Abstract
Paired, simultaneous recordings were made in anaesthetized cats from the peripheral and central axons of individual tactile and kinaesthetic sensory fibres. The aim was to determine whether failure of spike propagation occurred at any of the three major axonal branch points in the path to their cuneate target neurones, and whether propagation failure may contribute, along with synaptic transmission failures, to limitations in transmission security observed for the cuneate synaptic relay. No evidence for propagation failure was found at the two major axonal branch points prior to the cuneate nucleus, namely, the T-junction at the dorsal root ganglion, and the major branch point near the cord entry point, even for the highest impulse rates (∼400 impulses s−1) at which these fibres could be driven. However, at the highest impulse rates there was evidence at the central, intra-cuneate recording site of switching between two states in the terminal axonal spike configuration. This appears to reflect a sporadic propagation failure into one of the terminal branches of the sensory axon. In conclusion, it appears that central impulse propagation over group II sensory axons occurs with complete security through branch points within the dorsal root ganglion and at the spinal cord entry zone. However, at high rates of afferent drive, terminal axonal propagation failure may contribute to the observed decline in transmission security within the cuneate synaptic relay.
Transmission characteristics for the synaptic linkages between single sensory fibres and their target neurones of the dorsal column nuclei (DCN) have been investigated for several tactile fibre classes, including the Pacinian corpuscle (PC)-related class, the slowly adapting type I (SAI) and type II (SAII) classes, and the hair follicle afferent (HFA)-related class (Ferrington et al. 1986, 1987a,b; Vickery et al. 1994; Gynther et al. 1995; Zachariah et al. 2001; Rowe, 2002), and for kinaesthetic afferent fibres that originate in the joints (Coleman et al. 2003). For each class, a single impulse arising in a single afferent fibre could produce spike output in one or more DCN neurones. Furthermore, trains of impulses were also relayed reliably across this synaptic linkage, at rates up to ≈200-400 impulses s−1, with the pattern of activity in the DCN neurone reflecting that in the single input fibre. For all classes, a remarkably potent synaptic connection was revealed in the one-to-one linkages formed by single fibres with individual DCN neurones.
Nevertheless, despite the high synaptic security, as the discharge rate in the input fibre increased, a point was reached where there was a breakdown in transmission security reflected in a decline in the percentage of input spikes that generated spike output from the DCN neurone. The most likely explanation is in terms of limitations in the capacity of the synapse to transmit high-frequency input signals to the postsynaptic element (Ferrington et al. 1986, 1987a,b; Vickery et al. 1994; Gynther et al. 1995; Zachariah et al. 2001; Rowe, 2002; Coleman et al. 2003). However, an additional explanation could be that there has been a conduction failure along the afferent axon, in particular, at branch points, where there may be a low safety factor (Swadlow et al. 1980). There are three principal branch points for sensory fibres whose axons ascend in the dorsal columns (Fig. 1). The first is in the dorsal root ganglion, where there is a T-junction connection of the soma with the parent axon. A second major branch point occurs upon entry to the spinal cord (Wall & Shortland, 1991) and a third occurs near the terminal where synaptic contacts are formed with central target neurones.
Figure 1. Experimental arrangement for paired, simultaneous recording from peripheral and central segments of single sensory nerve fibres.
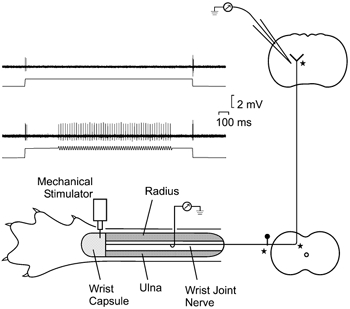
Schematic representation of the preparation for paired recording from a single afferent fibre in the wrist joint nerve and within the cuneate division of the dorsal column nuclei (DCN). Major sites of axonal branching are indicated by stars. The responses of individual wrist joint afferents were monitored via a hook electrode placed under the intact wrist joint nerve, and via a microelectrode inserted into the cuneate division of the DCN. Inset, impulse activity recorded from a single afferent fibre in the wrist joint nerve when mechanical stimuli were delivered to its receptive field on the joint capsule. This fibre responded only to the dynamic components of a 1.5 s long static indentation (400 μm; upper traces), but could be driven in a sustained manner (lower traces) by a 1 s long vibration train (50 Hz) superimposed on the background step. Negativity upwards in impulse traces in this and other figures.
As propagation failures have been reported at axonal branch points in both invertebrate (e.g. see Parnas, 1972; Grossman et al. 1973, 1979; Smith & Hatt, 1976; Westerfield et al. 1978) and vertebrate species (e.g. see Dun, 1955; Wall et al. 1956; Lüscher et al. 1994b,a; for review, see Swadlow et al. 1980), the purpose of the present study was to investigate whether propagation failure within tactile and kinaesthetic sensory axons, in particular, at the dorsal root ganglion or at the major intraspinal branch point, might contribute, along with synaptic transmission failures, to the observed decline in transmission security at DCN synapses. The analysis involved simultaneous recording from peripheral and central segments of single afferent fibres, and has been reported in abstract form (Coleman et al. 1999).
METHODS
Animal preparation
Experiments were conducted on adult cats of either sex. All experiments conformed with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Cats were anaesthetized initially by an intraperitoneal injection of sodium pentobarbitone (Nembutal, 40 mg kg−1, Abbot), and maintained during the course of the experiment by infusion of the anaesthetic agent (≈2 mg kg−1 h−1) via a cannula inserted into the femoral vein. In addition, atropine sulfate (Atropine Injection BP, 80 μg kg−1, Astra) was routinely administered at the commencement of the experiment to reduce respiratory secretions. The femoral artery was cannulated to monitor heart rate and blood pressure, by means of a pressure transducer. A tracheostomy was performed and a tracheal cannula inserted. Rectal temperature was maintained at 38 ± 0.5 °C with a feedback-controlled heating blanket and infrared heating lamp. Autonomic indices of anaesthetic level, including heart rate, blood pressure and pupillary aperture, were monitored throughout the experiment. In addition, the animal's hind paw was periodically pinched to test for the presence of a withdrawal reflex. When necessary, the rate of anaesthetic infusion was adjusted to maintain a stable plane of anaesthesia. Experiments were terminated with an overdose of sodium pentobarbitone.
The nerves supplying the distal forelimb, with the exception of either the deep radial (for joint afferent fibres) or the superficial radial nerve (for hair follicle afferent (HFA) fibres), were sectioned (including the median, ulnar, musculocutaneous and median musculocutaneous nerves) to eliminate contaminating afferent signals that could arise from sources other than the monitored nerves.
Recording and stimulation procedures
Recordings were made from single wrist joint afferent fibres in the articular branch of the dorsal interosseous nerve (the wrist joint nerve) that supplied the dorsal aspect of the wrist joint capsule, or from single HFA fibres in intact fascicles of the lateral branch of the superficial radial nerve. Preparation of these nerves for the purpose of monitoring the activity of individual, identified group II sensory fibres has been described elsewhere (Vickery et al. 1994; Coleman et al. 1998, 2003).
Impulse activity in the peripheral nerve or nerve fascicle was recorded with a hook electrode placed under the nerve, as illustrated schematically in Fig. 1 for the wrist joint nerve recording. Simultaneous recordings were made from the centrally projecting axon with a microelectrode inserted into the cuneate division of the DCN, which had been exposed by removal of a small part of the atlas and occiput. Peripherally and centrally recorded signals were displayed on oscilloscopes and stored on magnetic tape. Single afferent fibres could be selectively activated from the skin (for HFA fibres) or from the wrist joint capsule, by means of a fine (0.25 mm diameter) stimulator probe, driven by a feedback-controlled mechanical stimulator. Afferent fibres of both classes (wrist joint afferent and HFA fibres) were activated most effectively by applying maintained forms of dynamic mechanical disturbances, in particular, sinusoidal vibration, to the focal point of their receptive fields (see Fig. 1 impulse traces).
Latency histograms (for details see Coleman et al. 2003) were constructed (e.g. in Fig. 2B and Fig. 5A) to examine the temporal relations of impulse occurrences at the peripheral and central recording sites. These histograms allowed the calculation of conduction latency and the reliability of impulse propagation, by giving a measure of the percentage of peripheral spikes that were followed by correlated spike activity in the central recording trace.
Figure 2. Paired recordings from peripheral and central segments of a single joint afferent fibre.
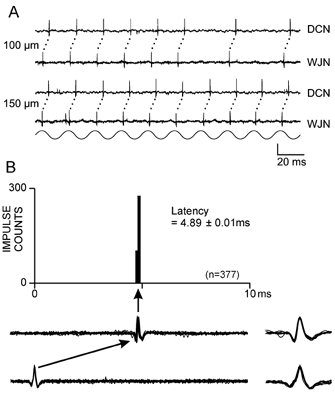
A, impulse activity recorded simultaneously from the wrist joint nerve (WJN) and the dorsal column nuclei (DCN) in response to a 50 Hz (150 μm) vibration train applied to the joint afferent fibre's receptive field on the exposed wrist joint capsule. The response traces in A and the latency histogram in B (see Methods) illustrate the close correlation between the spike output of the central unit and preceding impulse activity in the peripheral fibre, with a latency of 4.89 ± 0.01 ms (mean ± S.D., n = 377). The ten superimposed impulse traces beneath the graph in B, from the central (upper) and peripheral (lower) recordings, are on the same time scale as the latency histogram. The peripherally recorded spike is aligned at time zero on the histogram. Superimposed, expanded spike waveforms (1 ms long traces) are shown to the right. Vertical scale bar represents 100 μV for the DCN traces, and 50 μV for the WJN traces for both A and B. The histogram contains 100 addresses, each of 100 μs.
Figure 5. Analysis of the timing relations between the peripheral and central axonal responses for a single, identified sensory nerve fibre.
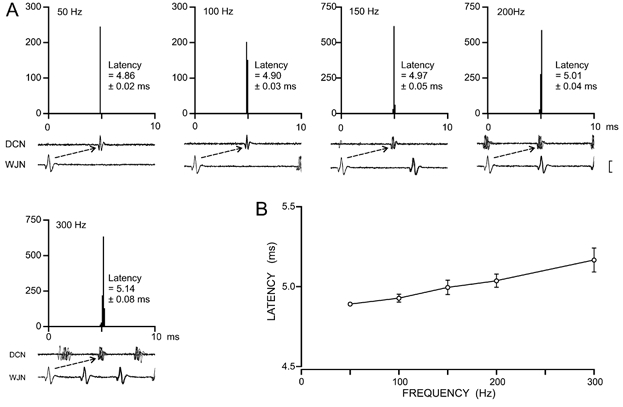
A, latency histograms constructed to obtain measures of the timing relations (latency ± S.D.) between peripheral and central axonal responses for a single wrist joint afferent fibre when activated by focal stimulation of the joint capsule at five vibration frequencies (50–300 Hz). In the associated paired impulse traces the vertical scale bar represents 400 μV for lower traces and 100 μV for upper traces in each pair. Each histogram contains 100 addresses, each of 100 μs width. B, the plot of mean latency ± S.D. for the central response as a function of vibration frequency shows that response latency at each frequency was extremely consistent, although there was a small but significant slowing of conduction with increasing frequency (P < 0.0005).
Cycle histograms (CHs) were constructed to evaluate the capacity of the afferent fibres to encode the temporal features of dynamic forms of mechanical stimuli, in particular, controlled vibratory stimuli, in their pattern of discharge (e.g. Ferrington et al. 1987a,b; Vickery et al. 1994; Zachariah et al. 2001; Coleman et al. 2003). Furthermore, in the present analysis, comparison of CHs obtained for peripheral and central responses provided a measure of the fidelity with which the temporal pattern of discharge in the peripheral fibre segment was preserved in the course of propagation to the central axon segment. The CHs use a pulse associated with the onset of each vibration cycle as a stimulus marker and show the time of occurrence of impulses during successive cycles. They were usually constructed from five or ten repetitions of the 1 s vibration stimulus. The tendency of the fibre to respond preferentially during a particular phase of the vibration cycle was reflected in the clustering of impulse counts in a restricted segment of the CH, and was quantified by calculating the resultant vector (R; see Mardia, 1972; Greenstein et al. 1987; Vickery et al. 1994; Coleman et al. 2003) for the CH distribution. The value of R ranges from 0 (indicating an absence of phase preference) to a theoretical maximum of 1 (indicating perfect phase-locking; Durand & Greenwood, 1958; Zar, 1984).
RESULTS
Simultaneous peripheral and central recording from the one sensory nerve fibre
Paired simultaneous recordings from the peripheral and central segments of sensory nerves were carried out for five afferent fibres, three identified as HFA fibres in fascicles of the superficial radial nerve, and two as joint afferent fibres recorded from the wrist joint nerve. In each case the central recording was made from the axon within the cuneate nucleus. All were isolated caudal to, and within 2 mm of the obex, at distances of 0.8 to 1.8 mm lateral to the midline, and at depths of 0.65 to 1.1 mm below the brainstem surface. This small sample comprised all the recordings of this kind that could be gathered in the course of over 50 experiments for two major studies of cuneate transmission properties (Zachariah et al. 2001; Coleman et al. 2003).
Before establishing criteria for determining whether the central and peripheral recordings arose from the one sensory nerve fibre it was necessary to examine the spike configuration for the centrally recorded element. This provides a guide for distinguishing cuneate soma responses from those of peripheral axons recorded within the cuneate nucleus or its entry zone, as the putative axonal spikes are shorter in duration (< 0.5 ms) than those of central neurones, and generally have a triphasic waveform with a large negative-going component (see expanded waveforms in Fig. 2 and Fig. 3; and Winter, 1965). Having established that the centrally recorded responses were consistent with an axonal origin it was then essential, before concluding that central and peripheral responses arose from the one sensory fibre, that the centrally recorded spike followed the peripheral spike with a latency that was highly stable in comparison with that of central neurone responses (see below). Furthermore, at least up to moderate rates of afferent drive, there should be a correspondence in the presence or absence of the peripherally and centrally recorded spikes.
Figure 3. Impulse traces and stimulus-response relations for a single wrist joint afferent fibre recorded at both peripheral (WJN) and central (DCN) sites.
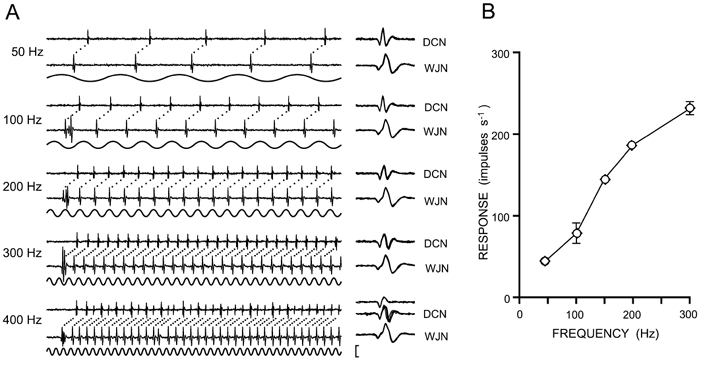
Vibratory stimuli were applied within the fibre's receptive field at a range of frequencies (50–400 Hz). A, each paired impulse trace shows the response to the first 100 ms of a 1 s stimulus, at a vibration amplitude that produced 1:1 following in the fibre (i.e. one impulse on every vibration cycle). Ten expanded, superimposed spike waveforms (1 ms long) are shown to the right in A. In response to the 400 Hz stimulus, the spike waveform recorded centrally began to switch between two alternative configurations, suggesting (in the approximately two-thirds of cases in the illustrated traces) that there is propagation block in either the entire terminal axon, or in one axonal branch at this high impulse frequency. B, response levels (impulses s−1 ± S.D.) at the central (diamonds) and peripheral (circles) segments of the same wrist joint afferent fibre plotted as a function of vibration frequency, when 1 s vibration trains were applied to the joint capsule at amplitudes that produced near 1:1 following in the fibre. However, at vibration frequencies of 100 and 300 Hz, the vibration amplitude was below that needed to generate a consistent 1:1 pattern of response and this contributes to the greater standard deviation seen in the response levels (for other vibration frequencies, the very low S.D. values fell within the symbols on the graph). The vertical scale bar represents 400 μV for WJN traces and 100 μV for DCN traces.
Latency histograms were constructed to reveal the temporal precision in the correlated responses obtained from the peripheral and central elements in the paired recording arrangement. For each sensory fibre examined, the centrally recorded response occurred at a fixed latency with respect to the peripherally recorded spike, and with a very small standard deviation. In the example illustrated in Fig. 2, the mean latency ± S.D. values were 4.89 ± 0.01 ms and in the examples in Fig. 5, the S.D. values for the latency were usually less than 0.05 ms. In contrast, even the most secure synaptic linkages formed between identified tactile afferents and cuneate neurones display a greater latency variability, as measured by the standard deviation values, in particular, in response to higher rates (>100 impulses s−1) in the peripheral input (see, e.g. Fig. 5 and Fig. 6C in Vickery et al. 1994; and Fig. 6 and Fig. 7 in Coleman et al. 2003).
For each sensory fibre examined, it was also found that following each peripheral spike, the central response unfailingly consisted of a single action potential (Fig. 2A and Fig. 3), in contrast to the frequent occurrence of paired spikes or spike bursts in the responses of central target neurones to individual peripheral spikes (e.g. Figs 2–4 in Ferrington et al. 1987a; Fig. 1 in Vickery et al. 1994; and Fig. 3 and Fig. 4 in Zachariah et al. 2001). The exact one-to-one correspondence between central and peripheral spikes apparent in the representative impulse records of Fig. 2 and Fig. 3 was confirmed for the wrist joint afferent fibre of Fig. 3 in the quantified stimulus-response relation in Fig. 3B in which response level in the peripheral recording is plotted as open circles and that of the central neurone as open diamonds. Each point represents the mean response (impulses s−1) to five repetitions of the 1 s-long vibration stimulus, obtained when the peripherally recorded joint afferent was responding in a near one-to-one manner (i.e. one impulse per vibration cycle) to a 1 s-long sinusoidal vibration train at 50, 150 and 200 Hz. The S.D. values, as well as mean response levels, were identical for the peripheral and central responses at all five frequencies from 50 to 300 Hz (the 400 Hz responses are considered below), further corroborating the conclusion that the central and peripheral recordings came from one and the same sensory axon.
Similarity of phase-locking in peripheral and central responses to vibratory stimuli
Further evidence for the reliable propagation of peripheral sensory spikes to the DCN comes from the comparison of the tightness of phase-locking of peripheral and central responses to focal vibration applied to the receptive field focus, whether on the skin or the joint capsule. Cycle histograms (CHs) constructed (Fig. 4) from the responses of the peripheral and central axonal segments of a wrist joint afferent at five vibration frequencies from 50 to 300 Hz showed identical, or near-identical, distributions, at any given frequency for the peripheral and central elements. Quantitative comparison of the phase coherence, based on calculation of the resultant vector (R) or the standard deviation (S.D.) derived from these cycle histogram distributions, confirmed the near identity in phase dispersion, in contrast to the substantial increase in phase dispersion for DCN neurones compared with that for the input fibres (e.g. Fig. 7 in Ferrington et al. 1987a; Fig. 12 in Ferrington et al. 1987b; and Fig. 8 and Fig. 9 in Coleman et al. 2003). At the vibration frequency of 50 Hz (upper pair of CHs in Fig. 4) the R value was 0.98 for both peripheral (left side) and central (right side) response distributions, and remained almost identical at the higher frequencies (100-300 Hz) where the central neurones are known to show a marked decline in the tightness of phase-locking (Douglas et al. 1978; Ferrington et al. 1987a,b; Vickery et al. 1994; Gynther et al. 1995; Coleman et al. 2003). Although in Fig. 4 the R values at 200 and 300 Hz were fractionally higher for the central responses (e.g. at 300 Hz, 0.91 compared with 0.89 for the peripheral responses) the difference is attributable, first, to slight errors of counting resulting from the switching in the central spike configuration at high frequencies (reflected in the lower counts for the right hand CHs at 200 and 300 Hz in Fig. 4; see below), and second, to a slight contamination of the peripheral recording (e.g. see onset of spike trains in Fig. 3A).
Figure 4. Cycle histograms constructed to display the phase-locking in the vibration-induced responses recorded at the peripheral and central sites for a single wrist joint afferent fibre.
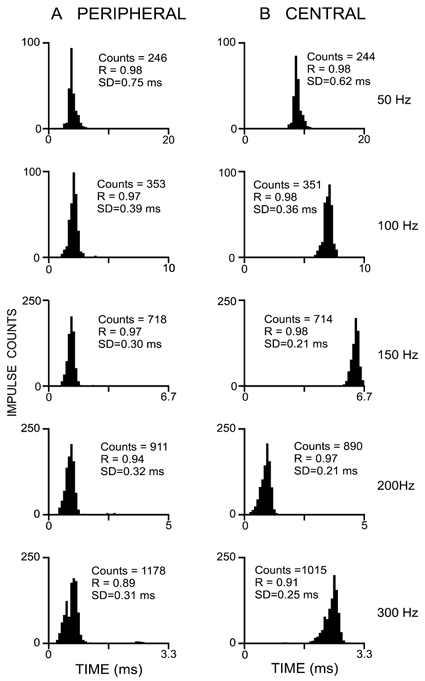
Data come from the fibre whose responses are illustrated in Fig. 3. At each vibration frequency (50–300 Hz) the shapes of the cycle histogram distributions for peripheral and central recording sites are nearly identical, as would be expected if the cuneate unit is the centrally projecting axon of the fibre recorded in the peripheral nerve. Slight discrepancies in the impulse counts and measures of phase-locking (the resultant vector, R) and the standard deviation (S.D.) for the distribution in the peripheral and central responses of the fibre were due to small errors of counting, primarily related to the switching in the configuration of the central spike at high frequencies (see Fig. 3).
Evidence for intra-cuneate propagation failure in primary sensory axons
In the sensory fibre that displayed the highest impulse rate in response to peripheral vibratory stimuli (Fig. 3) it was found that when activated by a 400 Hz vibratory stimulus to respond at rates of 400 impulses s−1 (as monitored at the peripheral recording site), the central axon responded unfailingly at this same rate and with a precise spike-to-spike temporal correlation (Fig. 3). However, the centrally recorded spike underwent a sporadic switching in its configuration between two states. In approximately one-third of cases in the 400 Hz response, the central spike resembled the predominantly triphasic configuration displayed at lower rates of drive generated by vibration at 50-300 Hz (Fig. 3). This spike waveform, shown in the expanded superimposed DCN records on the right hand side in Fig. 3A, consisted of an initial positive (downgoing phase, then a large negative, upgoing phase) that was followed by another phase of positivity (and a small later negativity). However, approximately two-thirds of the central spikes at 400 Hz had a simpler biphasic (positive-negative) configuration. It is unlikely that the more complex form of this central spike is attributable to a fused spike complex from both a presynaptic axon and its cuneate target neurone, because the temporal link between components within this spike complex is far too precise and stable to include a synaptic transmission step. Our tentative hypothesis for the switching of configuration is that the recording microelectrode was located close to a central axonal branch point near the axon terminals in the cuneate nucleus. The more complex spike configuration seen in the expanded waveforms of Fig. 3 may reflect the superimposition of spike waveforms produced in adjacent axon branches of this one sensory fibre. The change to the biphasic spike waveform at the high discharge rate could therefore result from failure of conduction through one of the intra-cuneate branches of the axon at high impulse rates (in this case in excess of 300 impulses s−1). However, an alternative explanation for the switching of spike shape could be a propagation block, not just in one or other of the terminal branches, but perhaps in the terminal axon as a whole.
Stability of response latency in the central axonal response as a function of impulse rate
The paired, simultaneous recording from the peripheral and central segments of each of the five sensory fibres sampled, enabled the overall conduction velocity for each fibre to be calculated for the axon segment between the forearm peripheral recording site and the central intra-cuneate recording site, a distance that, on average, was ≈25 cm. The values for both the wrist joint afferent fibres were ≈50 m s−1, while those for the three HFA fibres were ≈62, ≈55 and ≈53 m s−1, all of which fall within the group II range.
As indicated above (Fig. 2), the latency stability for the central response in the five fibres was too great to involve a synaptic junction, leading to the conclusion that the response was from the central component of the sensory axon. Nevertheless, a small prolongation in latency and decline in latency stability occurs as a function of increases in response levels in the fibre. This is illustrated for a wrist joint afferent fibre in Fig. 5 where the latency histograms (Fig. 5A) show the latency distributions of the central spike response (in relation to the peripheral spike occurrences at time zero on the abscissa), when the joint afferent fibre was activated at progressively higher impulse rates in response to focal vibration (50-300 Hz) applied to the receptive field on the wrist joint capsule. Regression analysis confirmed that the increase in latency as a function of vibration frequency (Fig. 5A and B) was significant (P < 0.0005). At the highest impulse rate (≈230 impulses s−1 generated by the 300 Hz stimulus) the latency was ≈0.3 ms longer than at the 50 s−1 rate and had greater variability (5.14 ± 0.08 ms at 300 Hz compared with 4.86 ± 0.02 ms at 50 Hz; mean ± S.D., Fig. 5). The same tendency was also observed for HFA fibres as the vibration stimulus frequency was increased. However, as was the case for the joint afferent fibres, the latency variation for the central HFA responses was still very much less at all driving frequencies than that observed for postsynaptic responses from cuneate neurones (Vickery et al. 1994; Coleman et al. 2003).
DISCUSSION
Transmission security at DCN synapses for tactile and kinaesthetic afferent fibres
High transmission security within the DCN appears to be a general attribute for all classes of tactile and kinaesthetic sensory nerve fibres (Ferrington et al. 1986, 1987a,b; Vickery et al. 1994; Gynther et al. 1995; Zachariah et al. 2001; Coleman et al. 2003). These studies have revealed that inputs arriving over individual sensory fibres may be transmitted with amplification across the DCN synapse and with a preservation of temporal precision in the signalling. Nevertheless, a point is reached, often at afferent fibre driving rates of > 100-200 impulses s−1, where there is a decline in transmission security, usually ascribed to failure in the process of synaptic transmission. However, it remains unclear whether part of this breakdown in transmission security may be attributed to failure of impulse propagation over the sensory axon itself. Failure of axonal propagation has been observed at regions of low safety factor such as axonal branching points (Dun, 1955; Wall et al. 1956) or regions of demyelination (Swadlow et al. 1980; Bostock & Grafe, 1985). Thus, axons may not always conduct impulses faithfully, but ‘might, in fact, function in some systems as variable filters modulating the spatial and temporal relations between impulses' (Swadlow et al. 1980). These authors have emphasized that the ‘classical view' of axonal conduction, where ‘impulses initiated near the soma are faithfully distributed along the axonal arbors and reproduced at the axon terminals', may be too simplistic.
The isolation of responses from both peripheral and central segments of individual tactile and kinaesthetic afferent fibres in the present study provided an opportunity to investigate whether there is reliable impulse propagation from the periphery to the DCN. Evidence that the simultaneously recorded central and peripheral responses came from the one sensory axon was based (see Results) upon the spike-to-spike correlation in the peripheral and central recording (Fig. 2 and Fig. 3), the latency stability for the central response following the peripheral spike occurrence (Fig. 2B and Fig. 5), and the near-identity in measures of phase-locking obtained from central and peripheral responses to the vibromechanical stimuli (Fig. 4).
Secure impulse propagation for tactile and kinaesthetic afferents across the axonal branch points in the dorsal root ganglion and at the spinal cord entry zone
In none of the group II tactile and kinaesthetic afferent fibres studied was there evidence of propagation failure at either of the two major axonal branch points prior to the DCN, namely, the T-junction at the dorsal root ganglion (DRG) and the major branch point near the entry point to the spinal cord. This was the case even for the highest impulse rates (up to 400 impulses s−1; see Fig. 2 and Fig. 3) at which we could drive these fibres with 1 s-long natural stimuli, although conduction block has been reported previously at both these sites (Dun, 1955; Wall et al. 1956). However, the propagation failure at the DRG T-junction, observed by Dun (1955), was found in amphibia and may reflect a species difference or simply a propagation failure related to the lower temperature in these species. Propagation failure in the DRG of mammals has also been reported although this was in cultured DRGs from fetal rats (Lüscher et al. 1994b), and occurred at inter-impulse intervals below ≈12 ms for paired impulses, and at longer intervals for trains of impulses. The marked difference between this observation and our demonstration of reliable propagation to the central axon at impulse rates up to at least 400 s−1 is almost certainly attributable, first, to the absence of myelination in the immature (fetal) DRG neurones which had conduction velocities below 1 m s−1, and second, to the non-physiological temperature of 22 ± 2 °C used for the in vitro study (Lüscher et al. 1994b).
Earlier arguments for propagation failure at the major axon bifurcation point near the entry zone of the spinal cord have been based upon a decline in the amplitude of thoracic dorsal column evoked potentials when sciatic nerve stimulation rates exceeded 100 s−1 (Wall et al. 1956). It was argued that this branch point may impose a limit on the rate of information transmission to the terminal arborizations in the DCN that falls far below the maximum impulse rate observable in the peripheral segments of nerve fibres. However, the evoked potential decline may have reflected some decrease in amplitude of individual action potentials contributing to the evoked potential, as the relative refractory period of the nerve fibres was reached. Other contributing factors may include, for example, the accumulation of extracellular K+, associated with whole sciatic nerve stimulation at this rate, or a varying postsynaptic contribution to the observed evoked potential. In the present study, based on single unit recording, we found no evidence for propagation failure at this branch point at discharge rates up to ≈400 impulses s−1.
The present observations therefore provide no evidence for propagation failure at either of the two major axonal branch points in the tactile and kinaesthetic sensory axons. However, some slowing of axonal conduction as a consequence of prior activity was apparent in the present study (Fig. 5), a phenomenon observed earlier, for example, in visual (George et al. 1984) and tactile afferent fibres (Wall & McMahon, 1994) and in unmyelinated afferent axons (Schmelz et al. 1995; Grafe et al. 1997; Serra et al. 1999). The magnitude of this change was small, < 5 % in the most marked example (Fig. 5), and occurred when the peripheral-to-central conduction time increased from ≈4.9 to 5.1 ms as the impulse rate in this joint afferent fibre was increased from 50 to 300 impulses s−1 in response to focal vibration. This prolongation of conduction time, and an accompanying slight increase in the variability of the conduction time (Fig. 5) at high impulse rates may be related to extracellular K+ accumulation around the axon, in particular, at axonal branch points (Vyskocil et al. 1972), leading to a decreased membrane potential and increased sodium channel inactivation (Hodgkin & Huxley, 1952). However, this explanation is speculative.
It should be emphasized that these small changes in axonal conduction time that occur with increasing impulse rates in the sensory fibre have negligible impact on the tightness of phase-locking in the central axon segment compared with that observed in the peripheral segment (Fig. 4). Thus, the capacity of the sensory fibre to reliably encode temporal features of peripheral stimuli, such as the vibratory type used in the present study, was apparently unaffected by the observed changes in conduction occurring over the centrally projecting component of the axon.
Propagation failure in terminal axonal branches within the cuneate nucleus
Although the intra-cuneate responses of the axons studied showed an unfailing correlation with the simultaneously monitored peripheral spike activity, even at the maximum rates (up to 400 impulses s−1) of peripheral drive, there was evidence at high impulse rates (Fig. 3) for switching in the central response between two spike configurations. As the primary sensory axons are known to branch extensively upon entering the cuneate nucleus to supply central target neurones (Fyffe et al. 1985, 1986) it is possible, at these terminal branch points where the axon diameter is narrowing, that the safety factor for propagation declines. At high discharge rates (e.g. > 300 impulses s−1), other influences, such as relative refractoriness, may further diminish the safety factor and lead to failure of the action potential to invade some or all of the terminal axonal branches. Thus, in circumstances such as those of Fig. 3, propagation failure into one or all branches at high impulse rates may explain the observed switching of the spike configuration between the two states.
It is improbable that the sporadic terminal propagation failure of the type encountered in Fig. 3 is a consequence of damage to the axonal branch from the recording electrode as any such damage would probably also give rise to the sporadic switching of configuration at low frequencies of afferent drive. Furthermore, differential conduction block of a similar kind has been observed at points of axonal division in other circumstances (e.g. Parnas, 1972; Grossman et al. 1973, 1979; Smith & Hatt, 1976; Westerfield et al. 1978). In addition, the propagation failure we report is reminiscent of that reported for some peripheral branches of human C-nociceptive afferent fibres of the peroneal nerve (Weidner et al. 2003).
In conclusion, it appears that the central impulse propagation over tactile and kinaesthetic sensory axons occurs with complete security through the branch points within the dorsal root ganglion and at the spinal cord entry zone. However, at high rates of afferent drive (> ≈300 Hz), successful invasion of each axon terminal branch within the cuneate nucleus is less certain, with inevitable consequences for the number of activated transmitter release sites for the presynaptic axon (Redman & Walmsley, 1983a,b; Walmsley, 1991). The present observations therefore indicate that terminal axonal propagation failure could contribute to the observed decline in transmission security within the DCN relay nucleus at high rates of afferent input generated in single tactile or kinaesthetic afferent fibres (Ferrington et al. 1986, 1987a,b; Vickery et al. 1994; Gynther et al. 1995; Zachariah et al. 2001; Rowe, 2002; Coleman et al. 2003). However, as the decline in central neurone responsiveness, or transmission security, often becomes apparent at lower rates of afferent drive (e.g. 100 and 200 Hz), we may infer that the principal component of the decline in transmission security is attributable to synaptic transmission failures, rather than to propagation failures in the terminal segments of the afferent axon. We should also emphasize that in physiological circumstances, in which multiple afferent fibres might be active, whether as a consequence of flexion-extension movements at the wrist joint or, in the case of cutaneous afferents, as a result of an object brushing over the skin, the convergent actions of the recruited fibres could compensate for failures associated with individual afferent fibres, whether in synaptic transmission or propagation failure within the terminal axon.
Acknowledgments
We acknowledge the technical assistance of C. Riordan and D. Sarno. The work was supported by the National Health and Medical Research Council of Australia and the Australian Research Council.
REFERENCES
- Bostock H, Grafe P. Activity-dependent excitability changes in normal and demyelinated rat spinal root axons. J Physiol. 1985;365:239–257. doi: 10.1113/jphysiol.1985.sp015769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman GT, Zachariah MK, Rowe MJ. Reliable propagation through the dorsal root ganglion of impulses arising in single mechanoreceptive afferent fibers of the cat. Proc Aust Neurosci Soc. 1999;10:135. [Google Scholar]
- Coleman GT, Zhang HQ, Mackie PD, Rowe MJ. An intact peripheral nerve preparation for examining the central actions of single kinesthetic afferent fibers arising in the wrist joint nerve of the cat. Prim Sensory Neuron. 1998;3:61–70. [Google Scholar]
- Coleman GT, Zhang HQ, Rowe MJ. Transmission security for single kinesthetic afferent fibers of joint origin and their target cuneate neurons in the cat. J Neurosci. 2003;23:2980–2992. doi: 10.1523/JNEUROSCI.23-07-02980.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas PR, Ferrington DG, Rowe MJ. Coding of information about tactile stimuli by neurones of the cuneate nucleus. J Physiol. 1978;285:493–513. doi: 10.1113/jphysiol.1978.sp012585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun FT. The delay and blockage of sensory impulses in the dorsal root ganglion. J Physiol. 1955;127:252–264. doi: 10.1113/jphysiol.1955.sp005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D, Greenwood JA. Modifications of the Rayleigh test for uniformity in analysis of two-dimensional orientation data. J Geol. 1958;60:229–238. [Google Scholar]
- Ferrington DG, Rowe MJ, Tarvin RPC. High gain transmission of single impulses through dorsal column nuclei of the cat. Neurosci Lett. 1986;65:227–282. doi: 10.1016/0304-3940(86)90274-0. [DOI] [PubMed] [Google Scholar]
- Ferrington DG, Rowe MJ, Tarvin RPC. Actions of single sensory fibres on cat dorsal column nuclei neurones: vibratory signalling in a one-to-one linkage. J Physiol. 1987a;386:293–309. doi: 10.1113/jphysiol.1987.sp016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrington DG, Rowe MJ, Tarvin RPC. Integrative processing of vibratory information in cat dorsal column nuclei neurones driven by identified sensory fibers. J Physiol. 1987b;386:311–331. doi: 10.1113/jphysiol.1987.sp016536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe REW, Cheema SS, Light AR, Rustioni R. The organization of neurons and afferent fibers in the cat cuneate nucleus. In: Rowe MJ, Willis WD, editors. Development, Organization, and Processing in Somatosensory Pathways. New York: Alan R. Liss; 1985. pp. 163–173. [Google Scholar]
- Fyffe REW, Cheema SR, Rustioni A. Intracellular staining study of the feline cuneate nucleus. I. Terminal patterns of primary afferent fibers. J Neurophysiol. 1986;56:1268–1283. doi: 10.1152/jn.1986.56.5.1268. [DOI] [PubMed] [Google Scholar]
- George SA, Mastronarde DN, Dubin MW. Prior activity influences the velocity of impulses in frog and cat optic nerve fibers. Brain Res. 1984;304:121–126. doi: 10.1016/0006-8993(84)90867-9. [DOI] [PubMed] [Google Scholar]
- Grafe P, Quasthoff S, Grosskreutz J, Alzheimer C. Function of the hyperpolarization-activated inward rectification in nonmyelinated peripheral rat and human axons. J Neurophysiol. 1997;77:421–426. doi: 10.1152/jn.1997.77.1.421. [DOI] [PubMed] [Google Scholar]
- Greenstein J, Kavanagh P, Rowe MJ. Phase coherence in vibration-induced responses of tactile fibers associated with Pacinian corpuscle receptors in the cat. J Physiol. 1987;386:263–275. doi: 10.1113/jphysiol.1987.sp016533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y, Parnas I, Spira ME. Differential conduction block in branches of a bifurcating axon. J Physiol. 1979;295:283–305. doi: 10.1113/jphysiol.1979.sp012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y, Spira ME, Parnas I. Differential flow of information into branches of a single axon. Brain Res. 1973;64:379–386. doi: 10.1016/0006-8993(73)90191-1. [DOI] [PubMed] [Google Scholar]
- Gynther BD, Vickery RM, Rowe MJ. Transmission characteristics for the 1:1 linkage between SAII fibers and their cuneate target neurons in cat. Exp Brain Res. 1995;105:67–75. doi: 10.1007/BF00242183. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952;116:497–506. doi: 10.1113/jphysiol.1952.sp004719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Streit J, Lipp P, Lüscher H-R. Action potential propagation through embryonic dorsal root ganglion cells in culture. II. Decrease of conduction reliability during repetitive stimulation. J Neurophysiol. 1994b;72:634–643. doi: 10.1152/jn.1994.72.2.634. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Streit J, Quadroni R, Lüscher H-R. Action potential propagation through embryonic dorsal root ganglion cells in culture. I. Influence of the cell morphology on propagation properties. J Neurophysiol. 1994a;72:622–633. doi: 10.1152/jn.1994.72.2.622. [DOI] [PubMed] [Google Scholar]
- Mardia KV. Statistics of Directional Data. London: Academic Press; 1972. [Google Scholar]
- Parnas I. Differential block at high frequency of branches of a single axon innervating two muscles. J Neurophysiol. 1972;35:903–914. doi: 10.1152/jn.1972.35.6.903. [DOI] [PubMed] [Google Scholar]
- Redman S, Walmsley B. The time course of synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983a;343:117–133. doi: 10.1113/jphysiol.1983.sp014884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman S, Walmsley B. Amplitude fluctuations in synaptic potentials evoked in cat spinal motoneurones at identified group Ia synapses. J Physiol. 1983b;343:135–145. doi: 10.1113/jphysiol.1983.sp014885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MJ. Synaptic transmission between single tactile and kinaesthetic sensory nerve fibres and their central target neurones. Proceedings of a Wenner-Gren international conference on brain mechanisms of tactile perception. Behav Brain Res. 2002;135:197–212. doi: 10.1016/s0166-4328(02)00166-3. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Forster C, Schmidt R, Ringkamp M, Handwerker HO, Torebjörk HE. Delayed responses to electrical stimuli reflect C-fibre responsiveness in human microneurography. Exp Brain Res. 1995;104:331–336. doi: 10.1007/BF00242018. [DOI] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J Physiol. 1999;515:799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DO, Hatt H. Axon conduction block in a region of dense connective tissue in crayfish. J Neurophysiol. 1976;39:794–801. doi: 10.1152/jn.1976.39.4.794. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Kocsis JD, Waxman SG. Modulation of impulse conduction along the axonal tree. Ann Rev Biophys Eng. 1980;9:143–179. doi: 10.1146/annurev.bb.09.060180.001043. [DOI] [PubMed] [Google Scholar]
- Vickery RM, Gynther BD, Rowe MJ. Synaptic transmission between slowly adapting type I fibers and their cuneate target neurones in cat. J Physiol. 1994;474:379–392. doi: 10.1113/jphysiol.1994.sp020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyskočil F, Křiž N, Bureš J. Potassium-selective microelectrodes used for measuring the extracellular brain potassium during spreading depression and anoxic depolarization in rats. Brain Res. 1972;39:255–259. doi: 10.1016/0006-8993(72)90802-5. [DOI] [PubMed] [Google Scholar]
- Wall PD. Do nerve impulses penetrate terminal arborizations? A presynaptic control mechanism. Trends Neurosci. 1995;18:99–103. doi: 10.1016/0166-2236(95)93883-y. [DOI] [PubMed] [Google Scholar]
- Wall PD, Lettvin JY, McCulloch WS, Pitts W. Factors limiting the maximum impulse transmitting ability of an afferent system of nerve fibers. In: Cherry C, editor. Information Theory. London: Butterworth; 1956. pp. 329–344. 3rd London Symposium. [Google Scholar]
- Wall PD, McMahon SB. Long range afferents in rat spinal cord. III. Failure of impulse transmission in axons and relief of the failure after rhizotomy of dorsal roots. Philos Trans R Soc Lond B Biol Sci. 1994;343:211–223. doi: 10.1098/rstb.1994.0022. [DOI] [PubMed] [Google Scholar]
- Wall PD, Shortland P. Long-range afferents in the rat spinal cord. 1. Numbers, distances and conduction velocities. Philos Trans R Soc Lond B Biol Sci. 1991;334:85–93. doi: 10.1098/rstb.1991.0098. [DOI] [PubMed] [Google Scholar]
- Walmsley B. Central synaptic transmission: studies at the connection between primary afferent fibers and dorsal spinocerebellar tract (DSCT) neurones in Clarke's column of the spinal cord. Prog Neurobiol. 1991;36:391–423. doi: 10.1016/0301-0082(91)90017-u. [DOI] [PubMed] [Google Scholar]
- Weidner C, Schmidt R, Schmelz M, Torebjörk HE, Handwerker HO. Action potential conduction in the terminal arborisation of nociceptive C-fibre afferents. J Physiol. 2003;547:931–940. doi: 10.1113/jphysiol.2002.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M, Joyner RW, Moore JW. Temperature-sensitive conduction failure at axon branch points. J Neurophysiol. 1978;41:1–8. doi: 10.1152/jn.1978.41.1.1. [DOI] [PubMed] [Google Scholar]
- Winter DL. N. gracilis of cat. Functional organization and corticofugal effects. J Neurophysiol. 1965;28:48–70. doi: 10.1152/jn.1965.28.1.48. [DOI] [PubMed] [Google Scholar]
- Zachariah MK, Coleman GT, Mahns DA, Zhang HQ, Rowe MJ. Transmission security for single, hair follicle-related tactile afferent fibers and their target cuneate neurons in cat. J Neurophysiol. 2001;86:900–911. doi: 10.1152/jn.2001.86.2.900. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical Analysis. 2. New Jersey: Prentice-Hall; 1984. [Google Scholar]


