Abstract
Voltage-gated K+ channels activating close to resting membrane potentials are widely expressed and differentially located in axons, presynaptic terminals and cell bodies. There is extensive evidence for localisation of Kv1 subunits at many central synaptic terminals but few clues to their presynaptic function. We have used the calyx of Held to investigate the role of presynaptic Kv1 channels in the rat by selectively blocking Kv1.1 and Kv1.2 containing channels with dendrotoxin-K (DTX-K) and tityustoxin-Kα (TsTX-Kα) respectively. We show that Kv1.2 homomers are responsible for two-thirds of presynaptic low threshold current, whilst Kv1.1/Kv1.2 heteromers contribute the remaining current. These channels are located in the transition zone between the axon and synaptic terminal, contrasting with the high threshold K+ channel subunit Kv3.1 which is located on the synaptic terminal itself. Kv1 homomers were absent from bushy cell somata (from which the calyx axons arise); instead somatic low threshold channels consisted of heteromers containing Kv1.1, Kv1.2 and Kv1.6 subunits. Current-clamp recording from the calyx showed that each presynaptic action potential (AP) was followed by a depolarising after-potential (DAP) lasting around 50 ms. Kv1.1/Kv1.2 heteromers had little influence on terminal excitability, since DTX-K did not alter AP firing. However TsTX-Kα increased DAP amplitude, bringing the terminal closer to threshold for generating an additional AP. Paired pre- and postsynaptic recordings confirmed that this aberrant AP evoked an excitatory postsynaptic current (EPSC). We conclude that Kv1.2 channels have a general presynaptic function in suppressing terminal hyperexcitability during the depolarising after-potential.
Voltage-gated K+ conductances play multiple roles in regulating neuronal excitability. High threshold channels (such as Kv3) shape the action potential waveform and facilitate rapid repolarisation (Rudy & McBain, 2001). Low threshold K+ channels which activate close to resting potentials (such as Kv1) regulate firing threshold and excitability (Brew & Forsythe, 1995) and their mutation or deletion may result in epilepsy and ataxia (Smart et al. 1998). Six of the seven Shaker-related Kv1 family members (Kv1.1–1.6) are expressed throughout the mammalian brain and there is extensive evidence for Kv1 localisation adjacent to nodes of Ranvier and at many central synaptic terminals (Wang et al. 1994).
Owing to the difficulties in directly recording from nerve terminals, the function of presynaptic Kv1 channels remains elusive. Blockade of channels containing Kv1.1, Kv1.2 or Kv1.6 subunits by dendrotoxin-I caused spontaneous firing at the neuromuscular junction (Anderson & Harvey, 1988). Similar observations have been made at central synapses such as inhibitory cerebellar basket cell terminals where blockade of channels containing Kv1.1 and Kv1.2 subunits increased the rate and amplitude of spontaneous inhibitory postsynaptic currents (sIPSCs) (Southan & Robertson, 1998, 2000; Zhang et al. 1999). However the sensitivity of these sIPSCs to tetrodotoxin and the absence of a rise in presynaptic [Ca2+]i (Tan & Llano, 1999), suggested that the firing rate in basket cell terminals was increased rather than APs broadened. Similarly, in neurones of the medial nucleus of the trapezoid body (MNTB) DTX-I has no effect on the amplitude of evoked EPSCs (Brew & Forsythe, 1995) suggesting that the low threshold currents at the calyx of Held do not contribute to AP broadening.
We have used the calyx of Held to examine the role of Kv1 channels at central excitatory synapses by making direct pre- and postsynaptic recordings, combined with subunit-specific pharmacology and immunohistochemistry. We show that Kv1 channels do not influence terminal AP waveform but instead suppress axonal excitability, ensuring that aberrant APs are not generated.
METHODS
Electrophysiology
Cochlear nucleus and brainstem slices (100–150 μm thick) were prepared as previously described (Rusznak et al. 1997; Dodson et al. 2002). 8–14 day old Wistar and Lister Hooded rats were killed by decapitation in accordance with the UK Animals (Scientific Procedures) Act 1986. Whole-cell patch recordings were made from visually identified presynaptic terminals using an Optopatch amplifier (Cairn, Faversham, UK). Bushy cells were identified by location and morphology when filled with Lucifer yellow. During recording, slices were perfused with artificial cerebrospinal fluid (aCSF; ≈1 ml min−1 at 25 °C). Patch pipettes were pulled from thick-walled borosilicate glass (GC150F-7.5, Harvard Apparatus, UK). Presynaptic series resistances were 19.1 ± 2.2 MΩ (n = 11) and compensated by 70 % with 10 μs lag; recordings with a change exceeding ± 2 MΩ were excluded from analysis. Input resistances were 529 ± 100 MΩ (n = 11) and did not correlate with axon length. Trapezoid axons were stimulated (4–10 V, 0.2 ms, DS2A isolated stimulator, Digitimer, Welwyn Garden City, UK) at the midline using a bipolar platinum electrode. For simultaneous pre- and postsynaptic recordings, synaptic connections were detected using postsynaptic calcium imaging (Billups et al. 2002). Postsynaptic EPSCs were recorded using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA); series resistances were < 10 MΩ and 5 mM QX-314 was included in the intracellular patch solution. IPSCs were blocked by 10 μM bicuculline and 1 μM strychnine. Data were acquired with a CED1401 interface using Patch v6.39 software (Cambridge Electronic Design Ltd, Cambridge, UK), filtered at 2 kHz and digitised at 5–40 kHz. The current amplitude for current-voltage (I-V) relationships was measured 10 ms into the test step for the presynaptic recordings but delayed until 180 ms for bushy cells, to avoid contamination by the transient outward K+ current (IA). Leak currents, estimated from a linear fit between −90 mV and −75 mV, were subtracted from the I-V curve. Example I-V curves from single cells are presented but similar results were observed in at least four cells, averaged data for which are presented in the text as means ±s.e.m. Stated voltages exclude a −7 mV junction potential.
Immunohistochemistry
Sections were prepared from postnatal day (P) 9 Lister Hooded rats, as described previously (Dodson et al. 2002). Primary antibodies were applied overnight at 4 °C at a dilution of 1:100 and incubated with a fluorescein isothiocyanate (FITC) -labelled secondary antibody (1:1000) at 20 °C or, for co-localisation studies, FITC and Texas Red (both 1:500) applied together. Slides were prepared using ProLong Antifade (Molecular Probes, Eugene, OR, USA) and examined on an Olympus Fluoview confocal microscope (IX70) with a × 60 (NA 1.4) objective.
Solutions and toxins
aCSF contained (mM): 125 NaCl, 2.5 KCl, 10 glucose, 1.25 NaH2PO4, 26 NaHCO3, 2 sodium pyruvate, 3 myo-inositol, 0.5 ascorbic acid, 2 CaCl2, and 1 MgCl2, gassed with 95 % O2:5 % CO2 (pH 7.4). Except for synaptic studies, 0.5 mM [Ca2+]o and 2.5 mM [Mg2+]o were used to minimize Ca2+-dependent currents, ZD7288 (10 μM, Tocris Cookson, Bristol, UK) was used to block the hyperpolarisation-activated cation current (IH) and tetrodotoxin (1 μM TTX, Latoxan, Valence, France) was used to block voltage-gated sodium channels. Intracellular patch solution contained (mM): 97.5 potassium gluconate, 32.5 KCl, 10 Hepes, 5 EGTA, 1 MgCl2 (pH 7.2 with KOH) and Lucifer yellow or sulforhodamine 101 (1 mg ml−1; Molecular Probes, Eugene, OR, USA). Chemicals were obtained from Sigma (Poole, UK) except: DTX-I, Kv1.1, Kv1.2, Kv1.4, Kv1.6 and Kv3.1b antibodies, Alomone Labs (Jerusalem, Israel); Texas Red and FITC (goat anti-rabbit), Jackson ImmunoResearch Laboratories (West Grove, PA, USA); Kv1.1, Kv1.2 and Kv1.6 antibodies, Upstate Biotechnology (NY, USA), used for co-localisation; TsTX-Kα, Peptide Institute (Osaka, Japan); and DTX-K, a kind gift from Brian Robertson, University of Strathclyde, Glasgow, UK.
RESULTS
Presynaptic low threshold current is dominated by Kv1.2 homomers
Presynaptic outward K+ currents were found to consist of both high and low threshold components (Fig. 1A1). TsTX-Kα (100 nM), which blocks channels containing Kv1.2 subunits (Hopkins, 1998), blocked 87 ± 4.8 % of the current evoked at −45 mV (Fig. 1C3; n = 7). The remaining current (13 %) was high threshold K+ current, which activates at around −45 mV, is blocked by tetraethylammonium (TEA) and is DTX-I insensitive (P. D. Dodson & I. D. Forsythe, unpublished observations). DTX-K (100 nM), which blocks channels containing Kv1.1 subunits (Robertson et al. 1996) blocked 32 ± 4.1 % of the current (Fig. 1C3; n = 6). Since only one toxin-sensitive subunit is required for these toxins to block and Kv1.1 and Kv1.2 are the only Kv1 subunits present in presynaptic axons in the MNTB (Dodson et al. 2002), these data suggest that two-thirds of presynaptic low threshold current is mediated by Kv1.2 homomers and the remaining third by Kv1.1/Kv1.2 heteromers.
Figure 1. Kv1.2 homomers dominate the presynaptic low threshold current.
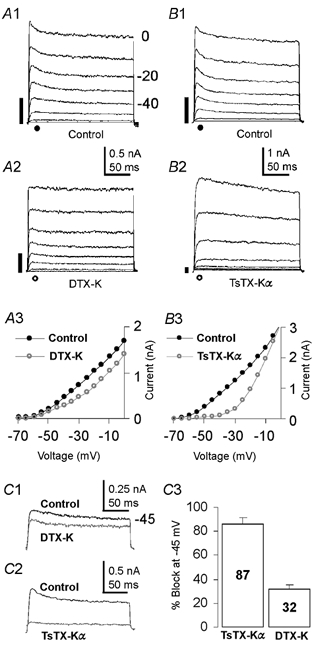
Outward currents were evoked by voltage step from −70 mV to 0 mV. A 750 ms pre-pulse to −100 mV was followed by 5 ms at −70 mV, before the 200 ms test step. The filled bar (left of traces) represents the magnitude of the current at −40 mV. A1, control traces after leak subtraction. A2, DTX-K (100 nM) partially blocked the low threshold current. A3, current-voltage relationship (I-V) of the data in A1, (•) and A2, (○). B1, control traces from a different presynaptic terminal. B2, TsTX-Kα, (100 nM) blocked the low threshold current, leaving only the high threshold current. B3, I-V of the data in B1 (•) and B2 (○). C, superimposed traces showing the current during a step to −45 mV in control and DTX-K (C1) or TsTX-Kα (C2). C3, Bar chart showing the percentage block of the current evoked at −45 mV. The terminals in A and B had axons cut within 20 μm of the terminal during slicing; identical currents were measured in terminals with long axons.
Kv1.1 and Kv1.2 are concentrated in the axonal region just preceding the terminal
The distribution of Kv1.1 and Kv1.2 containing channels at the calyx was investigated by co-localisation with Kv3.1b, which is expressed in the calyceal fingers and proximal region of the axon (Fig. 2A1). Kv3.1 is also expressed at the postsynaptic MNTB soma (Elezgarai et al. 2003) but is clearly distinguishable from presynaptic immunofluorescence (Fig. 2A1). Kv1.1 and Kv1.2 are concentrated in a 20 μm region of the presynaptic axon adjacent to the terminal, but not in the calyx itself (Fig. 2A3 and 2B). Kv1.6 was not expressed in the presynaptic axon or terminal (data not shown).
Figure 2. Kv1.1 and Kv1.2 are localised to the final 20 μm of the presynaptic axon but are excluded from the terminal.
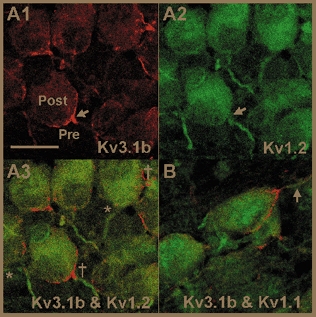
A1, Kv3.1b is concentrated in the calyceal fingers of the terminal and the proximal region of the presynaptic axon (arrow) and is also expressed at lower levels in MNTB neurones. A2, Kv1.2 is located in a 20 μm region of presynaptic axons in the same section as A1 but is absent from the calyceal fingers (arrow). A3, co-localisation of Kv3.1b (red) and Kv1.2 (green), confirming that Kv1.2 is localised to presynaptic (†) as well as postsynaptic (*) axons. B, co-localisation of Kv3.1b and Kv1.1 shows that Kv1.1 is located in an axonal region similar to Kv1.2 (arrow). Images in A and B are single confocal optical sections; n = 3 animals. Scale bar in A1 is 20 μm and applies to all parts.
Heteromeric channels containing Kv1.1, Kv1.2 and Kv1.6 are expressed at the Bushy cell soma
To examine whether K+ currents expressed at the terminal were similar to somatic currents, we compared the calyx data with somatic recordings from bushy cells, which give rise to the calyx of Held. Immunostaining for Kv1.1, Kv1.2 and Kv1.6 but not Kv1.4 was detected in neurones of the anteroventral cochlear nucleus (aVCN) (Fig. 3A). In whole-cell recordings from bushy neurones, DTX-K (100 nM) blocked 83.7 ± 13 % of the somatic current at −45 mV (Fig. 3B; n = 4). TsTX-Kα (100 nM) blocked 69.3 ± 15.2 % of the low threshold current in four neurones, (Fig. 3C), whereas in the remaining three neurones TsTX-Kα had no effect (< 5 % block). This disparity in TsTX-Kα sensitivity is consistent with two bushy cell populations (spherical and globular cells), expressing different complements of Kv1 channels. Taken together, these data suggest that low threshold somatic currents are mediated by heteromers including Kv1.1, Kv1.2 and Kv1.6 subunits. In contrast to the calyx (Fig. 1), bushy cell somata possess a transient outward current (IA), which is insensitive to TsTX-Kα and DTX-K (Fig. 3B2 and 3C2), activates at around −45 mV, rapidly inactivates and is TEA insensitive (Z. Rusznak and G. Szucs, unpublished observations). IA currents are either mediated by Kv4 channels, Kv3.4, Kv1.4 or other Kv1 channels with inactivating Kvβ subunits (Coetzee et al. 1999). As this IA is a high threshold TEA-insensitive current, we can exclude Kv3 and Kv1 channels. Since Kv4.2 is the only Kv4 subunit expressed in bushy cells (Fitzakerley et al. 2000), we conclude that the bushy cell IA is probably composed of Kv4.2 homomers.
Figure 3. Heteromers containing Kv1.1, Kv1.2 and Kv1.6 subunits compose bushy cell low threshold somatic currents.
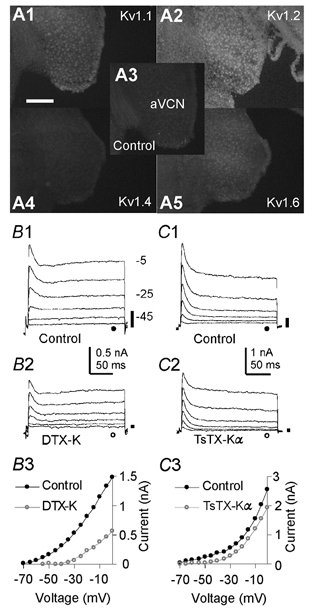
A, neurones of the aVCN express Kv1.1 (A1), Kv1.2 (A2) and Kv1.6 (A5) at their soma. Kv1.4 is not expressed (A4), since immunofluorescence is similar to the background level (A3). Immunofluorescence was visualised using a × 10 objective on an epifluorescence microscope (n = 3 animals). Scale bar represents 200 μm. B1, voltage-clamp recordings from bushy cells. Voltage protocols were as in Fig. 1 without leak subtraction. B2, DTX-K (100 nM) blocked 83.7 ± 13 % of the low threshold current, leaving the high threshold current and a rapidly inactivating transient current, both of which activate around −45 mV. B3, I-V for the data in B1 (•) and B2 (○). C1, control currents recorded in a different bushy cell. C2, TsTX-Kα (100 nM) blocked 69.3 ± 15.2 % of the low threshold current in 4 neurones. In the remaining 3 neurones TsTX-Kα had no effect (< 5 % block). C3, I-V for the currents in C1 (•) and C2 (○).
Kv1.2-containing channels regulate presynaptic AP firing
To investigate the role of Kv1 channels at the calyx we applied subunit-specific toxins whilst recording from terminals under current clamp. In brain slices, calyces with less than 20 μm of intact axon fired a single AP in response to sustained depolarisation (Fig. 4A1; n = 10). Axon length was assessed under epifluorescent illumination by inclusion of sulphorhodamine in the intracellular solution. DTX-K (100 nM) had no effect on AP firing (Fig. 4A1 inset, n = 3), suggesting that Kv1.1/Kv1.2 heteromers make little or no contribution to terminal excitability. In contrast, 100 nM TsTX-Kα caused AP firing throughout the current injection (Fig. 4A2, n = 4), suggesting that Kv1.2 homomers regulate AP firing at the terminal. Calyces with more than 20 μm of intact axon will typically fire multiple APs during sustained current injection (Forsythe, 1994), however such long depolarisations are not physiological in axons. To produce a more physiological stimulus we triggered orthodromic APs at the midline. Simultaneous dual recordings were made of presynaptic APs under current clamp along with the resulting MNTB neurone EPSCs under voltage clamp. In control conditions each electrical stimulus evoked a single presynaptic AP which generated one EPSC in the postsynaptic neurone (Fig. 4B1). From a membrane potential of −75 mV, evoked APs peaked at 23 ± 6.8 mV, had a half-width of 414 ± 30 μs and repolarised to −71.2 ± 2 mV (n = 10). APs were followed by a depolarising after-potential (DAP), which peaked at −62.5 ± 1.6 mV, had a half-width of 15.1 ± 2.4 ms and is attributable to passive discharge from the myelin sheath (Barrett & Barrett, 1982). Single presynaptic APs were maintained when the terminal membrane potential was changed over a range of −90 to −25 mV (data not shown). Following TsTX-Kα (100 nM) however, two APs were fired after a single stimulus (Fig. 4B2; 10 of 10 terminals), resulting in two EPSCs in the postsynaptic neurone. Similarly aberrant firing was observed during trains of stimuli in the presence of TsTX-Kα (Fig. 4C2). Aberrant firing in response to orthodromic AP propagation has never been observed under control conditions, but following TsTX-Kα aberrant APs were observed at potentials between −90 and −25 mV. TsTX-Kα had no effect on EPSC amplitude in the postsynaptic neurone (P = 0.93, two-tailed, paired t test, n = 5), confirming that low threshold K+ currents do not influence AP waveform or release probability (Brew & Forsythe, 1995). The AP half-width in the presence of TsTX-Kα was unchanged from control (P = 0.59, two-tailed, paired t test), but the DAP was significantly larger (P = 0.005) peaking at −55.4 ± 1.5 mV (n = 7). This suggests that the presynaptic low threshold K+ conductance serves to shunt and reduce the DAP; hence, when Kv1 currents are blocked, threshold is exceeded and a second AP is generated during the DAP. These data suggest that presynaptic Kv1.2 channels prevent terminal hyperexcitability following AP invasion and thus preserve the timing and pattern of AP firing.
Figure 4. Kv1.2-containing channels prevent aberrant presynaptic action potential firing.
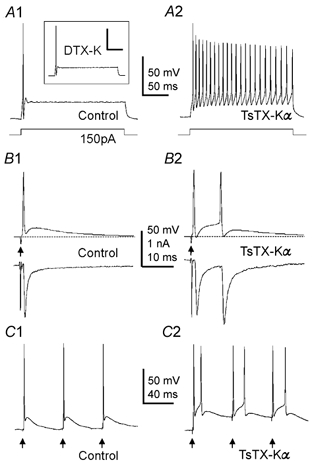
A1, control response to 150 pA current injection recorded from a presynaptic terminal under current clamp. DTX-K (100 nM) had no effect on AP firing (inset; n = 3). A2, TsTX-Kα (100 nM) caused firing throughout the current injection (n = 3). Scale bars in A represent 50 mV and 50 ms. B1, simultaneous recording of the presynaptic AP (under current-clamp, upper trace) and the postsynaptic EPSC (under voltage-clamp, lower trace). APs were evoked by electrical stimulation at the midline (arrows). In control conditions, a single AP and corresponding EPSC are generated by each stimulus (n = 13; upper portion of the postsynaptic stimulus artefact was removed). B2, following Kv1.2 block with 100 nM TsTX-Kα, an aberrant AP is fired in the terminal during the DAP, which triggered a second EPSC in the MNTB neurone (n = 10). C1, 20 Hz train under control conditions. C2, in the presence of TsTX-Kα aberrant APs are fired during the DAPs at 20 Hz stimulation (n = 3).
DISCUSSION
Kv1 channels are present in axons and presynaptic terminals throughout the brain (Wang et al. 1994; Rhodes et al. 1997; Southan & Robertson, 2000). We show that presynaptic Kv1 channels make little contribution to action potential repolarisation at the calyx of Held, but promote transmission fidelity by preventing aberrant firing following an action potential. All presynaptic Kv1 channels contain Kv1.2 subunits; Kv1.2 homomers contribute two-thirds of the current and Kv1.1/Kv1.2 heteromers the remaining third. This contrasts with somatic Kv1 currents that are exclusively mediated by heteromeric channels, composed of Kv1.1, Kv1.2 and Kv1.6 subunits. Our results suggest that presynaptic Kv1 channels play a pivotal role in preventing nerve terminal hyperexcitability.
The presynaptic low threshold current is dominated by Kv1.2 homomers; these channels are largely responsible for ensuring that each AP propagating from the bushy cell results in a single AP arriving at the terminal. Kv1.1/Kv1.2 heteromers are also present presynaptically, but blocking these channels does not influence AP firing (Fig. 4A1). This contrasts with the postsynaptic MNTB neurones, which have no homomeric channels but in which Kv1.1/Kv1.2 heteromers, located at the initial segment of the axon, are dominant in regulating AP firing (Dodson et al. 2002). Immunohistochemical localisation shows that bushy cell bodies express Kv1.1/Kv1.2/Kv1.6 subunits similar to MNTB somata (Dodson et al. 2002; Brew et al. 2003). Subunit composition can influence localisation of channels by association of certain subunits with Kvβ or Caspr (Poliak et al. 1999; Manganas et al. 2001). A general hypothesis consistent with our observations is that Kv1.6-containing channels are restricted to somatic regions whereas channels containing Kv1.1 and Kv1.2 are permitted access to axonal regions and contribute to initial segment, juxtaparanodal and terminal low threshold K+ conductances. Heteromers contribute at all sites, with Kv1.2 homomers located exclusively at the transition zone between the axon and terminal. This observation may have therapeutic implications for selective regulation of presynaptic versus postsynaptic excitability.
Immunohistochemical studies demonstrate that most Kv1 channels isolated from brain synaptic membranes contain Kv1.2 subunits, with Kv1.2 homomers being prevalent (Shamotienko et al. 1997). Our results show that Kv1.2 channels are located at the transition zone between the axon and terminal (the last 20 μm) but are excluded from the terminal itself (Fig. 2). Kv1 channels are also localised at transition zones at the neuromuscular junction (Zhou et al. 1998), thus allowing greatest influence over AP invasion of the terminal and minimising antidromic reflection by raising threshold following an AP. Kv1 channels also exert control over axonal APs in the initial segment (observed in recordings from MNTB neurones, Dodson et al. 2002).
In some nerve terminals, such as hippocampal mossy fibre boutons (Geiger & Jonas, 2000) and neurohypophysis terminals of the pituitary (Jackson et al. 1991), inactivating K+ currents are responsible for activity-dependent AP broadening and facilitation of transmitter release. For example in hippocampal mossy fibre boutons, low threshold DTX-sensitive channels, presumed to contain Kv1.1, Kv1.4 and Kvβ1 subunits, inactivate during repetitive stimulation causing AP broadening (Rhodes et al. 1997; Geiger & Jonas, 2000).
In other terminals, however, Kv1 channels are involved in preventing hyperexcitability. In the frontal cortex, blockade of putative Kv1.2 homomers causes local spiking in thalamocortical axon terminals (Lambe & Aghajanian, 2001) and in the entorhinal cortex, α-DTX block of Kv1.2-containing channels increases the frequency of sIPSCs (Cunningham & Jones, 2001). Kv1.1 and Kv1.2 are highly concentrated in cerebellar basket cell terminals (Wang et al. 1994; Rhodes et al. 1997) and block of the presynaptic Kv1 current by α-DTX increases both frequency and amplitude of sIPSCs in Purkinje cells (Southan & Robertson, 1998, 2000). Kv1.1-null mice also exhibit increased spontaneous activity at cerebellar basket cell synapses (Zhang et al. 1999), hyperexcitability of peripheral axons (Zhou et al. 1998) and epilepsy (Smart et al. 1998). We have demonstrated that Kv1.2 channels prevent hyperexcitability at the calyx of Held, preserving AP fidelity, which is critical for sound source localisation (Trussell, 1999). Our results also highlight the general importance of Kv1 channels in preventing errors during repetitive firing. The widespread distribution of presynaptic Kv1 channels, combined with their importance in preventing aberrant firing, suggests a general role for presynaptic Kv1 channels in preventing hyperexcitability in nerve terminals throughout the central and peripheral nervous system.
Acknowledgments
We would like to thank Dr L. Kaczmarek for his comments on the manuscript. This work was supported by the Wellcome Trust, the MRC and the Hungarian National Science Foundation (OTKA T-031824). P.D.D. is a Wellcome Trust Prize PhD student.
REFERENCES
- Anderson AJ, Harvey AL. Effects of the potassium channel blocking dendrotoxins on acetylcholine release and motor nerve terminal activity. Br J Pharmacol. 1988;93:215–221. doi: 10.1111/j.1476-5381.1988.tb11424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol. 1982;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups B, Wong AY, Forsythe ID. Detecting synaptic connections in the medial nucleus of the trapezoid body using calcium imaging. Pflugers Arch. 2002;444:663–669. doi: 10.1007/s00424-002-0861-6. [DOI] [PubMed] [Google Scholar]
- Brew HM, Forsythe ID. Two voltage-dependent K+ conductances with complementary functions in postsynaptic integration at a central auditory synapse. J Neurosci. 1995;15:8011–8022. doi: 10.1523/JNEUROSCI.15-12-08011.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew HM, Hallows JL, Tempel BL. Hyperexcitability and reduced low threshold potassium currents in auditory neurons of mice lacking the channel subunit Kv1. 1. J Physiol. 2003;548:1–20. doi: 10.1113/jphysiol.2002.035568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz De Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Jones RS. Dendrotoxin sensitive potassium channels modulate GABA but not glutamate release in the rat entorhinal cortex in vitro. Neuroscience. 2001;107:395–404. doi: 10.1016/s0306-4522(01)00361-x. [DOI] [PubMed] [Google Scholar]
- Dodson PD, Barker MC, Forsythe ID. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J Neurosci. 2002;22:6953–6961. doi: 10.1523/JNEUROSCI.22-16-06953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elezgarai I, Diez J, Puente N, Azkue JJ, Benitez R, Bilbao A, Knopfel T, Donate-Oliver F, Grandes P. Subcellular localization of the voltage-dependent potassium channel Kv3. 1b in postnatal and adult rat medial nucleus of the trapezoid body. Neuroscience. 2003;118:889–898. doi: 10.1016/s0306-4522(03)00068-x. [DOI] [PubMed] [Google Scholar]
- Fitzakerley JL, Star KV, Rinn JL, Elmquist BJ. Expression of Shal potassium channel subunits in the adult and developing cochlear nucleus of the mouse. Hear Res. 2000;147:31–45. doi: 10.1016/s0378-5955(00)00118-0. [DOI] [PubMed] [Google Scholar]
- Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J Physiol. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca(2+) inflow by fast-inactivating K(+) channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Hopkins WF. Toxin and subunit specificity of blocking affinity of three peptide toxins for heteromultimeric, voltage-gated potassium channels expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;285:1051–1060. [PubMed] [Google Scholar]
- Jackson M, Konnerth A, Augustine G. Action potential broadening and frequency-dependent facilitation of calcium signals in pituitary nerve terminals. Proc Natl Acad Sci U S A. 1991;88:380–384. doi: 10.1073/pnas.88.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambe EK, Aghajanian GK. The Role of Kv1. 2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J Neurosci. 2001;21:9955–9963. doi: 10.1523/JNEUROSCI.21-24-09955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Wang Q, Scannevin RH, Antonucci DE, Rhodes KJ, Trimmer JS. Identification of a trafficking determinant localized to the Kv1 potassium channel pore. Proc Natl Acad Sci U S A. 2001;98:14055–14059. doi: 10.1073/pnas.241403898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–1047. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvβ1 and Kvβ2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B, Owen D, Stow J, Butler C, Newland C. Novel effects of dendrotoxin homologues on subtypes of mammalian Kv1 potassium channels expressed in Xenopus oocytes. FEBS Lett. 1996;383:26–30. doi: 10.1016/0014-5793(96)00211-6. [DOI] [PubMed] [Google Scholar]
- Rudy B, Mcbain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Rusznak Z, Forsythe ID, Brew HM, Stanfield PR. Membrane currents influencing action potential latency in granule neurons of the rat cochlear nucleus. Eur J Neurosci. 1997;9:2348–2358. doi: 10.1111/j.1460-9568.1997.tb01652.x. [DOI] [PubMed] [Google Scholar]
- Shamotienko OG, Parcej DN, Dolly JO. Subunit combinations defined for K+ channel Kv1 subtypes in synaptic membranes from bovine brain. Biochemistry. 1997;36:8195–8201. doi: 10.1021/bi970237g. [DOI] [PubMed] [Google Scholar]
- Smart SL, Lopantsev V, Zhang CL, Robbins CA, Wang H, Chiu SY, Schwartzkroin PA, Messing A, Tempel BL. Deletion of the K(V)1. 1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–819. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- Southan AP, Robertson B. Patch-clamp recordings from cerebellar basket cell bodies and their presynaptic terminals reveal an asymmetric distribution of voltage-gated potassium channels. J Neurosci. 1998;18:948–955. doi: 10.1523/JNEUROSCI.18-03-00948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan AP, Robertson B. Electrophysiological characterization of voltage-gated K(+) currents in cerebellar basket and Purkinje cells: Kv1 and Kv3 channel subfamilies are present in basket cell nerve terminals. J Neurosci. 2000;20:114–122. doi: 10.1523/JNEUROSCI.20-01-00114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YP, Llano I. Modulation by K+ channels of action potential-evoked intracellular Ca2+ concentration rises in rat cerebellar basket cell axons. J Physiol. 1999;520:65–78. doi: 10.1111/j.1469-7793.1999.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO. Synaptic mechanisms for coding timing in auditory neurons. Annu Rev Physiol. 1999;61:477–496. doi: 10.1146/annurev.physiol.61.1.477. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL. Localization of Kv1. 1 and Kv1.2, 2 K-channel proteins, to synaptic terminals, somata, and dendrites in the mouse-brain. J Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C-L, Messing A, Chiu SY. Specific alteration of spontaneous GABAergic inhibition in cerebellar Purkinje cells in mice lacking the potassium channel Kv1. 1. J Neurosci. 1999;19:2852–2864. doi: 10.1523/JNEUROSCI.19-08-02852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhang CL, Messing A, Chiu SY. Temperature-sensitive neuromuscular transmission in Kv1. 1 null mice: role of potassium channels under the myelin sheath in young nerves. J Neurosci. 1998;18:7200–7215. doi: 10.1523/JNEUROSCI.18-18-07200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


