Abstract
The present study examined the effect of short-term submaximal training on plasma acid–base balance during exercise. The influence of water and ion exchange between plasma, active muscles and erythrocytes in the response to training were also studied. The contributions of independent physicochemical variables (i.e. strong ion difference ([SID]), total concentration of weak acids ([Atot]) and PO2) to changes in arterial (a) and femoral venous (v) plasma [H+] were examined in six subjects (age 24 ± 1.5 years; maximum oxygen consumption rate ( ), 3.67 ± 0.24 l min−1) during steady-state cycling for 15 min at each of 30, 65 and 75 % of
), 3.67 ± 0.24 l min−1) during steady-state cycling for 15 min at each of 30, 65 and 75 % of  before (pre) and after (post) training for 7 days on a cycle ergometer (2 h daily at 60 %
before (pre) and after (post) training for 7 days on a cycle ergometer (2 h daily at 60 %  ). The rise in [H+]a during exercise was attenuated post-training by 3 and 5 nequiv l−1 (P < 0.05) at 65 and 75 %
). The rise in [H+]a during exercise was attenuated post-training by 3 and 5 nequiv l−1 (P < 0.05) at 65 and 75 %  , respectively, due first to less decrease in [SID]a, secondary to lower [Cl−]a and [Lac−]a; and second, to a reduction in [Atot]a, due to greater plasma volume and less plasma water flux (Jv) into leg muscle (P < 0.05). The rise in [H+]v was also less in post-training by 4.5 and 6 nequiv l−1 (P < 0.05) at 65 and 75 %
, respectively, due first to less decrease in [SID]a, secondary to lower [Cl−]a and [Lac−]a; and second, to a reduction in [Atot]a, due to greater plasma volume and less plasma water flux (Jv) into leg muscle (P < 0.05). The rise in [H+]v was also less in post-training by 4.5 and 6 nequiv l−1 (P < 0.05) at 65 and 75 %  , respectively, and attributed solely to lower [Atot]v (P < 0.05). Attenuation of exercise induced decreases in plasma [SID]a and [SID]v from rest to 75 %
, respectively, and attributed solely to lower [Atot]v (P < 0.05). Attenuation of exercise induced decreases in plasma [SID]a and [SID]v from rest to 75 %  was accompanied by reductions in erythrocyte Lac− and Cl− uptake (P < 0.05), and smaller increases in erythrocyte K+ release (P < 0.05). We conclude that the training-induced attenuation of the rise in plasma [H+]a and [H+]v during incremental exercise resulted from adaptive changes within muscles (less Lac− production and less water uptake) and erythrocytes (less uptake of Lac−, Cl− and K+), leading to greater [SID] and lower [Atot] in both arterial and femoral venous plasma.
was accompanied by reductions in erythrocyte Lac− and Cl− uptake (P < 0.05), and smaller increases in erythrocyte K+ release (P < 0.05). We conclude that the training-induced attenuation of the rise in plasma [H+]a and [H+]v during incremental exercise resulted from adaptive changes within muscles (less Lac− production and less water uptake) and erythrocytes (less uptake of Lac−, Cl− and K+), leading to greater [SID] and lower [Atot] in both arterial and femoral venous plasma.
Moderate to heavy exercise induces ionic changes within contracting muscles that contribute to the development of acidosis (McCartney et al. 1983a,b; Kowalchuk et al. 1984; Lindinger et al. 1995). The exchange of strong ions, CO2 and water between the intracellular and extracellular compartments helps to restore acid–base homeostasis (Lindinger et al. 1992, 1994). Lactate (Lac−) produced during exercise is released from the active muscles into the plasma compartment with K+, while Na+ and Cl− are taken up. The resulting changes in the relative intracellular balance of the anions and cations serves to reduce some of the increase in intracellular [H+] via their influence on the strong ion difference ([SID]) (Stewart, 1983). In addition, the influx of fluid from plasma into the active intracellular muscle compartment lowers the concentration of intracellular total weak acids ([Atot]), but results in increased concentration of plasma Atot, of ions that do not move into the intracellular compartment, and of ions added to the plasma compartment, which further contributes to increases in plasma [H+].
Recent studies have examined the effects of long-term (1 month or more) high-intensity exercise training on plasma acid–base and ion balance in human subjects during exercise of varied intensities. Improvements in fatigue resistance were ascribed to attenuation of plasma concentrations of H+ and K+ (McKenna et al. 1996, McKenna 1997). By comparison, studies that have investigated the effects of low- to moderate-intensity short-term training (1 week or less) on plasma acid–base and ion balance in man during exercise are limited (Green et al. 1987a, 1993). However, studies of low- to moderate-intensity short-term training have reported metabolic (Green et al. 1991b; Phillips et al. 1995b, 1996; Putman et al. 1998; Chesley et al. 1996) and ionic (Green et al. 1993; Phillips et al. 1995b) adaptations. Submaximal short-term training has been shown to result in lower rates of net muscle Lac− production (Chesley et al. 1996; Putman et al. 1998) and greater rates of muscle Lac− extrusion into the venous plasma compartment (Bonen et al. 1998), as well as increased Lac− clearance from plasma (Phillips et al. 1995b). In addition, short-term training studies have reported lower venous PCO2 (Phillips et al. 1995a), plasma volume expansion (Sawka et al. 2000) and a reduction in exercise-induced venous hyperkalaemia (Green et al. 1993).
Changes in erythrocyte ion exchange may also be important in the adaptive response to short-term training. Lindinger et al. (McKelvie et al. 1991; Lindinger et al. 1995, 1999) have shown that the erythrocyte aids in regulating plasma acid–base and ion balance in response to high-intensity sprint cycling and during recovery, by actively participating in the systemic redistribution of Lac−, K+ and other strong ions. Thus, it is possible that adaptive changes associated with short-term training may also extend to the erythrocyte and contribute to the accompanying improvements in plasma acid–base balance.
Using the integrated physicochemical systems approach described by Stewart (1983), it is possible to quantify the contributions of linked physiological systems to the regulation of plasma acid–base balance (for reviews see Jones, 1990; Heigenhauser, 1995; Jones & Heigenhauser, 1996). The important elements include the control of electrolyte concentrations (i.e. strong ions), fluid shifts, as well as physiological and biochemical events that occur within the muscular, respiratory and circulatory systems. Within each fluid compartment, [H+] and [HCO3−] are considered dependent variables that are determined by three independent variables - [SID], PCO2and [Atot]. The behaviour of each independent variable is governed by its equilibrium constant in water, and further constrained by the laws of conservation of mass and electrical neutrality. The purpose of the present study was to test the hypothesis that short-term submaximal training would result in attenuation of the steady-state exercise-induced increase in arterial and femoral venous plasma [H+], and would be associated with alterations in one or more of the independent variables in plasma and erythrocytes as they passed through exercising muscle.
METHODS
Subjects
Six male subjects participated in the present study (mean ± s.e.m.: age, 24 ± 1.5 years; weight, 82 ± 4.8 kg;  , 3.7 ± 0.24 l min−1). Additional blood samples were collected from six of the seven subjects that took part in our previous study (Putman et al. 1998) and formed the basis for the present study. The calculated active muscle mass (Lindinger et al. 1994) for the subjects was 9.2 ± 0.5 kg before and after training. Approval was obtained from the ethics committees of McMaster University and McMaster University Medical Centre. Written informed consent was obtained from all subjects after a complete description of the risks associated with the study. All procedures used conformed to the Declaration of Helsinki.
, 3.7 ± 0.24 l min−1). Additional blood samples were collected from six of the seven subjects that took part in our previous study (Putman et al. 1998) and formed the basis for the present study. The calculated active muscle mass (Lindinger et al. 1994) for the subjects was 9.2 ± 0.5 kg before and after training. Approval was obtained from the ethics committees of McMaster University and McMaster University Medical Centre. Written informed consent was obtained from all subjects after a complete description of the risks associated with the study. All procedures used conformed to the Declaration of Helsinki.
Determination of  and training protocol
and training protocol
Two weeks before training was initiated and the day after completion of the training protocol, subjects completed a progressive exercise test to determine their  and work capacity (Putman et al. 1998). Subjects trained on a cycle ergometer for 2 h daily at 60 % of their pre-training
and work capacity (Putman et al. 1998). Subjects trained on a cycle ergometer for 2 h daily at 60 % of their pre-training  for 7 (five subjects) or 8 (one subject) consecutive days, and were allowed to stop cycling at any time. The total cycling time, however, remained constant at 2 h daily.
for 7 (five subjects) or 8 (one subject) consecutive days, and were allowed to stop cycling at any time. The total cycling time, however, remained constant at 2 h daily.
Experimental protocol
One week before beginning the training protocol and within 48 h of completing the last training session, each subject reported to the laboratory in the morning after consuming a light carbohydrate meal. Subjects abstained from consuming caffeine or alcohol for 48 h prior to each experiment. After subcutaneous infiltration of the antecubital region with 0.5 ml of 2 % xylocaine, without epinephrine (adrenaline), the brachial artery was catheterised percutaneously (20 gauge, 3.2 cm; Parke Davis and Co., Sandy, UT, USA). The femoral vein was subsequently catheterised percutaneously with a radiopaque Teflon catheter by the Seldinger technique, after local anaesthesia. Catheters were kept patent by slow infusion (0.2 ml min−1) of non-heparinised isotonic saline (0.9 % NaCl). After insertion of the catheters, subjects rested for 20 min in a seated position and were studied at rest. Arterial and venous samples were drawn into airtight heparinised plastic syringes and immediately placed on ice. Leg blood flow was then measured by thermodilution (Andersen & Saltin, 1985). Subjects were then studied during steady-state cycling at work loads corresponding to 30, 65 and 75 % of  , each maintained for 15 min; corresponding workloads were 83 ± 6, 180 ± 12 and 208 ± 14 W, respectively. Arterial and venous blood samples were obtained during steady-state cycling at 9-11 and 13-15 min of cycling, at each workload. Because measured parameters did not differ between samples collected at these two time points, the data were averaged at each of the three cycling intensities. Immediately after blood sampling, leg blood flow was measured followed by a needle biopsy of the vastus lateralis, using the Bergström method, without the use of suction. Biopsy samples were immediately frozen in liquid nitrogen. The methods and results of measurements made on the biopsy samples have been provided in a previous publication (Putman et al. 1998).
, each maintained for 15 min; corresponding workloads were 83 ± 6, 180 ± 12 and 208 ± 14 W, respectively. Arterial and venous blood samples were obtained during steady-state cycling at 9-11 and 13-15 min of cycling, at each workload. Because measured parameters did not differ between samples collected at these two time points, the data were averaged at each of the three cycling intensities. Immediately after blood sampling, leg blood flow was measured followed by a needle biopsy of the vastus lateralis, using the Bergström method, without the use of suction. Biopsy samples were immediately frozen in liquid nitrogen. The methods and results of measurements made on the biopsy samples have been provided in a previous publication (Putman et al. 1998).
Blood sampling
Arterial (a) and venous (v) blood samples (6 ml) were drawn simultaneously and placed on ice; samples were subsequently divided into five aliquots. The first was centrifuged immediately at 15 500 g for 2 min and the plasma supernatant was removed. A portion was frozen for later analyses of total protein, Na+, K+ and Cl−, while another portion (400 μl) was deproteinised in 2 vol. of 6 % perchloric acid (PCA) and used for analysis of plasma lactate concentration ([Lac−]). A second aliquot of whole blood was placed in 100 μl heparinised capillary tubes for haematocrit determination. A third aliquot was frozen and thawed three times in liquid nitrogen to lyse the erythrocyte cell membranes and used for determination of whole blood, [Na+], [K+] and [Cl−]. A fourth aliquot of was deproteinised in 2 vol. of 6 % PCA and used for analysis of [Lac−]. The fifth aliquot remained within the airtight heparinised syringes on ice and was immediately analysed for plasma pH, PCO2, PO2 and O2 saturation.
Blood analyses
Blood [Lac−] was determined in plasma and whole blood PCA extracts according to Bergmeyer (1983). [Na+] and [K+] were measured in plasma and lysed whole blood extracts using ion-selective electrodes (Radiometer KNA1 Na+-K+ analyser) (McKelvie et al. 1991; Lindinger et al. 1992). [Cl−] was measured by a Buchler-Cotlove chloridometer (model 4-2008). Plasma pH, PCO2, PO2, HCO3− and base excess (BE) were measured with a Corning 178 pH/blood gas analyser, corrected to 37 °C. [H+] was calculated from pH measurements. Haematocrit was measured in duplicate after centrifugation of heparinised capillary tubes for 15 min at 15 000 g (IEC MB micro-hematocrit centrifuge). Haemoglobin concentration ([Hb]) and O2 saturation (SO2) were determined photometrically, in duplicate, using a Radiometer OSM2 Hemoximeter. Analysers were calibrated immediately before and at regular intervals throughout the analyses using precision standards that were in the range of the measurements. Plasma protein concentrations ([PPr−]) were assayed according to the method of Lowry (Lowry et al. 1951).
Calculations
The total concentration of weak acids ([Atot]) was calculated as 2.45 ×[PPr−] (McKenna et al. 1997). Plasma [SID] was calculated as the sum of the strong cations minus the sum of the strong anions (Stewart, 1983).
| (1) |
Erythrocyte ion concentration was determined according to McKelvie et al. (1991; see also Harrison, 1985):
| (2) |
where [ion]ery is the ion concentration in the erythrocyte (mequiv (l cell water)−1), [ion]wb is the ion concentration of the haemolysed whole blood sample, [ion]pl is the ion concentration of the plasma sample, and h is the correction for trapped plasma and peripheral sampling (Lindinger et al. 1994).
The percentage changes in plasma volume (ΔPV) (Lindinger et al. 1994), blood volume (ΔBV) (McKenna et al. 1997) and erythrocyte volume (ΔEV) (Lindinger et al. 1994) were calculated from rest (r) and exercise (e) measurements of [Hb] and Hct to assess the impact of fluid shifts on ion concentrations in plasma, whole blood and in the erythrocyte compartment. The effects of training on PV, BV and EV were calculated using Hb and Hct measurements (Harrison, 1985). Hct (%) was corrected for trapped plasma and peripheral sampling by multiplying by 0.96 and 0.91, and expressed as ‘H’, according to Lindinger et al. (1994):
| (3) |
| (4) |
| (5) |
The net transcapillary water flux (Jv) into or out of the plasma compartment was calculated according to Lindinger et al. (1994), including correction for trapped plasma and peripheral sampling errors as above:
| (6) |
The net ion flux (Fion) was calculated across the active muscle bed from the concentrations of plasma ions, corrected for changes in Jv, where Q is the plasma flow and calculated as leg blood flow × (1 - Hcta):
| (7) |
With the aid of a computer program, free plasma [H+] was calculated using the empirical relationship derived by Stewart (1983), which describes the dependency of [H+] on the three independent physicochemical variables, as well as on mass action equilibriums, conservation of mass and electrical neutrality.
| (8) |
Values used for the equilibrium constants KA, KC, K'W and K3 were as follows: KA = 3.0 × 10−7 equiv l−1, KC = 2.45 × 10−11 (equiv l−1)2, K3 = 6.0 × 10−11 equiv l−1 and K'W = 4.4 × 10−14 (equiv l−1)2.
Muscle water content was determined from the frozen muscle wet-to-dry weight ratio after lyophilisation. Muscle Lac− accumulation and phosphocreatine (PCr) hydrolysis were calculated (Putman et al. 1998) and related to changes in muscle water content.
Contributions of the independent variables to increases in [H+]
Contributions of the three independent variables, [SID], PCO2and [Atot], to changes in plasma [H+] were calculated using a computer program (Kowalchuk & Scheuermann, 1995). Each of the independent variables was individually changed to the corresponding exercise value while the remaining two were held constant at resting levels (Table 7). The calculated resting [H+] was then subtracted from the resulting [H+] and the difference expressed as a percentage of the total Δ[H+] (Table 7).
Table 7.
Contributions of the independent variables PCO2, [SID] and [Atot] to increases in plasma [H+] during steady-state cycling at 65% and 75% 
| Concentration | Contributions of independent variables [H+] (nmol l−1) | Δ[H+] exercise–rest (nmol l−1) | Percentage contribution to Δ[H+] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Rest | 65% | 75% | 65% | 75% | 65% | 75% | 65% | 75% |
| Arterial pre-training | |||||||||
| [H+]meas. (nmol l−1) | 37.5 | 43.5 | 45.0 | — | — | +6.0 | +7.5 | — | — |
| [H+]calc. (nmol l−1) | 33.7 | 40.6 | 43.0 | — | — | +6.9 | +9.3 | — | — |
| Pa,CO2 (mmHg) | 37.8 | 37.9 | 34.1 | 33.8 | 30.6 | +0.1 | −3.1 | +1% | −33% |
| [SID] (mequiv l−1) | 41.2 | 37.1 | 35.3 | 39.2 | 42.2 | +5.5 | +8.5 | +80% | +91% |
| [Atot] (mequiv l−1) | 15.1 | 16.0 | 18.0 | 34.7 | 37.0 | +1.0 | +3.3 | +14% | +35% |
| Arterial post-training | |||||||||
| [H+]meas. (nmol l−1) | 35.5 | 40.6 | 40.9 | — | — | +5.1 | +5.4 | — | — |
| [H+]calc. (nmol l−1) | 33.4 | 39.7 | 38.4 | — | — | +6.3 | +5.0 | — | — |
| Pa,CO2 (mmHg) | 36.0 | 38.7 | 36.3 | 35.7 | 33.6 | +2.3 | +0.2 | +37% | +4% |
| [SID] (mequiv l−1) | 40.1 | 37.7 | 36.8 | 36.5 | 37.8 | +3.1 | +4.4 | +49% | +88% |
| [Atot] (mequiv l−1) | 15.0 | 15.5 | 15.2 | 33.9 | 33.6 | +0.5 | +0.2 | +8% | +4% |
| Venous pre-training | |||||||||
| [H+]meas. (nmol l−1) | 41.9 | 55.1 | 58.6 | — | — | +13.2 | +16.7 | — | — |
| [H+]calc. (nmol l−1) | 44.7 | 59.1 | 61.9 | — | — | +14.4 | +17.2 | — | — |
| Pv,CO2 (mmHg) | 45.9 | 61.5 | 60.9 | 58.5 | 58.0 | +13.8 | +13.3 | +96% | +77% |
| [SID] (mequiv l−1) | 41.2 | 40.5 | 38.5 | 45.9 | 49.6 | +1.2 | +4.9 | +8% | +28% |
| [Atot] (mequiv l−1) | 18.3 | 17.8 | 17.2 | 44.0 | 43.2 | −0.7 | −1.5 | −5% | −9% |
| Venous post-training | |||||||||
| [H+]meas. (nmol l−1) | 41.0 | 50.6 | 52.9 | — | — | +9.6 | +11.9 | — | — |
| [H+]calc. (nmol l−1) | 42.6 | 55.0 | 57.1 | — | — | +12.6 | +14.5 | — | — |
| Pv,CO2 (mmHg) | 46.8 | 60.9 | 61.2 | 54.6 | 54.8 | +12.0 | +12.2 | +95% | +84% |
| [SID] (mequiv l−1) | 40.1 | 40.1 | 39.0 | 42.6 | 44.3 | 0.0 | +1.7 | 0% | +12% |
| [Atot] (mequiv l−1) | 14.9 | 15.2 | 15.0 | 43.0 | 42.7 | +0.4 | +0.1 | +3% | +1% |
Statistical analyses
Data are reported as means ± s.e.m. and were analysed by analysis of variance (ANOVA) with repeated measures. When a significant F-ratio was observed, mean values were compared using the least significant difference post hoc test for planned comparisons. The slopes of linear regression data were compared between conditions using Student's t test. Data in which a priori hypotheses were established, and the direction of changes predicted in advance, were analysed by Student's one-tailed t test. These included BV, PV, Jv, [Na+], [K+], [Cl−], [SID], ion fluxes and respiratory exchange ratio (RER), all at matched time points. Differences were considered significant at P < 0.05.
RESULTS
Respiratory responses to incremental exercise and training
Training did not result in changes to  , being 3.67 ± 0.24 l min−1 before, and 3.76 ± 0.30 l min−1 after training. While
, being 3.67 ± 0.24 l min−1 before, and 3.76 ± 0.30 l min−1 after training. While 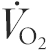 did not differ between conditions at any time point studied,
did not differ between conditions at any time point studied, 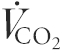 and RER were lower during cycling at 30 and 65 %
and RER were lower during cycling at 30 and 65 %  after training. Expiratory flow (
after training. Expiratory flow (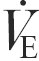 ) was similar at all time points, except during cycling at 75 %
) was similar at all time points, except during cycling at 75 %  , where it was lower post-training (Table 1).
, where it was lower post-training (Table 1).
Table 1.
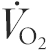 ,
, 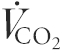 , RER,
, RER, 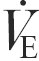 , PO2, SO2, leg blood flow, leg plasma flow, plasma water flux and leg
, PO2, SO2, leg blood flow, leg plasma flow, plasma water flux and leg 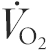 at rest and during steady-state incremental cycling exercise
at rest and during steady-state incremental cycling exercise
| Measurement | Rest | 30% 
|
65% 
|
75% 
|
|---|---|---|---|---|
| Pre-training | ||||
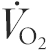 (1 min−1 STPD) (1 min−1 STPD) |
0.36 ± 0.02 | 1.49 ± 0.08* | 2.60 ± 0.16*† | 3.07 ± 0.18*†‡ |
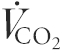 (1 min−1 STPD) (1 min−1 STPD) |
0.32 ± 0.03 | 1.39 ± 0.08* | 2.54 ± 0.15*† | 3.00 ± 0.15*†‡ |
| RER (1 min−1 STPD) | 0.89 ± 0.05 | 0.94 ± 0.01 | 0.98 ± 0.02 | 0.98 ± 0.02 |
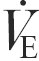 (1 min−1 STPD) (1 min−1 STPD) |
10.7 ± 0.6 | 31.5 ± 1.9* | 56.5 ± 2.9*† | 77.0 ± 5.6*†‡ |
| Pa,O2 (mmHg) | 111.3 ± 6.7 | 105.6 ± 8.8 | 104.3 ± 4.3 | 103.1 ± 6.6 |
| Pv,O2 (mmHg) | 40.1 ± 3.0 | 29.7 ± 1.2* | 26.3 ± 1.1* | 25.3 ± 1.2* |
| Sa,O2 (%) | 98.3 ± 0.4 | 98.3 ± 0.2 | 97.7 ± 0.3 | 97.2 ± 0.5*† |
| Sv,O2 (%) | 71.9 ± 4.2 | 46.8 ± 2.0* | 35.4 ± 1.5*† | 31.7 ± 1.9*† |
| Leg blood flow (1 min−1) | 1.79 ± 0.45 | 7.41 ± 1.08* | 8.05 ± 1.18* | 9.60 ± 1.08*† |
| Leg plasma flow (1 min−1) | 1.02 ± 0.25 | 4.26 ± 0.61* | 4.45 ± 0.62* | 5.25 ± 0.56*† |
| Jv (ml min−1) | 19 ± 30 | 123 ± 90 | 201 ± 73* | 113 ± 45 |
Leg 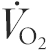 (mmol min−1) (mmol min−1) |
3.5 ± 0.6 | 32.3 ± 4.4* | 44.0 ± 7.3* | 55.8 ± 7.8* |
| Post-training | ||||
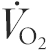 (1 min−1 STPD) (1 min−1 STPD) |
0.40 ± 0.02 | 1.48 ± 0.08* | 2.52 ± 0.11*† | 3.04 ± 0.10*†‡ |
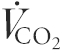 (1 min−1 STPD) (1 min−1 STPD) |
0.38 ± 0.03§ | 1. 32 ± 0.06*§ | 2.37 ± 0.09*†§ | 2.92 ± 0.10*†‡ |
| RER (1 min−1 STPD) | 0.94 ± 0.06§ | 0.89 ± 0.01§ | 0.94 ± 0.01§ | 0.96 ± 0.01 |
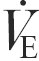 (1 min−1 STPD) (1 min−1 STPD) |
11.8 ± 1.1 | 29.8 ± 0.9* | 52.8 ± 1.7*† | 68.9 ± 4.0*†‡§ |
| Pa,O2 (mmHg) | 129.0 ± 5.8§ | 117.9 ± 4.5§ | 111.4 ± 8.8* | 106.5 ± 7.2* |
| Pv,O2 (mmHg) | 37.7 ± 2.8 | 27.6 ± 0.8* | 25.6 ± 1.8* | 24.5 ± 0.9* |
| Sa,O2 (%) | 99.1 ± 0.1§ | 98.8 ± 0.2 | 98.3 ± 0.3* | 98.0 ± 0.3*†§ |
| Sv,O2 (%) | 67.6 ± 3.9 | 46.3 ± 1.6* | 38.1 ± 3.0*† | 33.1 ± 1.2*† |
| Leg blood flow (1 min−1) | 1.45 ± 0.45 | 6.18 ± 0.56* | 8.19 ± 0.52*† | 10.69 ± 0.68*†‡ |
| Leg plasma flow (1 min−1) | 0.84 ± 0.26 | 3.70 ± 0.31* | 4.81 ± 0.28*† | 6.30 ± 0.46*†§ |
| Jv (ml min−1) | −4 ± 14 | 58 ± 49 | 58 ± 6§ | 138 ± 54* |
Leg 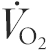 (mmol min−1) (mmol min−1) |
2.8 ± 0.3 | 26.2 ± 3.1* | 41.0 ± 4.7*† | 56.8 ± 5.6*†‡ |
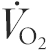 , O2 consumption rate;
, O2 consumption rate; 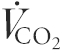 , CO2 production rate; RER, respiratory exchange ratio;
, CO2 production rate; RER, respiratory exchange ratio; 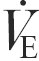 , respiratory flow; Pa,O2, arterial partial pressure of O2; Pv,O2, venous partial pressure of O2; Sa,O2, arterial O2 saturation; Sv,O2, venous O2 saturation; Jv, plasma water flux; STPD, standard temperature and pressure, dry.
, respiratory flow; Pa,O2, arterial partial pressure of O2; Pv,O2, venous partial pressure of O2; Sa,O2, arterial O2 saturation; Sv,O2, venous O2 saturation; Jv, plasma water flux; STPD, standard temperature and pressure, dry.
Significantly different from rest
significantly different from 30%
significantly different from 65%
significantly different from pre-training. Leg blood flow, leg plasma flow and leg 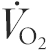 are calculated for 2 legs. For Jv, a positive value indicates ml min−1 water moving into the active muscles.
are calculated for 2 legs. For Jv, a positive value indicates ml min−1 water moving into the active muscles.
Oxygenation, leg blood flow and leg gas exchange
Pre-training arterial partial pressure of oxygen (Pa,O2) remained unchanged throughout exercise and differed from the post-training condition only at rest and 30 %  , where it was lower (Table 1). Arterial (Sa,O2) and venous (Sv,O2) oxygen saturation were unaffected by training (Table 1). Leg plasma flow increased similarly in both conditions in response to incremental exercise, with the exception of 75 %
, where it was lower (Table 1). Arterial (Sa,O2) and venous (Sv,O2) oxygen saturation were unaffected by training (Table 1). Leg plasma flow increased similarly in both conditions in response to incremental exercise, with the exception of 75 %  where it was elevated by 1.1 l min−1 after training (P < 0.05, Table 1); leg blood flow was similarly elevated. Exercise-induced increases in leg
where it was elevated by 1.1 l min−1 after training (P < 0.05, Table 1); leg blood flow was similarly elevated. Exercise-induced increases in leg 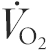 were similar before and after training (Table 1).
were similar before and after training (Table 1).
Haemoconcentration
Before training, incremental exercise resulted in an approximately 5 % increase in both arterial and femoral venous Hct at 65 and 75 %  (P < 0.05, Table 2). Exercise-induced increases in [Hb] were qualitatively similar but slightly smaller in magnitude. Before training [PPr−]a was also elevated by 7 and 19 % during cycling at 65 and 75 %
(P < 0.05, Table 2). Exercise-induced increases in [Hb] were qualitatively similar but slightly smaller in magnitude. Before training [PPr−]a was also elevated by 7 and 19 % during cycling at 65 and 75 %  , respectively (Table 2). After training, arterial and femoral venous [Hct] and [PPr−] were lower at all time points and did not change in response to incremental cycling (Table 2).
, respectively (Table 2). After training, arterial and femoral venous [Hct] and [PPr−] were lower at all time points and did not change in response to incremental cycling (Table 2).
Table 2.
Haemoglobin, haematocrit and plasma protein concentration at rest and during steady-state incremental cycling exercise
| Measurement | Rest | 30% 
|
65% 
|
75% 
|
|---|---|---|---|---|
| Pre-training | ||||
| [Hb]a (g(100 ml)−1) | 14.3 ± 0.8 | 14.4 ± 0.8 | 14.5 ± 0.8* | 14.6 ± 0.8* |
| [Hb]v (g(100 ml)−1) | 14.3 ± 0.8 | 14.5 ± 0.8 | 14.6 ± 0.8 | 14.6 ± 0.9 |
| Hcta (%) | 44.7 ± 2.2 | 44.1 ± 1.9 | 46.1 ± 1.9 | 46.9 ± 1.9† |
| Hctv (%) | 45.9 ± 2.2 | 45.6 ± 2.2 | 48.3 ± 2.6*† | 48.3 ± 2.4*† |
| [PPr−]a (mg ml−1) | 61.5 ± 3.5 | 65.1 ± 5.1 | 65.5 ± 4.4* | 73.3 ± 5.7*†‡ |
| [PPr−]v (mg ml−1) | 74.9 ± 7.9 | 70.8 ± 6.4 | 72.6 ± 6.3 | 70.2 ± 5.4 |
| [PPr−]a-v (mg ml−1) | −13.4 ± 6.3 | −5.7 ± 2.8 | −7.1 ± 2.6 | 3.2 ± 1.7*‡ |
| Post-training | ||||
| [Hb]a (g(100 ml)−1) | 13.3 ± 0.8§ | 13.4 ± 0.8§ | 13.8 ± 0.8*‡§ | 13.7 ± 0.8*‡§ |
| [Hb]v (g(100 ml)−1) | 13.4 ± 0.8§ | 13.4 ± 0.8§ | 13.8 ± 0.8*‡§ | 13.9 ± 0.8*‡§ |
| Hcta (%) | 43.5 ± 1.4 | 41.4 ± 1.4§ | 42.6 ± 2.0§ | 42.9 ± 2.0§ |
| Hctv (%) | 42.8 ± 2.0§ | 42.7 ± 1.8§ | 43.5 ± 1.9§ | 43.5 ± 1.8§ |
| [PPr−]a (mg ml−1) | 61.2 ± 6.2 | 60.8 ± 4.6 | 63.4 ± 4.2 | 62.1 ± 4.8§ |
| [PPr−]v (mg ml−1) | 60.7 ± 5.3§ | 62.2 ± 5.2§ | 62.2 ± 4.4§ | 61.3 ± 3.7§ |
| [PPr−]a-v (mg ml−1) | 0.5 ± 4.1§ | -1.4 ± 3.5 | 1.2 ± 2.8 | 0.9 ± 3.5 |
Hb, haemoglobin; Hct, haematocrit; PPr−, plasma protein.
Significantly different from rest
significantly different from 30%
significantly different from 65%
significantly different from pre-training.
Plasma and erythrocyte volumes
Training resulted in increases in resting BVa and BVv that were almost entirely accounted for by corresponding increases in resting PV (Table 3). In contrast, training did not significantly alter resting EVa or EVv. Before training, exercise-induced reductions in BVa and BVv were similar in direction and magnitude but did not differ from rest (Table 3). After training, however, both BVa and BVv decreased by up to 3.4 % in response to exercise (P < 0.05, Table 3).
Table 3.
Percentage changes in whole blood (ΔBV), plasma (ΔPV) and erythrocyte (ΔEV) volumes at rest and from rest to steady-state cycling of varying intensities
| Measurement | Rest | 30% 
|
65% 
|
75% 
|
|---|---|---|---|---|
| Pre-training | ||||
| ΔBVa | — | − 0.42 ± 0.53 | −1.26 ± 0.52 | −1.55 ± 0.71 |
| δBVv | — | −1.24 ± 1.20 | −2.12 ± 1.41 | −2.00 ± 1.36 |
| ΔPVa | — | 0.62 ± 1.32 | −3.10 ± 1.22‡ | −4.36 ± 2.36*‡ |
| ΔPVv | — | −0.67 ± 2.37 | −5.61 ± 2.11*‡ | −5.50 ± 1.51*‡ |
| ΔEVa | — | −1.68 ± 1.46 | 2.06 ± 2.26 | 3.81 ± 4.32‡ |
| ΔEVv | — | −1.96 ± 1.32 | 2.87 ± 1.68‡ | 3.19 ± 1.86‡ |
| Post-training | ||||
| ΔBVa | 8.4 ± 2.6§ | −0.92 ± 1.42 | −3.42 ± 1.33*‡§ | −2.75 ± 1.48*‡ |
| ΔBVv | 7.4 ± 2.7§ | −0.31 ± 1.16 | −2.77 ± 1.34*‡ | −3.37 ± 1.63*‡ |
| ΔPVa | 11.3 ± 3.9§ | 2.09 ± 2.42 | −2.27 ± 2.09‡ | −1.89 ± 2.22‡ |
| ΔPVv | 14.3 ± 6.6§ | −0.14 ± 1.23 | −3.68 ± 1.43*‡ | −4.25 ± 1.74*‡ |
| ΔEVa | 6.0 ± 3.4 | −5.53 ± 3.12* | −5.51 ± 2.46*§ | −4.35 ± 2.63*§ |
| ΔEVv | 0.2 ± 1.5 | −0.42 ± 1.40 | −1.11 ± 1.33 | −1.64 ± 2.06 |
Significantly different from rest
significantly different from 30%
significantly different from 65%
significantly different from pre-training.
PVa and PVv decreased (P < 0.05) similarly in response to incremental exercise at matched time points. However, because resting PVa and PVv were elevated after training, the absolute PVa and PVv remained elevated throughout cycling post-training. Before training, exercise induced increases in EVa and EVv of the order of 3.8 and 3.2 % at 75 %  , respectively (P < 0.05, Table 3); after training the EV decreased (P < 0.05) in both compartments at matched time points (Table 3).
, respectively (P < 0.05, Table 3); after training the EV decreased (P < 0.05) in both compartments at matched time points (Table 3).
Water flux during exercise
Before training there was progressive net water flux (Jv) from the vascular compartment into the active leg muscles, reaching 123, 201 and 113 ml min−1 at 30, 65 and 75 % of  , respectively (Table 1). Training resulted in attenuation of the exercise-induced Jv, reaching 58 ml min−1 at 30 and 65 %
, respectively (Table 1). Training resulted in attenuation of the exercise-induced Jv, reaching 58 ml min−1 at 30 and 65 %  (P < 0.05); at 75 %
(P < 0.05); at 75 %  , Jv was similar between conditions. Muscle water content was lower at 75 %
, Jv was similar between conditions. Muscle water content was lower at 75 %  after training (pre vs. post: 3.65 ± 0.08 vs. 3.44 ± 0.07 ml (g dry muscle weight)−1, P < 0.05) and associated with reduced muscle Lac− accumulation and PCr hydrolysis (Fig. 1).
after training (pre vs. post: 3.65 ± 0.08 vs. 3.44 ± 0.07 ml (g dry muscle weight)−1, P < 0.05) and associated with reduced muscle Lac− accumulation and PCr hydrolysis (Fig. 1).
Figure 1. Coordinate changes in total tissue water, and the accumulation of muscle Lac− and phosphocreatine (PCr) hydrolysis in the active leg muscles.
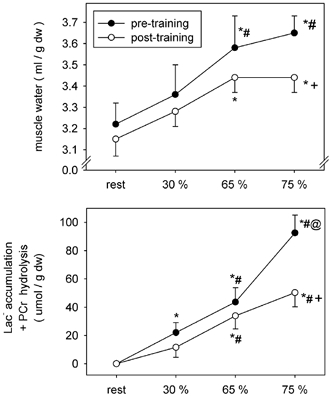
* Significantly different from rest; # significantly different from 30 %  ; © significantly different from 65 %
; © significantly different from 65 %  ; + significantly different from pre-training at matched times points.
; + significantly different from pre-training at matched times points.
Whole blood, plasma and erythrocyte ions that determine [SID]
Lactate
Training resulted in attenuation of the exercise-induced increase in [Lac−] in the whole blood, plasma and erythrocyte compartments at 65 and 75 %  (Table 4). Arterial and femoral venous whole blood [Lac−] were lower by 1.1 and 1.5 mequiv l−1 at 65 %, and by 2.0 and 2.2 mequiv l−1 at 75 %
(Table 4). Arterial and femoral venous whole blood [Lac−] were lower by 1.1 and 1.5 mequiv l−1 at 65 %, and by 2.0 and 2.2 mequiv l−1 at 75 %  , respectively. Plasma [Lac−]a and [Lac−]v were similarly reduced by 1.4 and 2.4 mequiv l−1 at 65 and 75 %
, respectively. Plasma [Lac−]a and [Lac−]v were similarly reduced by 1.4 and 2.4 mequiv l−1 at 65 and 75 %  after training (Table 4). Before training, erythrocyte [Lac−]a and [Lac−]v (Table 4) increased substantially in response to exercise, whereas after training erythrocyte [Lac−]a and [Lac−]v did not differ from resting values (Table 4).
after training (Table 4). Before training, erythrocyte [Lac−]a and [Lac−]v (Table 4) increased substantially in response to exercise, whereas after training erythrocyte [Lac−]a and [Lac−]v did not differ from resting values (Table 4).
Table 4.
Arterial and femoral venous ion concentrations in whole blood, plasma and erythrocytes
| Measurement | Condition | Rest | 30% 
|
65% 
|
75% 
|
|---|---|---|---|---|---|
| Whole blood | |||||
| [Lac−]a (mequiv l−1) | Pre | 0.7 ± 0.1 | 1.1 ± 0.2 | 3.6 ± 0.5*‡ | 5.7 ± 0.8*‡‡ |
| Post | 0.7 ± 0.1 | 0.9 ± 0.1 | 2.5 ± 0.4*‡§ | 3.7 ± 0.5*‡§ | |
| [Lac−]v (mequiv l−1) | Pre | 0.7 ± 0.1 | 1.1 ± 0.2 | 3.9 ± 0.6*‡ | 6.0 ± 0.9*‡§ |
| Post | 0.8 ± 0.1 | 0.9 ± 0.2 | 2.4 ± 0.4*‡§ | 3.8 ± 0.6*‡‡ | |
| [Cl−]a (mequiv l−1) | Pre | 82.2 ± 1.3 | 85.8 ± 0.9 | 90.8 ± 2.2*‡ | 90.3 ± 1.0*‡ |
| Post | 86.7 ± 1.1 | 85.9 ± 0.9 | 87.5 ± 1.8§ | 89.5 ± 1.3 | |
| [Cl−]v (mequiv l−1) | Pre | 85.7 ± 1.9 | 85.8 ± 1.1 | 88.3 ± 1.1 | 92.0 ± 1.8*‡‡ |
| Post | 86.5 ± 0.8 | 87.8 ± 0.6 | 85.9 ± 1.5 | 90.2 ± 0.8*‡ | |
| [Na+]a (mequiv l−1) | Pre | 100.8 ± 2.1 | 101.3 ± 3.2 | 104.5 ± 2.6 | 105.8 ± 2.4*‡ |
| Post | 106.6 ± 1.6§ | 106.1 ± 1.8§ | 106.4 ± 3.0 | 107.9 ± 3.8 | |
| [Na+]v (mequiv l−1) | Pre | 103.6 ± 3.5 | 105.8 ± 1.4 | 105.2 ± 4.0 | 106.6 ± 3.9 |
| Post | 103.3 ± 3.2 | 104.8 ± 2.4 | 104.6 ± 2.5 | 106.9 ± 3.2 | |
| [K+]a (mequiv l−1) | Pre | 49.3 ± 1.4 | 48.2 ± 1.3 | 48.8 ± 1.7 | 48.1 ± 2.2 |
| Post | 47.0 ± 1.8§ | 46.9 ± 1.8§ | 47.9 ± 2.1 | 46.6 ± 2.4 | |
| [K+]v (mequiv l−1) | Pre | 48.3 ± 1.5 | 48.5 ± 1.7 | 48.4 ± 2.2 | 43.0 ± 3.6*‡‡ |
| Post | 47.2 ± 1.8 | 46.1 ± 1.8§ | 48.0 ± 1.8 | 47.4 ± 2.3§ | |
| Plasma | |||||
| [Lac−]a (mequiv l−1) | Pre | 1.2 ± 0.2 | 1.4 ± 0.2 | 5.2 ± 0.9*‡ | 8.1 ± 1.1*‡‡ |
| Post | 0.8 ± 0.1 | 1.1 ± 0.2 | 3.9 ± 0.8*§ | 5.8 ± 1.1*‡‡§ | |
| [Lac−]v (mequiv l−1) | Pre | 1.2 ± 0.2 | 1.5 ± 0.3 | 5.4 ± 0.9*‡ | 8.8 ± 1.2*‡‡ |
| Post | 0.9 ± 0.1 | 1.2 ± 0.2 | 4.0 ± 0.9*§ | 6.4 ± 1.2*‡‡§ | |
| [Cl−]a (mequiv l−1) | Pre | 106.1 ± 1.2 | 106.8 ± 0.9 | 109.2 ± 0.7*‡ | 109.3 ± 0.5*‡ |
| Post | 108.6 ± 0.8§ | 109.2 ± 0.7§ | 109.8 ± 0.6* | 109.9 ± 0.7*§ | |
| [Cl−]v (mequiv l−1) | Pre | 105.3 ± 1.0 | 106.1 ± 0.6 | 106.5 ± 0.5 | 107.0 ± 0.5* |
| Post | 107.9 ± 0.5§ | 107.6 ± 0.5§ | 108.8 ± 0.9§ | 108.4 ± 0.6 | |
| [Na+]a (mequiv l−1) | Pre | 144.2 ± 0.6 | 143.4 ± 0.9 | 146.4 ± 0.8*‡ | 147.1 ± 0.7*‡ |
| Post | 145.1 ± 1.5 | 146.7 ± 1.17§ | 146.2 ± 0.4 | 147.2 ± 0.8* | |
| [Na+]v (mequiv l−1) | Pre | 143.7 ± 1.0 | 145.7 ± 0.7* | 147.2 ± 0.8* | 148.6 ± 0.7*‡ |
| Post | 144.7 ± 1.0 | 145.9 ± 0.7 | 147.7 ± 1.1* | 148.5 ± 0.9*‡ | |
| [K+]a (mequiv l−1) | Pre | 4.22 ± 0.12 | 4.59 ± 0.06* | 5.16 ± 0.08*‡ | 5.52 ± 0.15*‡‡ |
| Post | 4.39 ± 0.13 | 4.72 ± 0.10* | 5.19 ± 0.18*‡ | 5.32 ± 0.13*‡‡ | |
| [K+]v (mequiv l−1) | Pre | 4.03 ± 0.08 | 4.62 ± 0.06* | 5.15 ± 0.09*‡ | 5.62 ± 0. 13*‡‡ |
| Post | 4.21 ± 0.13 | 4.67 ± 0.10* | 5.10 ± 0.10*‡ | 5.37 ± 0.13*‡‡§ | |
| Erythrocytes | |||||
| [Lac−]a (mequiv l−1) | Pre | 0.04 ± 0.4 | 0.7 ± 0.1 | 1.4 ± 0.4* | 2.8 ± 0.6*‡‡ |
| Post | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.6 ± 0.7 | 0.9 ± 0.9§ | |
| [Lac−]v (mequiv l−1) | Pre | 0.1 ± 0.3 | 0.6 ± 0.1 | 2.2 ± 0.6*‡ | 2.8 ± 0.8*‡ |
| Post | 0.6 ± 0.1 | 0.4 ± 0.2 | 0.4 ± 0.7§ | 0.3 ± 0.9§ | |
| [Cl−]a (mequiv l−1) | Pre | 51.5 ± 1.0 | 57.8 ± 1.5 | 67.3 ± 5.4*‡ | 67.3 ± 3.3*‡ |
| Post | 57.0 ± 2.7§ | 51.2 ± 4.1§ | 56.2 ± 4.2§ | 60.5 ± 5.0‡§ | |
| [Cl−]v (mequiv l−1) | Pre | 61.2 ± 4.9 | 59.8 ± 3.7 | 67.2 ± 3.2 | 75.0 ± 4.5*‡‡ |
| Post | 56.6 ± 2.3 | 59.8 ± 2.3 | 55.0 ± 3.8§ | 65.4 ± 2.4§ | |
| [Na+]a (mequiv l−1) | Pre | 44.8 ± 2.7 | 46.3 ± 3.3 | 53.6 ± 5.1 | 56.8 ± 4.7* |
| Post | 54.8 ± 2.2§ | 47.9 ± 4.6 | 51.1 ± 5.3 | 54.1 ± 7.2 | |
| [Na+]v (mequiv l−1) | Pre | 54.7 ± 6.4 | 55.1 ± 5.2 | 58.1 ± 7.8 | 60.6 ± 7.2 |
| Post | 46.8 ± 5.3 | 47.7 ± 4.2 | 46.9 ± 3.4 | 51.8 ± 4.9 | |
| [K+]a (mequiv l−1) | Pre | 107.5 ± 2.2 | 105.9 ± 2.1 | 102.4 ± 3.2 | 98.8 ± 5.0* |
| Post | 104.4 ± 2.5 | 109.0 ± 3.5 | 107.4 ± 1.3§ | 104.0 ± 4.2§ | |
| [K+]v (mequiv l−1) | Pre | 103.4 ± 4.4 | 103.6 ± 2.9 | 97.5 ± 5.5 | 85.2 ± 7.8*‡‡ |
| Post | 106.9 ± 1.9 | 104.0 ± 3.0 | 105.9 ± 1.7§ | 104.1 ± 3.6§ | |
Significantly different from rest
significantly different from 30%
significantly different from 65%
significantly different from pre-training.
Lower circulating plasma [Lac−]a and [Lac−]v after training (Table 4) were accompanied by lower net muscle Lac− efflux at 30 and 65 %  (Table 5). Maintenance of similar plasma arteriovenous concentration differences in the pre- and post-training conditions at 65 and 75 %
(Table 5). Maintenance of similar plasma arteriovenous concentration differences in the pre- and post-training conditions at 65 and 75 %  occurred despite greater whole blood Lac− release before training (Fig. 2). At 65 %
occurred despite greater whole blood Lac− release before training (Fig. 2). At 65 %  , most of the pre-training Lac− load (i.e. ≥ 85 %) was taken up by the erythrocyte compartment and resulted in significant erythrocyte Lac− accumulation (Fig. 2; Table 4). At 75 %
, most of the pre-training Lac− load (i.e. ≥ 85 %) was taken up by the erythrocyte compartment and resulted in significant erythrocyte Lac− accumulation (Fig. 2; Table 4). At 75 %  there was no net erythrocyte [Lac−]a-v before training but there was an accumulation of Lac− within the erythrocyte compartment (Table 4), indicating considerable erythrocyte Lac− uptake occurred in the period that preceded sampling. Continued whole blood Lac− release at 75 %
there was no net erythrocyte [Lac−]a-v before training but there was an accumulation of Lac− within the erythrocyte compartment (Table 4), indicating considerable erythrocyte Lac− uptake occurred in the period that preceded sampling. Continued whole blood Lac− release at 75 %  before training was, therefore, entirely accounted for by the quantity released directly into the plasma compartment (Fig. 2). After training there was a small net uptake of Lac− from whole blood at 65 %
before training was, therefore, entirely accounted for by the quantity released directly into the plasma compartment (Fig. 2). After training there was a small net uptake of Lac− from whole blood at 65 %  and net release was less at 75 %
and net release was less at 75 %  (Fig. 2); a portion of the Lac− released into the plasma compartment appears to have been derived from the erythrocyte compartment (Fig. 2). Lac− efflux from the active muscles was lower during cycling after training (Table 5).
(Fig. 2); a portion of the Lac− released into the plasma compartment appears to have been derived from the erythrocyte compartment (Fig. 2). Lac− efflux from the active muscles was lower during cycling after training (Table 5).
Table 5.
Net plasma ion and H+ fluxes across contracting skeletal muscles at rest and during incremental cycling exercise
| Measurement | Rest | 30% 
|
65% 
|
75% 
|
|---|---|---|---|---|
| Pre-training | ||||
| [Lac−] (mequiv min−1) | 0.06 ± 0.41 | 0.69 ± 0.28 | 1.58 ± 0.77 | 2.91 ± 0.53*‡ |
| [Cl−] (mequiv min−1) | −2.5 ± 3.3 | −15.6 ± 10.9 | −30.2 ± 9.1* | −24.4 ± 4.9* |
| [Na+] (mequiv min−1) | −3.4 ± 4.7 | −8.8 ± 15.4 | −24.3 ± 12.8 | −8.8 ± 5.9 |
| [K+] (mequiv min−1) | 0.09 ± 0.15 | 0.82 ± 0.47 | 1.50 ± 0.65* | 1.49 ± 0.58* |
| [H+] (nequiv min−1) | 4.62 ± 1.29 | 38.47 ± 8.33* | 54.04 ± 9.83* | 62.36 ± 7.04*‡ |
| Post-training | ||||
| [Lac−] (mequiv min−1) | 0.04 ± 0.04 | 0.15 ± 0.15§ | -0.39 ± 0.52§ | 0.95 ± 1.15 |
| [Cl−] (mequiv min−1) | 0.3 ± 1.9 | −11.9 ± 6.0 | −10.6 ± 2.5§ | −25.3 ± 9.9* |
| [Na+] (mequiv min−1) | −0.2 ± 2.2 | −7.8 ± 7.6 | −0.4 ± 4.5§ | −13.7 ± 14.4 |
| [K+] (mequiv min−1) | −0.11 ± 0.06 | 0.02 ± 0.20§ | 0.29 ± 0.23§ | 0.30 ± 0.31§ |
| [H+] (nequiv min−1) | 4.06 ± 1.04 | 22.98 ± 5.12* | 43.02 ± 3.34*‡ | 92.52 ± 11.99*‡‡§ |
Significantly different from rest
significantly different from 30%
significantly different from 65%
significantly different from pre-training. Data were calculated for 2 legs. A positive value indicates net efflux from the active muscle mass, while a negative value indicates net influx; n = 5 for all analyses.
Figure 2. Arteriovenous (A-V) Lac− concentration differences for whole blood, plasma and erythrocyte compartments.
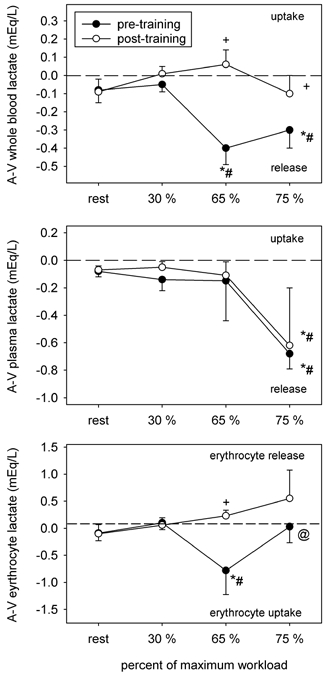
Symbols as per Fig. 1.
To further examine the relationship between the erythrocyte and plasma [Lac−] before and after training, regression analyses were completed for both conditions, within the same range of venous plasma [Lac−] (i.e. < 7.6 mequiv l−1) (Fig. 3). Training resulted in lower erythrocyte concentration for any given plasma [Lac−] (in mequiv l−1), as indicated by the reduced slope after training (P < 0.02).
Figure 3. Regression analyses of erythrocyte and plasma Lac− concentrations before and after training.
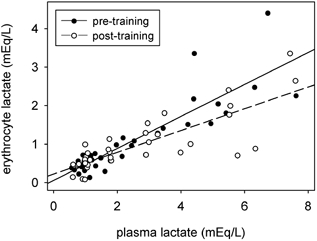
Pre-training:
| (9) |
r = 0.874, P < 0.0001, S.E. slope = 0.041, S.E.(y0) = 0.131 mequiv l−1, n = 34.
Post-training:
| (10) |
r = 0.814, P < 0.0001, S.E. slope = 0.033, S.E.(y0) = 0.106 mequiv l−1, n = 39.
Chloride
An increase in whole blood [Cl−]a from rest to 65 and 75 % of  (8.6 and 8.1 mequiv l−1 respectively) before training, was less after training (-0.6 and +2.8 mequiv l−1, respectively; Table 4). Whole blood [Cl−]v remained unchanged during incremental cycling in the pre-training condition until reaching 75 % of
(8.6 and 8.1 mequiv l−1 respectively) before training, was less after training (-0.6 and +2.8 mequiv l−1, respectively; Table 4). Whole blood [Cl−]v remained unchanged during incremental cycling in the pre-training condition until reaching 75 % of  , where it was elevated by 6.3 mequiv l−1 over resting levels; after training the rise was attenuated by 2.6 mequiv l−1. Changes in plasma [Cl−]a were similar in the pre- and post-training conditions, with small increases at 65 and 75 % of
, where it was elevated by 6.3 mequiv l−1 over resting levels; after training the rise was attenuated by 2.6 mequiv l−1. Changes in plasma [Cl−]a were similar in the pre- and post-training conditions, with small increases at 65 and 75 % of  (Table 4); post-training plasma [Cl−]a was greater than pre-training values by 0.6-2.5 mequiv l−1. Before training, plasma [Cl−]v was elevated only in response to cycling at 75 %
(Table 4); post-training plasma [Cl−]a was greater than pre-training values by 0.6-2.5 mequiv l−1. Before training, plasma [Cl−]v was elevated only in response to cycling at 75 %  (1.7 mequiv l−1). After training, plasma [Cl−]v was not altered by incremental cycling but was elevated over pre-training values by 1.5-2.6 mequiv l−1 (Table 4).
(1.7 mequiv l−1). After training, plasma [Cl−]v was not altered by incremental cycling but was elevated over pre-training values by 1.5-2.6 mequiv l−1 (Table 4).
Erythrocyte [Cl−]a and [Cl−]v progressively increased in response to incremental cycling in both conditions, but increases were attenuated after training by ≈5 to 12 mequiv l−1 (Table 4). Net movement of whole blood Cl− across the active muscles was similar between conditions (Fig. 4), while net uptake from the plasma compartment was attenuated after training (Fig. 4). Differences in Cl− movement into or out of the erythrocyte compartment between conditions were limited to rest where a significant uptake was observed after training (Fig. 5). Muscle Cl− influx was similar between conditions except during cycling at 65 %  , where it was lower (Table 5).
, where it was lower (Table 5).
Figure 4. A–V Cl− concentration differences for whole blood, plasma and erythrocyte compartments.
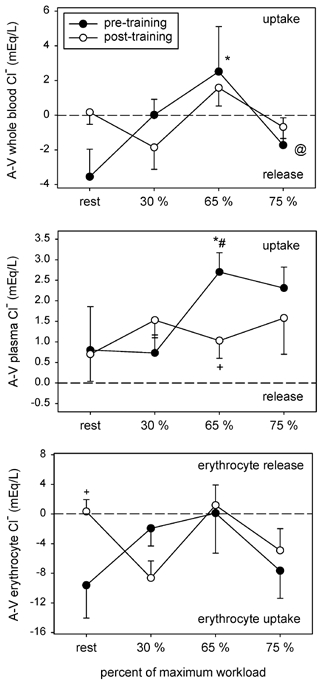
Symbols as per Fig. 1.
Figure 5. A–V Na+ concentration differences for whole blood, plasma and erythrocyte compartments.
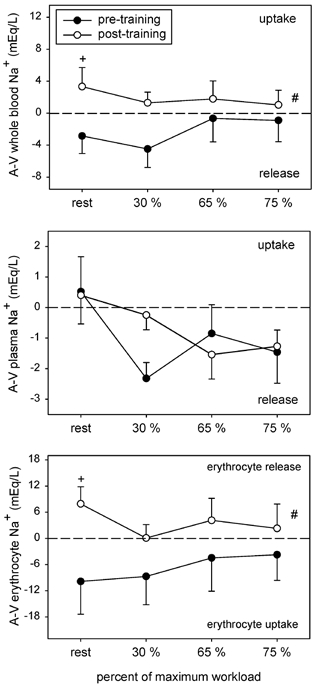
Symbols as per Fig. 1.
Sodium
Before training whole blood [Na+]a increased in response to incremental cycling reaching significance at 75 %  , where it was elevated by 5.0 mequiv l−1 over rest (Table 4). After training, whole blood [Na+]a was not altered by incremental cycling but was elevated by ≈5.8 and 4.8 mequiv l−1 at rest and at 30 %
, where it was elevated by 5.0 mequiv l−1 over rest (Table 4). After training, whole blood [Na+]a was not altered by incremental cycling but was elevated by ≈5.8 and 4.8 mequiv l−1 at rest and at 30 %  . No differences were detected in whole blood [Na+]v between conditions (Table 4). Before training, plasma [Na+]a and [Na+]v increased in response to incremental cycling at 65 and 75 % of
. No differences were detected in whole blood [Na+]v between conditions (Table 4). Before training, plasma [Na+]a and [Na+]v increased in response to incremental cycling at 65 and 75 % of  , by 2.9 and 4.9 mequiv l−1, respectively (Table 4). After training the rise was attenuated by 1 mequiv l−1 in the arterial samples. Erythrocyte [Na+]a increased gradually in response to incremental cycling in the pre-training condition, reaching significance at 75 %
, by 2.9 and 4.9 mequiv l−1, respectively (Table 4). After training the rise was attenuated by 1 mequiv l−1 in the arterial samples. Erythrocyte [Na+]a increased gradually in response to incremental cycling in the pre-training condition, reaching significance at 75 %  when it was elevated by 12 mequiv l−1 (Table 4). The only difference between conditions occurred at rest where erythrocyte [Na+]a was elevated by 10 mequiv l−1 after training.
when it was elevated by 12 mequiv l−1 (Table 4). The only difference between conditions occurred at rest where erythrocyte [Na+]a was elevated by 10 mequiv l−1 after training.
Before training the whole blood [Na+]a-v (Fig. 5) indicated a release from the leg muscles at rest and during cycling, and corresponded to an increase in Na+ uptake by the erythrocyte compartment (Fig. 5). After training, whole blood Na+ movement switched to a net uptake (training main effect, P < 0.02, Fig. 5), and was associated with net Na+ release from the erythrocyte compartment. Net Na+ release into the plasma compartment remained unchanged by training (Fig. 5). Steady-state plasma Na+-flux into the active leg muscles (Table 5) increased in response to incremental cycling in both conditions but was attenuated (P < 0.05) at 65 %  after training.
after training.
Potassium
Whole blood [K+]a remained unchanged during incremental cycling in both conditions (Table 4), but was somewhat lower at rest and at 30 %  post-training. Whole blood [K+]v was reduced by 5.3 mequiv l−1 in response to cycling at 75 %
post-training. Whole blood [K+]v was reduced by 5.3 mequiv l−1 in response to cycling at 75 %  before training but was not altered during steady-state incremental cycling after training (Table 4). In comparison with the pre-training condition, post-training whole blood [K+]v was attenuated by 2.4 mequiv l−1 at 30 % and elevated by 4.4 mequiv l−1 at 75 %
before training but was not altered during steady-state incremental cycling after training (Table 4). In comparison with the pre-training condition, post-training whole blood [K+]v was attenuated by 2.4 mequiv l−1 at 30 % and elevated by 4.4 mequiv l−1 at 75 %  . Plasma [K+]a was remarkably similar between the pre- and post-training conditions, rising to the same extent in response to incremental cycling; [K+]v was 0.25 mequiv l−1 lower (P < 0.05) at 75 %
. Plasma [K+]a was remarkably similar between the pre- and post-training conditions, rising to the same extent in response to incremental cycling; [K+]v was 0.25 mequiv l−1 lower (P < 0.05) at 75 %  after training (Table 4). In contrast, erythrocyte [K+]a and [K+]v decreased in response to cycling at 75 %
after training (Table 4). In contrast, erythrocyte [K+]a and [K+]v decreased in response to cycling at 75 %  before training (Table 4) and both were higher at this work load post-training, by 12 and 19 mequiv l−1, respectively.
before training (Table 4) and both were higher at this work load post-training, by 12 and 19 mequiv l−1, respectively.
Examination of whole blood [K+]a-v revealed a large uptake of K+ by the active muscles during steady-state cycling at 75 %  before training, which corresponded to increased erythrocyte K+ release (Fig. 6). At all other cycling intensities the whole blood [K+]a-v was similar between conditions. The plasma [K+]a-v displayed a shift from net release toward increased uptake or reduced release after training (Fig. 6). When corrected for fluid shifts, the K+-flux data showed a reduction in net efflux from the active muscles after training (Table 5).
before training, which corresponded to increased erythrocyte K+ release (Fig. 6). At all other cycling intensities the whole blood [K+]a-v was similar between conditions. The plasma [K+]a-v displayed a shift from net release toward increased uptake or reduced release after training (Fig. 6). When corrected for fluid shifts, the K+-flux data showed a reduction in net efflux from the active muscles after training (Table 5).
Figure 6. A–V K+ concentration differences for whole blood, plasma and erythrocyte compartments.
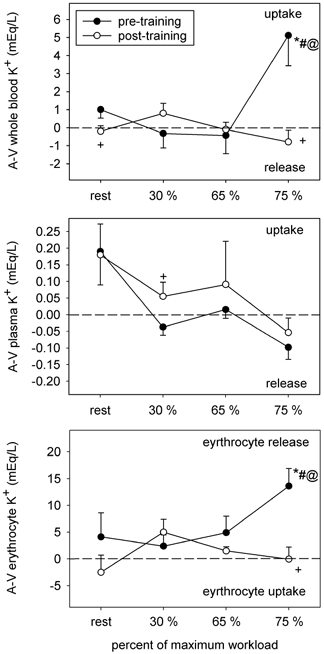
Symbols as per Fig. 1.
Arterial and venous H+
[H+]a and [H+]v increased in response to incremental exercise in both the pre- and post-training conditions but were lower after training (Fig. 7). After training, [H+]a and [H+]v were ≈3-5 mequiv l−1 and ≈4.5-6 mequiv l−1 lower, respectively. The net steady-state [H+]a-v (Fig. 7) decreased and apparent H+ efflux from the active leg muscles increased (Table 5) in response to incremental cycling in both conditions. Apparent H+ efflux was similar at all cycling intensities between the pre- and post-training conditions, except at 75 %  , where it was 50 % greater (P < 0.05) in post-training (Table 5).
, where it was 50 % greater (P < 0.05) in post-training (Table 5).
Figure 7. Arterial and venous [H+], and the arteriovenous [H+] difference before and after training.
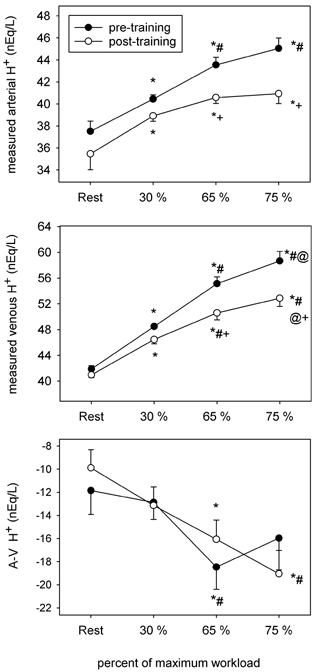
Symbols as per Fig. 1.
HCO3−
[HCO3−]a decreased progressively during incremental cycling before training, and the decrease was attenuated after training (Fig. 8). Resting [HCO3−]v rose transiently at 30 %  and declined continuously thereafter. Post-training values were higher in both the arterial and venous compartments during cycling at 65 and 75 %
and declined continuously thereafter. Post-training values were higher in both the arterial and venous compartments during cycling at 65 and 75 %  . The net apparent [HCO3−]a-v was increasingly negative across the active leg muscles during cycling but did not differ between conditions.
. The net apparent [HCO3−]a-v was increasingly negative across the active leg muscles during cycling but did not differ between conditions.
Figure 8. Arterial and venous [HCO3−], and the arteriovenous [HCO3−] difference before and after training.
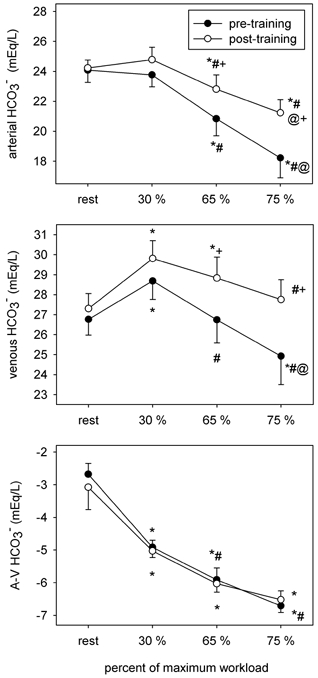
Symbols as per Fig. 1.
Independent and dependent acid–base variables
To assess the individual contributions of the three independent variables (i.e. [SID], PCO2and [Atot], Table 6), plasma [H+] was calculated as one of the three variables was changed to the corresponding exercise value while the others were held constant at resting levels (Table 7).
Table 6.
Independent variables [SID], PCO2 and [Atot] in arterial and femoral venous plasma
| Measurement | Condition | Rest | 30% 
|
65% 
|
75% 
|
|---|---|---|---|---|---|
| [SID]a (mequiv l−1) | Pre | 41.2 ± 0.7 | 39.8 ± 0.3* | 37.1 ± 0.9*‡ | 35.3 ± 1.1*‡‡ |
| Post | 40.1 ± 1.0 | 40.1 ± 0.8 | 37.7 ± 0.9*‡ | 36.8 ± 1.2*‡‡ | |
| [SID]v (mequiv l−1) | Pre | 41.2 ± 0.6 | 42.7 ± 0.6 | 40.5 ± 1.0 | 38.5 ± 1.5*‡ |
| Post | 40.1 ± 0.9 | 41.8 ± 0.3 | 40.1 ± 1.2 | 39.0 ± 1.4 | |
| Pa,CO2 (mmHg) | Pre | 37.8 ± 1.7 | 40.2 ± 1.3 | 37.9 ± 1.6 | 34.1 ± 1.9*‡‡ |
| Post | 36.0 ± 2.0 | 40.4 ± 1.7* | 38.7 ± 1.5* | 36.3 ± 1.4‡ | |
| Pv,C02 (mmHg) | Pre | 45.9 ± 1.3 | 58.1 ± 1.7* | 61.5 ± 1.8*‡ | 60.9 ± 2.6*‡ |
| Post | 46.8 ± 1.5 | 57.9 ± 2.1* | 60.9 ± 1.9*‡ | 61.2 ± 1.8*‡ | |
| [Atot]a (mequiv l−1) | Pre | 15.1 ± 0.9 | 15.9 ± 1.2 | 16.0 ± 1.0* | 18.0 ± 1.4*‡‡ |
| Post | 15.0 ± 1.5 | 14.9 ± 1.1 | 15.5 ± 1.0 | 15.2 ± 1.2§ | |
| [Atot]v (mequiv l−1) | Pre | 18.3 ± 1.9 | 17.3 ± 1.6 | 17.8 ± 1.5 | 17.2 ± 1.3 |
| Post | 14.9 ± 1.3 | 15.2 ± 1.3§ | 15.2 ± 1.1§ | 15.0 ± 0.9§ |
[SID], strong ion difference; Pa,Co2, arterial partial pressure of CO2; Pv,CO2, venous partial pressure of CO2; [Atot], total concentration of weak acids.
Significantly different from rest
significantly different from 30%
significantly different from 65%
significantly different from pre-training.
Arterial plasma
The greater increase in [H+]a from rest to 65 %  before training (Fig. 7) resulted from the cumulative effect of a 4.1 mequiv l−1 decrease in [SID]a (P < 0.05) and a 1 mequiv l−1 increase in [Atot]a (Table 6), which accounted for 80 and 14 % of the increase in [H+]a, respectively (Table 7). The increase in [H+]a from rest to 75 %
before training (Fig. 7) resulted from the cumulative effect of a 4.1 mequiv l−1 decrease in [SID]a (P < 0.05) and a 1 mequiv l−1 increase in [Atot]a (Table 6), which accounted for 80 and 14 % of the increase in [H+]a, respectively (Table 7). The increase in [H+]a from rest to 75 %  (Fig. 7) resulted from a 5.9 mequiv l−1 decrease in [SID]a (P < 0.05) and a 2.9 mequiv l−1 increase in [Atot]a (P < 0.05, Table 6), which contributed up to 91 and 35 % of the increase in [H+]a (Table 7); those changes were counteracted by a 3.7 mmHg decrease (P < 0.05) in Pa,CO2(Table 6) that corresponded to greater
(Fig. 7) resulted from a 5.9 mequiv l−1 decrease in [SID]a (P < 0.05) and a 2.9 mequiv l−1 increase in [Atot]a (P < 0.05, Table 6), which contributed up to 91 and 35 % of the increase in [H+]a (Table 7); those changes were counteracted by a 3.7 mmHg decrease (P < 0.05) in Pa,CO2(Table 6) that corresponded to greater 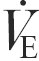 (Table 1), and prevented a potential 33 % increase in [H+] (Table 7).
(Table 1), and prevented a potential 33 % increase in [H+] (Table 7).
Lower plasma [H+]a at 65 %  after training resulted from a small increase in [SID]a and a small decrease in [Atot]a (Table 6). Exercise-induced increases in [H+]a from rest to 65 %
after training resulted from a small increase in [SID]a and a small decrease in [Atot]a (Table 6). Exercise-induced increases in [H+]a from rest to 65 %  post-training (Fig. 7) resulted from a 2.4 mequiv l−1 decrease in [SID]a (P < 0.05) and a 2.7 mmHg increase in Pa,CO2(P < 0.05, Table 6). These changes accounted for 49 and 37 % of the increase in [H+]a, respectively (Table 7); an ≈1 mequiv l−1 increase in [Atot]a accounted for up to 8 % of the increase in [H+].
post-training (Fig. 7) resulted from a 2.4 mequiv l−1 decrease in [SID]a (P < 0.05) and a 2.7 mmHg increase in Pa,CO2(P < 0.05, Table 6). These changes accounted for 49 and 37 % of the increase in [H+]a, respectively (Table 7); an ≈1 mequiv l−1 increase in [Atot]a accounted for up to 8 % of the increase in [H+].
Lower plasma [H+]a at 75 %  in the post-training condition (Fig. 7) resulted from a 1.5 mequiv l−1 increase in the [SID]a (P < 0.05) and a 2.8 mequiv l−1 decrease in [Atot]a (P < 0.05) (Table 6). Exercise-induced increases in [H+]a from rest to 75 %
in the post-training condition (Fig. 7) resulted from a 1.5 mequiv l−1 increase in the [SID]a (P < 0.05) and a 2.8 mequiv l−1 decrease in [Atot]a (P < 0.05) (Table 6). Exercise-induced increases in [H+]a from rest to 75 %  post-training (Fig. 7) primarily resulted from a 3.3 mequiv l−1 decrease in [SID]a (P < 0.05, Table 6) and accounted for 88 % of the increase in [H+]a (Table 7); the remainder was attributed to small increases in Pa,CO2and [Atot]a.
post-training (Fig. 7) primarily resulted from a 3.3 mequiv l−1 decrease in [SID]a (P < 0.05, Table 6) and accounted for 88 % of the increase in [H+]a (Table 7); the remainder was attributed to small increases in Pa,CO2and [Atot]a.
Venous plasma
From rest to cycling at 65 %  , in the pre-training condition, 96 % of the increase in plasma [H+]v (Fig. 7) was attributed to a 15.6 mmHg increase in Pv,CO2(P < 0.05) (Tables 6 and 7); reductions in the [SID]v and [Atot]v (Table 6) accounted for +8 and −5 % of the change in plasma [H+]v (Table 7). In contrast from rest to cycling at 75 %
, in the pre-training condition, 96 % of the increase in plasma [H+]v (Fig. 7) was attributed to a 15.6 mmHg increase in Pv,CO2(P < 0.05) (Tables 6 and 7); reductions in the [SID]v and [Atot]v (Table 6) accounted for +8 and −5 % of the change in plasma [H+]v (Table 7). In contrast from rest to cycling at 75 %  , 28 % of the increase in plasma [H+]v resulted from a 2.7 mequiv l−1 decrease in the [SID]v (P < 0.05), while 77 % of the increase was due to a 15 mmHg increase in Pv,CO2(P < 0.05) (Tables 6 and 7); a 1.1 mequiv l−1 reduction in [Atot]v (Table 6) contributed to a potential 9 % decrease in plasma [H+]v (Table 7). In contrast, exercise-induced increases in [H+]v during cycling at 65 and 75 %
, 28 % of the increase in plasma [H+]v resulted from a 2.7 mequiv l−1 decrease in the [SID]v (P < 0.05), while 77 % of the increase was due to a 15 mmHg increase in Pv,CO2(P < 0.05) (Tables 6 and 7); a 1.1 mequiv l−1 reduction in [Atot]v (Table 6) contributed to a potential 9 % decrease in plasma [H+]v (Table 7). In contrast, exercise-induced increases in [H+]v during cycling at 65 and 75 %  after training primarily resulted from ≈14 and 15 mmHg increases (P < 0.05) in Pv,CO2(Table 6), accounting for 95 and 84 % of the increase in [H+]v, respectively (Table 7). At 75 %
after training primarily resulted from ≈14 and 15 mmHg increases (P < 0.05) in Pv,CO2(Table 6), accounting for 95 and 84 % of the increase in [H+]v, respectively (Table 7). At 75 %  , a 0.9 mequiv l−1 decrease in [SID]v (Table 6) accounted for an additional 12 % increase in plasma [H+]v. The training-induced reduction in plasma [H+]v at 65 and 75 %
, a 0.9 mequiv l−1 decrease in [SID]v (Table 6) accounted for an additional 12 % increase in plasma [H+]v. The training-induced reduction in plasma [H+]v at 65 and 75 %  (Fig. 7) was entirely attributed to respective 2.6 and 2.2 mequiv l−1 reductions in [Atot]v (P < 0.05, Table 6).
(Fig. 7) was entirely attributed to respective 2.6 and 2.2 mequiv l−1 reductions in [Atot]v (P < 0.05, Table 6).
Erythrocyte [SID]
Before training incremental exercise was associated with increases in erythrocyte [Lac−], [Cl−] and [Na+] and lower erythrocyte [K+] (Table 4) that resulted in reductions in both the erythrocyte [SID]a and [SID]v (Fig. 9). These changes were associated with greater net erythrocyte Lac− (Fig. 2) and Cl− (Fig. 4) uptake, and increased net erythrocyte K+ release (Fig. 6). After training, greater erythrocyte [SID] (Fig. 9) resulted from lower [Lac−] and [Cl−] and higher [K+] (Table 4); these changes were associated with greater net erythrocyte Lac− release (Fig. 2) and lower net erythrocyte K+ release (Fig. 6).
Figure 9. Arterial and venous erythrocyte [SID] before and after training.
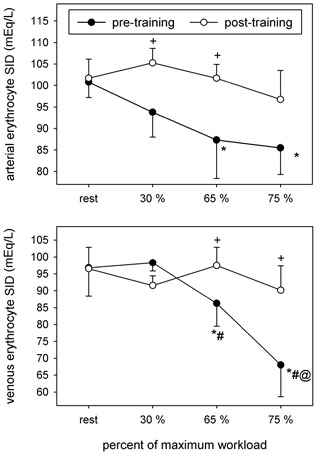
Symbols as per Fig. 1.
DISCUSSION
An integrated physicochemical systems approach was taken to examine the effects of short-term submaximal training on arterial and femoral venous plasma acid–base balance during steady-state exercise at three submaximal intensities. This was chosen in the light of its ability to quantify the important variables contributing to changes in [H+] in various fluid compartments; as in previous studies that used the approach, we found excellent agreement between measured plasma [H+], and plasma [H+] calculated by the equation derived by Stewart (1983) (r = 0.806, P < 0.0001). The short-term (1 week) training protocol led to attenuation of the rise in plasma [H+]a and [H+]v in response to incremental steady-state cycling (Fig. 7) and proved to be a good experimental model to study the associated early adaptive changes in [SID], PCO2 and [Atot]. Whilst in the past, exercise-induced acid- base changes, and the effects of training upon them, have been ascribed mainly to changes in plasma [Lac−], the results suggested a more complex picture, to which several factors contributed. Recent studies have shown that changes in erythrocyte ion concentrations occur during heavy exercise (McKelvie et al. 1991; Lindinger et al. 1995, 1999); such changes were less following training. Whilst some of this effect may be ascribed to less Lac− efflux from the exercising muscle, there may also be changes that occur following training on erythrocyte ion exchange mechanisms.
Fluid volume changes
Short-term submaximal training induced 11 and 14 % increases in resting PVa and PVv, respectively, and accounted for all of the increase in total BV (Table 3). Our findings are in accordance with previous reports that used similar short-term (Green et al. 1987b, 1991a; Sawka et al. 2000) and long-term (Green et al. 1991c; Sawka et al. 2000) exercise training protocols. The observed increases in PV were most probably associated with hormonal changes that are known to accompany exercise training, such as increases in vasopressin, plasma renin, aldosterone, atrial naturetic peptide and catecholamines (Roy et al. 2001). For the group of subjects examined, it could be calculated (Sawka et al. 2000) that training induced an absolute increase in resting PV that amounted to ≈500 ml. Greater PVa and PVv did not, however, result in lower resting concentrations of PPr− (Table 2) or electrolytes (Table 4). Despite initial differences in resting arterial BV and PV, the relative exercise-induced reductions in these measurements during steady-state cycling exercise were similar in magnitude between the pre- and post-training conditions (Table 3) and thus cannot account for any of the electrolyte concentration changes between the two conditions.
Following training there was a marked reduction in water flux from the vascular compartment into muscle. As the present subjects underwent needle muscle biopsy samples, these changes may be compared with the accompanying changes in intramuscular water content. Changes in the wet weight/dry weight ratio indicated a lower water content at 75 %  (pre vs. post: 3.65 ± 0.08 vs. 3.44 ± 0.07 ml (g dry weight)−1, P < 0.05). Considering an active muscle mass of 9.2 kg wet weight and a difference of ≈0.21 ml of water per gram of dry muscle (Fig. 1) this amounted to an absolute reduction in muscle water content of the order of ≈630 ml, and was similar to a previous report (Lindinger et al. 1994). It has been suggested that the main driving force to muscle water accumulation is the osmotic effect accompanying the breakdown of glycogen to lactate and of creatine phosphate. Training reduced the increase in muscle [Lac−] from rest to 75 %
(pre vs. post: 3.65 ± 0.08 vs. 3.44 ± 0.07 ml (g dry weight)−1, P < 0.05). Considering an active muscle mass of 9.2 kg wet weight and a difference of ≈0.21 ml of water per gram of dry muscle (Fig. 1) this amounted to an absolute reduction in muscle water content of the order of ≈630 ml, and was similar to a previous report (Lindinger et al. 1994). It has been suggested that the main driving force to muscle water accumulation is the osmotic effect accompanying the breakdown of glycogen to lactate and of creatine phosphate. Training reduced the increase in muscle [Lac−] from rest to 75 %  from 40 to 13 mmol (kg dry weight)−1, and the decrease in creatine phosphate from 46 to 30 mmol (kg dry weight)−1 (Putman et al. 1998). However, the absence of relative exercise-induced differences in PV between conditions, suggests that greater fluid movement into the active muscles before training may have been compensated by a proportional shift from comparatively inactive tissues into the intravascular space (Lindinger et al. 1990).
from 40 to 13 mmol (kg dry weight)−1, and the decrease in creatine phosphate from 46 to 30 mmol (kg dry weight)−1 (Putman et al. 1998). However, the absence of relative exercise-induced differences in PV between conditions, suggests that greater fluid movement into the active muscles before training may have been compensated by a proportional shift from comparatively inactive tissues into the intravascular space (Lindinger et al. 1990).
Greater EV observed during cycling at 75 %  of the pre-training condition (Table 3) probably resulted from an increase in the osmolarity of the erythrocyte compartment. Van Beaumont et al. (1981) reported that increases in EV were proportional to the degree of acidification and the increase in plasma osmolarity; it was further reported that changes in EV became detectable when osmolarity, resulting from chloride shift and Lac− entry into the erythrocyte compartment, was elevated by at least 5 mosmol l−1. Calculation of the erythrocyte osmolarity from data in Table 4 indicates that an approximate 10 mosmol l−1 increase occurred in our subjects, supporting a similar mechanism regulating EV during exercise. Our findings, in the pre-training condition, differed from those of Lindinger et al. (1994) who reported a decrease of 1-3 % in EV. This was probably related to the widely different training status of the two groups of subjects. The relative (45 ml kg min−1) and absolute (3.7 l min−1)
of the pre-training condition (Table 3) probably resulted from an increase in the osmolarity of the erythrocyte compartment. Van Beaumont et al. (1981) reported that increases in EV were proportional to the degree of acidification and the increase in plasma osmolarity; it was further reported that changes in EV became detectable when osmolarity, resulting from chloride shift and Lac− entry into the erythrocyte compartment, was elevated by at least 5 mosmol l−1. Calculation of the erythrocyte osmolarity from data in Table 4 indicates that an approximate 10 mosmol l−1 increase occurred in our subjects, supporting a similar mechanism regulating EV during exercise. Our findings, in the pre-training condition, differed from those of Lindinger et al. (1994) who reported a decrease of 1-3 % in EV. This was probably related to the widely different training status of the two groups of subjects. The relative (45 ml kg min−1) and absolute (3.7 l min−1)  of our subjects was significantly lower than in those studied by Lindinger et al. (i.e. relative
of our subjects was significantly lower than in those studied by Lindinger et al. (i.e. relative  = 63 ml kg min−1 and absolute
= 63 ml kg min−1 and absolute  = 4.9 l min−1), and probably formed the basis for fundamental qualitative and quantitative differences in ion regulation. In contrast, after training the decrease in EV of our subjects was similar to that reported by Lindinger et al. (1994).
= 4.9 l min−1), and probably formed the basis for fundamental qualitative and quantitative differences in ion regulation. In contrast, after training the decrease in EV of our subjects was similar to that reported by Lindinger et al. (1994).
Effects of training on the concentrations of strong ions
Lactate
In the present study, increases in plasma [Lac−]a and [Lac−]v contributed to reductions in plasma [SID]a and [SID]v. The reduction in plasma [Lac−] in both compartments after training (Table 4) was thus central to improved acid–base homeostasis, contributing to greater plasma [SID]a at 75 %  , and to lower rates of change for plasma [SID]a and [SID]v during incremental cycling (Fig. 10).
, and to lower rates of change for plasma [SID]a and [SID]v during incremental cycling (Fig. 10).
Figure 10. Contributions of [SID], [Lac−] and [Atot] to changes in the non-volatile acid concentrations in arterial and venous plasma before and after training.
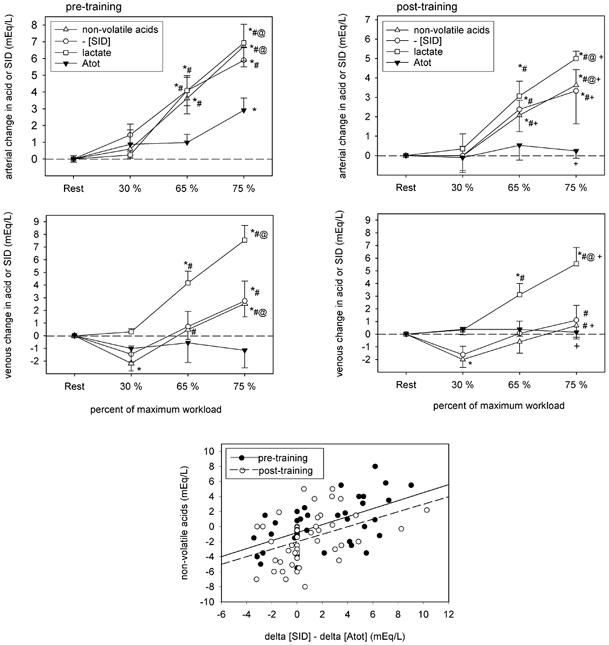
Symbols as per Fig. 1.
During exercise the prevailing plasma [Lac−] results from the balance of production and removal, with each component being highly dependent on exercise intensity (Lindinger et al. 1995; Brooks, 1999). In a previously published investigation (Putman et al. 1998), in which the same subjects were studied, muscle glycogen utilisation, the accumulation of glycolytic intermediates, glucose uptake and pyruvate oxidation were found to be similar before and after training during steady-state cycling at 65 %  , indicating that the rates of Lac− production were similar. Thus lower circulating [Lac−]a and [Lac−]v at 65 %
, indicating that the rates of Lac− production were similar. Thus lower circulating [Lac−]a and [Lac−]v at 65 %  after training must have resulted from an increased rate of Lac− clearance. These findings are in accordance with a previous study (Phillips et al. 1995b), which used isotopic tracer methods and showed increased Lac− clearance during steady-state exercise of a similar intensity (i.e. 59 %
after training must have resulted from an increased rate of Lac− clearance. These findings are in accordance with a previous study (Phillips et al. 1995b), which used isotopic tracer methods and showed increased Lac− clearance during steady-state exercise of a similar intensity (i.e. 59 %  ) after short-term training. In contrast, at 75 %
) after short-term training. In contrast, at 75 %  factors underlying the post-training reduction in plasma [Lac−]a and [Lac−]v were twofold. First, lower net muscle Lac− production occurred as the direct result of a reduction in the glycogenolytic rate (Chesley et al. 1996; Putman et al. 1998); second, similar rates of Lac− efflux were observed between conditions (Table 5), while post-training plasma and erythrocyte [Lac−] values were reduced, and plasma flow (Table 1) and erythrocyte Lac− extrusion (Fig. 2 and Fig. 3) were increased, indicating that Lac− clearance was also increased at this higher intensity.
factors underlying the post-training reduction in plasma [Lac−]a and [Lac−]v were twofold. First, lower net muscle Lac− production occurred as the direct result of a reduction in the glycogenolytic rate (Chesley et al. 1996; Putman et al. 1998); second, similar rates of Lac− efflux were observed between conditions (Table 5), while post-training plasma and erythrocyte [Lac−] values were reduced, and plasma flow (Table 1) and erythrocyte Lac− extrusion (Fig. 2 and Fig. 3) were increased, indicating that Lac− clearance was also increased at this higher intensity.
An important, but unexpected, finding was a reduction in erythrocyte [Lac−]. Previous studies established that erythrocyte [Lac−] increased by 0.4 in proportion to plasma [Lac−], as found pre-training in the present study. Post-training the proportion fell to 0.28. After short-term training, a lower plasma [Lac−] has been correlated with an increased capacity for monocarboxylate transporter-1 (MCT-1) carrier-mediated Lac− transport (Bonen et al. 1998). Similar to findings of that study, the post-training reduction in erythrocyte [Lac−] (Fig. 3; Table 4) formed a unique component of the adaptive response, and represents a novel finding of the present study. In the presence of a lower erythrocyte [H+]i/[H+]o transmembrane gradient after training, as reflected in the higher erythrocyte [SID] (Fig. 9), these adaptive changes may be interpreted as an increase in erythrocyte Lac− transporter content (Juel & Halestrap, 1999). Indeed greater erythrocyte Lac− transport capacity has been observed in trained individuals (Skelton et al. 1998). Such changes would serve to facilitate the bi-directional movement of Lac− between the erythrocyte, plasma and intracellular compartments, and to facilitate more rapid redistribution of Lac− to active and inactive tissues, thus contributing to increased post-training Lac− clearance.
Sodium
The movement of Na+ into the muscular compartment has been previously reported in response to cycling at 75 %  (Lindinger et al. 1994) and maximal exercise (McKenna et al. 1997). In those reports it was suggested that increased interstitial Na+ attenuated the fall of intracellular [SID] at the expense of the plasma [SID], and facilitated the re-uptake of intracellular K+. Additionally, a study by McKelvie et al. (1991) reported significant uptake of Na+ by the erythrocyte compartment during and after maximal exercise that was similar in magnitude to that observed in the pre-training condition of the present study (Fig. 5). This too was consistent with attenuating the fall in erythrocyte [SID], precipitated by the large accumulation of erythrocyte Lac−.
(Lindinger et al. 1994) and maximal exercise (McKenna et al. 1997). In those reports it was suggested that increased interstitial Na+ attenuated the fall of intracellular [SID] at the expense of the plasma [SID], and facilitated the re-uptake of intracellular K+. Additionally, a study by McKelvie et al. (1991) reported significant uptake of Na+ by the erythrocyte compartment during and after maximal exercise that was similar in magnitude to that observed in the pre-training condition of the present study (Fig. 5). This too was consistent with attenuating the fall in erythrocyte [SID], precipitated by the large accumulation of erythrocyte Lac−.
In the present study, the rate of Na+ flux into the muscular compartment was elevated in response to incremental steady-state cycling and attenuated by training (Table 5). The stepwise increases in Na+ influx (Table 5), and erythrocyte Na+ uptake (Fig. 5) in response to incremental cycling are consistent with a role in attenuating the reduction in [SID] within the plasma and erythrocyte compartments. Lower Na+ influx into the muscular compartment, and a lessening of erythrocyte Na+ uptake after training seem to have occurred secondary to adaptive changes in these compartments with regard to lower [Lac−] and greater [SID] (Tables 4 and 6; Fig. 2 and Fig. 9).
Potassium
Exercise-induced decreases in intramuscular [K+] contribute to increases in intracellular [H+] via associated reductions in intracellular [SID] and may underlie some of the muscular fatigue associated with exercise (Lindinger et al. 1995). In addition, corresponding increases in the extracellular-to-intracellular K+ ratio (i.e. [K+]o/[K+]i) are thought to underlie the aetiology of both local muscular fatigue, via its effects on membrane depolarisation, as well as on the development of central fatigue (Sejersted & Sjogaard, 2000). As shown by McKelvie et al. (1991), during exercise the erythrocyte compartment plays an active role in regulating extracellular plasma [K+]. This has the effect of preventing large and rapid increases in [K+]o/[K+]i when release is increased and of also counteracting reductions in plasma [SID] and associated increases in [H+], which have also been associated with fatigue.
In the present study, plasma [K+]a was similar before and after training (Table 4); [K+]v was also similar, with the exception of 75 %  , where [K+]v was lower by 0.25 mequiv l−1 (Table 4). It appears, thus, that during steady-state cycling the erythrocyte compartment played an active role in re-establishing K+ homeostasis, which was achieved much earlier after training, as indicated by a reduction in the apparent whole blood [K+] uptake, erythrocyte release, plasma release (Fig. 6) and net K+ efflux (Table 5). The primary underlying adaptive change in this regard was most probably greater muscle Na+,K+-ATPase pump content leading to an associated increase in sarcolemmal K+ transport capacity (Green et al. 1993, 2000) and therefore more rapid muscle K+ uptake early during the rest-to-work transition phase of each cycling intensity.
, where [K+]v was lower by 0.25 mequiv l−1 (Table 4). It appears, thus, that during steady-state cycling the erythrocyte compartment played an active role in re-establishing K+ homeostasis, which was achieved much earlier after training, as indicated by a reduction in the apparent whole blood [K+] uptake, erythrocyte release, plasma release (Fig. 6) and net K+ efflux (Table 5). The primary underlying adaptive change in this regard was most probably greater muscle Na+,K+-ATPase pump content leading to an associated increase in sarcolemmal K+ transport capacity (Green et al. 1993, 2000) and therefore more rapid muscle K+ uptake early during the rest-to-work transition phase of each cycling intensity.
Chloride
Whilst the changes in plasma [Cl−] were relatively minor both with exercise and following training, large changes occurred in erythrocyte [Cl−]. Before training, erythrocyte [Cl−] increased by about 16 mequiv l−1 between rest and 75 %  in both arterial and venous blood, whereas following training the increase was reduced, to about 9 mequiv l−1. Deuticke et al. (Deuticke, 1982; Deuticke et al. 1982) demonstrated that that a non-specific anion exchanger for Cl− on the erythrocyte membrane has an affinity for Lac− ions, and thus may be partially responsible for transport of Lac− into erythrocytes. A specific Lac− transporter sensitive to changes in plasma H+ may have an important role in modulating plasma [Lac−]. Movement of Lac− and Cl− from plasma into the erythrocyte tends to lower plasma [H+] by increasing plasma [SID]; because the buffer capacity (dependent on [Atot]) in plasma alone is so much less than in erythrocytes, the ion exchange is a powerful mechanism preventing large increases in plasma [H+] in heavy exercise. To what extent the changes following training merely accompany reductions in lactate production in muscle, or are due to alterations in the function of the ion exchangers remains to be established. However, the changes in the relationship between plasma and erythrocyte [Lac−] may be an indication that control of transporters has been modified as a result of training.
in both arterial and venous blood, whereas following training the increase was reduced, to about 9 mequiv l−1. Deuticke et al. (Deuticke, 1982; Deuticke et al. 1982) demonstrated that that a non-specific anion exchanger for Cl− on the erythrocyte membrane has an affinity for Lac− ions, and thus may be partially responsible for transport of Lac− into erythrocytes. A specific Lac− transporter sensitive to changes in plasma H+ may have an important role in modulating plasma [Lac−]. Movement of Lac− and Cl− from plasma into the erythrocyte tends to lower plasma [H+] by increasing plasma [SID]; because the buffer capacity (dependent on [Atot]) in plasma alone is so much less than in erythrocytes, the ion exchange is a powerful mechanism preventing large increases in plasma [H+] in heavy exercise. To what extent the changes following training merely accompany reductions in lactate production in muscle, or are due to alterations in the function of the ion exchangers remains to be established. However, the changes in the relationship between plasma and erythrocyte [Lac−] may be an indication that control of transporters has been modified as a result of training.
Independent variables, [SID], [Atot] and PCO2
[SID]
In the present study, changes in plasma [SID] represented a significant component of the adaptive response in both arterial and femoral venous blood; greater [SID] post-training accounted for up to 31 and 16 % of the reduction in plasma [H+]a and [H+]v, respectively, during heavy exercise (Tables 6 and 7). The smaller fall in arterial [SID] with increasing exercise intensity, following training, paralleled the smaller increase in plasma [Lac−]. Although, as widely appreciated, increases in plasma [Lac−] play a important role in reducing plasma [SID] in heavy exercise, the results of the present study raise the possibility that their effects may be lessened through transfer of both Lac− and Cl− from plasma into erythrocytes. Large increases in the erythrocyte concentrations of both ions, together with falls in K+ were observed before training, thus sparing the plasma dramatic reductions in [SID], at the expense of a large fall in erythrocyte [SID]. Improved plasma acid–base status following training was associated with less accumulation of Cl−, Lac− and Na+, and higher K+ in erythrocytes. Before training, erythrocyte [SID] fell from 101 to 86; after training it fell from 102 to 96. It seems possible that if we consider the defence of plasma homeostasis to be of great importance during exercise, the erythrocyte may play a larger role than previously appreciated.
[Atot]
Plasma [Atot] is primarily composed of plasma proteins, and is an important consideration when examining the control of plasma acid–base balance. Although usually considered in relation to buffering capacity, they act as weak acids; thus, hyperproteinaemia increases plasma [H+], while hypoproteinaemia results in the opposite response (Rossing et al. 1986). In the present study, lower [Atot]a and [Atot]v after training contributed to lower plasma [H+]a and [H+]v (Tables 6 and 7). Pre-training exercise-induced increases in [PPr−]a (Table 2) and [Atot]a (Table 6) at 65 %  corresponded to an equivalent decrease in PVa (Table 3), and accounted for 14 % of the total increase in [H+]a (Table 7; Fig. 8). In contrast during cycling at 75 %
corresponded to an equivalent decrease in PVa (Table 3), and accounted for 14 % of the total increase in [H+]a (Table 7; Fig. 8). In contrast during cycling at 75 %  before training, [PPr−]a (Table 2) and [Atot]a (Table 6) were elevated by ≈19 %, and accounted for 35 % of the increase in [H+]a (Table 7; Fig. 8). At this higher intensity, however, the decrease in PVa could account for only 4 % of the change in [PPr−]a and [Atot]a. This indicates a relative gain of protein within the arterial plasma compartment of the order of ≈15 %, or an absolute gain of ≈28 g. While it is not possible to determine from the present data the source of the increased intravascular protein, it was probably derived from an increased rate of albumin clearance from the interstitial space of the active muscle mass (Havas et al. 2000) or from cutaneous tissues (Harrison & Edwards, 1975; Freund et al. 1987). The calculated absolute gain in plasma protein in our study was within the range of a previous report that quantified increases in plasma albumin in response to exercise (Hayes et al. 2000). By comparison, after training there were no exercise-induced alterations in [PPr−]a (Table 2) or [Atot]a (Table 6). Thus it appears that fluid shifts and greater albumin clearance rate contributed to changes in [Atot]a in the untrained state, and ultimately to the exercise-induced increase in plasma [H+]a. By comparison, lower arterial and venous [Atot] after training (Table 6) resulted solely from greater PV, and was a major adaptive response contributing to lower plasma arterial and venous [H+].
before training, [PPr−]a (Table 2) and [Atot]a (Table 6) were elevated by ≈19 %, and accounted for 35 % of the increase in [H+]a (Table 7; Fig. 8). At this higher intensity, however, the decrease in PVa could account for only 4 % of the change in [PPr−]a and [Atot]a. This indicates a relative gain of protein within the arterial plasma compartment of the order of ≈15 %, or an absolute gain of ≈28 g. While it is not possible to determine from the present data the source of the increased intravascular protein, it was probably derived from an increased rate of albumin clearance from the interstitial space of the active muscle mass (Havas et al. 2000) or from cutaneous tissues (Harrison & Edwards, 1975; Freund et al. 1987). The calculated absolute gain in plasma protein in our study was within the range of a previous report that quantified increases in plasma albumin in response to exercise (Hayes et al. 2000). By comparison, after training there were no exercise-induced alterations in [PPr−]a (Table 2) or [Atot]a (Table 6). Thus it appears that fluid shifts and greater albumin clearance rate contributed to changes in [Atot]a in the untrained state, and ultimately to the exercise-induced increase in plasma [H+]a. By comparison, lower arterial and venous [Atot] after training (Table 6) resulted solely from greater PV, and was a major adaptive response contributing to lower plasma arterial and venous [H+].
P CO2
Due to the steady-state nature of the exercise protocol employed, the production of CO2 probably resulted from increased aerobic metabolism and was not influenced by movement of CO2 from stores (Jones & Heigenhauser, 1996). Neither Pa,CO2 nor Pv,CO2 differed between conditions at matched time points (Table 1). Consequently, the effects of PCO2 on [H+] in arterial or venous plasma must not have differed between conditions (Table 6). As expected, the primary effect of plasma CO2 accumulation on the exercise-induced increase in plasma [H+] was observed in the venous compartment where it accounted for 77-96 % of the exercise-induced increase in [H+]v (Table 7). This was not surprising especially during heavy exercise when the venous compartment behaves as a closed system, with CO2 content increasing greatly before being eliminated by the lungs. In contrast, the arterial compartment is considered an open system and, as such, changes in PaCO2 played a comparatively smaller role. The only time point in which PaCO2 influenced the plasma [H+]a was during cycling at 75 %  before training, when a relative hyperventilation of 8.1 l min−1 (Table 1) prevented an additional 33 % increase in [H+]a.
before training, when a relative hyperventilation of 8.1 l min−1 (Table 1) prevented an additional 33 % increase in [H+]a.
Muscle H+ efflux
The sarcolemmal MCT-1 Lac−-H+ co-transporter is considered the dominant acid-extruding system during intense exercise and has been shown to be upregulated in response to exercise training (Juel, 1998). Increases in the content and activity of the MCT-1 Lac−-H+ co-transporter after training have been suggested to underlie increases in H+ efflux from the active muscle bed during heavy exercise, and greater Lac−-H+ uptake during recovery from activity (Juel, 1998). While MCT-1 and other membrane transporters are undoubtedly central to the regulation of ion exchange between fluid compartments, the means by which they mediate the putative co-transfer of H+ remains uncertain. This has, to some extent, confounded our understanding of the in vivo regulation of plasma acid–base balance. According to the unifying theory of acid–base regulation proposed by Stewart (1983), the transport of H+per se does not necessarily alter [H+] on either side of a membrane, mainly due to the huge capacity of water to provide a ‘sink’ for H+ and OH−. The prevailing [H+] within a fluid compartment results from changes in the concentration of the independent variables whose movements occur by facilitated transport or by diffusion (i.e. strong ions and CO2, respectively), thus creating only the appearance of H+ movement. In the present study, the apparent post-training extrusion of H+ from active muscles was elevated by 1.5-fold (P < 0.05, Table 5) during steady-state cycling at 75 %  , and could not be attributed entirely to greater plasma flow (Table 1). Furthermore, greater post-training H+-extrusion corresponded to threefold and fivefold reductions (P < 0.05) in Lac− efflux and K+ influx, respectively, and a 1.6-fold increase in Na+ influx (Table 5). Thus the increase in H+ efflux from muscle post-training could not be explained by increased Lac−-H+ co-transport, and suggests the independent movement of Lac−.
, and could not be attributed entirely to greater plasma flow (Table 1). Furthermore, greater post-training H+-extrusion corresponded to threefold and fivefold reductions (P < 0.05) in Lac− efflux and K+ influx, respectively, and a 1.6-fold increase in Na+ influx (Table 5). Thus the increase in H+ efflux from muscle post-training could not be explained by increased Lac−-H+ co-transport, and suggests the independent movement of Lac−.
Conclusions
The present study examined the effect of short-term submaximal training on [SID], PCO2and [Atot] in plasma arterial and venous blood samples during incremental exercise. The major finding of the present study is that short-term submaximal exercise training induced adaptive changes in two of the three independent physicochemical variables resulting in the attenuation of exercise-induced plasma [H+] during heavy exercise. Lower steady-state [H+]a post-training was attributed to: (1) attenuation of the absolute exercise-induced decrease in [SID]a; and (2) attenuation of the exercise-induced increase in [Atot]a. By comparison, lower steady-state [H+]v after training primarily resulted from the abolition of exercise-induced increases in [Atot]v. Changes in [SID] resulted from altered Lac−, K+, Cl− and Na+ kinetics within the muscular and erythrocyte compartments, while alterations in [Atot] resulted from greater initial PV and reduced water fluxes post-training. The results confirm and amplify the findings of previous studies, which have emphasised the importance of ion transfer between plasma and erythrocytes.
Acknowledgments
The authors thank T. Bragg, Dr M. Ganagaragah and G. Obminski for excellent technical assistance with these experiments. This study was funded in part by research grants from the Canadian Institutes for Health Research (to G. J. F. Heigenhauser and N. L. Jones), the Heart and Stroke Foundation of Ontario (to G. J. F. Heigenhauser), the Natural Sciences and Engineering Council of Canada (to C. T. Putman) and the Alberta Heritage Foundation for Medical Research (AHFMR, to C. T. Putman). G. J. F. Heigenhauser is a career investigator of the Heart and Stroke Foundation of Ontario. C. T. Putman is a Heritage Medical Scholar of AHFMR.
REFERENCES
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmeyer HU. Methods of Enzymatic Analysis. 3. New York: Academic Press; 1983. [Google Scholar]
- Bonen A, McCullagh KJ, Putman CT, Hultman E, Jones NL, Heigenhauser GJ. Short-term training increases human muscle MCT1 and femoral venous lactate in relation to muscle lactate. Am J Physiol. 1998;274:E102–107. doi: 10.1152/ajpendo.1998.274.1.E102. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Are arterial, muscle and working limb lactate exchange data obtained on men at altitude consistent with the hypothesis of an intracellular lactate shuttle. Adv Exp Med Biol. 1999;474:185–204. doi: 10.1007/978-1-4615-4711-2_16. [DOI] [PubMed] [Google Scholar]
- Deuticke B. Monocarboxylate transport in erythrocytes. J Membr Biol. 1982;70:89–103. doi: 10.1007/BF01870219. [DOI] [PubMed] [Google Scholar]
- Deuticke B, Beyer E, Forst B. Discrimination of three parallel pathways of lactate transport in the human erythrocyte membrane by inhibitors and kinetic properties. Biochim Biophys Acta. 1982;684:96–110. doi: 10.1016/0005-2736(82)90053-0. [DOI] [PubMed] [Google Scholar]
- Freund BJ, Claybaugh JR, Dice MS, Hashiro GM. Hormonal and vascular fluid responses to maximal exercise in trained and untrained males. J Appl Physiol. 1987;63:669–675. doi: 10.1152/jappl.1987.63.2.669. [DOI] [PubMed] [Google Scholar]
- Green HJ. Adaptations in the muscle cell to training: role of the Na+-K+-Atpase. Can J Appl Physiol. 2000;25:204–216. doi: 10.1139/h00-016. [DOI] [PubMed] [Google Scholar]
- Green HJ, Chin ER, Ball-Burnett M, Ranney D. Increases in human skeletal muscle Na(+)-K(+)-ATPase concentration with short-term training. Am J Physiol. 1993;264:C1538–1541. doi: 10.1152/ajpcell.1993.264.6.C1538. [DOI] [PubMed] [Google Scholar]
- Green HJ, Coates G, Sutton JR, Jones S. Early adaptations in gas exchange, cardiac function and haematology to prolonged exercise training in man. Eur J Appl Physiol. 1991a;63:17–23. doi: 10.1007/BF00760795. [DOI] [PubMed] [Google Scholar]
- Green HJ, Hughson RL, Thomson JA, Sharratt MT. Supramaximal exercise after training-induced hypervolemia. I. Gas exchange and acid–base balance. J Appl Physiol. 1987a;62:1944–1953. doi: 10.1152/jappl.1987.62.5.1944. [DOI] [PubMed] [Google Scholar]
- Green HJ, Jones LL, Hughson RL, Painter DC, Farrance BW. Training-induced hypervolemia: lack of an effect on oxygen utilization during exercise. Med Sci Sports Exerc. 1987b;19:202–206. [PubMed] [Google Scholar]
- Green HJ, Jones S, Ball-Burnett M, Smith D, Livesey J, Farrance B. Early muscular and metabolic adaptations to prolonged exercise training in humans. J Appl Physiol. 1991b;70:2032–2038. doi: 10.1152/jappl.1991.70.5.2032. [DOI] [PubMed] [Google Scholar]
- Green HJ, Sutton JR, Coates G, Ali M, Jones S. Response of red cell and plasma volume to prolonged training in humans. J Appl Physiol. 1991c;70:1810–1815. doi: 10.1152/jappl.1991.70.4.1810. [DOI] [PubMed] [Google Scholar]
- Harrison MH. Effects on thermal stress and exercise on blood volume in humans. Physiol Rev. 1985;65:149–209. doi: 10.1152/physrev.1985.65.1.149. [DOI] [PubMed] [Google Scholar]
- Harrison MH, Edwards RJ, Leitch DR. Effect of exercise and thermal stress on plasma volume. J Appl Physiol. 1975;39:925–931. doi: 10.1152/jappl.1975.39.6.925. [DOI] [PubMed] [Google Scholar]
- Havas E, Lehtonen M, Vuorela J, Parviainen T, Vihko V. Albumin clearance from human skeletal muscle during prolonged steady-state running. Exp Physiol. 2000;85:863–868. [PubMed] [Google Scholar]
- Hayes PM, Lucas JC, Shi X. Importance of post-exercise hypotension in plasma volume restoration. Acta Physiol Scand. 2000;169:115–124. doi: 10.1046/j.1365-201x.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- Heigenhauser GJ. A quantitative approach to acid–base chemistry. Can J Appl Physiol. 1995;20:333–340. doi: 10.1139/h95-026. [DOI] [PubMed] [Google Scholar]
- Jones NL. A quantitative physicochemical approach to acid–base physiology. Clin Biochem. 1990;23:189–195. doi: 10.1016/0009-9120(90)90588-l. [DOI] [PubMed] [Google Scholar]
- Jones NL, Heigenhauser GJ. Getting rid of carbon dioxide during exercise. Clin Sci. 1996;90:323–335. doi: 10.1042/cs0900323. [DOI] [PubMed] [Google Scholar]
- Juel C. Muscle pH regulation: role of training. Acta Physiol Scand. 1998;162:359–366. doi: 10.1046/j.1365-201X.1998.0305f.x. [DOI] [PubMed] [Google Scholar]
- Juel C, Halestrap AP. Lactate transport in skeletal muscle-role and regulation of the monocarboxylate transporter. J Physiol. 1999;517:633–642. doi: 10.1111/j.1469-7793.1999.0633s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalchuk JM, Heigenhauser GJF, Jones NL. Effect of pH on metabolic and cardiorespiratory responses during progressive exercise. J Appl Physiol. 1984;57:1558–1563. doi: 10.1152/jappl.1984.57.5.1558. [DOI] [PubMed] [Google Scholar]
- Kowalchuk JM, Scheuermann BW. acid–base balance: origin of plasma [H+] during exercise. Can J Appl Physiol. 1995;20:341–356. doi: 10.1139/h95-027. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, Heigenhauser GJF, McKelvie RS, Jones NL. Role of nonworking muscle on blood metabolites and ions with intense intermittent exercise. Am J Physiol. 1990;258:R1486–1494. doi: 10.1152/ajpregu.1990.258.6.R1486. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, Heigenhauser GJF, McKelvie RS, Jones NL. Blood ion regulation during repeated maximal exercise and recovery in humans. Am J Physiol. 1992;262:R126–136. doi: 10.1152/ajpregu.1992.262.1.R126. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, Horn PL, Grudzien SP. Exercise-induced stimulation of K+ transport in human erythrocytes. J Appl Physiol. 1999;87:2157–2167. doi: 10.1152/jappl.1999.87.6.2157. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, McKelvie RS, Heigenhauser GJ. K+ and Lac− distribution in humans during and after high-intensity exercise: role in muscle fatigue attenuation. J Appl Physiol. 1995;78:765–777. doi: 10.1152/jappl.1995.78.3.765. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randell RJ. Protein measurements with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McCartney N, Heigenhauser GJF, Jones NL. Effects of pH on maximal power output and fatigue during short- term dynamic exercise. J Appl Physiol. 1983a;55:225–229. doi: 10.1152/jappl.1983.55.1.225. [DOI] [PubMed] [Google Scholar]
- McCartney N, Heigenhauser GJF, Jones NL. Power output and fatigue of human muscle in maximal cycling exercise. J Appl Physiol. 1983b;55:218–224. doi: 10.1152/jappl.1983.55.1.218. [DOI] [PubMed] [Google Scholar]
- McKelvie RS, Lindinger MI, Heigenhauser GJ, Jones NL. Contribution of erythrocytes to the control of the electrolyte changes of exercise. Can J Physiol Pharmacol. 1991;69:984–993. doi: 10.1139/y91-148. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Harmer AR, Fraser SF, Li JL. Effects of training on potassium, calcium and hydrogen ion regulation in skeletal muscle and blood during exercise. Acta Physiol Scand. 1996;156:335–346. doi: 10.1046/j.1365-201X.1996.199000.x. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Heigenhauser GJ, McKelvie RS, MacDougall JD, Jones NL. Sprint training enhances ionic regulation during intense exercise in men. J Physiol. 1997;501:687–702. doi: 10.1111/j.1469-7793.1997.687bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, MacDonald MJ, Hughson RL. Progressive effect of endurance training on VO2 kinetics at the onset of submaximal training. J Appl Physiol. 1995a;79:1914–1920. doi: 10.1152/jappl.1995.79.6.1914. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnopolsky MA, Grant SM. Increased clearance of lactate after short-term training in men. J Appl Physiol. 1995b;79:1862–1869. doi: 10.1152/jappl.1995.79.6.1862. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GJ, Grant SM. Progressive effect of endurance training on metabolic adaptations in working skeletal muscle. Am J Physiol. 1996;270:E265–272. doi: 10.1152/ajpendo.1996.270.2.E265. [DOI] [PubMed] [Google Scholar]
- Putman CT, Jones NL, Hultman E, Hollidge-Horvat MG, Bonen A, McConachie DR, Heigenhauser GJ. Effects of short-term submaximal training in humans on muscle metabolism in exercise. Am J Physiol. 1998;275:E132–139. doi: 10.1152/ajpendo.1998.275.1.E132. [DOI] [PubMed] [Google Scholar]
- Rossing TH, Maffeo N, Fencl V. acid–base effects of altering plasma protein concentration in human blood in vitro. J Appl Physiol. 1986;61:2260–2265. doi: 10.1152/jappl.1986.61.6.2260. [DOI] [PubMed] [Google Scholar]
- Roy BD, Green HJ, Grant SM, Tarnopolsky MA. Acute plasma volume expansion in the untrained alters the hormonal response to prolonged moderate-intensity exercise. Horm Metab Res. 2001;33:238–245. doi: 10.1055/s-2001-14943. [DOI] [PubMed] [Google Scholar]
- Sawka MN, Convertino VA, Eichner ER, Schnieder SM, Young AJ. Blood volume: importance and adaptations to exercise training, environmental stresses, and trauma/sickness. Med Sci Sports Exerc. 2000;32:332–348. doi: 10.1097/00005768-200002000-00012. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Sjogaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- Skelton MS, Kremer DE, Smith EW, Gladden LB. Lactate influx into red blood cells from trained and untrained human subjects. Med Sci Sports Exerc. 1998;30:536–542. doi: 10.1097/00005768-199804000-00011. [DOI] [PubMed] [Google Scholar]
- Stewart PA. Modern quantitative acid–base chemistry. Can J Physiol Pharmacol. 1983;61:1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- Van Beaumont W, Underkofler S, Van Beaumont S. Erythrocyte volume, plasma volume, and acid–base changes in exercise and heat hydration. J Appl Physiol. 1981;50:1255–1262. doi: 10.1152/jappl.1981.50.6.1255. [DOI] [PubMed] [Google Scholar]


