Abstract
We have examined the influence of cortico-tectal projections from one of the pattern-processing extrastriate visual cortical areas, area 21a, on the responses to visual stimuli of single neurones in the superior colliculi of adult cats. For this purpose area 21a was briefly inactivated by cooling to 10 °C using a Peltier device. Responses to visual stimuli before and during cooling as well as after rewarming ipsilateral area 21a were compared. In addition, in a subpopulation of collicular neurones we have studied the effects of reversible inactivation of ipsilateral striate cortex (area 17, area V1). When area 21a was cooled, the temperature of area 17 was kept at 36 °C and vice versa. In the majority of cases (41/65; 63 %), irrespective of the velocity response profiles of collicular neurones, inactivation of area 21a resulted in a significant decrease in magnitude of responses of neurones in the ipsilateral colliculus and only in a small proportion of cells (2/65; 3.1 %) was there a significant increase in the magnitude of responses. Inactivation of area 21a resulted in significant changes in the magnitude of responses of collicular cells located not only in the retino-recipient layers but also in the stratum griseum intermediale. In most cases, reversible inactivation of area 17 resulted in a greater reduction in the magnitude of responses of collicular cells than inactivation of area 21a. Reversible inactivation of area 21a also affected the direction selectivity indices and length tuning of most collicular cells tested.
In highly ‘visual’ mammals such as virtually all primates (including the most extensively studied macaque monkeys) and carnivores with frontally positioned eyes (e.g. domestic cats), information about the visual world is relayed from the retina to the primary visual cortex through the dorsal lateral geniculate nucleus (LGNd) by a number of largely parallel information channels (cf. for recent reviews Burke et al. 1998; Vidyasagar et al. 2002). In the retino-geniculo-cortical pathway of the cat, the parallel streams consist of the so-called W, X and Y channels while in macaque monkeys they consist of the analogous if not homologous streams, respectively koniocellular, parvocellular and magnocellular channels (cf. for reviews Burke et al. 1998; Vidyasagar et al. 2002). In primates, the parallel information channels apparent in the retino-geniculo-primary visual cortex pathway continue to a large extent in the two parallel information-processing streams in the extrastriate cortices, the so-called ‘what’ and ‘where’ streams (Ungerleider & Mishkin, 1982) or their respective equivalents the ‘form/pattern' ('ventral' or ‘perception’) and ‘motion’ ('dorsal' or ‘action’) streams (for reviews see Goodale & Milner, 1992; Young 1992; Burke et al. 1998). The existence of distinct form/pattern and motion streams in the cat's visual cortex has recently been challenged on the basis of global analysis of cortical connectivity (cf. Scannell et al. 1999; Hilgetag et al. 2000). However, analysis of cortical connectivity combined with functional analysis (e.g. receptive field properties, effects of selective reversible inactivation of distinct cortical areas on visual discriminations) strongly supports the notion that there are two distinct parallel information-processing streams in visual cortices of cats (Dreher, 1986; Dreher et al. 1996; Lomber et al. 1996; for reviews see Burke et al. 1998; Lomber, 2001). In the cat, the principal input to the form/pattern cortical stream derives from the X-channel while that to the motion cortical stream derives mainly from the Y channel (for review see Burke et al. 1998).
The principal retino-recipient nucleus of the mammalian midbrain, the superior colliculus (SC; cf. Berson, 1988; Rhoades et al. 1991; Stein & Meredith, 1991; Waleszczyk et al. 1999; Wang et al. 2001) appears to be mainly involved in directing visual attention to novel stimuli of any modality, multimodal integration and spatial location of the object of interest, followed by the initiation and termination of saccadic eye movements to bring the image of the object of interest onto the high spatial resolution area of the retina - the fovea centralis or area centralis (cf. for reviews Stein & Meredith, 1991; Schiller & Tehovnik, 2001). Thus, the SC appears to be mainly involved in motion detection rather than form/pattern analysis (cf. however, Sprague et al. 1970; Tunkl & Berkley, 1977, 1985; Sprague, 1991; Lomber, 2002). Consistent with this, in the cat the superficial (retino-recipient) collicular layers receive their principal direct retinal inputs via the W and Y channels (Berson 1988; Tamamaki, et al. 1995; Waleszczyk et al. 1999; Wang et al. 2001) and very little or no input via the X-channel (cf. Hoffmann, 1973; Tamamaki, et al. 1995; for reviews see Berson, 1988; Stein & Meredith, 1991). Although the retino-recipient, as well as the deep collicular layers, receive also indirect Y-like input relayed via cortico-tectal projections from layer 5 of the ipsilateral visual cortex (Hoffmann, 1973), there is no clear functional or morphological evidence indicating that the cortico-tectal projection from the ipsilateral visual cortex relays X-type information (Berson 1988). On the other hand, it has been clearly demonstrated that the SC receives a substantial direct input (cf. for review Harting et al. 1992) not only from the motion-processing extrastriate cortical areas (e.g area 18 and areas located around the lateral suprasylvian sulcus) but also from the form/pattern-processing extrastriate areas such as area 21a and areas 20a and 20b in the ventral temporal cortex (cf. for review Burke et al. 1998).
Area 17 of the cat appears to be involved both in form/pattern and motion analysis (e.g. Pasternak et al. 1995). Consistent with this, in addition to strong X-type input and at least some W-type input, area 17 receives a substantial Y-type input from the LGNd (cf. Stone & Dreher, 1973; Dreher et al. 1980; for review see Burke et al. 1998). Therefore, reported clear effects of reversible inactivation (Wickelgren & Sterling, 1969; Ogasawara et al. 1984) or permanent removal (Wickelgren & Sterling, 1969; Rosenquist & Palmer, 1971) of area 17 on the response magnitude and/or receptive field properties of neurones in the retino-recipient layers of the ipsilateral SC could be related to the Y-type nature of area 17 neurones projecting to the SC (Palmer & Rosenquist, 1974). The bulk of cortical input to another of the form/pattern-processing visual areas, area 21a (Fig. 1A and B; Dreher, 1986; Mizobe et al. 1988; Wimborne & Henry, 1992; Dreher et al. 1993, 1996; Morley et al. 1997; cf. for reviews Burke et al. 1998; Lomber, 2001) originates from ipsilateral area 17 (Rosenquist, 1985; Dreher, 1986; Dreher et al. 1996). However, in contrast to area 17, area 21a, despite the fact that a substantial proportion (15-25 %) of its cortical input originates from ipsilateral area 18 (the principal target of Y-type LGNd input; cf. Stone et al. 1973; Dreher et al. 1980, 1996), appears not to receive a significant excitatory input from the Y channel (Dreher et al. 1993). In view of the fact that: (1) the superficial layers of the SC project to laminae C of the LGNd (containing W cells) as well as to the lateral posterior–pulvinar complex (LP-pulvinar) of the dorsal thalamus, which in turn, project to cortical areas 17, 18, 19 and 21a and (2) cells in laminae 5 of areas 17, 18, 19 and 21a project directly to the superficial, retino-recipient layers of the ipsilateral SC (Fig. 1B), the cortico-tectal projections could also be considered as ‘feedback’ projections.
Figure 1. Visual cortical areas and principal connections of superior colliculus of the cat.
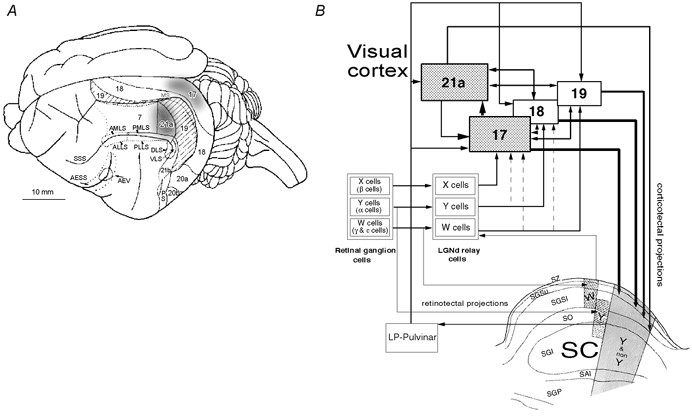
A, dorsolateral view of the surface anatomy of the left cerebral hemisphere of the cat with cortical visual areas outlined. In addition to visuotopically organised cytoarchitectonic areas 7, 17, 18, 19, 21a, 21b, 20a and 20b there are several visuotopically organised lateral suprasylvian areas (anteromedial, anterolateral, posteromedial, posterolateral, dorsal and ventral; AMLS, ALLS, PMLS, PLLS, DLS and VLS, respectively), posterior suprasylvian (PS) and anterior ectosylvian visual area (AEV). AESS, anterior ectosylvian sulcus: MS, marginal sulcus; SSS, suprasylvian sulcus. The borders outlined by interrupted lines are buried in sulci (after Palmer et al. 1978; Tusa et al. 1978, 1979; Updyke, 1986). The shaded part of area 17 corresponds visuotopically to area 21a and the part of the superior colliculus we recorded from. Location of cytoarchitectonic area 7 is also indicated. Area 21a, or the shaded part of area 17 were briefly inactivated by cooling them to 10 °C. When one of the areas was cooled the other was warmed to 36 °C. B, simplified neuronal circuitry of retino-geniculo-cortical, retino-collicular, cortico-cortical and cortico-tectal pathways. A schematic diagram of a coronal section through the superior colliculus (SC) is also incorporated in the diagram. The anatomical subdivisions of the SC are: SZ, stratum zonale; SGSu, stratum griseum superficiale upper; SGSl, stratum griseum superficiale lower; SO, stratum opticum; SGI, stratum griseum intermediale; SAI, stratum album intermediale; SGP, stratum griseum profundum. Note that while the dorsal lateral geniculate nucleus (LGNd) and indirectly the visual cortices are innervated by three distinct functional and morphological types of retinal ganglion cells (X, Y, W), the SC is innervated only by Y and W-type retinal ganglion cells. The primary visual cortices (areas 17 and 18) and area 19 all receive direct W-type geniculate input. Direct Y-type geniculate input is restricted to areas 18 and 17 while direct X-type geniculate input is restricted to area 17. Area 21a constitutes part of the form/pattern- rather than motion-processing stream (cf. Burke et al. 1998; Lomber, 2001). The superficial layers of the SC project to laminae C of the LGNd (containing W cells) as well as to the lateral posterior–pulvinar complex (LP-pulvinar) of the dorsal thalamus which in turn projects to cortical areas 17, 18, 19 and 21a. The cortico-tectal projections from primary visual cortices are more numerous and tend to terminate more superficially than those from areas 19 and 21a. Furthermore, some terminals from areas 19 and 21a, unlike these from areas 17 and 18, terminate in the SGI (after Dreher, 1986; Berson, 1988; Harting et al. 1992; Dreher et al. 1996; Burke et al. 1998; Waleszczyk et al. 1999).
In the present study we have attempted to examine the functional role of the cortico-tectal projections from area 21a by determining the magnitude of responses as well as some of the receptive field properties of collicular neurones before and during reversible inactivation and after reactivation of the ipsilateral area 21a. In addition, in a subpopulation of collicular cells we compared the effects of reversible inactivation of ipsilateral area 21a with those of reversible inactivation of ipsilateral area 17. Our data indicate that area 21a exerts a strong, mostly excitatory, effect on the magnitude of responses of most neurones in the retino-recipient layers and the stratum griseum intermediale of the SC. However, in the retino-recipient layers at least, the influence of area 21a tends to be weaker than that of area 17 (cf. Ogasawara et al. 1984). Furthermore, area 21a appears to ‘fine-tune' some specific receptive field properties of the collicular neurones such as direction- and length-selectivities. A preliminary report describing some of our findings has already been published in the form of an abstract (Hashemi-Nezhad et al. 2001).
METHODS
Experimental procedures and husbandry followed the guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes and were approved by the Animal Ethics Committee of the University of Sydney.
Animals, anaesthesia and surgical procedures
Nine adult cats of either sex weighing 2.4-4.5 kg provided by the Laboratory Animal Services of Sydney University were used. Initial surgery involving cannulation of the trachea and left cephalic vein as well as bilateral cervical sympathectomy (to minimise eye movements) was carried out under ketamine anaesthesia (20 mg kg−1, I.M.). Throughout the rest of the experiment, anaesthesia was maintained with a gaseous mixture of 0.4-0.7 % halothane in N2O-O2 (67 %-33 %). Heart rate and electroencephalogram (EEG) were monitored continuously and maintained, respectively, at less than 180 beats min−1 and at a slow-wave synchronised activity by adjusting the halothane level in the anaesthetic mixture. Neuromuscular blockade was induced with an intravenous injection of 40 mg of gallamine triethiodide in 1 ml of sodium lactate (Hartmann's) solution and maintained with a continuous intravenous infusion of gallamine triethiodide at a rate of 7.5 mg kg−1 h−1 in a mixture of equal parts of 5 % dextrose and Hartmann's solution. Animals were artificially ventilated with a pulmonary pump, their body temperature monitored continuously and maintained at around 37 °C with an electric heating blanket. Expired CO2 was also monitored continuously and maintained at 3.5-4.0 % by adjusting the rate or stroke volume of the pulmonary pump. Each day, antibiotic (amoxycillin trihydrate, 75 mg), dexamethasone phosphate (4 mg) and atropine sulphate (0.3 mg) were injected I.M. Furthermore, to prevent brain oedema, every alternate day 5 ml of 25 % solution of D-mannitol was injected intravenously.
The corneas were protected with zero-power, air-permeable plastic contact lenses. Pupils were dilated and accommodation was paralysed with 1 % atropine sulphate solution. The nictitating membranes were retracted with 0.01 % phenylephrine hydrochloride. Corrective lenses were used if necessary (as determined with streak retinoscope), to focus the eyes on the tangent screen placed 57 cm in front of the animal. To reduce spherical aberrations, artificial pupils, 3 mm in diameter were placed in front of the eyes. The location of the optic discs and the areae centrales were plotted twice daily using a fibre-optic projector (Pettigrew et al. 1979).
Recording
A plastic cylinder was mounted and glued with dental acrylic around a craniotomy made over area 17/superior colliculus (Horsley-Clarke coordinates A4 to P10 and L0-5) and area 21a (P0-8; L8-16). To gain microelectrode access to the SC a small incision in the dura was made over area 17. The vertically oriented microelectrode was advanced fairly rapidly until the tip was positioned 14 mm below the cortical surface. Further advance was then made with a stepping micro-drive. To reduce brain pulsation, the well over the craniotomy was covered with 4 % agar in physiological saline, and sealed with warm wax (melting point 40 °C). For reversible inactivation of the cortices, fitted Peltier probes were placed over areas 17 and 21a (for details see below and Fig. 1A).
The action potentials of single neurones were isolated, amplified, window-discriminated and used to trigger standard pulses which were fed into a microcomputer to generate online peristimulus time histograms (PSTHs). The signal was also acoustically amplified and broadcast over a loudspeaker.
Photic simulation and data analysis
The classical excitatory receptive fields (RFs) were identified and plotted by hand-held light and black spots. The size of the excitatory receptive field was defined as the area of visual space within which moving photic stimuli elicited an increase in the cell's firing rate that was detectable by ear (minimum discharge field; cf. Barlow et al. 1967). A quantitative analysis followed this qualitative protocol. For this purpose, stimulation of the cell was carried out using light slits from the slide projector. The light slits were projected onto a mirror attached to a galvanometer which was controlled by a microcomputer. The light slits had a luminance of 15 cd m−2 against the background of 0.9 cd m−2. Alternatively, the cells were stimulated via a monitor screen (BARCO) with custom-made software. To determine the optimal stimulus parameters (axis and direction preferences, optimal velocity, length tuning), elongated light bars of various dimensions moving over a wide range of velocities (2-2000 deg s−1) were swept across the cell's receptive field.
The PSTHs were compiled by summing the responses to a number of sweeps of the same velocity. The temporal base of each histogram was divided into 150 bins. The bin width varied depending on stimulus velocity. The peak discharge rates are the weighted averages of spike frequencies across five bins centred on the bin containing the maximal number of action potentials. The PSTHs were smoothed by second-order convolving with a Gaussian function.
For a given cell the direction selectivity index (DI) was based on the peak discharge rate of the cell to photic stimuli presented via the dominant eye, moving at optimal velocity and stimulus size parallel to the horizontal meridian of the visual field. The DI was calculated according to the following formula:
where Rp and Rnp are the peak discharge rates at the preferred and non-preferred direction, respectively (Dreher et al. 1993).
Reversible inactivation of areas 21a and 17
Once the properties of the dominant RF were established, area 21a (or area 17) was inactivated by reducing the temperature of the cooling probes to 10 °C. The cooling probes were gold-plated metal probes attached to a Peltier elements (9 W, Marlow, Dallas, TX, USA) controlled by a laboratory-built device (Wang et al. 2000). The probe placed over area 21a was quadrant-shaped with radius of 7 mm, the probe placed over area 17 was rectangular, 3 × 6 mm. The contact surfaces of the probes were shaped to match the curvatures of the cortical surfaces on which they were placed. Temperatures over areas 21a and 17 were continuously monitored by the thermocouples built into the foot of each probe. In addition, in a number of control experiments the temperature at different depths and different distances from the temperature-controlling metal probes was monitored (using thermocouples), both during the cooling and rewarming of areas 21a and 17. In the present series of experiments the temperature of the superficial layers 1 and 2 of areas 21a or 17 dropped to 10 °C within 30 s after the temperature of the Peltier probe covering the area was lowered to 10 °C (cf. Jasper et al. 1970; Wang et al. 2000). However, the temperature of the deeper layers continued to decline up to 1-2 min after the temperature of the Peltier device dropped to 10 °C (cf. Ogasawara et al. 1984; Lomber et al. 1999). In both area 21a and area 17, the temperature in layers 5, that is, the layers in which all cortico-tectal cells are located (see for review Rhoades et al. 1991) stabilised at 15-17 °C. After the temperature of the cortical surface reached 10 °C, we waited for 5 min before we started to conduct the tests. The magnitude of responses and some of the receptive field properties of collicular neurones were examined quantitatively before and during the cooling of area 21a. The spike activity was recorded by the microcomputer. To prevent irreversible damage to the cortex, the cortex was kept cool only for further 8-10 min during which the tests were conducted (13-15 min from the time the temperature of the cooling probe dropped to 10 °C). After completion of data acquisition during cortical cooling, the cortex was reactivated by slow (over a period of 1-2 min) rewarming to 36 °C. To monitor the cell's recovery, the responses were re-examined for the next 30-60 min at 10 min intervals.
Localisation of recording sites
At the end of most penetrations, small amounts of current (10-20 μA for 20 s) were passed through the electrode. The resulting small lesions allowed precise reconstruction of the recording tracks. At the end of the recording session (lasting usually 3-5 days) the animal was deeply anaesthetised with an intravenous injection of 120-220 mg of sodium pentobarbitone. The amount of sodium pentobarbitone necessary for deep anaesthesia was determined by monitoring the heart rate of the animal and discontinuing injection when the heart rate dropped to less than 30 beats min−1. Overall, the amount required correlated positively with the weight of animals. Once the animals were deeply anaesthetised they were perfused transcardially with 800 ml Hartmann's solution at 37 °C (with 2000 u of anticoagulant, heparin) followed by 1200 ml of 4 % solution of paraformaldehyde in 0.2 M phosphate buffer (pH 7.4). For cryoprotection the brains were kept in 30 % sucrose in 0.2 M phosphate buffer until they sank. The colliculi were stereotactically blocked and sectioned along the coronal plane at 50 μm on a freezing microtome. The sections were mounted on gelatinised slides and counterstained for Nissl substance with cresyl violet.
Statistics
Statistical significance of differences was obtained using two non-parametric tests. For paired comparisons, the Wilcoxon matched-pairs signed-rank test (Siegel, 1956; it will be referred to as the Wilcoxon test) was used while for non-paired comparisons the Mann–Whitney U test was used (Siegel, 1956). Statistical significance of the differences between the two sets of data was accepted if the probability (P) was < 0.05 at the two-tailed criterion. For individual collicular cells, the effect of inactivation of the cortex (area 21a or area 17) was deemed significant if a difference between the initial control state (when the cortex was at 36 °C) and the cooled state (when the cortex was at 10 °C) was statistically significant (Wilcoxon test). The recovery after inactivation was deemed significant if there was statistically significant difference (Wilcoxon test) between the cooled state and the subsequent rewarmed state (area 21a or area 17 again at 36 °C).
RESULTS
Receptive field sizes, binocularity and velocity tuning of collicular neurones
Centres of RFs of all but three collicular cells were located within 10 deg of the areae centrales (Fig. 2A). Despite the topographic restriction of RFs to the central part of the visual field, there was a clear positive correlation (n = 45; r2 = 0.514; P < 0.001) between the eccentricity of the RF positions and their sizes.
Figure 2. Receptive field properties of collicular neurones encountered in the present study.
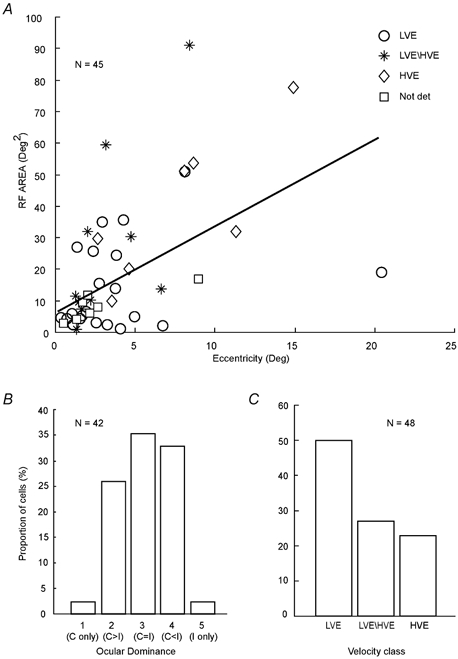
A, receptive field (RF) area of collicular neurones vs. their eccentricity from area centralis. Note that consistent with numerous previous reports (cf. Waleszczyk et al. 1999) there is a clear positive correlation between eccentricity of the RF position and size of the RF area. B, percentage histogram of eye dominance classes of collicular neurones. Class 1 cells are monocular cells which respond only to photic stimuli presented via the contralateral eye; class 2 cells are binocular cells which respond more strongly to photic stimuli presented via the contralateral eye; class 3 cells are binocular neurones which responds equally well to stimuli presented through either eye; class 4 cells are also binocular but respond more vigorously to stimuli presented through the ipsilateral eye; class 5 cells are monocular and respond only to stimuli presented via the ipsilateral eye. C, velocity profiles of collicular cells encountered. The cells were classified according to criteria established by Waleszczyk et al. (1999). LVE (low velocity excitatory) cells respond optimally to stimuli moving at velocities not exceeding 40 deg s−1; LVE/HVE (low-velocity excitatory/high-velocity excitatory) cells give clear-cut excitatory responses over a very wide range of velocities (1-1000 deg s−1), while the HVE (high-velocity excitatory) cells give clear-cut responses at velocities 20-1000 deg s−1; Not det, not determined.
As indicated in Fig. 2B and consistent with numerous previous reports (for reviews see Stein & Meredith, 1991; Waleszczyk et al. 1999) most of the cells for which we determined their eye dominance were binocular (40/42; 95.2 %) and over a third of the sample (15/42; 35.7 %) were class 3 cells, that is, cells that responded equally well to stimuli presented via the contralateral and ipsilateral eyes. The majority of cells in our sample (48/65; 73.8 %) were also classified into distinct classes based on their velocity response profiles to photic stimuli moving across their RFs (Waleszczyk et al. 1999; Fig. 2C). Thus: (1) half of the sample (24/48) were identified as low-velocity excitatory or LVE cells, since they exhibited their strongest excitatory responses to stimuli moving at velocities not exceeding 40 deg s−1 and responded poorly or not at all at velocities exceeding 100 deg s−1; (2) low-velocity excitatory/high-velocity excitatory or LVE/HVE cells, that is cells that gave clear-cut excitatory responses to stimuli moving at slow- medium (1-100 deg s−1) as well as fast (100-2000 deg s−1) velocities, constituted over a quarter of the sample (13/48; 27.1 %); and (3) the so-called high-velocity excitatory or HVE cells, that is cells that did not respond at low stimulus velocities (1-10 deg s−1) but gave clear-cut excitatory responses to stimuli moving at moderate (20-40 deg s−1) and high velocities (100-2000 deg s−1) constituted over one-fifth of the sample (11/48; 22.9 %). Our sample did not include any LVE/HVS (low-velocity excitatory/high-velocity suppressive) cells.
Effects of cooling area 21a on the magnitude of responses of collicular cells
We examined the effect of cooling of ipsilateral area 21a on the magnitude of responses of 65 collicular neurones recorded in nine cats (3-10 cells per cat). The peristimulus time histograms in Fig. 3A illustrate a fairly typical effect of reversible inactivation of ipsilateral area 21a on the responses of collicular neurones. Indeed, as in the case illustrated in Fig. 3A, and irrespective of their eye dominance class and velocity response profile, in a substantial majority of cells tested (41/65; 63 % of the sample), reversible inactivation of area 21a resulted in a significant decrease (P < 0.05, Wilcoxon test) in the magnitude of their responses to visual stimuli (Fig. 3B). Furthermore, in a substantial proportion of these cells (6/41; 14.6 % or 6/65, 9.2 % of the whole sample) area 21a appeared to provide a principal visual drive to collicular neurones as indicated by the fact that inactivation of ipsilateral area 21a resulted in a complete (albeit reversible) loss of response to the visual stimuli used in the investigation (Fig. 3B).
Figure 3. Effects of reversible inactivation of area 21a on the magnitude of responses and background (spontaneous) activity of collicular neurones.
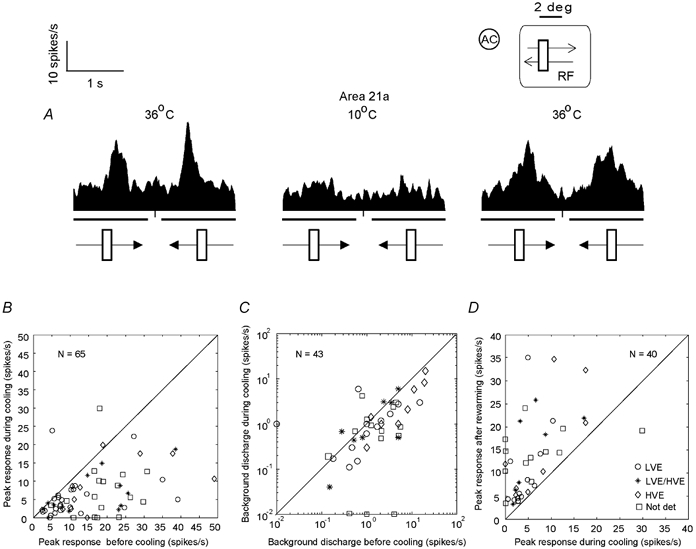
A, peristimulus time histograms (PSTHs) constructed on the basis of responses of a collicular neurone to visual stimuli before and during inactivation (by cooling) of area 21a as well as after rewarming area 21a. Each PSTH was constructed on the basis of responses to 10 presentations of a 2.7 × 0.9 deg light bar moving across the cell's RF at 5 deg s−1 parallel to the horizontal meridian. The heavy lines below the abscissae indicate the periods when the stimuli moved across the RF. The directions of movement are indicated by the arrowed bars at the bottom of the PSTHs. The inset in the upper righthand corner indicates location (in relation to the area centralis, AC) and size of the cell's RF. The length of the visual stimulus in relation to the size of the cell's RF is also indicated. Note that during cooling of the Peltier probe located over area 21a to 10 °C the cell displayed a substantial (71 %) decrease in the magnitude of response and that rewarming of area 21a resulted in a substantial (60 %) recovery of the response. B, peak discharge rates of our sample of collicular neurones during inactivation of area 21a vs. those before inactivation of area 21a. Overall, for the whole sample there was a significant drop (P < 0.0001; Mann–Whitney U test) in the magnitude of responses during cortical inactivation. C, background discharge rates of collicular neurones during inactivation of area 21a vs. background discharge rates of collicular neurones before inactivation of area 21a. For graphical purposes 0.01 spikes s−1 were added to the cells which showed zero background activity. The values are plotted only for those cells in which inactivation of area 21a resulted in significant changes in the magnitude of responses. For this sample the mean background activity was 3.36 ± 4.66 spikes s−1 (range 0.01-20 spikes s−1). During cooling of area 21a, the mean background activity of the sample decreased to 1.96 ± 2.82 spikes s−1; (range 0.01-15 spikes s−1). This effect was not significant (P = 0.06; Mann-Whiney U test). D, peak discharge rates of responses of collicular neurones after rewarming of area 21a vs. those during cooling. The values are plotted only for those cells in which rewarming of area 21a resulted in a significant recovery of the responses to pre-cooling levels. Note that in all but one cell the magnitude of response to visual stimuli was increased after rewarming of area 21a (P < 0.0001; Mann–Whitney U test). The exception was a cell that increased its responses when area 21a was cooled. Not det, not determined.
By contrast, only a small proportion of collicular cells (2/65; 3.1 %) exhibited a significant (P < 0.0001, Wilcoxon test) increase in the magnitude of their responses to visual stimuli following inactivation of area 21a.
In the remaining third of the sample (22/65; 33.8 %) inactivation of area 21a did not affect significantly (P > 0.05, Wilcoxon test) the magnitude of responses to visual stimuli. Characteristically, cells whose responses were not significantly affected by cortical inactivation, tended to respond rather weakly (peak magnitude of responses of less than 10 spikes s−1) to any visual stimuli presented.
Not surprisingly, the principal trend for the whole sample following reversible inactivation of area 21a was a highly significant (P < 0.0001; Mann–Whitney U test) reduction (mean for the whole sample 52.26 ± 28.66 %) in the magnitude of the responses to visual stimuli (Fig. 3B).
There was no clear correlation between the magnitude of changes induced by inactivation of area 21a and the velocity response classes of collicular cells. The mean reduction in the magnitude of responses of HVE cells, that is, cells which receive their principal excitatory input from the Y channel (cf. Waleszczyk et al. 1999) was lower (mean reduction 42.32 %; range 15-78 %; n = 11) than that for LVE cells (mean reduction 56.42 %; range 8-100 %; n = 24), that is, cells which receive their principal excitatory input from the W channel (cf. Waleszczyk et al. 1999). However, the difference between the two populations was not significant (P = 0.31; Mann–Whitney U test).
There was no clear correlation between the magnitude of changes induced by inactivation of area 21a and the eye dominance class of binocular cells. Thus, although the mean reduction in the magnitude of responses of binocular class 2 cells, that is, cells dominated by the input from the contralateral eye, was lower (mean reduction 54.5 %; range 9.5-86.5 %; n = 11) than that for binocular class 4 cells (mean reduction 61.7 %; range 9-100 %; n = 14), that is, cells dominated by the input from ipsilateral eye, the difference between the two populations was not significant (P > 0.06; Mann–Whitney U test).
As indicated in Fig. 3C for cells in which inactivation of area 21a resulted in significant changes in the magnitude of responses, cooling of area 21a resulted usually in a reduction in their background (spontaneous) discharge rates (mean 3.36 ± 4.66 spikes s−1 before inactivation vs. mean 1.96 ± 2.82 spikes s−1 during inactivation). However, the effect of inactivation of area 21a on the background activity was only marginal (P = 0.06; Mann–Whitney U test).
Recovery of responsiveness of collicular neurones following rewarming of area 21a
While the effects of cortical inactivation on the magnitude of responses of collicular neurones were clearly apparent within 3 min from cooling area 21a to 10 °C, the recovery following rewarming of area 21a to 36 °C had a much slower time course. Thus, the time for a complete or a partial but steady level of recovery varied from 30 to 60 min and the mean recovery time for the whole sample was 43.6 ± 12 min.
In all but one cell significantly affected by cooling area 21a (39/43; 93 %; Fig. 3D), there was a significant increase (P < 0.05 Wilcoxon test) in responsiveness following rewarming of area 21a. In one cell, rewarming of area 21a resulted in a significant decrease (P < 0.05; Wilcoxon test) in responsiveness. Furthermore, only in less than a fifth of the sample of cells in which the magnitude of responses to visual stimuli was significantly affected by cooling area 21a (8/43; 18.6 %), were the magnitudes of responses to optimal visual stimuli after rewarming area 21a not significantly different (P > 0.05; Wilcoxon test) from those before cooling area 21a. Of the cells which, after rewarming area 21a, exhibited a full ‘recovery’ to the pre-cooling level of responsiveness, three were LVE cells (two located in the stratum zonale or SZ and one in the lower stratum griseum superficiale or SGSl; see Fig. 1B), two were LVE/HVE cells (one located in the SGSl and one in the stratum griseum intermediale or SGI; see Fig. 1B), and three cells (located either in the SGSl or SGI; see Fig. 1B), were not identified in terms of our classification scheme (Waleszczyk et al. 1999).
For the whole sample of cells significantly affected by cooling area 21a, 30 min after rewarming area 21a the mean magnitude of responses of the cells reached only 71.42 ± 21.32 %) of the pre-cooling control values. Nevertheless, the increase in the magnitude of responses to visual stimuli following rewarming was highly significant (P < 0.001; Mann–Whitney U test).
Effects of inactivation of area 21a vs. laminar location of collicular neurones
We were able to determine histologically the laminar location of all but four (39/43; 90.7 %) of the collicular neurones significantly affected by inactivation of area 21a (Fig. 4A and B). As indicated in Fig. 4B, most of them (29/39; 74.4 %) were located in the superficial (retino-recipient) layers with the remainder (10/39; 25.6 %) located in the most superficial of the deep layers - the SGI. Virtually all cells recorded from the SZ or the upper part of stratum griseum superficiale (SGSu) were LVE cells. In the lower part of the SGSl, LVE cells were intermingled with LVE/HVE and HVE cells.
Figure 4. Laminar location of collicular neurones and changes in the magnitude of responses during reversible inactivation of area 21a.
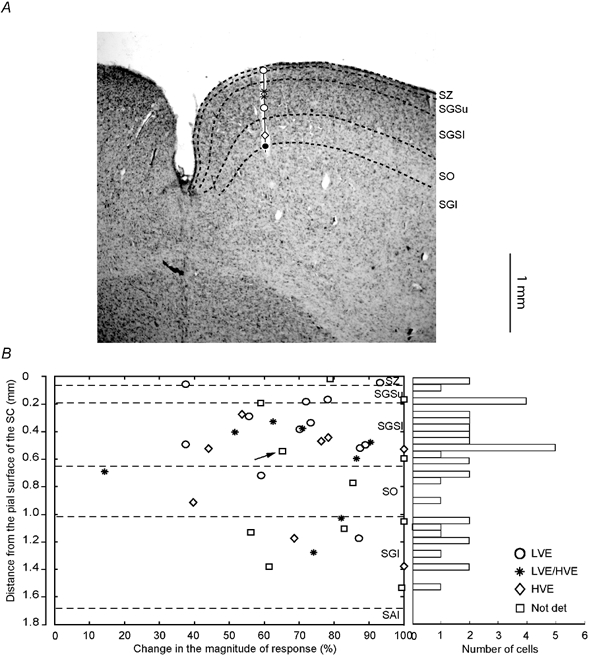
A, reconstruction of an electrode penetration through the superior colliculus. Laminar boundaries are indicated by black interrupted lines. Type and location of collicular cells are indicated by appropriate symbols (see lower right corner in B). Large black dot at the end of the track indicates the location of electrolytic lesion. B, changes in the magnitude of response during cooling area 21a vs. distance from the pial surface of the SC cells. Right, frequency histogram indicating the number of cells encountered in each lamina. Only cells which exhibited significant change in their magnitude of response during cooling are indicated. Note that in almost all the cells (38/39; 97 % of sample) the change was 38 % or more and that there is no correlation between the degree of change in the magnitude of response and the laminar position of the cell. Not det, not determined.
It is apparent from Fig. 4B that there was no clear correlation between the laminar location of collicular cells and the strength of effect of inactivation of area 21a on the magnitude of their responses to visual stimuli. Although the mean reduction in the magnitude of responses of cells located in the retino-recipient layers (SZ, SGSu, SGSl and SO), was lower (68.4 ± 22.3 %; range 14.4-100 %; n = 29) than that for cells located in the SGI(81.2 ± 16 %; range 56.2-100 %; n = 10), the difference between the two populations was not significant (P = 0.3597; Mann- Whitney U test).
Effects of inactivation of area 21a vs. effects of inactivation of area 17 on the magnitude of responses of collicular cells
In the cat, unlike in primates, area 17 (striate cortex, area V1) constitutes only a part of the primary visual cortex and is the almost exclusive direct cortical target of the X-type information relayed from the retina by the LGNd neurones (cf. Stone & Dreher, 1973; Dreher et al. 1980). In three cats, the effects of inactivation of areas 21a and area 17 were tested sequentially (after a complete or a partial but steady level of recovery). Figure 5A shows again a fairly typical effect of an inactivation of area 21a on the magnitude of response of a collicular neurone (cf. also Fig. 3A) while Fig. 5B shows for the same cell a fairly typical effect of selective cooling of area 17 (which followed, after a sufficient recovery period, rewarming of area 21a). In this case, inactivation of area 21a resulted in over 70 % reduction in the magnitude of response while inactivation of area 17 resulted in almost complete abolition (95 % reduction in magnitude) of the response.
Figure 5. Effects of inactivation of area 21a on the magnitude of responses of collicular neurones vs. effects of inactivation of area 17.
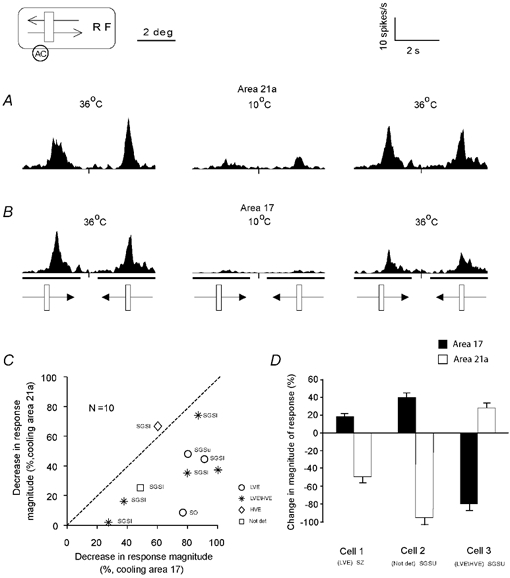
A, the PSTHs of a collicular neurone before and during cooling and after rewarming of area 21a. Each PSTH was constructed on the basis of responses to 10 presentations of a 1.6 × 0.6 deg light bar moving across the cell's RF at 5 deg s−1 parallel to the horizontal meridian. Other conventions as in Fig. 3. Note that cooling the Peltier probe located over area 21a to 10 °C resulted in a dramatic reduction (over 70 %) in the magnitude of responses and 30 min after rewarming area 21a to 36 °C the magnitude of the response was largely restored (over 84 % of the original response). B, the PSTHs of responses of the same cell before and during cooling and after rewarming of area 17. Note that the response was almost abolished (94 % reduction) during cooling the Peltier probe located over area 17 to 10 °C and 30 min after rewarming area 17 to 36 °C partially recovered (over 56 % of the pre-cooling magnitude). C, the standardised decrease in the magnitude of response of 10 collicular cells in the preferred direction during inactivation of area 21a vs. decrease in the magnitude of responses of the same cells following sequential inactivation of area 17. The cells were recorded in three animals. The laminar location (see Fig. 1) of all but one cell (Not det) is indicated. Note that inactivation of area 17 resulted in a more profound decrease in the magnitude of response of the collicular neurones than inactivation of area 21a. This difference was highly significant (P < 0.001; Wilcoxon test). D, in three of our population cells (two of them recorded in one animal), inactivation of areas 21a and 17 had opposite effects; detailed description in the text.
Indeed, in the great majority of collicular cells tested (10/13 cells; 77 % of sample), we found that inactivation of either of the cortical areas resulted in a significant (P < 0.05; Wilcoxon test) reduction in the magnitude or even abolition of responses to visual stimuli of the SC neurones. However, in all but one of these (9/10; Fig. 5C), inactivation of area 17 resulted in a more profound decrease in the magnitude of responses than that caused by inactivation of area 21a (P < 0.001; Wilcoxon test).
In the remaining cells (3/13; 23 % of sample, two of them in one animal), the effects of inactivation of area 17 were opposite to those of inactivation of area 21a (Fig. 5D). Thus, while two collicular cells displayed significant increases (18 % and 38 %, respectively) in the magnitude of responses to visual stimuli during reversible inactivation of area 17, inactivation of area 21a resulted in significant (P < 0.05; Wilcoxon test) and substantial (40 % and 100 %, respectively) reduction. By contrast, one cell displayed a 25 % increase in the magnitude of response during inactivation of area 21a and an 80 % reduction when area 17 was inactivated.
Overall, inactivation of area 17 resulted in a mean decrease in the magnitude of response by 53.5 %, while inactivation of area 21a resulted in a mean decrease of only 24.2 %. All but two cells in which the effects of inactivation of areas 21a and 17 were tested separately were located in the SGS (Fig. 5C).
Effects of cooling area 21a or area 17 on the directional selectivity of collicular neurones
It has been well established that in the cat most collicular neurones located in the retino-recipient layers exhibit a substantial degree of directional selectivity, that is, when stimuli move along a particular axis across the cell's excitatory receptive field, many cells respond well to stimuli moving in one (preferred) direction and poorly or not at all to stimuli moving in the opposite (non-preferred) direction (for reviews see Dreher & Hoffmann 1973; Ogasawara et al. 1984; Stein & Meredith, 1991; Mendola & Payne, 1993). Preferred/non-preferred axes of movement vary quite substantially depending on the position of the receptive field, and for about 60 % of collicular cells the responses to motion along the axis orthogonal to the preferred/non-preferred axis were not direction-selective (cf. Straschill & Hoffmann, 1968; Dreher & Hoffmann, 1973). For most cells in our sample we did not explore systematically the preferred/non-preferred axes of movements and determined direction selectivity indices (DI; see Methods) only for movements along the horizontal axis. Nevertheless, with this caveat, a majority of cells in the present sample (36/65; 55.4 %) exhibited DI values of over 20 % and in about a quarter of the sample (16/65; 24.6 %), the magnitude of the response to the stimulus moving in one direction was at least twice the magnitude of the response to the stimulus moving in the opposite direction (DI > 50 %). Finally, a substantial proportion (9/65; 13.8 %) of cells exhibited a very strong directional selectivity (DI > 70 %).
It has been reported that in the cat there is a positive correlation between the strength of input from ipsilateral area 17 and direction selectivity of collicular neurones (Ogasawara et al. 1984). However, in the present study there was no positive correlation between the strength of the input from area 21a and the DI of the collicular cells. In particular, for cells with DI < 50 % the mean reduction in the magnitude of response (62.5 %; range 18.2-100 %; n = 49) was not significantly different (P < 0.1476; Mann- Whitney U test) from that (50.6 %; range 14.4-94 %; n = 16) for cells with DI > 50 %.
Nevertheless, in a proportion of cells, inactivation of area 21a resulted in substantial changes in their DI. Figure 6A illustrates the effect of inactivation of area 21a on one such cell. In this case the reduction in the magnitude of the response during cooling of area 21a was largely direction-dependent, resulting in a substantial degree of directional selectivity which was not apparent before inactivation of area 21a (DI changed dramatically from 20 % to 73 %). Although after rewarming area 21a to 36 °C, the DI was higher than that before cooling area 21a (38 % vs. 20 %), it was substantially lower than that when area 21a has been cooled to 10 °C (38 % vs. 73 %).
Figure 6. Effect of inactivation of area 21a on the direction selectivity indices (DI) of collicular neurones.
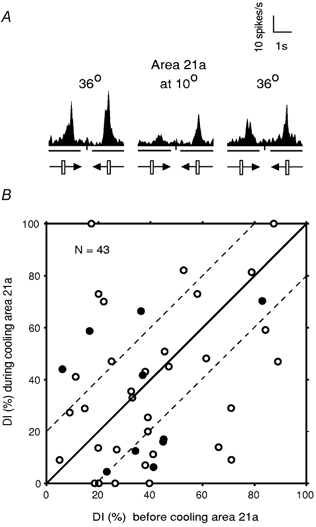
A, the PSTHs illustrating the direction selectivity of a collicular unit before and during cooling as well as after rewarming of area 21a. Each PSTH was constructed on the basis of responses to 10 presentations of a 3.3 × 0.8 deg light bar moving across the cell's RF at 5 deg s−1 parallel to the horizontal meridian. The neurone exhibited DIs of 0.2 and 0.73 before and during inactivation of area 21a, respectively. After rewarming area 21a, the DI was reduced to 0.38. B, DIs of collicular neurones with significant change in the magnitude of response before and during cooling area 21a. Inactivation of area 21a resulted in some change in DI in almost all of the cells (42/43; 98 % of sample). The filled symbols indicate cells which changed their preferred direction of response during cooling (10/43; 23 % of sample). The parallel dashed lines separate the cells with a DI change greater than 20 % from those with smaller changes. Thus, in a majority of cells (26/43; 60.5 %) the change in their DIs was 20 % or more.
For the entire sample of collicular cells in which the magnitude of responses to visual stimuli was significantly affected by inactivation of ipsilateral area 21a, we plotted the DIs for the movement along the horizontal axis during inactivation of area 21a against their DIs before inactivation of area 21a (Fig. 6B). Figure 6B shows that in a majority of cells in our sample (26/43; 60.5 %), inactivation of area 21a resulted in a substantial (20 % or more) increase (9/26 cells) or decrease (17/26 cells) in the DI. Overall, it is apparent from Fig. 6B that: (1) four of the cells which, before inactivation of area 21a, exhibited only a weak directional asymmetry (DIs 12-20 %), became either very strongly (DI 60-70 %; see also Fig. 6A) or completely (DI 100 %) direction-selective during inactivation of area 21a and (2) in another four cells, inactivation of area 21a resulted in complete abolition of direction selectivity (DI 0 %). Furthermore, in almost a quarter of cells in which inactivation of area 21a significantly affected the magnitude of responses to visual stimuli (10/43; 23.3 %), there was also a reversal of the cell's preferred direction during cooling of area 21a (Fig. 6B; filled symbols).
In almost half of the small sample of collicular neurones in which we examined the effect of reversible inactivation of area 17 (6/13; 46 %), the DI was changed by more than 20 %. In two of those cells (15 % of the sample), the inactivation of area 17 resulted in the reversal of the cell's preferred direction.
Effects of cooling area 21a on length tuning of collicular neurones
Generally, most collicular neurones prefer stimuli much smaller than their RFs and this size specificity includes length specificity (cf. McIlwain & Buser, 1968; Dreher & Hoffmann, 1973; Waleszczyk et al. 1999; for review see Stein & Meredith, 1991). In control conditions, that is, before cooling area 21a, most of the small sample of cells (5/8; 62.5 %) tested quantitatively for length selectivity, exhibited some length summation (magnitude of responses increased with increase in the length) for stimuli shorter than 2-3 deg and a clear reduction in the magnitude of responses for stimuli longer than 2-3 deg (Fig. 7A-C). When area 21a was inactivated, three of these cells (3/5; 60 %) exhibited much sharper length tuning than that before inactivation of area 21a (Fig. 7A and B) while the other two cells (2/5; 40 %) ceased to be length-selective (Fig. 7C). In all five cases there was virtually complete recovery to the original pattern of length tuning when area 21a was rewarmed to 36 °C (Fig. 7A-C). Two of the other three cells were very broadly tuned for length when area 21a was kept at 36 °C but during inactivation of area 21a, they became very length selective responding only to bars of very specific length but not to stimuli of any other length tested (Fig. 7D). Finally, one cell (1/8; 12.5 %) which was not length selective before inactivation of area 21a, remained so during inactivation of area 21a (not illustrated).
Figure 7. Effects of inactivation of area 21a on the length-tuning properties of collicular cells.
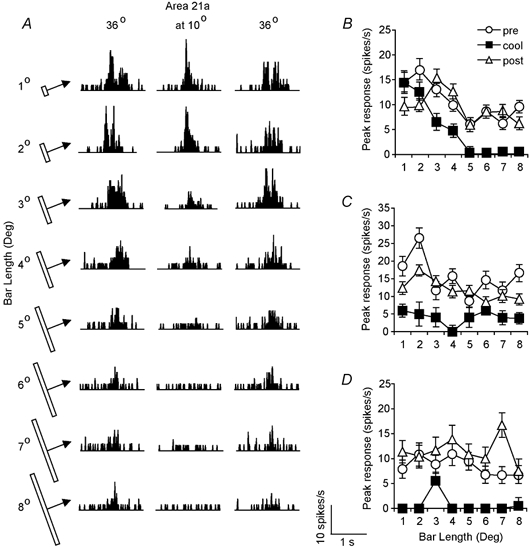
A, the PSTHs illustrating the length selectivity of a collicular unit before and during cooling as well as after rewarming of area 21a. Each PSTH was constructed on the basis of responses to 10 presentations of a 0.7 deg light bar of different length (indicated on the left) moving across the cell's RF at 5 deg s−1 parallel to the horizontal meridian. Note that although there is a clear end-stopping before inactivation of area 21a and after rewarming area 21a, the cell responds quite well to bars longer than 4 deg (weak end-stopping). Inactivation of area 21a affects relatively weakly the magnitude of responses to bars not exceeding 4 deg but the longer bars do not produce any response (strong end-stopping). B, the peak discharge rate vs. bar length of the collicular cell whose PSTHs are illustrated in A. C, the peak discharge rate of another collicular cell vs. bar length before and during inactivation of area 21a and after rewarming. Note that inactivation of area 21a also results in reversible changes in length tuning. D, another example of the effect of inactivation of area 21a on length tuning of collicular neurones.
DISCUSSION
The effectiveness and selectivity of inactivation of areas 21a or 17
It has been demonstrated in several previous studies that cooling cat's area 17 (Michalski et al. 1993, 1994) or 21a (Wang et al. 2000) to 10 °C results in virtually complete abolition of both spontaneous and visually evoked activity in the cooled areas (cf. also Jasper et al. 1970; Ogasawara et al. 1984; Volgushev et al. 2000). Furthermore, it has been clearly established in the past that the visually evoked activity in cat's cortical areas is completely abolished at cortical temperatures of 20 °C or less (Lomber et al. 1999). Since in the present study when the temperature of the Peltier device at the surface of area 17 or 21a was 10 °C, the temperature in layers 5, where cortico-tectal cells are located (see for review Rhoades et al. 1991), was 15-17 °C, it appears that cooling surfaces of areas 17 or 21a to 10 °C prevented relay of the visually evoked activity from the areas to the SC.
At the same time, it is very unlikely that the changes in responses of collicular neurones during cooling of areas 21a or 17 observed by us were due to the direct spread of cooling to the superior colliculus. Thus, Wickelgren & Sterling (1969) noted that when frozen Ringer's solution (0 °C) was placed over the visual cortices, the temperature at the surface of the colliculus ipsilateral to the cooled cortex was 35 °C. Consistent with this, the background activity of collicular neurones whose responsiveness to visual stimuli was dramatically decreased by cooling of the ipsilateral visual cortices was unaffected. Furthermore, Ogasawara et al. (1984) measured the temperature of the SC during cooling of area 17 or the posterior suprasylvian areas (PSSC) to 10 °C, and in both cases found no change in temperature of either ipsilateral or contralateral colliculi.
On the other hand, the question arises: how selective was our inactivation of area 21a or area 17 in cortical terms? In the present study, only area 21a or area 17 were intentionally cooled, to about 10 °C and the drops in the temperature of area 21a during cooling of area 17 or vice versa were counterbalanced by the presence of a second ‘warming’ Peltier probe located on visuotopically corresponding parts of the non-cooled cortical area. This dual temperature control approach allowed us to maintain the physiological temperature during cooling of one of the areas not only at the visuotopically corresponding parts of areas 17 and 21a covered by our warming probe, but also in the areas immediately adjacent to them (e.g. area 18 when area 17 was warmed during the cooling of area 21a or area 19 when area 21a was warmed during cooling of area 17). However, due to the temperature gradient of 4-5 °C mm−1 produced by the cooling probe (cf. Girard & Bullier, 1989; Michalski et al. 1993; Wang et al. 2000), during cooling of area 17 or 21a to 10 °C, the temperature of segments of the cortical areas immediately adjacent to the cooled area (area 18 in the case of cooling area 17; in the case of cooling area 21a visuotopically organised areas such as area 19, the PMLS area, the VLS area and to a lesser extent area 21b as well as area 7; see Fig. 1A) must have been lowered even when there was no significant encroachment of the cooling probe onto these areas. Furthermore, even a small temperature drop appears to have some direct effects on the responsiveness of cells in the cooled area. Indeed, during moderate cooling, both hyper-excitability (Klee et al. 1974; for review see Brooks, 1983; Michalski et al. 1993; Volgushev et al. 2000) and reduction in responsiveness of neurones in the mammalian central nervous system (Michalski et al. 1993) have been reported.
In our experimental set-up, spread of cooling which might have contributed significantly to the ‘collicular effects' observed in this investigation was probably limited to area 18 (from the cooling probe in area 17) and to area 19 (from the cooling probe in area 21a). Thus, parts of both areas 18 and 19 immediately adjacent to our cooling probes in areas 17 and 21a contain representation of the central part of the visual field (Hubel & Wiesel, 1965; Tusa et al. 1979; Mulligan & Sherk, 1993). Furthermore, both areas 18 and 19, like areas 17 and 21a, send strong projections to the retino-recipient layers of the ipsilateral SC (cf. Harting et al. 1992). It is unlikely that the spread of cooling to the remaining visuotopically organised areas in the vicinity of area 21a, that is the PMLS area, VLS area, area 21b and area 20a, contributed significantly to the ‘collicular effects' observed. Thus, parts of the PMLS area or area 21b immediately adjacent to area 21a contain representations of peripheral rather than central parts of the contralateral visual hemifield (Turlejski & Michalski, 1975; Palmer et al. 1978; Tusa & Palmer, 1980; Djavadian & Harutiunian-Kozak, 1983). It is also unlikely that collicular effects observed in this investigation were related to the spread of cooling from area 21a to areas 20a or area 7, since area 20a is located over 5 mm away from area 21a (Tusa & Palmer, 1980) while area 7, unlike the visuotopically organised areas in the vicinity of area 21a, does not project to the retino-recipient collicular layers (cf. Harting et al. 1992).
There are additional complicating factors in ascribing the collicular effects observed in this study during cooling of area 21a or area 17 to inactivation of specific cortical areas. Thus, inactivation or reduction in the temperature of a given cortical area might also exert an indirect effect on the responses of collicular cells by affecting the activity of other cortical areas projecting to the SC. In particular, reversible bilateral inactivation of cat's areas 17 and 18 dramatically reduces (or occasionally significantly increases) both spontaneous and visually evoked activity of most cells in area 21a (Michalski et al. 1993). The effect on both spontaneous and visually evoked activity of most cells in area 21a is similar (albeit weaker) when only the ipsilateral or contralateral areas 17 and 18 are inactivated (Michalski et al. 1994). Similarly, despite maintaining areas 17 and 18 at physiological temperature, reversible inactivation of area 21a significantly reduces (or occasionally significantly increases) both spontaneous and visually evoked activity of almost a third of cells in the part of ipsilateral area 17 corresponding visuotopically to area 21a (Wang et al. 2000). Furthermore, reversible inactivation of areas 20a and 20b either significantly reduces or significantly increases both spontaneous and visually evoked activity of a high proportion of cells in ipsilateral area 17 (Huang et al. 2002). In view of the fact that area 19, like area 21a, is strongly interconnected both directly and indirectly with areas 17 and 18 (for reviews see Rosenquist, 1985; Dreher, 1986; Salin & Bullier, 1995; Dreher et al. 1996), partial inactivation of this area when area 21a was cooled to 10 °C might have an appreciable influence on activity of areas 17 and 18.
Overall it appears that cooling of area 21a or area 17 to 10 °C: (1) did not affect the temperature of the SC, and (2) resulted in a substantial lowering of the temperature of visuotopically corresponding regions in very close proximity to the cooled areas (area 18 when cooling area 17 and area 19 when cooling area 21a). However, since only area 21a or area 17 were cooled to a temperature as low as 10 °C, that is, to the temperature at which neuronal activity ceases (for references see above), we believe that inactivation of area 21a or area 17 are fairly selective major factors contributing to our results.
Effects of inactivation of area 21a vs. effects of inactivation of area 17 on the magnitude of responses of collicular neurones
Area 17
In the present study, cooling of area 17 to 10 °C resulted in significant reduction in the magnitude of responses or a complete abolition of the responses to visual stimuli in almost 85 % of a small sample of neurones in the ipsilateral colliculus. In the remaining 15 % of collicular cells, inactivation of ipsilateral area 17 resulted in significant increases in the magnitude of responses to visual stimuli. This result is consistent with several previous reports (Wickelgren & Sterling, 1969; Ogasawara et al. 1984). It agrees also with the demonstration of an excitatory input from areas 17 and 18 to the superficial layers of the SC (cf. Hoffmann & Straschill, 1971; McIlwain, 1977; Berson, 1988), probably mediated via the N-methyl-D-aspartate (NMDA) glutamate receptors (Binns & Salt, 1996), and with the ultramicroscopic evidence (Sterling, 1971; Behan, 1984).
The suppressive effect of the cortico-tectal input from area 17 observed in a small proportion of collicular cells (the present study; see also a small number of cells in the retino-recipient layers of the SC which responded to visual stimuli only when ipsilateral areas 17-18 were cooled to 10-12 °C (Ogasawara et al. 1984)), might be exerted via inhibitory neurones intrinsic to the SC. Indeed, there are numerous γ-amino-butyric acid (GABA)-accumulating (presumably inhibitory) neurones and terminals in the superficial layers of cat's SC and at least some of them receive synaptic input from the ipsilateral primary visual cortex (for review see Mize, 1992).
Area 21a
Cooling area 21a to 10 °C resulted in a significant reduction in the magnitude of responses or a complete abolition of the responses to visual stimuli in about 63 % of neurones in the ipsilateral colliculus. In a small proportion (about 3 %), inactivation of area 21a resulted in significant increases in the magnitude of responses to visual stimuli, and in about one-third of the sample inactivation of ipsilateral area 21a did not result in significant changes in magnitude of the responses. Ogasawara et al. (1984) reported that inactivation of the PSSC affected mainly the magnitude of responses and receptive field properties of cells in the deep rather than superficial layers of the ipsilateral SC. However, cooling of the PSSC region which included also a small lateral portion of area 21a, resulted in 30 % or greater reduction in the magnitude of responses to visual stimuli in 59 % of binocular neurones located in the retino-recipient layers of the ipsilateral SC (Ogasawara et al. 1984). As far as we know no electromicroscopic data concerning the nature of synapses between terminals originating from area 21a and the appendages or somata of neurones in the SC are available. If one assumes that the nature of synaptic contacts between terminals originating from area 21a and the appendages of neurones in the ipsilateral SC is similar to those between terminals originating from neighbouring area 19, our functional data will be reasonably consistent with morphology. Thus, about 75 % of the tectal terminals originating in area 19 contain round synaptic vesicles and form asymmetric (presumably excitatory) synapses on the dendrites or dendritic spines of the neurones in the retino-recipient collicular layers, while the remaining 25 % of area 19 terminals contain pleomorphic vesicles and form symmetric (presumably inhibitory) synapses on the dendrites or dendritic spines (Behan, 1984).
Area 17 vs. area 21a
In the present study the effects of inactivation of area 17 on the magnitude of responses of collicular cells to optimal visual stimuli tended to be substantially greater than those due to inactivation of area 21a. Several factors might contribute to these differences. First, the density of the cortico-tectal terminals originating in area 17 (especially in the SZ and SGSl) appears to be substantially greater than that of terminals originating from area 21a (Harting et al. 1992). A similar argument, albeit to a substantially lesser extent, applies to the differences in the density of cortico-tectal terminals originating from area 18 (which is likely to be partially inactivated when area 17 is cooled to 10 °C) and those originating from area 19 (which is likely to be partially inactivated when area 21a is cooled to 10 °C). Second, strong excitatory Y-type indirect input to collicular neurones (Hoffmann, 1973; cf. Berson, 1988) is relayed via areas 17 and 18 (Stone & Dreher, 1973; Dreher et al. 1980; Berson, 1988) but not via areas 21a (Dreher et al. 1993) or 19 (Dreher et al. 1980). Third, inactivation of feedforward excitatory connections from primary visual cortices (areas 17 and 18) to area 21a during cooling of the primary visual cortex appears to affect both spontaneous and visually evoked activity of area 21a neurones very strongly. Thus, cooling areas 17 and 18 to 10-12 °C resulted in significant reduction in the magnitude of responses in most cells and virtual cessation of visually evoked activity in about 30 % of cells (including, presumably, neurones projecting to the SC) in ipsilateral area 21a (Michalski et al. 1994). The effect of inactivation of the ‘feedback’ pathway from area 21a to area 17 (by cooling area 21a to 10 °C) on the activity of area 17 neurones appears to be much more moderate; the magnitude of responses to visual stimuli was significantly affected in about 30 % of cells in the visuotopically corresponding region of ipsilateral area 17 and in none of them did the visually evoked activity cease (Wang et al. 2000).
The question arises: could the collicular effect of inactivation of area 21a be entirely due to the effect which area 21a exerts on area 17? Several lines of reasoning argue against it. Thus, while the magnitude of responses to visual stimuli was significantly affected (mostly reduced) in almost two-thirds of cells in the SC ipsilateral to inactivated area 21a (the present study), a significant change (almost invariably reduction) in the magnitude of responses to visual stimuli was observed in less than 30 % of area 17 neurones in the visuotopically corresponding region of ipsilateral area 17 (Wang et al. 2000). Second, as mentioned above, in none of area 17 cells tested did inactivation of area 21a result in cessation of responses to visual stimuli (Wang et al. 2000). By contrast, reversible inactivation of area 21a resulted in a reversible but complete cessation of visually evoked activity in almost 10 % of neurones in the ipsilateral SC (the present study). Third, in a proportion of collicular cells, reversible inactivation of area 17 vs. reversible inactivation of area 21a produced opposite effects (increase vs. decrease) on the responses to visual stimuli (the present study). Fourth, inactivation of area 17 affects the magnitude of responses of collicular cells located in the retino-recipient layers but not of those located in the deep layers (Ogasawara et al. 1984). This result is consistent with reported richness of area 17 terminals in the retino-recipient layers and their paucity in the deep collicular layers (Harting et al. 1992). By contrast, inactivation of area 21a affects not only the responses of cells located in the retino-recipient layers but also those located in the SGI (the present study). This result is partially consistent with the fact that at least some cortico-tectal terminals originating from area 21a, unlike those from areas 17 and 18, terminate in the SGI (Fig. 1; Harting et al. 1992). On the other hand, in view of the fact that the cortico-tectal terminals originating from area 21a are much more numerous in the superficial layers (especially in the SGSl) than in the SGI (Harting et al. 1992), the strength of effect of inactivation of area 21a on the responses of cells in the SGI is rather puzzling. Finally, in cats in which areas 17 and 18 were ablated, cooling the posterior region of the suprasylvian cortex (which included also a small lateral portion of area 21a), resulted in 30 % or greater reduction in the magnitude of responses to visual stimuli in 60 % of neurones located in the retino-recipient layers of the ipsilateral SC (Ogasawara et al. 1984).
Influence of area 21a on direction and length selectivities of collicular neurones: comparison with area 17
In most of the ablation studies it has been reported that, in the cat at least, area 17 plays an important role in determining the binocular convergence, direction selectivity and interactions between the different parts of receptive fields of neurones in the retino-recipient layers of the SC (Wickelgren & Sterling, 1969; Rosenquist & Palmer, 1971; Ogasawara et al. 1984). Similarly, it has been reported that reversible inactivation of areas 17-18 affects the binocular convergence and directional selectivity of neurones in the superficial layers of the ipsilateral SC (Ogasawara et al. 1984). We have no data concerning the role of area 17 in relation to the degree of excitatory binocular convergence in the SC. However, on the basis of effects observed in a small sample of collicular cells studied by us, and consistent with reported high proportion of directionally selective cells among corticotectal area 17 neurones (Palmer & Rosenquist, 1974; Weyand & Gafka, 2001), it appears that area 17 indeed contributes to the directional selectivity mechanism of neurones in the superficial layers of the ipsilateral SC.
Rosenquist & Palmer (1971) reported that: (1) unilateral ablation of area 17 resulted in a dramatic reduction in the proportion of directionally selective cells in the ipsilateral SC and (2) when area 17 was spared, ablations of areas 18 and 19 or a large area around the lateral suprasylvian sulcus (likely to include area 21a) did not affect the proportions of directionally selective cells in the ipsilateral colliculus. On the other hand, the present data indicate that despite the relative paucity of strongly directionally selective cells in area 21a (Mizobe et al. 1988; Wimborne & Henry, 1992; Dreher et al. 1993) this area also contributes to the direction selectivity of neurones in the ipsilateral SC. The discrepancy is probably more apparent than real since the present data indicate that area 17 provides a much stronger excitatory drive to most of the cells in the superficial collicular layers. Presumably the presence of the input from area 17 can easily compensate for the loss of input from extrastriate area 21a. There is an additional confounding factor here. It has been reported in a number of studies that ablation of areas 17 and 18 (with usually at least partial involvement of area 19) in adult (Rizzolatti et al. 1970; Hoffmann & Straschill, 1971; Mendola & Payne, 1993) or one-day-old kittens (Mendola & Payne, 1993) results in only minor or no changes in the proportion of direction-selective cells in the retino-recipient layers of the ipsilateral SC. Indeed, it has been argued that the principal mechanism underlying direction selectivity in the collicular neurones might (1) be intrinsic to the SC (Rizzolatti et al. 1970; Hoffmann & Straschill, 1971; Dreher & Hoffmann, 1973), (2) originate from retina (Binns & Salt, 1996), and/or (3) in the absence of the input from areas 17, 18 and 19, include some sort of physiological compensation based on the projections from the extrastriate cortices (Mendola & Payne, 1993). Present data suggest that area 21a might be one of the areas involved in such a compensatory mechanism.
In the present study reversible inactivation of area 21a resulted also in substantial reversible changes in length selectivity of most collicular neurones in which length selectivity has been examined. Whatever the mechanism of this effect is, area 21a, like area 17 (Rosenquist & Palmer, 1971; cf. Ogasawara et al. 1984; Stein & Meredith, 1991), appears to strongly influence the spatial properties of collicular receptive fields. The fact that length selectivity is pronounced in collicular neurones (at least those located in the retino-recipient layers; for review see Stein & Meredith, 1991), combined with the fact that there is often a clear effect on length selectivity of collicular neurones when form/pattern-processing area 21a is inactivated is consistent with the reported involvement of the SC in pattern discrimination (e.g. Sprague et al. 1970; Tunkl & Berkley, 1977, 1985; Sprague, 1991; Lomber, 2002).
Conclusion
The present results indicate that one of the extrastriate form/pattern-processing areas, area 21a, (which receives very little if any of its visual input via the Y channel) exerts a significant, mainly excitatory, influence on the neuronal activity of cells located in the ipsilateral SC. In most cases the effect exerted by area 21a is substantially weaker than that exerted by the striate cortex. However, unlike area 17, area 21a strongly modulates the neuronal activity of cells located not only in the superficial (retino-recipent) layers but also those located in the SGI. This in turn suggests some influence of area 21a on the integrative and motor functions of the SC. In a proportion of collicular neurones the ‘feedback’ projections from area 21a appear to affect the specific receptive field properties such as direction selectivity and length tuning.
Acknowledgments
This research was supported by grant from the National Health and Medical Research Council. We also thank two anonymous reviewers for their insightful comments.
REFERENCES
- Barlow HB, Blakemore C, Pettigrew JD. The neural mechanism of binocular depth discrimination. J Physiol. 1967;193:327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M. An EM-autoradiographic analysis of the projection from cortical areas 17, 18, and 19 to the superior colliculus in the cat. J Comp Neurol. 1984;225:591–604. doi: 10.1002/cne.902250409. [DOI] [PubMed] [Google Scholar]
- Berson DM. Retinal and cortical inputs to cat superior colliculus: composition, convergence and laminar specificity. Prog Brain Res. 1988;75:17–26. doi: 10.1016/s0079-6123(08)60462-8. [DOI] [PubMed] [Google Scholar]
- Binns KE, Salt TE. Corticofugal influences on visual responses in cat superior colliculus: The role of NMDA receptors. Vis Neurosci. 1996;13:683–694. doi: 10.1017/s0952523800008579. [DOI] [PubMed] [Google Scholar]
- Brooks DM. Study of brain function by local, reversible cooling. Rev Physiol, Biochem Pharmacol. 1983;95:1–109. [Google Scholar]
- Burke W, Dreher B, Wang C. Selective block of conduction in Y optic nerve fibres: significance for the concept of parallel processing. Eur J Neurosci. 1998;10:8–19. doi: 10.1046/j.1460-9568.1998.00025.x. [DOI] [PubMed] [Google Scholar]
- Djavadian RL, Harutiunian-Kozak BA. Retinotopic organization of the lateral suprasylvian area of the cat. Acta Neurobiol Exp (Warsaw) 1983;43:251–262. [PubMed] [Google Scholar]
- Dreher B. Thalamocortical and corticocortical interconnections in the cat visual system: relation to the mechanisms of information processing. In: Pettigrew JD, Sanderson KJ, Levick WR, editors. Visual Neuroscience. Cambridge: Cambridge University Press; 1986. pp. 290–314. [Google Scholar]
- Dreher B, Djavadian RL, Turlejski KJ, Wang C. Areas PMLS and 21a of cat visual cortex are not only functionally but also hodologically distinct. Prog Brain Res. 1996;112:251–276. doi: 10.1016/s0079-6123(08)63334-8. [DOI] [PubMed] [Google Scholar]
- Dreher B, Hoffmann K-P. Properties of excitatory and inhibitory regions in the receptive fields of single units in the cat's superior colliculus. Exp Brain Res. 1973;16:333–353. doi: 10.1007/BF00233427. [DOI] [PubMed] [Google Scholar]
- Dreher B, Leventhal AG, Hale PT. Geniculate input to cat visual cortex: a comparison of area 19 with areas 17 and 18. J Neurophysiol. 1980;44:804–826. doi: 10.1152/jn.1980.44.4.804. [DOI] [PubMed] [Google Scholar]
- Dreher B, Michalski A, Ho RHT, Lee CWF, Burke W. Processing of form and motion in area 21a of cat visual cortex. Vis Neurosci. 1993;10:93–115. doi: 10.1017/s0952523800003254. [DOI] [PubMed] [Google Scholar]
- Girard P, Bullier J. Visual activity in area V2 durung reversible inactivation of area 17 in the macaque monkey. J Neurophysiol. 1989;62:1287–1302. doi: 10.1152/jn.1989.62.6.1287. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Harting JK, Updyke BV, Van Lieshout DP. Corticotectal projections in the cat: anterograde transport studies of twenty-five cortical areas. J Comp Neurol. 1992;324:379–414. doi: 10.1002/cne.903240308. [DOI] [PubMed] [Google Scholar]
- Hashemi-Nezhad M, Wang C, Burke W, Dreher B. Influence of corticotectal projections on neuronal activities in the retinorecipient layers in the superior colliculus of the cat. Proc Austral Neurosci Soc. 2001;12:208. [Google Scholar]
- Hilgetag C-C, Burns GAPC, O'Neill MA, Scannell JW, Young MP. Anatomical connectivity defines the organization of clusters of cortical areas in the macaque monkey and the cat. Phil Trans Roy Soc Lond. 2000;355:91–110. doi: 10.1098/rstb.2000.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K-P. Conduction velocity in pathways from retina to superior colliculus in the cat: a correlation with receptive-field properties. J Neurophysiol. 1973;36:409–424. doi: 10.1152/jn.1973.36.3.409. [DOI] [PubMed] [Google Scholar]
- Hoffmann K-P, Straschill M. Influences of cortico-tectal and intertectal connections on visual responses in the cat's superior colliculus. Exp Brain Res. 1971;12:120–131. doi: 10.1007/BF00234310. [DOI] [PubMed] [Google Scholar]
- Huang JY, Wang C, Fitzgibbon T, Dreher B. Areas 20a and 20b modulate neuronal activity in area 17 of the cat. Proc Austral Neurosci Soc. 2002;13:220. [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture in two nonstriate visual areas (18 and 19) of the cat. J Neurophysiol. 1965;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Shacter DG, Montplaisir J. The effect of local cooling and evoked electrical activity of cerebral cortex. Can J Physiol Pharmacol. 1970;48:640–652. doi: 10.1139/y70-094. [DOI] [PubMed] [Google Scholar]
- Klee MR, Pierau F-K, Faber DS. Temperature effects on resting potential and spike parameters of cat motoneurons. Exp Brain Res. 1974;19:478–492. doi: 10.1007/BF00236112. [DOI] [PubMed] [Google Scholar]
- Lomber SG. Behavioral cartography of visual functions in cat parietal cortex: areal and laminar dissociations. Prog Brain Res. 2001;134:265–284. doi: 10.1016/s0079-6123(01)34018-9. [DOI] [PubMed] [Google Scholar]
- Lomber SG. Learning to see the trees before the forest: reversible deactivation of the superrior colliculus during learning of local and global visual features. Proc Natl Acad Sci U S A. 2002;99:4049–4054. doi: 10.1073/pnas.062551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Cornwell P, Long KD. Perceptual and cognitive visual functions of parietal and temporal cortices in the cat. Cereb Cortex. 1996;6:673–695. doi: 10.1093/cercor/6.5.673. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Horel JA. The cryoloop: an adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neuronal function. J Neurosci Meth. 1999;86:179–194. doi: 10.1016/s0165-0270(98)00165-4. [DOI] [PubMed] [Google Scholar]
- McIlwain JT. Topographic organization and convergence in corticotectal projections from areas 17, 18 and 19 in the cat. J Neurophysiol. 1977;40:189–198. doi: 10.1152/jn.1977.40.2.189. [DOI] [PubMed] [Google Scholar]
- McIlwain JT, Buser P. Receptive fields of single cells in the cat's superior colliculus. Exp Brain Res. 1968;5:314–325. doi: 10.1007/BF00235906. [DOI] [PubMed] [Google Scholar]
- Mendola JD, Payne BR. Direction selectivity and physiological compensation in the superior colliculus following removal or areas 17 and 18. Vis Neurosci. 1993;10:1019–1026. doi: 10.1017/s0952523800010129. [DOI] [PubMed] [Google Scholar]
- Michalski A, Wimborne BM, Henry GH. The effect of reversible cooling of cat's primary visual cortex on the responses of area 21a neurones. J Physiol. 1993;466:133–156. [PMC free article] [PubMed] [Google Scholar]
- Michalski A, Wimborne BM, Henry GH. The role of ipsilateral and contralateral inputs from primary cortex in responses of area 21a neurons in cats. Vis Neurosci. 1994;11:839–849. doi: 10.1017/s0952523800003801. [DOI] [PubMed] [Google Scholar]
- Mize RR. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res. 1992;90:219–248. doi: 10.1016/s0079-6123(08)63616-x. [DOI] [PubMed] [Google Scholar]
- Mizobe K, Itoi M, Kaihara T, Toyama K. Neuronal responsiveness in area 21a of the cat. Brain Res. 1988;438:307–310. doi: 10.1016/0006-8993(88)91353-4. [DOI] [PubMed] [Google Scholar]
- Morley JW, Yuan L, Vickery RM. Corticocortical connections between area 21a and primary visual cortex in the cat. Neuroreport. 1997;8:1263–1266. doi: 10.1097/00001756-199703240-00041. [DOI] [PubMed] [Google Scholar]
- Mulligan K, Sherk H. A comparison of magnification functions in area 19 and the lateral suprasylvian area in the cat. Exp Brain Res. 1993;97:195–208. doi: 10.1007/BF00228689. [DOI] [PubMed] [Google Scholar]
- Ogasawara K, McHaffie JG, Stein BE. Two visual corticotectal systems in cat. J Neurophysiol. 1984;52:1226–1245. doi: 10.1152/jn.1984.52.6.1226. [DOI] [PubMed] [Google Scholar]
- Palmer LA, Rosenquist AC. Visual receptive fields of single striate cortical units projecting to the superior colliculus of the cat. Brain Res. 1974;67:27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Palmer LA, Rosenquist AC, Tusa RJ. The retinotopic organization of lateral suprasylvian visual areas in the cat. J Comp Neurol. 1978;177:237–256. doi: 10.1002/cne.901770205. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Tompkins J, Olson CR. The role of striate cortex in visual function of the cat. J Neurosci. 1995;15:1940–1950. doi: 10.1523/JNEUROSCI.15-03-01940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew JD, Cooper ML, Blasdel GG. Improved use of tapetal reflection for eye-position monitoring. Invest Ophthalmol Vis Sci. 1979;18:490–495. [PubMed] [Google Scholar]
- Rhoades RW, Mooney RD, Fish SE. Retinotopic and visuotopic representations in the mammalian superior colliculus. In: Dreher B, Robinson SR, editors. Vision and Visual Dysfunction, Neuroanatomy of the Visual Pathways and their Development. Vol. 3. London: MacMillan; 1991. pp. 150–175. [Google Scholar]
- Rizzolatti G, Tradardi V, Camarda R. Unit responses to visual stimuli in the cat's superior colliculus after removal of the visual cortex. Brain Res. 1970;24:336–339. doi: 10.1016/0006-8993(70)90114-9. [DOI] [PubMed] [Google Scholar]
- Rosenquist AC. Connections of visual cortical areas in the cat. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 3. New York: Plenum Publishing; 1985. pp. 81–117. [Google Scholar]
- Rosenquist AC, Palmer LA. Visual receptive field properties of cells of the superior colliculus after cortical lesions in the cat. Exp Neurol. 1971;33:629–652. doi: 10.1016/0014-4886(71)90133-6. [DOI] [PubMed] [Google Scholar]
- Salin P-A, Bullier J. Corticocortical connections in the visual system: structure and function. Physiol Rev. 1995;75:107–154. doi: 10.1152/physrev.1995.75.1.107. [DOI] [PubMed] [Google Scholar]
- Scannell JW, Burns GAPC, Hilgetag C-C, O'Neill MA, Young MP. The connectional organization of the cortico-thalamic system of the cat. Cereb Cortex. 1999;9:277–299. doi: 10.1093/cercor/9.3.277. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Tehovnik EJ. Look and see: how the brain moves your eyes about. Prog Brain Res. 2001;134:127–142. doi: 10.1016/s0079-6123(01)34010-4. [DOI] [PubMed] [Google Scholar]
- Siegel S. Nonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill; 1956. [Google Scholar]
- Sprague JM. The role of the superior colliculus in facilitating visual attention and form perception. Proc Natl Acad Sci U S A. 1991;88:1286–1290. doi: 10.1073/pnas.88.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JM, Berlucchi G, Di Berardino A. The superior colliculus and pretectum in visually guided behavior. Brain Behav Evol. 1970;3:285–294. doi: 10.1159/000125478. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. Functional organization of the superior colliculus. In: Leventhal AG, editor. Vision and Visual Dysfunction. The Neural Basis of Visual Function. Vol. 4. London: MacMillan; 1991. pp. 85–110. [Google Scholar]
- Sterling P. Receptive fields and synaptic organization of the superficial gray layer of the cat superior colliculus. Vis Res Suppl. 1971;3:309–328. doi: 10.1016/0042-6989(71)90048-4. [DOI] [PubMed] [Google Scholar]
- Stone J, Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973;36:551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- Straschill M, Hoffmann K-P. Relationship between localization and functional properties of movement sensitive neurones of the cat's tectum opticum. Brain Res. 1968;8:382–385. doi: 10.1016/0006-8993(68)90059-0. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Uhlrich DJ, Sherman SM. Morphology of physiologically identified retinal X and Y axons in the cat's thalamus and midbrain as revealed by intraaxonal injection of biocytin. J Comp Neurol. 1995;354:583–607. doi: 10.1002/cne.903540408. [DOI] [PubMed] [Google Scholar]
- Tunkl JE, Berkley MA. The role of superior colliculus in vision: visual form discrimination in cats with superior colliculus ablations. J Comp Neurol. 1977;176:575–588. doi: 10.1002/cne.901760408. [DOI] [PubMed] [Google Scholar]
- Tunkl JE, Berkley MA. Stimulus-dependent form discrimination deficits in cats with superior colliculus lesions. Behav Brain Res. 1985;15:51–61. doi: 10.1016/0166-4328(85)90017-8. [DOI] [PubMed] [Google Scholar]
- Turlejski K, Michalski A. Clare-Bishop area in the cat: location and retinotopical projection. Acta Neurobiol Exp (Warsaw) 1975;35:179–188. [PubMed] [Google Scholar]
- Tusa RJ, Palmer LA. Retinotopic organization of areas 20 and 21 in the cat. J Comp Neurol. 1980;193:147–164. doi: 10.1002/cne.901930110. [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Palmer LA, Rosenquist AC. The retinotopic organization of area 17 (striate cortex) in the cat. J Comp Neurol. 1978;177:213–236. doi: 10.1002/cne.901770204. [DOI] [PubMed] [Google Scholar]
- Tusa RJ, Rosenquist AC, Palmer LA. Retinotopic organization of areas 18 and 19 in the cat. J Comp Neurol. 1979;185:657–678. doi: 10.1002/cne.901850405. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual areas. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of the Visual Behavior. Cambridge, MA, USA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Updyke BV. Retinotopic organization within the cat's posterior suprasylvian sulcus and gyrus. J Comp Neurol. 1986;246:265–280. doi: 10.1002/cne.902460210. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR, Kulikowski JJ, Lipnicki DM, Dreher B. Convergence of parvocellular and magnocellular information channels in the primary visual cortex of the macaque. Eur J Neurosci. 2002;16:945–956. doi: 10.1046/j.1460-9568.2002.02137.x. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, Yousef T, Eysel UT. Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J Physiol. 2000;522:59–76. doi: 10.1111/j.1469-7793.2000.0059m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waleszczyk WJ, Wang C, Burke W, Dreher B. Velocity response profiles of collicular neurons: Parallel and convergent visual information channels. Neurosci. 1999;93:1063–1076. doi: 10.1016/s0306-4522(99)00190-6. [DOI] [PubMed] [Google Scholar]
- Wang C, Waleszczyk WJ, Benedek G, Burke W, Dreher B. Convergence of Y and non-Y channels onto single neurons in the superior colliculi of the cat. NeuroReport. 2001;12:2927–2933. doi: 10.1097/00001756-200109170-00035. [DOI] [PubMed] [Google Scholar]
- Wang C, Waleszczyk WJ, Burke W, Dreher B. Modulatory influence of feedback projections from area 21a on neuronal activities in striate cortex of the cat. Cereb Cortex. 2000;10:1217–1232. doi: 10.1093/cercor/10.12.1217. [DOI] [PubMed] [Google Scholar]
- Weyand TG, Gafka AC. Visuomotor properties of corticotectal cells in area 17 and posteromedial lateral suprasylvian (PMLS) cortex of the cat. Vis Neurosci. 2001;18:77–91. doi: 10.1017/s0952523801181071. [DOI] [PubMed] [Google Scholar]
- Wickelgren BG, Sterling P. Influence of visual cortex on receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969;32:16–23. doi: 10.1152/jn.1969.32.1.16. [DOI] [PubMed] [Google Scholar]
- Wimborne BM, Henry GH. Responses characteristics of neurones of cortical area 21a of the cat with special reference to orientation specificity. J Physiol. 1992;449:457–478. doi: 10.1113/jphysiol.1992.sp019096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MP. Objective analysis of the topological organization of the primate visual cortical system. Nature. 1992;358:152–154. doi: 10.1038/358152a0. [DOI] [PubMed] [Google Scholar]


