Abstract
During swallowing, the airway is protected from aspiration of ingested material by brief closure of the larynx and cessation of breathing. Mechanoreceptors innervated by the internal branch of the superior laryngeal nerve (ISLN) are activated by swallowing, and connect to central neurones that generate swallowing, laryngeal closure and respiratory rhythm. This study was designed to evaluate the hypothesis that the ISLN afferent signal is necessary for normal deglutition and airway protection in humans. In 21 healthy adults, we recorded submental electromyograms, videofluoroscopic images of the upper airway, oronasal airflow and respiratory inductance plethysmography. In six subjects we also recorded pressures in the hypopharynx and upper oesophagus. We analysed swallows that followed a brief infusion (4–5 ml) of liquid barium onto the tongue, or a sip (1–18 ml) from a cup. In 16 subjects, the ISLN was anaesthetised by transcutaneous injection of bupivacaine into the paraglottic compartment. Saline injections using the identical procedure were performed in six subjects. Endoscopy was used to evaluate upper airway anatomy, to confirm ISLN anaesthesia, and to visualise vocal cord movement and laryngeal closure. Comparisons of swallowing and breathing were made within subjects (anaesthetic or saline injection vs. control, i.e. no injection) and between subjects (anaesthetic injection vs. saline injection). In the non-anaesthetised condition (saline injection, 174 swallows in six subjects; no injection, 522 swallows in 20 subjects), laryngeal penetration during swallowing was rare (1.4 %) and tracheal aspiration was never observed. During ISLN anaesthesia (16 subjects, 396 swallows), all subjects experienced effortful swallowing and an illusory globus sensation in the throat, and 15 subjects exhibited penetration of fluid into the larynx during swallowing. The incidence of laryngeal penetration in the anaesthetised condition was 43 % (P < 0.01, compared with either saline or no injection) and of these penetrations, 56 % led to tracheal aspiration (without adverse effects). We further analysed the swallow cycle to evaluate the mechanism(s) by which fluid entered the larynx. Laryngeal penetration was not caused by premature spillage of oral fluid into the hypopharynx, delayed clearance of fluid from the hypopharynx, or excessive hypopharyngeal pressure generated by swallowing. Furthermore, there was no impairment in the ability of swallowing to halt respiratory airflow during the period of pharyngeal bolus flow. Rather, our observations suggest that loss of airway protection was due to incomplete closure of the larynx during the pharyngeal phase of swallowing. In contrast to the insufficient closure during swallowing, laryngeal closure was robust during voluntary challenges with the Valsalva, Müller and cough manoeuvres under ISLN anaesthesia. We suggest that an afferent signal arising from the ISLN receptor field is necessary for normal deglutition, especially for providing feedback to central neural circuits that facilitate laryngeal closure during swallowing. The ISLN afferent signal is not essential for initiating and sequencing the swallow cycle, for co-ordinating swallowing with breathing, or for closing the larynx during voluntary manoeuvres.
In mammals, closure of the larynx during swallowing is a vital protective mechanism for preventing aspiration of ingested materials into the lungs (Negus, 1949). The human larynx closes during deglutition at three levels: the true vocal cords adduct to close the glottis, the false cords adduct, and the arytenoids fold anteriorly against the epiglottis to close the laryngeal aditus (Anderson Stuart, 1891–1892; Pressman, 1941; Fink, 1974; Logemann et al. 1992). The larynx also elevates and arcs anteriorly under the tongue, which helps expand the hypopharyngeal space and open the upper oesophageal sphincter. The epiglottis folds posteriorly over the laryngeal aditus and directs the ingested bolus into the piriform recesses towards the now relaxed upper oesophageal sphincter (Ardran & Kemp, 1952). Furthermore, the respiratory rhythm is strongly reset by swallowing. Airflow ceases during bolus flow through the hypopharynx, and resumes usually in the expiratory phase after completion of the pharyngeal phase of swallowing (Paydarfar et al. 1995). Thus, the airway is protected from aspiration during swallowing by the reconfiguration of the larynx and pharynx, and the resetting of the respiratory rhythm. The initiation and co-ordination of these events are controlled by the central nervous system. However, despite over a century and a half of debate and experimental work (Reid, 1838; Månsson & Sandberg 1974; Miller, 1982; Ali et al. 1994; Bastian & Riggs, 1999; Sulica et al. 2002), it is not certain whether sensory feedback is also necessary for airway protection once the swallowing sequence is initiated.
It is well established that laryngeal closure in humans and other mammals can be evoked by electrical stimulation of the internal branch of the superior laryngeal nerve (ISLN) (Ogura & Lam, 1953; Murakami & Kirchner, 1972), which contains afferents from the supraglottic larynx and epiglottis (Sanders & Mu, 1998). In addition to laryngeal closure, stimulation of the ISLN can induce swallowing movements (Ogura & Lam, 1953; Doty & Bosma, 1956), central apnoea (Iscoe et al. 1979), and strong resetting of the respiratory rhythm (Paydarfar et al. 1986). Moreover, experiments in decerebrate or anaesthetised animals have demonstrated that mechanoreceptors innervated by the ISLN are activated by swallowing (Sumi, 1964), and that stimulation of the A-alpha fibre sub-type within the ISLN (from proprioceptors) optimally triggers reflex swallowing (Miller & Loizzi, 1974). Taken together, these studies clearly establish the existence of ISLN afferents that are activated by swallowing, and that connect to central circuits involved in laryngeal closure, swallowing and breathing. However, it is not known whether ISLN afferents are necessary for normal swallowing and, in particular, for airway protection during swallowing in humans.
If a closed-loop feedback pathway involving the ISLN maintains swallow integrity and ensures airway protection, then opening the feedback loop by blocking ISLN afferent signals should lead to a derangement of swallowing and an increase in the incidence of aspiration. The present study was designed to evaluate this hypothesis in humans. We found that during ISLN anaesthesia, the subjects experienced effortful swallowing and an illusory perception of swelling or obstruction in the throat. Videofluoroscopy revealed that during swallowing ingested liquid frequently leaked into the larynx and entered the trachea. In order to examine the mechanism(s) of dysphagia and aspiration induced by ISLN anaesthesia, we analysed the oral, pharyngeal and early oesophageal phases of swallowing. We tested for defects in bolus transport, pharyngeal contractility, upper oesophageal sphincter function, laryngeal closure, and the co-ordination between swallowing and breathing. A preliminary report of some of our results has been presented (Jafari et al. 2001).
METHODS
Human subjects
This study was approved by the Committee for the Protection of Human Subjects in Research at the University of Massachusetts Medical School, which conforms to the standards set by the Declaration of Helsinki. All prospective subjects completed a health questionnaire and those with a history of medical illness, neck surgery, smoking within 10 years of the study or suspected pregnancy, were excluded. We obtained written informed consent from all subjects who entered the study.
Selective ISLN anaesthesia
The ISLN was anaesthetised bilaterally by transcutaneous injection of 0.5 % bupivacaine into the right and left paraglottic compartments (Gaskill & Gillies, 1966; Stockwell et al. 1995). A 1.5 in (3.8 cm), 22 gauge short bevel needle was inserted through the skin at a point between the hyoid bone and the thyroid cartilage, and 1 cm anterior to the greater cornu of the thyroid cartilage. The needle was advanced until increased resistance was encountered. The needle was then withdrawn 1–2 mm and 3 ml of the anaesthetic solution was injected. The plunger of the syringe was withdrawn prior to injection to ensure that the needle tip was not in a blood vessel. We used the identical procedure to inject the vehicle (3 ml normal saline) into the left and right paraglottic compartments. The percutaneous technique described above has been shown in studies of fresh cadavers to confine the injected fluid to the paraglottic compartment, and the ISLN is the only nerve that courses through the paraglottic compartment (Stockwell et al. 1995).
Flexible endoscopy of the upper airway
A fibreoptic endoscope (1.8 mm o.d., no. S1002, Mitsubishi Cable America, NY, USA) was inserted through the nasal passage and into the hypopharynx of each subject. Topical anaesthesia was not used. Endoscopy was used to evaluate the anatomy of the upper airway, laryngeal and hypopharyngeal sensation, and laryngeal motor function before and after the neck injections.
Evaluation of upper airway sensation
Sensation was tested by touching the tip of the endoscope to the base of the tongue, vallecula, lingual and laryngeal surfaces of the epiglottis, arytenoids and piriform sinuses. The anterior and posterior pillars of the fauces were tested perorally with a cotton-tipped applicator. Sensation was rated on a 0–3 scale: 0, no sensation (anaesthetic); 1, probing is detected but the sensation is not unpleasant; 2, probing evokes unpleasant sensation; 3, probing evokes unpleasant sensation with gagging or coughing. Initially the laryngeal or hypopharyngeal structure being tested was touched lightly with the tip of the scope, or, in the case of the fauces, rubbed lightly with the cotton-tipped applicator. If this did not provoke a rating of 2 or 3, then the same site was tested more firmly to elicit a final response from the subject. This method of graded stimulation was used to avoid extreme reactions in highly sensitive individuals. We recorded the maximum rating for each structure during each test. The structure was deemed anaesthetic only if the subject reported no sensation. Partial anaesthesia of a structure was determined by comparing the rating before and after neck injection.
Evaluation of laryngeal motor function
We tested the efferent function of the larynx during voluntary manoeuvres. Using the endoscope we examined vocal cord movement while the subject vocalised. The subjects were then asked to maintain laryngeal closure during maximum expiratory effort (Valsalva manoeuvre), and to execute a cough. The ability to maintain and abruptly terminate laryngeal closure was visualised. Subsequently, after the facemask was placed, oronasal airflow was recorded during the Valsalva manoeuvre, cued cough, and while the subjects maintained an airtight seal against maximum inspiratory effort (Müller manoeuvre). All laryngeal manoeuvres were performed while the mouth was open.
Swallow induction
All subjects were studied in the upright, seated position. Swallows were induced using three methods.
Automated bolus infusion
This method was developed in order to deliver boluses in a reproducible manner. We used a custom-built device to specify the timing, duration and volume of liquid infusions. The device consisted of a pressurised chamber containing liquid barium, which was mixed continuously by a magnetically driven stir bar. The outflow port of the chamber was connected to a solenoid-controlled valve. A timing circuit opened the valve for a preset duration and transmitted a signal that marked the bolus infusion interval (Fig. 1A). The duration of bolus infusion was set at 0.50 s in seven subjects (nos 1 and 3–8), 0.75 s in 13 subjects (nos 9–21) and 1.00 s in subject no. 2. Fluid was delivered via a flexible tube (3 mm o.d.) that was attached to the outlet of the valve. The pressure of the chamber was kept constant using a regulator which could be adjusted to specify the bolus volume. Once the pressure was set, the volume of repeated infusions remained constant to within ± 0.2 ml (range). The set volume for all subjects was between 4.0 and 4.8 ml (mean 4.5 ml).
Figure 1. Example of physiological recordings and definitions.
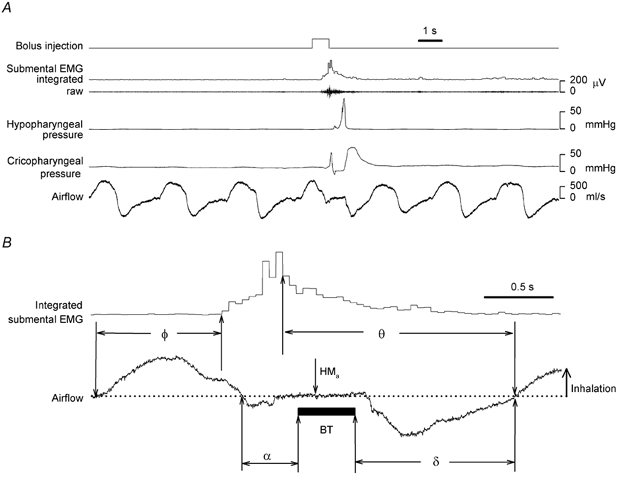
A, a single run including a swallow induced by automated bolus infusion in subject no. 11 in the control arm. B, definitions of old phase (Φ), cophase (Θ), and the latencies, α and δ, between hypopharyngeal bolus transit (BT) and oronasal airflow. HMa: onset of hyoid movement in the anterior direction. Φ and Θ are normalised to the mean period of 3 breaths before the swallow.
The outflow tube was threaded through a facemask, and advanced until the tip was placed onto the surface of the subject's tongue. Infusion of fluid onto the tongue (rather than posteriorly into the pharynx) was assured because the tip of the tube was sealed and fluid flowed only through a port near the tip that was oriented downward onto the tongue. Once positioned on the tongue, the tube was fixed to the mask using a lock nut, which prevented the tube from twisting or advancing. The tube was otherwise flexible and moved freely with tongue movements. Each subject was instructed to swallow the bolus as soon as it was collected on the tongue. Using videofluoroscopy and debriefing of the subjects, we confirmed that the bolus infusions did not spray into the pharynx.
Sip from a cup
Sips through a straw from a 40 ml cup were alternated with sips taken directly from the cup. The subject was instructed to begin the sip when cued by the investigator with a red light-emitting diode, positioned 1 m in front of the subject. The volume of fluid consumed with each sip was recorded. The median bolus volume was 7.5 ml (range 1.0–17.5 ml)
Spontaneous swallows
We recorded spontaneous swallows during continuous infusion of water at the rate of 1.7–7.2 ml min−1 onto the tongue through the flexible tube attached to the facemask. The infusion rate was adjusted to reach a spontaneous swallowing rate of ≤10 min−1.
Swallow recordings
Videofluoroscopy
Fluoroscopy was used to evaluate swallowing of liquid barium (54 % w/v; E-Z-EM, Inc. Westbury, NY, USA) induced by automated bolus infusion and sipping from a cup. A portable C-arm fluoroscope (BV 300 Philips Medical Systems, N.A., Bothel, WA, USA) was positioned to view subjects in the lateral plane. Male subjects wore a lap shield and female subjects wore a shield that covered the chest, abdomen and pelvis. Total radiation exposure time did not exceed 5 min for each subject (3 mSv (300 mrem)). Fluoroscopic images were recorded at 30 frames s−1 on a VHS videocassette recorder, which allowed frame-by-frame playback for subsequent analysis. Fluoroscopic recording was started when each automated infusion was triggered or the light cue was turned on. Recording continued without interruption for up to 5 s. If tracheal aspiration was visualised during this period, the fluoroscope was activated intermittently to evaluate the subject's ability to clear the barium from the upper airway by coughing. A digital timer (Thalner Electronics, Ann Arbor, MI, USA) was used to display time in hundredths of a second onto the video images. The timer was reset to zero and restarted at the beginning of each run. The timer simultaneously transmitted a 5 ms square wave pulse at 1 s intervals to the digital data acquisition system that recorded respiratory and other swallow-related signals. In this way we were able to synchronise the fluoroscopic events with the other physiological recordings.
Submental electromyography
We placed a surface electrode 1 cm posterior to the genu of the mandible over the midline suprahyoid muscle complex. This signal was differentially amplified (Bioamp 100, Axon Instruments, Union City, CA, USA) relative to a surface recording over the left zygomatic arch. An electrode over the right zygomatic arch served as the ground lead. The signal was further amplified and filtered (band pass 100 Hz to 10 kHz, CyberAmp 380, Axon Instruments).
Manometry
Intrapharyngeal and upper oesophageal manometry was performed using a catheter (2.2 mm diameter, model 16CT/S-4, Gaeltec/MMI, Hackensack, NJ, USA) with four pressure transducers mounted at 3 cm intervals. The catheter was inserted in a sealed chamber and calibrated with a mercury manometer. Following calibration, the tip of the catheter was lubricated and inserted through the nasopharynx, with the transducers facing posteriorly. Topical anaesthetic was not used. The catheter's position was adjusted to obtain the characteristic pressure recordings (e.g. see Fig. 1A) at the level of the hypopharynx and cricopharyngeus, recorded from the middle two sensors. Once placement was achieved, the catheter was secured in position by taping it to the nasal bridge and the cheek. We monitored the position of the catheter throughout the experiment by noting the location of the radio-opaque transducer sleeves with respect to the arytenoids and the cervical vertebrae on the fluoroscopic images. At the end of the experiment, the catheter was removed and recalibrated. We found no significant change in the calibration at the end of the experiment.
Respiratory and cardiac recordings
Airflow through the mouth and nose was measured using a facemask (Hans Rudolph, Inc., Kansas City, MO, USA), Fleisch pneumotachograph (90 % rise time < 10 ms), and a differential pressure transducer (model 5551; 90 % rise time < 100 μs, Silicon Microstructures, Inc., Fremont, CA, USA). The dead space of the system was 150 ml.
The airflow signal was filtered (low pass 100 Hz) and amplified (CyberAmp 380, Axon Instruments). Zero flow was established by voluntary breath holding or by removing the pneumotachograph from the facemask. To calibrate the volume, 3 l of air were passed through the pneumotachograph. We also recorded abdomen and chest wall inductance plethysmography (Ambulatory Monitoring Inc., Ardsley, NY, USA). The chest movement signal was multiplied by two and added to the abdomen signal. The combined signal was calibrated to provide an estimate of tidal volume (Banzett et al. 1995). Airway PCO2 was monitored throughout the study using an infrared CO2 monitor (Capnogard, Novamatrix Medical Systems Inc., Wallingford, CT, USA) that continuously analysed gas sampled from a nasal cannula placed in the subject's nares. We monitored cardiac rhythm (Physio-Control, Lifepak 9P, Redmond, WA, USA) using conductive adhesive electrodes that were placed on the left and right upper chest, and the left lower chest.
Inhalation of carbon dioxide
Because any potential role for the ISLN in regulating swallowing and airway protection could involve fibres sensitive to airway CO2 (Bartlett & Knuth, 1992), we studied four subjects during hypercapnoeic hyperpnoea. The inspired CO2 fraction (FI,CO2) was raised every 15 min, using a gas-mixing unit in series with a heater and humidifier. The inspired O2 fraction (FI,O2 was 50 %, and the balance of the mixture was N2. The mixture was fed through the inspiratory port of a T-valve that was attached to the pneumotachograph. Every 15 min after changing the FI,CO2, the subjects were asked to rate their level of air hunger using a scale described by Banzett et al. (1996). The target FI,CO2was the level at which the subject reported the maximum air hunger that could be tolerated for the remainder of the study (45–60 min). Once this level of air hunger was achieved, the FI,CO2 was not raised further. All other subjects breathed room air.
Masking of sound cues
In order to avoid sound cues that might provoke swallowing or cause a change in respiration, the subjects were exposed to filtered white noise (50–1000 Hz) through headphones. The noise level was gradually increased and set to a level that the subject reported to be the maximum level that was not uncomfortable. During the debriefing sessions the subjects reported no auditory cues that led them to anticipate the automated infusions.
Experimental protocol
Twenty-six healthy subjects (nine female, 17 male; age 19–51 years) entered the study. Of these, 21 (eight female, 13 male, age 19–51 years) completed the study and are the basis for our results. Four subjects were excluded because of incomplete laryngeal anaesthesia (laryngeal epiglottis and/or arytenoids). A fifth subject was excluded because he experienced unexpected loss of sensation in the lower face bilaterally, which resolved within 10 h of the injections without complications.
Table 1 summarises the protocols that were used. Twenty subjects (nos 1–15 and 17–21) were studied during a control period (no intervention), and during a period that followed the experimental intervention (injection of vehicle or bupivacaine). In 15 of these subjects (nos 1–10 and 17–21), the two conditions were studied in separate sessions ≥ 2 days apart. The session with ISLN anaesthesia was first in subjects no. 2–4, 7, 8 and 10, and second in subjects no. 1, 5, 6 and 9. In the remaining five subjects (nos 11–15) the control condition preceded ISLN blockade on the same day because removal and reinsertion of the manometry catheter on separate days would have compromised the reproducibility of the recordings. Subject no. 16 was studied following injection of the vehicle in one session, and following injection of bupivacaine in a later session. In this case the two sessions were 2 weeks apart.
Table 1.
Experimental protocols
| Subject no. | Control | Bupivacaine | Vehicle | Manometry |
|---|---|---|---|---|
| 1–10 | X | X | — | — |
| 11–15 | X | X | — | X |
| 16 | — | X | X | — |
| 17–21 | X | — | X | — |
All procedures and their risks were fully described to the subjects but they were not informed about the specific aims of the study. Subjects no. 11–21, and the investigator performing the endoscopy and neck injections during their experiments, were masked to the content of the injected solution (i.e. vehicle vs. bupivacaine).
Sequence of procedures and recordings in a session
The following steps were followed in each experimental session, with the exception of steps (2) and (10), which were omitted during the control session. Each procedure was performed in all subjects unless stated otherwise.
Sensors for respiratory recordings, submental EMG and ECG were placed. The manometry catheter was placed in subjects nos 11–15.
We performed upper airway endoscopy (to evaluate anatomy, sensation and laryngeal motor function). Then we injected 3 ml of fluid (bupivacaine or saline) into the paraglottic compartment bilaterally. Fifteen minutes later we repeated the endoscopy.
Ventilation using inductance plethysmography and nasally expired PCO2 was recorded for 5 min.
The C-arm was positioned for fluoroscopic recording. subjects no. 6–11 and 16 were cued to take a sip from a cup of barium up to five times, and each sip and subsequent swallow(s) were recorded.
The facemask was attached. Subjects no. 1, 2, 4 and 5 breathed a mixture of CO2 that was titrated to the target level. The remaining subjects breathed room air.
Oronasal airflow during Valsalva and Müller manoeuvres, and during a voluntary cough was recorded.
A series of automated bolus infusions was given, and the swallow(s) subsequent to each infusion was recorded. At least three full breaths preceded the bolus infusion, and at least three breaths were allowed to elapse after infusion before the next run (Fig. 1A). The bolus infusions were given at various times throughout the respiratory cycle. In order to avoid any regularity in the presentation of the bolus that might be anticipated by the subject, the number of control breaths was varied between 3 and 10, and the time of infusion within the respiratory cycle was changed randomly from one run to the next.
The facemask was removed, and in subjects no. 6–11 and 16 step (4) was repeated.
In subjects nos 4, 5, 7, 9 and 16 a series of spontaneous swallows during slow infusion of water onto the tongue was recorded. This was not captured on fluoroscopy.
Upper airway endoscopy was repeated in subjects no. 2, 5–7, 9–11 and 14–16 within 30 min (range 3.7–29.8 min) after their last fluoroscopically recorded swallow.
Criteria for ending a session
A session was terminated when the subject had received the maximum allowable radiation exposure, or if the subject developed a bout of coughing that lasted > 1 min.
Debriefing session and follow-up
We noted each subject's comments during the experiments, and during a debriefing session immediately following completion of each experimental session. In the debriefing session, we allowed the subject to describe sensations related to their swallowing and breathing in the various conditions without prompting, and then we asked specific questions. All subjects were contacted on the day after the experiment in which they received neck injections. Subjects were instructed to report any symptoms that might develop during the 2 weeks that followed the experiments, including difficulty swallowing or breathing, sore throat, fever, cough or chest pain.
Data handling
We reviewed each swallow captured on videofluoroscopy at normal speed, then at slow speed, stopping for frame-by-frame analysis of specific markers of swallowing phases and suspected abnormalities. At least two investigators reviewed the tapes independently. One of the reviewers was masked with respect to the subject's condition at the time of the study (i.e. control, bupivacaine injection or saline injection). If there was disagreement between the investigators, the event in question was omitted from analysis (< 1 % of observations).
All analog signals were digitised (sample rate 1 kHz, AT CODAS, DATAQ Instruments, Akron, OH, USA), displayed online and saved on digital storage media for subsequent replay using playback software (WinDaq Waveform Browser, DATAQ Instruments). The submental EMG signal was whole-wave rectified, and integrated in 50 ms intervals (Advanced CODAS, DATAQ Instruments). The airflow signal was also integrated to obtain inhaled and exhaled volumes. A computer program was written to determine for each swallow the onset, peak and offset of EMG activity if the value of the integrated signal exceeded two standard deviations above the baseline activity over the preceding 10 s. The onsets and peaks of expired and inspired volumes were also determined by the program if the volume in either direction was > 5 ml. If the signal remained within ± 5 ml for more than 0.25 s the program labelled this period as apnoea and marked the onset and offset of zero airflow. Apnoea that coincided with a swallow was designated as deglutition apnoea.
An experimental run was excluded from analysis if there was a burst of EMG activity during the control breaths before the beginning of an automated bolus infusion. This exclusion was rare (16 out of a total of 259 runs in 11 subjects). Manometer recordings were excluded from analysis if the catheter moved during the study by a distance > 1 mm relative to the position at the beginning of the study. This occurred in one subject, resulting in exclusion of 6 out of a total of 11 runs.
Definitions
Fluoroscopic events
We determined the following times for each swallow: onset of hyoid movement in the anterior direction, peak excursion of the hyoid bone in the anterior and superior direction, initial contact of barium with the vallecula, arrival of the head of the bolus in the hypopharynx (bolus arrival, BA), and departure of the tail of the bolus from the hypopharynx at the level of the arytenoids (bolus departure, BD). On occasion, barium trickled around the vallecula and entered the piriform sinus before BA. In these cases, the time of piriform contact was determined. Bolus transit time in the hypopharynx was defined as the interval between BA and BD. The time of laryngeal closure was the first frame in which the larynx was completely closed, having tissue density with no air density in the larynx. The time of laryngeal opening was the first frame with a continuous air density from the pharynx to the trachea. Sometimes laryngeal closure and anterior hyoid movement occurred without transport of barium through the pharynx. These movements were not considered a swallow. The onset of laryngeal penetration was defined as the time at which barium entered the larynx past a line across the laryngeal introitus at the most anterior point of the piriform recess, parallel to the axis of the cervical spine. If barium was seen entering the trachea (below the vocal folds) the time of tracheal aspiration was determined.
Phase resetting analysis
Figure 1B illustrates the definitions that we used to quantify phase resetting of respiratory rhythm by swallowing. The time within the respiratory cycle at which swallowing was initiated was defined as old phase (Φ), measured as the interval from the onset of inspiration to the onset EMG activity (Paydarfar et al. 1995). The effect of the swallow on the timing of the subsequent breaths was defined by cophase (Θ), measured as the time from EMG peak to the onset of the subsequent inspirations following the swallow (Θ1, Θ2, Θ3). The resulting data were normalised by assigning a value of 1 to the average period of three control breaths preceding the swallow. Thus, the old phase and cophases were expressed as fractions of one respiratory cycle rather than units of time.
If the swallow had no effect on respiratory timing of the first breath, then as the swallow was initiated progressively later in the respiratory cycle (increasing Φ), the latency from the swallow to the next breath (i.e. Θ) would become progressively smaller; Θvs.Φ would be defined by a line with a slope of −1. Deviations from this line are due to phase resetting, and the amount of deviation from the ‘no resetting’ line provides a quantitative index of the strength of phase resetting. In order to prove that such deviations are due to phase resetting of rhythm, they must persist beyond the first cycle after the swallow (Paydarfar et al. 1995).
Latencies between hypopharyngeal bolus flow and oronasal airflow (Fig. 1B)
For each swallow induced by automated bolus infusion we measured the latency from the end of inspiration to BA, defined as α. The latency from BD to the onset of the next inspiration is defined as δ. If α or δ are < 0, inspiration is concurrent with bolus transit through the hypopharynx.
Statistical analyses
To compare the incidences of penetration or of aspiration, we used the Wilcoxon test (paired, control vs. vehicle or bupivacaine injection) and the Mann-Whitney test (unpaired, vehicle injection vs. bupivacaine injection). We calculated mean values ±s.e.m. of measurements related to swallowing and breathing for each condition (control, vehicle injection, bupivacaine injection). For single comparisons of these variables we used Student's t test (paired or unpaired). Multiple comparisons were necessary for analysing the swallow sequence because a given swallow event is also computed within a swallow interval in the sequence, and therefore events and intervals cannot be considered as independent measurements. Therefore, to test for effects of anaesthetic injection compared to no intervention, we used Analysis of Variance for Mixed Models (McLean et al. 1991) by Maximum Likelihood (REML) using PROC MIXED from the SAS Statistical Analysis System (SAS Institute Inc, Cary, NC, USA). The distributional characteristics of the data were evaluated by inspection of frequency histograms of residuals from the linear model. For all statistical tests, differences were considered significant if the null hypothesis was rejected at P < 0.05.
RESULTS
Globus sensation and effortful swallowing during ISLN anaesthesia
Within 15 min after neck injection with bupivacaine, all 16 subjects described a constant foreign body sensation, like ‘something was stuck’ in the throat, and/or a sensation of fullness, like the throat was ‘swollen’. They described an urge to clear their throats and to swallow repeatedly to relieve the foreign body sensation. In addition, they reported symptoms of swallowing difficulty, i.e. dysphagia. On specific questioning, none of the subjects experienced difficulty collecting the bolus in the mouth and delivering it to the throat, but they all needed to use greater effort to ‘push’ the bolus through the throat. For example, they described the ‘need to give more force to swallow’, ‘coddle’ and ‘exaggerate’ the swallow. Most subjects reported a gradual improvement of these symptoms over the course of the experiment. Six subjects with ISLN anaesthesia were aware that they had aspirated (e.g. ‘something went down the wrong way’).
In the non-anaesthetised condition, all subjects reported that their swallowing felt normal. Some subjects described a transient ‘lump in the throat’ or ‘swelling’ sensation immediately after vehicle injection, which always resolved within 10 min after injection.
Anatomical examination of the upper airway
Endoscopic examination revealed normal pharyngeal and laryngeal anatomy prior to neck injection in all subjects. Fifteen minutes after neck injection, we found a slight unilateral distortion of the piriform mucosa suggestive of submucosal swelling in two subjects (nos 7 and 18), and similar changes in the left aryepiglottic fold in one subject (no. 4). Upper airway anatomy was normal after neck injections in the remaining subjects.
Sensory loss associated with ISLN anaesthesia
Prior to neck injection, all 21 subjects detected endoscopic probing of all sites in the pharynx and larynx. The sensation was most frequently described as an unpleasant feeling (e.g. ‘like a jab’) in the throat, sometimes with concomitant gagging. There was no change in laryngeal or pharyngeal sensation after vehicle injection (six subjects).
Fifteen minutes after bilateral bupivacaine injections, both arytenoids and the laryngeal surface of the epiglottis were completely anaesthetised in 16 subjects. Further testing in these subjects revealed that the piriform sinuses were completely anaesthetised in 12 subjects, partially anaesthetised in one subject (no. 16), and fully sensate in three subjects (nos 7–9). The base of the tongue was anaesthetised in 12 subjects, partially anaesthetised in three subjects (nos 6, 8 and 9), and fully sensate in one subject (no. 15). The lingual surface of the epiglottis was anaesthetised in 7 of 12 subjects tested (it could not be reached in four subjects), and the vallecula was anaesthetised in 7/7 subjects in whom it could be reached. The pillars of the fauces were tested in two subjects, and the posterior hypopharynx in one subject. The sensation of these areas was unaffected by ISLN anaesthesia. We avoided contact of these areas in the remaining subjects to minimise discomfort due to gagging.
Voluntary laryngeal closure was not impaired by ISLN anaesthesia
Using endoscopy, we visualised complete glottic closure with the Valsalva manoeuvre and voluntary cough before and after neck injection in all subjects. Similarly, vocal cord movement was symmetric with vocalisation in all conditions. We also recorded apnoea with no air leaks when we repeated these manoeuvres, as well as the Müller manoeuvre, while recording oronasal airflow.
Airway protection during swallowing was impaired by ISLN anaesthesia
Figure 2 is an example, in a subject with ISLN anaesthesia, of barium penetration into the larynx followed by aspiration into the trachea on videofluoroscopy. Overall, 43 % of all swallows after ISLN anaesthesia led to penetration. Of these penetrations, 56 % were followed by aspiration. In the non-anaesthetised condition, penetration was seen with less than 2 % of the swallows, and aspiration was never observed. Table 2 shows the breakdown of the number of swallows, penetrations and aspiration for each of the 16 subjects in the anaesthetised condition. Table 3 shows the total number of runs, swallows and penetrations in the non-anaesthetised condition. The laryngeal penetrations enumerated in Tables 2 and 3 were associated with swallowing. We observed 11 additional penetrations with ISLN anaesthesia that were not related to swallowing. Some of these occurred during other laryngeal movements, e.g. those related to coughing. Others occurred without clear laryngeal movement while the larynx was open. In these instances, the source of penetration was residual barium in the vallecula or the piriform recess. Ten of these penetrations led to tracheal aspiration. In all subjects with ISLN anaesthesia, spontaneous cough was observed only after entry of fluid into the trachea.
Figure 2. Example of penetration and aspiration in a subject (no. 7) with ISLN anaesthesia.
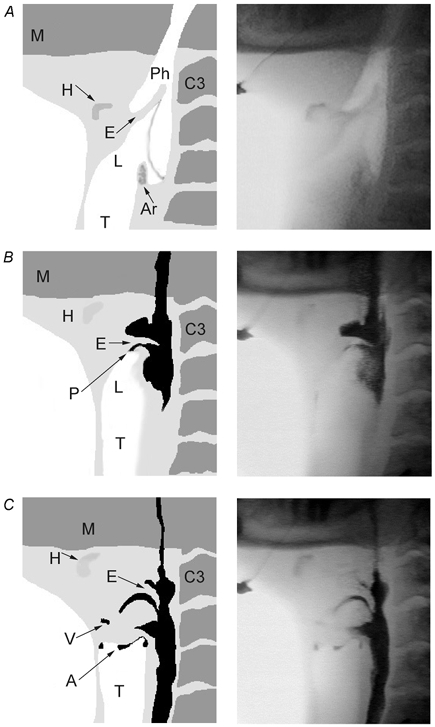
Three fluoroscopic images (right panels) and the corresponding schematic rendition (left panels) of the upper airway just before (A) and during (B and C) a single swallow in the ISLN-anaesthetised condition. A, prior to swallowing, the hyoid (H) bone is in the resting position, and the epiglottis (E) is pointing rostrally. The pharynx (Ph) and larynx (L) are open. B, the swallow is in the pharyngeal phase, 1.13 s after the onset of an automated bolus infusion into the mouth. The hyoid is blurry as it moves anteriorly. Barium, shown in black, is now mostly in the pharynx with a small amount penetrating (P) into the larynx between the horizontal epiglottis and the tip of the arytenoids. C, the larynx appears closed but there is barium in the vestibule (V) and aspiration (A) just beneath the vocal cords, 1.18 s after the onset of bolus infusion. Ar: arytenoids; C3: third cervical vertebra; M: mandible; T: trachea.
Table 2.
Number of events in subjects with ISLN anaesthesia
| Subject no. | Runs | Swallows | Penetrations | Aspirations |
|---|---|---|---|---|
| 1 | 22(22,0) | 27(27,NA) | 8(8,NA) | 5(5,NA) |
| 2 | 30(30,0) | 30(30,NA) | 4(4,NA) | 0(0,NA) |
| 3 | 5(5,0) | 5(5,NA) | 3(3,NA) | 2(2,NA) |
| 4 | 20(20,0) | 39(39,NA) | 12(12,NA) | 10(10,NA) |
| 5 | 35(35,0) | 35(35,NA) | 13(13,NA) | 11(11,NA) |
| 6 | 29(21,8) | 33(21,12) | 6(0,6) | 0(0,0) |
| 7 | 17(9,8) | 20(10,10) | 13(9,4) | 5(4,1) |
| 8 | 9(1,8) | 17(1,16) | 7(0,7) | 4(0,4) |
| 9 | 18(10,8) | 47(29,18) | 43(29,14) | 28(21,7) |
| 10 | 25(15,10) | 27(15,12) | 0(0,0) | 0(0,0) |
| 11 | 17(10,7) | 18(10,8) | 14(8,6) | 10(5,5) |
| 12 | 15(15,0) | 27(27,NA) | 9(9,NA) | 3(3,NA) |
| 13 | 5(5,0) | 5(5,NA) | 4(4,NA) | 2(2,NA) |
| 14 | 30(30,0) | 32(32,NA) | 14(14,NA) | 2(2,NA) |
| 15 | 13(13,0) | 16(16,NA) | 7(7,NA) | 2(2,NA) |
| 16 | 9(5,4) | 18(12,6) | 13(9,4) | 11(8,3) |
| Total: 16 | 299(246,53) | 396(314,82) | 170(129,41) | 95(75,20) |
ISLN, internal branch of the superior laryngeal nerve. Values in parentheses are: (number associated with automated bolus infudion, number associated with sip from a cup). Most subjects swallowed multiple times during some of their runs. NA, not applicble because sip from cup was not studied in these subjects.
Table 3.
Number of events in subjects without ISLN anaesthesia
| Protocol | Subject no. | Runs | Swallows | Penetrations | Aspirations |
|---|---|---|---|---|---|
| Control | 1–15, 17–21 | 492(407,85) | 522(426,96) | 8(6,2), subject nos 3,7,9,21 | 0(0,0) |
| Vehicle | 16–21 | 169(121,48) | 174(124,50) | 2(1,1), subject no. 17 | 0(0,0) |
| Total | 21 | 661(528,133) | 696(550,146) | 10(7,3) | 0(0,0) |
Values in parentheses are: (number associated with automated bolus infusion, number associated with sip from a cup). Most subjects swallowed multiple times during some of their runs.
There was a significant increase in the incidence of penetration (paired, P < 0.0005) and aspiration (P < 0.002) with ISLN anaesthesia compared with control. Compared with vehicle injection, bupivacaine injection was associated with a significant increase in penetration (unpaired, P < 0.005) and aspiration (P < 0.02). Subjects who received vehicle injection in one arm and no intervention in the other arm, exhibited no increase in the incidence of penetration, and no aspirations in either arm.
In the anaesthetised condition, we found no clustering of the incidence of penetration or aspiration related to the method of bolus presentation, the bolus volume, the level of ventilation, whether the subject was masked to the content of neck injection, or the extent of extra-laryngeal anaesthesia at the beginning of the experiment. Likewise, our findings were not influenced by whether the control experiment was performed before or after the experiment with neck injection. In 12 subjects, penetrations were observed uniformly throughout their recordings, which ended 48–200 min after neck injection. In three subjects (nos 6, 7 and 14), penetrations ceased to occur late in the study, and in subject no. 10 penetration did not occur at all. These findings were not accompanied by recovery of laryngeal mucosal sensation at the end of the experiment. In contrast, subject no. 21 continued to have penetration and aspiration throughout her experiment, despite the fact that when she was tested within 5 min after the end of her recordings, she had recovered mucosal sensation in all her upper airway structures except the arytenoids, which remained completely anaesthetised.
Timing of penetration and aspiration relative to swallowing and breathing
Penetration usually coincided with the pharyngeal phase of swallowing. Figure 3A is an example of airflow and EMG recordings following automated injection of barium. Both laryngeal penetration and aspiration occurred during the pharyngeal phase, which is marked by peak integrated submental EMG activity, apnoea, anterior hyoid movement and bolus transit through the pharynx. Indeed, 91 % of the penetrations were clustered during the pharyngeal phase (Fig. 3B). Furthermore, penetration occurred prior to peak pharyngeal contraction, at less than 20 % of peak pharyngeal pressure (Fig. 4). The distribution of aspirations on the other hand was bimodal (Fig. 3C), with 68 % falling during the pharyngeal phase, while 28 % occurred within 5 s after the end of the pharyngeal phase.
Figure 3. The incidence of laryngeal penetration peaks during the pharyngeal phase of swallowing.
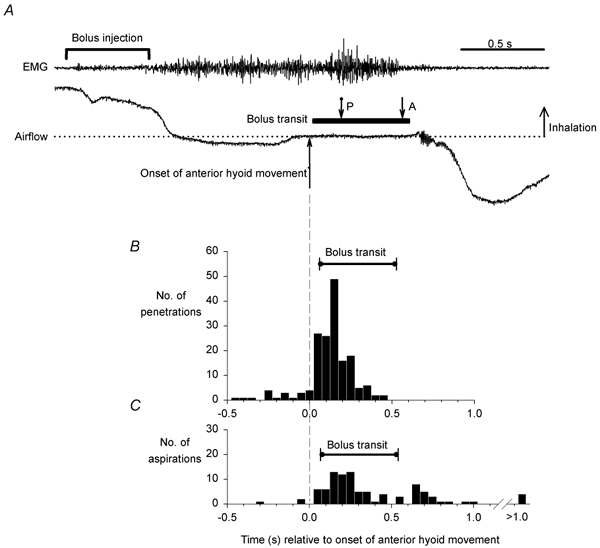
A, a single swallow following automated bolus infusion onto the tongue in subject no. 5 (ISLN-anaesthetised). Submental EMG activity begins after the onset of barium infusion, and marks the onset of the oral phase of swallowing. The onset of deglutition apnoea, marked by zero airflow (dotted line), is followed by the onset of anterior hyoid movement and bolus transit through the hypopharynx, all of which mark the pharyngeal phase of swallowing. Both penetration (P) and aspiration (A) occur during the pharyngeal phase. B, the incidence of all penetrations (n = 170) and associated bolus transits and, C, all aspirations (n = 95) and associated bolus transits, in subjects with ISLN anaesthesia relative to the time of onset of anterior hyoid movement.
Figure 4. The timing of penetration relative to pharyngeal pressure.
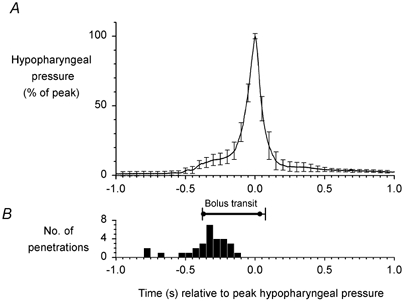
A, mean pharyngeal pressure in 29 swallows associated with penetration across 5 subjects with ISLN anaesthesia. Time = 0 is the time of peak pharyngeal pressure in each swallow. Mean peak hypopharyngeal pressure in each subject was set to 100 % to normalise mean pressure across subjects. B, the timing of penetration and bolus transit associated with the swallows depicted in A. All penetrations occurred before peak pharyngeal pressure and most were observed in the early phase of pharyngeal contraction and bolus transport through the hypopharynx.
A few penetrations (9 %) occurred prior to the onset of the pharyngeal phase (Fig. 3B). All of these were associated with premature spillage of barium into the pharynx prior to anterior hyoid movement or bolus transit. In the non-anaesthetised state, two penetrations were associated with premature spillage and occurred early. The remaining eight occurred during the pharyngeal phase. Overall, 23 % of the swallows with ISLN anaesthesia, and 38 % of the swallows without anaesthesia, were associated with premature spillage of barium into the piriform recesses.
Oronasal airflow was negligible around the time of penetration
In order to assess whether inspiratory airflow contributed to the penetration of barium into the larynx, we analysed oronasal airflow recordings around the time of penetration. We found that oronasal airflow rarely occurred within ±200 ms of laryngeal penetration (Fig. 5A), and when it did, it was of very low amplitude (Fig. 5B).
Figure 5. Direction and magnitude of airflow around the time of penetration.
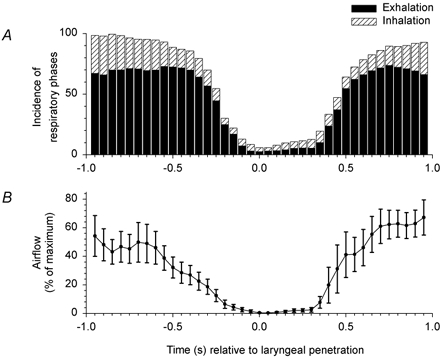
A, the incidence of inhalation and exhalation was determined ±1 s around the time of penetration (time = 0) in 81 swallows in 7 subjects with ISLN anaesthesia, including the 4 subjects studied during hyperpnoea. B, mean absolute airflow (inhalation and exhalation), normalized to maximum airflow for each of the 7 individuals represented in A. Oronasal airflow rarely occurred within ±200 ms of laryngeal penetration, and when it did, it was of very low amplitude.
Pressure waves generated by swallowing were blunted during ISLN anaesthesia
Figure 6 shows an example of pharyngeal and cricopharyngeal pressures associated with swallowing before and during ISLN anaesthesia. Peak hypopharyngeal pressure was diminished during ISLN anaesthesia compared with control in all five subjects who had manometry recordings (81.4 ± 20.4 vs. 119.6 ± 17.3 mmHg, P < 0.02), and the cricopharyngeal pressure trough was blunted (−5.7 ± 2.4 vs. −10.5 ± 1.2 mmHg, P < 0.05). The mean resting tonic cricopharyngeal pressure was decreased but the values did not reach statistical significance (15.3 ± 6.2 vs. 20.9 ± 5.7 mmHg, P = 0.064). Similarly, the pharyngeal pressure at bolus arrival was not affected by ISLN anaesthesia (7.2 ± 3.2 vs. 4.1 ± 1.7 mmHg, P > 0.4).
Figure 6. Pharyngeal and upper oesophageal pressures generated by swallowing in subject no. 12.
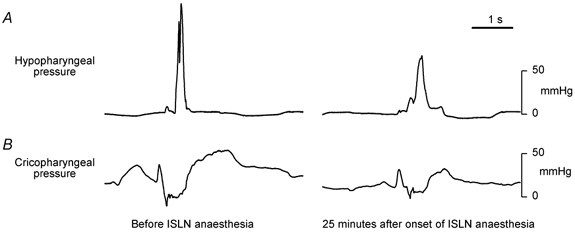
The peak hypopharyngeal and trough upper oesophageal sphincter pressures are blunted in the ISLN-anaesthetised condition, compared with the control tracings.
The effect of ISLN anaesthesia on the timing of the swallow sequence
We analysed the first swallow that followed each automated bolus injection in all subjects who received the anaesthetic injection. Figure 7 depicts the timing (expressed as mean ±s.e.m. between subjects) of all swallow events that were recorded for these subjects in the ISLN-anaesthetised condition (closed circles and bars) and in the control condition (open circles and bars). The discrepancy in number of subjects in Tables 1–3 is because Fig. 7 shows data only from the first swallow after automated bolus infusion, and in some subjects all events were not recorded.
Figure 7. The effect of ISLN anaesthesia on the timing of the swallow sequence.
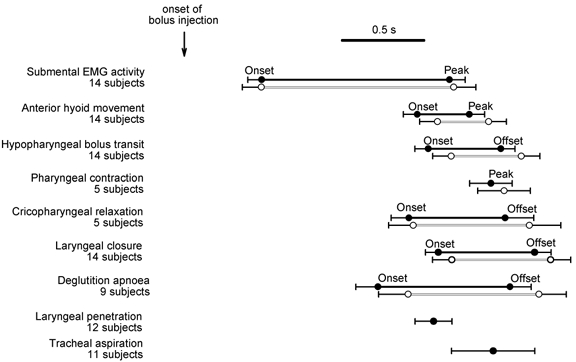
Measurements were taken from the first swallow induced by automated bolus infusion, with ISLN anaesthesia (closed circles and bars), and with no intervention (open circles and bars). Error bars depict s.e.m. The mean time after onset of bolus infusion of all swallow events (weighted to the number of subjects) was 1.638 s in the anaesthetised condition and 1.741 s in the control condition (no intervention). The difference in the means (103 ms) is significant (F = 10.18, P = 0.002). The intervals between events within the swallow sequence were unaffected by ISLN anaesthesia.
In the anaesthetised condition all events and intervals after onset of submental EMG activity occurred earlier than the corresponding events and intervals in the control condition. The mean time after onset of bolus infusion of swallow events (weighted to the number of subjects) was 1.638 s in the anaesthetised condition and 1.741 s in the control condition. Analysis of variance (see Methods section) reveals that the difference in the means (103 ms) is significant (F value = 10.18, P = 0.002). The analysis also suggests that the intervals between events within the swallow sequence were unaffected by ISLN anaesthesia, since we found that the relationship among the swallow events was unaffected by ISLN anaesthesia (F = 0.26, P > 0.5). In particular, there was no change with ISLN anaesthesia in the duration of laryngeal closure, deglutition apnoea or hyoid movement. The latency from barium arrival at the vallecula to bolus arrival was not significantly changed with ISLN anaesthesia compared with control. Furthermore, there was no significant difference in the timing from the onset of anterior hyoid movement to laryngeal closure, bolus arrival to laryngeal closure, or the onset of anterior hyoid movement to bolus arrival. In other words, analysis of the timeline suggests that ISLN anaesthesia reduced the latency from bolus infusion to activation of the swallow sequence, without affecting the timing of events within the sequence.
Figure 7 also shows the timing of penetration and aspiration in the anaesthetised condition. In the control condition, there were only three penetrations (in two subjects), with latencies of 1.91, 0.80 and 1.00 s after onset of infusion.
Lack of effect of ISLN anaesthesia on ventilation, CO2-induced air hunger, and the co-ordination between breathing and swallowing
While breathing room air, the subjects’ mean minute ventilation, tidal volume, respiratory rate and end-tidal PCO2 were not changed after the injection of anaesthetic compared with the control study (7.2 ± 1.2 vs. 7.6 ± 1.5 l min−1, 0.53 ± 0.08 vs. 0.49 ± 0.09 l, 15.2 ± 1.5 vs. 16.7 ± 1.2 breaths min−1, 39.1 ± 2.2 vs. 41.4 ± 1.2 mmHg, respectively). ISLN anaesthesia did not cause dyspnoea in the subjects studied on room air, and in the subjects studied during hypercapnoeic hyperpnoea there was no difference in the inhaled FI,CO2 that induced moderate air hunger in the anaesthetised vs. non-anaesthetised conditions. Furthermore, during CO2-induced hypercapnoea there were no differences in the mean minute ventilation, tidal volume, respiratory rate or end-tidal PCO2 in the anaesthetised vs. non-anaesthetised conditions (49.4 ± 8.3 vs. 45.7 ± 6.0 l min−1, 2.1 ± 0.3 vs. 2.0 ± 0.3 l, 24.8 ± 0.5 vs. 23.9 ± 1.4 breaths min−1, 51.2 ± 1.7 vs. 52.7 ± 1.2 mmHg, respectively).
ISLN anaesthesia did not affect the co-ordination between breathing and swallowing in any of the subjects. Figure 8 shows an example in one subject (no. 5) during hyperpnoea. Figure 8A shows that the initiation of spontaneous swallows (during continuous infusion of water onto the tongue) was clustered during the middle to late inspiratory phase, a property that was unaffected by ISLN anaesthesia. Figure 8B-D depicts data related to swallows initiated by automated bolus infusion (0.5 s) given at various times throughout the respiratory cycle. ISLN anaesthesia did not affect the strong resetting of the respiratory rhythm induced by swallowing (Fig. 8B). Both α and δ were positive, with the exception of three control runs, indicating that inspiration did not coincide with bolus transit in most instances (Fig. 8C and D). Indeed, α and δ were positive in all the runs that were associated with laryngeal penetration. Finally, we analysed the swallows associated with a laryngeal penetration in all subjects. We constructed a histogram of the incidence of laryngeal penetration as a function of the respiratory phase of swallow initiation (Φ). The incidence of laryngeal penetration was evenly distributed (not shown), i.e. penetration was not more likely for swallows initiated at a particular phase in the respiratory cycle.
Figure 8. The co-ordination between swallowing and respiration in subject no. 5.
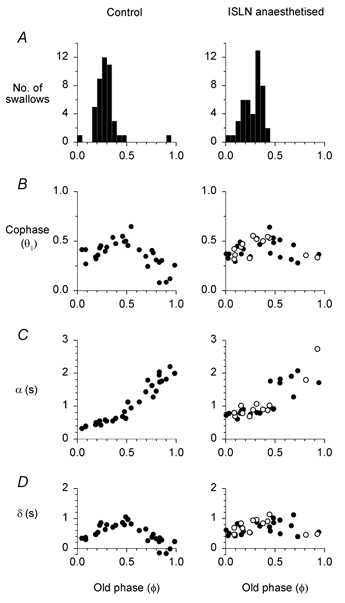
A, incidence of spontaneous swallows during continuous water infusion over the tongue. B, C and D are all derived from swallows induced by automated bolus infusion for 0.50 s. Open circles refer to swallows that were associated with penetration into the trachea. B, swallowing induced strong resetting of the respiratory cycle, which persisted beyond the first respiratory cycle (Θ2, Θ3 plots are not shown), and was unaffected by ISLN anaesthesia. C and D, the latencies from the end of inspiration and bolus arrival in the pharynx (α) and from bolus departure to onset of the next inspiration (δ) are unaffected by ISLN anaesthesia. See Fig. 1 for definitions of Φ, Θ, α and δ.
Safety of ISLN anaesthesia
Every subject who had an aspiration during an experiment coughed within seconds after aspirating. In most, coughing resolved within 1 min. If coughing persisted for longer than 1 min, the experiment was ended (subjects no. 3, 8, 11 and 19; in these cases coughing resolved within 5 min). All subjects reported that their dysphagia resolved within 5 h after ISLN anaesthesia. Six subjects who received the anaesthetic and one subject who received vehicle injection reported a mild ‘sore throat’ or ‘scratchy throat’ the day after the experiment, which resolved spontaneously within 2 days. During the 2 week follow-up period no subject had any other adverse effects or complications.
DISCUSSION
The major finding of this study was that anaesthetic blockade of the ISLN in healthy subjects induced effortful swallowing, an illusory sensation of throat swelling or obstruction, and a loss of airway protection due to insufficient closure of the larynx during swallowing. We further analysed the swallow cycle to evaluate the mechanism(s) by which fluid entered the larynx. Laryngeal penetration was not caused by premature spillage of oral fluid into the hypopharynx, delayed clearance of fluid from the hypopharynx, or excessive hypopharyngeal pressure generated by swallowing. Furthermore, there was no impairment in the ability of swallowing to halt respiratory airflow during the period of pharyngeal bolus flow. Rather, our observations suggest that loss of airway protection was due to incomplete closure of the larynx during the pharyngeal phase of swallowing. In contrast to the insufficient closure during swallowing, laryngeal closure was robust during voluntary challenges with the Valsalva, Müller and cough manoeuvres. We propose that an afferent signal arising from the ISLN receptor field is necessary for normal deglutition, especially for providing feedback to central neural circuits that facilitate laryngeal closure during swallowing. The ISLN afferent signal is not essential for initiating and sequencing the swallow cycle, for co-ordinating swallowing with breathing, or for closing the larynx during voluntary manoeuvres.
Dysphagia, globus sensation and impaired airway protection during swallowing
All subjects reported a sensation of laboured or effortful swallowing during ISLN anaesthesia, a symptom noted in previous studies of swallowing during upper airway anaesthesia (Pommerenke, 1928; Månsson & Sandberg, 1974; Tanabe et al. 1975; Horner et al. 1991; Fitzpatrick et al. 1995; Bastian & Riggs, 1999). We further defined the sensory experience associated with ISLN blockade and found that there were no problems executing the oral phase of swallowing. Rather, the dysphagia was isolated to the pharyngeal phase, during which the subjects felt the need to generate a more forceful swallow in order to move the bolus down the throat. They also experienced a persistent sensation of fullness or swelling in the throat, and the urge to swallow repeatedly in order to relieve the perceived obstruction. These are the symptoms of globus pharyngis (e.g. Deary et al. 1995), which in our subjects were illusory because endoscopy and videofluoroscopy revealed no significant swelling, narrowing or obstruction of the pharynx or larynx during ISLN anaesthesia. Furthermore, the bolus flowed through the pharynx without delay and there was no evidence of cricopharyngeal hypertonia.
Using videofluoroscopy, we found that ISLN anaesthesia impaired the closure of the larynx during the pharyngeal phase of swallowing. The key observation was leakage of the bolus from the pharynx into the laryngeal vestibule (e.g. Fig. 2B and C). We interpret this as a sign of incomplete closure of the laryngeal aditus and false cords. Laryngeal closure was defined as the obliteration of the laryngeal air column on the radiographic images. Although this is an easily recognised marker of laryngeal closure, its presence does not guarantee that closure is robust, as highlighted by our observation that loss of the air column usually occurred just before laryngeal penetration. In just over a half of the laryngeal penetrations, the fluid proceeded to enter the trachea, a sign of incomplete closure of the glottis. While there was variability in the timing of laryngeal penetration and tracheal aspiration, the majority occurred during the pharyngeal phase of swallowing (Fig. 3). It is noteworthy that in the ISLN-anaesthetised condition, spontaneous coughing occurred only after tracheal aspiration rather than earlier when fluid was confined to the larynx. Therefore, it is possible that a delay in the triggering of the cough reflex contributed to the aspiration process by allowing fluid to enter the trachea during the swallow if glottic closure was incomplete, or after the swallow when the glottis normally reopens.
We analysed the timing of laryngeal closure and of other components of the pharyngeal swallow. In the anaesthetised condition, the pharyngeal sequence appeared ≈0.1 s earlier than expected following bolus infusion. However, the intervals within the sequence were unaffected by ISLN blockade. Notably, we found no derangement in the timing of the swallow cycle that would lead to aspiration. For example, laryngeal penetration cannot be explained by premature contractions of the base of the tongue or hypopharynx that would propel the bolus into the hypopharynx before closure of the larynx. We also found no evidence that blocking the ISLN increases the incidence of premature spillage of liquid into the hypopharynx, delays the relaxation of the upper oesophageal sphincter, or slows the transport of the bolus through the pharynx. The only manometric abnormalities that were consistently detected during ISLN anaesthesia were reductions in the peak constriction pressure in the hypopharynx and the trough relaxation pressure in the upper oesophageal sphincter. It is possible that these pressure waves were reduced because of diminished activation of the pharyngeal constrictors and of the cricopharyngeus or other muscles that influence upper oesophageal sphincter relaxation. Experiments in anaesthetised animals have shown that ISLN afferents activate pharyngeal swallow neurones within the medulla (Jean, 1972). Our observations raise the possibility that removal of the ISLN facilitatory input to central swallow neurones can diminish the motor output of the pharyngeal swallow programme. However, another possibility is that the peak and trough pressure waves were blunted by dissipation of pressure through an incompletely sealed larynx. In any case, the observed blunting of pressure waves did not appear to contribute to the penetration of fluid into the larynx.
ISLN regulation of swallowing and airway protection
Our findings support the hypothesis that an afferent signal arising from the ISLN receptor field is essential for normal swallowing function, especially for ensuring the effective closure of the larynx and prevention of aspiration during normal swallowing. Silencing the ISLN activity by anaesthetic blockade caused a failure in the motor control of laryngeal closure during the pharyngeal phase of swallowing. In contrast to the insufficient closure during swallowing, laryngeal closure was normal during voluntary challenges with the Valsalva, Müller and cough manoeuvres. The robustness of laryngeal closure during these voluntary manoeuvres under ISLN anaesthesia is consistent with previous physiological studies that suggest the ISLN lacks efferent motor function in dogs (Reid, 1838; Lemere, 1932,1933; Mårtensson, 1963), goats (Murtagh, 1945) and humans (Tanabe et al. 1975).
It is likely that laryngeal mechanoreceptors are normally stimulated by laryngeal motor activities during the oral and pharyngeal phases of swallowing. These movements include early laryngeal elevation (Ardran & Kemp, 1952) and vocal cord adduction (Shaker et al. 1990; Flaherty et al. 1995). Therefore, a burst of ISLN activity that begins early in the swallow cycle, before entry of the bolus into the hypopharynx, could intensify laryngeal closure by afferent facilitation of central neural circuits. Tonic ISLN activity may also be important for maintaining the proper level of excitability of these circuits, similar to tonic afferent facilitation of spinal segmental reflexes (Nathan & Sears, 1960).
In most of our subjects, sensory testing during ISLN anaesthesia revealed that the ISLN receptor field extended beyond the supraglottic larynx and included the lingual surface of the epiglottis, the vallecula and the piriform sinuses. The presence of extra-laryngeal sensory innervation of the ISLN is consistent with anatomical studies (Sanders & Mu, 1998). It is not possible, therefore, to clearly resolve which components of the subjective and objective findings are due solely to deafferentation of the larynx. Some of our subjects with laryngeal anaesthesia had normal extra-laryngeal mucosal sensation, and developed the complete syndrome of dysphagia, globus sensation and aspiration. However we cannot exclude the possibility that submucosal extra-laryngeal ISLN afferents were blocked in these subjects.
We are aware of only a few animal studies that have looked for signs of dysphagia and aspiration after bilateral transection of the ISLN. Reid (1838) observed that following transection in two dogs and one rabbit, ‘the animals readily swallowed both solids and fluids without exciting the slightest cough or the least difficulty of breathing. The lungs were carefully examined after death, and none of the food taken could be detected in the air-passages’. Blumin et al. (1999) monitored 20 dogs for a 6 month period after bilateral ISLN transection. None of the animals exhibited cough, fever, pneumonia, or a significant change in feeding behaviour or weight. On the other hand, Venker-van Haagen et al. (1999) suspected aspiration in two of three dogs with bilaterally transected ISLNs because the dogs produced ‘wet sounds from the trachea’. However, interpretation of the animal studies is limited by the lack of a direct method for visualising the upper airway and detecting tracheal aspiration during feeding. In addition, it is not known whether these animal models are applicable to airway vulnerability during swallowing in humans. For example, there are large differences between species in the position and orientation of the larynx relative to the pharynx (Negus, 1949). Radiographic (Ardran & Kemp, 1952; Logemann et al. 1992; Kahrilas et al., 1997) and endoscopic (Langmore et al. 1988; Shaker et al. 1990; Bastian & Riggs, 1999; Sulica et al. 2002) techniques have been established for visualising the upper airway during swallowing in humans. Yet to our knowledge, there has been no previous study that uses these techniques to study airway protection during selective ISLN anaesthesia, or in patients with isolated ISLN damage.
In a recent endoscopic study of swallowing in healthy subjects (Bastian & Riggs, 1999), application of lidocaine (lignocaine) to the mucosa of the entire upper aero-digestive tract, including the larynx, produced little or no laryngeal penetration or aspiration. Topical anaesthesia probably spares mechanoreceptors from the submucosa, laryngeal muscles and articular structures. In a subsequent study (Sulica et al. 2002), swallowing dysfunction and aspiration were found in subjects with mucosal anaesthesia of the pharynx, larynx and trachea, combined with subcutaneous injections of anaesthetic drug into the larynx (through the thyroid notch and around the ISLN bilaterally) and trachea (via the cricothyroid membrane). Although this study used widespread upper airway anaesthesia, it is possible that ISLN blockade, which anaesthetises afferents from both mucosal and deep receptors, was the critical procedure that led to aspiration.
Hypothetical alterations in central neural processing induced by sensory blockade
We propose that blockade of the ISLN causes globus sensation, dysphagia and aspiration by changing the function of central neural circuits that normally receive afferent information from the ISLN receptor field.
The laryngeal closure reflex is mediated centrally by a multisynaptic circuit (Sasaki & Suzuki, 1976; Ludlow et al. 1992). Subnuclei of the tractus solitarius in the dorsal medulla receive the afferent impulses from the ISLN, and vagal motor neurones in the ventral medulla provide the efferent output to the laryngeal muscles (Miller, 1982; Jean, 2001). We propose that deafferentation reduces the excitability of vagal motoneurones that mediate laryngeal closure during swallowing. While the medulla is required for activation of the laryngeal closure reflex, full closure during swallowing may require long feedback loops involving suprabulbar circuits. For example, the ISLN may facilitate swallowing through a ponto-cortico-medullary loop in addition to a purely bulbar mechanism (Sumi, 1972; Narita et al. 1999).
The failure in laryngeal closure appears to be specific to the task of swallowing because in our study, the subjects with ISLN blockade were able to maintain an airtight laryngeal seal against maximum transglottic pressures generated by the Valsalva, Müller and voluntary cough manoeuvres. This suggests that voluntary motor commands can activate full closure of the deafferented larynx, possibly via corticofugal projections to the vagal motor neurones within the nucleus ambiguus (Kuypers, 1958), or to neurones in the nucleus of the tractus solitarius (Jean & Car, 1979). It is possible, therefore, that the failure in ‘automatic’ laryngeal closure during swallowing can be reversed by voluntary facilitation of the closure mechanism. Other adaptive mechanisms may function to restore the ‘gain’ of deafferented central circuits, analogous to the compensatory increase in gain of the vestibulo-ocular reflex that is the basis for recovery of head-eye co-ordination after acute labyrinthectomy (Dichgans et al. 1973). It is noteworthy that following ISLN anaesthesia, laryngeal penetrations ceased to occur late in the study of three subjects. This finding was not explained by early recovery of ISLN-mediated sensation. We speculate that adaptive mechanisms could provide an important source of recovery of airway protection in the deafferented condition.
A striking symptom during ISLN blockade was laboured swallowing. The central neural substrate underlying the sensation of pharyngeal dysphagia is not known. However, perhaps our observations can be framed in a broader context, namely the perception of motor effort. The intensity of perceived effort associated with a motor task can be modified by peripheral afferents from the corresponding segmental level (for review, see McCloskey, 1981). For example, a weight lifted by flexion of the index finger feels heavier when the thumb is anaesthetised (Gandevia & McCloskey, 1977). One hypothesis on the origin of perceived effort is that the sensation is directly coupled to the centrally generated voluntary motor command that produces the motor activity (Gandevia & McCloskey, 1977; McCloskey, 1981). If mechanoreceptors provide excitatory drive to their corresponding motor neurone pool, removal of such afferent excitation would require a compensatory increase in descending central command (and hence an increase in perceived effort) needed to generate a given level of muscle output. An analogous interplay between peripheral feedback and central command signals could explain our subjects’ sensation of effortful swallowing if we assume that ISLN afferent activity provides excitatory drive to the neurones that generate pharyngeal swallowing activity, as suggested by studies in anaesthetised animals (Jean, 1972). Blocking the afferent signal would require a more intense or widespread cortical command signal to evoke the same swallowing motor output. Another possibility is that ISLN blockade causes dysphagia through pathways that are independent of the motor command to swallow, for example by removal of peripheral afferent input to cortical sensory circuits. At present there is insufficient knowledge of the neural substrate for swallowing sensation to distinguish between these possibilities or to suggest an alternative.
Illusory enlargement of a deafferented body part is a well-known curiosity, often described following amputation, injury or anaesthesia of a digit, and by subjects receiving oral anaesthesia for dental procedures. Gandevia & Phegan (1999) provided a systematic description of the phenomenon for the digit and lip in humans, and they proposed that increases in perceptual size could be explained by the enlargement in cortical somatosensory maps that are known to occur with acute peripheral deafferentation (Calford & Tweedale, 1991). Our subjects’ illusory perception of a ‘swollen throat’ following ISLN anaesthesia is consistent with these previous observations on other deafferented body parts.
Ventilation and the co-ordination of swallowing with breathing
In anaesthetised animals, laryngeal afferent fibres exhibit impulses from receptors that are sensitive to transmural pressure (Sant'Ambrogio et al. 1983), cooling (Sant'Ambrogio et al. 1985), carbon dioxide (Bartlett & Knuth, 1992) and mechanical distortion of laryngeal structures (Sampson & Eyzaguirre, 1964). Therefore, swallowing and breathing should affect laryngeal receptor activity. Our experiments provided us with an opportunity to test the hypothesis that chemo- or mechano-responsive laryngeal afferent activity is important in setting the level of ventilation or in co-ordinating swallowing with breathing in awake humans.
We found that ISLN blockade resulted in no significant change in the respiratory rate, or in the ventilation at rest or during hypercapnoeic hyperpnoea. Furthermore, blockade did not alter the level of ‘air hunger’ sensation associated with moderate hypercarbia.
Our analysis, over a large range of ventilation, revealed no shift in the normal phase relationships between swallowing and breathing. Specifically we found that swallowing induced apnoea and strong resetting of respiratory rhythm with quantitative relationships that were unaffected by ISLN blockade. The incidence of spontaneous swallowing was also modulated by the phase of ongoing respiration (Fig. 8A), a property that was not affected by ISLN blockade.
Laryngeal penetration almost always occurred during deglutition apnoea, excluding an important role for inspiratory airflow in the pathogenesis of aspiration. However, we cannot rule out the possibility that inspiratory effort during deglutition apnoea contributes to laryngeal penetration in the setting of inadequate laryngeal closure. Indeed, swallowing is normally associated with brief diaphragmatic contraction (Vantrappen & Hellemans, 1967), and it is not known whether laryngeal deafferentation affects the magnitude or timing of this contraction. If present, suction pressure in the larynx during deglutition apnoea could contribute to the transfer of material from the pharynx into the insufficiently closed larynx.
Relevance to clinical dysphagia and aspiration
We propose that ISLN damage alone, without additional lesions in the airway or brain, can produce dysphagia and aspiration. Our observations support the suggestion that ISLN damage is a major risk for post-operative swallowing dysfunction and aspiration following conservation surgery of the larynx (Ward et al. 1977). However, laryngeal insufficiency in the deafferented condition is not absolute. Rather, voluntary facilitation of the closure mechanism along with other neural adaptive mechanisms may promote the recovery of normal airway protection in the deafferented condition. These adaptive mechanisms could be the basis of recovery that is evident clinically in patients with dysphagia and aspiration following supraglottic laryngectomy (Ward et al. 1977).
Cerebral damage can result in neurogenic dysphagia and aspiration, which is most commonly seen in patients with stroke (Veis & Logemann, 1985; Mann et al. 1999), and the ingested material often penetrates into the larynx during the pharyngeal phase of swallowing (Kahrilas et al. 1997). In stroke patients with dysphagia, the presence of sensory loss in the larynx and pharynx is a poor prognostic factor for the development of aspiration pneumonia (Aviv et al. 1997). There has been some success in the use of sensory enhancement of swallowing in patients with cerebral lesions, for example by applying a cold tactile stimulus to the anterior pillars of the fauces (Lazzara et al. 1986) or by ingesting sour boluses (Logemann et al. 1995). These clinical studies encourage the view that activation of peripheral neural pathways can facilitate swallowing and airway protection when suprabulbar mechanisms are damaged, and complement animal studies that show parallel activation of the swallowing pattern generator by peripheral and cortical inputs (Sumi, 1969). Since our findings suggest that the ISLN regulates the central swallowing mechanism, especially the circuits responsible for laryngeal closure, it may be possible to treat neurogenic dysphagia and prevent aspiration in patients with cerebral lesions by stimulation of ISLN afferent fibres.
Acknowledgments
We are grateful to the research subjects for their participation in this study. We also thank William Schwartz and Peter Grigg for valuable comments on the manuscript, Stephen Baker and Joan Swearer for guidance on statistical analyses, and Arthur Allard and Thomas Marchese for constructing the automated bolus injector. This work was supported in part by a grant (R01 HL49848) from the National Heart and Lung Institute of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
REFERENCES
- Ali GN, Laundl TM, Wallace KL, Shaw DW, Decarle DJ, Cook IJ. Influence of mucosal receptors on deglutitive regulation of pharyngeal and upper esophageal sphincter function. Am J Physiol. 1994;267:G644–649. doi: 10.1152/ajpgi.1994.267.4.G644. [DOI] [PubMed] [Google Scholar]
- Anderson TP. On the mechanism of the closure of the larynx. A preliminary communication. Proc R Soc Lond B Biol Sci. 1891;50:323–339. –1892. [Google Scholar]
- Ardran GM, Kemp FH. The protection of the laryngeal airway during swallowing. Br J Radiol. 1952;25:406–416. doi: 10.1259/0007-1285-25-296-406. [DOI] [PubMed] [Google Scholar]
- Aviv JE, Sacco RL, Mohr JP, Thompson JLP, Levin B, Sunshine S, Thomson J, Close LG. Laryngopharyngeal sensory testing with modified barium swallow as predictors of aspiration pneumonia after stroke. Laryngoscope. 1997;107:1254–1260. doi: 10.1097/00005537-199709000-00018. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Lansing RW, Evans KC, Shea SA. Stimulus-response characteristics of CO2-induced air hunger in normal subjects. Resp Physiol. 1996;103:19–31. doi: 10.1016/0034-5687(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Mahan ST, Garner DM, Brughera A, Loring SH. A simple and reliable method to calibrate respiratory magnetometers and Respitrace. J Appl Physiol. 1995;79:2169–2176. doi: 10.1152/jappl.1995.79.6.2169. [DOI] [PubMed] [Google Scholar]
- Bartlett D, Jr, Knuth SL. Responses of laryngeal receptors to intralaryngeal CO2 in the cat. J Physiol. 1992;457:187–193. doi: 10.1113/jphysiol.1992.sp019372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian RW, Riggs LC. Role of sensation in swallowing function. Laryngoscope. 1999;109:1974–1977. doi: 10.1097/00005537-199912000-00014. [DOI] [PubMed] [Google Scholar]
- Blumin JH, Ye M, Berke GS, Blackwell KE. Recovery of laryngeal sensation after superior laryngeal nerve anastomosis. Laryngoscope. 1999;109:1637–1641. doi: 10.1097/00005537-199910000-00017. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate expansion of receptive fields of neurons in area 3b of Macaque monkeys after digit denervation. Somatosens Motor Res. 1990;8:249–260. doi: 10.3109/08990229109144748. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Wilson JA, Harris MB, MacDougall G. Globus pharyngis: development of a symptom assessment scale. J Psychosom Res. 1995;39:203–213. doi: 10.1016/0022-3999(94)00104-d. [DOI] [PubMed] [Google Scholar]
- Dichgans J, Bizzi E, Morasso P, Tagliasco V. Mechanisms underlying recovery of eye-head coordination following bilateral labyrinthectomy in monkeys. Exp Brain Res. 1973;18:548–562. doi: 10.1007/BF00234137. [DOI] [PubMed] [Google Scholar]
- Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19:44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- Fink BR. Folding mechanism of the human larynx. Acta Otolaryngol. 1974;78:124–128. doi: 10.3109/00016487409126336. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick MF, Zintel T, Stockwell M, Mink J, Gallagher CG. Superior laryngeal nerve blockade and inspiratory resistive load detection in normal subjects. J Appl Physiol. 1995;79:1567–1570. doi: 10.1152/jappl.1995.79.5.1567. [DOI] [PubMed] [Google Scholar]
- Flaherty RF, Seltzer S, Campbell T, Weisskoff RM, Gilbert RJ. Dynamic magnetic resonance imaging of vocal cord closure during deglutition. Gastroenterology. 1995;109:843–849. doi: 10.1016/0016-5085(95)90393-3. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McCloskey DI. Effects of related sensory inputs on motor performances in man studied through changes in perceived heaviness. J Physiol. 1977;272:653–672. doi: 10.1113/jphysiol.1977.sp012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Phegan CML. Perceptual distortions of the human body image produced by local anaesthesia, pain and cutaneous stimulation. J Physiol. 1999;514:609–616. doi: 10.1111/j.1469-7793.1999.609ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill JR, Gillies DR. Local anesthesia for peroral endoscopy. Using superior laryngeal nerve block with topical application. Arch Otolaryngol. 1966;84:654–657. doi: 10.1001/archotol.1966.00760030656012. [DOI] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol. 1991;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscoe S, Feldman JL, Cohen MI. Properties of inspiratory termination by superior laryngeal and vagal stimulation. Resp Physiol. 1979;36:353–366. doi: 10.1016/0034-5687(79)90047-1. [DOI] [PubMed] [Google Scholar]
- Jean A. Localisation et activité des neurones deglutiteurs bulbairs. J Physiol Paris. 1972;64:227–268. [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Jean A, Car A. Inputs to the swallowing medullary neurons from the peripheral afferent fibers and the swallowing cortical area. Brain Res. 1979;178:567–572. doi: 10.1016/0006-8993(79)90715-7. [DOI] [PubMed] [Google Scholar]
- Jafari S, Prince R, Kim D, Paydarfar D. Laryngeal afferent control of swallowing: anaesthesia of the internal superior laryngeal nerve causes glottic dysfunction and aspiration in humans. J Physiol. 2001;536.P:146P. [Google Scholar]
- Kahrilas PJ, Lin S, Rademaker AW, Logemann JA. Impaired deglutitive airway protection: A videofluoroscopic analysis of severity and mechanism. Gastroenterology. 1997;113:1457–1464. doi: 10.1053/gast.1997.v113.pm9352847. [DOI] [PubMed] [Google Scholar]
- Kuypers HGJM. Corticobulbar connexions to the pons and lower brain-stem in man. An anatomical study. Brain. 1958;81:364–388. doi: 10.1093/brain/81.3.364. [DOI] [PubMed] [Google Scholar]
- Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia. 1988;2:216–219. doi: 10.1007/BF02414429. [DOI] [PubMed] [Google Scholar]
- Lazzara G, Lazarus C, Logemann JA. Impact of thermal stimulation on the triggering of the swallowing reflex. Dysphagia. 1986;1:73–77. [Google Scholar]
- Lemere F. Innervation of the larynx: I. Innervation of laryngeal muscles. Am J Anat. 1932;51:417–438. [Google Scholar]
- Lemere F. Innervation of the larynx: III. Experimental paralysis of the laryngeal nerve. Arch Otolaryngol. 1933;18:413–424. [Google Scholar]
- Logemann JA, Kahrilas PJ, Cheng J, Pauloski BR, Gibbons PJ, Rademaker AW, Lin S. Closure mechanisms of the laryngeal vestibule during swallowing. Am J Physiol. 1992;262:G338–344. doi: 10.1152/ajpgi.1992.262.2.G338. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Pauloski BR, Colangelo L, Lazarus C, Fujiu M, Kahrilas PJ. Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. J Speech Lang Hear Res. 1995;38:556–563. doi: 10.1044/jshr.3803.556. [DOI] [PubMed] [Google Scholar]
- Ludlow C, Van Pelt F, Koda J. Characteristics of late responses to superior laryngeal nerve stimulation in humans. Ann Otol Rhinol Laryngol. 1992;101:127–135. doi: 10.1177/000348949210100204. [DOI] [PubMed] [Google Scholar]
- McCloskey DI. Corollary discharges: Motor commands and perception. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology, section 1, The Nervous System, vol. II, Motor control. Bethesda: American Physiological Society; 1981. pp. 1415–1447. part 2. [Google Scholar]
- McLean RA, Sanders WL, Stroup WW. A unified approach to mixed linear models. Am Stat. 1991;45:54–64. [Google Scholar]
- Mann G, Dip PG, Hankey GJ, Cameron D. Swallowing function after stroke-prognosis and prognostic factors at 6 months. Stroke. 1999;30:744–748. doi: 10.1161/01.str.30.4.744. [DOI] [PubMed] [Google Scholar]
- Månsson I, Sandberg N. Effects of surface anesthesia on deglutition in man. Laryngoscope. 1974;84:427–437. doi: 10.1288/00005537-197403000-00006. [DOI] [PubMed] [Google Scholar]
- Mårtensson A. Reflex responses and recurrent discharges evoked by stimulation of laryngeal nerves. Acta Physiol Scand. 1963;57:248–269. [Google Scholar]
- Miller AJ. Deglutition. Physiol Rev. 1982;62:129–184. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Loizzi RF. Anatomical and functional differentiation of superior laryngeal nerve fibers affecting swallowing and respiration. Exp Neurol. 1974;42:369–387. doi: 10.1016/0014-4886(74)90033-8. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Kirchner JA. Mechanical and physiological properties of reflex laryngeal closure. Ann Otol. 1972;81:59–71. doi: 10.1177/000348947208100106. [DOI] [PubMed] [Google Scholar]
- Murtagh JA. The respiratory function of the larynx: some observations on laryngeal innervation, preliminary report. Ann Otol Rhinol Laryngol. 1945;54:307–321. [Google Scholar]
- Narita N, Yamamura K, Yao D, Martin RE, Sessle BJ. Effects of functional disruption of lateral pericentral cerebral cortex on primate swallowing. Brain Res. 1999;824:140–145. doi: 10.1016/s0006-8993(99)01151-8. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Sears TA. Effects of posterior root section on the activity of some muscles in man. J Neurol Neurosurg Psychiat. 1960;23:10–22. doi: 10.1136/jnnp.23.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus VE. The Comparative Anatomy and Physiology of the Larynx. London: Heinemann; 1949. [Google Scholar]
- Ogura JH, Lam RL. Anatomical and physiological correlations on stimulating the human superior laryngeal nerve. Laryngoscope. 1953;63:947–959. doi: 10.1288/00005537-195310000-00003. [DOI] [PubMed] [Google Scholar]
- Paydarfar D, Eldridge FL, Kiley JP. Resetting of mammalian respiratory rhythm: existence of a phase singularity. Am J Physiol. 1986;250:R721–727. doi: 10.1152/ajpregu.1986.250.4.R721. [DOI] [PubMed] [Google Scholar]
- Paydarfar D, Gilbert RJ, Poppel CS, Nassab PF. Respiratory phase resetting and airflow changes induced by swallowing in humans. J Physiol. 1995;483:273–288. doi: 10.1113/jphysiol.1995.sp020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommerenke WT. A study of the sensory areas eliciting the swallowing reflex. Am J Physiol. 1928;84:36–41. [Google Scholar]
- Pressman JJ. Sphincter action of the larynx. Arch Otolaryngol. 1941;33:351–377. [Google Scholar]
- Reid J. An experimental investigation into the functions of the eighth pair of nerves, or the glossopharyngeal, pneumogastric, and spinal accessory. Edinburgh Med Surg J. 1838;134:109–176. [PMC free article] [PubMed] [Google Scholar]
- Sampson S, Eyzaguirre C. Some functional characteristics of mechanoreceptors in the larynx of the cat. J Neurophysiol. 1964;27:464–480. doi: 10.1152/jn.1964.27.3.464. [DOI] [PubMed] [Google Scholar]
- Sanders I, Mu L. Anatomy of the human internal superior laryngeal nerve. Anat Rec. 1998;252:646–656. doi: 10.1002/(SICI)1097-0185(199812)252:4<646::AID-AR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G, Mathew OT, Fisher JT, Sant'Ambrogio FB. Laryngeal receptors responding to transmural pressure, airflow and local muscle activity. Resp Physiol. 1983;54:317–330. doi: 10.1016/0034-5687(83)90075-0. [DOI] [PubMed] [Google Scholar]
- Sant'Ambrogio G, Mathew OT, Sant'Ambrogio FB, Fisher JT. Laryngeal cold receptors. Resp Physiol. 1985;59:35–44. doi: 10.1016/0034-5687(85)90016-7. [DOI] [PubMed] [Google Scholar]
- Sasaki CT, Suzuki M. Laryngeal reflexes in cat, dog, and man. Arch Otolaryngol. 1976;102:400–402. doi: 10.1001/archotol.1976.00780120048004. [DOI] [PubMed] [Google Scholar]
- Shaker R, Dodds WJ, Dantas RO, Hogan WJ, Arndorfer RC. Coordination of deglutitive glottic closure with oropharyngeal swallowing. Gastroenterology. 1990;98:1478–1484. doi: 10.1016/0016-5085(90)91078-k. [DOI] [PubMed] [Google Scholar]
- Stockwell M, Lozanoff S, Lang SA, Nyssen J. Superior laryngeal nerve block: an anatomical study. Clin Anat. 1995;8:89–95. doi: 10.1002/ca.980080202. [DOI] [PubMed] [Google Scholar]
- Sulica L, Hembree A, Blitzer A. Swallowing and sensation: evaluation of deglutition in the anesthetized larynx. Ann Otol Rhinol Laryngol. 2002;111:291–294. doi: 10.1177/000348940211100402. [DOI] [PubMed] [Google Scholar]
- Sumi T. Neuronal mechanisms in swallowing. Pflugers Arch. 1964;278:467–477. doi: 10.1007/BF00363579. [DOI] [PubMed] [Google Scholar]
- Sumi T. Some properties of cortically-evoked swallowing and chewing in rabbits. Brain Res. 1969;15:107–120. doi: 10.1016/0006-8993(69)90313-8. [DOI] [PubMed] [Google Scholar]
- Sumi T. Reticular ascending activation of frontal cortical neurons in rabbits, with special reference to the regulation of deglutition. Brain Res. 1972;46:43–54. doi: 10.1016/0006-8993(72)90004-2. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Kitajima K, Gould WJ. Laryngeal phonatory reflex. The effect of anesthetization of the internal branch of the superior laryngeal nerve: acoustic aspects. Ann Otol. 1975;84:206–212. doi: 10.1177/000348947508400212. [DOI] [PubMed] [Google Scholar]
- Vantrappen G, Hellemans J. Studies on the normal deglutition complex. Am J Digestive Diseases. 1967;12:255–266. doi: 10.1007/BF02233643. [DOI] [PubMed] [Google Scholar]
- Veis SL, Logemann JA. Swallowing disorders in persons with cerebrovascular accident. Arch Phys Med Rehabil. 1985;66:372–375. [PubMed] [Google Scholar]
- Venker-van Haagen AJ, Van den Brom WE, Hellebrekers LJ. Effect of superior laryngeal nerve transection on pharyngeal muscle contraction timing and sequence of activity during eating and stimulation of the nucleus solitarius in dogs. Brain Res Bull. 1999;49:393–400. doi: 10.1016/s0361-9230(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Ward PH, Berci G, Calcaterra TC. New insights into the causes of postoperative aspiration following conservation surgery of the larynx. Ann Otol. 1977;96:724–736. doi: 10.1177/000348947708600603. [DOI] [PubMed] [Google Scholar]


