Abstract
Delta9-tetrahydrocannabinol (Δ9-THC) is an effective anti-emetic; however, other potential gastrointestinal therapeutic effects of Δ9-THC are less well-known. Here, we report a role of Δ9-THC in a vago-vagal reflex that can result in gastro-oesophageal reflux, that is, gastric distension-evoked lower oesophageal sphincter (LOS) relaxation. Oesophageal, LOS and gastric pressures were measured using a miniaturized, manometric assembly in decerebrate, unanaesthetized ferrets. Gastric distension (30 ml) evoked LOS relaxation (70 ± 8 % decrease from baseline). Δ9-THC administered systemically (0.2 mg kg−1, I.V.) or directly to the dorsal hindbrain surface (0.002 mg), significantly attenuated the nadir of the gastric distention-evoked LOS relaxation, and time to reach maximal response. Similar increases to maximal effect were observed after treatment with the cannabinoid receptor agonist WIN 55,212–2 (0.2 mg kg−1, I.V.). The effect of systemic Δ9-THC on gastric distention-evoked LOS relaxation was reversed by a selective cannabinoid1 (CB1) receptor antagonist, SR141617A (1 mg kg−1, I.V.). Since this reflex is vagally mediated, we used a CB1 receptor antiserum and immunocytochemistry to determine its distribution in ferret vagal circuitry. CB1 receptor staining was present in cell bodies within the area postrema, nucleus tractus solitarius (NTS) and nodose ganglion. Intense terminal-like staining was noted within the NTS and dorsal motor vagal nucleus (DMN). Neither nodose ganglionectomy nor vagotomy altered the CB1 receptor terminal-like staining in the dorsal vagal complex. Retrogradely labelled gastric- or LOS-projecting DMN neurones did not express CB1 receptors within their soma. Therefore, CB1 receptor staining in the NTS and DMN is not due to primary vagal afferents or preganglionic neurones. These novel findings suggest that Δ9-THC can modulate reflex LOS function and that the most likely site of action is via the CB1 receptor within the NTS. This effect of Δ9-THC may have implications in treatment of gastro-oesophageal reflux and other upper gut disorders.
Delta9-tetrahydrocannabinol (Δ9-THC), the active constituent of marijuana, and endogenous cannabinoid receptor ligands (anandamide and 2-arachidonylglycerol) mediate their effects through specific receptors designated as the cannabinoid 1 (CB1) and cannabinoid 2 (CB2) receptors. The CB1 receptor and its mRNA are seen throughout the central nervous system (Matsuda et al. 1993; Tsou et al. 1998), whereas the CB2 receptor is found primarily in immune tissues (Berdyshev, 2000).
The distribution of CB1 receptor in the central and enteric nervous systems accounts for the profound effects of Δ9-THC on gastrointestinal function. Indeed, cannabinoids have been effectively used as anti-emetics in humans undergoing chemotherapy (Tramer et al. 2001). In the stomach, CB1 receptor agonists reduce stress-induced gastric ulceration (Germano et al. 2001), slow gastric emptying (Izzo et al. 1999), decrease pentagastrin-induced gastric acid secretion (Coruzzi et al. 1999) and inhibit gastric contractility (Krowicki et al. 1999). Although CB1 receptor activation can inhibit intestinal contractile activity directly by reducing excitatory neurotransmission to the smooth muscle (Pertwee, 2001), the inhibition of gastric motility by Δ9-THC is due primarily to activation of CB1 receptor in the vagal circuitry of the dorsal hindbrain. Two lines of evidence support this notion. Firstly, a dose of Δ9-THC that is lower than the effective systemic dose, applied directly to the dorsal surface of the medulla, reduces gastric motility and, secondly, vagotomy completely abolishes the gastric motor effects of systemically administered Δ9-THC (Krowicki et al. 1999). In addition, recent work suggests that the anti-emetic effects of Δ9-THC and the cannabinoid receptor agonist WIN 55,212–2 in ferrets may be due to a site of action in the hindbrain (Van Sickle et al. 2001).
The dorsal vagal complex in the hindbrain medulla modulates vago-vagal reflexes. Primary afferents from the gut enter the nucleus tractus solitarius (NTS) and one or more interneurones relay information to preganglionic vagal motor neurones in the dorsal motor nucleus of the vagus (DMN). One example of a vago-vagal reflex is gastric distension-evoked LOS relaxation (Franzi et al. 1990; Blackshaw et al. 1998). This reflex is clinically relevant because improper relaxation of the LOS can compromise the gastro-oesophageal barrier and allow reflux of gastric contents (Holloway et al. 1985; Holloway & Dent, 1990). Pharmacological manipulation of this reflex is a possible therapeutic avenue for the prevention of gastro-oseophageal reflux disease. Therefore, based on the central vagal effects of CB1 receptor agonists on emesis and gastric motor function, we investigated whether Δ9-THC could modulate gastric distension-evoked LOS relaxation in ferrets. In addition, we wanted to elucidate the distribution of the CB1 receptor in the ferret hindbrain and nodose ganglion using immunocytochemistry and a well-characterized CB1 receptor antibody (Tsou et al. 1998).
METHODS
General
Experiments were performed on male ferrets (Mustelae putorius furo, 0.5–1.5 kg, Marshall Farms, NY, USA), which were fed a standard carnivore diet with free access to water, but were deprived of food for at least 12 h prior to surgery. All studies were conducted within the guidelines of the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee. Animals used in functional studies were killed at the end of the studies by intravenous injection of 10 ml of saturated KCl.
Functional studies
Animals were initially anaesthetized with isoflurane (4 % induction with 2 % maintenance) using a facial mask. Adequate anaesthesia was confirmed by the loss of the hindlimb-pinch withdrawal reflex.The jugular vein and carotid artery were cannulated for intravenous drug and fluid administration and for monitoring of arterial blood pressure, respectively. Anaesthetic was administered during surgery through a tracheal cannula: this was also used to monitor respiration rate via a thermoprobe, which measured changes in temperature caused by inhalation and exhalation during the respiratory cycle, throughout the study. The cervical vagal trunks were exposed and isolated with a silk suture snare for later nerve transection during the experiment. A midline incision was made in the abdomen and the stomach exteriorized and divided at the level of the incisura angularis with a ligature. A cannula was inserted immediately proximal to the ligature and directed orally for drainage of the proximal stomach. The abdominal incision was then sutured closed. Animals were then placed in a stereotaxic frame with ferret adaptor ear bars (David Kopf Instruments, Tujunga, CA, USA). A midcollicular incision was made and the cerebral hemispheres and underlying brainstem removed rostral to the superior colliculus. The dorsal surface of the hindbrain and obex was exposed through a partial occipital craniotomy for the topical administration of drugs at the level of obex. Anaesthesia was discontinued when the animals were stable, since the decerebration procedure had rendered the animal insentient. When necessary, respiration was aided by a small animal respirator.
A miniaturized manometric assembly (Dent sleeve, Wayville, South Australia) was inserted perorally and positioned such that the sleeve lay across the LOS. Oesophageal, LOS and gastric pressures together with blood pressure and respiration rate were recorded via a Macintosh PowerMac computer using data acquisition software (PowerLab, AD Instruments, Melbourne, Australia). Distension of the proximal stomach (25–40 ml isotonic saline depending on the body weight of the animal) was performed via the central channel of the manometric assembly, which was positioned within the proximal stomach just distal of the LOS. The bolus of saline was administered over 1–2 min and retained within the stomach for 5 min, before being drained via the gastric cannula.
Three separate sets of studies were conducted. First, gastric distension was performed (in consecutive order): under control conditions, after vehicle (1 ml kg−1, I.V.) and after either Δ9-THC (0.02, 0.2, and 2.0 mg kg−1, I.V.) or WIN 55,212–2 (0.2 mg kg−1, I.V.). Secondly, distension was performed (in consecutive order): under control conditions, after vehicle (1 ml kg−1, I.V.), after SR141716A (1 mg kg−1, I.V.), and after THC (0.2 mg kg−1, I.V.). A third series of studies was conducted with gastric distention performed (in consecutive order): under control conditions, after vehicle (2 μl kg−1 applied topically on the dorsal surface of the hindbrain at the level of the obex) and after Δ9-THC (0.002 mg kg−1 applied topically on the dorsal surface of the hindbrain at the level of the obex). Gastric distension was repeated at least twice under each treatment condition with at least 15 min allowed between the drainage of the previous distention and the start of the next distention. Gastric distention was performed at least 5 min after intravenous administration of Δ9-THC, WIN 55,212–2 or SR141716A, and 15 min after topical administration of Δ9-THC.
Immunocytochemical studies
Ferrets were anaesthetized with either halothane or isoflurane (4 % induction with 2 % maintenance) and then intubated before the following surgical procedures were performed. Adequate anaesthesia was confirmed by the loss of the hindlimb-pinch withdrawal reflex. Animals received buprenorphine (0.01–0.05 mg kg−1, I.P.) twice daily for 2 days. They were monitored closely for 2 h after recovery from anaesthetic and at least twice daily after that by trained veterinarians for signs of post-operative distress. Additionally, tetracycline (1g (100 ml)−1) was added to drinking water for a period of 4 days. They were also observed to recover their appetites and gain weight normally in the post-operative period.
Nodose ganglionectomy (n = 4)
The left vagal nerve trunk was isolated from the adjacent carotid artery and traced rostrally until the nodose ganglion enlargement was identified immediately prior to the nerve entering into the jugular foramen. The nerve was transected on either side of the nodose ganglion and removed. The right nodose ganglion remained intact as a control. Animals recovered for at least 7 days, then were deeply anaesthetized with sodium pentobarbital (60 mg kg−1, I.P.) prior to intracardiac perfusion with saline and fixative (4 % paraformaldehyde in phosphate buffered saline (PBS, pH 7.4).
Vagotomy (n = 2)
A 5 mm section of the left vagus nerve was removed at the mid-cervical level. The right vagus nerve remained intact as a control. These animals recovered for at least 7 days, then they were deeply anaesthetized and perfused as described above.
Retrograde labelling (n = 4)
A paracostal approach was used to expose the LOS and proximal stomach region. Cholera toxin B subunit conjugated to tetramethyl rhodamine isothiocyanate (CTB-TRITC) (50 μl of 0.1 % solution), was injected in 5–10 μl aliquots via a Hamilton syringe into the muscular wall of the LOS (n = 2) or proximal stomach (n = 2). Animals were allowed to recover for 4 days and then they were deeply anaesthetized and perfused as described above.
The brains and nodose ganglia were removed and post-fixed in 4 % paraformaldehyde and 20 % sucrose for 24 h. Nodose ganglia were embedded in gelatin and post-fixed in the same solution and sectioned longitudinally at 40 μm thickness using a cryostat. The brainstem was cut into 40 μm thick coronal sections using a cryostat. All sections were collected in PBS and were then incubated for 48 h with rat CB1 receptor antibody diluted to 1 part in 1000–8000 parts PBS. The CB1 receptor antibody and its immunizing protein were a gift of Dr Kenneth Mackie (Department of Anaesthesiology, University of Washington, Seattle, WA, USA). The antiserum has been thoroughly characterized previously for use in immunocytochemistry (Tsou et al. 1998). Brain sections were then stained with a tyramide amplification system (TSA direct kit, NEN, Boston, MA, USA) with fluorescein-labelled tyramine. Nodose ganglion sections were stained with a routine avidin-biotin complex (ABC Elite, Vector Laboratory, Burlingame, CA, USA) immunocytochemistry, with 0.1 % 3,3 diaminobenzidine (Sigma, St Louis, MO, USA) and 1 % hydrogen peroxide. Control experiments were performed by preincubating the antibody against the rat CB1 receptor with its immunizing protein at 4 μg ml−1 for 1 h before applying CB1 receptor antibody to nodose ganglion (n = 2) and hindbrain (n = 2) sections of untreated animals.
Photomicrograph images were taken using a digital camera (Magnafire, Optronics) attached to a Nikon Optiphot 2 microscope and a computer (Dell Pentium 3). For the fluorescence images, excitation wavelengths of 540–580 nm and 490–525 nm were used for the rhodamine and fluorescein probes, respectively. Both colour and black and white images were imported directly into Adobe Photoshop, where they were unmodified except for minor adjustments for brightness and contrast. For colocalization studies, colour images of CTB-TRITC and CB1 receptor tagged with fluorescein were combined in Photoshop using the ‘screen’ command.
Data analysis
LOS and gastric pressures were measured for 1 min before each gastric distension and during the 5 min gastric distension period. LOS pressure was always analysed relative to gastric pressure, i.e. the LOS pressure used in calculations was raw LOS pressure minus raw gastric pressure. The depth of LOS relaxation caused by gastric distension was measured as a percentage of the nadir LOS pressure compared with LOS pressure measured immediately prior to each distension period. Arterial blood pressure was measured for 1 min at least 5 min after administration of each drug. Statistical analysis was performed by a one-way ANOVA followed by Dunnett's post hoc test. P < 0.05 was considered to be statistically significant.
Drugs
Δ9-THC was obtained from the National Institute on Drug Abuse. SR1417l6A [N-(piperidin-lyl)-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxy hydrochloride] was synthesized at Pfizer Central Research (Groton, CT, USA) as a free base. WIN 55,212–2 was purchased from Sigma. The intravenous doses of Δ9-THC and SR141716A were based on an earlier study investigating the effects of Δ9-THC on gastric motility in rats (Krowicki et al. 1999). Δ9-THC, WIN 55,212–2 and SR141716A were dissolved in 1:1:18 by volume emulphor:ethanol:saline and administered at a standard minimum volume of 0.5 ml kg−1. Emulphor (Alkamuls EL-620L), a polyoxyethylated vegetable oil, was obtained from Rhone-Poulenc (Princeton, NJ, USA).
RESULTS
Functional studies
Under control conditions, distension of the proximal stomach decreased LOS pressure, as illustrated by the sample experimental recordings (upper panels, Fig. 1A-D). The decrease in LOS pressure, or LOS relaxation, generally commenced during the gastric infusion period and was maintained throughout the duration of the distension. When the stomach was drained at the end of the distension period, LOS pressure increased slowly until it returned to basal pre-distension levels. This data was compiled in two ways; firstly, we quantified the change in LOS pressure evoked by gastric distention as a percentage change from the basal level, i.e. depth of LOS relaxation (−71 ± 4 % under control conditions, Fig. 2A). Secondly, we measured the time taken for LOS pressure to reach peak levels during the gastric distention period (40 ± 12 s under control conditions, Fig. 2B). Mean arterial blood pressure was also calculated after each treatment prior to gastric distention. Vehicle administration did not significantly alter the depth or the time to peak of the gastric distention evoked LOS relaxation or mean arterial pressure (data not shown).
Figure 1. Examples of chart recordings of the effects of Δ9-THC, WIN 55,212–2 and SR141716A on gastric distension-evoked LOS relaxation.
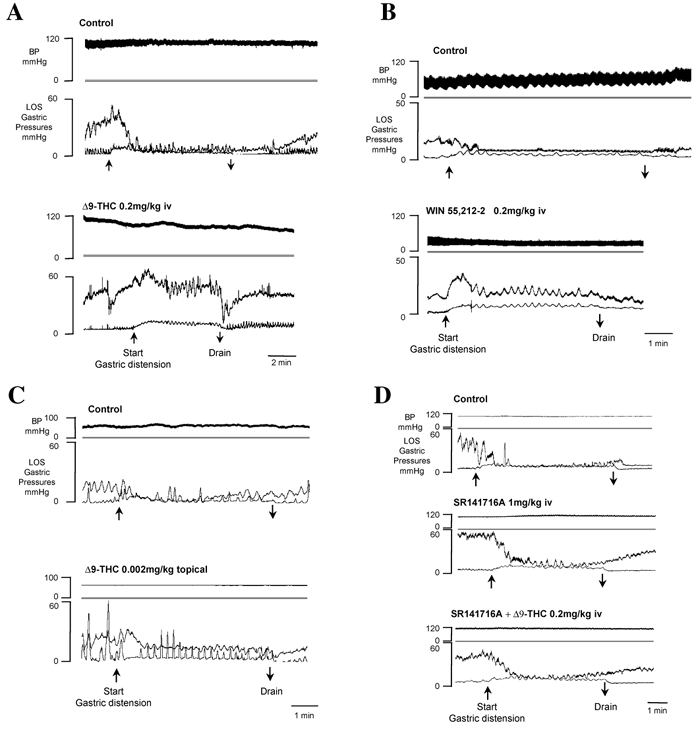
Under control conditions in A-D (top panels), gastric distension (by 30 ml saline, upward arrow) dramatically reduced LOS tone. The LOS relaxation was maintained throughout the duration of the distension until the stomach was drained (downward arrow), after which LOS pressure slowly returned to pre-distension levels. A, systemic Δ9-THC (0.2 mg kg−1 I.V.; bottom panel) inhibited the LOS relaxation evoked by gatsric distention compared to that evoked under control conditions (top panel). B, the cannabinoid agonist WIN 55,212–2 (0.2 mg kg−1 I.V., bottom panel) attenuated the LOS relaxation response to gastric distention. C, central Δ9-THC (0.002 mg kg−1 on dorsal surface of hindbrain) elicited an inhibition of the LOS relaxation response to gastric distension. This animal had an active gastric motility as is evident by the contractile activity seen in the tracings. D, CB1 receptor antagonist SR141716A, administered alone (1 mg kg−1 I.V., middle panel) or in combination with Δ9-THC (0.2 mg kg−1 I.V., bottom panel), did not affect the gastric distension-evoked LOS relaxation.
Figure 2. Group data showing the effects of the cannabinoid agonists Δ9-THC, WIN 55,212–2 and the CB1 receptor antagonist SR141716A on depth and time to nadir of gastric distension-evoked LOS relaxation and on arterial blood pressure.
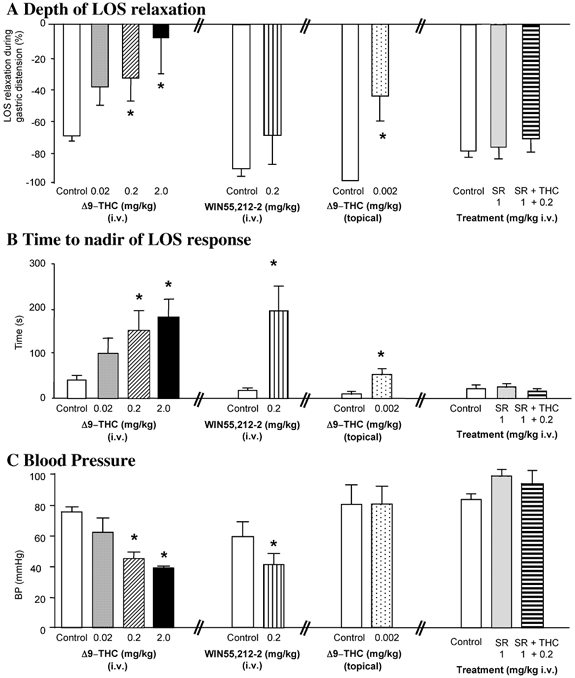
A, the depth (percentage change) of LOS relaxation evoked by gastric distension was attenuated by systemic Δ9-THC (0.02–2.0 mg kg−1 I.V., n > 6) in a dose-related manner, by systemic WIN 55,212–2 (0.2 mg kg−1 I.V., n = 5) and by central Δ9-THC (0.002 mg kg−1 topical on dorsal hindbrain surface, n = 4). The specific CB1 receptor antagonist SR141716A, administered alone (1 mg kg−1 I.V., n > 6) or in combination Δ9-THC (0.2 mg kg−1 I.V., n > 4), did not affect the depth of the gastric distension-evoked LOS relaxation. B, the time taken to reach nadir LOS pressure during gastric distension was increased by systemic Δ9-THC (0.02–2.0 mg kg−1 I.V.) in a dose-dependent manner, by WIN 55,212–2 (0.2 mg kg−1 I.V.) and by central Δ9-THC (0.002 mg kg−1 topical). SR141716A, administered alone (1 mg kg−1 I.V.) or together with Δ9-THC (0.2 mg kg−1 I.V.), did not affect the time to peak response of the LOS relaxation. C, blood pressure was decreased by systemic Δ9-THC (0.02–2.0 mg kg−1 I.V.) in a dose-dependent manner and by WIN 55,212–2 (0.2 mg kg−1 I.V.). Neither Δ9-THC (0.002 mg kg−1 topical) nor SR1417l6A, administered alone (1 mg kg−1 I.V.) or together with Δ9-THC (0.2 mg kg−1 I.V.), altered arterial blood pressure. Data are represented as means ±s.e.m. *P > 0.05 compared to effect under control conditions.
The cannabinoid agonist Δ9-THC (0.02–2.0 mg kg−1, I.V.; n > 6) dose-dependently decreased the depth of LOS relaxation evoked by the gastric distension (example Fig. 1A, group data Fig. 2A) with a significant reduction occurring at a dose of 0.2 mg kg−1, I.V. (−35 ± 15 % at 0.2 mg kg−1, I.V. vs. −70 ± 4 % under control conditions, P < 0.05, n > 6). The time taken to reach the nadir of the LOS response to gastric distention was also dose-dependently increased (Fig. 2C), with a significant increase occurring at a dose of 0.2 mg kg−1, I.V. (148 ± 43 s at 0.2 mg kg−1, I.V. vs. 40 ± 12 s under control conditions, P < 0.05). Systemic Δ9-THC also dose-dependently decreased blood pressure (46 ± 5 mmHg at 0.2 mg kg−1, I.V. vs. 76 ± 4 mmHg under control conditions, P < 0.05).
The synthetic cannabinoid agonist WIN 55,212–2 (0.2 mg kg−1, I.V., n = 5) also altered the LOS response to gastric distention (example in Fig. 1B, group data Fig. 2A). While the depth of the LOS relaxation, i.e. percentage LOS relaxation, was reduced by WIN 55,212–2 (−70 ± 18 % vs. −91 ± 6 % under control conditions, Fig. 2A), it did not reach statistical significance compared to control. However, the time to reach peak response was significantly increased (145 ± 42 s vs.14 ± 4 s under control conditions, P < 0.05, Fig. 2B). In addition, the cannabinoid receptor agonist significantly decreased mean arterial pressure (41 ± 8 mmHg vs. 59 ± 10 mmHg under control conditions, P < 0.05; Fig. 2C).
The effect of centrally administered Δ9-THC was also investigated in this model. Δ9-THC was administered topically on the dorsal surface of the hindbrain at the level of the obex at a dose that was one hundreth the effective intravenous dose to determine its effects on the gastric distention-evoked LOS relaxation. Under control conditions, gastric distention evoked a complete relaxation of the LOS (example Fig. 1C). After Δ9-THC application on the hindbrain surface (0.002 mg in 2 μl), gastric distention-evoked LOS relaxation was markedly reduced (−44 ± 15 % Δ9-THC topical vs. −100 ± 2 % under control conditions, P < 0.05, Fig. 2A, n = 4). The time taken to reach peak response was also significantly increased (53 ± 12 s vs. 10 ± 5 s under control conditions, P < 0.05, Fig. 2B). There was no effect of central Δ9-THC on mean arterial blood pressure (80 ± 13 mmHg vs. 80 ± 12 mmHg under control conditions, Fig. 2C).
The selective CB1 receptor antagonist SR141716A (1 mg kg−1, I.V.) administered alone did not alter the gastric distension-evoked LOS relaxation (example Fig. 1D) using the two parameters that we measured, i.e. neither the depth of relaxation (−87 ± 3 % vs. 85 ± 5 % under control conditions, Fig. 2A, n > 4) nor the time taken to reach peak response (25 ± 8 s vs. 21 ± 9 s under control conditions, Fig. 2B) was significantly changed compared with control. SR141716A alone did not noticeably alter mean arterial pressure (101 ± 5 mmHg vs. 85 ± 4 mmHg under control conditions, Fig. 2C).
On the other hand, SR141716A (1 mg kg−1, I.V.) prevented the effects of Δ9-THC (0.2 mg kg−1, I.V., Fig. 1D) and WIN 55,212–2 (0.2 mg kg−1, I.V., data not shown) on the gastric distension-evoked LOS relaxation. This is manifested in the depth of LOS relaxation (−76 ± 8 % after SR14176A ±Δ9-THC vs. −85 ± 5 % under control conditions, n > 4, Fig. 2A), time to peak of the LOS response (16 ± 5 s after SR14176A +Δ9-THC vs. 21 ± 9 s under control conditions, Fig. 2B). There was also no change in mean arterial pressure when SR141716A was administered together with Δ9-THC or WIN 55,212–2 (96 ± 8 mmHg after SR141716A +Δ9-THC, 101 ± 5 mmHg after SR141716A alone and 85 ± 4 mmHg under control conditions; Fig. 2C, data on WIN 55,212–2 + SR141716A not shown).
Immunocytochemical studies
A low-power view of CB1 receptor immunostaining in the dorsal vagal complex is illustrated in Fig. 3A. CB1 receptor immunostained cell bodies are seen throughout the area postrema (arrowhead) and the NTS (arrows). There is intense diffuse ‘terminal-like’ staining throughout the dorsal vagal complex, with the greatest intensity within the DMN and in the border between the DMN and NTS. No staining is visualized in the hypoglossal nucleus.
Figure 3. Distribution of CB1 receptor in the ferret hindbrain.
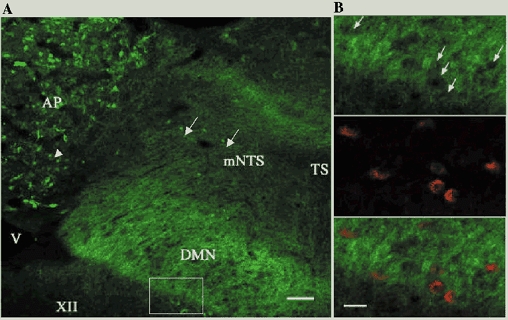
A, low power montage of CB1 receptor-immunoreactive staining in the dorsal vagal complex. Immunostained cell bodies are noted in the area postrema (arrowheads) and NTS (arrows) but not in the DMN, which has an intense diffuse terminal-like staining. B, higher magnification views of the dorsal motor nucleus of the vagus (equivalent to the boxed area indicated in A) in sections that were double stained with CB1 receptor antibody labelled with FITC (upper panel) and CTB-TRITC (middle panel) injected into the LOS and their combined image (bottom panel). Arrows indicate the ‘holes’ noted in the CB1 receptor staining, which match perfectly with the location of retrogradely labelled vagal motor neurones. This is confirmed in the combined image, in which the absence of yellow indicates a complete absence of colocalization of the markers (this would appear as yellow). Abbreviations: AP, area postrema; DMN, dorsal motor vagal nucleus; mNTS, medial NTS; TS, tractus solitarius; V, ventricle; XII, hypoglossal nucleus. Scale bar = l00 μm in A and 50 μm in B.
To determine whether CB1 receptor is expressed in the soma of vagal motor neurones we performed double labelling studies using CTB-TRITC injected into either the LOS (n = 2) or gastric fundus (n = 2). In Fig. 3B, the location of LOS-projecting labelled neurones in the DMN is illustrated (middle panel) and when this image is superimposed onto the CB1 receptor staining (bottom panel) there is no evidence that these markers are colocalised. In none of the four cases did we observe the presence of CB1 receptor immunostaining in vagal motor neurones. Consistent with this finding, unilateral vagotomy 7 days prior to perfusion did not result in any attenuation of the CB1 receptor staining in the dorsal vagal complex (data not shown). This procedure has been previously shown to allow vagal motor neurones to degenerate and results in reduction of immunocytochemical visualization of receptors expressed by these neurones (for example, GABAB receptors (McDermott et al. 2001).
In the nodose ganglion, most cells are moderately stained with the CB1 receptor (Fig. 4A, left side). The specificity of the CB1 receptor immunostaining in ferret nodose is confirmed by the absence of specific CB1 receptor immunostaining in nodose ganglion sections incubated in CB1 antiserum that had been preadsorbed with its immunizing protein construct (Fig. 4A, right side). We therefore determined the contribution of CB1 receptor expression in primary vagal afferents in the NTS by performing a unilateral nodose ganglionectomy, 7 days prior to perfusion (n = 4). This procedure results in degeneration of central projections of both vagal afferents and vagal efferents. To maximize our ability to detect a reduction in staining in the dorsal vagal complex on the operated side, we used a wide range of concentrations of CB1 receptor of the antiserum. At 1:4000, the terminal-like CB1 receptor staining is just perceptible and yet no difference in density of CB1 receptor staining is noted for control and nodosectomized sides of the dorsal vagal complex (Fig. 4B and C).
Figure 4.
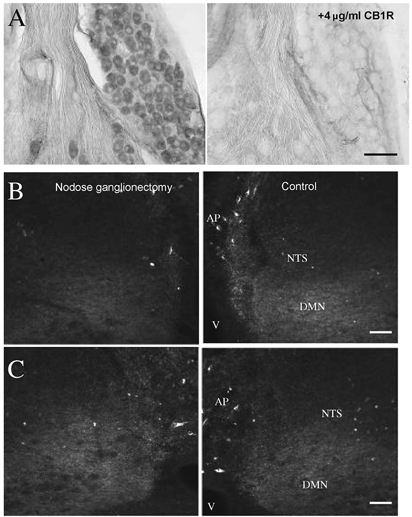
A, adjacent sections of nodose ganglion were immunocytochemically stained with rat CB1 receptor antiserum (left) and with antiserum that has been preadsorbed with the immunizing peptide construct (right). Scale bar = 50 μm. B and C, examples in two different animals of CB1 receptor staining in the dorsal vagal complex after nodose ganglionectomy (left) compared to control (right). Nodose ganglionectomy 1 week prior to perfusion did not result in noticeable differences in CB1 receptor staining compared to the corresponding control side even though sections were incubated with CB1 receptor antiserum at a concentration (1:4000) that resulted in just-detectable levels of fluorescence to attempt to detect differences in staining resulting from the treatment. Scale bars = 100 μm. Abbreviations: AP, area postrema; DMN, dorsal motor vagal nucleus; NTS, nucleus tractus solitarius; V, ventricle.
DISCUSSION
This study shows that CB1 receptor activation modulates a vago-vagal pathway that has been linked with acid reflux and the development of gastro-oesophageal reflux disease. This finding expands our knowledge of the existing therapeutic gastrointestinal effects of cannabinoids, such as for appetite stimulation and prevention of emesis/nausea. Our functional data show that systemic and central hindbrain treatment with Δ9-THC and systemic WIN 55,212–2 can attenuate gastric distension-evoked LOS relaxation. This effect is prevented by a selective CB1 receptor antagonist, SR141716A. In addition, immunocytochemical data show that CB1 receptor is present in vagal sensory afferent cells in the nodose ganglion and in punctate terminal field-like fibres in the dorsal vagal complex. However, neither vagal primary afferents nor preganglionic motor neurones are a major source of CB1 receptor in the dorsal vagal complex. Here we will discuss these data in terms of the choice of model used, the potential site of action of CB1 receptor on vagal circuitry associated with gastro-oesophageal reflux, and in comparison with other agents that have effects on these circuits.
We developed a decerebrate ferret model for these experiments in order to readily evoke gastric distension evoked-LOS relaxation in an immobilized animal without the reported dampening effects of anaesthesia on this reflex (Altschuler et al. 1985). Conscious ferrets have been used to investigate both the mechanisms involved in triggering LOS relaxations in response to gastric insufflation (Blackshaw et al. 1998) and agents that can alter this reflex (Blackshaw et al. 1999). Use of the decerebrate model allows more invasive measurements, with fewer recording artifacts, such as movement distortion of the manometric recording device, than can be made in conscious ferrets. Transient lower oesophageal sphincter relaxations in ferrets are similar to those in human (Blackshaw et al. 1998) suggesting that this is a relevant animal model to use to study this reflex.
The effects of both Δ9-THC and WIN 55,212–2 to attenuate the depth of the LOS relaxation and increase the time to maximal effect are prevented by CB1 receptor antagonist SR 141716A. This agrees with recent data in conscious dogs where WIN 55,212–2 reduced the number of transient LOS relaxations triggered by gastric distension; this too was preventable by CB1 receptor antagonism with SR 141716A (Lehmann et al. 2002). In both conscious dogs (Martin et al. 1986) and decerebrate ferrets (Abrahams et al. 2002), the LOS response to gastric distension is vagally mediated because it is abolished by bilateral vagal cooling or vagotomy at the cervical level. Thus, the most likely site of action of CB1 receptor is on vagal circuitry. Cannabinoid receptor binding is present in the DMN and NTS of human brain (Glass et al. 1997), which concurs with the high level of CB1 receptor staining observed in the dorsal vagal complex in the present study. In addition, we noted CB1 receptor expression in cells of the nodose ganglion. The presence of CB1 receptor immunostaining in central vagal circuitry suggests that CB1 receptor activation attenuates the gastric distension-evoked LOS response at either the peripheral terminals of vagal afferents or in the NTS. Since nodose ganglionectomy did not decrease the level of CB1 receptor staining in the NTS, the receptor is unlikely to be transported centrally from the soma within the nodose ganglia to the central terminals in the NTS.
This is perhaps surprising in light of the CB1 receptor immunostaining in cells in the nodose ganglion; however, cannabinoid receptors are transported peripherally from the dorsal root ganglion (Hohmann & Herkenham, 1999). In addition, CB1 receptor staining was prevalent in the myenteric plexus of the stomach and duodenum (Van Sickle et al. 2001). Other ligands, such as substance P, are preferentially transported from the nodose ganglion to the periphery (Brimijoin et al. 1980), with only 6–8 % of the total peptide content exported from the nodose ganglion being transport centrally. Likewise, the CB1 receptor synthesized in cell bodies of the nodose ganglion may be preferentially transported to peripheral, rather than central, endings. However, WIN 55,212–2 does not alter the firing pattern of gastric afferents in response to gastric distention in the ferret (Lehmann et al. 2002). It may be that despite the presence of the CB1 receptor in the periphery, they may not have any functionality. In addition, CB1 receptors from the nodose ganglia may project to other organs such as the cardiovascular system where they may have a role in modulating blood pressure.
The absence of CB1 receptor expression in preganglionic vagal motor neurones projecting to the gastric fundus or LOS and the lack of any diminution of staining in DMN after vagotomy makes it unlikely that the receptor is modulating vagal motor output directly. Therefore, the most likely site of action of Δ9-THC is via the CB1 receptor on interneurones in the NTS that ultimately input onto vagal motor neurones. The majority of reports in the literature indicate that the CB1 receptor acts by presynaptic modulation of neurotransmitter release. For example, in the hippocampus the CB1 receptor presynpatically inhibits both GABAergic (Katona et al. 2000) and glutamatergic (Shen et al. 1996) synaptic transmission. Therefore, we would predict that the CB1 receptor modulates the known excitatory and inhibitory inputs (Travagli et al. 1991) that control tonic firing rate of vagal motor neurones in the DMN.
The long-lasting hypotensive effect of Δ9-THC in our experiments is similar to that seen in normotensive and spontaneously hypertensive rats (Lake et al. 1997), where the cannabinoid receptor agonist produced an SR141716A-sensitive decrease in blood pressure using similar doses of each compound. This effect of Δ9-THC was observed under urethane anaesthesia and in conscious rats. Here, we report that the synthetic cannabinoid WIN 55,212–2 also has similar effects on mean arterial pressure, which are also blocked by SR141716A (data not shown). This decrease in blood pressure is thought to be due to an inhibition of sympathetic tone via a presynaptic site of CB1 receptors on postganglionic sympathetic nerves innervating the heart and vasculature. This would also explain why centrally administered Δ9-THC in our experiment failed to elicit a hypotensive response.
The attenuating effects of Δ9-THC on gastric distension-evoked LOS relaxation are very similar to those obtained by peripheral administration of the GABAB agonist baclofen in humans (Lidums et al. 2000), ferrets (Blackshaw et al. 1999) and dogs (Lehmann et al. 1999). GABAB activation reduces the firing of vagal afferents (Page & Blackshaw, 1999) and preynaptically inhibits vagal preganglionic neurones (Blackshaw et al. 2000; McDermott et al. 2001). It is therefore interesting that the primary site of action of Δ9-THC appears to be in the NTS in this study. This suggests that combinations of these drugs might be additive in preventing gastric distension-evoked LOS relaxation which might permit a combination at lower doses than would be necessary for either agent alone and thus reduce the adverse effects.
Acknowledgments
The advice and invaluable assistance given by Anders Lehmann, AstraZeneca, Molndal, Sweden is gratefully acknowledged. Support for these studies was provided by an American Digestive Health Foundation Research Scholar Award (T.P.A.) and a National Institutes of Health grant (DDK 42714, P.J.H.).
REFERENCES
- Abrahams TP, Partosoedarso ER, Hornby PJ. Lower esophageal sphincter relaxation evoked by stimulation of the dorsal motor nucleus of the vagus in ferrets. Neurogastroenterol Motil. 2002;14:295–304. doi: 10.1046/j.1365-2982.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Boyle JT, Nixon TE, Pack AI, Cohen S. Simultaneous reflex inhibition of lower esophageal sphincter and crural diaphragm in cats. Am J Physiol. 1985;249:G586–591. doi: 10.1152/ajpgi.1985.249.5.G586. [DOI] [PubMed] [Google Scholar]
- Berdyshev EV. Cannabinoid receptors and the regulation of immune response. Chem Phys Lipids. 2000;108:169–190. doi: 10.1016/s0009-3084(00)00195-x. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Smid SD, O'Donnell TA, Dent J. GABA(B) receptor-mediated effects on vagal pathways to the lower oesophageal sphincter and heart. Br J Pharmacol. 2000;130:279–288. doi: 10.1038/sj.bjp.0703244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw LA, Staunton E, Dent J, Holloway RH, Malbert CH. Mechanisms of gastro-oesophageal reflux in the ferret. Neurogastroenterol Motil. 1998;10:49–56. doi: 10.1046/j.1365-2982.1998.00085.x. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Staunton E, Lehmann A, Dent J. Inhibition of transient lower esophageal sphincter relaxations and reflux in ferrets by GABA B receptor ligands. Am J Physiol. 1999;277:G867–874. doi: 10.1152/ajpgi.1999.277.4.G867. [DOI] [PubMed] [Google Scholar]
- Brimijoin S, Lundberg JM, Brodin E, Hokfelt T, Nilsson G. Axonal transport of substance P in the vagus and sciatic nerves of the guinea pig. Brain Res. 1980;191:443–457. doi: 10.1016/0006-8993(80)91293-7. [DOI] [PubMed] [Google Scholar]
- Coruzzi G, Adami M, Coppelli G, Frati P, Soldani G. Inhibitory effect of the cannabinoid receptor agonist WIN 55, 212–2 on pentagastrin-induced gastric acid secretion in the anaesthetized rat. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:715–718. doi: 10.1007/s002109900135. [DOI] [PubMed] [Google Scholar]
- Franzi SJ, Martin CJ, Cox MR, Dent J. Responses of canine lower esophageal sphincter to gastric distension. Am J Physiol. 1990;259:G380–385. doi: 10.1152/ajpgi.1990.259.3.G380. [DOI] [PubMed] [Google Scholar]
- Germano MP, D'Angelo V, Mondello MR, Pergolizzi S, Capasso F, Capasso R, Izzo AA, Mascolo N, De Pasquale R. Cannabinoid CB1-mediated inhibition of stress-induced gastric ulcers in rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:241–244. doi: 10.1007/s002100000360. [DOI] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Cannabinoid receptors undergo axonal flow in sensory nerves. Neuroscience. 1999;92:1171–1175. doi: 10.1016/s0306-4522(99)00220-1. [DOI] [PubMed] [Google Scholar]
- Holloway RH, Dent J. Pathophysiology of gastroesophageal reflux: lower esophageal sphincter dysfunction in gastroesophageal reflux disease. Gastroenterol Clin N Amer. 1990;19:517–535. [PubMed] [Google Scholar]
- Holloway RH, Hongo M, Berger K, McCallum RW. Gastric distension: a mechanism for postprandial gastroesophageal reflux. Gastroenterology. 1985;89:779–784. doi: 10.1016/0016-5085(85)90572-4. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Capasso R, Germano MP, De Pasquale R, Capasso F. Inhibitory effect of cannabinoid agonists on gastric emptying in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:221–223. doi: 10.1007/s002109900054. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, Czirjak S, Mackie K, Vizi ES, Freund TF. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Krowicki ZK, Moerchbaecher JM, Winsauer PJ, Sivarao DV, Hornby PJ. Delta9-tetrahydrocannabinol inhibitis gastric motility in the rat through cannabinoid CB1 receptors. Eur J Pharmacol. 1999;371:187–196. doi: 10.1016/s0014-2999(99)00165-x. [DOI] [PubMed] [Google Scholar]
- Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Antonsson M, Bremner-Danielsen M, Flardh M, Hansson-Branden L, Karrberg L. Activation of the GABA(B) receptor inhibits transient lower esophageal sphincter relaxations in dogs. Gastroenterology. 1999;117:1147–1154. doi: 10.1016/s0016-5085(99)70400-2. [DOI] [PubMed] [Google Scholar]
- Lehmann A, Blackshaw LA, Branden L, Carlsson A, Jensen J, Nygren E, Smid SD. Cannabinoid receptor agonism inhibits transient lower esophageal sphincter relaxations and reflux in dogs. Gastroenterology. 2002;123:1129–1134. doi: 10.1053/gast.2002.36025. [DOI] [PubMed] [Google Scholar]
- Lidums I, Lehmann A, Checklin H, Dent J, Holloway RH. The GABA B agonist baclofen inhibits transient lower esophageal sphincter relaxations and gastroesophageal reflux in normal human subjects. Gastroenterology. 2000;118:7–13. doi: 10.1016/s0016-5085(00)70408-2. [DOI] [PubMed] [Google Scholar]
- McDermott CM, Abrahams TP, Partosoedarso E, Hyland NP, Ekstrand J, Monroe M, Hornby PJ. Site of action of GABA B receptor for vagal motor control of the lower esophageal sphincter. Gastroenterology. 2001;120:1749–1762. doi: 10.1053/gast.2001.24849. [DOI] [PubMed] [Google Scholar]
- Martin CJ, Patrikios J, Dent J. Abolition of gas reflux and transient lower esophageal sphincter relaxation by vagal blockade in the dog. Gastroenterology. 1986;91:890–896. doi: 10.1016/0016-5085(86)90691-8. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- Page AJ, Blackshaw LA. GABA(B) receptors inhibit mechanosensitivity of primary afferent endings. J Neurosci. 1999;19:8597–8602. doi: 10.1523/JNEUROSCI.19-19-08597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoids and the gastrointestinal tract. Gut. 2001;48:859–867. doi: 10.1136/gut.48.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramer MR, Carroll D, Campbell FA, Reynolds DJ, Moore RA, McQuay HJ. Cannabinoids for control of chemotherapy induced nausea and vomiting: quantitative systematic review. Br Med J. 2001;323:16–21. doi: 10.1136/bmj.323.7303.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol. 1991;260:G531–536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JS, Sharkey KA. Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology. 2001;121:767–774. doi: 10.1053/gast.2001.28466. [DOI] [PubMed] [Google Scholar]


