Abstract
We investigated the neurobiological basis of variation in sensitization between three aplysiid species: Aplysia californica, Phyllaplysia taylori and Dolabrifera dolabrifera. We tested two different forms of sensitization induced by a noxious tail shock: local sensitization, expressed near the site of shock, and general sensitization, tested at remote sites. Aplysia showed both local and general sensitization, whereas Phyllaplysia demonstrated only local sensitization, and Dolabrifera lacked both forms of learning. We then investigated a neurobiological correlate of sensitization, heterosynaptic modulation of sensory neuron excitability by tail-nerve stimulation. We found (1) an increase in sensory neuron (SN) excitability after both ipsilateral and contralateral nerve stimulation in Aplysia, (2) a smaller and shorter-lasting increase in Phyllaplysia, and (3) no effect in Dolabrifera. Because sensitization in Aplysia is strongly correlated with serotonergic (5-HT) neuromodulation, we hypothesized that the observed interspecific variation in sensitization and SN neuromodulation might be correlated with variation in the anatomy and/or functional response of the serotonergic system. However, using immunohistochemistry, we found that all three species showed a similar pattern of 5-HT innervation. Furthermore, they also showed comparable 5-HT release evoked by tail-nerve shock, as measured with chronoamperometry. These observations indicate that interspecific variation in learning is correlated with differences in SN heterosynaptic plasticity within a backgound of evolutionary conservation in the 5-HT neuromodulatory pathway. We thus hypothesize that evolutionary changes in learning phenotype do not involve modifications of the 5-HT pathway per se, but rather, changes in the response of SNs to the activation of this or other neuromodulatory pathways upon noxious stimulation.
The aplysiid molluscs provide a useful model lineage with which to study the evolution of neurobiological mechanisms of learning. Members of the aplysiid family (Fig. 1) exhibit various defensive reflexes that are controlled by homologous sets of identified neurons (Wright et al. 1996; Erixon et al. 1999). Furthermore, these species can adapt their behaviour to their past experience through simple forms of learning such as sensitization, a generalized increase in responsiveness after a novel or noxious stimulus (Wright, 1998; Erixon et al. 1999). Of particular interest is the existence of evolutionary differences between the expression of sensitization in the three aplysiid species, Aplysia californica, Phyllaplysia taylori and Dolabrifera dolabrifera. Stimuli that readily induce general sensitization in Aplysia, have no effect on Phyllaplysia and Dolabrifera (Wright, 1998; Erixon et al. 1999). To understand how the neurobiological mechanisms of these simple forms of learning changed across evolution, we have taken advantage of the detailed mechanistic analyses of learning in Aplysia to study homologous mechanisms in Phyllaplysia and Dolabrifera.
Figure 1. Evolution of opistobranch species.
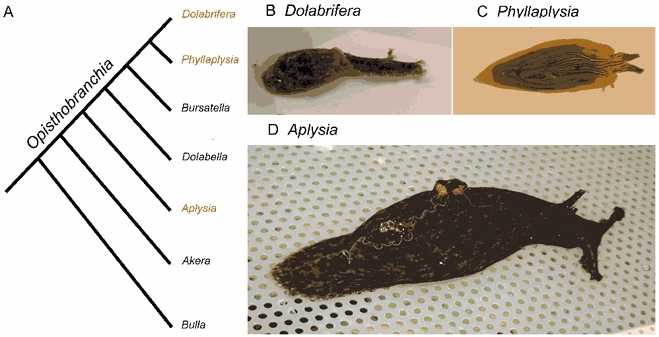
A, cladogram of the evolution of opistobranch species (adapted from a recent cladistic analysis of mDNA sequence and conventional character traits; Medina & Walsh, 2000). All species except Bulla are in the family Aplysiidae. B-D, pictures of the different species used in the present study: Dolabrifera (B), Phyllaplysia (C) and Aplysia (D). Wild-caught adult Aplysia individuals are usually much larger (about 250 g body weight, 15 cm long) than their Dolabrifera and Phyllaplysia counterparts (2–3 g body weight, 3 cm long).
In Aplysia, sensitization is thought to rely on the activation of one or several ‘instructor’ loci that respond to the learning experience and release neuromodulatory substances in the central nervous system (CNS). This neuromodulation contributes to the adaptive changes in the behavioural output of various neural circuits. For example, several defensive reflexes are enhanced by a noxious stimulation such as an electric shock applied to the tail or the head (Castellucci & Kandel, 1976; Walters et al. 1983b). At the cellular level, the tail-mantle withdrawal reflex is mediated by a population of tail sensory neurons (SNs) located in the pleural ganglion. These SNs directly excite tail motor neurons (MNs) responsible for tail withdrawal, and indirectly contribute to the excitation of different siphon MNs, through several interneurons located in the pleural and abdominal ganglia (Frost et al. 1988; Trudeau & Castellucci, 1993; Cleary & Byrne, 1993; Cleary et al. 1995; Frost & Kandel, 1995). During sensitization, a serotonergic instructor locus is activated and releases serotonin (5-HT) onto a variety of reflex circuits including the tail SNs (Mackey et al. 1989; Marinesco & Carew, 2002a). This evoked 5-HT initiates heterosynaptic changes in a variety of loci, including enhancement of transmitter release of SNs onto their many follower neurons, which increase the strength and/or duration of withdrawal reflexes (Brunelli et al. 1976; Glanzman et al. 1989; Mercer et al. 1991; review by Byrne & Kandel, 1996). Thus, generalized sensitization is a coordinated response to noxious stimuli that is initiated by a serotonergic ‘instructor’ locus and is expressed as an enhancement in the response of the neurons that mediate defensive withdrawal responses.
In the present study, we asked whether the response of this serotonergic locus varies across three species that show variation in their sensitization phenotype. A cladistic analysis has shown that SNs in Dolabrifera but not in Phyllaplysia have lost two ancestral modulatory responses to 5-HT that are thought to underlie sensitization in Aplysia: increased excitability and spike broadening (Wright et al. 1996; Erixon et al. 1999). Although these findings suggest that the lack of sensitization observed in Dolabrifera (Wright, 1998) might be attributable to this loss of neuromodulation, they do not explain the lack of sensitization in Phyllaplysia (Erixon et al. 1999). Most importantly, they leave open the possibility that evolutionary changes in serotonergic pathways might have had a critical role in the evolution of learning phenotype.
We first sought to better characterize the behavioural deficits showed by Phyllaplysia and Dolabrifera. It is now commonly accepted that sensitization exists in different forms depending on the proximity between the test site eliciting the reflex and the sensitizing site where the noxious stimulus is applied. Thus, sensitization induced by a noxious tail shock is usually stronger and longer-lasting when tested near the site of shock (local) compared to more remote sites (general). Moreover, some molecular mechanisms required for general sensitization seem different from those involved in local sensitization (Walters & Byrne, 1985; Walters, 1987a; Sutton & Carew, 2002).
We found significant variation in local and general sensitization in the three species. This variation was paralleled by interspecific variation in the heterosynaptic changes to SN excitability induced by nerve stimulation. By contrast, neither the anatomical patterns, nor the amperometrically measured response of the serotonergic instructor locus, showed any major interspecific variation. Thus, interspecific variation in learning seems correlated with changes in the response of defensive reflex circuits to modulatory signals triggered by noxious stimulation, but does not appear to involve any changes in the anatomy or function of the pathways releasing these signals.
METHODS
Animals
We performed these studies on adult Aplysia californica, Phyllaplysia taylori, and Dolabrifera dolabrifera of similar size (3–6 cm in length). This size range was near the maximum size for both Phyllaplysia and Dolabrifera, and although small for adult Aplysia, was nevertheless 3–5 times larger than the earliest developmental stage known to show sensitization (Rankin & Carew, 1988). Because of seasonal constraints, in the chronoamperometric studies of 5-HT release, we were obliged to use larger sizes for Aplysia species (5–20 cm) than for the other two species (3–6 cm). This size difference should not account for the observed differences in 5-HT release. The development of the Aplysia serotonergic system is usually over at much earlier juvenile stages (between stages 9 and 12; 0.1–0.25 cm; Nolen & Carew, 1994; Marois & Carew, 1997). Furthermore, among these Aplysia we saw no correlation between release and the size of the animal being tested. Individuals of Aplysia, Phyllaplysia, and Dolabrifera were supplied by the University of Miami Aplysia facility, Alacrity Marine, Marinus (Long Beach, CA, USA), Dave Duggins (Friday Harbor, WA, USA), and Curt Fiedler (University of Hawaii). Individuals of Aplysia and Phyllaplysia were maintained in a cold salt-water tank (14–16 °C). Dolabrifera specimens were kept in a warm salt-water tank (22–25 °C). These temperatures are near the ambient temperatures typical for each species (Aplysia, 13–25 °C, W. G. Wright, personal observation; Phyllaplysia, 10–22 °C, D. Duggins, Friday Harbor, WA, USA, personal communication; Dolabrifera, 22-28 °C, C. Fiedler, University of Hawaii, personal communication).
Behaviour
Animals were placed in petri dishes (9 cm diameter × 1.5 cm deep) approximately 30 min prior to the start of each experiment. Water temperature was maintained at 14–16 °C for Aplysia and Phyllaplysia and room temperature (22–25 °C) for Dolabrifera. Our testing and training protocols were adapted from Walters (1987b). We measured the duration of the tail-mantle withdrawal (Wright, 1998; Erixon et al. 1999) in response to test stimuli delivered with a pressure activated plunger (1 mm diameter; 0.5 s of 25 Hz pulses) applied to both left and right sides of the tail of all three species before and after application of a strong electric shock to one (randomly determined) of the two test sites. Each test was video-taped and scored at a later time by a ‘blind’ observer, who did not know the animal's treatment. Test stimuli were delivered to the tail approximately 75 % of the way between the tip of the tail and the posterior end of the mantle and half-way between the midline and the lateral edge of the tail. Two pre-test stimuli on each side were given at 10 min intervals (5 min between stimuli to alternate sides) prior to the training stimuli. Four post-test stimuli on each side were delivered at 10 min intervals beginning 20 min after the training stimuli.
The training stimulus (10 × 500 ms trains of shock; 1 mA, 25 Hz; every 5 s was administered to either the test site on the left or right side (randomly determined) of the tail via a suction electrode (capacitance-coupled). This stimulus readily induced ink and/or opaline release. The post-test tail-mantle reflex duration from each side was divided by the mean of that side's two pre-test responses. Significant increases indicated ‘local sensitization’ if observed on the shocked side, ‘general sensitization’ if observed on the unshocked side, and ‘site-specific sensitization’ if greater on the shocked than the unshocked sides. Statistical analysis was performed using either ANOVA on normalized reflex responses, followed by a LSD (least significant difference; Sokal & Rohlf, 1981) post hoc test, or a Student's t test when only two data groups were compared (significance level: P < 0.05).
Excitability of tail SNs
Individuals of all three species were dissected after anaesthesia (injection of isotonic MgCl2; approximately 1 ml (g body weight)−1). The cerebral, pleural, and pedal ganglia and peripheral nerves were removed intact and pinned in a Sylgard-coated dish. Both pleural ganglia were desheathed in a 50:50 mixture of isotonic MgCl2 and artificial sea water (ASW; 460 mM NaCl, 55 mM MgCl2, 11 mM CaCl2, 10 mM KCl, 10 mM Trizma, pH 7.6). Experiments were performed at room temperature (22–25 °C) to allow comparison with previous neuromodulation experiments (Wright et al. 1996; Erixon et al. 1999). ASW perfused the preparation at a rate of ca 6 ml min−1. Tail-sensory homologues were identified by their size and location (Wright et al. 1996; Erixon et al. 1999) and their membrane potential was recorded intracellularly with microelectrodes (10–20 MΩ for Aplysia and Phyllaplysia; 30–40 MΩ for Dolabrifera) filled with 3 M KCl.
To test SN excitability, a 500 ms pulse of depolarizing current (0.2–1.5 nA; similar for all three species) was adjusted to fall between the thresholds for eliciting one versus two spikes (≈20 % above threshold for a single spike). Three baseline tests were made at 1 min intervals. Only experiments with single spikes during each of the three baseline tests were continued. Two SNs from each of the two pleural ganglia were often recorded simultaneously. Five seconds after the third baseline test, 4–10 blocks of shocks were delivered via suction electrodes to either the left or right P9 (tail) nerve (interblock interval 5 s). Each block consisted of a 1 s train of pulses (40 ms, 12 Hz, 27 V; capacitance-coupled). The first post-training test was delivered 10 s after the end of the training period and every 1 min thereafter. Three to four experiments, each using a different SN, were performed for ipsi- and contralateral nerve stimulation for each animal (time between experiments was > 20 min) and averaged for statistical analysis. We observed no systematic increase or decrease in the excitability response across trials, nor was there any bias due to which nerve (ipsi- or contralateral) was stimulated first. An ANOVA followed by a LSD post hoc analysis was used to compare the number of spikes before and after nerve shock, as well as the number of post-shock spikes on the ipsilateral versus contralateral sides.
Immunohistochemistry
Immunohistochemical labelling of 5-HT fibres was performed through the following steps: the intact ganglia were fixed overnight with 4 % formaldehyde (in 0.01 M PBS with 20 % sucrose) at 4 °C, the tissue was then permeabilized with 4 % Triton (in 0.01 M PBS) for 1 h and non-specific binding was blocked by immersion in 10 % goat serum (Jackson Immunoresearch Laboratories, West Grove, PA, USA) with 0.5 % Triton for 1 h before exposure to the rabbit polyclonal anti-serotonin antibody (Immunostar, Stillwater, MN, USA; 1/1000 for 2.5 days at 4 ° C), and finally, visualization of the primary antibody to 5-HT was performed with a goat anti-rabbit secondary antibody directly coupled to Alexa fluor 488 (Molecular Probes, Eugene, OR, USA; 20 μl ml−1 for 3 h). Fluorescence images were acquired with a BioRad confocal microscope (Bio Rad, Hercules, CA, USA). Excitation was performed at 488 nm by a Kr/Ar mixed gas laser. We used standard T1/E2 filters with a detection filter at 522 /35 nm. Fluorescence images shown in Fig. 4 were obtained by projection of 10 to 15 optical sections spaced by 10 μm using the Lasersharp software.
Figure 4. Immunohistochemical labelling of serotonin-immunoreactive fibres in the vicinity of tail sensory neurons.
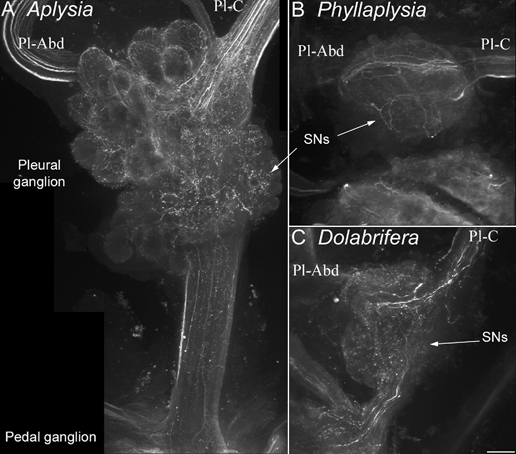
Confocal photomicrographs of clusters of tail SNs in the pleural ganglion, stained with an anti-5-HT antibody were taken from Aplysia (A), Phyllaplysia (B) and Dolabrifera (C, n = 2). The orientation is the same for all species: pleural ganglion (top), pedal ganglion (bottom), pleural-abdominal nerve (Pl-Abd, left) and pleural-cerebral nerve (Pl-C, right). The three species show a similar pattern of 5-HT innervation: numerous 5-HT fibres are present in the cerebral-pleural nerve, they branch over the cluster of tail SNs (arrows) and/or travel to the abdominal ganglion through the pleural-abdominal nerve. Some 5-HT processes also travel towards the pedal ganglion through the pleural-pedal nerve. No 5-HT-immunoreactive cell bodies were evidenced in the pleural ganglion. Scale bar = 100 μm.
Chronoamperometric detection of neuronal 5-HT release
Neuronal 5-HT release was detected using carbon fibre electrodes covered with cellulose as described by Marinesco & Carew (2002b). Briefly, carbon fibre electrodes were dipped five times in a solution 5 % cellulose with 10 min intervals to allow the film to dry. Before the experiment, the tip of the electrode was hydrolysed for 20 min in a solution of KOH 0.08 M and rinsed in ASW. This method allowed a more sensitive detection of biogenic amines in the Aplysia brain with a detection limit as low as 10–20 nm for 5-HT (Marinesco & Carew, 2002a). Recordings were performed with a VA-10 voltammeter (NPI Electronic, Tamm, Germany) connected to a 3-electrode potentiostat. Reference and auxiliary electrodes were both made of a chlorided silver wire (Medwire, Mount Vernon, NY, USA). Data acquisition was achieved through a 16-bit acquisition card (Instrutech Corp., Greatneck, NY, USA) run with a homemade software based on IGOR 4.03 procedures (Wavemetrics Inc., Lake Oswego, OR, USA).
Carbon fibre electrodes allow selective oxidation of 5-HT in living ganglia, the measured oxidation current being an index of the 5-HT concentration at the vicinity of the electrode. To detect 5-HT release in the nervous system of Aplysia and its related species, we used chronoamperometry, which allows detection of oxidation currents at high temporal resolution (2 Hz). Chronoamperometry was performed with four successive voltage steps (80 mV, 40 ms; 230 mV, 15 ms; 250 mV, 40 ms; 400 mV, 15 ms) applied between the working and reference electrodes every 500 ms. 5-HT oxidation currents were estimated as the difference in current between the fourth and third pulse. We have previously shown that this technique allows reliable estimates of 5-HT concentrations in the Aplysia CNS (Marinesco & Carew, 2002a,b). The recorded current was filtered with a 1 kHz low-pass filter and measured by averaging its value over the last 5 ms of the pulse, to improve the signal-to-noise ratio.
Tail SNs were visually identified as for intracellular recordings, and carbon fibre electrodes were inserted in the neuropil, under the SN cell bodies in the pleural ganglion, or in the putative region of SN-MN synapses, in the pedal ganglion, immediately above the junction of the pleural-pedal nerve with the pedal ganglion and under tail MN cell bodies (Marinesco & Carew, 2002a). For chronoamperometry experiments, tail nerves were shocked with a 2 s train of 5 ms pulses at 40 Hz (≈30 V), a procedure known to induce reliable 5-HT release in Aplysia (Marinesco & Carew, 2002a). At the end of the experiment, the carbon fibre electrode was calibrated in a flow-injection chamber, by injection of a solution of 5-HT 1 μM for 1 min. Because the 5-HT oxidation current is linear with 5-HT concentration (Marinesco & Carew, 2002b), we could estimate the maximal concentration released into the neuropil in each experiment, using the standard value obtained with 1 μM 5-HT.
RESULTS
Behaviour
Tail-mantle withdrawal reflexes before training were somewhat different between the three species, but were statistically indistinguishable in the shocked versus unshocked side for each species: 6.4 ± 0.4 s in Aplysia, 9.2 ± 0.8 s in Phyllaplysia and 8.1 ± 0.6 s in Dolabrifera. As previously observed (Walters, 1987a), Aplysia showed both general and local sensitization (Fig. 2A). Statistically enhanced reflexes (P ≤ 0.05) were observed in all but the 40 min post test on the unshocked side (Fig. 2A). The shocked-side reflex was significantly longer than the unshocked reflex at the 40 min test (P ≤ 0.05). Combining the data from the 20 and 30 min post tests for shocked and unshocked sides indicated significant short-term sensitization on the shocked (+103 ± 27 % above baseline; P = 0.003, see Fig. 2D) and unshocked (+75 ± 20 %; P = 0.04) sides. Short-term sensitization was significantly different between the shocked and unshocked side when combining the data from all four post tests (20 to 50 min after shock, P < 0.05) but not when combining the 20 and 30 min post tests only (P = 0.43, Fig. 2D). Indeed, the reflex evoked by testing the ipsilateral side stayed sensitized for a longer time than the one evoked from the contralateral side, allowing site-specific sensitization to appear at later time points (30–50 min) in this species. This finding stands in contrast with previous studies from Walters (1987b), which demonstrated site-specific sensitization of the tail-induced tail withdrawal reflex as soon as 15 min after tail shock. This discrepancy might reside in the differences in the neuronal networks underlying tail or mantle withdrawal reflexes.
Figure 2. Sensitization of the tail withdrawal reflex in the three species.
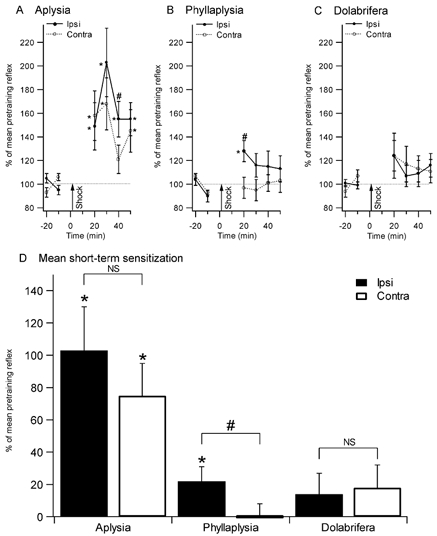
Reflex duration (% of mean pretraining reflex) after training is shown in the top panels (A, B, and C). Tail withdrawal was tested every 10 min before and after tail shock in Aplysia (n = 11, A), Phyllaplysia (n = 12, B) and Dolabrifera (n = 11, C). Combined post-shock data are displayed in D. Aplysia showed significant sensitization both ipsilateral and contralateral to the shock. Phyllaplysia showed only local (ipsilateral) sensitization. Dolabrifera showed a transient, reduced form of sensitization on both sides. Analysis of short-term sensitization (mean between 20 and 30 min tests) confirmed the presence of both local and general sensitization in Aplysia, and local sensitization only in Phyllaplysia. However, the small enhancement in tail withdrawal in Dolabrifera was not significant (D). *Significant difference between pre- and post-test values (P < 0.05); # significant difference between ipsilateral and contralateral sides (P < 0.05).
Phyllaplysia showed modest, short-lasting local sensitization but no general sensitization (Fig. 2B). At the first (20 min) post test, the shocked-side reflex was significantly elevated above its preshock levels (P = 0.02), indicating local sensitization, and significantly above the reflex of the unshocked side (P < 0.01), indicating site-specific sensitization. The reflex of the unshocked side was unchanged, suggesting a lack of general sensitization, as shown by previous studies (Wright, 1998; Erixon et al. 1999). The averaged data for short-term sensitization (20–30 min) showed a significant enhancement on the shocked side (22 ±9 % above baseline, P = 0.02). In addition, the shocked side reflex was significantly longer than the unshocked one (P ≤ 0.01), confirming the presence of site-specific sensitization.
Although mean reflex duration in Dolabrifera appeared to increase immediately after ipsilateral shock as much as that observed in Phyllaplysia (Fig. 2C, +24 % and +28 %, respectively), the large variability associated with this increase prevented statistical significance. None of the four post tests on the shocked side were significantly elevated above baseline. Similarly, none of the shocked-side post tests were greater than the equivalent test on the unshocked side. Combining the data from 20–30 min post tests for shocked and unshocked sides gave no significant sensitization measured on either side (shocked side, 14 ± 13 %, P = 0.28, n.s.; unshocked side, 18 ± 14 %, P = 0.19, n.s.), and no significant difference between the two sides. Taken together with previous behavioural experiments (Wright, 1998; Erixon et al. 1999), these results suggest that sensitization in Phyllaplysia is limited to areas near the site of the sensitizing stimulus, whereas Dolabrifera may be deficient in all forms of sensitization.
Effects of tail-nerve shock on SN excitability
Sensitization in Aplysia is believed to rely, at least in part, on heterosynaptic plasticity of SNs mediating defensive reflexes such as gill, siphon, or tail withdrawal (reviews by Byrne & Kandel, 1996; Kandel, 2001). This heterosynaptic plasticity includes an increase in SN excitability and facilitation of SN-MN synapses, and is thought to be mediated by 5-HT released after noxious stimulation (Brunelli et al. 1976; Glanzman et al. 1989; Mackey et al. 1989; Marinesco & Carew, 2002a). Recently, Wright et al. (1996) demonstrated that Dolabrifera tail SNs are unresponsive to exogenously applied 5-HT, suggesting that their neuromodulatory responses might have been lost across evolution. To examine heterosynaptic plasticity in tail SNs we measured SN excitability before and after electrical stimulation of either the ipsilateral or contralateral tail nerve in all three species. Tail-nerve shock mimics the effects of a noxious stimulus (Mercer et al. 1991).
In Aplysia, tail-nerve stimulation increased excitability much more in ipsilateral than in contralateral sensory neurons (Fig. 3A). The group data (Fig. 3D) showed significantly elevated excitability for 2 min after nerve shock in ipsilateral sensory neurons and 1 min after nerve shock in contralateral sensory neurons. The significant difference between ipsi- and contralateral excitability lasted 2 min subsequent to shock. These results are similar to previous research on Aplysia (e.g. Walters et al. 1983b). In Phyllaplysia, excitability of sensory homologues was also increased by ipsilateral and contralateral nerve shocks (Fig. 3B). The magnitude of the increase in excitability 10 s after shock was reduced compared to Aplysia (5.5 spikes vs. 7.1 on the ipsilateral side; 2.3 spikes vs. 3.7 on the contralateral side). Moreover, the duration of the effect was much reduced in Phyllaplysia compared to Aplysia (1 min vs. 2 min ipsilateral; 10 s vs. 1 min contralateral; Fig. 3E). The difference between ipsi- and contralateral nerve stimulation was significant at the 10 s (P ≤ 0.01) and 1 min tests (P ≤ 0.01). Finally, Dolabrifera showed no excitability increase, regardless of which nerve was stimulated (Fig. 3C and F). These results indicated that interspecific differences in SN heterosynaptic plasticity were correlated with interspecific differences in sensitization.
Figure 3. Comparison of the effect of nerve shock on excitability of tail sensory neurons in the three study species.
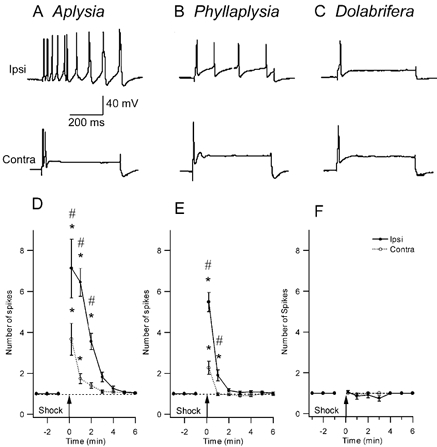
A-C, representative examples of intracellular recordings from homologous SNs in three species. The sensory neuron was injected with a 500 ms intracellular pulse of depolarizing current, 1 min after electrical stimulation of a peripheral tail nerve (top trace: ipsilateral tail-nerve shock; bottom trace: contralateral tail-nerve shock). Current amplitude was set prior to nerve stimulation to give a single action potential (traces not shown). D-F, SN excitability across time after nerve stimulation shows the change in the number of evoked spikes after nerve shock. D, Aplysia (n = 17) shows a significant increase in excitability both ipsi- and contralateral to the shock, that lasts about 3–5 min. E, in Phyllaplysia (n = 17), nerve shock increased excitability only in the ipsilateral SNs for 1 min after nerve stimulation. F, in Dolabrifera (n = 11), no excitability increase was observed after nerve shock. * Significant difference between pre- and post-test values (P < 0.05); # significant difference between ipsilateral and contralateral sides (P < 0.05).
Immunohistochemical labelling of serotonin-immunoreactive fibres
Both sensitization and SN heterosynaptic modulation in Aplysia are thought to rely on 5-HT release in the CNS evoked by noxious stimulation (Mackey et al. 1989; Levenson et al. 1999; Marinesco & Carew, 2002a). Therefore, the interspecific differences we identified might depend on variations in the anatomical projections of the 5-HT system or in its responsiveness to noxious stimulation. To test this hypothesis, we first labelled serotonin-immunoreactive fibres with fluorescent antibodies and observed 5-HT fibres projecting to the tail SN cluster in the pleural ganglion using confocal microscopy. In Aplysia, numerous 5-HT fibres are known to branch over the ventral cluster of the pleural ganglion, where the tail SNs are located (Zhang et al. 1991; Wright et al. 1995; Marinesco & Carew, 2002a). These fibres establish numerous synapses onto SN cell bodies (Zhang et al. 1991). Our immunohistochemical labelling revealed a similar pattern of serotonin-immunoreactive innervation in all three species (Fig. 4, n = 2 for each species). Numerous serotonin-immunoreactive fibres were observed coming from the cerebral ganglion through the cerebral-pleural connective, with extensive branching in the area of tail SN cell bodies (arrows in Fig. 4). Some fibres travelled longitudinally through the pleural ganglion and into the pleural-abdominal (pleural- visceral) nerve. Numerous fibres were also observed in the pleural-pedal connective, travelling towards the pedal ganglion, where tail SNs synapse onto tail MNs. These data show that in all three species, tail SNs receive a significant 5-HT innervation, suggesting that the architecture of the 5-HT modulatory pathway is evolutionarily conserved. Is it possible that these serotonergic fibres do not release 5-HT in response to noxious stimulation in Phyllaplysia and Dolabrifera?
Detection of 5-HT release evoked by tail-nerve stimulation
We measured the concentration of 5-HT released into the neuropil in the vicinity of tail SN cell bodies in the pleural ganglion, and SN-MN synapses in the pedal ganglion using chronoamperometry. Carbon fibre microelectrodes implanted in the vicinity of tail SN cell bodies or SN-MN synapses can readily detect 5-HT released in response to tail-nerve stimulation in Aplysia (Marinesco & Carew, 2002a). Using this technique, we could successfully detect 5-HT release evoked by different patterns of tail-nerve stimulation in Aplysia. After a single tail-nerve shock (2 s train of 5 ms pulses at 40 Hz, 20–50 V), 5-HT release was detected as a transient increase of the 5-HT oxidation current, that peaked 2 to 6 s after the end of the stimulation and returned to its baseline value in about 30–40 s (Fig. 5A). When the tail nerve was shocked repetitively 10 times at 6 s intervals (500 ms trains of 5 ms pulses at 40 Hz, 20–50 V), in a pattern similar to the one used for behavioural and SN excitability studies, 5-HT release was slightly enhanced and prolonged, lasting about 60–80 s (Fig. 5B). However, there was a severe depression in the amplitude of the 5-HT response evoked by individual shocks. This response was maximal at the first shock, decreased at the second shock and was undetectable at the third and subsequent shocks (Fig. 5B). Because the amplitude of 5-HT signals decreased when shocks were repeated at short intervals, we chose to compare 5-HT release in all three species, using one single shock applied to each tail nerve at 10 min intervals (interval between two shocks of the same nerve was 20 min). When shocks are applied at this rate, no depression in 5-HT release is evidenced (Marinesco & Carew, 2002a).
Figure 5. 5-HT release evoked by one or repeated tail-nerve shocks in Aplysia.
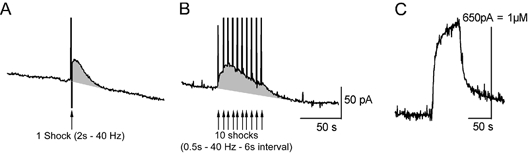
The tail nerve was stimulated using one single shock (A, 2 s train of 5 ms/30 V pulses at 40 Hz) or 10 shocks (B, 500 ms train of 5 ms/30 V pulses at 40 Hz) repeated at 6 s intervals. Time of shocks is shown by arrows. The vertical deflections in the 5-HT signal are caused by the stimulus artifact. C, calibration of the electrode used for the experiment in 5-HT 1 μM, using a flow injection chamber. 5-HT release is only slightly greater with repeated stimulation because of a severe depression in the 5-HT signals evoked by each successive stimulus.
Serotonin release was detected as a similar increase of the 5-HT oxidation current in all three species (Fig. 6). However, the amplitude of the signal varied between species. Using the standard current value obtained in 5-HT 1 μM after each electrode had been implanted in a ganglion, we could assess the maximal concentration that was released in each particular experiment. We found that the maximal 5-HT concentration after ipsilateral tail-nerve shock was greater for Aplysia (72 ± 16 nM pleural, 79 ± 11 nM pedal), intermediate for Phyllaplysia (51 ± 9 nM pleural, 58 ± 11 nM pedal) and lowest for Dolabrifera (32 ± 12 nM pleural, 31 ± 5 nM pedal, Fig. 6A, B and C, Fig. 7A and B). 5-HT release evoked by contralateral nerve shock was smaller than for ipsilateral stimulation in all three species. However, this contralateral release was greatest for Phyllaplysia (15 ± 3 nM pleural, 27 ± 8 nM pedal), intermediate for Aplysia (10 ± 4 nM pleural, 15 ± 5 nM pedal) and lowest for Dolabrifera (3 ± 2 nM pleural, 4 ± 2 nM pedal, Fig. 7A and B), suggesting possible differences in the ratio between 5-HT release evoked by ipsilateral and contralateral stimulation. To examine the degree of lateralization of 5-HT release in the three species, we computed their lateralization ratio as (Ipsi - Contra)/ (Ipsi + Contra), in which 1.0 indicates complete lateralization to the ipsilateral side, 0.0 for equal release in ipsi- and contralateral sides, −1.0 for complete lateralization to the contralateral side (Fig. 7C and D). This ratio was lowest in Phyllaplysia (0.54 ± 0.09 pleural, 0.44 ± 0.15 pedal), with one individual showing greater release on the contralateral side than on the ipsilateral side (Fig. 7D), intermediate in Aplysia (0.79 ± 0.08 pleural, 0.67 ± 0.11 pedal) and highest in Dolabrifera (0.88 ± 0.08 pleural, 0.87 ± 0.08 pedal).
Figure 6. Examples of 5-HT release evoked by tail-nerve stimulation in Aplysia (A), Phyllaplysia (B) and Dolabrifera (C).
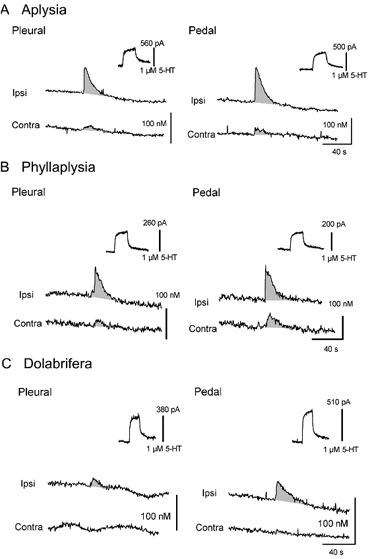
Chronoamperometric recordings obtained from carbon fibre electrodes implanted underneath tail SNs in the pleural ganglion (left) and in the ventral portion of the pedal ganglion proximal to the pleural-pedal nerve, in the region of tail SN-MN synapses (right). Serotonin release was evoked by ipsilateral (Ipsi) and contralateral (Contra) tail-nerve stimulation and 5-HT concentrations were estimated using the calibration value obtained in 5-HT 1 μM for each electrode (upper right of each recording). Consistent 5-HT release was found in all species, lasting 30–40 s after tail-nerve stimulation. However the maximal 5-HT concentration as well as the ratio between the amounts of 5-HT released by ipsilateral and contralateral stimulation varied between the three species. Increases in 5-HT oxidation currents have been underlined by shading, and stimulation artifacts have been removed.
Figure 7. Summary data and statistical analysis of 5-HT release evoked by tail-nerve stimulation.
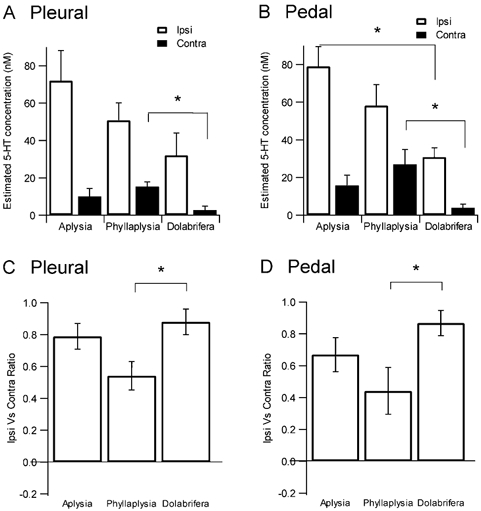
Top, maximal concentration of 5-HT release evoked by ipsilateral or contralateral tail-nerve stimulation in the pleural (A) and pedal (B) ganglia. Ipsilateral stimulation released a greater concentration of 5-HT into the extracellular space in Aplysia than in Phyllaplysia and Dolabrifera. However, 5-HT release evoked by contralateral stimulation was maximal in Phyllaplysia, intermediate in Aplysia and minimal in Dolabrifera. Bottom, a ratio of lateralization was computed as (Ipsi - Contra)/(Ipsi + Contra); 1.0 for complete lateralization to the ipsilateral side, 0.0 for equal release in ipsi- and contralateral sides, −1.0 for complete lateralization to the contralateral side. The lateralization ratio for both pleural (C) and pedal (D) ganglia was maximal for Dolabrifera, intermediate for Aplysia and minimal for Phyllaplysia. Histograms show mean ±s.e.m. *P ≤ 0.05 as assessed by a one-way ANOVA followed by a LSD post hoc test.
Statistical analysis using ANOVA followed by a LSD post hoc test confirmed these interspecies differences. Serotonin release on the ipsilateral side of the stimulation varied significantly over the three species (F2/16= 3.8, P = 0.04, pleural, pedal not significant) with Aplysia having significantly more release than Dolabrifera (P = 0.01). On the contralateral side, Phyllaplysia had significantly more 5-HT release than Dolabrifera (P = 0.03 pleural and pedal). The apparent differences in lateralization were also confirmed by the statistical analysis, which showed that 5-HT release was less lateralized to the ipsilateral side in Phyllaplysia than in Dolabrifera (P = 0.04 pleural, P = 0.03 pedal). There was no significant difference in lateralization between Phyllaplysia and Aplysia.
Overall, these data show that the 5-HT fibres projecting to tail SNs can indeed release 5-HT in response to tail-nerve stimulation in all three species, with similar kinetics (30 to 40 s duration). Although there is some interspecific variation in the details of this 5-HT release, these data suggest that the 5-HT system is essentially conserved across these three species.
DISCUSSION
In the present study, we sought to better characterize the behavioural differences in sensitization between Aplysia, Phyllaplysia and Dolabrifera, and examine possible differences in neuronal mechanisms underlying this form of memory. Phyllaplysia and Dolabrifera have been shown to lack the general sensitization found in Aplysia (Wright, 1998; Erixon et al. 1999). We confirmed this difference in generalized sensitization. Furthermore, we demonstrated that local sensitization, a stronger form of sensitization expressed when testing a site close to the noxious stimulation, has also undergone evolutionary variations: it is strong in Aplysia, weak in Phyllaplysia and virtually absent in Dolabrifera. Therefore, both forms of sensitization appear deficient in Phyllaplysia and Dolabrifera, relative to their expression in Aplysia. The Phyllaplysia learning phenotype, however, seems closer to that of Aplysia, in that it shows some form of local sensitization. We next found a clear correspondence between these behavioural patterns and the increase in SN excitability after noxious stimuli: the increase was robust in Aplysia, weak (and largely ipsilateral) in Phyllaplysia, and non-existent in Dolabrifera. Finally, we asked whether these behavioural and modulatory differences are related to evolutionary variation in the anatomical architecture of the 5-HT system and/or its responsiveness to noxious stimulation. Immunohistochemical staining of 5-HT fibres in the pleural and pedal ganglia and 5-HT release evoked by tail-nerve shock were essentially conserved across all three species, except for a few minor differences: (1) Dolabrifera showed significantly less release than Aplysia and (2) Phyllaplysia showed significantly more generalized 5-HT signals than Dolabrifera. These minor variations cannot fully account for the differences in SN heterosynaptic plasticity and sensitization between Aplysia, Phyllaplysia and Dolabrifera. For example, Phyllaplysia showed greater 5-HT release than Aplysia on the side contralateral to the stimulation, whereas SN excitability was much reduced, and general sensitization was absent. Also, Dolabrifera did not show any SN excitability changes or sensitization whereas 5-HT release was only reduced by half. Given that the interspecific differences in modulatory pathways are small, what neuronal mechanisms could account for the loss of SN neuromodulation and behavioural sensitization in Phyllaplysia and Dolabrifera?
In Aplysia, 5-HT released within the CNS after noxious stimuli is strongly correlated with sensitization of defensive reflexes and SN neuromodulation (Brunelli et al. 1976; Mackey et al. 1989; Mercer et al. 1991; Marinesco and Carew, 2002a; review by Byrne & Kandel, 1996). In particular, 5-HT is known to be necessary for the expression of sensitization (Glanzman et al. 1989). Interestingly, our data indicate that the magnitude of sensitization is not always correlated with the amount of 5-HT released in the vicinity of tail SNs. For example, sensitization was similar on the ipsilateral and contralateral sides of the animal (at least soon after the shock), whereas 5-HT release in the ring ganglia was clearly lateralized to the ipsilateral side. It is possible that the relationship between 5-HT release and sensitization is non-linear. Indeed, previous experiments, suggest that the relationship between facilitation of sensory to motor neurons synapses and 5-HT release is s-shaped, in distinction to the linear increase in SN excitability seen when increasing 5-HT release (Marinesco & Carew, 2002a). Other possibilities include the role of other neuromodulators (e.g. small cardiac peptide, SCP, Abrams et al. 1984) or offsetting plasticity in interneurons (Cleary et al. 1995).
Combining the present study's findings - that SNs in Dolabrifera lack a modulatory response to noxious stimuli in spite of an essentially conserved serotonergic modulatory pathway - with previous research showing that the neuromodulatory response of SNs to 5-HT is absent in Dolabrifera, suggests that a learning-related loss of function occurred in the SN response to 5-HT released by noxious stimuli. Perhaps one or several mutations in the 5-HT receptors involved in SN heterosynaptic plasticity and/or in the second messenger pathways transducing the 5-HT signal, may have occurred, resulting in a loss of SN neuromodulation, and possibly of behavioural sensitization.
By contrast, the absence of generalized sensitization and reduction in local sensitization observed in Phyllaplysia is much more puzzling. Unlike in Dolabrifera, Phyllaplysia tail SNs have retained the ancestral neuromodulatory responses to exogenously applied 5-HT (i.e. increased excitability and spike broadening; Erixon et al. 1999). Because 5-HT release in the vicinity of tail SN cell bodies and synapses in Phyllaplysia is not significantly different from that of Aplysia, one would expect similar SN heterosynaptic plasticity following tail-nerve shock in both species. Instead, we observed reduced heterosynaptic plasticity in Phyllaplysia SNs. Two possible explanations can be proposed: first, there may be evolutionary changes in 5-HT receptors expressed by tail SNs and/or in the second messenger pathways activated by 5-HT. Interestingly, increased SN excitability lasts no more than 1 min in Phyllaplysia, which is close to the duration of 5-HT release evoked by tail-nerve shock. By contrast, SN excitability increase lasts for 2–3 min after shock in Aplysia and is thought to be mediated by protein kinases A (PKA) and C (Klein et al. 1982; Baxter & Byrne, 1989; Walsh & Byrne, 1989; Critz & Byrne, 1992) and terminated by different phosphatases. Thus, Phyllaplysia SNs could possess a different balance between kinases and phosphatases, thereby leading to a shorter duration of the 5-HT effect. This hypothesis could be examined using pharmacological treatments aimed at the 5-HT/cAMP/PKA pathway. Second, tail SNs in Phyllaplysia may have become more sensitive to a separate inhibitory pathway that can block 5-HT effects. Among possible inhibitory transmitters present in the Aplysia CNS, Phe-Met-Arg-Phe-amide (FMRFamide) has been shown to inhibit SN-MN transmission (Mackey et al. 1987; Dale & Kandel, 1990; Critz et al. 1991; Pieroni & Byrne, 1992; Sun et al. 1996), increase the SN firing threshold (Billy & Walters, 1989) and modulate specific postsynaptic MNs involved in defensive reflexes (Belkin & Abrams, 1993). In addition, dopamine is also known to increase the firing threshold of tail SNs (Billy & Walters, 1989) and inhibit SN-MN synapses (Abrams et al. 1984). Finally, acetylcholine might also inhibit SN-MN transmission because the cholinergic neuron L16 has been shown to evoke slow inhibitory postsynaptic potentials (IPSPs) inhibiting siphon SNs (Storozhuk & Castellucci, 1999), and exogenous acetylcholine increases the firing threshold of tail SNs (Billy & Walters, 1989).
Collectively, our data suggest that evolutionary variations in learning are correlated with changes in the response of defensive reflex circuits to modulatory signals triggered by noxious stimulation, but do not appear to involve any changes in the anatomy or the function of the modulatory pathways themselves. However, although increased SN excitability is commonly considered as an important neurobiological correlate of sensitization in Aplysia (Klein et al. 1982; Walters et al. 1983b; Walters, 1987b; Baxter & Byrne, 1989; Walsh & Byrne, 1989; Mercer et al. 1991; Critz & Byrne, 1992), its time course is much shorter than the actual duration of sensitization. This feature of SN heterosynaptic plasticity lasts 2–3 min in Aplysia (Liao et al. 1999) and 1 min in Phyllaplysia, far shorter than the enhancement in the reflex measured 20–30 min after noxious stimulation. However, increase in SN excitability is not the only aspect of SN heterosynaptic plasticity following noxious stimulation. Other cellular changes in SNs includes presynaptic facilitation of transmitter release by SNs onto their followers, increased input resistance and spike broadening (Castellucci & Kandel, 1976; Klein et al. 1982; Walters et al. 1983b). In Aplysia, short-term facilitation of SN-MN synapses lasts about 20 min, and matches better the time course of behavioural sensitization (Brunelli et al. 1976; Castellucci & Kandel, 1976). The amplitude and duration of cellular changes besides SN excitability in Phyllaplysia and Dolabrifera are currently unknown. In particular, synaptic facilitation is especially difficult to study in Phyllaplysia and Dolabrifera due to their small size. Our data suggest that examining these aspects of SN heterosynaptic plasticity might help clarify the exact neurobiological mechanisms that have been modified across phylogeny.
It is also important to remember that the tail-mantle withdrawal reflex involves a polysynaptic circuit (Frost et al. 1988; Trudeau & Castellucci, 1993; Cleary et al. 1995; Frost & Kandel, 1995). Interneurons in the pleural ganglia (possibly Pl17, see Cleary & Byrne, 1993; Cleary et al. 1995) receive monosynaptic connections from tail SNs and transmit this information to the interneurons and MNs that withdraw the mantle. Synaptic plasticity at such interneuronal sites, either SN-interneuron or interneuron- MN synapses, is much more poorly understood. However, it is clear, in Aplysia, that noxious stimuli and 5-HT produce changes at these synapses that can contribute to the modulation of defensive reflexes (Frost et al. 1988; Cleary et al. 1995; Storozhuk & Castellucci, 1999; Bristol et al. 2001). The cells homologous to the interneurons used in these studies have yet to be identified, to address the evolutionary dimensions of this important question.
To conclude, the serotonergic ‘instructor’ locus involved in heterosynaptic modulation of specific SNs during sensitization appears to be essentially conserved across species that nevertheless show substantial variation in their learning phenotypes. Although activation of this instructor locus by noxious stimulation releases 5-HT in the CNS of all three aplysiid species studied here, the effects of this 5-HT release differ tremendously: the facilitatory effect of 5-HT on SN-MN transmission, well-known in Aplysia, seems virtually non-existent in Dolabrifera, probably because its SNs lack any modulatory response to 5-HT, and greatly reduced in Phyllaplysia, possibly through modifications in the transduction of the 5-HT signal, and/or the opposing action of an unknown inhibitory pathway.
Acknowledgments
This work was supported by the National Science Foundation (IBN-9632069). We would like to thank Laura de Martini and Barbara Maynard for assistance with the behavioural experiments. We are grateful to Adam S. Bristol for comments on an earlier version of this manuscript and to Thomas J. Carew and David L. Glanzman for helpful scientific discussions.
S. Marinesco and K. L. Duran contributed equally to this work.
REFERENCES
- Abrams TW, Castelluci VF, Camardo JS, Kandel ER, Lloyd PE. Two endogenous neuropeptides modulate the gill and siphon withdrawal reflex in Aplysia by presynaptic facilitation involving cAMP-dependent closure of a serotonin-sensitive potassium channel. Proc Natl Acad Sci U S A. 1984;81:7956–7960. doi: 10.1073/pnas.81.24.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter D, Byrne J. Serotonergic modulation of two potassium currents in the pleural sensory neurons of Aplysia. J Neurophysiol. 1989;62:665–679. doi: 10.1152/jn.1989.62.3.665. [DOI] [PubMed] [Google Scholar]
- Belkin K, Abrams T. FMRFamide produces biphasic modulation of the LFS motor neurons in the neural circuit of the siphon withdrawal reflex of Aplysia by activating Na+ and K+ currents. J Neurosci. 1993;13:5139–5152. doi: 10.1523/JNEUROSCI.13-12-05139.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billy A, Walters E. Modulation of mechanosensory threshold in Aplysia by serotonin, small cardioactive peptide B (SCPB), FMRFamide, acetylcholine and dopamine. Neurosci Lett. 1989;105:200–204. doi: 10.1016/0304-3940(89)90037-2. [DOI] [PubMed] [Google Scholar]
- Bristol A, Fischer T, Carew T. Combined effects of intrinsic facilitation and modulatory inhibition of identified interneurons in the siphon withdrawal circuitry of Aplysia. J Neurosci. 2001;21:8990–9000. doi: 10.1523/JNEUROSCI.21-22-08990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: possible role for serotonin and cyclic AMP. Science. 1976;194:1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellucci V, Kandel E. Presynaptic facilitation as a mechanism for behavioral sensitization in Aplysia. Science. 1976;194:1176–1178. doi: 10.1126/science.11560. [DOI] [PubMed] [Google Scholar]
- Cleary L, Byrne J. Identification and characterization of a multifunction interneuron contributing to defensive arousal in Aplysia. J Neurophysiol. 1993;70:1767–1776. doi: 10.1152/jn.1993.70.5.1767. [DOI] [PubMed] [Google Scholar]
- Cleary L, Byrne J, Frost W. Role of interneurons in defensive withdrawal reflexes in Aplysia. Learn Mem. 1995;2:133–151. doi: 10.1101/lm.2.3-4.133. [DOI] [PubMed] [Google Scholar]
- Critz S, Baxter D, Byrne J. Modulatory effects of serotonin, FMRFamide, and myomodulin on the duration of action potentials, excitability, and membrane currents in tail sensory neurons of Aplysia. J Neurophysiol. 1991;66:1912–1926. doi: 10.1152/jn.1991.66.6.1912. [DOI] [PubMed] [Google Scholar]
- Critz S, Byrne J. Modulation of IK,Ca by phorbol ester-mediated activation of PKC in pleural sensory neurons of Aplysia. J Neurophysiol. 1992;68:1079–1086. doi: 10.1152/jn.1992.68.4.1079. [DOI] [PubMed] [Google Scholar]
- Dale N, Kandel E. Facilitatory and inhibitory transmitters modulate spontaneous transmitter release at cultured Aplysia sensorimotor synapses. J Physiol. 1990;421:203–222. doi: 10.1113/jphysiol.1990.sp017941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erixon N, Demartini L, Wright W. Dissociation between sensitization and learning-related neuromodulation in an Aplysiid species. J Comp Neurol. 1999;408:506–514. doi: 10.1002/(sici)1096-9861(19990614)408:4<506::aid-cne5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Frost W, Clark G, Kandel E. Parallel processing of short-term memory for sensitization in Aplysia. J Neurobiol. 1988;19:297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- Frost W, Kandel E. Structure of the network mediating siphon-elicited siphon withdrawal in Aplysia. J Neurophysiol. 1995;73:2413–2427. doi: 10.1152/jn.1995.73.6.2413. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Lloyd PE, Kandel ER. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci. 1989;9:4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Klein M, Camardo J, Kandel E. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982;79:5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J, Byrne JH, Eskin A. Levels of serotonin in the hemolymph of Aplysia are modulated by light/dark cycles and sensitization training. J Neurosci. 1999;19:8094–8103. doi: 10.1523/JNEUROSCI.19-18-08094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Brou C, Walters E. Limited contributions of serotonin to long-term hyperexcitability of Aplysia sensory neurons. J Neurophysiol. 1999;82:3223–3235. doi: 10.1152/jn.1999.82.6.3223. [DOI] [PubMed] [Google Scholar]
- Mackey S, Glanzman D, Small S, Dyke A, Kandel E, Hawkins R. Tail-shock produces inhibition as well as sensitization of the siphon-withdrawal reflex of Aplysia: possible behavioral role for presynaptic inhibition mediated by the peptide Phe-Met-Arg-Phe-NH2. Proc Natl Acad Sci U S A. 1987;84:8730–8734. doi: 10.1073/pnas.84.23.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey SL, Kandel ER, Hawkins RD. Identified serotonergic neurons LCB1 and RCB1 in the cerebral ganglia of Aplysia produce presynaptic facilitation of siphon sensory neurons. J Neurosci. 1989;9:4227–4236. doi: 10.1523/JNEUROSCI.09-12-04227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco S, Carew TJ. Serotonin release evoked by tail-nerve stimulation in Aplysia: characterization and relationship to heterosynaptic plasticity. J Neurosci. 2002a;22:2299–2312. doi: 10.1523/JNEUROSCI.22-06-02299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinesco S, Carew T. Improved electrochemical detection of biogenic amines in Aplysia using base-hydrolyzed cellulose-coated carbon fiber microelectrodes. J Neurosci Methods. 2002b;117:87–97. doi: 10.1016/s0165-0270(02)00093-6. [DOI] [PubMed] [Google Scholar]
- Marois R, Carew T. Ontogeny of serotonergic neurons in Aplysia californica. J Comp Neurol. 1997;386:477–490. [PubMed] [Google Scholar]
- Medina M, Walsh PJ. Molecular systematics of the order Anaspidea based on mitochondrial DNA sequence (12S, 16S and COI) Molec Phylogenet Evol. 2000;15:41–58. doi: 10.1006/mpev.1999.0736. [DOI] [PubMed] [Google Scholar]
- Mercer AR, Emptage NJ, Carew TJ. Pharmacological dissociation of modulatory effects of serotonin in Aplysia sensory neurons. Science. 1991;254:1811–1813. doi: 10.1126/science.1662413. [DOI] [PubMed] [Google Scholar]
- Nolen TG, Carew TJ. Ontogeny of serotonin-immunoreactive neurons in juvenile Aplysia californica: implications for the development of learning. Behav Neural Biol. 1994;61:282–295. doi: 10.1016/s0163-1047(05)80011-1. [DOI] [PubMed] [Google Scholar]
- Pieroni J, Byrne J. Differential effects of serotonin, FMRFamide and small cardioactive peptide on multiple, distributed processes modulating sensorimotor synaptic transmission in Aplysia. J Neurosci. 1992;12:2633–2647. doi: 10.1523/JNEUROSCI.12-07-02633.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin C, Carew T. Dishabituation and sensitization emerge as separate processes during development in Aplysia. J Neurosci. 1988;8:197–211. doi: 10.1523/JNEUROSCI.08-01-00197.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York: WH Freeman and Co.; 1981. [Google Scholar]
- Storozhuk M, Castellucci V. Modulation of cholinergic transmission in the neuronal network of the gill and siphon withdrawal reflex in Aplysia. Neuroscience. 1999;90:291–301. doi: 10.1016/s0306-4522(98)00458-8. [DOI] [PubMed] [Google Scholar]
- Sun Z, Kauderer B, Schacher S. Differential distribution of functional receptors for neuromodulators evoking short-term heterosynaptic plasticity in Aplysia californica sensory neurons. J Neurosci. 1996;16:7540–7549. doi: 10.1523/JNEUROSCI.16-23-07540.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton M, Carew T. Behavioral, cellular and molecular analysis of memory in Aplysia I: Intermediate-term memory. Integ Comp Bio. 2002;42:728–735. doi: 10.1093/icb/42.4.725. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Castellucci V. Sensitization of the gill and siphon withdrawal reflex of Aplysia: multiple sites of change in the neuronal network. J Neurophysiol. 1993;70:1210–1220. doi: 10.1152/jn.1993.70.3.1210. [DOI] [PubMed] [Google Scholar]
- Walsh J, Byrne J. Modulation of a steady-state Ca2+ -activated, K+ current in tail sensory neurons of Aplysia: role of serotonin and cAMP. J Neurophysiol. 1989;61:32–44. doi: 10.1152/jn.1989.61.1.32. [DOI] [PubMed] [Google Scholar]
- Walters ET. Site-specific sensitization of defensive reflexes in Aplysia: a simple model of long-term hyperalgesia. J Neurosci. 1987a;7:400–407. doi: 10.1523/JNEUROSCI.07-02-00400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET. Multiple sensory neuronal correlates of site-specific sensitization in Aplysia. J Neurosci. 1987b;7:408–417. doi: 10.1523/JNEUROSCI.07-02-00408.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Byrne JH. Long-term enhancement produced by activity-dependent modulation of Aplysia sensory neurons. J Neurosci. 1985;5:662–672. doi: 10.1523/JNEUROSCI.05-03-00662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters ET, Byrne JH, Carew TJ, Kandel ER. Mechanoafferent neurons innervating tail of Aplysia. II Modulation by sensitizing stimulation. J Neurophysiol. 1983b;50:1543–1559. doi: 10.1152/jn.1983.50.6.1543. [DOI] [PubMed] [Google Scholar]
- Wright W. Evolution of nonassociative learning: behavioral analysis of a phylogenetic lesion. Neurobiol Learn Mem. 1998;69:326–337. doi: 10.1006/nlme.1998.3829. [DOI] [PubMed] [Google Scholar]
- Wright W, Jones K, Sharp P, Maynard B. Widespread anatomical projections of the serotonergic modulatory neuron, CB1, in Aplysia. Invert Neurosci. 1995;1:173–183. doi: 10.1007/BF02331914. [DOI] [PubMed] [Google Scholar]
- Wright W, Kirschman D, Rozen D, Maynard B. Phylogenetic analysis of learning-related neuromodulation in molluscan mechanosensory neurons. Evolution. 1996;50:2248–2263. doi: 10.1111/j.1558-5646.1996.tb03614.x. [DOI] [PubMed] [Google Scholar]
- Zhang ZS, Fang B, Marshak DW, Byrne JH, Cleary LJ. Serotonergic varicosities make synaptic contacts with pleural sensory neurons of Aplysia. J Comp Neurol. 1991;311:259–270. doi: 10.1002/cne.903110207. [DOI] [PubMed] [Google Scholar]


