Abstract
Cajal-Retzius (CR) cells are among the earliest generated population of neurons in the developing neocortex and have been implicated in regulating cortical lamination. In rodents, CR cells are transient, being present only up to 2–3 weeks after birth. Although previous electrophysiological studies have demonstrated the presence of NMDA and GABAA receptors in CR cells, little is known about the functional properties of these receptors. Using whole-cell patch-clamp techniques in neocortical slices, we confirmed the presence of D-aminophosphonovaleric acid (APV)- and ifenprodil-sensitive NMDA receptors, and found that the functional expression of this receptor subtype is strain specific. The NMDA-induced response was consistently accompanied by overriding current transients that were blocked by APV and ifenprodil. In addition, bicuculline readily abolished these transients without affecting the NMDA-induced current response. The generation of these overriding current transients was dependent upon intracellular Ca2+ and was prevented by dialysis with the high-affinity Ca2+-chelator BAPTA. Overall, this study uncovered a synergistic interaction between these receptors, whereby activation of NMDA receptors leads to enhanced GABAA receptor-mediated activity through a Ca2+-dependent mechanism.
Cajal-Retzius (CR) cells are amongst the earliest generated neurons of the developing cerebral cortex and represent a unique group of cells found exclusively within the marginal zone (MZ; future layer I). Cajal-Retzius cells play a vital role during cortical development. By synthesizing and releasing the extracellular glycoprotein reelin, they regulate the inside-out migration of newly generated neurons to their destined positions within the cortical plate (D'Arcangelo et al. 1995; Ogawa et al. 1995; Curran & D'Arcangelo, 1998). Their absence results in disruption of normal neuronal migration and laminar formation (Ogawa et al. 1995; Mallamaci et al. 2000; Super et al. 2000). Despite the importance of CR cells in regulating cortical development, little is known about their functional and physiological properties. It is clear that excitatory and inhibitory activity are important factors in regulating many diverse aspects of development (Behar et al. 1999, 2000; Owens & Kriegsteina2002, b; Wong & Ghosh, 2002). Yet little is known about such activity in CR cells. In rodent CR cells, sensitivity to γ-aminobutyric acid (GABA), mediated via the GABAA receptor, has been demonstrated to be excitatory and does not switch postnatally to being inhibitory (Mienville, 1998) as has been observed in other cell types (Ben-Ari et al. 1989, 1997, 2002). Glutamate responses in CR cells have also been shown, mainly through the activation of N-methyl-D-aspartate (NMDA) receptors, although some reports have demonstrated the presence of an α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) mediated response in rats (Kim et al. 1995; Schwartz et al. 1998) and in humans (Lu et al. 2001).
Given the apparent differences in glutamate sensitivity, it is interesting to note that human and rodent CR cells differ in another important aspect. Specifically, during the course of neocortical development, CR cells in rodents begin to disappear soon after birth, usually within the first 2 postnatal weeks, whereas in humans many persist through to adulthood. Whether the disappearance of these cells is due to degeneration or transformation into other cells types (Edmunds & Parnavelas, 1982; Parnavelas & Edmunds, 1983; Derer & Derer, 1990, 1992) is still controversial, although it has been suggested that activation of NMDA receptors may contribute to unfavourable physiological conditions for their survival (Mienville & Pesold, 1999). Using patch-clamp recording techniques, we characterized the electrophysiological properties of CR cells in the mouse, focusing on the functional properties of the NMDA receptor. We report that functional expression of this receptor is strain dependent, being notably absent in C57BL/6J mice. In addition, we demonstrate for the first time that activation of NMDA receptors synergistically triggers in CR cells GABAA receptor-mediated current transients via a Ca2+-dependent mechanism.
METHODS
Preparation of cortical slices
All procedures involving animals were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Neonatal C57BL/6J and ICR mouse pups between postnatal days 4–10 (postnatal day 0 is the day of birth) were killed by rising concentrations of carbon dioxide, the brains were quickly isolated and used to prepare cortical slices for electrophysiological recording. Briefly, coronal brain slices (200 μm) containing neocortex were prepared using an oscillating tissue slicer (model OTS-4000, Electron Microscopy Sciences, Fort Washington, PA, USA). Slices were cut in ice-cold oxygenated artificial cerebrospinal fluid (aCSF) solution containing (mM): 125 NaCl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 1.25 NaHPO4, 25 NaHCO3 and 25 glucose. The slices were maintained in oxygenated aCSF at room temperature and allowed to recover for at least 30 min prior to electrophysiological recording.
Patch-clamp recording and drug application
Slices were transferred to a recording chamber and maintained at 37 °C on the heated stage of an upright microscope (BXWI50, Olympus, Melville, NY, USA) and continuously perfused with oxygenated Mg2+-free aCSF supplemented with 10 μM glycine. Where applicable, 0.5 μM tetrodotoxin (TTX) was perfused through the recording chamber to suppress voltage-gated sodium channel activation. Individual CR cells were visualized under Hoffman modulation optics (HMO) (Modulation Optics, Greenvale, NY, USA) using a ×40 water immersion objective (3 mm working distance; Olympus). Real-time images were captured using an analogue video camera attached to a video framegrabber board (Integral Technologies, Indianapolis, IN, USA) and displayed on a computer monitor, which aided the navigation and placement of the drug and recording pipettes.
Whole-cell patch-clamp recordings were performed with an Axopatch 200A patch-clamp amplifier (Axon Instruments, Foster City, CA, USA). Pipette solutions used contained (mM): either (1) 140 CsCl, 1 CaCl2, 5 EGTA, 10 Hepes, 3 MgATP, 0.3 NaGTP; or (2) 75 CsCl, 1 CaCl2, 75 potassium gluconate, 5 EGTA, 10 Hepes, 3 MgATP, 0.3 NaGTP; or (3) 75 CsCl, 1 CaCl2, 30 BAPTA, 10 Hepes, 3 MgATP, 0.3 NaGTP. Data were acquired and digitized using pCLAMP 8 software via a Digidata 1320 A/D interface board (Axon Instruments). Lucifer Yellow (0.1 %) was included in the recording solution to reveal the morphology of recorded cells during electrophysiological recording and to aid identification of recorded cells following subsequent immunohistochemical processing.
Glutamate and its agonists were loaded into individual barrels of an 8-channel multi-barrel pipette assembly connected to a 4-channel picospritzer (General Valve Co., Fairfield, NJ, USA) and delivered to the immediate vicinity of the cell using regulated pressure (≤ 2 p.s.i., 0.5–3 s duration at 20 s intervals). Between drug applications, aCSF was applied to facilitate clearance of the agonist and control for mechanical artifacts. A similar protocol was used to test the action of the GABAA receptor antagonist bicuculline (100 μM), the NMDA receptor antagonists 2-amino-5-phosphonovaleric acid (APV; 50 μM; Sigma, St Louis, MO, USA) and ifenprodil (50 μM; Tocris Cookson, Ellisville, MO, USA), and the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX, 50 μM; Sigma).
Immunohistochemistry
Following electrophysiological recording, slices were fixed in 4 % paraformaldehyde dissolved in 0.1 M phosphate buffer (PB) overnight and subsequently washed three times for 10 min each in PB. The slices were then incubated in blocking solution consisting of 10 % normal goat serum (NGS), 0.2 % Triton X-100 in 0.1 M PB for 1 h at room temperature (RT) before incubation with reelin antibody E4 (diluted 1:1000 in 1 % NGS, 0.2 % TX-100 in 0.1 M PB; de Bergeyck et al. 1998) overnight at 4 °C. The slices were then washed with PB and incubated with a secondary antibody conjugated to Alexa-568 (Molecular Probes, Eugene, OR, USA) for 2 h at RT. Images were captured using a laser-scanning confocal microscope (FV300, Olympus) and analysed using the ImageJ software package (NIH).
RESULTS
Cajal-Retzius cells in coronal neocortical slices were identified on the basis of the following morphological criteria. (1) Their location in proximity to the pial surface. (2) They had a large oval somata. (3) The long processes often extended parallel to the pial surface (Fig. 1A). To further confirm the identity of cells during and after recording, Lucifer Yellow (0.1 %) was routinely included in the pipette solution which readily dialysed cells during whole-cell recording (Figs 1B, 2A and D). Following recording, slices were processed for immunohistochemistry with an antibody against reelin, and Lucifer Yellow-filled cells were examined for reelin immunoreactivity (Fig. 2B and E). As expected, the majority of cells identified based on morphological criteria were found to be immunopositive for reelin (Fig. 2C). However, a small population of the recorded cells with morphology resembling CR cells (2 of 15 cells) did not display reelin immunoreactivity (Fig. 2F).
Figure 1. Identification of Cajal-Retzius cells in a live cortical slice.
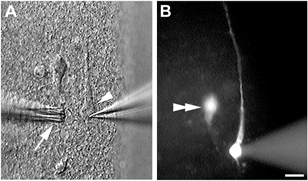
A, CR cell (arrowhead) in a 200 μm slice from postnatal day 7 mouse during recording. Drugs were delivered by means of a multi-barrel drug assembly (arrow). B, Lucifer Yellow was routinely included in the internal recording solution to aid identification of the cell type following recording. A second Lucifer Yellow-filled cell that was recorded separately can be seen in the same field (double arrowhead). Scale bar: 10 μm.
Figure 2. Reelin expression in electrophysiologically recorded CR cells.

Cells were filled with Lucifer Yellow during recording and the slice was processed for immunohistochemistry with an antibody against reelin. A-C, laser scanning confocal-microscope images of two cells that were recorded separately and filled with Lucifer Yellow (A), one of which was reelin immunopositive (B, arrow). B, a number of other reelin-immunopositive cells can be seen residing in layer I in this slice. C, overlay of A and B showing a reelin-positive CR cell (arrow) and a nearby, multipolar non-CR cell filled with Lucifer Yellow but is reelin-negative (arrowhead). D-F, a CR cell filled with Lucifer Yellow (D, arrow) and reelin immunohistochemical staining in the same slice (E). F, overlay of D and E shows an occasional Lucifer Yellow-filled cell, with morphology typical of CR cells but is negative for reelin immunoreactivity. Scale bar: 20 μm.
Responses of CR cells to glutamate agonists
Figure 3 summarizes whole-cell current responses of CR cells recorded from C57BL/6J and ICR mice following exogenous application of glutamate and NMDA. Patch clamp recordings were performed at a holding potential of - 60 mV and in Mg2+-free aCSF solution in order to relieve the Mg2+ block on NMDA receptors. Glutamate and NMDA evoked inward current responses in all CR cells recorded in ICR mice (Fig. 3A). The glutamate- and NMDA-evoked responses (n = 70 cells) were typically small in amplitude, ranging from 20–100 pA. In contrast, CR cells in C57BL/6J mice were insensitive to glutamate and NMDA (Fig. 3B) even when the agonists were applied at a high concentration (500 μM, n = 94). In agreement with previous studies (Mienville & Pesold, 1999; Lu et al. 2001), application of AMPA (200 μM) failed to elicit any current response in CR cells of either strain, although robust current responses were routinely observed in layer II/III pyramidal cells from the same cortical slice (data not shown). In the same slices from both strains, application of GABA (25 μM) to CR cells evoked robust current responses that were blocked by bicuculline and potentiated by diazepam, typical of GABAA receptor activation (data not shown).
Figure 3. Strain-dependent sensitivity of CR cells to glutamate.

A, small current responses were elicited in CR cells from ICR mice following application of 500 μM glutamate or NMDA. However, (B) CR cells in C57BL/6J were insensitive to either glutamate or NMDA.
The pharmacological specificity of the glutamate response was further examined using NMDA and AMPA receptor-specific antagonists. Exposure to CNQX (50 μM), an antagonist of the AMPA receptor, had no effect on the glutamate-induced current response (8/8 cells, Fig. 4A). On the other hand, APV (50 μM), a NMDA receptor antagonist, blocked the glutamate-induced current (8/8 cells, Fig 4B), indicating that the glutamate-induced current is mediated by activation of NMDA receptors. Indeed, application of NMDA evoked current responses that were blocked by APV (12/12 cells, Fig. 4C and E). In addition, ifenprodil (50 μM), a NMDA receptor antagonist selective for receptors containing the NR2B subunit, blocked the current response induced by application of NMDA (11/11 cells, Fig. 4D and E). Thus glutamate-evoked responses in CR cells are predominantly mediated via NR2B-containing NMDA receptors and the response is strain specific.
Figure 4. Current responses to glutamate agonists in CR cells of ICR mice.
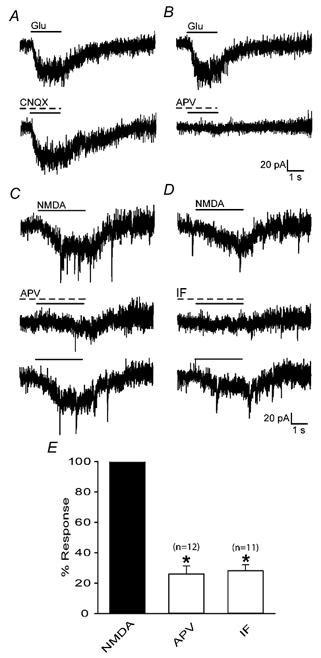
Response to application of 500 μM glutamate is insensitive to blockade by CNQX (A), but is blocked by APV (B). C, response to application of 500 μM NMDA can be partially and reversibly blocked by the NMDA receptor antagonist APV. D, reversible suppression of NMDA response by the NR2B subunit selective antagonist ifenprodil. E, effect of APV and ifenprodil on peak amplitude of NMDA-induced current response. Values were normalized to control NMDA responses. * Statistical significance (Student's paired t test, P < 0.001). Error bars show s.e.m.
Spontaneous postsynaptic events in CR cells
Previous studies have reported the presence of GABAA receptor mediated spontaneous postsynaptic currents (sPSCs) in CR cells in the rat (Kilb & Luhmann, 2001; Radnikow et al. 2002). In this study sPSCs were observed in 37/60 cells, the majority of which occurred at a low frequency (0.11 ± 0.07 Hz, n = 28), although some cells displayed a higher frequency of activity (0.99 ± 0.46 Hz, n = 9). In addition, the sPSCs were insensitive to APV (50 μM) and CNQX (50 μM), but were completely abolished by application of the GABAA receptor antagonist bicuculline (50 μM). The same responses were observed in both C57BL/6J and ICR mice, suggesting that in each strain the sPSCs are mediated by GABAA receptors. Such activity was observed at all postnatal ages examined and stands in contrast to previous studies in the rat in which sPSCs were not detected after P4 (Kilb & Luhmann, 2001).
NMDA-induced current responses trigger overriding depolarizing currents transients
In approximately 60 % of the CR cells, application of NMDA (300–500 μM) produced small but clear-cut inward current responses that were often accompanied by overriding depolarizing current transients (Fig. 5Aa). The overriding current transients were not affected by bath application of the voltage-gated sodium channel blocker TTX (0.5 μM, Fig. 5Ac), indicating that they occurred independently of action potential generation. A unique and novel finding was that application of bicuculline, a GABAA receptor antagonist, consistently abolished the overriding current transients while leaving the NMDA-induced current unaltered (Fig. 5Ab). Thus, activation of NMDA receptors led to an increase in GABAA receptor-mediated activity in CR cells, a synergistic interaction that was not observed in cells examined in deeper cortical layers which displayed bicuculline-insensitive, TTX-sensitive spikes (Fig. 5Bb and c) upon exposure to NMDA.
Figure 5. NMDA-induced current responses are accompanied by overriding transients.
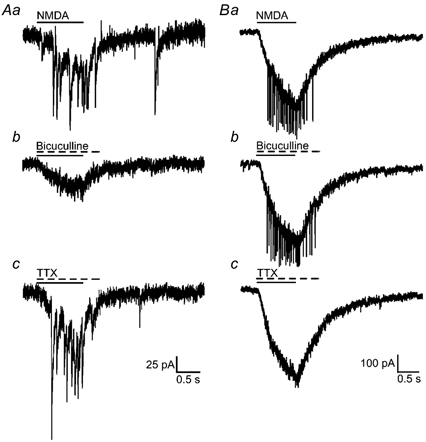
A shows NMDA-induced current responses in CR cells, while B demonstrates the same response in a layer 2/3 pyramidal cell. Aa, application of NMDA induced small responses that were accompanied by overriding current transients. Ab, the overriding current transients were suppressed by co-application of bicuculline (100 μM), but were insensitive to TTX (0.5 μM; Ac). In contrast, deep layer pyramidal neurons responded to NMDA with a robust current response (Ba) and overriding spiking activity that was bicuculline insensitive (Bb) and TTX sensitive (Bc).
A synergistic interaction between NMDA and GABAA receptors could occur via a number of pre- and postsynaptic mechanisms. To establish whether the mechanism underlying the generation of the overriding current transients in CR cells involved a presynaptic component, we examined the frequency and amplitude of sPSC (Fig. 6Aa). In some CR cells, brief (≤ 3 s) applications of NMDA (500 μM) increased the number of spontaneous events as compared to that recorded prior to NMDA application, from 0.36 ± 0.22 to 0.69 ± 0.43 Hz (n = 7, P < 0.05; Fig. 6Ab an 6B). The amplitude of the sPSCs remained unaltered (data not shown). These sPSCs were completely and reversibly blocked by bicuculline, indicating that the increase reflected enhanced GABAA receptor-mediated events rather than the emergence of NMDA receptor-mediated EPSCs (Fig. 6Ac). In addition, application of APV, which blocked the NMDA-induced current, prevented the increase in the frequency of sPSCs (Fig. 6Ad).
Figure 6. Spontaneous postsynaptic currents recorded from CR cells of ICR mice.
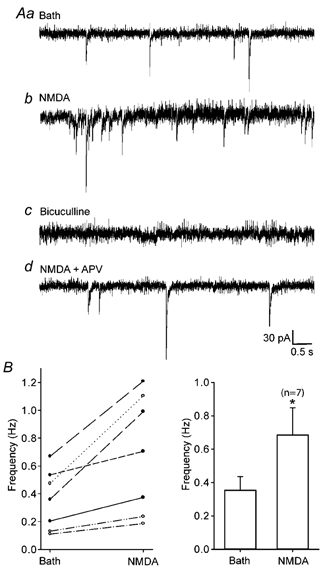
Aa, sample trace to show sPSCs prior to drug application. Ab, brief applications (≤ 3 s of NMDA increased the frequency of spontaneous events observed. Ac, spontaneous activity was completely and reversibly blocked by application of bicuculline. Ad, co-application of the NMDA receptor antagonist APV prevented the increase in frequency of spontaneous events. B, summary of NMDA-induced changes in GABAA receptor-mediated sPSCs. The left panel shows a scatterplot representing changes in frequency of sPSCs in CR cells. Each pair of connected points represent data derived from one cell. The right panel shows a histogram summarizing the changes in the frequency of sPSCs following NMDA application. * Statistical significance (Student's paired t test, P < 0.05, n = 7). Error bars show s.e.m.
We also examined whether a postsynaptic mechanism may be involved in generating the overriding current transients. Previous studies have demonstrated that activation of NMDA receptors can modulate positively (Llano et al. 1991) or negatively (Isokawa, 1998) GABAA receptor-mediated inhibitory synaptic currents. Influx of Ca2+ through NMDA receptors appears to be a key component underlying both mechanisms, and can be prevented by calcium channel blockers, such as cadmium, or calcium chelators such as EGTA or BAPTA (Llano et al. 1991; Isokawa, 1998). In this light, we asked whether Ca2+-dependent changes in postsynaptic GABAA receptor function could account for the overriding current transients that accompanied the NMDA response. BAPTA, a high affinity Ca2+ chelator, was introduced intracellularly during whole-cell recording by inclusion in the pipette solution. As illustrated in Fig. 7, the overriding current transients were not observed in cells recorded with 30 mM BAPTA (8/8 cells, Fig. 7B), but were present in control cells from the same slices (7/11 cells, Fig. 7A). In one experiment, under control conditions, the current transients were abolished when slices were perfused with Ca2+-free aCSF. These observations suggest that the activation of NMDA receptors in CR cells leads to influx of Ca2+ which, in turn, enhances GABAA receptor-mediated activity and the generation of the observed current transients.
Figure 7. Lack of GABAA receptor-mediated overriding spikes following intracellular dialysis of BAPTA.

A, bicuculline sensitive overriding current transients are present in control cells but (B) not in cells recorded with pipette solutions containing 30 mM BAPTA. Scale bar applies to both A and B.
DISCUSSION
In this study, we examined glutamate-induced current responses by whole-cell patch-clamp recording in mouse CR cells during early postnatal development. We report that glutamate responses in CR cells are mediated by the NMDA receptor, with no detectable AMPA receptor component. Such responses are prevalent in ICR mice but were never observed in C57BL/6J mice, and thus, are strain dependent. In addition, we demonstrate the presence of GABAA receptor-mediated overriding current transients, unique to CR cells, that accompany the NMDA-induced response.
Reelin and Cajal-Retzius cells
Reelin is synthesized and released by CR cells during pre- and postnatal development (Ogawa et al. 1995; Alcantara et al. 1998). It is widely used as a histochemical marker for this cell type. However, we have observed a small number of morphologically distinct CR cells that did not display reelin immunoreactivity. Although reelin-negative ‘pioneer neurons’ have been described in the MZ of the developing neocortex (Meyer et al. 1999), intracellular labelling of recorded CR cells indicate that their axons do not extend beyond layer I and, thus, are distinct from pioneer neurons (Meyer et al. 1999). Since CR cells are conventionally identified by morphology or reelin expression, it is conceivable that by combining both criteria, we have uncovered a small population of reelin-negative cells. Alternatively, the lack of reelin immunoreactivity may reflect a temporal regulation in the expression of this protein. Since our experiments were conducted during the second postnatal week, a period when CR cells begin to disappear, downregulation of reelin expression may be a consequence of, or prelude to, cellular death or transformation into other cell types (Edmunds & Parnavelas, 1982; Parnavelas & Edmunds, 1983; Derer & Derer, 1990, 1992). Indeed, reelin expression decreases during postnatal development (Schiffmann et al. 1997) just prior to the disappearance of CR cells during the second and third postnatal weeks (Derer & Derer, 1990). Pertinent to this idea, reelin expression is downregulated by increasing levels of brain-derived neurotrophic factor (BDNF), and transgenic mice overexpressing BDNF have reduced reelin levels and abnormalities in CR cell morphology (Ringstedt et al. 1998). These observations suggest that there indeed may be a window of time, just prior to their disappearance, when reelin is no longer expressed by CR cells.
Strain-specific response to glutamate
While the presence of functional NMDA receptors has been demonstrated (Kim et al. 1995; Schwartz et al. 1998; Mienville & Pesold, 1999; Lu et al. 2001), there remains some discrepancy as to whether CR cells express AMPA and kainate receptors (reviewed in Mienville, 1999). A recent study by Lu et al. (2001) demonstrated the presence of an AMPA receptor-mediated response in human CR cells, which was absent in mouse CR cells recorded under identical conditions, providing the first direct evidence that receptor expression may be species dependent. Our results confirm the absence of AMPA receptors in mouse CR cells. Additionally, we provide evidence that expression of functional NMDA receptors may be strain dependent, being notably absent in CR cells from C57BL/6J mice. Thus, glutamate receptor expression may be strain and species dependent, and could account for the disparity in reported AMPA receptor expression.
A synergistic NMDA-GABAA receptor interaction
Exogenous application of NMDA resulted in a small, but clear current response that was reversibly blocked by APV and ifenprodil. The NMDA-induced responses were occasionally accompanied by small overriding depolarizing current transients or action potentials and have not been described in previous studies (Mienville & Pesold, 1999; Lu et al. 2001). An unexpected finding was that these current transients were completely blocked by bicuculline, and thus, are mediated by the GABAA receptor. These observations suggest a synergistic interaction between these two classes of receptors resulting in the generation of overriding current transients.
Application of NMDA increased the frequency of sPSCs without affecting their amplitude, indicative of a presynaptic mechanism possibly involving increased neurotransmitter release as a result of influx of Ca2+ into the presynaptic terminal. Nevertheless, it is difficult to reconcile this with the generation of overriding current transients, especially in the light of the observation that chelation of postsynaptic intracellular Ca2+ can negate the effect of NMDA. This raises two interesting possibilities that could account for these observations. Firstly, since coupling via gap junctions has been reported between CR cells and adjacent cells (Radnikow et al. 2002), BAPTA could leak from recorded CR cell and buffer Ca2+ in the presynaptic cell, thereby blocking the effect of NMDA. However, this is unlikely since intracellular loading of recorded CR cells with Lucifer Yellow did not reveal any coupled cells. Secondly, we considered the possibility that CR cells may form autapses (reviewed in Bekkers, 1997). However, depolarizing pulses in the recorded cell (Pouzat & Marty, 1998) did not elicit autaptic currents or sPSCs similar to those observed with NMDA application (data not shown).
Several key observations argue against the possibility that the generation of overriding current transients is due to increased neurotransmitter release. Firstly, we were able to record overriding current transients in CR cells that displayed little or no spontaneous activity. In addition, the current transients were TTX insensitive, unlike those observed in deeper layer cortical neurons, and thus were action potential-independent events. These observations suggest that presynaptic inputs may not play a major role in the generation of the overriding current transients. Thus, our results are more consistent with a postsynaptic mechanism, perhaps mediated by changes in intracellular Ca2+ concentration ([Ca2+]i). GABAA receptors in CR cells may be subject to modulation by [Ca2+]i and by NMDA receptor activation, as has been demonstrated in hippocampal and cerebellar neurons (Inoue et al. 1986; Marchenko, 1991; Llano et al. 1991; Chen & Wong, 1995). In support of this, buffering of intracellular Ca2+ by BAPTA prevented the generation of the overriding current transients. However, the mechanism through which [Ca2+]i modulates GABAA-receptor function in CR cells remains to be elucidated.
GABA, glutamate and cell death?
It has been proposed that activation of NMDA receptors in response to ambient levels of glutamate leads to an overload of intracellular Ca2+ ions and results in cell death (Mienville & Pesold, 1999). This hypothesis is supported by evidence that in vivo blockade of NMDA receptors with dizocilpine increases the number of surviving CR cells in older postnatal rats (Mienville & Pesold, 1999). Based on these observations, it may be postulated that CR cells in C57BL/6J mice are protected from the normal process of degeneration as they possess neither AMPA nor NMDA receptors, and thus would persist beyond the second postnatal week. However, we found no significant difference between numbers of reelin immunopositive cells at P21 in ICR, which express NMDA receptors, and C57BL/6J mice, which do not (C.-H. Chan & H. H. Yeh, unpublished observations). This suggests that NMDA receptors may not be the only factor in the disappearance of CR cells.
In the developing neocortex, ambient GABA and glutamate depolarize neurons by tonic activation of GABAA and NMDA receptors (LoTurco et al. 1991, 1995; Kim et al. 1995). However, such tonic activation has not been observed in CR cells (Mienville & Pesold, 1999; C.-H. Chan & H. H. Yeh, unpublished observations), arguing against the presence of ambient glutamate or GABA in vitro. Despite these observations, it is conceivable that in vivo these neurotransmitters may be released into the extracellular milieu of CR cells by deep layer pyramidal and nonpyramidal cells, or by other CR cells which are known to display immunoreactivity for glutamate (Del Rio et al. 1995) and GABA (Imamoto et al. 1994). In addition, during neonatal development, a GABAergic plexus is present in layer I, consisting of axons of layer I neurons and projections from the zona incerta that form synaptic contacts (Dammerman et al. 2000), further contributing to the release and accumulation of an ambient level of GABA in the extracellular space. Thus, in the presence of ambient GABA, NMDA receptor activation could lead to enhancement of GABAA receptor-mediated activity and the generation of overriding current transients. Given the persistent depolarizing action of GABA on CR cells, we postulate that GABAA receptors, either alone or in synergy with NMDA receptors, could contribute to the disappearance of CR cells.
Acknowledgments
The authors thank Drs John Parnavelas, Peter Shrager and Qing Cheng for critical reading of this manuscript and Ms Pamela Yeh for technical assistance. E4 was a kind gift from Dr Andre Goffinet. This work was in part supported by PHS grants NS41489 and NS24830.
REFERENCES
- Alcantara S, Ruiz M, D'Arcangelo G, Ezan F de L, Curran T, Sotelo C, Soriano E. Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cereb Cortex. 2000;10:899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- Behar TN, Scott CA, Greene CL, Wen X, Smith SV, Maric D, Liu QY, Colton CA, Barker JL. Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration. J Neurosci. 1999;19:4449–4461. doi: 10.1523/JNEUROSCI.19-11-04449.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM. Are autapses prodigal synapses? Curr Biol. 1997;8:R52–55. doi: 10.1016/s0960-9822(98)70033-8. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y. Developing networks play a similar melody. TrendsNeurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- Chen QX, Wong RK. Suppression of GABAA receptor responses by NMDA application in hippocampal neurones acutely isolated from the adult guinea-pig. J Physiol. 1995;482:353–362. doi: 10.1113/jphysiol.1995.sp020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, D'Arcangelo G. Role of reelin in the control of brain development. Brain Res Brain Res Rev. 1998;26:285–294. doi: 10.1016/s0165-0173(97)00035-0. [DOI] [PubMed] [Google Scholar]
- Dammerman RS, Flint AC, Noctor S, Kriegstein AR. An excitatory GABAergic plexus in developing neocortical layer 1. J Neurophysiol. 2000;84:428–434. doi: 10.1152/jn.2000.84.1.428. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- de Bergeyck V, Naerhuyzen B, Goffinet AM, Lambert de Rouvroit C. A panel of monoclonal antibodies against reelin, the extracellular matrix protein defective in reeler mutant mice. J Neurosci Methods. 1998;82:17–24. doi: 10.1016/s0165-0270(98)00024-7. [DOI] [PubMed] [Google Scholar]
- Del Rio J, Martinez A, Fonseca M, Auladell C, Soriano E. Glutamate-like immunoreactivity and fate of Cajal-Retzius cells in the murine cortex as identified with calretinin antibody. Cereb Cortex. 1995;5:13–21. doi: 10.1093/cercor/5.1.13. [DOI] [PubMed] [Google Scholar]
- Derer P, Derer M. Cajal-Retzius cell ontogenesis and death in mouse brain visualized with horseradish peroxidase and electron microscopy. Neuroscience. 1990;36:839–856. doi: 10.1016/0306-4522(90)90027-2. [DOI] [PubMed] [Google Scholar]
- Derer P, Derer M. Development of Cajal-Retzius cells in vivo and in vitro. In: Sharma S, Goffinet A, editors. Development of the Central Nervous System in Vertebrates. New York: Plenum Press; 1992. pp. 113–119. [Google Scholar]
- Edmunds SM, Parnavelas JG. Retzius-Cajal cells: an ultrastructural study in the developing visual cortex of the rat. J Neurocytol. 1982;11:427–446. doi: 10.1007/BF01257987. [DOI] [PubMed] [Google Scholar]
- Imamoto K, Karasawa N, Isomura G, Nagatsu I. Cajal-Retzius neurons identified by GABA immunohistochemistry in layer I of the rat cerebral cortex. Neurosci Res. 1994;20:101–105. doi: 10.1016/0168-0102(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Inoue M, Oomura Y, Yakushiji T, Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986;324:156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- Isokawa M. Modulation of GABAA receptor-mediated inhibition by postsynaptic calcium in epileptic hippocampal neurons. Brain Res. 1998;810:241–250. doi: 10.1016/s0006-8993(98)00922-6. [DOI] [PubMed] [Google Scholar]
- Kilb W, Luhmann HJ. Spontaneous GABAergic postsynaptic currents in Cajal-Retzius cells in neonatal rat cerebral cortex. Eur J Neurosci. 2001;13:1387–1390. doi: 10.1046/j.0953-816x.2001.01514.x. [DOI] [PubMed] [Google Scholar]
- Kim HG, Fox K, Connors BW. Properties of excitatory synaptic events in neurons of primary somatosensory cortex of neonatal rats. Cereb Cortex. 1995;5:148–157. doi: 10.1093/cercor/5.2.148. [DOI] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–574. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Loturco JJ, Blanton MG, Kriegstein AR. Initial expression and endogenous activation of NMDA channels in early neocortical development. J Neurosci. 1991;11:792–799. doi: 10.1523/JNEUROSCI.11-03-00792.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loturco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Lu SM, Zecevic N, Yeh HH. Distinct NMDA and AMPA receptor-mediated responses in mouse and human Cajal-Retzius cells. J Neurophysiol. 2001;86:2642–2646. doi: 10.1152/jn.2001.86.5.2642. [DOI] [PubMed] [Google Scholar]
- Mallamaci A, Mercurio S, Muzio L, Cecchi C, Pardini CL, Gruss P, Boncinelli E. The lack of Emx2 causes impairment of Reelin signaling and defects of neuronal migration in the developing cerebral cortex. J Neurosci. 2000;20:1109–1118. doi: 10.1523/JNEUROSCI.20-03-01109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko SM. Mechanism of modulation of GABA-activated current by internal calcium in rat central neurons. Brain Res. 1991;546:355–357. doi: 10.1016/0006-8993(91)91502-r. [DOI] [PubMed] [Google Scholar]
- Meyer G, Goffinet AM, Fairen A. What is a Cajal-Retzius cell? A reassessment of a classical cell type based on recent observations in the developing neocortex. Cereb Cortex. 1999;9:765–775. doi: 10.1093/cercor/9.8.765. [DOI] [PubMed] [Google Scholar]
- Mienville JM. Persistent depolarizing action of GABA in rat Cajal-Retzius cells. J Physiol. 1998;512:809–817. doi: 10.1111/j.1469-7793.1998.809bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mienville JM. Cajal-Retzius cell physiology: just in time to bridge the 20th century. Cereb Cortex. 1999;9:776–782. doi: 10.1093/cercor/9.8.776. [DOI] [PubMed] [Google Scholar]
- Mienville JM, Pesold C. Low resting potential and postnatal upregulation of NMDA receptors may cause Cajal-Retzius cell death. J Neurosci. 1999;19:1636–1646. doi: 10.1523/JNEUROSCI.19-05-01636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002a;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Developmental neurotransmitters? Neuron. 2002b;36:989–991. doi: 10.1016/s0896-6273(02)01136-4. [DOI] [PubMed] [Google Scholar]
- Parnavelas JG, Edmunds SM. Further evidence that Retzius-Cajal cells transform to nonpyramidal neurons in the developing rat visual cortex. J Neurocytol. 1983;12:863–871. doi: 10.1007/BF01258156. [DOI] [PubMed] [Google Scholar]
- Pouzat C, Marty A. Autaptic inhibitory currents recorded from interneurones in rat cerebellar slices. J. Physiol. 1998;509:777–783. doi: 10.1111/j.1469-7793.1998.777bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radnikow G, Feldmeyer D, Lubke J. Axonal projection, input and output synapses, and synaptic physiology of Cajal-Retzius cells in the developing rat neocortex. J Neurosci. 2002;22:6908–6919. doi: 10.1523/JNEUROSCI.22-16-06908.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstedt T, Linnarsson S, Wagner J, Lendahl U, Kokaia Z, Arenas E, Ernfors P, Ibanez CF. BDNF regulates reelin expression and Cajal-Retzius cell development in the cerebral cortex. Neuron. 1998;21:305–315. doi: 10.1016/s0896-6273(00)80540-1. [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Bernier B, Goffinet AM. Reelin mRNA expression during mouse brain development. Eur J Neurosci. 1997;9:1055–1071. doi: 10.1111/j.1460-9568.1997.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Rabinowitz D, Unni V, Kumar VS, Smetters DK, Tsiola A, Yuste R. Networks of coactive neurons in developing layer 1. Neuron. 1998;20:541–552. doi: 10.1016/s0896-6273(00)80993-9. [DOI] [PubMed] [Google Scholar]
- Super H, Del R, Martinez A, Perez-Sust P, Soriano E. Disruption of neuronal migration and radial glia in the developing cerebral cortex following ablation of Cajal-Retzius cells. Cereb Cortex. 2000;10:602–613. doi: 10.1093/cercor/10.6.602. [DOI] [PubMed] [Google Scholar]
- Wong RO, Ghosh A. Activity-dependent regulation of dendritic growth and patterning. Nat Rev Neurosci. 2002;3:803–812. doi: 10.1038/nrn941. [DOI] [PubMed] [Google Scholar]


