Abstract
Using whole-cell recording techniques we compared effects of the environmental cerebellar neurotoxicant methylmercury (MeHg) on spontaneous IPSCs (sIPSCs) of both Purkinje and granule cells in cerebellar slices of the rat. In Purkinje cells, bath application of 10, 20 or 100 μM MeHg initially increased then suppressed the frequency of sIPSCs to zero. In granule cells, the initial increase in frequency was not observed in ≈50 % of cells examined, but suppression of sIPSCs by MeHg occurred in every cell tested. For both cells, time to onset of effects of MeHg was inversely related to the concentration; moreover, the pattern of changes in mIPSCs induced by MeHg in the presence of tetrodotoxin was similar to that in sIPSCs. For each concentration of MeHg, it took 2–3 times longer to block sIPSCs in Purkinje cells than it did in granule cells. MeHg also initially increased then decreased amplitudes of sIPSCs to block in both cells; again the response was more variable in granule cells. In most Purkinje and some granule cells, MeHg induced a giant, slow inward current during the late stages of exposure. Appearance of this current appeared to be MeHg concentration dependent, and the direction of current flow was reversed by changing the holding potentials. Reduction of the [Cl−] in the internal solution caused inwardly directed, but not outwardly directed giant currents to disappear, suggesting that this current is a Cl−-mediated response. However, bicuculline and picrotoxin failed to block it. MeHg apparently acts at both presynaptic and postsynaptic sites to alter GABAA receptor-mediated inhibitory synaptic transmission. GABAA receptors in granule cells appear to be more sensitive to block by MeHg than are those in Purkinje cells, although the general patterns of effects on the two cells are similar.
Methylmercury (MeHg) is a prominent environmental neurotoxicant, which induces marked toxicity in the cerebellum in both humans and animals. Poisoning following acute or chronic exposure to MeHg is characterized by a series of neurotoxic symptoms and signs that routinely include cerebellar-based ataxia and sensory disturbances. Pathological examination of patients and experimental animals with acute and chronic MeHg poisoning indicates that the granule cells in the cerebellar cortex are particularly sensitive to MeHg whereas the neighbouring Purkinje cells are relatively refractory to MeHg-induced damage despite accumulating as much or more MeHg (Chang, 1977; Leyshon-Sorland & Morgan, 1991). The cellular and molecular mechanisms underlying this differential sensitivity of cerebellar granule and Purkinje cells to MeHg remain unclear.
One factor that may contribute to the differential effects of MeHg on cerebellar granule and Purkinje cells is differential expression of GABAA receptor subunits, particularly α subunits, in the two types of cerebellar cells. α6 GABAA receptor subunits are only expressed in the mature postmigratory cerebellar granule cells (Fritschy et al. 1992; Laurie et al. 1992; Thompson et al. 1992; Thompson & Stephenson, 1994; Gao & Fritschy, 1995; Wisden et al. 1996; Mäkeläet al. 1999); the adjacent Purkinje cells express the α1 subunit. Pharmacological and electrophysiological studies of native and recombinant GABAA receptors have shown that differential expression of individual α1 or α6 subunits confers GABAA receptors with unique properties and characteristics including differential sensitivity to several agonists, antagonists or modulators such as benzodiazepines, barbiturates, furosemide, zinc and lanthanum (Pritchett et al. 1989; Draguhn et al. 1990; Korpi et al. 1995; Tia et al. 1996; Saxena & MacDonald, 1996; Fisher et al. 1997; Fisher & MacDonald, 1998; Zhu et al. 1998; Sigel & Baur, 2000). Whether or not this differential expression of GABAA receptor α subunits in granule and Purkinje cells contributes to their different sensitivity to MeHg or more specifically, whether expression of α6 subunits in cerebellar granule cells makes the cerebellar granule cells more sensitive to MeHg remains unclear.
GABAergic-mediated inhibitory synaptic transmission is markedly affected by MeHg in isolated slices of rat hippocampus (Yuan & Atchison, 1997). Furthermore, in dorsal root ganglion neurons, high concentrations of MeHg suppress GABA-mediated chloride currents and induce a slowly, but continuously developing non-specific cation-mediated inward current (Arakawa et al. 1991). MeHg also shortens hexobarbital-induced sleep time in mice by an action which is presumed to involve the ‘barbiturate receptor’ on GABAA receptors (Su & Okita, 1986). Moreover, in primary cultures of cerebellar granule cells, the GABAA receptors appear to be highly sensitive to MeHg because acute exposure to 0.1 μM MeHg rapidly and markedly suppressed GABAA receptor-mediated whole cell currents (Xu & Atchison, 1998). Thus MeHg prominently affects the function of GABAA receptors in the CNS. However, to date no direct comparison has been made of the effects of MeHg on GABAA receptor-mediated inhibitory synaptic responses in cerebellar granule and Purkinje cells. Thus, as a first step, the present study was designed specifically to test the hypothesis that MeHg differentially affects GABAA receptor-mediated responses in granule and Purkinje cells.
Inhibitory postsynaptic responses evoked by either spontaneous or stimulus-elicited release of GABA from the presynaptic terminals can be recorded in both Purkinje and granule cells. The spontaneous IPSCs (sIPSCs) or miniature IPSCs (mIPSCs) reflect most accurately activity occurring during normal functioning of GABAergic synapses, and are thought to be better suited for pharmacological studies, than are evoked IPSCs because of the normal lack of synchrony of individual IPSCs originating from several synapses activated by a stimulus (Mody et al. 1994). Moreover, we have demonstrated a potent Ca2+ channel blocking action of MeHg in both granule cells (Sirois & Atchision, 2000) and Purkinje cells in culture (Peng & Atchison, 2002) which would clearly affect nerve-evoked release of GABA. As such, we limited our study to comparison of the effects of MeHg primarily on sIPSCs and to a limited extent on mIPSCs recorded from Purkinje or granule cells in slice.
The present study had several goals. First, we sought to characterize the effects of MeHg on sIPSCs in both Purkinje and granule cells. Second, we compared the concentration-response relationship and time courses of effects of MeHg on sIPSCs to determine whether or not the GABAA receptor-mediated responses in cerebellar granule cells are more sensitive to MeHg than are those in Purkinje cells. Third, we sought to determine if the effects of MeHg on central synapses were similar to or different from those reported for peripheral cholinergic synapses (Atchison & Narahashi, 1982). Parts of these results have been published in abstract form (Yuan & Atchison, 2001, 2002).
METHODS
Preparation of cerebellar slices
All animal procedures complied with the National Institutes of Health of the USA guidelines on animal care and use, and the UK Animal (Scientific Procedures) Act 1986 and were approved by Michigan State University Laboratory Animal Use and Care Committee. Cerebellar slices were prepared as described previously (Yuan & Atchison, 1999). In brief, the cerebellum was removed quickly from the brain of a young Sprague-Dawley rat (10–21 days postnatal, either sex, Harlan Industries, Verona, WI, USA) and immersed immediately in cold, oxygenated ‘slicing’ solution (see below for composition). A portion of vermis isolated by two cuts was glued onto the tissue pedestal of an OTS-3000-05 Automatic Oscillating Slicer (FHC, Inc. Brunswick, ME, USA) with cyanoacrylate glue. The tissue block was then transected sagittally to produce five to eight slices with a thickness of approximately 150–200 μm. Slices were then transferred to a home-made holding chamber, aerated continuously with 95 % O2-5 % CO2, and incubated at 35 °C for 60 min and then at a room temperature of 22–25 °C until use.
Whole-cell recording in cerebellar slices
One slice was transferred to a modified RC-26 recording chamber (Warner Instruments Corp., Hamden, CT, USA) assembled with an SS-3 slice support and mechanically fixed using a home-made ‘U’-shaped anchor made of a platinum wire frame with nylon mesh. The slice was superfused (2–4 ml min−1) continuously with artificial cerebrospinal fluid (ACSF, see below) by gravity force. Granule and Purkinje cells in slices were identified by a combination of their electrophysiological properties and a visual assessment based on their size, shape and location, using a Nikon E600FN upright microscope (Nikon Optics, Tokyo, Japan) equipped with Nomarski optics (× 40 water immersion objective) and Sony IR-1000 infrared charge coupled device (CCD) video camera system (DAGE MTI, Michigan City, IN, USA). Recording electrodes were pulled from 7052 glass capillaries (o.d. = 1.5 mm, i.d. = 1.0 mm, Garner Glass Co., Claremont, CA, USA) using a P-97 puller (Sutter Instrument Co., Novato, CA, USA), coated with Sylgard resin 184 (Dow Corning, Midland, MI, USA) and fire-polished to a resistance of 6–10 MΩ for granule cells or 1.5–3 MΩ for Purkinje cells when filled with the standard pipette solution (see below). Whole-cell recordings of spontaneous postsynaptic currents from Purkinje or granule cells were made using an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA) and the ‘blow and seal’ technique described by Stuart et al. (1993). Except where noted otherwise, sIPSCs were recorded continuously at a holding potential of −60 mV in the presence of 10 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 50–100 μM amino-5-phosphonopentanoic acid (APV) in the external solution to block glutamate receptor-mediated excitatory postsynaptic currents. In some cells, mIPSCs were isolated using the same recording conditions but in the presence of 0.5 μM tetrodotoxin (TTX) in addition to CNQX and APV in the external solution, to block the presynaptic, action potential-evoked release of GABA. A concentration of 0.5 μM TTX appeared to block effectively the presynaptic, action potential-evoked IPSCs within 2–5 min after application in our recordings. This was tested in each cell after application of TTX by using voltage ramp changes from −100 to +60 mV at a holding potential of −60 mV and/or voltage step changes from −100 to +60 mV at a holding potential of −80 mV. No voltage-dependent Na+ current components were elicited. No Ca2+ channel blockers such as Cd2+ were applied during recordings of spontaneous currents, because they may interfere with the effect of MeHg (see Sirois & Atchison, 2000; Peng et al. 2002). After compensation of capacitance and series resistance at 60–80 %, series resistances were usually less than 10 MΩ for Purkinje cells or less than 20 MΩ for granule cells and were monitored throughout the experiments to ensure their constancy. The liquid junction potential was estimated to be less than 4 mV and was ignored. All experiments were carried out at room temperature of 22–25 °C. Only one slice per rat was used for any given experiment and only one concentration of MeHg was applied per slice. Each experiment was replicated with slices from at least five rats.
Data acquisition and analysis
Data were acquired using a PC-compatible computer equipped with a Digidata 1200B interface and pCLAMP8.1 software (Axon Instruments, Union City, CA, USA). Whole-cell currents were filtered at 1–2 kHz with an 8-pole low-pass Bessel filter and digitized at 20 kHz for later off-line analysis using pCLAMP8.1 program and MiniAnalysis program 5.2.8 (Synaptosoft Inc., Decatur, GA, USA). Spontaneous synaptic currents were first screened automatically using MiniAnalysis software with a set of the prespecified parameters and then accepted or rejected manually with an event detection amplitude threshold at 10 pA for Purkinje cells or 5 pA for granule cells, and based on the shape (kinetic properties) of the spontaneous events. Unless specified otherwise, at least 100 events per cell were averaged for analysis. Peak amplitudes of currents were measured at the absolute maximum after subtraction of the baseline noise.
Data were collected continuously before and during application of MeHg and analysed statistically using Student's paired t test or one-way analysis of variance (ANOVA) for time-dependent measures unless otherwise specified. Dunnett's procedure was used for post hoc comparison. For comparison of differences between two cumulative amplitude distribution curves obtained before and after exposure to MeHg at a given time, the Komogorov-Smirnov (K-S) test was used. Values were considered statistically significant at P < 0.05. The data are presented as means ±s.e.m. unless otherwise specified, and the number of replicates is given.
Solutions and chemicals
Methylmercuric chloride, purchased from ICN Biomedical, Inc. (Costa Mesa, CA, USA), was dissolved in deionized water to a final concentration of 10 mM to serve as stock solution. The applied solutions (10, 20 or 100 μM) were diluted just before perfusion with modified ACSF consisting of (mM): 125, NaCl; 2.5, KCl; 1, MgCl2; 1.25, KH2PO4; 26, NaHCO3; 2, CaCl2; and 25, D-glucose (pH 7.35–7.4 saturated with 95 % O2-5 % CO2 at room temperature). Three different MeHg concentrations (10, 20 and 100 μM) were used in the present studies. Clinical studies of MeHg-induced toxicity indicate that following acute exposure to the toxicant, generalized weakness of the extremities and ataxia of cerebellar origin occurred in humans with a frequency of incidence of 70–100 % at body burdens of 200–312 mg of Hg (Bakir et al. 1973) or 0.997–1.56 mmol of Hg. Taking the upper range in an 80 kg adult male and assuming an approximate tissue density of 1.0 kg l−1, this converts to 19.5 μM. Hence, in this study, the concentrations of 10 and 20 μM MeHg are within the range of those reported to be found in the blood of patients poisoned with MeHg in Iraq in the 1970s (Bakir et al. 1973). The higher concentration of MeHg, 100 μM, was used to condense the time course of effect of MeHg and examine effects on the concentration-response curve that may have occurred at higher concentrations. The ‘slicing’ solution contained (mM): 125, NaCl; 2.5, KCl; 4, MgCl2; 1.25, KH2PO4; 26, NaHCO3; 1, CaCl2; and 25, D-glucose (pH 7.35–7.4 saturated with 95 % O2-5 % CO2 at room temperature). The standard pipette solution consisted of (mM): 140, CsCl; 4, NaCl; 0.5, CaCl2; 10, Hepes; 5, EGTA; 2, Mg-ATP; and 0.4, GTP (pH 7.3 adjusted with CsOH). For experiments to characterize the giant slow inward currents, a reduced chloride-containing pipette solution was used; CsCl of the standard pipette solution was replaced with equimolar caesium gluconate in the pipette solution and was prepared freshly just before recording. D-Penicillamine, CNQX, APV, (−)-bicuculline methobromide and picrotoxin (PTX) were all purchased from Sigma Chemical Co. (St. Louis, MO, USA). TTX was purchased from either Sigma or Alomone Labs Ltd (Jerusalem, Israel).
RESULTS
MeHg increases and then decreases both amplitude and frequency of sIPSCs and mIPSCs in Purkinje and granule cells
Purkinje cell sIPSCs
Consistent with previous reports (Konnerth et al. 1990; Puia et al. 1994; Zhang et al. 1999), the spontaneous postsynaptic currents recorded from Purkinje cells in cerebellar slices using the CsCl-based internal solution are virtually all GABAA receptor-mediated sIPSCs at a holding potential of −60 mV, because bath application of 10 μM bicuculline completely and reversibly abolished all spontaneous postsynaptic currents even in the absence of the glutamate receptor antagonists CNQX and APV (data not shown). Nonetheless, 10 μM CNQX and 50–100 μM APV were added routinely to the external solutions in all experiments to ensure that the spontaneous postsynaptic responses recorded from these cells were monosynaptic sIPSCs in isolation. Under these conditions, amplitudes of control sIPSCs in Purkinje cells varied from 10 pA to several hundreds of picoamps, with some responses even in the nanoamp range; the averaged mean amplitude was 116.0 ± 13.9 pA (n = 41 cells) and the frequency varied from 1 to 16 Hz with a mean frequency of 6.9 ± 0.8 Hz (n = 41 cells).
Bath application of MeHg (10, 20, or 100 μM) caused a complex series of effects on inhibitory synaptic function. Figure 1A depicts a continuous recording from a cerebellar Purkinje cell in the presence of MeHg (100 μM), which was applied to the slice beginning at the point denoted by the arrow. Use of a relatively high concentration of MeHg allowed the entire sequence of action to be readily apparent by shortening the latency to onset. Temporally, MeHg first increased the frequency of occurrence of sIPSCs as compared to the pre-MeHg control level (Fig. 1A, a and b). This effect occurred after a short latent period and is seen at points c-e, and as individual traces taken at these time points in Fig. 1B (c-e). As sIPSC frequency increased, the amplitude of sIPSCs also increased, and some very large amplitude and prolonged inward currents occurred (Fig. 1Ac and Bc). With further increases in sIPSC frequency, repetitive firing was occasionally triggered (Fig. 1Af and Bf). Coincidentally with the increase in sIPSC frequency, a slowly developing, tonic inward current was induced. This is seen as the continuous downward deflection of the recording baseline beginning at e (Fig. 1Ae). Ultimately, the amplitude of sIPSCs and their frequency diminished (Fig. 1Ag and Bg), until no further sIPSCs were seen (Fig. 1Ah-i and Bh-i). At this time very slow and large amplitude inward currents - or ‘giants’ - appeared.
Figure 1. MeHg-induced changes in amplitude and frequency of sIPSCs in a cerebellar Purkinje cell in slice.
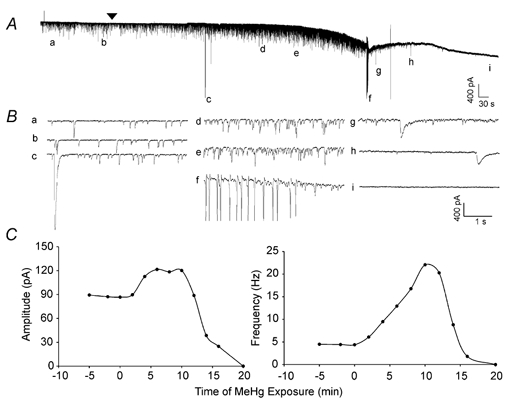
A, continuous recording of the time course of effects of bath-applied MeHg (100 μM) on spontaneous inhibitory currents (sIPSCs) recorded from a representative Purkinje cell in a slice at a holding potential of −60 mV. Note, the arrowhead indicates the starting point of MeHg exposure. Prior to that time, the cell had been held continuously for approximately 15 min to ensure that the recording was stable (only ≈5 min trace is shown here). All recordings were made in the presence of CNQX (10 μM) and APV (50 μM) in the external solution to block glutamatergic synaptic function. The lower case letters (a-i) indicate specific changes in spontaneous events before and during MeHg exposure. B, the same sampling points as in A are shown on an expanded time scale: a and b, control; c, g and h, MeHg-induced giant slow inward currents; d and e, MeHg-induced initial peak increase in sIPSC amplitude and frequency; f, spontaneous repetitive firing of Purkinje cell; i, complete cessation of whole-cell currents. C, time courses of effects of MeHg on Purkinje cell sIPSC amplitude (left) and frequency (right). MeHg was applied at time = 0 min. Data were sampled over 2 min periods and represent the average for that 2 min block.
Time courses of changes induced by MeHg in sIPSC amplitude and frequency are depicted for this cell in Fig. 1C. For both parameters, the effects of MeHg were biphasic; initially they increased before ultimately decreasing to complete block. Prior to MeHg exposure, the mean frequency and amplitude of sIPSCs recorded from this Purkinje cell over a 2 min period were 4.3 Hz and −87.2 ± 83.5 pA (mean ± S.D., averaged from 532 events (Fig. 1Aa and Ba), respectively. After exposure of the slice to 100 μM MeHg, both frequency and amplitude of sIPSCs increased markedly. However the two effects were not necessarily correlated, because the times to maximum increases in amplitude and frequency were different. For example, the peak increase in amplitude of sIPSCs (121.5 ± 94.9 pA, mean ± S.D. of 1552 events) occurred at 6–8 min, whereas the increase in frequency of sIPSCs did not reach the peak (22.1 Hz) until 12 min after exposure (Fig. 1Ad,e, Bd,e and C), before subsequently declining to zero. We did not attempt to apply a GABAA receptor agonist to the cell iontophoretically at this point, so it is not certain whether the block in sIPSCs reflects solely a postsynaptic action of MeHg at GABAA receptors, a cessation of release, or a combination of the two effects.
Figure 2 depicts the concentration dependence of the actions of MeHg. The biphasic nature of action of MeHg on both sIPSC frequency and amplitude seen at the high concentration, as well as the complex sequence of events elicited also occurred at the lower concentrations (10 and 20 μM) of MeHg, albeit with different time courses. The time to onset, time of maximum increases and time to disappearance in Purkinje cell sIPSC frequency were all inversely related to the MeHg concentration applied. At 10, 20 and 100 μM MeHg, the mean times to maximum increases in frequency were 45.0 ± 5.4, 13.8 ± 3.0 and 4.3 ± 0.4 min (n = 5–11), respectively. However, the magnitude of these increases, while all significantly greater than pre-MeHg control (P < 0.05, Student's paired t test), were not statistically different from one another (P > 0.05, one way ANOVA); at 10, 20 and 100 μM, peak frequency was increased to 268 ± 101 %, 333 ± 61 % and 365 ± 138 % of control (n = 5–11), respectively. Times to disappearance of sIPSCs in Purkinje cells were 97.5 ± 3.3 min, 53.1 ± 5.4 min and 19.7 ± 1.0 min (n = 5–11) at 10, 20 and 100 μM MeHg, respectively. As with the effects of MeHg on sIPSC frequency, the magnitude of the increase in sIPSC amplitude caused by MeHg was not concentration dependent. At 10, 20 and 100 μM, MeHg increased the mean amplitude of sIPSCs to 143 ± 30 %, 162 ± 22 % and 161 ± 20 % of their pretreatment control values (P < 0.05, Student's paired t test, n = 5–11), respectively.
Figure 2. MeHg causes a biphasic effect on frequency and amplitude of sIPSCs of cerebellar Purkinje cells which is concentration and time dependent.
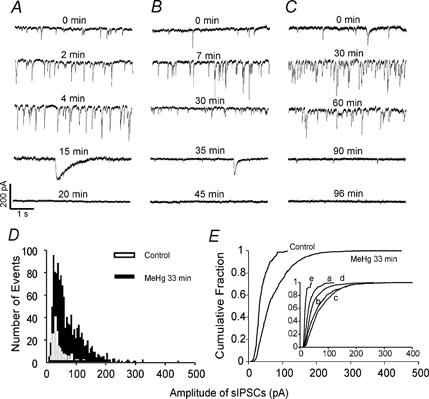
sIPSCs were recorded from Purkinje cells in three sagittal cerebellar slices, following continuous perfusion with MeHg at 100 (A), 20 (B) and 10 μM (C). The holding potential was −60 mV, and all recordings were made in the presence of 10 μM CNQX and 50 μM APV in the external solution. A-C, time courses of effects of MeHg on sIPSCs. Data were collected before (control) and at different time points after exposure to MeHg. Each trace is a representative depiction of 5–11 individual experiments. D, histogram of amplitude distribution of sIPSCs recorded from a Purkinje cell before (control, open bar) and after exposure to 10 μM MeHg for 33 min (filled bar). E, cumulative amplitude distribution of sIPSCs in control (220 events) and at 30 min after exposure to 10 μM MeHg (1252 events). MeHg shifted the cumulative amplitude distribution curve to the right after exposure for 30 min (P < 0.05, Kolmogorov-Smirnov test). Inset, 10 μM MeHg-induced shifting of the cumulative amplitude distribution curves in the same cell at different time points of exposure: a, control, 220 events; b, 15 min, 645 events; c, 30 min, 1252 events; d, 60 min, 747 events; e 90 min, 76 events.
Figure 2D-E demonstrates a representative example of the early effect of MeHg on sIPSC amplitude distribution of a Purkinje cell. At 10 μM and after 33 min exposure for this cell, MeHg markedly increased both frequency and amplitude of sIPSCs, and as shown in Fig. 2E, shifted the cumulative amplitude distribution curve to the right (P < 0.05). However, as shown in the inset of Fig. 2E, as the time of exposure of the cell to MeHg increased, the cumulative amplitude distribution curve gradually returned to that of the original control (inset curve d) and subsequently shifted to the left (inset curve e) as the large amplitude sIPSCs disappeared during the later phase of exposure (see inset). Possibly because of postsynaptic effects of MeHg, the large amplitude sIPSCs generally disappeared earlier, and thus may have already disappeared while the frequency of sIPSCs was still increasing due to the increased number of small amplitude sIPSCs. Taken together, the results of the effects of MeHg on sIPSC amplitude and frequency imply that MeHg may initially alter both the average probability of release and the quantal size of GABA at the basket cell-Purkinje cell synapse and/or stellate cell-Purkinje cell synapse.
Also shown in Fig. 2 (A and B) are the giant, slow inward currents that occurred in 19 of 25 Purkinje cells during later times of exposure to 10–100 μM MeHg. Figure 2C shows a representative example of a cell which lacked these giant events after exposure to 10 μM MeHg. However in three of the five cells tested these giant events occurred after exposure to 10 μM MeHg as well. These anomalous currents usually emerged after most or all of the sIPSCs had disappeared, although occasionally a giant slow inward current was also seen at earlier times of MeHg exposure (see for example, Fig. 1Ac and Bc). Time to appearance of these giant, slow inward currents appeared to be MeHg concentration dependent as well. At 10, 20 and 100 μM MeHg, the giant slow inward currents first appeared at 37.3 ± 9.3 min (n = 3), 28.4 ± 5.1 min (n = 8) and 6.6 ± 1.0 min (n = 8), respectively. In addition, MeHg caused a constant, and very slow inward current (Fig. 1A), which is consistent with previous reports by Arakawa et al. (1991) and Xu & Atchison (1998).
Granule cell sIPSCs
In granule cells, the frequency of sIPSCs in the absence of MeHg was much lower than in Purkinje cells; it varied from 0.03–4.0 Hz with a mean of 1.5 ± 0.4 Hz, (n = 24). Furthermore, the amplitudes of sIPSCs were relatively smaller (16.9 ± 3.4 pA, n = 24) than those of Purkinje cells. However, like Purkinje cells, exposure to 10, 20 or 100 μM MeHg generally caused a similar pattern of biphasic changes in both frequency and amplitude of sIPSCs, although not all granule cells expressed the initial increase in sIPSC frequency (Fig. 3). Considering all concentrations of MeHg, in 13 of 24 granule cells the early increases in sISPC frequency never occurred. In these cells, MeHg only suppressed and blocked sIPSCs. These data imply that the GABAA receptors in granule cells may respond to MeHg exposure differently. Figure 3 depicts two examples of the 11 preparations in which MeHg increased sIPSC frequency. Exposure of slices to 100 (Fig. 3A) or 10 μM MeHg (Fig. 3C) for 2 and 20 min, respectively, increased the frequency and amplitude of sIPSCs, albeit apparently not as dramatically as occurred in Purkinje cells. However the sIPSCs rapidly disappeared after 5, 23 or 37 min, respectively, at 100, 20 or 10 μM MeHg.
Figure 3. MeHg causes a similar biphasic effect on frequency and amplitude of sIPSCs of cerebellar granule cells.
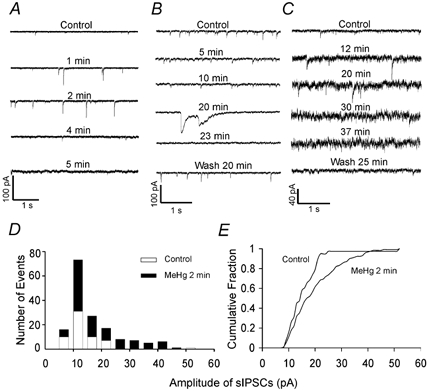
sIPSCs were recorded from granule cells in three sagittal cerebellar slices, following continuous perfusion with MeHg at 100 (A), 20 (B) and 10 μM (C). The holding potential was −60 mV, and all recordings were made in the presence of 10 μM CNQX and 50 μM APV in the external solution. A-C, time courses and reversibility of effects of MeHg on sIPSCs of granule cells. Data were collected in the absence (control) and presence of 100 (A), 20 (B) and 10 μM MeHg (C) at different time points. Note, in B and C, after sIPSCs were blocked completely, washing slices with 1 mM D-penicillamine for 20–25 min caused recovery of the sIPSCs. Also shown particularly well in panel C is the recovery of the background electrical ‘noise’ to its pretreatment level following wash with D-penicillamine. Each trace is a representative depiction of 4–8 individual experiments. D, histogram of amplitude distribution of sIPSCs recorded from a cerebellar granule cell before (control, white bar) and after exposure to 100 μM MeHg for 2 min (black bar). E, cumulative amplitude distribution of sIPSCs from the same granule cell as in D prior to (109 events), and at 2 min after starting perfusion with 100 μM MeHg (181 events). MeHg significantly shifted the cumulative amplitude distribution curve to the right after exposure for 2 min (P < 0.05, Kolmogorov-Smirnov test).
Also shown in Fig. 3C, in many granule cells the background ‘noise’ increased after exposure to MeHg, particularly during the later phase of exposure. This may be a result of MeHg-induced membrane damage to cause loss of seal resistance, it may be due to tonic activation of extrasynaptic GABAA receptors by increased spillover, or it may result from induction of an unknown background ion conductance. To determine if the rapid disappearance of sIPSCs in granule cells was due to a direct action of MeHg, in some experiments after sIPSCs were blocked completely by 10–20 μM MeHg, we washed the cells with ACSF containing 1 mM D-penicillamine, a MeHg chelator. As shown in Fig. 3B and C, in four of six granule cells washed with D-penicillamine for 20–30 min, sIPSCs were recovered completely to the pretreatment control level. For the other two cells, penicillamine was completely ineffective. Interestingly, washing with D-penicillamine also reduced the background noise to nearly pretreatment control level (Fig. 3C). Thus the relatively rapid disappearance of sIPSCs in granule cells was due to a direct action of MeHg, and apparent changes in membrane noise did not reflect irreversible loss of seal resistance. In contrast, in Purkinje cells after complete block of transmission occurred, washing with D-penicillamine was ineffective at inducing recovery of sIPSCs (data not shown). The irreversibility of sIPSCs in Purkinje cells by washing may be due to irreversible membrane damage resulting from the longer time of MeHg exposure typically needed to cause complete block of sIPSCs in Purkinje cells.
In those granule cells that showed the early increases in sIPSC frequency and amplitude, MeHg also shifted the amplitude histogram distribution (Fig. 3D) and cumulative amplitude distribution curve to the right (Fig. 3E). The giant slow inward currents were also observed in eight granule cells during the late phase of exposure to MeHg (Fig. 3B), as was the MeHg-induced slowly developing inward current (data not shown). Thus, the responses of sIPSCs recorded from granule cells are superficially similar to those in Purkinje cells in terms of pattern of responses. However, for many granule cells, the time course of response to MeHg exposure appeared to be more compressed and granule cells in general were more varied in their responses to MeHg.
Figure 4 summarizes and compares times to block of sIPSCs recorded from Purkinje and granule cells. In some preparations, we recorded mIPSCs in the presence of TTX. The effects of MeHg on these mIPSCs were similar to those on sIPSCs (data not shown). Because the time courses and pattern of effects of MeHg on sIPSCs and mIPSCs were generally similar, we pooled both sets of data as sIPSCs. At each concentration of MeHg, sIPSCs recorded from granule cells were blocked at earlier times than were sIPSCs recorded from Purkinje cells (P < 0.05, Student's t test).
Figure 4. MeHg blocks sIPSCs in granule cells more rapidly than it does those in Purkinje cells.
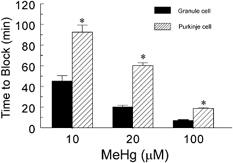
Comparison of times to block by 10, 20 or 100 μM MeHg of sIPSCs recorded from Purkinje and granule cells. All values are the mean ±s.e.m. of 7–18 individual experiments (pooled sIPSCs and mIPSCs). The asterisks indicate significant differences between times to block of sIPSCs of Purkinje and granule cells at 10, 20 or 100 μM MeHg (P < 0.05, Student's t test).
Are the giant slow inward currents GABAergic?
Two anomalous types of current accompanied exposure of Purkinje and granule cells in cerebellar slices to MeHg. The first of these was the slowly but constantly developing inward current occurring during the later phase of exposure to MeHg, which was reported in primary cultures of dorsal root ganglion neurons (Arakawa et al. 1991) and cerebellar granule cells (Xu & Atchison, 1998). In addition, as shown in Fig. 1–3 and Fig. 5, and described above, MeHg induced transient, giant slow inward currents in most Purkinje cells and some granule cells as well. In contrast to those slowly developing but constant inward currents, these giant currents presented as transient changes in membrane current without a regular rhythm. Time of onset and frequency of occurrence of these currents varied considerably in individual cells, ranging from an occasional to more frequently occurring events (from one to more than 10 events in a given preparation), and typically occurring during the later stages of MeHg exposure. Table 1 summarizes the kinetic characteristics of the giant slow inward currents recorded from Purkinje cells. Apparently, this giant slow inward current could be divided superficially into two groups - fast and slow responses based on the rate of rise. Additionally, though this was not an absolute correlation, the so-called ‘fast’ currents were sometimes of much larger amplitude than the slower events (see for example the giant currents depicted in Fig. 1). However, as evidenced by the extremely large s.e.m. value, there was considerable variability in this observation. The fast responses also usually appeared earlier than did the slow ones, but sometimes were intermingled among several slow responses. Conversely, in some cells, only the slow responses could be observed. Thus, whether or not the two types of responses are identical events remains unclear. Furthermore, these giant slow inward currents and sIPSCs or mIPSCs occur independently; sIPSCs or mIPSCs were occasionally superimposed on giant currents (see for example Fig. 5B), suggesting that they are distinct events. Interestingly, this giant current reversed direction in a manner similar to sIPSCs or mIPSCs when membrane holding potentials were changed (Fig. 5).
Figure 5. The giant slow inward currents can be reversed in direction at positive holding potentials.
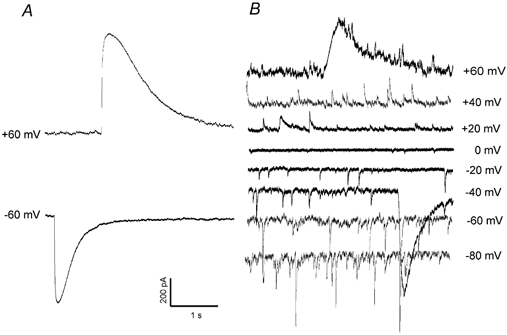
A the giant slow inward current induced by MeHg (20 μM, at 45 min of exposure) in a Purkinje cell at a holding potential of −60 mV became an outward current at +60 mV. B, a family of mIPSCs were recorded from another Purkinje cell at a set of holding potentials of −80 to +60 mV after exposure to 20 μM MeHg for 50 min. Note that the giant slow inward current superimposed upon the mIPSCs recorded at −40 mV became outward current at +60 mV(again with mIPSCs superimposed upon it).
Table 1.
Kinetic analysis of MeHg-induced giant slow inward currents recorded from cerebellar Purkinje cells in slices
| Giant response | Fast* | Slow |
|---|---|---|
| Rise time (ms)† | 43 ± 25 | 157 ± 168 |
| Peak amplitude (pA) | −620 ± 537 | −193 ± 120 |
| Rate of rise (pA ms−1)‡ | −17 ± 16 | −2 ± 1.44 |
| Duration (ms)‖ | 821 ± 576 | 1447 ± 921 |
| τ (pA ms−1)¶ | 182 ± 200 | 460 ± 538 |
| Number of events | 15 | 15 |
The giant slow inward currents were arbitarily divided into two groups: fast and slow responses based on their rising rate. Responses with rates of rise < 5 pA ms−1 were identifies as slow responses, while those with rates of rise > 5 pA ms−1 were classifies as fast responses. Responses were pooled for all three concentrations of MeHg tested (10, 20 and 100 μm). All values are expressed as means ±s.e.m.
Rise time is defined as the time requires from the baseline to the peak amplitude.
Rate of rise is defined as peak amplitude/rise time.
Duration of response is defined as the time from the start point to the end point of response.
Decay of gaint responses was fitted using Clampfit fitting program with a single exponential.
Several experiments were undertaken subsequently in attempts to identify these giant currents and their possible origin. To determine if they are chloride-mediated responses, Cl− (140 mM CsCl) in the standard pipette solution was replaced with a membrane-impermeant anion (140 mM caesium gluconate), yielding a low (≈5 mM) [Cl−] solution. Figure 6A depicts the time course of the effect of 100 μM MeHg on sIPSCs recorded from a representative Purkinje cell with the low chloride internal solution at a holding potential of 0 mV. MeHg still caused the same pattern of effects on frequency and amplitude of sIPSCs and induced the giant slow currents, which were now outwardly directed (Fig. 6A and B for 100 μM MeHg; Fig. 6Ca for 20 μM MeHg). However, with reduced intracellular Cl− concentration, the transient, giant slow inward currents disappeared when cell membranes were held at potentials from −40 to −100 mV (Fig. 6D). Under these same conditions, the sIPSCs remained observable and their frequency and amplitude changed as the holding potentials change from −100 to +60 mV (data not shown). Also shown in Fig. 6A and D are the MeHg-induced slowly and constantly developing outward and inward currents, respectively. Thus, the transient giant slow current appears to be a Cl−-mediated response.
Figure 6. The giant slow inward current does not appear in low [Cl−] solutions.
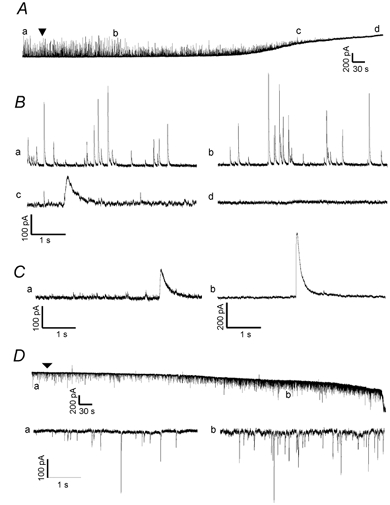
A, continuous recording of the time course of effects of bath-applied MeHg (100 μM) on spontaneous currents recorded from a representative Purkinje cell in slice at a holding potential of 0 mV with low Cl−-containing pipette solution (140 mM caesium gluconate). Note, the arrowhead indicates the starting point of MeHg exposure. The lower case letters (a-d) indicate specific noteworthy changes in spontaneous events after MeHg exposure. B, portions of the traces from the same sampling points indicated in A are shown on an expanded time scale: a, control; b, MeHg-induced changes in amplitude and frequency of spontaneous currents; c, MeHg-induced giant slow outward currents and induction of the slow tonic current, which is now outward at this holding potential; d, complete block of all spontaneous events, but continuation of the tonic slow current. C, two examples demonstrate specifically the presence of MeHg-induced giant slow outward currents in two other Purkinje cells with low [Cl−] pipette solution at different holding potentials and with different MeHg concentrations. Ca, a giant slow outward response recorded from a Purkinje cell at a holding potential of 0 mV at 25 min after exposure to 20 μM MeHg. Cb, a giant slow outward current recorded from another Purkinje cell at +60 mV after exposure to 100 μM MeHg for 12 min. D, continuous recording of the time course of effects of MeHg (100 μM) on spontaneous currents recorded from a Purkinje cell in another slice at a holding potential of −70 mV using the low Cl−-containing pipette solution. The lower case labels (a, b) again indicate the amplitude and frequency of spontaneous events before (a) and after (b)14 min of MeHg exposure. In B, the portions of the same sampling points indicated in D are shown on an expanded time scale: a, control; b, MeHg-induced initial increases in amplitude and frequency of the spontaneous currents. The arrowhead indicates the starting point of MeHg exposure. Note that now in response to MeHg no slow giant currents are seen, although large amplitude rapid mIPSCs still occur, as do the increases in mean frequency and amplitude of mIPSCs and the tonic slowly developing current. Each trace is a representative depiction of 4–6 individual experiments.
To determine if the giant slow inward currents are GABAergic responses, we measured sIPSCs or mIPSCs in the presence of 20–100 μM MeHg until the frequency and amplitude of sIPSCs or mIPSCs reached their level of maximum increase. The GABAA receptor antagonist bicuculline (10 μM) was then added in addition to MeHg. As shown in Fig. 7, after MeHg caused apparent increases in sIPSC frequency (7 and 25 min at 100 and 20 μM MeHg, respectively), adding 10 μM bicuculline rapidly blocked sIPSCs, but failed to prevent the appearance of the giant slow inward current. Similarly, picrotoxin (PTX) a GABAA receptor channel blocker, failed to prevent this MeHg-induced giant slow inward current (data not shown). Thus, these giant slow inward currents do not appear to be GABAA receptor-mediated responses.
Figure 7. The giant slow inward current is insensitive to the GABAA receptor antagonist bicuculline.
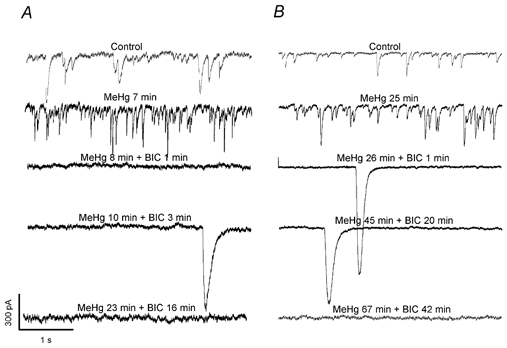
After treatment of Purkinje cells with 100 (A) and 20 μM MeHg (B) for 7 and 25 min, respectively, induced apparent increases in amplitude and frequency of mIPSCs, application of 10 μM bicuculline rapidly blocked mIPSCs, but failed to prevent the appearance of the giant slow inward currents in either cell. Each trace is a representative depiction of 5 individual experiments.
DISCUSSION
The cerebellum is a primary target of MeHg-induced neurotoxicity and the granule cell layer is especially sensitive. One particular question we are interested in is why cerebellar granule cells are so much more sensitive to the effects of MeHg than are their neighbouring Purkinje cells. Many mechanisms such as differential expression of the calcium binding protein, calbindin D28K (Celio, 1990; Kadowaki et al. 1993; Amenta et al. 1994), different subtypes of voltage-gated Ca2+ channels (Hillman et al. 1991; Tanaka et al. 1995; Randall & Tsien, 1995) and different cellular defence systems could contribute to this differential sensitivity between granule and Purkinje cells. However, because of the effects of MeHg on inhibitory synaptic function that we have demonstrated previously (Yuan & Atchison, 1997) in conjunction with the well known differences in phenotype of GABAA receptors between granule and Purkinje cells, a hypothesis that we are pursuing is that expression of different GABAA receptor α subunits in these two cells plays a role in this differential sensitivity to MeHg. If this is true, then responses of GABAA receptor-mediated inhibitory postsynaptic currents in Purkinje and granule cells to MeHg exposure should differ. As a first step in testing this, we examined and compared the effects of MeHg on the spontaneous inhibitory postsynaptic currents recorded from Purkinje and granule cells in rat cerebellar slices using whole-cell recording techniques.
A range of MeHg concentrations was used. Irrespective of the concentration of MeHg used, the pattern of response was similar - typically biphasic changes occurred in the frequency and amplitude of sIPSCs or mIPSCs recorded from Purkinje or granule cells. However the changes induced were concentration and time dependent in that higher concentrations reduced the time needed to evoke an effect. Surprisingly, the magnitude of changes in frequency or amplitude of postsynaptic current was independent of the concentration of MeHg. This suggests either that a fairly constant series of events is initiated once an effective concentration of MeHg is attained, or that the concentrations tested were all at the high end of the concentration-response relationship. Unfortunately because of the concentration-dependent latent period preceding time to onset, it is unrealistic to reduce the range of MeHg concentrations used and still maintain high quality continuous whole-cell recordings.
A biphasic change in spontaneous current frequency is a general pattern of the effects of MeHg on synaptic transmission
A common characteristic of the effects of acute exposure to MeHg on excitatory synaptic transmission is that MeHg causes an initial stimulation followed by depression of synaptic transmission. At neuromuscular preparations, MeHg initially stimulated then depressed to block the spontaneous release of ACh (Atchison & Narahashi, 1982; Atchison, 1986; Traxinger & Atchison, 1987; Levesque & Atchison 1987, 1988). A similar general pattern of effects of MeHg on central excitatory synaptic transmission was observed in our previous studies of the hippocampal CA1 region and cerebellar cortex (Yuan & Atchison, 1993, 1995, 1999). In the present study, although acting at different inhibitory synapses on Purkinje and granule cells, MeHg generally repeated this pattern of effects on frequency and amplitudes of sIPSCs or mIPSCs recorded from both cells. The effect was particularly noticeable in Purkinje cells. Thus, at least for the presynaptic effects on spontaneous current frequency, the pattern of effects of acute MeHg exposure on synaptic transmission appears to be universal, irrespective of whether it is of peripheral or central origin, or excitatory or inhibitory in nature.
The mechanisms by which these changes in sIPSC frequency occur are yet unknown. The MeHg-induced early increase in sIPSC frequency is consistent with the observation that MeHg increased the spontaneous release of [3H]GABA from synaptosomes derived from specific regions of rat brain - including the cerebellum - in the absence of extracellular Ca2+ (Minnema et al. 1989). At neuromuscular junctions, the increase in frequency of MEPPs caused by MeHg is thought to result from release of intracellular Ca2+ from nerve terminal mitochondria (Atchison, 1986; Levesque & Atchison 1987, 1988; Traxinger & Atchison, 1987). Because mIPSCs are analogous to the MEPPs recorded at neuromuscular junctions in the presence of TTX and are relatively independent of extracellular Ca2+ entry (Mody et al. 1994), it is reasonable to assume that release of Ca2+ from intracellular stores is also responsible for the MeHg-induced early increase in frequency of sIPSCs. However, our preliminary results showed that pretreatment of cells with membrane permeant BAPTA/AM, a Ca2+ chelator, plus reduced extracellular Ca2+ concentration failed to prevent the MeHg-induced increase in frequency of mIPSCs (Yuan & Atchison, 2002). If this is true, it suggests that either other mechanisms are involved or MeHg may induce release directly much like Ca2+ and Pb2+ do, perhaps by increasing the probability of presynaptic GABA release.
Spontaneous current amplitudes also change in a complex way in response to MeHg
Coincident with the early increase in sIPSC frequency, amplitudes of sIPSCs were also changed and the cumulative amplitude distribution curve was shifted to the right, suggesting that MeHg induced changes in quantal size or quantal amplitude. Altered quantal size could result from an alteration of the amount of GABA packaged into each presynaptic vesicle (vesicle content), altered density of the postsynaptic receptors, or altered affinity or efficacy of GABA at these postsynaptic receptors. In the CNS, the quantal size is often determined not by the vesicle contents but by the number of receptors available (Zucker et al. 1999). This is especially true when the concentration of GABA released into the synaptic cleft is normally at a saturated level under typical synaptic activity (Mody et al. 1994). Thus, increasing release of GABA from the presynaptic terminals does not appear to be a major factor for MeHg-induced early increased amplitude of sIPSCs. Inasmuch as synaptic currents result from summation of openings and closures of a number of transmitter-occupied receptors, the peak synaptic current will depend on the number of channels opening synchronously. Thus, the most likely explanation for MeHg-induced early increases in sIPSC amplitude is a transient increase in sensitivity or availability of postsynaptic GABAA receptors after acute exposure to MeHg. MeHg increases the total number of benzodiazepine binding sites of GABAA receptors in the retina and some areas of the rat brain including the cerebellum (Corda et al. 1981; Concas et al. 1983; Komulainen et al. 1995). Another possible explanation for this effect is that MeHg may initially stimulate the rate of GABA binding to receptors and/or accelerate the transition from GABA-bound receptor channels in the closed state to GABA-bound receptor channels in the open state.
Effects of MeHg on spontaneous current amplitude and frequency are not necessarily linked
The times to maximum increases in frequency and amplitude of sIPSCs or mIPSCs induced by MeHg did not always correlate well. Sometimes, the averaged amplitude of sIPSCs or mIPSCs began to decline while the frequency was still increasing because of an increased number of small amplitude sIPSCs or mIPSCs. The increased number of small-amplitude sIPSCs could result from inadequate vesicle filling, incomplete vesicle release or decreased receptor sensitivity. At this point it is unclear whether the ultimate block of sIPSCs or mIPSCs in Purkinje and granule cells is due primarily to a pre- or postsynaptic effect; perhaps both contribute.
The MeHg-induced giant slow inward current is not a GABAergic response
An unexpected observation in this study was the appearance of the MeHg-induced giant and transient slow inward current, which behaved like a MeHg exposure-related, time- and voltage-dependent, Cl−-mediated response. This current, which had very slow kinetics and large amplitude, was observed in most Purkinje and some granule cells after exposure to MeHg. The current could be simply an ‘artefact’ because it occurred randomly without a regular rhythm or fixed amplitude size. However, the time of emergence of this current appeared to be related to MeHg exposure and was MeHg concentration dependent, suggesting that it is a MeHg exposure-related response. Moreover, this current also appeared to be voltage dependent in terms of the current flow direction, which could be reversed as the membrane holding potentials changed from negative to positive. Thus, we think it is unlikely to be an ‘artefact'. Interestingly, after reducing the intracellular [Cl−] to a very low level, this giant slow inward current disappeared at holding potentials more negative than −40 mV. However, the outwardly directed giant slow currents remained inducible at more positive membrane holding potentials (0 to +60 mV). This suggests that this transient, giant slow inward current may be a Cl−-mediated response. However, this current was insensitive to both bicuculline and PTX, suggesting that it is not a GABAA receptor-mediated response. Perhaps it is a non-specific cation-mediated response. However, our preliminary results obtained from experiments in which application of Gd3+, a non-specific cation channel blocker, failed to prevent appearance of these giant slow inward currents (Yuan & Atchison, 2003), imply that this current probably does not result from a MeHg-induced activation of non-specific cation channels. In all likelihood, it is an intracellular Ca2+-activated Cl− current because MeHg causes increases in intracellular Ca2+ (Hare et al. 1993). It is unclear whether this current is a response induced specifically by MeHg exposure, or an intrinsic response unmasked and stimulated by MeHg exposure. These giant currents are not the only anomalous type of response induced by MeHg. In dorsal root ganglion neurons, both inorganic mercury and MeHg induced a slowly developing but steady-state inward current, which was not blocked by bicuculline, PTX or lanthanum (Arakawa et al. 1991; Narahashi et al. 1994). A similar slow inward current was also observed in cerebellar granule cells in culture. This current was independent of extracellular Ca2+, Na+ and K+, in addition to being insensitive to bicuculline and PTX (Xu & Atchison, 1998). This suggests that this current may be a non-specific cation-mediated response, and could be correlated to MeHg-induced membrane depolarization. This MeHg-induced slowly developing steady-state inward current was also observed in both Purkinje and granule cells in the present studies.
GABAA receptor-mediated inhibitory synaptic responses in granule cells are more sensitive to MeHg than are those in Purkinje cells
As a test of the hypothesis that granule cell GABAA receptors were more sensitive to MeHg than were Purkinje cell receptors, we compared the time to block of responses in the two cells. Consistent with the notion that the cerebellar granule cell is extremely sensitive to MeHg-induced neurotoxicity (Hunter & Russell, 1954; Leyshon-Sorland et al. 1994) or cytotoxicity (Marty & Atchison, 1998), the sIPSCs in granule cells were blocked more rapidly by MeHg than were those in Purkinje cells. Also consistent with this notion, preliminary data obtained from cerebellar granule cells in culture showed that a very low concentration of MeHg (0.1 μM) inhibited GABAA receptor-mediated currents significantly (Xu & Atchison, 1998), which suggests that the sIPSCs in granule cells may be indeed highly sensitive to MeHg exposure. In the present study, it took about 3-fold less time for MeHg (10, 20 and 100 μM) to block sIPSCs in granule cells than to block those in Purkinje cells. This lends further credence to the conclusion that GABAA receptor-mediated responses in granule cells in cerebellar brain slices are highly sensitive to MeHg, albeit at considerably higher concentrations than were required in isolated single cells in culture.
The onset of the presynaptic effect - increased sIPSC/mIPSC frequency - induced by MeHg in granule cells was also hastened as compared to the effect in Purkinje cells. Hence, heightened sensitivity to MeHg appears to be a more general characteristic of cerebellar granule cells. It is unclear yet whether or not the block of sIPSCs in granule cells is a direct effect of MeHg exposure or a secondary effect due to MeHg-induced membrane damage, because background electrical noise, a sign of membrane damage, usually increased during the late phase of exposure. However, it is likely to be a direct effect because in some cases the blocked responses re-emerged after washing cells with the MeHg chelator D-penicillamine, and the noise level reverted to pre-exposure levels. Moreover, it took a longer time to block sEPSCs recorded from granule cells under similar recording conditions than it did sIPSCs (Yuan & Atchison, 2001).
Conclusion
In summary, acute bath application of MeHg to Purkinje or granule cells in rat cerebellar slices caused prominent alterations of inhibitory GABAergic transmission. Both pre- and postsynaptic mechanisms appeared to be involved. However, spontaneous GABAergic currents in granule cells were blocked at an earlier time by MeHg than were those in Purkinje cells. Whether or not this differential sensitivity is due to a differential expression of GABAA receptor α subunits in these two cells remains to be determined. If the differential expression of GABAA receptor α subunits in these two cells does play a role in causing differential sensitivity of Purkinje or granule cells to MeHg, this could provide new insight into the mechanisms by which MeHg causes selective neurotoxicity in the cerebellum.
Acknowledgments
Supported by NIH grants ES03299 and ES11662. The authors gratefully acknowledge the expert word processing assistance of Kelly O'Brien and Mallory Koglin.
REFERENCES
- Amenta F, Cavalotta D, Del Valle ME, Mancini M, Sabbatini M, Torres JM, Vega JA. Calbindin D-28k immunoreactivity in the rat cerebellar cortex, age-related changes. Neurosci Lett. 1994;178:131–134. doi: 10.1016/0304-3940(94)90307-7. [DOI] [PubMed] [Google Scholar]
- Arakawa O, Nakahiro M, Narahashi T. Mercury modulation of GABA-activated chloride channel and non-specific cation channels in rat dorsal root ganglion neurons. Brain Res. 1991;551:58–63. doi: 10.1016/0006-8993(91)90913-g. [DOI] [PubMed] [Google Scholar]
- Atchison WD. Extracellular calcium-dependent and independent effects of methylmercury on spontaneous and potassium-evoked release of acetylcholine at the neuromuscular junction. J Pharmacol Exp Ther. 1986;237:672–680. [PubMed] [Google Scholar]
- Atchison WD, Narahashi T. Methylmercury-induced depression of neuromuscular transmission in the rat. Neurotoxicology. 1982;3:37–50. [PubMed] [Google Scholar]
- Bakir F, Damluji S, Amin-Zaki L, Murtadha M, Khalidi A, Al-Rawi NY, Tikriti S, Dahir HI, Clarkson TW, Smith JC, Doherty RA. Methylmercury poisoning in Iraq. Science. 1973;181:230–240. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Celio MR. Calbindin D-28k and parvalbumin in the rat nervous system. Neuroscience. 1990;35:375–475. doi: 10.1016/0306-4522(90)90091-h. [DOI] [PubMed] [Google Scholar]
- Chang LW. Neurotoxic effects of mercury - a review. Environ Res. 1977;14:329–373. doi: 10.1016/0013-9351(77)90044-5. [DOI] [PubMed] [Google Scholar]
- Concas A, Corda MG, Salis M, Mulas ML, Milia A, Corongiu FP, Biggio G. Biochemical changes in the rat cerebellar cortex elicited by chronic treatment with methyl mercury. Toxicol Lett. 1983;18:27–33. doi: 10.1016/0378-4274(83)90066-8. [DOI] [PubMed] [Google Scholar]
- Corda MG, Concas A, Rossetti Z, Guarneri P, Corongiu FP, Biggio G. Methyl mercury enhances [3H]diazepam binding in different areas of the rat brain. Brain Res. 1981;229:264–269. doi: 10.1016/0006-8993(81)90769-1. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Fisher JL, MacDonald RL. The role of an α subtype M2-M3 His in regulating inhibition of GABAA receptor current by zinc and other divalent cations. J Neurosci. 1998;18:2944–2953. doi: 10.1523/JNEUROSCI.18-08-02944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Zhang J, MacDonald RL. The role of α1 and α6 subtype amino-terminal domains in allosteric regulation of γ-aminobutyric acida receptors. Mol Pharmacol. 1997;52:714–724. doi: 10.1124/mol.52.4.714. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Benke D, Mertens S, Oertel WH, Bachi T, Mohler H. Five subtypes of type A gamma-aminobutyric acid receptors identified in neurons by double and triple immunofluorescence staining with subunit-specific antibodies. Proc Natl Acad Sci U S A. 1992;89:6726–6730. doi: 10.1073/pnas.89.15.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Fritschy JM. Cerebellar granule cells in vitro recapitulate the in vivo pattern of GABAA-receptor subunit expression. Brain Res Develop Brain Res. 1995;88:1–16. doi: 10.1016/0165-3806(95)00062-i. [DOI] [PubMed] [Google Scholar]
- Hare MF, McGinnis KM, Atchison WD. Methylmercury increases intracellular concentrations of Ca++ and heavy metals in NG 108–15 cells. J Pharmacol Exp Ther. 1993;266:1626–1635. [PubMed] [Google Scholar]
- Hillman D, Chen S, Aung TT, Cherksey B, Sugimori M, Llinas RR. Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci U S A. 1991;88:7076–7080. doi: 10.1073/pnas.88.16.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter D, Russell DS. Focal cerebellar atrophy in a human subject due to organic mercury compounds. J Neurol Neurosurg Psych. 1954;17:235–241. doi: 10.1136/jnnp.17.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki K, McGowan E, Mock G, Chandler S, Emson PC. Distribution of calcium binding protein mRNAs in rat cerebellar cortex. Neurosci Lett. 1993;153:80–84. doi: 10.1016/0304-3940(93)90082-v. [DOI] [PubMed] [Google Scholar]
- Komulainen H, Keränen A, Saano V. Methylmercury modulates GABAA receptor complex differentially in rat cortical and cerebellar membranes in vitro. Neurochem Res. 1995;20:659–662. doi: 10.1007/BF01705532. [DOI] [PubMed] [Google Scholar]
- Konnerth A, Llano I, Armstrong CM. Synaptic currents in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1990;87:2662–2665. doi: 10.1073/pnas.87.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Kuner T, Seeburg PH, Lüddens H. Selective antagonist for the cerebellar granule cell-specific GABAA receptor. Mol Pharmacol. 1995;47:283–289. [PubMed] [Google Scholar]
- Laurie DJ, Seeburg PH, Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992;12:1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque PC, Atchison WD. Interaction of mitochondrial inhibitors with methylmercury on spontaneous quantal release of acetylcholine. Toxicol Appl Pharmacol. 1987;87:315–324. doi: 10.1016/0041-008x(87)90293-6. [DOI] [PubMed] [Google Scholar]
- Levesque PC, Atchison WD. Effect of alteration of nerve terminal Ca2+ regulation on increased spontaneous quantal release of acetylcholine by methylmercury. Toxicol Appl Pharmacol. 1988;94:55–65. doi: 10.1016/0041-008x(88)90336-5. [DOI] [PubMed] [Google Scholar]
- Leyshon-Sorland K, Jasani B, Morgan AJ. The location of mercury and metallothionein in the cerebellum of rats experimentally exposed to methylmercury. Histochem J. 1994;26:161–169. doi: 10.1007/BF00157965. [DOI] [PubMed] [Google Scholar]
- Leyshon-Sorland K, Morgan AJ. An integrated study of the morphological and gross-elemental consequences of methyl mercury intoxication in rats, with particular attention on the cerebellum. Scanning Microsc. 1991;5:895–904. [PubMed] [Google Scholar]
- Mäkelä R, Wisden W, Korpi ER. Loreclezole and La3+ differentiate cerebellar granule cell GABAA receptor subtypes. Eur J Pharmacol. 1999;367:101–105. doi: 10.1016/s0014-2999(98)00944-3. [DOI] [PubMed] [Google Scholar]
- Marty MS, Atchison WD. Elevation of intracellular Ca2+ as a probable contributor to decreased viability in cerebellar granule cells following acute exposure to methylmercury. Toxicol Appl Pharmacol. 1998;150:98–105. doi: 10.1006/taap.1998.8383. [DOI] [PubMed] [Google Scholar]
- Minnema DJ, Cooper GP, Greenland RD. Effects of methylmercury on neurotransmitter release from rat brain synaptosomes. Toxicol Appl Pharmacol. 1989;99:510–521. doi: 10.1016/0041-008x(89)90158-0. [DOI] [PubMed] [Google Scholar]
- Mody I, Koninck YD, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Ma JY, Arakawa O, Reuveny E, Nakahiro M. GABA receptor-channel complex as a target site of mercury, copper, zinc, and lanthanides. Cell Molec Neurobiol. 1994;14:599–621. doi: 10.1007/BF02088671. [DOI] [PubMed] [Google Scholar]
- Peng S-Q, Atchison WD. Methylmercury-induced block of voltage-gated Ca2+ channels in acutely isolated cerebellar Purkinje neurons of rat. The Toxicologist. 2002;61:208. (abstract) [Google Scholar]
- Peng S-Q, Hajela RK, Atchison WD. Effects of methylmercury on human neuronal L-type calcium channels transiently expressed in human embryonic kidney cells. J Pharmacol Exp Ther. 2002;302:424–432. doi: 10.1124/jpet.102.032748. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Puia G, Costa E, Vicini S. Functional diversity of GABA-activated Cl− currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994;12:117–126. doi: 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, MacDonald RL. Properties of putative cerebellar γ-aminobutyric acidA receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Sigel E, Baur R. Electrophysiological evidence for the coexistence of α1 and α6 subunits in a single functional GABAA receptor. J Neurochem. 2000;74:2590–2596. doi: 10.1046/j.1471-4159.2000.0742590.x. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Atchison WD. Methylmercury affects multiple subtypes of Ca2+ channels in cerebellar granule neurons. Toxicol Appl Pharmacol. 2000;167:1–11. doi: 10.1006/taap.2000.8967. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dolt H-U, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Su MO, Okita GT. Effect of methylmercury on hypnotic action of hexobarbital, liver hydroxylase and cytochrome P-450 in mice. Toxicology. 1986;39:233–245. doi: 10.1016/0300-483x(86)90025-9. [DOI] [PubMed] [Google Scholar]
- Tanaka O, Sakagami H, Kondo H. Localization of mRNAs of voltage-dependent Ca2+-channels, four subtypes of α1- and β-subunits in developing and mature rat brain. Brain Res Mol Brain Res. 1995;30:1–16. doi: 10.1016/0169-328x(94)00265-g. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Bodewitz G, Stephenson FA, Turner JD. Mapping of GABAA receptor α-5 and α-6 subunit-like immunoreactivity in rat brain. Neurosci Lett. 1992;144:53–56. doi: 10.1016/0304-3940(92)90714-i. [DOI] [PubMed] [Google Scholar]
- Thompson CL, Stephenson FA. GABAA receptor subtypes expressed in cerebellar granule cells, a developmental study. J Neurochem. 1994;62:2037–2044. doi: 10.1046/j.1471-4159.1994.62052037.x. [DOI] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental change of inhibitory synaptic currents in cerebellar granule neurons. Role of GABAA receptor α6 subunit. J Neurosci. 1996;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traxinger DL, Atchison WD. Comparative effects of divalent cations on the methylmercury-induced alteration of acetylcholine release. J Pharmacol Exp Ther. 1987;240:451–459. [PubMed] [Google Scholar]
- Wisden W, Korpi ER, Bahn S. The cerebellum, a model system for studying GABAA receptor diversity. Neuropharmacology. 1996;35:1139–1160. doi: 10.1016/s0028-3908(96)00076-7. [DOI] [PubMed] [Google Scholar]
- Xu YF, Atchison WD. Methylmercury blocks gamma-aminobutyric acid (GABAA) current and induces a nonspecific inward current in rat cerebellar granule cells. Soc Neurosci Abst. 1998;23:373. [Google Scholar]
- Yuan Y, Atchison WD. Disruption by methylmercury of membrane excitability and synaptic transmission in hippocampal slices of the rat. Toxicol Appl Pharmacol. 1993;120:203–215. doi: 10.1006/taap.1993.1104. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury acts at multiple sites to block hippocampal synaptic transmission. J Pharmacol Exp Ther. 1995;275:1308–1316. [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Action of methylmercury on GABAA receptor-mediated inhibitory synaptic transmission is primarily responsible for its early stimulatory effects on hippocampal CA1 excitatory synaptic transmission. J Pharmacol Exp Ther. 1997;282:64–73. [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Comparative effects of methylmercury on parallel-fiber and climbing-fiber response of rat cerebellar slices. J Pharmacol Exp Ther. 1999;288:1015–1025. [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Characterization of effects of methylmercury on synaptic currents at mossy fiber-granule cell, parallel fiber- and climbing fiber-Purkinje cell synapses in rat cerebellar slices) Soc Neurosci Abstr. 2001;27:713–4. [Google Scholar]
- Yuan Y, Atchison WD. Multiple factors are involved in the methylmercury (MeHg)-induced early stimulatory effects on spontaneous synaptic currents of Purkinje cells in cerebellar slices of rat. The Toxicologist. 2002;61:208. (abstract) [Google Scholar]
- Yuan Y, Atchison WD. Methylmercury induces an unidentified transient giant slow inward current in Purkinje cells in cerebellar slices of rat. Biophys J. 2003;84:555A. doi: 10.1124/jpet.104.080721. [DOI] [PubMed] [Google Scholar]
- Zhang C, Messing A, Chiu S. Specific alteration of spontaneous GABAergic inhibition in cerebellar Purkinje cells in mice lacking the potassium channel Kv1. 1. J Neurosci. 1999;19:2852–2864. doi: 10.1523/JNEUROSCI.19-08-02852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu WJ, Wang JF, Corsi L, Vicini S. Lanthanum-mediated modification of GABAA receptor deactivation, desensitization and inhibitory synaptic currents in rat cerebellar neurons. J Physiol. 1998;511:647–661. doi: 10.1111/j.1469-7793.1998.647bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Kullmann DM, Bennett M. Release of neurotransmitters. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. San Diego: Academic Press; 1999. pp. 155–192. [Google Scholar]


