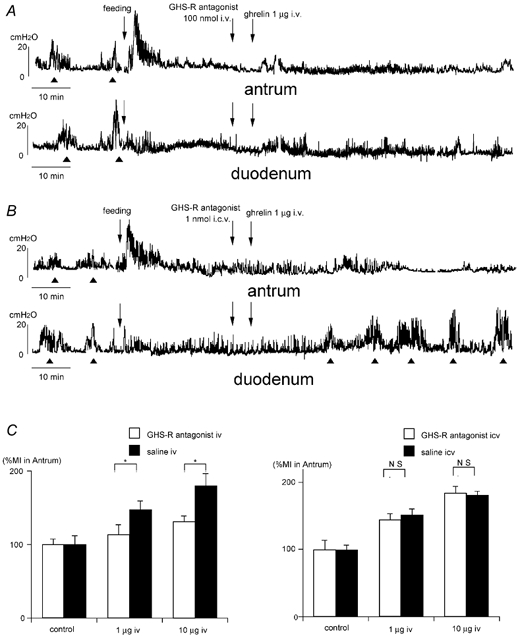
A and B, effects of i.v. (A) and i.c.v. (B) injection of GHS-R antagonist on the action of ghrelin injected i.v. in the fed animals. i.v. injection of GHS-R antagonist completely blocks the effect of i.v. injection of ghrelin seen in Fig. 1B (A), however i.c.v. injection of GHS-R antagonist does not alter the effect of i.v. injection of ghrelin seen in Fig. 1B (B). C, comparison of %MI in the antrum induced by GHS-R antagonist (i.v.) + ghrelin (i.v.) and GHS-R antagonist (i.c.v.) + ghrelin (i.v.). Data are compared to saline injected controls. *P <0.05.
Involvement of autonomic pathways and the site of action of ghrelin
We examined the involvement of the vagal pathway in the effects of ghrelin on gastrointestinal motility using rats that had received truncal vagotomy (Table 3). In vagotomized animals, the fasted motor pattern was replaced by the fed motor pattern within 7.5 ± 0.7 min (n = 3)of food intake in the antrum and in 38.5 ± 1.8 min (n = 3)of food intake in the duodenum (Fig. 4A and B). The frequency of the phase III-like contractions in the antrum (15.3 ± 2.0 h−1, n = 6) and that in the duodenum (9.7 ± 1.9 h−1, n = 6) in vagotomized animals was significantly higher than that of the antrum (5.3 ± 0.5 h−1, n = 6) and the duodenum (5.6 ± 0.8 h−1, n = 6) in normal animals (Table 1). Ghrelin was injected I.C.V. or I.V. when the fed pattern was observed in the antrum and duodenum in vagotomized animals (Fig. 4A and B). Changes in gastroduodenal motility induced by I.C.V. injection of ghrelin seen in Fig 1A and C were completely blocked by truncal vagotomy (Fig. 4A and C). On the other hand, changes in duodenal motility induced by I.V. injection of ghrelin seen in Fig. 1B were not altered by truncal vagotomy (Fig. 4B). More interestingly, I.V. injection of ghrelin induced fasted motor patterns in the antrum in vagotomized animals (Fig. 4B).
Table 3.
Body weight change
| Before the operation (g) | On the day of experiments (g) | |
|---|---|---|
| Control rats | 244.8 ± 4.9a | 238.8 ± 2.0 |
| Vagotomized rats | 241.7 ± 2.0 | 208.3 ± 8.7** |
| Sympathextomized rats | 238.5 ± 5.1 | 238.5 ± 3.4 |
Comparison of body weight change before the operation and on the day that all rats received brain or abdomial operation for catheter infusion. Values are means ±s.d. from 6 animals.
P < 0.01 compared to a.
Figure 4.
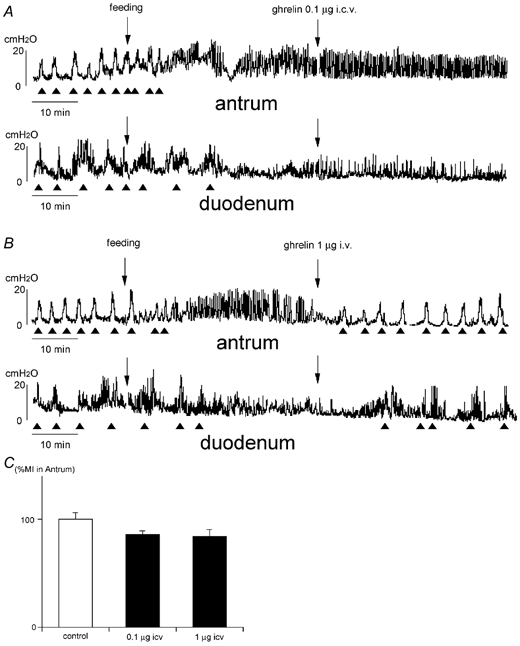
A and B, effects of i.c.v. (A) and i.v. (B) injection of ghrelin on the fed motor activity in vagotomized rats. The frequency of the phase III-like contractions in the fasted pattern is increased in vagotomized rats (A and B) compared to that seen in normal rats (Fig. 2). The fasted motor pattern is replaced by the fed motor pattern in both the antrum and duodenum several minutes after food intake (A and B). i.v. injection of ghrelin induces the fasted-like motor pattern in both the antrum and duodenum (B). However i.c.v. injection of ghrelin does not affect motor activity (A). C, comparison of %MI in the antrum induced by i.c.v. injection of ghrelin in vagotomized rats.
To examine the effects of endogenous ghrelin on gastrointestinal motor activity, GHS-R antagonist was given I.C.V. or I.V. to animals in the fasted state with and without truncal vagotomy (Fig. 5 and Fig. 6). GHS-R antagonist injected I.C.V. did not alter the fasted motor activities in the antrum and duodenum in either normal or vagotomized animals (Fig. 5A and B, Table 1). GHS-R antagonist injected I.V. did not alter the fasted motor pattern in the antrum or duodenum in normal animals (Fig. 6A, Table 1). However, it completely disrupted the fasted motor pattern in both the antrum and duodenum in vagotomized animals (Fig. 6B).
Figure 5. Effect of i.c.v. injection of GHS-R antagonist on the fasted motor activity in normal (A) and vagotomized (B) rats.
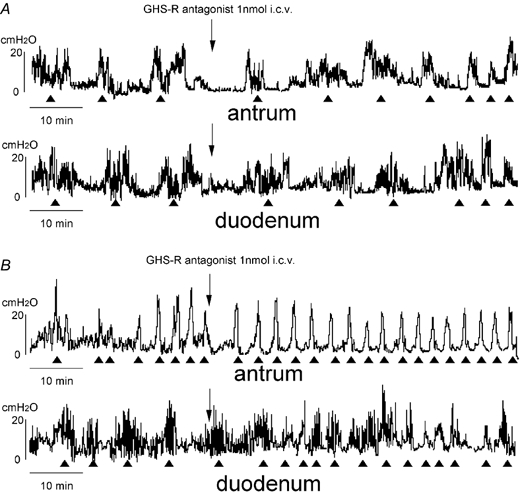
The fasted motor activities in both the antrum and duodenum in normal (A) and vagotomized (B) rats are not altered by i.c.v. injection of GHS-R antagonist.
Figure 6. Effect of i.v. injection of GHS-R antagonist on the fasted motor activity in normal (A) and vagotomized (B) rats.
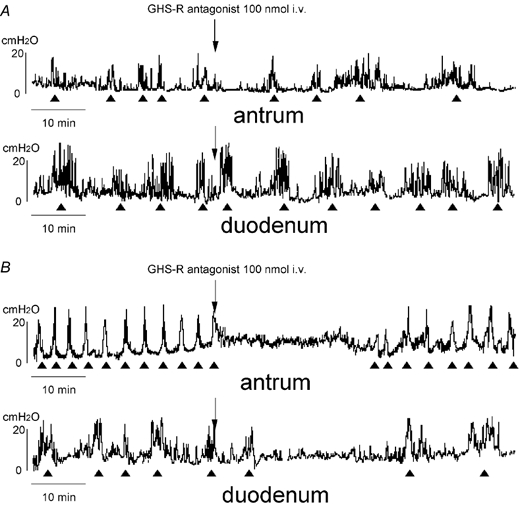
The fasted motor patterns in both the antrum and duodenum are disrupted by i.v. injection of GHS-R antagonist in vagotomized rats (B), however they are not altered by i.v. injection of GHS-R antagonist in normal rats (A).
We also examined the involvement of the sympathetic pathway in the effects of ghrelin on gastrointestinal motility using rats that had undergone surgical sympathectomy (Table 3). The frequency of the phase III-like contractions in the antrum (5.7 ± 1.2 h−1, n = 3) and duodenum (6.0 ± 1.0 h−1, n = 3) in the fasted state of sympathectomized rats was not changed from normal controls (Table 1). Sympathectomy did not alter the effects of I.V. injected ghrelin on gastroduodenal motility (Fig. 7A); that is I.V. injection of ghrelin increased % MI of fed motor activity in the antrum (Fig. 7A and B) and induced the fasted motor activity in the duodenum (Fig. 7A, Tables 1, 2).
Figure 7.
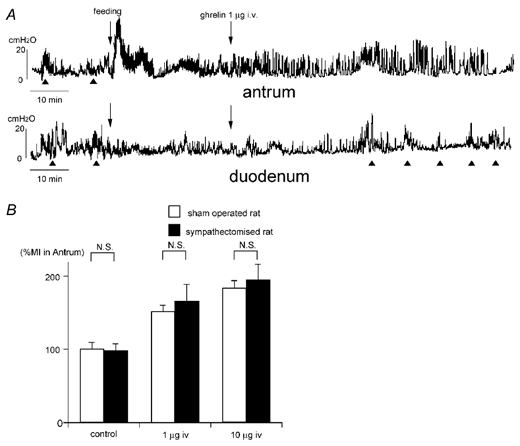
A, effect of i.v. injection of ghrelin on the fed motor activity in sympathectomized rats. Changes in the motor activities in the antrum and duodenum seen in Fig. 1B are not altered in sympathectomized rats. B, comparison of %MI in the antrum induced by i.v. injection of ghrelin in normal and sympathectomized rats.
Brain mechanism responsible for mediating the action of ghrelin
We examined the brain mechanism responsible for mediating the effect of I.C.V. and I.V. injection of ghrelin on gastroduodenal motility, by immunoneutralization by NPY antiserum injected into the brain. Changes in motor activity in both antrum and duodenum induced by I.C.V. and I.V. injection of ghrelin were completely blocked by I.C.V. injection of NPY antiserum (Fig. 8A, B and C). However normal serum did not alter the effects of I.C.V. and I.V. injection of ghrelin on gastroduodenal motility (Fig. 8C).
Figure 8.
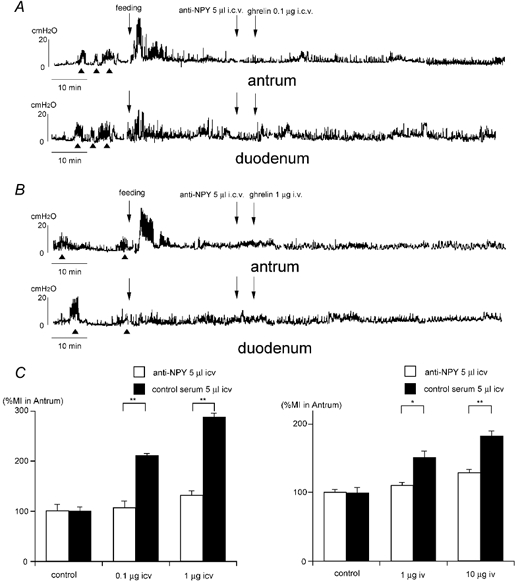
Effect of i.c.v. injection of NPY antiserum on the action of ghrelin injected i.c.v. (A) or i.v. (B) in fed animals. NPY antiserum completely blocked the effects of ghrelin injected i.c.v. and i.v.C, comparison of %MI in the antrum induced by anti-NPY antiserum (i.c.v.) + ghrelin (i.c.v.) and anti-NPY antiserum (i.c.v.) + ghrelin (i.v.). Data are compared to normal serum-injected controls. *P < 0.05; ** P < 0.01.
To examine the site of action of peripherally administered ghrelin on the brain NPY neurons, GHS-R antagonist was injected I.C.V. 5 min before I.V. injections of ghrelin in vagotomized animals (Fig. 9). Changes in motor activity induced by I.V. injections of ghrelin seen in Fig. 4B were not altered by I.C.V. injections of GHS-R antagonist (Fig. 9).
Figure 9. Effects of i.c.v. injection of GHS-R antagonist on the action of ghrelin injected i.v. into vagotomized rats in the fed state.
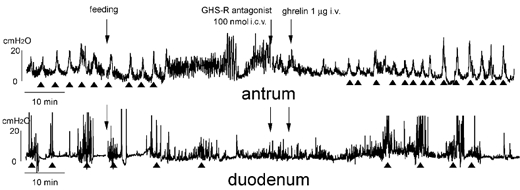
i.c.v. injection of GHS-R antagonist does not alter the effect of i.v. injection of ghrelin seen in Fig. 4B.
Intragastric pH and action of ghrelin
The relationship between intragastric pH and the effects of ghrelin on gastroduodenal motility was examined. Intragastric pH in animals in the fasted state was 4.3 ± 0.6 (n = 3) and it decreased to 2.5 ± 0.2 (n = 3) within 30 min after the start of feeding (4 g laboratory chow), returning to 5.4 ± 0.6 (n = 3) 60 min after the start of feeding (Table 4). In famotidine-treated animals, intragastric pH kept high levels (6≈7) throughout the fasted and fed states (Table 4). The effects of I.V. injection of ghrelin on gastrointestinal motility was examined in the famotidine-treated animals (Fig. 10, Table 1). The time lag before the initiation of the fasted pattern in the duodenum induced by I.V. injection of ghrelin seen in Fig. 1B disappeared in famotidine-treated animals and the fasted pattern appeared immediately after I.V. injection of ghrelin (Fig. 10, Table 2).
Table 4.
pH of gastric juice
| Normal rat | Famotodine-treated rat | |
|---|---|---|
| Fasting state | 4.3 ± 0.6a | 6.7 ± 0.1** |
| 30 min after start of feeding | 2.5 ± 0.2** | 6.0 ± 0.3* |
| 60 min after start of feeding | 5.4 ± 0.6 | 6.6 ± 0.2** |
Values are means ±s.d. from 3 animals.
P < 0.05
P < 0.01 compared witha.
Figure 10. Effect of i.v. injection of ghrelin on the fed motor activity in rats pretreated with famotidine.
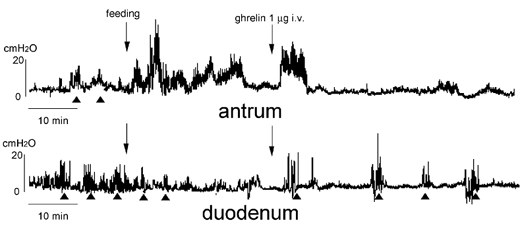
Changes in the motor activities in the antrum and duodenum seen in Fig. 1B appear immediately after i.v. injection of ghrelin.
DISCUSSION
The present experiments using freely moving conscious rat models permit the measurement of gastroduodenal motility in animals in the physiological fed and fasted states (Fujimiya et al. 2000; Kihara et al. 2001). The present results demonstrated that ghrelin given I.C.V. and I.V. in animals in the fed state induced fasted motor activity in the duodenum and increased motor activity in the gastric antrum. The effect of ghrelin in inducing the fasted motor activities to the duodenum is consistent with that observed in NPY as shown in our previous paper (Fujimiya et al. 2000). However the sites of action seem to differ, because NPY is effective only after I.C.V. administration, whereas ghrelin is effective following either I.C.V. or I.V. administration.
We used ghrelin doses of 0.1 and 1 μg (0.03 and 0.3 nmol) for I.C.V. injection and 1 and 10 μg (0.3 and 3 nmol) for I.V. injection. Although plasma levels of ghrelin in physiological conditions in rats are 500–2000 fmol ml−1 (Murakami et al. 2002), previous studies used doses of 0.1–3 μg for I.C.V. injections to stimulate food intake (Shintani et al. 2001, Wren et al. 2000) and 0.2–10 μg for I.V. injection to stimulate gastric motility and secretion (Masuda et al. 2000). Therefore, we choose the above doses according to the previous studies. In our preliminary study, we measured ghrelin concentration in arterial blood in rats (n = 10) after I.V. injection of 5 μg (1.5 nmol) ghrelin. Ghrelin concentration of the arterial blood was 159 ± 24 fmol ml−1 in the basal state which increased to 603 ± 110 fmol ml−1 when measured 20 min after I.V. injection. We also confirmed that ghrelin concentration of the gastric vein of 24 h-fasted rats (n = 10) was 487 ± 140 fmol ml−1. Therefore the dose of ghrelin we used in this study seems to be relevant to the physiological state.
In the present study the site of action of ghrelin was examined by using GHS-R antagonist. Changes in gastroduodenal motility induced by I.V. injection of ghrelin were not affected by I.C.V. injection of GHS-R antagonist at a dose which was sufficient to block the effects of I.C.V. injection of ghrelin. The effect of I.V. ghrelin, on the other hand, was completely blocked by GHS-R antagonist given by the same route. These results suggest that ghrelin whether administered I.C.V. or I.V. may exert effects on gastroduodenal motility via ghrelin receptors in the brain and periphery, respectively. It has been shown that GHS-R is located in the hypothalamus as well as in the stomach (Guan et al. 1997; Shuto et al. 2001) and such location supports our hypothesis. Although GHS-Rs are located in both brain and periphery, the presence of endogenous ghrelin has been shown in the stomach (Date et al. 2000b) with much smaller amounts in the brain (Kojima et al. 1999). Therefore, it seems possible that endogenous ghrelin released from the stomach regulates gastroduodenal motility via GHS-Rs in the stomach or duodenum, or via GHS-Rs in the brain.
Involvement of the vagal nerve in mediating the action of ghrelin was examined in the present study. Results show that truncal vagotomy blocked the effects of I.C.V. injection of ghrelin on antral and duodenal motility, suggesting that the vagal pathway may mediate the action of centrally administered ghrelin on gastroduodenal motility. On the other hand, truncal vagotomy did not alter the effects of I.V. injection of ghrelin on the duodenal motility. More characteristically, the effects of I.V. injection of ghrelin on the antral motility in vagotomized rats were quite different from those in normal rats. I.V. Injection of ghrelin induced the fasted motor activity in both antrum and duodenum in vagotomized rats, while it induced fasted motility only in the duodenum in normal rats. This result suggests that peripherally administered ghrelin may act directly on the ghrelin receptor located in the stomach and duodenum, and induce fasted motor activity in vagally denervated animals.
In the present study we found that the frequency of phase III-like contractions of the fasted motility in the stomach and duodenum in vagally denervated animals was significantly higher than that in normal animals. However sympathectomy did not alter the frequency of phase III contractions. These results were inconsistent with the previous findings obtained in dogs, in which the incidence of phase III contraction was reduced by vagotomy (Marik et al. 1975) and the frequency of the phase III contraction was decreased by sympathectomy (Marlett & Code, 1979). The difference between the present and previous data might be due to a species difference.
The effects of intrinsic ghrelin on gastroduodenal motility were examined by the experiments using GHS-R antagonist. The results showed that I.V. injection of GHS-R antagonist blocked fasted motor activity in both the stomach and duodenum in vagotomized rats, but did not alter the fasted motor activity in vagally innervated normal rats. Therefore, the fasted motor activity in the stomach and duodenum in vagally denervated animals might be under the regulation of ghrelin originating from the stomach. On the other hand, in normal animals with an intact vagal pathway, neither I.V. nor I.C.V. injection of GHS-R antagonist affected the fasted motor activity as shown in the present study. However a previous study has shown that immunoneutralization of NPY in the brain completely blocked the fasted motor activity in the duodenum (Fujimiya et al. 2000). The present and previous findings indicate that fasted motor activity in the gastrointestinal tract in normal rats is under the control of NPY neurons in the brain; however, once brain control is eliminated by truncal vagotomy, ghrelin might be primarily involved in the regulation of fasted motor activity.
The present results showed that immunoneutralization of NPY in the brain blocked the effects of I.C.V. and I.V. injection of ghrelin on gastroduodenal motility. Since the NPY antiserum completely blocks the NPY pathway in the brain (Inui, 1999), these results suggest that NPY neurons might be involved in activation of a ghrelin-sensitive mechanism in the brain to induce the fasted motor activity in the gastrointestinal tract. To examine the pathways mediating the peripherally administered ghrelin on the NPY neurons in the brain, we injected GHS-R antagonist I.C.V. combined with an I.V. injection of ghrelin in vagotomized animals. Changes in the gastroduodenal motility induced by I.V. injection of ghrelin were not altered by GHS-R antagonist injected I.C.V., suggesting that peripheral ghrelin may primarily act on peripheral GHS-Rs but not directly on the central GHS-Rs on the NPY neurons. Involvement of the vagal afferent neuron in mediating this action seems highly possible, because c-fos expression in the arcuate nucleus induced by I.V. injection of ghrelin was blocked by afferent vagal nerve blockade and ghrelin receptor mRNA expression was demonstrated on the vagal afferent neurons by an in situ hybridization study (Date et al. 2002).
As human ghrelin has a structural resemblance to human motilin, and human ghrelin receptors exhibit a 50 % identity with human motilin receptors (Asakawa et al. 2000), the role of ghrelin in gastrointestinal function is comparable with that of motilin which has been widely investigated previously (Itoh 1997; Siadati & Sarr, 1998; Sarna et al. 2000). Although motilin originates from the endocrine cells in the duodenum (Itoh, 1997) while ghrelin originates from the endocrine cells of the stomach (Date et al. 2000b), both of them are involved in the regulation of phase III contractions in the gastrointestinal tract. Motilin induces fasted motility in the stomach and duodenum when it is given peripherally (Siadati & Sarr, 1998; Sarna et al. 2000) but not when given centrally (Hashmonai et al. 1987). Since it is known that gastric acidification modulates the action of motilin (Yamamoto et al. 1994a,1994b), we examined the relationship between the effects of ghrelin on motor activity and intragastric pH. The results showed that within 30 min after feeding low intragastric pH (pH 2.5 ± 0.2) inhibited the effect of I.V. injected ghrelin on gastroduodenal motility, and that this effect was reversed by an increase of intragastric pH (pH 5.4 ± 0.6) within 60 min after feeding, or by pretreatment of famotidine (intragastric pH 6.0–6.7). These results suggest that the sensitivity of the GHS-R in the gastrointestinal tract might be inhibited by low intragastric pH.
In conclusion, the present study indicates that ghrelin originating from the stomach is involved in the regulation of fasted motor activity of the gastrointestinal tract. The fasted motor activity in the duodenum of vagally innervated normal rats is under the regulation of central NPY neurons which are activated by ghrelin. Ghrelin released from the stomach may act on the ghrelin receptor on vagal afferent nerve terminals and then activate NPY neurons in the brain. However once brain regulation is eliminated by vagal denervation, ghrelin may be primarily involved in the regulation of fasted-like motor activity in both the stomach and duodenum via ghrelin receptors possibly located on the stomach and duodenum. The action of ghrelin to induce fasted motor activity is strongly affected by intragastric pH; low pH inhibits the action.
REFERENCES
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2000;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Momose K, Ueno N, Fujino MA, Kasuga M. Motilin increases food intake in mice. Peptides. 1998;19:987–990. doi: 10.1016/s0196-9781(97)00477-4. [DOI] [PubMed] [Google Scholar]
- Banks WA, Tschop M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barriers is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Pumell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000a;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- Date Y, Murakmi N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- Date Y, Nakazato M, Murakami N, Kojima M, Kangawa K, Matsukura S. Ghrelin acts in the central nervous system to stimulate gastric acid secretion. Biochem Biophys Res Commun. 2000b;280:904–907. doi: 10.1006/bbrc.2000.4212. [DOI] [PubMed] [Google Scholar]
- Djurhuus CB, Hansen TK, Gravholt C, Orskov L, Hosoda H, Kangawa K, Jorgensen JOL, Holst JJ, Schmitz O. Circulation levels of ghrelin and GLP-1 are inversely related during glucose ingestion. Horm Metab Res. 2002;34:411–413. doi: 10.1055/s-2002-33475. [DOI] [PubMed] [Google Scholar]
- Fujimiya M, Itoh E, Kihara N, Yamamoto I, Fujimura M, Inui A. Neuropeptide Y induces fasted pattern of duodenal motility via Y2 receptors in conscious fed rats. Am J Physiol Gastrointest Liver Physiol. 2000;279:G32–38. doi: 10.1152/ajpgi.2000.278.1.G32. [DOI] [PubMed] [Google Scholar]
- Guan XM, Yu H, Palyha OC, Mackee KK, Feighner SD, Sirinath-Singhji DJ, Smith RG, Van Der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 1997;48:23–29. doi: 10.1016/s0169-328x(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Hashmonai M, Go VLW, Yaksh T, Szurszewski JH. Effect of central administration of motilin on migrating complexes in the dog. Am J Physiol. 1987;252:G195–199. doi: 10.1152/ajpgi.1987.252.2.G195. [DOI] [PubMed] [Google Scholar]
- Hashmonai M, Szurszewski JH. Effect of cerebroventricular perfusion of bombesin on gastrointestinal myoelectrical activity. Am J Physiol. 1998;274:G677–686. doi: 10.1152/ajpgi.1998.274.4.G677. [DOI] [PubMed] [Google Scholar]
- Inui A. Neuropeptide Y feeding receptors are multiple subtypes involved? Trends Pharmacol Sci. 1999;20:43–46. doi: 10.1016/s0165-6147(99)01303-6. [DOI] [PubMed] [Google Scholar]
- Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci. 2001;2:551–560. doi: 10.1038/35086018. [DOI] [PubMed] [Google Scholar]
- Inui A, Inoue T, Nakajima M, Okita M, Sakatani N, Okimura Y, Chihara K, Baba S. Brain neuropeptide Y in the control of adrenocorticotropic hormone secretion in the dog. Brain Res. 1990;510:211–215. doi: 10.1016/0006-8993(90)91369-r. [DOI] [PubMed] [Google Scholar]
- Itoh Z. Motilin and clinical application. Peptides. 1997;18:593–608. doi: 10.1016/s0196-9781(96)00333-6. [DOI] [PubMed] [Google Scholar]
- Kihara N, Fujimura M, Yamamoto I, Itoh E, Inui A, Fujimiya M. Effect of central and peripheral urocortin on fed and fasted gastroduodenal motor activity in conscious rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G406–419. doi: 10.1152/ajpgi.2001.280.3.G406. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Marik F, Code CF. Control of the interdigestive myoelectric activity in dogs by the vagus nerves and pentagastrin. Gastroenterology. 1975;69:387–395. [PubMed] [Google Scholar]
- Marlett JA, Code CF. Effects of celiac and superior mesenteric ganglionectomy on interdigestive myoelectric complex in dogs. Am J Physiol. 1979;6:E432–436. doi: 10.1152/ajpendo.1979.237.5.E432. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Tanaka T, Inomata N, Ohmura N, Tanaka S, Itoh Z, Hososa H, Kojima M, Kangawa K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–908. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- Murakami N, Hayashida T, Kuroiwa T, Nakahara K, Ida T, Mondal MS, Nakazato M, Kojima M, Kangawa K. Role of central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol. 2002;174:283–288. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Membrilla MA, Martinz V, Vergara P. Peripheral and central cholecystokinin receptors regulate postprandial intestinal motility in the rat. J Pharmacol Exp Therap. 1996;275:486–493. [PubMed] [Google Scholar]
- Sarna SK, Gonzalez A, Ryan R. Enteric locus of action of prokinetics: ABT-229, motilin, and erythromycin. Am J Physiol Gastrointest Liver Physiol. 2000;278:G744–752. doi: 10.1152/ajpgi.2000.278.5.G744. [DOI] [PubMed] [Google Scholar]
- Shintani M, Ogawa Y, Ebihara K, Abe AM, Miyagawa F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, Kangawa K, Nakao K. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- Shuto Y, Shibasaki T, Wada K, Parhar I, Kamegai J, Suhihara H, Okikawa S, Wakabayashi I. Generation of polyclonal antiserum against the growth hormone secretagogue receptor (GHS-R): Evidence that the GHS-R exists in the hypothalamus, pituitary and stomach of the rats. Life Sci. 2001;68:991–996. doi: 10.1016/s0024-3205(00)01001-8. [DOI] [PubMed] [Google Scholar]
- Siadati M, Sarr MG. Role of extrinsic innervation in release of motilin and pattern of upper gut canine motility. J Gastrointest Surg. 1998;2:363–372. doi: 10.1016/s1091-255x(98)80076-0. [DOI] [PubMed] [Google Scholar]
- Tomasetto C, Karam SM, Ribieras S, Masson R, Lefebvre O, Staub A, Alexander G, Chenard MP, Rio MC. Identification and characterization of a novel gastric peptide hormone: The motilin-related peptide. Gastroenterology. 2000;119:395–405. doi: 10.1053/gast.2000.9371. [DOI] [PubMed] [Google Scholar]
- Willesen MG, Kristensen P, Romer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306–316. doi: 10.1159/000054491. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DGA, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kuwahara A, Yamamoto I, Fujimura M, Maeda T, Fujimiya M. Motor activity of vascular perfused rat duodenum. 1. Characteristics of spontaneous movement. Neurogastroenterol Motil. 1999;11:227–234. doi: 10.1046/j.1365-2982.1999.00153.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto O, Matsunaga Y, Haga N, Mizumoto A, Itoh Z. Inhibition of phase 3 activity by acidifying stomach in vagally denervated and innervated dogs with gastric pouches. Gastroenterology. 1994a;106:1533–1541. doi: 10.1016/0016-5085(94)90407-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto O, Matsunaga N, Haga N, Itoh Z. Vagovagal inhibition of motilin-induced phase 3 contractions by antral acidification in dog stomach. Am J Physiol. 1994b;273:G129–134. doi: 10.1152/ajpgi.1994.267.1.G129. [DOI] [PubMed] [Google Scholar]


