Abstract
Nutrients in the intestine initiate changes in secretory and motor function of the gastrointestinal (GI) tract. The nature of the ‘sensors’ in the intestinal wall is not well characterized. Intestinal lipid stimulates the release of cholecystokinin (CCK) from mucosal entero-endocrine cells, and it is proposed that CCK activates CCK A receptors on vagal afferent nerve terminals. There is evidence that chylomicron components are involved in this lipid transduction pathway. The aim of the present study was to determine (1) the pathway mediating reflex inhibition of gastric motility and (2) activation of duodenal vagal afferents in response to chylomicrons. Mesenteric lymph was obtained from awake rats fitted with lymph fistulas during intestinal perfusion of lipid (Intralipid, 170 μmol h−1, chylous lymph) or a dextrose and/or electrolyte solution (control lymph). Inhibition of gastric motility was measured manometrically in urethane-anaesthetized recipient rats in response to intra-arterial injection of lymph close to the upper GI tract. Chylous lymph was significantly more potent than control lymph in inhibiting gastric motility. Functional vagal deafferentation by perineural capsaicin or CCK A receptor antagonist (devazepide, 1 mg kg−1, I.V.) significantly reduced chylous lymph-induced inhibition of gastric motility. The discharge of duodenal vagal afferent fibres was recorded from the dorsal abdominal vagus nerve in an in vitro preparation of the duodenum. Duodenal vagal afferent nerve fibre discharge was significantly increased by close-arterial injection of CCK (1–100 pmol) in 43 of 83 units tested. The discharge of 88 % of CCK-responsive fibres was increased by close-arterial injection of chylous lymph; devazepide (100 μg, I.A.) abolished the afferent response to chylous lymph in 83 % of these units. These data suggest that in the intestinal mucosa, chylomicrons or their products release endogenous CCK which activates CCK A receptors on vagal afferent nerve fibre terminals, which in turn initiate a vago-vagal reflex inhibition of gastric motor function.
Perfusion of lipid into the intestine initiates changes in secretory and motor function of the gastrointestinal (GI) tract that are part of the response described as intestinal feedback inhibition of gastric function. Work from a number of groups over the last decade has established that intestinal feedback involves extrinsic pathways to the GI tract and release of paracrine or neurocrine substances from entero-endocrine cells in the gut mucosa. Inhibition of gastric emptying and gastric acid secretion in response to intestinal lipid is mediated by a cholecystokinin A (CCK A) receptor and vagal capsaicin-sensitive pathway (Lloyd et al. 1993, Holzer et al. 1994). Lipid in the small intestine stimulates the release of CCK from endocrine cells (Liddle, 1997); earlier observations suggesting that only long chain triglyceride is effective in releasing CCK have recently been confirmed and extended to show that only fatty acids of chain length C10 and above and with a free carboxy group will release CCK from intestinal mucosa endocrine cells (McLaughlin et al. 1998, 1999; Sidhu et al. 2000).
The mechanism of sensory transduction of long chain fatty acid in the intestinal lumen to activation of vagal afferent nerve activity is unclear. Lipid is digested intraluminally mainly to free fatty acid and 2-monoglyceride. The digestion products, with chain length equal to, or greater than, C12 are absorbed into enterocytes where they are resynthesized into triglyceride, packed into chylomicrons, removed from the enterocyte by exocytosis, and transported via the mesenteric lymph to the systemic circulation (Tso & Balint, 1986). Fatty acids with chain lengths of less than C10 diffuse out of the enterocyte and are transported via the portal blood to the liver, bound to albumin. There is evidence that long chain fatty acids can have a direct effect on CCK endocrine cells (McLaughlin et al. 1998), and this pool of CCK could activate CCK A receptors located on vagal afferent terminals in the intestinal mucosa. Recently, we have shown that the lipid-induced inhibition of gastric emptying is dependent on chylomicron formation (Raybould et al. 1998). Inhibition of chylomicron formation by the surfactant L-81 attenuated the ability of lumenal long chain triglyceride to inhibit gastric emptying. Further evidence for a role for chylomicrons comes from experiments in which donor lymph from lipid-fed animals was able to inhibit gastric motility in recipient rats, an effect that could not be accounted for by the triglyceride content of the lymph alone (Glatzle et al. 2002). Moreover, inhibition of chylomicron formation in the donor rats completely abolished the ability of lymph to inhibit gastric motor function in the recipient rats. These data suggest that the formation or secretion of chylomicrons from epithelial cells is important in the sensory transduction process.
The vagus nerve conveys information from the gut to the brainstem and this afferent information elicits vago-vagal reflexes important for the control of postprandial gastrointestinal function (Grundy & Scratcherd, 1989). Electrophysiological studies have confirmed the existence of vagal mucosal afferents that respond to a number of different stimuli, including mechanical, chemical and osmotic stimuli. In addition, vagal afferent activity can be activated by paracrine mediators, including CCK and 5-hydroxytryptamine (5-HT; Blackshaw & Grundy, 1990, 1993; Hillsley et al. 1998). These observations provide the electrophysiological support for the conclusions of functional experiments showing vagal afferent-mediated changes in gastrointestinal motility and secretion in response to mucosal stimulation and paracrine mediators. Recordings have been made from vagal afferents whose discharge is increased by lumenal perfusion of fatty acids (Lal et al. 2001). Intestinal oleate perfusion stimulated the discharge of vagal afferent fibres innervating both the jejunum and the ileum of the rat (Randich et al. 2000). This effect was dependent on the formation of chylomicrons, since L-81, a surfactant that inhibits chylomicron formation, blocked vagal afferent nerve discharge induced by intestinal oleate (Randich et al. 2000). Jejunal vagal afferent nerve discharge in response to intraluminal lipid infusion is mediated through different pathways, depending on the chain length of the infused lipid. Intraluminal long chain fatty acids activated vagal afferents of the rat jejunum via a CCK-dependent mechanism, whereas short chain fatty acids appeared to have a direct effect on vagal afferent terminals (Lal et al. 2001).
In the present study, we tested the hypothesis that chylomicrons contain the active component that is required for endogenous CCK release and activation of vagal capsaicin-sensitive afferent nerve fibres to initiate intestinal feedback in response to lipid. The hypothesis was tested by determining the pathway by which chylous lymph, obtained from rats actively absorbing lipid, produces reflex inhibition of gastric motility and by determining the effect of chylous lymph on the neuronal activity of duodenal vagal afferent fibres.
METHODS
Animals
Male Sprague Dawley rats (200-250 g and 300-350 g, Harlan Industries, San Diego, CA, USA) maintained on regular laboratory chow were housed under controlled conditions of illumination (12:12 h light:dark cycle starting at 07:00), humidity and temperature (21 °C). Rats were fasted overnight but allowed water ad libitum before all surgical and experimental procedures. Animals were killed by an overdose of sodium pentobarbital (100 mg kg−1 I.P.) followed by bilateral thoracotomy. The institutional guidelines for the care and use of laboratory animals (UC Davis Animal Use and Care Administrative Advisory Committee, Office of the Campus Veterinarian) were followed throughout the study.
Drugs and chemicals
For intra-arterial administration, CCK-8 (Research Biochemicals International, Natick, MA, USA) was dissolved in distilled water to make 20 pmol ml−1, 100 pmol ml−1 and 1 nmol ml−1 stock solutions, which were stored at −20 °C, and diluted immediately before use with phophate buffered saline (PBS). 2-Methyl-5-hydroxytryptamine (2-methyl-5-HT; Sigma, St Louis, MO, USA) was dissolved in physiological saline (0.9 % NaCl) and used at a final concentration from 0.1 to 100 nmol ml−1. The CCK A receptor antagonist devazepide (MK 329; Merck Sharp & Dohme, Rahway, NJ, USA), was prepared by dissolving 10 mg in 0.1 ml dimethyl sulfoxide (DMSO, Sigma), adding 0.1 ml Tween 80 (Sigma), followed by 0.8 ml physiological saline. This stock solution was diluted in physiological saline to achieve a final concentration of 1 mg ml−1. The 5-HT3R antagonist ondansetron (Glaxo, Greenford, Middx, UK) was dissolved in physiological saline to achieve a final concentration of 100 μg ml−1. Capsaicin (Sigma), 10 mg, was sonicated in 100 μl Tween 80 (Sigma) for 15 min, 0.9 ml of olive oil was added, and the suspension was sonicated for a further 10 min. Normal rat saline was made as previously described (mM, 140 NaCl, 5 KCl, 1 MgCl2, 1.3 Na2HPO4, 5 Hepes, 2 CaCl2 and 10 D-glucose; pH 7.38 ± 0.02) (Adelson et al. 1996).
Animal preparations
Perineural application of capsaicin to the vagus nerve
This method has been previously published (Raybould & Tache, 1988). Briefly, rats were anaesthetized with sodium pentobarbital (60 mg kg−1, I.P.). The carotid arteries were exposed by a midline neck incision and the vagus nerve was carefully separated from the carotid arteries, for a distance of 3-4 mm. A strip of parafilm (American National Can, Chicago, IL, USA) was placed under the nerve, the nerve was wrapped with a piece of cotton wool and the surrounding tissue was covered with parafilm to prevent spread of capsaicin. One drop of capsaicin 1 % or vehicle (10 %Tween 80 in olive oil) was applied on each vagus nerve for 30 min. At 10 min intervals the nerve was swapped and capsaicin was reapplied. After application the area was thoroughly rinsed with sterile saline and the incision was closed. Animals were used 10 days after treatment.
Collection of mesenteric lymph
Surgical preparation and lymph collection were performed as previously described (Glatzle et al. 2002). Briefly, rats (300-350 g) were anaesthetized with methohexital sodium (60 mg kg−1, I.P.; Brevital, Jones Pharma Inc., St Louis, MO, USA). The mesenteric lymph duct was cannulated with a PVC tube (Medical Grade, 0.50 mm i.d., 0.80 mm o.d., Dural Plastics and Engineering, Dural, Australia), fixed in place with a drop of ethyl cyanoacrylate glue (Krazy Glue, Elmers Products Inc., Columbus, OH, USA) and externalized through a insicion in the right flank. A second cannula (Silastic 1 mm i.d., 2.15 mm o.d.) was passed through the fundus of the stomach, extended 3 cm into the duodenum, secured in place with a silk suture and additionally fixed with a drop of ethyl cyanoacrylate glue and externalized. After surgery, rats were placed in Bollman cages and allowed to recover and a glucose-saline solution (glucose 0.2 M, NaCl 145 mM and KCL 4 mM) was perfused continuously through the duodenal cannula at a rate of 3 ml h−1 for 24 h during the recovery period, to ensure hydration, electrolyte and nutritional maintenance.
Control lymph was collected from rats after the 24 h recovery period for 2 h during glucose-saline infusion (control lymph) and chylous lymph was collected for 2 h during lipid-saline infusion (Intralipid 20 %, Baxter, Deerfield, IL, USA; 170 μmol triglyceride h−1, NaCl 145 mM, KCL 4 mM, at 3 ml h−1), in ice-chilled tubes, fractionated in time intervals of 2 h and frozen at −80 °C. Lymph samples were pooled within each group and centrifuged at 1000 g to eliminate cells and clots before being used in gastric motility experiments.
Measurement of gastric motility
Gastric motility measurement was performed in rats as previously described (Raybould & Tache, 1988). Briefly rats (200-250 g) were anaesthetized with urethane (1.25 g kg−1, I.P.; Sigma, St Louis, MO, USA), a catheter was placed into the trachea to ensure a clear airway (polyethylene tubing, PE 240, 1.67 mm i.d., 2.42 mm o.d.), the abdomen was opened, the pylorus was secured, gastric contents were gently flushed with warm physiological saline through an incision in the forestomach and a catheter (Silastic, 2 mm i.d., 3.2 mm o.d.) was placed through the incision to measure intraluminal gastric pressure (IGP). Catheters were placed in the jugular vein and femoral artery (PE 50, 0.58 mm i.d., 0.965 mm o.d.) for administration of drugs or lymph, respectively. The catheter in the femoral artery was advanced up to the junction with the coeliac artery for injections close-arterial to the upper gastrointestinal (GI) tract. The stomach was placed under 5-6 cmH2O pressure at the start of the recording period to standardize baseline intraluminal pressure. IGP was displayed and collected on-line for the duration of the experiment. Lymph samples were defrosted, stirred, centrifuged at 1000 g to eliminate cells and clots, and injected intra-arterially (I.A.). Changes of IGP were measured, analysed as the maximal decrease of IGP (cmH2O) and duration of inhibition (min).
Recording of vagal afferent nerve fibre discharge
The technique used is a modification of that previously published for recording from gastric vagal afferents (Wang et al. 1997). Rats were anaesthetized, decapitated and a segment of the thoracic oesophagus, stomach and proximal duodenum (≈4 cm from the pylorus to the common bile duct) was removed, and immersed in oxygenated normal rat saline containing 2 g l−1 D-glucose (Adelson et al. 1996). Using a dissecting microscope, the pancreas and stomach were removed, except for the pylorus and adjacent antrum, and the subdiaphragmatic dorsal vagus nerve was identified. A catheter was placed into the gastroduodenal artery and the hepatic, left and right gastric arteries were tied. The segment was pinned into the main chamber of a sylgard-coated organ bath that was perfused continuously with oxygenated normal rat saline at 2.0-2.5 ml min−1 flow rate and the temperature of the organ bath was maintained at 33 ± 1 °C (Wei & Wang, 2000). The isolated dorsal vagus nerve was placed into the recording chamber.
Recording of duodenal vagal afferent discharge
A thin nerve strand was isolated from the dorsal gastric vagus nerve trunk; the distal cut end was wrapped around one lead of a bipolar platinum recording electrode and a strip of neighbouring connective tissue was wrapped around another lead serving as the indifferent electrode. Action potentials were sent to a pre-amplifier (DAM-6 X100, 100-10 kHz band-pass filter; World Precision Instruments, Sarasota, FL, USA), displayed on a digital storage oscilloscope (model 2211, Tektronix) and recorded on-line using a digital tape recorder (Sony high-density linear A/D D/A optical digital audio tape deck, DTC-700). In addition, unit potentials were simultaneously sent to a PC computer equipped with an A/D board (DT2831, Data Translation, Marlboro, MA, USA).
Data acquisition and analysis
Using the acquisition module of WAVEFORM impulse analysis software (Adelson et al. 1996), units within upper and lower threshold settings of the pre-amplifier were acquired on-line onto the hard drive of a PC. Single units were discriminated from the multi-unit recordings based on the amplitude and waveform off-line (Adelson et al. 1996). The response pattern of different units was analysed and displayed separately. Response magnitudes were normalized by a response quotient (Q), where Q = 5 min of spike counts before being divided by the spike count after treatment. A value of Q > 1.25 was taken to indicate an excitatory response and Q = 1 ± 0.25 indicates no response.
Experimental protocols
Effect of mesenteric lymph on gastric motility
Gastric motility in response to injection of control or chylous lymph (1 ml) injected close-arterial to the upper GI tract was measured manometrically in three groups of recipient rats.
In one group of rats, functional vagal deafferentation by perineural capsaicin (10 mg ml−1, n = 10) or vehicle treatment (n = 8) was performed. In this group of rats, inhibition of gastric motility in response to injection of CCK (0.1 to 10 pmol in 0.1 ml, I.A.) served as a measure of the completeness of functional vagal deafferentation (Raybould & Tache, 1988). In the second group of rats, CCK A receptor antagonist devazepide (1 mg kg−1, I.V., n = 10) or vehicle (1 ml kg−1, I.V., n = 12) was administered 20 min prior to injection of lymph. Inhibition of gastric motility in response to injection of CCK (10 pmol in 0.1 ml, I.A.) before and after devazepide administration served as a control for the effectiveness of CCK A receptor blockade. In the third group, rats were treated with the 5-HT3R antagonist ondansetron (100 μg kg−1, I.V., n = 8) or vehicle (1 ml kg−1, I.V., n = 10) administered 20 min prior to injection of lymph. The 5-HT3R agonist 2-methyl-5-HT (3.3 nmol in 0.33 ml, I.A.) administered before and after ondansetron treatment served as a control for the effectiveness of the 5-HT3R blockade. In a preliminary set of experiments, a dose–response to 2-methyl-5-HT was performed in a group of rats (0.1-100 nmol rat−1, I.A., with (n = 4) and without (n = 8) pretreatment with the 5-HT3 receptor antagonist ondansetron). The dose of 2-methyl-5-HT used in subsequent experiments was selected on the basis of producing an effective inhibition of gastric motility and was completely blocked by ondansetron.
Recording of duodenal vagal afferent discharge
Electrophysiological recording from duodenal vagal afferents was started approximately 30 min after the preparation during stable recording conditions from two different experimental groups; (1) preparations investigating the effect of lymph injection on duodenal vagal afferent nerve discharge - data was analysed for single-unit activity from 49 units obtained in 18 preparations after close-arterial vehicle (0.1 ml NaCl), control lymph (0.3 and 0.5 ml), chylous lymph (0.3 and 0.5 ml) and CCK (10 pmol in 0.1 ml) injection and (2) preparations investigating the effect of devazepide on duodenal vagal afferent nerve fibre discharge after lymph injections - from 13 preparations, a total of 34 units were analysed for single-unit activity in response to close-arterial vehicle (0.1 ml NaCl), control lymph (0.5 ml), chylous lymph (0.5 ml) and CCK (10 pmol in 0.1 ml) injections before and after devazepide treatment. Devazepide was slowly administered close-arterially at a concentration of 100 μg (0.1 ml)−1, and 20 min were allowed before lymph or CCK injections were commenced.
Units were selected by the presence of spontaneous activity in the nerve strand. Each nerve strand containing spontaneously active units was tested for the response to intra-arterial injection of CCK. Each nerve strand was then tested with the described protocol to determine the response of both CCK-responsive and CCK-non- responsive units to lymph. In most circumstances, each nerve strand contained more than one analysable unit.
Statistical analysis
Data are presented as means ± standard error of the mean (s.e.m.). In gastric motility experiments, differences between groups were determined by ANOVA (analysis of variance) followed by Student's t test using the software package of JMP (Version 3.2.2, SAS, Institute Inc., Cary, NC, USA). A probability of P < 0.05 was taken as significant. In electrophysiological experiments, values were compared using Student's (paired or unpaired) t test and were considered significantly different if P <0.05.
RESULTS
Effect of functional vagal deafferentation on mesenteric lymph-induced inhibition of gastric motility
As previously described (Glatzle et al. 2002), both control lymph and chylous lymph significantly inhibited gastric motility when injected close-arterially to the GI tract in recipients rats; however, chylous lymph was significantly more potent than control lymph. Control lymph and chylous lymph, collected during intestinal lipid infusion, were less potent in inhibiting gastric motility after functional vagal deafferentation by capsaicin, representing a 63 and 72 % decrease of potency for control or chylous lymph respectively (Fig. 1). In vagal capsaicin-treated animals, the inhibition of gastric motility in response to CCK (0.1-1 pmol) was significantly reduced compared to vehicle-treated animals (decrease of IGP in cmH2O after CCK injection, 0.1-10 pmol, I.A., in vehicle- vs. capsaicin-treated rats; 0.1 pmol CCK, 0.23 ± 0.07 vs. 0.04 ± 0.03; 1 pmol CCK, 0.76 ± 0.11 vs. 0.12 ± 0.06; 10 pmol CCK, 1.00 ± 0.16 vs. 0.56 ± 0.12; P < 0.05 for all CCK concentrations).
Figure 1. Functional vagal deafferentation.
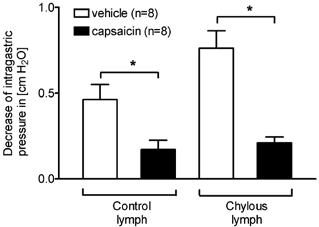
Functional vagal deafferentation by application of capsaicin to the cervical vagus nerve trunks significantly reduced the inhibitory effect of control and chylous lymph on gastric motility. Control lymph was 63 % less effective in inhibiting gastric motility in capsaicin-treated rats. Chylous lymph collected during intestinal lipid infusion was 72 % less effective in inhibiting gastric motility. *P < 0.01, vehicle vs. capsaicin.
Effect of CCK A receptor blockade on control- or chylous lymph-induced inhibition of gastric motility
CCK A receptor blockade by devazepide significantly reduced by 57 % the inhibition of gastric motility in response to chylous lymph (Fig. 2). The inhibition of gastric motility by control lymph, however, was unaffected by the CCK A receptor blockade. CCK injection in devazepide-treated animals served as a control for the CCK A receptor blockade (decrease of IGP in cmH2O after CCK injection, 10 pmol in 0.1 ml, I.A.; before vs. after devazepide treatment; 0.95 ± 0.15 vs. 0.11 ± 0.03, P < 0.01). Vehicle-treated animals responded to CCK (decrease of IGP in cmH2O in response to CCK, 10 pmol in 0.1 ml, I.A., before vs. after vehicle treatment; 0.97 ± 0.12 vs. 0.91 ± 0.13, not significant).
Figure 2. CCK A receptor blockade.
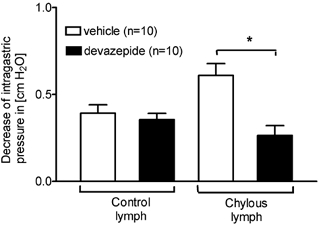
CCK A receptor blockade by devazepide significantly reduced the inhibitory effect on gastric motility of chylous lymph collected during intestinal lipid infusion. Inhibition of gastric motility in response to control lymph was unaltered by CCK A receptor blockade. *P < 0.01, vehicle vs. devazepide.
Effect of 5-HT3R blockade on mesenteric lymph induced-inhibition of gastric motility
5-HT3R blockade by ondansetron did not attenuate the inhibitory effect on gastric motility of either control or chylous lymph (data not shown). However, ondansetron significantly attenuated the inhibition of gastric motility in response to the 5-HT3 receptor agonist 2-methyl-5-HT (decrease of IGP in cmH2O after 2-methyl-5-HT injection, 3.3 nmol in 0.33 ml, I.A.; vehicle vs. ondansetron; 0.54 ± 0.10 vs. 0.03 ± 0.02, P < 0.001), indicating an effective blockade of 5-HT3 receptors.
Effect of CCK and mesenteric lymph on duodenal vagal afferent fibre discharge
Recordings were made from filaments of the dorsal vagus nerve in 18 preparations, and from these, 49 units were analysed for single-unit activity. The discharge of 23 out of these 49 units was significantly increased by intra-arterial injection of CCK (10 pmol; response Q = 3.21 ± 0.37, n = 23; Fig. 3 and Fig. 4). The discharge of 20 out of these 23 CCK-responsive afferents was significantly increased by intra-arterial injection of chylous lymph (0.3 ml, n = 13 and 0.5 ml, n = 10; Figs 3, 4 and 5). A total of 10 out of 13 CCK-responsive units tested with 0.3 ml of chylous lymph responded; the remaining three CCK-responsive units did not respond to control or chylous lymph (0.3 and 0.5 ml). The remaining 10 CCK-responsive units tested with 0.5 ml of chylous lymph responded (response Q = 2.44 ± 0.43, n = 10; Fig. 5). Equivalent volumes of saline had no significant effect on afferent discharge (0.3 and 0.5 ml, response Q = 0.90 ± 0.07, n = 23).
Figure 3. Histogram of single duodenal vagal afferent fibre discharge.
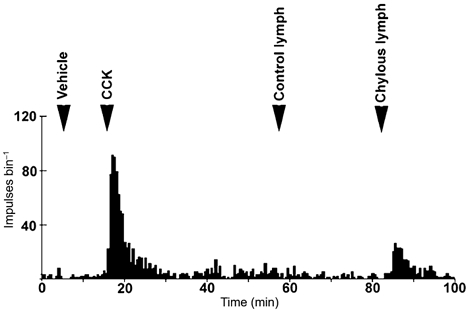
This unit showed an increase in firing rate in response to intra-arterial injection of CCK (10 pmol) and chylous lymph (0.3 ml). Note that this unit did not respond to injection of equivalent volumes of control lymph.
Figure 4. Histogram of multi-unit and single-unit duodenal vagal afferent fibre discharge.
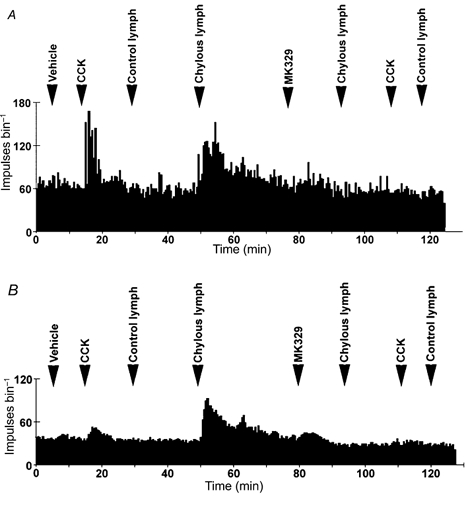
Multi-unit (A) and single-unit (B) histograms showing the duodenal vagal afferent fibre response to both CCK (10 pmol) and chylous lymph (0.3 ml) and the blockade by the CCK A receptor antagonist devazepide (MK 329; 100 μg (0.1 ml)−1).
Figure 5.
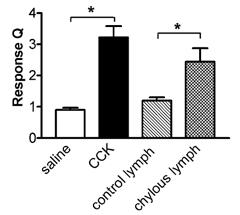
Response magnitude (Q) of CCK- responsive duodenal vagal afferent fibres (n = 23) to CCK (10 pmol, n = 23) and chylous lymph (0.3 ml, n = 10) but not control lymph (0.3 ml, n = 10). *P < 0.01, saline vs. CCK and control vs. chylous lymph.
Equivalent volumes of control lymph (0.3 and 0.5 ml), harvested during intestinal perfusion with maintenance solution, had no significant effect on duodenal vagal afferent fibre discharge in CCK-responsive units (1.04 ± 0.04 and 1.19 ± 0.11, n = 13 and 10, respectively; Fig. 5). However, six CCK-responsive units and one CCK-non-responsive unit showed a modest increase in afferent firing in response to 0.5 ml control lymph (response Q = 1.43 ± 0.05, n = 7). Only two out of the 49 CCK-non-responsive units responded to arterial injection of chylous lymph (0.3 and 0.5 ml).
Effect of devazepide on duodenal vagal afferent fibre discharge
A further 13 preparations were used for these experiments and single-unit activity was obtained from a total of 34 units. Of these 34 units, CCK stimulated the discharge of 20, increasing afferent discharge to response Q = 2.57 ± 0.36 (CCK 10 pmol, n = 20). Chylous lymph increased the discharge of 18 out of 20 of these CCK-responsive units tested (response Q = 2.45 ± 0.34, chylous lymph 0.5 ml, n = 18). Treatment of the preparation with devazepide (100 μg, I.A.) completely abolished the response to CCK in all 20 units (response Q = 0.95 ± 0.04, n = 20; Fig. 4 and Fig. 6), and abolished the response to chylous lymph in 15 out of 18 responsive units (response Q = 0.88 ± 0.04, n = 15; Fig. 4 and Fig. 6).
Figure 6.
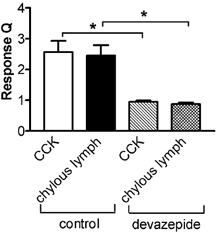
Response magnitude (Q) of duodenal vagal afferents to CCK (10 pmol; n = 20) and chylous lymph (0.5 ml; n = 18). Both responses were inhibited by devazepide (100 μg). *P < 0.01, control vs. devazepide.
DISCUSSION
The present study demonstrates that lymph containing the products of active lipid absorption inhibits gastric motility via a CCK A receptor and vagal capsaicin-sensitive pathway. Moreover, duodenal vagal afferent activity is increased by chylous lymph via a mechanism involving CCK A receptors. These data are consistent with the hypothesis that a constituent of post-absorptive chylomicrons releases endogenous CCK which then activates CCK A receptors on the terminals of vagal afferent fibres in the duodenal mucosa. This in turn initiates a vago-vagal reflex inhibition of gastric motility, which constitutes part of the post-prandial feedback inhibition of gastric function.
The concept that long chain triglycerides initiate CCK release and intestinal feedback inhibition of gastric function has been recognized for many years, yet the events that occur within the mucosa resulting in release of products from entero-endocrine cells are far from clear. The cellular transduction process by which long chain fatty acids in the lumen of the gut release CCK from entero-endocrine cells is not known. It is not clear if this is a direct or indirect effect of lipid on the cells, and if it is direct, whether this occurs at the apical membrane of the endocrine cell. This area of research has been aided by the use of tumour cell lines to study the mechanism of release of endocrine cell products, such as CCK. Long chain fatty acids have been shown to release CCK from STC-1 cells, suggesting a direct effect of lumenal free fatty acid (McLaughlin et al. 1998). However, this has not been demonstrated in vivo and because these tumour cells are not polarized, it is not clear if the lipid is acting on the apical or basolateral membrane. In contrast to this apparent direct effect on endocrine cells, data from in vivo experiments suggest that the effect of lipid on endocrine cells may not be solely direct since lipid-induced release of CCK was blocked by co-administration of Pluronic L-81 to block chylomicron formation (Raybould et al. 1998). This implies a post-absorptive site of action of lipid to release CCK.
The present studies provide further evidence that the stimulus to afferent nerve terminals is also post-absorptive to the epithelial cell layer. Previously we have demonstrated that inhibition of gastric emptying in response to lipid is dependent on chylomicron formation, implying a post-absorptive site of action of triglycerides (Raybould et al. 1998). Chylous lymph contains intact triglycerides, sterols and lipoproteins. The active factor could be intact triglyceride, apolipoproteins or another constituent of lymph. Previously published data have shown that it is not the triglyceride content of lymph that initiates reflex inhibition of gastric motor function (Glatzle et al. 2002). In addition, chylous lymph will also contain other factors that are released into the interstitium, including the products of endocrine cell secretion. Thus, it will contain CCK and it is possible that there is sufficient CCK in the lymph to stimulate afferent nerve terminals and initiate a vago-vagal inhibition of gastric motility. However, this is unlikely since preliminary data measuring CCK in chylous lymph showed a concentration of around 9 pM (J. R. Reeve, J. Glatzle & H. E. Raybould, unpublished observations). This represents an amount of CCK in each ml of lymph of 9 fmoles, well below the threshold for activation of vagal afferents (Blackshaw & Grundy, 1990) or initiation of vago-vagal reflex inhibition of gastric motility (Raybould et al. 1987; Blackshaw & Grundy, 1991). Thus it is unlikely to be a direct effect of CCK, but rather a constituent of the chylous lymph that is releasing CCK and activating vagal afferent nerve terminals. It is possible that this active constituent is apolipoprotein A-IV (Glatzle et al. 2002). However, it is also possible that it is more than one factor that may act synergistically together to initiate vagal afferent and reflex activity. In this case, even the very small amounts of CCK in lymph may be functionally important.
Our data show that 5-HT acting at 5-HT3 receptors is not involved in mediating the response to intestinal lipid. Administration of the specific and potent 5-HT3 receptor antagonist ondansetron abolished the ability of a 5-HT3 agonist to inhibit motility but had no effect on the response to chylous lymph. This is consistent with previous findings that the response to intestinal lipid is not mediated by 5-HT3 receptors (Holzer et al. 1994). In addition, there was no increase in 5-HT levels in chylous versus control lymph (H. Cooke, J. Glatzle & H. E. Raybould, unpublished observations).
Interestingly, control lymph harvested during infusion of a dextrose-electrolyte maintenance solution, also inhibited gastric motility via a capsaicin-sensitive vagal afferent pathway, but this effect was independent of CCK A receptors. It is possible that control lymph contains additional factors such as cytokines, hormones or catecholamines, since lymph samples were collected during the post-operative state. Recently, it has been shown that the GI tract can release pro-inflammatory cytokines such as tumor necrosis factor (TNF)α or interleukin (IL)-6 after an acute gastrointestinal insult, and that these cytokines can be drained into the mesenteric lymph (Mainous et al. 1995; Sakashita et al. 2000). The inhibitory effect of control and chylous lymph could therefore be partly cytokine related, since TNFα, under certain conditions is able to inhibit gastric motility (Hermann et al. 2002). The electrophysiological data supports the concept that at least part of this action is mediated via vagal afferents since control lymph increased the discharge of non-CCK-responsive vagal afferents. This fits with the observation that the functional response to control lymph is not altered in the presence of devazepide. The mechanisms and pathways by which control lymph inhibits gastric motility were not further investigated, but in addition to vagal pathways, spinal pathways or a direct activation of brain stem centres are possible.
The results from the electrophysiological experiments confirm that chylous lymph activates duodenal vagal afferent fibre discharge via a CCK A receptor-dependent mechanism. Chylous lymph was effective at increasing afferent fibre discharge predominately in CCK-responsive units and the response to chylous lymph was markedly reduced by devazepide. The majority of CCK-responsive duodenal vagal afferents responded to injection of either 0.3 or 0.5 ml of chylous lymph and this was unlikely to be volume related since injection of equivalent volumes of saline was without effect. Importantly, equivalent volumes of control lymph had no significant effect on CCK-responsive afferents, suggesting a specific effect of chylous lymph on afferents that express CCK A receptors.
In conclusion, these data support the hypothesis that in the intestinal mucosa, post-absorptive chylomicrons or their products, release endogenous CCK which activates CCK A receptors on vagal afferent nerve fibre terminals, which in turn initiates a vago-vagal reflex inhibition of gastric motor function. This pathway is important not only in the regulation of gastric motility and secretion in the post-prandial phase but also in the reduction of food intake in response to the presence of lipid in the small intestine.
Acknowledgments
This work was supported by grants of NIH DK 41004 (H.E.R.), DK 56910 (P.T.), DK 54504 (P.T.) and a grant of the Deutsche Forschungsgemeinschaft, Bonn, Germany, G.L. 311/3-1 (J.G.).
REFERENCES
- Adelson DW, Wei JY, Kruger L. H2O2 sensitivity of afferent splanchnic C fiber units in vitro. J Neurophysiol. 1996;76:371–380. doi: 10.1152/jn.1996.76.1.371. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990;31:191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Locally and reflexly mediated effects of cholecystokinin-octapeptide on the ferret stomach. J Auton Nerv Syst. 1991;36:129–137. doi: 10.1016/0165-1838(91)90109-g. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D. Effects of 5-hydroxytryptamine on discharge of vagal mucosal afferent fibres from the upper gastrointestinal tract of the ferret. J Auton Nerv Syst. 1993;45:41–50. doi: 10.1016/0165-1838(93)90360-7. [DOI] [PubMed] [Google Scholar]
- Glatzle J, Kalogeris TJ, Zittel TT, Guerrini S, Tso P, Raybould HE. Chylomicron components mediate intestinal lipid-induced inhibition of gastric motor function. Am J Physiol Gastrointest Liver Physiol. 2002;282:G86–91. doi: 10.1152/ajpgi.2002.282.1.G86. [DOI] [PubMed] [Google Scholar]
- Grundy D, Scratcherd T. Sensory afferents from the gastrointestinal tract. In: Wood JD, editor. Handbook of Physiology, Section 6, The Gastroinestinal System, Motility and Circulation. Vol. 1. Bethesda, MD: American Physiological Society; 1989. pp. 593–620. [Google Scholar]
- Hermann GE, Tovar CA, Rogers RC. LPS-induced suppression of gastric motility relieved by TNFR:Fc construct in dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol. 2002;283:G634–639. doi: 10.1152/ajpgi.00412.2001. [DOI] [PubMed] [Google Scholar]
- Hillsley K, Kirkup AJ, Grundy D. Direct and indirect actions of 5-hydroxytryptamine on the discharge of mesenteric afferent fibres innervating the rat jejunum. J Physiol. 1998;506:551–561. doi: 10.1111/j.1469-7793.1998.551bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer HH, Turkelson CM, Solomon TE, Raybould HE. Intestinal lipid inhibits gastric emptying via CCK and a vagal capsaicin-sensitive afferent pathway in rats. Am J Physiol. 1994;267:G625–629. doi: 10.1152/ajpgi.1994.267.4.G625. [DOI] [PubMed] [Google Scholar]
- Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;281:G907–915. doi: 10.1152/ajpgi.2001.281.4.G907. [DOI] [PubMed] [Google Scholar]
- Liddle RA. Cholecystokinin cells. Annu Rev Physiol. 1997;59:221–242. doi: 10.1146/annurev.physiol.59.1.221. [DOI] [PubMed] [Google Scholar]
- Lloyd KC, Holzer HH, Zittel TT, Raybould HE. Duodenal lipid inhibits gastric acid secretion by vagal, capsaicin-sensitive afferent pathways in rats. Am J Physiol. 1993;264:G659–663. doi: 10.1152/ajpgi.1993.264.4.G659. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Grazia LM, Jones MN, D'Amato M, Dockray GJ, Thompson DG. Fatty acid chain length determines cholecystokinin secretion and effect on human gastric motility. Gastroenterology. 1999;116:46–53. doi: 10.1016/s0016-5085(99)70227-1. [DOI] [PubMed] [Google Scholar]
- McLaughlin JT, Lomax RB, Hall L, Dockray GJ, Thompson DG, Warhurst G. Fatty acids stimulate cholecystokinin secretion via an acyl chain length-specific, Ca2+-dependent mechanism in the enteroendocrine cell line STC-1. J Physiol. 1998;513:11–18. doi: 10.1111/j.1469-7793.1998.011by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainous MR, Ertel W, Chaudry IH, Deitch EA. The gut: a cytokine-generating organ in systemic inflammation. Shock. 1995;4:193–199. [PubMed] [Google Scholar]
- Raybould HE, Meyer JH, Tabrizi Y, Liddle RA, Tso P. Inhibition of gastric emptying in response to intestinal lipid is dependent on chylomicron formation. Am J Physiol. 1998;274:R1834–1838. doi: 10.1152/ajpregu.1998.274.6.R1834. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Roberts ME, Dockray GJ. Reflex decreases in intragastric pressure in response to cholecystokinin in rats. Am J Physiol. 1987;253:G165–170. doi: 10.1152/ajpgi.1987.253.2.G165. [DOI] [PubMed] [Google Scholar]
- Raybould HE, Tache Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol. 1988;255:G242–246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- Sakashita Y, Hiyama E, Imamura Y, Murakami Y, Sugahara Y, Takesue Y, Matsuura Y, Yokoyama T. Generation of pro-inflammatory and anti-inflammatory cytokines in the gut in zymosan-induced peritonitis. Hiroshima J Med Sci. 2000;49:43–48. [PubMed] [Google Scholar]
- Sidhu SS, Thompson DG, Warhurst G, Case RM, Benson RS. Fatty acid-induced cholecystokinin secretion and changes in intracellular Ca2+ in two enteroendocrine cell lines, STC-1 and GLUTag. J Physiol. 2000;528:165–176. doi: 10.1111/j.1469-7793.2000.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso P, Balint JA. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am J Physiol. 1986;250:G715–726. doi: 10.1152/ajpgi.1986.250.6.G715. [DOI] [PubMed] [Google Scholar]
- Wang YH, Tache Y, Sheibel AB, Go VL, Wei JY. Two types of leptin-responsive gastric vagal afferent terminals: an in vitro single-unit study in rats. Am J Physiol. 1997;273:R833–837. doi: 10.1152/ajpregu.1997.273.2.R833. [DOI] [PubMed] [Google Scholar]
- Wei JY, Wang YH. Effect of CCK pretreatment on the CCK sensitivity of rat polymodal gastric vagal afferent in vitro. Am J Physiol Endocrinol Metab. 2000;279:E695–706. doi: 10.1152/ajpendo.2000.279.3.E695. [DOI] [PubMed] [Google Scholar]


