Abstract
Many smooth muscles display spontaneous electrical and mechanical activity, which persists in the absence of any stimulation. In the past this has been attributed largely to the properties of the smooth muscle cells. Now it appears that in several organs, particularly in the gastrointestinal tract, activity in smooth muscles arises from a separate group of cells, known as interstitial cells of Cajal (ICC), which are distributed amongst the smooth muscle cells. Thus in the gastrointestinal tract, a network of interstitial cells, usually located near the myenteric plexus, generates pacemaker potentials that are conducted passively into the adjacent muscle layers where they produce rhythmical membrane potential changes. The mechanical activity of most smooth muscle cells, can be altered by autonomic, or enteric, nerves innervating them. Previously it was thought that neuroeffector transmission occurred simply because neurally released transmitters acted on smooth muscle cells. However, in several, but not all, regions of the gastrointestinal tract, it appears that nerve terminals, rather than communicating directly with smooth muscle cells, preferentially form synapses with ICC and these relay information to neighbouring smooth muscle cells. Thus a set of ICC, which are distributed amongst the smooth muscle cells of the gut, are the targets of transmitters released by intrinsic enteric excitatory and inhibitory nerve terminals: in some regions of the gastrointestinal tract, the same set of ICC also augment the waves of depolarisation generated by pacemaker ICC. Similarly in the urethra, ICC, distributed amongst the smooth muscle cells, generate rhythmic activity and also appear to be the targets of autonomic nerve terminals.
Interstitial cells of Cajal (ICC) may be considered to be a specialised population of smooth muscle cells. Both arise from common mesenchymal cells (Lecoin et al. 1996; Young et al. 1996; Young, 1999). However, whereas smooth muscle cells develop an extensive array of contractile elements, ICC have few contractile elements but contain large numbers of mitochondria, an abundance of endoplasmic reticulum and distinct sets of channels in their membranes (Sanders, 1996). Many ICC express Kit, a tyrosine kinase receptor; this allows them to be recognised by their ability to bind antibodies to Kit (Sanders, 1996). Similarly ICC readily react with antibodies to vimentin whereas nearby smooth muscle cells do not (Rumessen & Thuneberg, 1996).
Many tissues, isolated from different regions of the gastrointestinal tract, contract rhythmically in the absence of neuronal or hormonal stimulation. When contractions and membrane potential are recorded simultaneously each contraction is seen to be triggered by a long lasting wave of depolarisation: because of their low frequency of occurrence and long duration, the waves of depolarisation have been termed slow waves (Tomita, 1981; Szurszewski, 1981). The origin and basis of the generation of slow waves have been debated for many years. It was initially thought that the generation of slow waves reflected some properties of gastrointestinal smooth muscle cells (Connor et al. 1974; El-Sharkaway & Daniel, 1975; Tomita, 1981) but studies on isolated smooth muscle cells have consistently failed to demonstrate a capability to generate slow wave activity (Farrugia, 1999). It has also long been recognised that the generation of slow waves does not rely on the sequential activation of voltage-dependent ion channels as do cardiac pacemaker cells. Rather, many early studies raised the possibility that rhythmical activity relied on the cycling of one or more metabolic processes within cells of the gut wall. Thus Prosser and his colleagues proposed that the generation of slow waves involved changes in the activity of the sodium pump (Connor et al. 1974). Subsequently Tomita and his colleagues suggested an involvement of glycolytic pathways, again assuming that pacemaking activity originated in smooth muscle cells (see Nakayama et al. 1997). Although ICC were first described in the intestine a century ago by Cajal, they were long viewed as an oddity. Their role in the generation of pacemaker activity in the gastrointestinal tract was suggested on the basis of histological studies (Fausonne-Pelligrini et al. 1977; Thuneberg, 1982). More recently, studies on mutants that lack subpopulations of ICC revealed their role in the generation of rhythmicity (Fig. 1A; Ward et al. 1994; Huizinga et al. 1995). Critically, whereas isolated smooth muscle cells rarely generate spontaneous electrical activity (Farrugia, 1999), isolated ICC invariably do (Langton et al. 1989; Tokutomi et al. 1995). Even more recently the key role played by ICC in communication between autonomic and enteric nerve terminals and many gut smooth muscle cells has become apparent (Burns et al. 1996; Ward et al. 2000a).
Figure 1. Pacemaker role of ICCMY in small intestine and gastric antrum.
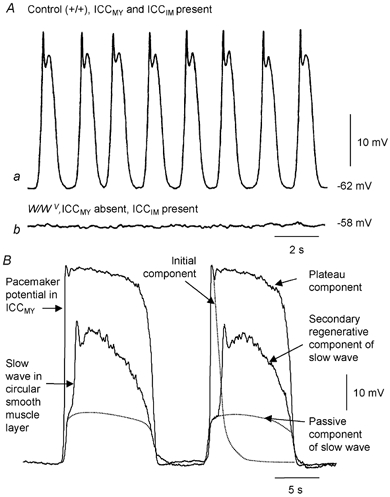
A shows intracellular recordings of electrical activity in the circular muscle of the small intestine of mice; both recordings are in the presence of nifedipine. Aa shows the regular discharge of slow waves recorded from a control tissue. Ab shows that slow waves are absent in the small intestine of W/WV mice: these tissues contain ICCDMP and normal smooth muscle cells but lack ICCMY. (Modified from Ward et al. 1994.) B shows simultaneous intracellular recordings from ICCMY and the circular muscle layer of guinea-pig gastric antrum. The onset of pacemaker depolarisation is seen to precede the onset of depolarisation in the circular muscle layer. Each pacemaker potential consist of two components, an initial component, followed by a plateau component. Furthermore each slow wave consists of two components, an initial passive component, whose time course is shown by a dotted line, and a secondary regenerative component, superimposed. (Modified from Hirst et al. 2002c.)
In the gastrointestinal tract at least three separate functional groups of ICC exist. In most regions of the gastrointestinal tract, a network of ICC lies in the myenteric region (ICCMY) between the circular and longitudinal muscle layers; ICCMY function as pacemaker cells and trigger slow waves. A second population of ICC (intramuscular ICC or ICCIM) are scattered amongst the smooth muscle cells. In the small intestine, ICCIM, rather than being widely distributed, are concentrated at the inner surface of the circular layer at the region of the deep muscular plexus, ICCDMP. In the antrum, ICCIM contribute to slow wave activity (Dickens et al. 2001) and throughout the stomach their principal role relates to the transfer of neuronal information to smooth muscle cells (Burns et al. 1996; Ward et al. 2000a). In larger animals a further population of ICC (ICCSEP) is distributed over the surface of muscle bundles and is said to have a septal location. ICCSEP serve, much like Pukinje fibres in the heart, to convey pacemaker activity deep into the muscle bundles making up the wall of the antrum (Horiguchi et al. 2001).
Experimental approaches used to study ICC
The electrical properties of ICC have been studied using several different techniques. Isolated ICC have been examined using conventional patch-clamp recording techniques. This approach, which has been applied to ICCMY, allows a description of the specific populations of ion channels present in their membranes (Langton et al. 1989; Lee & Sanders, 1993; Tokutomi et al. 1995) and an analysis of the cellular mechanisms which regulate the channels (Ward et al. 2000b; Koh et al. 2002). ICC are electrically coupled to neighbouring smooth muscle cells: any electrical activity ICC generate will produce a membrane potential change in the smooth muscle layers. With hindsight, simple intracellular recordings from smooth muscle cells in isolated segments of gastrointestinal tissues and isolated segments of urethra, after blocking smooth muscle L-type Ca2+ channels, record primarily the activity of the ICC in the tissues. The properties of ICCMY can be determined in situ, using sharp electrodes, allowing one to monitor the behaviour of populations of interconnected ICCMY and to determine how pacemaker potentials generate signals in adjacent smooth muscle layers (Dickens et al. 1999; Hirst & Edwards, 2001). A third method, used to study the properties of ICCIM, involves recording from small isolated segments of circular muscle; if dissected appropriately the preparations are isopotential and contain up to 2000 smooth muscle cells linked to up to 200 ICCIM. The membrane potential of both smooth muscle cells and ICCIM, can be varied over a limited range and the effects of nerve stimulation can be analysed (Suzuki & Hirst, 1999; Edwards et al. 1999; Hirst et al. 2002c; Suzuki et al. 2003). Finally, the use of mutant mice in which specific sets of ICC are either absent or dramatically reduced in numbers has allowed an evaluation of the physiological properties of tissues, with and without different sets of ICC. Taking W/WV mice as an example, in the small intestine, ICCMY are dramatically reduced in numbers whereas the distribution of ICCDMP remains little changed. Tissues containing ICCMY generate slow waves whereas those which lack ICCMY fail to generate slow waves (Fig. 1A; Ward et al. 1994, 1995; Huizinga et al. 1995). Conversely in the stomach of W/WV mice, ICCMY are retained but ICCIM are absent; in gastric tissues lacking ICCIM, neurotransmission is dramatically impaired (Burns et al. 1996; Ward et al. 2000a; Beckett et al. 2002; Suzuki et al. 2003).
Patterns of rhythmical electrical activity generated by ICC in the gastrointestinal tract
Slow waves, recorded from many regions of the gastrointestinal tract, persist when L-type Ca2+ channels are blocked (Ozaki et al. 1991; Suzuki & Hirst, 1999). Since gastrointestinal smooth muscle cells largely rely on L-type Ca2+ channels to generate inward currents (Ward & Sanders, 1992; Farrugia, 1999), the contribution made by smooth muscle cells themselves to slow wave activity is likely to be small.
In the antrum slow waves occur at 3-4 waves per minute (Dickens et al. 1999). Antral ICCMY, either in situ or isolated, generate large amplitude, long lasting pacemaker potentials (Dickens et al. 1999; Hirst et al. 2002a, Kim et al. 2002). Antral pacemaker potentials have two components, an initial rapid depolarising component which lasts for about 2 s, followed by a plateau component which lasts for about 8 s (Fig. 1B; Dickens et al. 1999; Hirst & Edwards, 2001; Hirst et al. 2002a; Kito et al. 2002a). Each pacemaker potential, generated by antral ICCMY, gives rise to an attenuated wave of depolarisation in the adjacent circular and longitudinal muscle layers (Dickens et al. 1999; Hirst & Edwards 2001). As the onset of depolarisation occurs in ICCMY before it occurs in either the circular or longitudinal muscle layers (Dickens et al. 1999; Hirst & Edwards, 2001), ICCMY clearly initiate slow waves in the antrum (Fig.1B). In the circular muscle layer each passive wave of depolarisation, originating in ICCMY, is augmented by a secondary regenerative component (Ohba et al. 1975; Dickens et al. 1999). In antral preparations, which contain ICCMY but lack ICCIM, the secondary component of the slow wave is absent (Fig. 2Ab; Dickens et al. 2001). Thus each antral slow wave reflects the sum of separate contributions from both ICCMY and ICCIM (Fig. 2Ab).
Figure 2. Generation of secondary regenerative component of antral slow waves by ICCIM located in the circular smooth muscle layer.

A schematic diagram showing a transverse section of the antral wall with an outer longitudinal layer (open circles at top), separated from the circular muscle layer (ellipsoids at bottom) by ICCMY. The circular layer consists of smooth muscle cells (dark shading) and ICCIM (light shading). Aa and b show intracellular recordings of electrical activity in the circular layer of the gastric antrum, from control and W/WV mice, respectively; both recordings are in the presence of nifedipine. Aa shows the regular discharge of slow waves recorded from a control tissue; each slow wave consists of a passive component, generated by ICCMY, and a secondary regenerative component (see Fig. 1). Ab shows that slow waves in the antrum of W/WV mice, which contain ICCMY but lack ICCIM, consist only of the passive component generated by ICCMY. (Modified from Dickens et al. 2001.) B shows traces which compare the time course of a regenerative potential (Ba), initiated by injecting depolarising current into a bundle of circular muscle (Bc), with the time course of the associated change in [Ca2+]i obtained by averaging 30 successive responses (Bb). Note that the onset of the increase in [Ca2+]i lags the onset of applied depolarisation by some 900 ms but corresponds with the start of the regenerative response. (Modified from Hirst et al. 2002b.)
In the mouse antrum the contributions from ICCMY and ICCIM show regional variation. Near the greater curvature, the density of ICCMY is very high and the depolarisation arising from ICCMY is large. The density of ICCMY falls as one moves from the greater to lesser curvature, and there is a corresponding fall in the depolarisation generated in the circular layer by ICCMY. However the regenerative contribution made by ICCIM is such that the amplitudes of slow waves remain unchanged over the region (Hirst et al. 2002a). Experimentally the regenerative component of the slow wave is initiated by depolarising single bundles of the circular muscle layer: these bundles contain ICCIM, but not ICCMY, which have been dissected away (Fig. 2B; Suzuki & Hirst, 1999). For convenience, these responses will be termed regenerative potentials. Regenerative potentials have a minimum latency of about 1 s after the onset of the applied depolarisation (Fig. 2Ba); each regenerative potential is associated with an increase in [Ca2+]i, whilst the preceding conditioning depolarisation itself fails to produce a detectable change in [Ca2+]i (Fig. 2Bb; Hirst et al. 2002b). Regenerative potentials are only initiated in bundles of circular muscle which contain ICCIM; they cannot be initiated in bundles taken from tissues which lack ICCIM (Suzuki et al. 2003).
Thus in summary, slow waves in the antrum of mice and guinea-pigs result from the combined activity of two separate populations of ICC. Pacemaker potentials are initiated in ICCMY. The wave of depolarisation conducts passively to the ICCIM and ICCIM respond to the pacemaker depolarisation by generating the secondary component of the slow wave which conducts to nearby circular smooth muscle cells.
In the small intestine slow waves occur at 10 to 40 waves per minute depending on species and location (Fig. 1A). In the small intestine of W/WV mice, where ICCMY are greatly reduced in number, slow waves are not detected (Fig. 1A; Ward et al. 1994). These observations suggest that in the small intestine, pacemaker activity is initiated by ICCMY and indicate that neither ICCDMP, which are retained in the small intestines of W/WV mice (Ward et al. 1994), nor smooth muscle cells are able to generate rhythmical activity. Furthermore if ICCDMP augment the depolarisation in the circular layer, as ICCIM do in the antrum, they must have different properties from those of antral ICCIM with antral regenerative potentials having refractory periods of some 5–10 s (Suzuki & Hirst, 1999). Recordings have been made from clusters of murine intestinal ICCMY, maintained in short term primary culture. Under these conditions intestinal ICCMY generate pacemaker currents with single monophasic components which last for some 1–2 s (Koh et al. 1998) and resemble the initial component of the antral pacemaker potential. Their waveforms and amplitudes are similar to those of slow waves recorded from intact tissue (Koh et al. 1998). This implies that, in situ, intestinal ICCMY generate sufficient current to passively depolarise the muscle layers without substantial change in waveform. These preliminary observations suggest that in the small intestine, unlike the antrum, slow waves may be generated by a single population of ICC, namely ICCMY. Unfortunately this idea has not been tested directly in the small intestine since recordings have not been made from intestinal ICCMYin situ. Indeed when changes in the internal Ca2+ levels ([Ca2+]i) have been monitored in murine ICCMY their time courses are often poorly correlated with those occurring in the longitudinal muscle layer (Yamazawa & Iino, 2002).
Cellular basis of electrical activity generated by ICC
At the outset, it should be stated that the cellular basis for the generation of electrical activity in ICC is controversial. This disagreement probably arises because ICC in different regions of the gut utilise different ion channels to generate electrical activity. In the mouse small intestine, pacemaker potentials, generated by cultured ICCMY, are monophasic (Koh et al. 1998). Intestinal pacemaker potentials are blocked by inhibitors of the mitochondrial Ca2+ transporter, by agents which block Ca2+ release from IP3-dependent Ca2+ stores (Ward et al. 2000b) and are partly blocked by low concentrations of agents which block Cl− channels (Koh et al. 1998). Intestinal ICCMY contain sets of cation-selective channels which are activated when [Ca2+]i falls to very low levels. These channels are blocked at normal [Ca2+]i levels and by Cl− channel antagonists, indicating that Cl− channel blockers cannot be used to distinguish between Ca2+-inhibited cation-selective channels and Cl− channels (Koh et al. 2002). It has been suggested that during each intestinal pacemaker potential, Ca2+ is released from an IP3-dependent store located at some distance from the ICC membrane. The local increase in [Ca2+]i near the inner mitochondrial membrane is thought to activate a generalised uptake of Ca2+ by the mitochondria so lowering the local concentration of Ca2+ near the ICC membrane. This local decrease in [Ca2+]i then activates cation-selective channels and a pacemaker potential ensues. As Ca2+ enters through the cation-selective channels, the local concentration of Ca2+ rises and the channels are inactivated, so terminating the pacemaker potential (Ward et al. 2000b).
Antral ICCMY and ICCIM generate spontaneous depolarisations in the absence of stimulation (Edwards et al. 1999; Hirst & Edwards 2001). In bundles of circular muscle containing ICCIM, the discharge of transient depolarisations is reflected as a ‘noisy’ resting membrane potential (Fig. 3Ba); when [Ca2+]i is buffered to low levels, individual depolarising potentials become readily detectable (Fig. 3Bb). These transient depolarising potentials have been termed unitary potentials (Edwards et al. 1999) or spontaneous transient depolarisations (van Helden et al. 2000) and are the membrane potential analogues in the antrum of the spontaneous transient inward currents detected in isolated urethral ICC (Sergeant et al. 2001b). For clarity the term unitary potential will be used to describe the subthreshold monophasic depolarisations generated spontaneously by antral ICC. It will be argued that much of the electrical activity generated by antral ICCMY or ICCIM results from changes in the frequency of occurrence of unitary potentials. Antral pacemaker potentials consist of two components, an initial component, which resembles the pacemaker potential generated by intestinal ICCMY, and a long lasting plateau component (Fig. 1A; Hirst & Edwards, 2001; Kim et al. 2002). The initial component of the pacemaker potential triggers the plateau component; the latter consists of a high frequency discharge of unitary potentials (Hirst & Edwards, 2001). Immediately after each pacemaker potential, the spontaneous discharge of unitary potentials falls to a very low rate and then gradually increases until it triggers the next pacemaker potential. The rate of generation of pacemaker potentials is slowed by chloride channel antagonists' at the same time the plateau component is abolished (Kito et al. 2002a). Similarly a decrease in [Cl−]o transiently increases both the rate of generation of pacemaker potentials and the amplitude of the plateau component (Hirst & Edwards, 2001). These observations suggest that unitary potentials, which both trigger the initial component of the pacemaker potential and give rise to the plateau component, result from the activation of Cl− channels (see Large & Wang, 1996). The membrane events underlying the initial component of antral pacemaker potentials are not understood. Antral ICCMY can be paced electrically and the rate of rise of the initial component is sensitive to small changes in [Ca2+]o (Hirst & Edwards, 2001; Hirst et al. 2002c). These observations suggest that the initial component is generated by voltage-dependent Ca2+ channels. Presumably they normally are activated when the discharge of unitary potentials causes the membrane potential ICCMY to reach threshold for the channels (Hirst & Edwards, 2001). The scheme does not explain the role of mitochondria, which clearly play a role in the maintenance of pacemaker activity in the antrum, with antral slow waves being abolished by inhibitors of mitochondrial Ca2+ uptake (Ward et al. 2000b).
Figure 3. Generation of electrical activity by antral ICC depends upon IP3 receptors and discharge of unitary potentials.
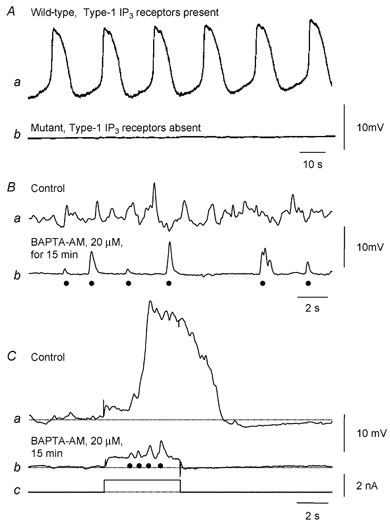
Aa and b show intracellular recordings of electrical activity in the circular layer of the gastric antrum, from control and mutant mice which lack type-1 IP3 receptors; both recordings were made in the presence of nifedipine. Aa shows the regular discharge of slow waves recorded from a control tissue. In contrast when recordings were made from tissues lacking type-1 IP3 receptors, slow waves were not detected (Ab). (Modified from Suzuki et al. 2001.) B shows membrane potential recordings from an isolated bundle of antral circular muscle from the guinea-pig. In control solution the resting potential displays an ongoing discharge of membrane noise (Ba); after buffering [Ca2+]i to low levels, individual unitary potentials (•) are detected (Bb). Ca shows a regenerative potential initiated in control solution. After buffering [Ca2+]i to low levels, depolarising currents (Cc) cause an increase in the frequency of occurrence of unitary potentials (•; Cb). (Modified from Edwards et al. 1999.)
Bundles of antral circular muscle, separated from ICCMY, generate regenerative responses spontaneously (Suzuki & Hirst, 1999; van Helden et al. 2000; Fukuta et al. 2002). The pattern is irregular and usually occurs at a lower frequency than that of ICCMY, indicating that ICCIM do not normally generate pacemaker activity in the intact tissue but are paced by ICCMY. A similar pattern of rhythmical activity is detected in the urethra (Hashitani & Edwards, 1999). It is not known whether the urethra has a dense anastomosing network of ICC, analogous to the ICCMY network found in the gastrointestinal tract. However isolated urethral ICC, but not smooth muscle cells, spontaneously generate unitary inward currents (Sergeant et al. 2000, 2001b). In the intact urethra each unitary potential increases the probability that another potential will occur. Consequently several depolarising potentials tend to occur in bursts and initiate a slow wave. Unlike the slow waves in most regions of the gastrointestinal tract, the slow wave in the urethra depends heavily upon the activation of L-type Ca2+ channels. Thus nifedipine abolishes the plateau component of the urethral slow wave and only the spontaneous potentials generated by ICC remain (Hashitani & Edwards, 1999).
In the circular muscle layer of the antrum, depolarisation transiently but dramatically increases the frequency of unitary potentials to give rise to a regenerative response (Fig. 3Ca). When [Ca2+]i is buffered to low levels so that the background frequency of unitary potentials is reduced, then the increase in frequency of unitary potentials produced by periods of depolarisation can be readily detected (Fig. 3Cb). The resting discharge of unitary potentials and regenerative responses are abolished by agents which interfere with mitochondrial Ca2+ uptake (Kito et al. 2002b), by 2-aminoethoxydiphenyl borate (2-APB) and by Cl− channel blockers, and by appropriate anion substitution (Hirst et al. 2002b), suggesting that Cl− channels are activated by Ca2+ released from IP3-dependent stores. In the urethra, unitary currents with pharmacological properties similar to unitary potentials generated by antral ICCIM, reverse at ECl and are similarly blocked by agents which block Ca2+-activated Cl− channels (Sergeant et al. 2001a,b). Together these observations suggest that ICCIM which can generate rhythmical activity rely upon Ca2+-activated Cl− channels to do so. It is to be stressed however that not all ICCIM generate rhythmical activity. In the fundus, ICCIM give rise to an ongoing discharge of unitary potentials (Beckett et al. 2002) but these unitary potentials show no tendency to co-ordinate their activity: moreover in the fundus the resting discharge of unitary potentials is not blocked by Cl− channel blockers (E. A. H. Beckett, S. M. Ward & G. D. S. Hirst, unpublished observations).
From the studies on different tissues, several common principles arise. Firstly rhythmical electrical activity generated by ICC, unlike the heart, does not depend entirely upon the activation of voltage-dependent ion channels. Rather the generation of electrical activity depends upon release into the cytoplasm of Ca2+ from IP3-dependent internal Ca2+ stores and the subsequent activation of ion-selective channels in the membranes of ICC. Thus the antrum is electrically silent in mutant mice lacking type-1 IP3 receptors (Fig. 3A; Suzuki et al. 2000), indicating that neither ICCMY nor ICCIM are functioning. Similarly slow waves are abolished by 2-APB (Hirst & Edwards, 2001), an inhibitor of IP3-induced Ca2+ release (Maruyama et al. 1997); by xestospongin, a membrane permeable inhibitor of IP3-dependent stores; and, in cultured ICCMY networks, by heparin placed in the patch pipette (Ward et al. 2000b). On the other hand, Ca2+ release into the cytoplasm from stores linked to ryanodine receptors appears to make little or no contribution to slow wave activity (Chowdhury et al. 1995; Ward et al. 2000b). Secondly cytosolic Ca2+ concentrations vary in a pulsatile manner and each local increase in [Ca2+]i is thought to either directly (Hirst & Edwards, 2001; Kito et al. 2002a) or indirectly (Koh et al. 2002) trigger the opening of sets of membrane channels, so giving rise to a unitary potential. Thirdly, mitochondria play a key part in regulating cytosolic [Ca2+]i in ICC. Thus during pacemaker activity in cultured networks of intestinal ICC, periodic increases in the concentration of Ca2+ within mitochondria are detected. Furthermore slow waves and regenerative potentials are abolished in several tissues by agents which impair Ca2+ handling by mitochondria (Ward et al. 2000b).
How individual ICC co-ordinate their activity with their neighbours is not understood. In the gastrointestinal tract, ICCMY form an interconnected network of cells, with numerous gap-junctions between neighbouring ICCMY (Sanders, 1996) that allow injected dyes to pass between cells (Dickens et al. 1999). It has been suggested that in antral ICCMY the initial voltage-dependent component co-ordinates activity between cells (Hirst & Edwards, 2001). In the urethra, although each unitary potential increases the probability that another will occur, the rate of discharge of unitary potentials in isolated cells appears to be independent of membrane potential (Sergeant et al. 2001b). The spread of pacemaker potentials in different regions of the GI tract occurs in an anisotropic manner, conducting more rapidly circumferentially than longitudinally. However the distribution of Kit immunoreactivity does not reveal a polarisation in ICC pacemaker networks and the mechanism underlying the behaviour is not understood.
Involvement of ICC in neuroeffector transmission
Most muscular organs containing smooth muscle cells are innervated by post-ganglionic axons which arise from either sympathetic, parasympathetic or enteric ganglion cells. In the gastrointestinal tract, muscle layers are often innervated by both excitatory and inhibitory projections. The traditional view has been that sympathetic, parasympathetic and enteric nerve varicosities release transmitter and the transmitter diffuses through the extracellular fluid directly activating any receptors present on the surface of the smooth muscle cells. However when this hypothesis has been tested directly it has only rarely been found to be correct. In many tissues, autonomic varicosities form organised neuroeffector junctions with nearby smooth muscle cells and neurally released transmitters appear to activate specific sets of postsynaptic receptors located close to the sites of transmitter release (Hirst et al. 1996). This also appears to be the case in the longitudinal muscle layer of the small intestine where neurally released and applied acetylcholine (ACh) activate different sets of muscarinic receptors on the smooth muscle cells (Cousins et al. 1995; Klemm, 1995). However several lines of evidence now suggest that, in several regions of the gastrointestinal tract, the specific sets of receptors activated by neurally released transmitter are not on smooth muscle cells but are located on ICCIM, in the stomach, and on ICCDMP, in the small intestine.
The idea that ICCIM were intermediaries in neural control pathways initially arose from studies which examined the relationship between enteric nerve terminals and ICCIM (Daniel, 1985). Their key role was demonstrated when neuroffector transmission in the stomach of W/WV mice was analysed. In the fundus of control mice, simultaneous stimulation of excitatory and inhibitory nerves evoked a complex response consisting of cholinergic excitatory and nitrergic inhibitory components. Surprisingly in the fundus of W/WV mice, where ICCIM were absent, both the excitatory and inhibitory components were greatly attenuated (Burns et al. 1996; Ward et al. 2000a) even though enteric varicosities were present and capable of releasing transmitters (Ward et al. 2000a). More recently a similar finding was made using tissues isolated from Steel mutant mice (Sl/Sld mice) where ICCIM are also absent from the stomach (Beckett et al. 2002). In the fundus of control mice, nerve stimulation evoked a biphasic response consisting of an excitatory junction potential (EJP), followed by an inhibitory junction potential (IJP; Fig. 4Aa). The IJP was abolished by Nω-nitro-L-arginine (L-NA), an inhibitor of NO production, suggesting that fundal IJPs largely result from the release of NO. In the presence of L-NA, nerve stimulation evoked an EJP (Fig. 4Ab) whose amplitude was only slightly increased by inhibiting cholinersterases with neostigmine (Fig. 4Ac). However in neostigmine, the cholinergic EJP was followed by a slower depolarisation (Fig. 4Ac). Both the slow response and the EJP were abolished by atropine (Fig. 4Ad). As with other tissues where ICCIM are present, the resting membrane potential displays an ongoing discharge of membrane noise, resulting from the random occurrence of unitary potentials (Fig. 4A). In contrast when similar recordings were made from the fundus of Sl/Sld mutants, nerve stimulation failed to evoke either an EJP or an IJP (Fig. 4Ba and b); at the same time membrane noise was absent (Fig. 4B). Clearly the cholinergic nerve terminals were functional, since in the presence of neostigmine an atropine-sensitive slow depolarisation was detected (Fig. 4Bc and d). The simplest explanation for these findings is that both inhibitory and excitatory nerve terminals selectively target ICCIM. When these cells are absent insufficient transmitter is released to activate ‘extra-junctional' receptors located on nearby smooth muscle cells. However, when the hydrolysis of ACh was inhibited then sufficient ACh diffused to the smooth cells where it activated muscarinic receptors, presumably with slower activation kinetics (see also Cousins et al. 1995). Qualitatively similar observations have been made on the circular layer of the mutant mouse antrum. In the control antrum, nerve stimulation evokes a complex IJP, consisting of an initial apamin-sensitive IJP and a slower nitrergic IJP; the inhibitory responses are followed by an atropine-sensitive excitatory response. In the antrum of W/WV mice, which lack ICCIM, although the initial apamin-sensitive component remained, both the nitrergic and cholinergic responses were absent (Suzuki et al. 2003).
Figure 4. Involvement of ICCIM in responses to inhibitory and excitatory nerve stimulation in the stomach.

Nerve stimulation evoked a biphasic response consisting of an EJP, followed by an IJP in the fundus of control mice (Aa). After abolishing the IJP with Nω-nitro-L-arginine (L-NA), nerve stimulation evoked an EJP (4Ab) whose amplitude was little changed by inhibiting cholinesterases with neostigmine (Ac); in neostigmine, the cholinergic EJP was followed by a slower depolarisation (Ac). Both excitatory responses were abolished by atropine (Ad). When similar recordings were made from Sl/Sld mutants, which lack ICCIM, nerve stimulation failed to evoke either an EJP or an IJP (Ba and b) but in the presence of neostigmine an atropine-sensitive slow depolarisation was detected (Bc and d; modified from Beckett et al. 2003). In an isolated bundle of circular muscle, dissected from guinea-pig antrum, after abolishing the effects of inhibitory nerve stimulation with apamin and L-NA, excitatory nerve stimulation evoked a regenerative potential (Ca). The regenerative potential, but not the response of smooth muscle cells to neurally released ACh, was abolished by hyperpolarisation (Ca). The excitatory response involving ICCIM was abolished by anthracene-9-carboxylic acid (9-AC) but the response of smooth muscle cells to neurally released ACh persisted (Cb). (Modified from Hirst et al. 2002c.)
The ideas derived from studies on murine tissues deficient in ICCIM are supported by analyses of neuroeffector transmission in guinea-pig antrum. In the circular layer of the gastric antrum, after the effects of inhibitory transmitters had been abolished pharmacologically, cholinergic nerve stimulation initiated regenerative responses identical to those initiated when ICCIM are depolarised (Fig. 4C; Hirst et al. 2002c). The responses, both to neurally released ACh and to direct depolarisation of ICCIM, depend upon the release of Ca2+ from IP3-dependent stores and the activation of Cl− channels (Fig. 4Cb). A small proportion of neurally released ACh appears to access the smooth muscle layer directly; there it triggers a rapid depolarisation that involves neither Cl− channels nor Ca2+ release from IP3-dependent stores (Fig. 4Cb). Furthermore the neural pathway which involved ICCIM included a voltage-dependent step, such that a moderate hyperpolarisation blocked activation of the pathway (Fig. 4Ca); in contrast the responses to neurally released ACh on smooth muscle were little affected by membrane hyperpolarisation (Fig. 4Ca). These experiments show that most functional excitatory neuronal connections in the antrum involve ICCIM, with neurally released ACh increasing the rate of discharge of unitary potentials so that a regenerative potential is initiated. Moreover in intact tissues excitatory neural activity increases the frequency of discharge of regenerative potentials so that ICCIM become the dominant pacemaker cells (Hirst et al. 2002c). Similarly neurally released nitric oxide selectively targets ICCIM (Suzuki et al. 2003). An analysis of nitrergic IJPs in the antrum indicates that neurally released NO suppresses the resting discharge of unitary potentials (Suzuki et al. 2003). Since unitary potentials result from an increase in chloride conductance (gCl), nitrergic hyperpolarisation manifests itself as a suppression of gCl (Suzuki et al. 2003; see also Crist et al. 1991a,b; Zhang & Paterson, 2002). In the urethra, ICCIM also appear to be intermediaries in the process of neuroeffector transmission. There the contractions evoked by noradrenergic nerve stimulation also involve Cl− channels (Sergeant et al. 2002) with added noradrenaline activating a different pathway presumably located in urethral smooth muscle cells (G. Sergeant personal communication).
Summary
The role of ICC in the control of gastrointestinal motility has been demonstrated using a range of different approaches. The studies suggest that although smooth muscle cells are the final mediators of contraction, their contribution to rhythmical electrical activity and their direct involvement in neuroeffector transmission is minimal. Recordings from mutant mice, in which selected sets of ICC are missing, have shown that in the small intestine ICCMY are essential for the generation of pacemaker activity. Similarly, recordings from the stomachs of such mice, where ICCMY are present but ICCIM are absent, have revealed roles for ICCIM in both excitatory and inhibitory neuroeffector transmission. Direct recordings of antral ICCMYin situ have shown that they generate pacemaker potentials which depolarise the adjacent muscle layers. Analyses of neuroeffector transmission in the stomach have shown that neurally released transmitters produce effects that can be attributed almost entirely to the transmitters acting solely on ICCIM.
Acknowledgments
We are grateful to Professors G. Campbell and R. A. R. Bywater, Drs N. J. Bramich, H. M. Cousins and F. R. Edwards for their helpful comments on the manuscript. This project was supported by a grant to G.D.S.H. from the Australian NH&MRC; S.M.W. received support from the NIH, research grant DK 57236.
REFERENCES
- Beckett EAH, Horiguchi K, Khoyi M, Sanders KM, Ward SM. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sld mice. J Physiol. 2002;543:871–887. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Acad Sci U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury JU, Pang YW, Huang SM, Tsugeno M, Tomita T. Sustained contraction produced by caffeine after ryanodine treatment in the circular muscle of the guinea-pig gastric antrum and rabbit portal vein. Br J Pharmacol. 1995;114:1414–1418. doi: 10.1111/j.1476-5381.1995.tb13363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Prosser CL, Weems WA. A study of pace-maker activity in intestinal smooth muscle. J Physiol. 1974;240:671–701. doi: 10.1113/jphysiol.1974.sp010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins HM, Edwards FR, Hirst GD. Neuronally released and applied acetylcholine on the longitudinal muscle of the guinea-pig ileum. Neuroscience. 1995;65:193–207. doi: 10.1016/0306-4522(94)00466-i. [DOI] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Chloride-mediated junction potentials in circular muscle of the guinea pig ileum. Am J Physiol. 1991a;261:G742–751. doi: 10.1152/ajpgi.1991.261.5.G742. [DOI] [PubMed] [Google Scholar]
- Crist JR, He XD, Goyal RK. Chloride-mediated inhibitory junction potentials in opossum esophageal circular smooth muscle. Am J Physiol. 1991b;261:G752–762. doi: 10.1152/ajpgi.1991.261.5.G752. [DOI] [PubMed] [Google Scholar]
- Daniel EE. Nonadrenergic, noncholinergic (NANC) neuronal inhibitory interactions with smooth muscle. In: Grover AK, Daniel EE, editors. Calcium and Contractility: Smooth Muscle. Clifton, NJ, USA: Humana Press; 1985. pp. 427–436. [Google Scholar]
- Dickens EJ, Edwards FR, Hirst GDS. Selective knockout of intramuscular interstitial cells reveals their role in the generation of slow waves in mouse stomach. J Physiol. 2001;531:827–833. doi: 10.1111/j.1469-7793.2001.0827h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GDS, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GDS, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. J Physiol. 1999;519:235–250. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkaway TY, Daniel EE. Ionic mechanisms of the control of intestinal electrical activity. Am J Physiol. 1975;229:1287–1298. doi: 10.1152/ajplegacy.1975.229.5.1287. [DOI] [PubMed] [Google Scholar]
- Farrugia G. Ionic conductances in gastrointestinal smooth muscles and interstitial cells of Cajal. Annu Rev Physiol. 1999;61:45–84. doi: 10.1146/annurev.physiol.61.1.45. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini M-S, Cortesini C, Romagnoli P. Sull' ultrastruttura della tunica muscolare della prozione cardiale dell' esofago e dello stomaco umano con particolare riferimento alle cosiddette cellule inerstiziali di Cajal. Arch Ital Anat Embriol. 1977;82:157–177. [PubMed] [Google Scholar]
- Fukuta H, Kito Y, Suzuki H. Spontaneous electrical activity and associated changes in calcium concentration in guinea-pig gastric smooth muscle. J Physiol. 2002;540:249–260. doi: 10.1113/jphysiol.2001.013306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. J Physiol. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Beckett EAH, Sanders KM, Ward SM. Regional variation in contribution of myenteric and intramuscular interstitial cells of Cajal to generation of slow waves in mouse gastric antrum. J Physiol. 2002a;540:1003–1012. doi: 10.1113/jphysiol.2001.013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Bramich NJ, Teramoto N, Suzuki H, Edwards FR. Regenerative component of slow waves in the guinea-pig gastric antrum involves a delayed increase in [Ca2+]i and Cl− channels. J Physiol. 2002b;540:907–919. doi: 10.1113/jphysiol.2001.014803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Choate JK, Cousins HM, Edwards FR, Klemm MF. Transmission by post-ganglionic axons of the autonomic nervous system: the importance of the specialized neuroeffector junction. Neuroscience. 1996;73:7–23. doi: 10.1016/0306-4522(96)00031-0. [DOI] [PubMed] [Google Scholar]
- Hirst GDS, Dickens EJ, Edwards FR. Pacemaker shift in the gastric antrum of guinea-pigs produced by excitatory vagal stimulation involves intramuscular interstitial cells. J Physiol. 2002c;541:917–928. doi: 10.1113/jphysiol.2002.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Edwards FR. Generation of slow waves in the antral region of guinea-pig stomach - a stochastic process. J Physiol. 2001;535:165–180. doi: 10.1111/j.1469-7793.2001.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi K, Semple GS, Sanders KM, Ward SM. Distribution of pacemaker function through the tunica muscularis of the canine gastric antrum. J Physiol. 2001;537:237–250. doi: 10.1111/j.1469-7793.2001.0237k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Kim TW, Beckett EA, Hanna R, Koh SD, Ordog T, Ward SM, Sanders KM. Regulation of pacemaker frequency in the murine gastric antrum. J Physiol. 2002;538:145–157. doi: 10.1113/jphysiol.2001.012765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito Y, Fukuta H, Suzuki H. Components of pacemaker potentials recorded from the guinea pig stomach antrum. Pflugers Arch. 2002a;445:202–217. doi: 10.1007/s00424-002-0884-z. [DOI] [PubMed] [Google Scholar]
- Kito Y, Fukuta H, Yamamoto Y, Suzuki H. Excitation of smooth muscles isolated from the guinea-pig gastric antrum in response to depolarization. J Physiol. 2002b;543:155–167. doi: 10.1113/jphysiol.2002.020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm MF. Neuromuscular junctions made by nerve fibres supplying the longitudinal muscle of the guinea-pig. J Auton Nerv Syst. 1995;55:155–164. doi: 10.1016/0165-1838(95)00036-w. [DOI] [PubMed] [Google Scholar]
- Koh SD, Jun JY, Kim TW, Sanders KM. A Ca2+-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002;540:803–814. doi: 10.1113/jphysiol.2001.014639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Sanders KM, ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of cajal from the murine small intestine. J Physiol. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci U S A. 1989;86:7280–7284. doi: 10.1073/pnas.86.18.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large WA, Wang Q. Charactersitics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol. 1996;271:C435–454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Lecoin L, Gabella G, Le Douarin N. Origin of the c-kit-positive interstitial cells in the avian bowel. Development. 1996;122:725–733. doi: 10.1242/dev.122.3.725. [DOI] [PubMed] [Google Scholar]
- Lee HK, Sanders KM. Comparison of ionic currents from interstitial cells and smooth muscle cells of canine colon. J Physiol. 1993;460:135–152. doi: 10.1113/jphysiol.1993.sp019463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Nakayama S, Chihara S, Clark JF, Huang SM, Horiuchi T, Tomita T. Consequences of metabolic inhibition in smooth muscle isolated from guinea-pig stomach. J Physiol. 1997;505:229–240. doi: 10.1111/j.1469-7793.1997.229bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M, Sakamoto Y, Tomita T. The slow wave in the circular muscle of the guinea-pig stomach. J Physiol. 1975;253:505–516. doi: 10.1113/jphysiol.1975.sp011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Stevens RJ, Blondfield DP, Publicover NG, Sanders KM. Simultaneous measurement of membrane potential, cytosolic Ca2+, and tension in intact smooth muscles. Am J Physiol. 1991;260:C917–925. doi: 10.1152/ajpcell.1991.260.5.C917. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ, Thuneberg L. Pacemaker cells in the gastrointestinal tract: interstitial cells of Cajal. Scand J Gastroenterol. 1996;216(suppl.):82–94. doi: 10.3109/00365529609094564. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, McHale NG, Thornbury KD. Role of IP3 in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am J Physiol Cell Physiol. 2001a;280:C1349–1356. doi: 10.1152/ajpcell.2001.280.5.C1349. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Spontaneous Ca2+ activated Cl− currents in isolated urethral smooth muscle cells. J Urol. 2001b;166:1161–1166. [PubMed] [Google Scholar]
- Sergeant GP, Thornbury KD, McHale NG, Hollywood MA. Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am J Physiol. 2002;283:C885–894. doi: 10.1152/ajpcell.00085.2002. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Hirst GDS. Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J Physiol. 1999;517:563–573. doi: 10.1111/j.1469-7793.1999.0563t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Ward SM, Bayguinov YR, Edwards FR, Hirst GDS. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J Physiol. 2003;546:751–763. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski JH. Electrical basis for gastrointestinal motility. In: Johnson R, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1981. pp. 1435–1465. [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells. Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- Tomita T. Electrical activity (spikes and slow waves) in gastrointestinal smooth muscles. In: Bulbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle: An Assessment of Current Knowledge. London: Edward Arnold; 1981. pp. 127–156. [Google Scholar]
- van Helden DF, Imtiaz MS, Nurgaliyeva K, Von Der Weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J Physiol. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Beckett EA, Wang X, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000a;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in Steel mutants. Am J Physiol. 1995;269:C1577–1585. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000b;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Upstroke component of electrical slow waves in canine colonic smooth muscle due to nifedipine-resistant calcium current. J Physiol. 1992;455:321–337. doi: 10.1113/jphysiol.1992.sp019304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazawa T, Iino M. Simultaneous imaging of Ca2+ signals in interstitial cells of Cajal and longitudinal smooth muscle cells during rhythmic activity in mouse ileum. J Physiol. 2002;538:823–835. doi: 10.1113/jphysiol.2001.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HM. Embryological origin of interstitial cells of Cajal. Microsc Res Tech. 1999;47:303–308. doi: 10.1002/(SICI)1097-0029(19991201)47:5<303::AID-JEMT1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Young HM, Ciampoli D, Southwell BR, Newgreen DF. Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol. 1996;180:97–107. doi: 10.1006/dbio.1996.0287. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Patterson WG. Role of Ca2+-activated Cl− channels and MLCK in slow IJP in opossum esophageal smooth muscle. Am J Physiol. 2002;283:G104–114. doi: 10.1152/ajpgi.00052.2002. [DOI] [PubMed] [Google Scholar]


