Abstract
Rho family GTPases are primary mediators of cytoskeletal reorganization, although they have also been reported to regulate cell secretion. Yet, the extent to which Rho family GTPases are activated by secretory stimuli in neural and neuroendocrine cells remains unknown. In bovine adrenal chromaffin cells, we found Rac1, but not Cdc42, to be rapidly and selectively activated by secretory stimuli using an assay selective for the activated GTPases. To examine effects of activated Rac1 on secretion, constitutively active mutants of Rac1 (Rac1-V12, Rac1-L61) were transiently expressed in adrenal chromaffin cells. These mutants facilitated secretory responses elicited from populations of intact and digitonin-permeabilized cells as well as from cells under whole cell patch clamp. A dominant negative Rac1 mutant (Rac1-N17) produced no effect on secretion. Expression of RhoGDI, a negative regulator of Rac1, inhibited secretory responses while overexpression of effectors of Rac1, notably, p21-activated kinase (Pak1) and actin depolymerization factor (ADF) promoted evoked secretion. In addition, expression of effector domain mutants of Rac1-V12 that exhibit reduced activation of the cytoskeletal regulators Pak1 and Partner of Rac1 (POR1) resulted in a loss of Rac1-V12-mediated enhancement of evoked secretion. These findings suggest that Rac1, in part, functions to modulate secretion through actions on the cytoskeleton. Consistent with this hypothesis, the actin modifying drugs phalloidin and jasplakinolide enhanced secretion, while latrunculin-A inhibited secretion and eliminated the secretory effects of Rac1-V12. In summary, Rac1 was activated by secretory stimuli and modulated the secretory pathway downstream of Ca2+ influx, partly through regulation of cytoskeletal organization.
The Rho family of small GTPases constitute a branch of the Ras superfamily that are essential regulators of actin cytoskeleton organization, gene expression, cell cycle progression and activation of NADPH oxidase (Hall, 1998; Mackay & Hall, 1998; Kaibuchi et al. 1999). The Rho family GTPases consist of more than 15 members that include isoforms of Rho, Rac, Cdc42 and TC10. Like related GTPases, Rho family GTPases are activated by guanine nucleotide exchange factors (GEFs) that stimulate GDP-GTP exchange, as well as inactivated by GTPase activation proteins (GAPs) and by nucleotide dissociation inhibitor proteins (RhoGDIs). Regulated by their GTP/GDP bound state, RhoA, Rac1 and Cdc42 act as molecular switches whose downstream effectors include serine/threonine kinases (e.g. p21 activated kinase, PKN and Rho kinase), tyrosine kinases (MLK3) and lipid kinases (e.g. phosphatidylinositol 4-phosphate 5-kinase). Rho family GTPases exhibit significant hierarchical and regulatory interactions, although Rho, Rac and Cdc42 promote notably different morphological changes of cells through selective actions on cytoskeletal reorganization (Ridley & Hall, 1992; Hall, 1994; Nobes & Hall, 1995).
A dense network of F-actin filaments, termed cortical actin, adjacent to the plasma membrane has been proposed to act as a barrier that prevents access of secretory vesicles to the membrane, but also to serve as a structural component that is essential to orchestrate recruitment and resupply of secretory vesicles to a readily releasable pool (Cheek & Burgoyne, 1986; Nakata & Hirokawa, 1992; Trifaro et al. 1992b; Trifaro & Vitale, 1993; Muallem et al. 1995; Chowdhury et al. 1999; Lang et al. 2000). Activation of exocytosis in several secretory cell types, including chromaffin cells (Vitale et al. 1995; Tchakarov et al. 1998), mast cells (Norman et al. 1994), synaptosomes (Bernstein & Bamburg, 1985), and pancreatic acinar cells (Jungermann et al. 1995) results in transient cortical F-actin disassembly followed by reassembly. The reorganizing of cortical actin structure during the secretory process has implicated Rho family GTPases as potentially critical regulators of calcium-dependent secretory pathway(s) (Pinxteren et al. 2000). Indeed, the effects of Rac, Cdc42 and Rho to regulate GTP- and/or Ca2+-dependent degranulation in mast cells and in the HL-60 mast cell line has been extensively investigated (Norman et al. 1996; O'Sullivan et al. 1996; Prepens et al. 1996; Guillemot et al. 1997; Brown et al. 1998; Hong-Geller & Cerione, 2000; Pinxteren et al. 2000). In neurons and neuroendocrine cells evidence for a role of these GTPases in neurosecretion is largely based on effects of clostridial toxins that inhibit RhoGTPase family members. Cdc42 and/or Rac have been implicated in regulation of pancreatic β cell (Kowluru et al. 1997), PC12 cell (Frantz et al. 2002) and adrenal chromaffin cell (Gasman et al. 1999) secretion and in acetylcholine release from Aplysia neurons (Doussau et al. 2000; Humeau et al. 2002). By comparison, most evidence suggests Rho exerts little regulatory influence on the secretory pathway (Kowluru et al. 1997; Gasman et al. 1999). However, to date there has been no evaluation of the extent to which these GTPases are activated by secretory stimuli.
A focus of the present investigation was to determine if the level of Rac1 or Cdc42 activation was altered in response to secretagogue activation of secretion, as would be expected of a dynamic mediator of the Ca2+-dependent neurosecretory pathway. As Rac1, but not Cdc42, was activated we further examined the relationship of Rac1 activation to functional effects on Ca2+-dependent neurosecretion by expression of mutants of Rac1 that are maintained in constitutively active (GTP-bound) or inactive (GDP-bound) conformations. Finally, we evaluated if Rac1-mediated regulation of the secretory pathway was mechanistically related to cytoskeletal reorganization by expression Rac1 effectors and effector domain mutants as well as by pharmacological alterations of cortical actin.
METHODS
Materials
Phalloidin, latrunculin-A, jasplakinolide, Alexa Fluor 568-conjugated phalloidin, tetramethylrhodamine-linked goat anti-mouse IgG, Alexa 488-linked goat anti-rabbit IgG and Alexa 488-conjugated goat anti-mouse IgG were from Molecular Probes. Monoclonal antibody against human Rac1 was from Upstate Biotechnology Inc. (Lake Placid, NY, USA) (c23A8). Anti-human RhoGDI antibody (c7) was from Transduction Laboratories (Lexington, KY, USA). Rabbit polyclonal anti-human Cdc42 (SC87) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal antibody against c-myc epitope tag (9E10) was from DSHB (Developmental Studies Hybridoma Bank, Iowa City, IA, USA) while anti-HA epitope tag antibody was from Berkeley Antibody Company (Richmond, CA, USA). The rabbit polyclonal dopamine-β-hydroxylase (DBH) antibody was from Dr Patrick J. Fleming (Georgetown University Medical Center, Washington, DC, USA). All the other chemicals were obtained from Sigma.
Plasmids
Mammalian expression plasmid pcDNA/HA-Rac1-V12, pEBG/GST-RacV12, pEBG/GST-RacN17 and pRK5/myc-RhoGDI, were kindly provided by Dr L.-H. Tsai (Harvard Medical School; Howard Hughes Medical Institute). pcDNA/3xHA-RacN17 was purchased from Guthrie Research Institute (Sayre, PA, USA). Effector domain mutants of pcDNA/HA-Rac1-V12 (T35S, Y40H, F37L) were constructed using the PCR-based Quickchange Site-Directed Mutagenesis kit from Stratagene (Cedar Creek, TX, USA). pCR3.1/ADF wild-type from chicken was from Dr James R. Bamburg. pcDNA/ANP–EmGFP, a granule-targeted ANP protein gene fused to an emerald version of green fluorescent protein (EmGFP) was from Dr Edwin Levitan (University of Pittsburgh, Pittsburgh, PA, USA). The GST-containing bacterial expression plasmid pGEX/GST-PAKPBD, which contains glutathione-S-transferase fused to the GTPase binding domain of human p21-activated kinase 1 (GST-PAKPBD) as well as pCMV6M/myc-hPAK1 has been described previously (Benard et al. 1999).
Preparation and culture of bovine chromaffin cells
Bovine adrenal glands were rinsed by gentle injection via the renal vein of a divalent metal-ion-free Locke's (DMF-Locke's) which contained (mM): 145 NaCl, 10 Hepes, 10 glucose, 5.6 KCl, 3.5 NaHCO3, with 100 units ml−1 penicillin, 100 μg ml−1 streptomycin, pH 7.4. Each gland was then injected with 5 ml of a pre-warmed 0.5 % collagenase-DMF-Locke's and gently shaken for 0.5 h at 37 oC. This collagenase digestion step was repeated once. The medullary tissue was then isolated into 15 ml of a 0.5 % (w/v) collagenase solution and the tissue minced prior to gentle shaking for 30 min at 37 oC. The digested tissue was then passed through a 250 μm gauge nylon mesh and diluted in cold DMF-Locke's. The cells were then pelleted (100 g, 3 min, 4 oC), washed twice in DMF-Locke's and resuspended into 100 ml of 14 % (v/v) sterile renograffin (Bracco Diagnostics, Princeton, NJ, USA). The cell suspension was loaded under 7.25 % renograffin in DMF-Locke's and centrifuged at 1500 g for 10 min at room temperature. The interface was collected and mixed with an equal volume of sterile saline containing (mM): 2.2 CaCl2, 1 MgCl2, 145 NaCl, 10 Hepes, 10 glucose, 5.6 KCl, 3.5 NaHCO3, with 100 units ml−1 penicillin, 100 μg ml−1 streptomycin, pH 7.4. The cell suspension was pelleted (500 g, 3 min, 20 oC), washed once and then resuspended in Dulbecco's modified Eagle's medium-F-12 containing 10 % fetal bovine serum (GIBCO BRL Life Technologies), 100 units ml−1 penicillin, 100 μg ml−1 streptomycin, 10 μg ml−1 gentamicin, 1 μM cytosine β-D-arabinofuranoside and 2.5 μg ml−1 fungizone. Cells were plated into collagen-coated 35 mm wells at a density of 3 × 106 cells per well. All experiments were performed on cells cultured between 3 and 8 days. One day prior to use, medium was replaced with fungizone-free DMEM-F-12.
Cell fractionation
Chromaffin cells (8 × 106 cells) were placed in Ca-PSS (mM: 118 NaCl, 25 NaHCO3, 1.13 MgCl2, 5 KCl, 1 Na2HPO4, 10 glucose and 2.2 CaCl2, pH 7.4) and stimulated with 20 μM of the nicotinic acetylcholine receptor agonist 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP) for 3 min at 30 °C. Control cells received no stimulation. The Ca-PSS was then removed, ice-cold fractionation buffer added (mM: 25 Tris–HCl, 50 NaCl, 1 EDTA, pH 7.5) and the cells rapidly collected. The cells were then sonicated on ice for 5 s at 7 W power output (Sonic Dismembrator Model 100, Fisher Scientific). The lysate was then subjected to centrifugation at 1000 g for 10 min and the resulting supernatant subjected to a further centrifugation at 100 000 g for 30 min. The 100 000 g pellet containing membrane fraction was solubilized with lysis buffer (mM: 25 Tris–HCl, 100 NaCl, 5 MgCl2, 1 dithiothreitol (DTT), with 5 % glycerol, 1 % Nonidet P-40 (Sigma), and a protease inhibitor cocktail consisting of 4 μg ml−1 leupeptin, 4 μg ml−1 aprotinin, 4 μg ml−1 pepstatin (Roche Diagnostics) and 1 mM phenylmethylsulfonyl fluoride (PMSF, Sigma)). Both the solubilized membrane fraction (particulate) and the 100 000 g supernatant containing the cytosol were subjected to SDS-PAGE and immunoblotting.
Cell transfection
Chromaffin cells were transfected with plasmid coated 1.0 μm diameter gold beads using a Helios Gene Gun (Bio-Rad Laboratories). The gold beads were prepared according to the manufacturer's instructions. The level of recombinant protein expression was achieved by controlling the plasmid concentration applied to the gold beads. For each of the constructs used we examined the effects of three plasmid concentrations on basal and evoked secretion. For the constitutively active constructs, the lowest plasmid concentration that gave an effect (0.5 μg per 20 mg beads) was used. For dominant negative Rac1-N17, plasmid concentrations up to 50 μg per 20 mg beads were tested. The time allowed for protein expression following transfection was held constant at 3 days.
GST-tagged fusion protein expression and purification
Recombinant GST-PAKPBD fusion protein was expressed in E. coli (BL21 codon plus RIL) and purified by glutathione-linked Sepharose 4B (Pharmacia Biotech Inc.) as previously described (Shuang et al. 1998). GST fusion protein was eluted from the Sepharose beads in 300 μl elution buffer (mM: 10 glutathione, 50 Tris–HCl, pH 8.0) and dialysed in a 10 kDa molecular weight cutoff cassette (Pierce, Rockford IL, USA) overnight against (mM): 25 Tris–HCl, 2 DTT, 1 MgCl2, with 2.5 % glycerol, pH 7.5. The GST-fusion protein was then collected, diluted in 1.7 × storage buffer (mM: 50 Hepes, 150 NaCl, 5 MgCl2, 5 DTT, with 50 % glycerol) and kept at −20 °C until use.
Rac and Cdc42 activation assay
Culture media were replaced with Ca-PSS and the cells preincubated (5 % CO2, 37 oC) for 1 h. Cells were then stimulated with 50 mM K+ in a modified Ca-PSS, or with 20 μM DMPP in Ca-PSS. For 50 mM K+ stimulation, an equal volume of a 2 × 50K/Ca-PSS solution (containing, mM: 23 NaCl, 25 NaHCO3, 1.13 MgCl2, 100 KCl, 1 Na2HPO4, 10 glucose and 2.2 CaCl2, pH 7.4) was added to the dish. For 20 μM DMPP stimulation, an equal volume of Ca-PSS containing 40 μM DMPP was added. The cells were then incubated for a given time period. Aspiration of the stimulation buffer and addition of 0.5 ml ice-cold lysis buffer containing 100 μg ml−1 GST-PAKPBD terminated stimulation. The cell lysate was collected and immediately centrifuged at 5000 g for 5 min at 4 oC. The supernatant was removed and mixed with 0.5 ml binding buffer (mM: 25 Tris–HCl, pH 7.5, 40 NaCl, 30 MgCl2, 1 DTT, with 0.5 % Nonidet P-40) to which 12 μl of glutathione-Sepharose 4B beads (Pharmacia Biotech) were added. The tubes were rotated for 45 min at 4 oC prior to collection of the beads (500 g, 3 min). The bead pellet was washed twice with 1 ml binding buffer followed by two washes with TNM buffer (mM: 50 Tris–HCl, pH 7.5, 50 NaCl and 5 MgCl2). The final bead pellet was resuspended in Laemmli sample buffer (Laemmli, 1970) and boiled. Proteins were separated by 10 % SDS-PAGE, transferred to nitrocellulose membrane and blots probed for the GTPase (Rac1 or Cdc42). Immunoreactivity was detected using the ECL chemiluminescence system (Amersham Pharmacia Biotech) in conjunction with a Fluor-S Max Multi-lmager system (Bio-Rad) running Quantity One software.
Chromaffin cell secretion assays
Human growth hormone (hGH) secretion was measured from transfected chromaffin cells using a highly sensitive chemiluminescence assay kit from Nichols Institute (San Juan Capistrano, CA, USA) as described previously (Wick et al. 1993). Secretion data were averaged between experiments or cell preparations by normalization of test responses to responses of control hGH-transfected cells within each experiment. In each case, cells were cotransfected with the hGH expression plasmid and an expression plasmid carrying the construct of interest and/or an empty vector parent plasmid (Neo). In experiments with the F-actin stabilizing drug jasplakinolide (100 nM), the drug was added to cell cultures 8 h prior to initiation of the hGH assay. Use of co-expressed hGH as a marker of the regulated secretory pathway allowed examination of recombinant Rac1 effects on secretion from only those cells expressing the recombinant GTPase protein. An assumption implicit with the approach, and well substantiated by prior studies, is that properties of hGH secretion closely mimic those of endogenous catecholamines (Wick et al. 1993; Holz et al. 1994).
Secretion from digitonin-permeabilized chromaffin cells was performed as previously described (Wick et al. 1993; Holz et al. 1994). Cells were permeabilized by incubation in KGEP solution (mM: 139 potassium glutamate, 20 Pipes (pH 6.6), 1 MgCl2, 5 EGTA, 2 Mg-ATP, 0.2 Li-GTP, with 2.5 mg ml−1 BSA, 20 μM digitonin) for 2 min at 30 oC. Cells were then stimulated for the time specified with Ca/KGEP solution that omitted digitonin and added 4.685 mM CaCl2 to set a final free calcium concentration (in combination with EGTA) to 30 μM. hGH secreted into the medium and total cell hGH content from transfected cells were determined from aliquots following centrifugation at 500 g, for 10 min at 4 oC. In experiments assessing effects of F-actin drugs (phalloidin and latrunculin-A) on permeabilized cells the drug was added to the KGEP during both the permeabilization and secretion phase of the protocol.
Electrophysiological recording of calcium current (ICa) and membrane capacitance (Cm)
Conventional whole cell patch-clamp recording methods were used to evoke and record ICa and measure time-resolved changes in Cm (ΔCm) from single adrenal chromaffin cells using an Axopatch 200A amplifier (Axon Instruments, Union City, CA, USA) and phase-tracking software (Pulse Control; Drs Jack Herrington and Richard Bookman, University of Miami Medical School, Miami, FL, USA). The ΔCm was measured by applying a sine wave (60 mV peak to peak at 1201 Hz) to a holding potential of −90 mV. Sixteen samples per sinusoidal period were used to compute one Cm point. Calibration pulses (100 fF) were generated at the beginning of each trace. Currents were filtered at 5 kHz and sampled at 20 μs. Series resistance and resting membrane capacitance were compensated electronically. Baseline leak current was subtracted prior to integration and recording was terminated when leak current exceeded 10 % of the depolarization-evoked calcium current. No corrections were made for linear leak during step depolarization. A train of eight step depolarizations from −90 to +10 mV at 0.2 s intervals was used to evoke ICa and ΔCm. The duration of the step depolarizations used for each train was generally 50 ms, although in two control cells longer pulse durations were applied (75 and 150 ms) to generate approximately 10 pC calcium influx per step depolarization. No significant differences were found between the treatments with respect to current density (A F−1) using 50 ms step depolarizations to +10 mV. Pipettes were constructed out of 1.5 mm outer diameter capillary glass (A-M Systems), coated with Sylgard and fire polished to resistances of 1.5–4 MΩ. The pipette soluion contained (mM): 128 N-methyl-D-glucamine-Cl, 40 Hepes, 10 NaCl, 2 Mg-ATP, 0.2 GTP, 0.25 Tris-EGTA, with pH adjusted to 7.1. The extracellular solution contained (mM): 137 tetraethylammonium chloride, 10 CaCl2, 2 MgCl2, 10 Hepes and 19 glucose, with the pH adjusted to 7.2 with Tris. Chromaffin cells were transfected 2–3 days prior to replating and electrophysiological recording.
Immunocytochemistry and phalloidin staining
Chromaffin cells cultured on collagen-coated coverglasses were fixed with 4 % paraformaldehyde-PBS for 30 min, quenched with 50 mM NH4Cl-PBS for 15 min, permeablized with 0.2 % Triton X-100-PBS for 8–10 min, and blocked with 2 % BSA-PBS for 60 min. Following incubation in primary antibody at 1:200 dilution in 2 % BSA-PBS for 1 h, the cells were incubated with fluorescein-labelled secondary antibody at 1:200 dilution for 1 h. For co-staining of F-actin, 0.25 U ml−1 Alexa-conjugated phalloidin was included in the secondary antibody solution. Digital fluorescence images from cell cultures were viewed either on a conventional Nikon epifluorescence microscope or with a Zeiss LSM 510 confocal microscope, and processed using Photoshop 6.0 software (Adobe Systems Inc., Mountain View, CA, USA). Two controls were used to ensure specificity of immunochemical reactivity. These include use of primary antibody against relevant recombinant epitope tags in non-transfected cells and use of secondary antibody alone on transfected and non-transfected cells. Immunoreactivity and fluorescent signals were quantified using NIH Image software.
Statistics
Data were generally calculated as a fraction of the control response observed within each experiment. All data are plotted as the mean ± s.e.m. All experiments were repeated on at least two, and usually several different cell preparations. The n number refers to the number of observations for each treatment/condition. For normalization within experiments, all values were normalized against the average value of the control group. Statistical significance of treatments relative to control was determined using Student's unpaired t tests or for multiple comparisons using ANOVA with Bartletts post hoc test on normally distributed data. In the case of non-parametric data, a Mann–Whitney test was performed using the original calculated values. Statistical analysis was performed using GraphPad Instat software (GraphPad Software Inc, San Diego, CA, USA).
RESULTS
Effect of secretagogue stimulation of chromaffin cells on activation of Rac1 and Cdc42
Figure 1A illustrates the presence of Rac1 and Cdc42 in chromaffin cells from adrenal medulla. Chromaffin cells were identified by co-localization of the catecholamine biosynthetic enzyme dopamine-β-hydroxylase. Rac1 and Cdc42 were predominantly localized to cytosol as well as the plasma membrane. To investigate the level of Rac1 and Cdc42 activation in chromaffin cells we used an assay based on the protein binding domain (PBD) of human p21-activated kinase 1 (PAK1) fused to GST. This protein demonstrates selective affinity for the GTP-bound form (i.e. active) of Rac1 and Cdc42, and does not bind Rho (Benard et al. 1999). Figure 1B shows that a rapid and transient activation of Rac1 occurred upon nicotinic ACh (nACh) receptor activation with DMPP (20 μM), or membrane depolarization with elevated K+ (50 mM). Control cells showed no change in Rac1 activation. Secretagogue-induced Rac1 activation peaked at approximately 3 min and was followed by a return toward baseline, where it remained elevated throughout a stimulus period lasting up to 30 min. DMPP stimulation of the cells also resulted in an increase in Rac1 immunoreactivity found in a particulate fraction and decrease in the cytosolic fraction relative to that of control cells (Fig. 1C). The shift in distribution is consistent with activation being coincident with translocation of activated Rac1 to membrane-delimited compartments. Moreover, Rac1 activation was found to be Ca2+ sensitive. Stimulation with elevated K+ (50 mM, 2 min) saline in the presence of Cd2+ (100 μM), a voltage-dependent Ca2+ channel blocker, limited Rac1 activation to 28 ± 5 % (n = 6) of the level observed without Cd2+ present. In comparison to Rac1, the levels of GTP-bound Cdc42 were found not to significantly increase in response to DMPP stimulation (Fig. 1D).
Figure 1. Effect of nicotinic acetylcholine receptor (nAChR) activation and membrane depolarization on the level of GTP-bound Rac1 and Cdc42 in chromaffin cells.

A, confocal images showing localization of endogenous α-Rac1 and α-Cdc42 immunoreactivity in chromaffin cells cultured for 3 days. Chromaffin cells were identified by anti-dopamine-β-hydroxylase reactivity as visualized with Alexa488-conjugated secondary antibody. Scale bar = 10 μm. B, time course of Rac1 activation in response to DMPP (20 μM)-mediated nAChR activation (•, n = 5) or elevated K+-induced membrane depolarization (▪, n = 9). Control cells (○, n = 8) from same cell preparations were handled identically, but without stimulation. C, distribution of Rac1 immunoreactivity among cytosol and membrane fractions (particulate) under control conditions and following stimulation with DMPP (20 μM, 2 min, n = 5). D, time course of Cdc42 activation in response to stimulation with DMPP (20 μM; •, n = 7) as compared to control (○, n = 7) cells. Asterisks represent significant differences (P < 0.05) from control.
Transfection and expression of recombinant Rac1
To investigate the role of Rac1 in regulation of chromaffin cell secretory responsiveness, we utilized transfection and overexpression of dominant active (V12 and L61) and dominant negative (N17) constructs of epitope-tagged Rac1. Immunocytochemistry against the epitope tag demonstrated successful transfection and expression of the recombinant GTPase constructs (Fig. 2A). Co-transfection of ANP–EmGFP, a fluorescent protein directed to dense core secretory granules, demonstrated that the recombinant Rac1 expression was localized to chromaffin cells. Overexpression of specific Rho family GTPases has the potential for binding upstream regulators (e.g. GEFs) as well as downstream effectors of corresponding members of this family. Therefore, care was taken to limit levels of overexpression by initially titrating the amount of plasmid DNA used for transfection in chromaffin cells. As representative, Fig. 2B shows that the concentration of plasmid DNA for RacV12 used in these investigations resulted in modest levels of overexpression (i.e. approximately 2.5-fold) of the recombinant protein over endogenous Rac1.
Figure 2. Transfection and expression of Rac1 mutants in chromaffin cells.

A, chromaffin cells were co-transfected with plasmids carrying HA epitope-tagged Rac1 mutants, and a reporter fusion gene (ANP–EmGFP) that is directed to secretory granules of the regulated exocytotic pathway. Co-expression was evaluated by fluorescence detection of the green fluorescent protein and immunofluorescent detection of the HA tag using an α-HA antibody. B, quantification of Rac1 immunofluorescence in non-transfected (control, n = 27) and Rac1-V12 (n = 10)-transfected chromaffin cells. Scale bars = 10 μm.
Expressed recombinant Rac1 protein alters secretory responsiveness in permeabilized chromaffin cells
The effect of Rac1 on secretory responsiveness in chromaffin cells was determined by expression of dominant active (Rac1-V12, Rac1-L61) or dominant negative (Rac1-N17) GTPases together with a plasmid encoding human growth hormone (hGH), which acts as a reporter for the regulated secretory pathway (Wick et al. 1993). A sensitive immunological assay for the detection of secreted hGH allowed the properties of the secretory response to be evaluated from the co-transfected cells alone, despite low transfection efficiency. The effects of mutant Rac1 protein on secretion were initially examined in digitonin-permeabilized chromaffin cells. In this way, effects of the overexpressed GTPases on secretion evoked by directly increasing the free Ca2+ to 30 μM reflect an effect of the mutant protein on the intracellular pathway governing exocytosis and avert effects potentially attributable to alteration in calcium signalling pathways. Figure 3A compares a representative time course of hGH secretion from cells expressing hGH alone or hGH in combination with dominant active Rac1-V12. Expression of Rac1-V12 enhanced the Ca2+-induced hGH secretory response, but had no effect on Ca2+-independent (i.e. basal) hGH secretion when compared to control cells cotransfected with a parent pcDNA (Neo.) plasmid. Figure 3B shows the averaged effects on evoked hGH secretion measured at 15 min from cells expressing the recombinant Rac1 proteins. Strong enhancement of Ca2+-dependent secretion was found for cells expressing Rac1-V12 and Rac1-L61 constructs. Rac1-N17, a dominant negative Rac1 mutant, was found not to alter Ca2+-dependent secretion relative to control. The enhancement of Ca2+-dependent secretion by constitutively active Rac1 constructs indicates that Rac1 can act as a positive regulator of an intracellular pathway leading to regulated exocytosis.
Figure 3. Effect of co-expression of mutant Rac1 mutants with human growth hormone (hGH) on hGH secretion in permeabilized chromaffin cells.
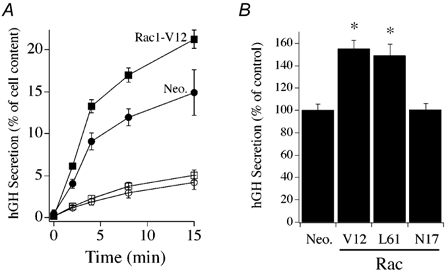
A, time course of hGH secretion from digitonin-permeabilized chromaffin cells transfected with Rac1-V12 (squares) or an empty parent plasmid (Neo., circles). Secretion was elicited by addition of 30 μM free Ca2+ (filled symbols) and compared to secretion in the absence of Ca2+ (open symbols; n = 3). Co-expression of hGH serves as a reporter of the regulated exocytotic pathway of transfected cells. B, effect of expression of Rac1 mutant proteins on Ca2+-evoked hGH secretion at 15 min (n = 9, Rac1-V12 and Rac1-L61; n = 6, Rac1-N17). Data were normalized to control (Neo.) for comparison between different cell preparations. Asterisks represent statistically significant difference (P < 0.05) from control group.
RhoGDI inhibits secretory responsiveness
The activity of the Rho family of GTPases is controlled in part by RhoGDIs, which inhibit release of GDP from the GTPase and also control partitioning of the GTPase between cytosol and membrane compartments. Figure 4A shows that RhoGDI and Rac1 interact in chromaffin cells, as immunoprecipitation of endogenous Rac1 from lysate of cultured chromaffin cells resulted in co-immunoprecipitation of RhoGDI. In addition, overexpression of recombinant RhoGDI was capable of inhibiting Ca2+-dependent secretory responses from digitonin-permeabilized chromaffin cells (Fig. 4B). The overexpression of RhoGDI resulted in an approximately 50 % inhibition of hGH secretion monitored at 2 min and 20 % inhibition at 15 min. Figure 4C demonstrates a time-dependent dialysis of RhoGDI from the permeabilized cells into the media and correlates with the time-dependent loss of inhibition by RhoGDI. The inhibitory effects of RhoGDI on hGH secretion provide additional evidence that Rac1 plays a regulatory role in the secretory pathway.
Figure 4. Effect of RhoGDI expression on Ca2+-dependent secretion from digitonin-permeabilized chromaffin cells.
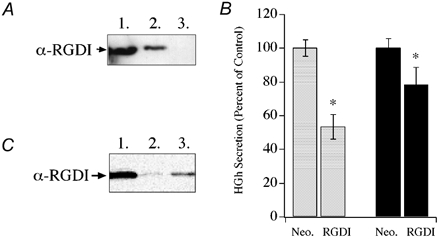
A, co-immunoprecipitation of RhoGDI (RGDI) with Rac1 from chromaffin cell lysate. Cell lysates were immunoprecipitated with Rac1 antibody and aliquots of samples subjected to SDS-PAGE and Western blotting. Blots were probed with a RhoGDI. Lane 1, lysate; 2, immunoprecipitate; 3, supernatant of final wash. B, effect of expression of RhoGDI on Ca2+-evoked (30 μM) hGH secretion, measured at 2 min (open bars) and 15 min (filled bars) from permeabilized cells (n = 6). Asterisks represent statistically significant difference (P < 0.05) from control (Neo.). C, dialysis of RhoGDI from permeabilized chromaffin cells. Immunoblot lanes, 1, cell lysate; 2 and 3, aliquot of medium collected from permeabilized chromaffin cells following 2 min and 15 min of Ca2+ stimulation (30 μM), respectively.
Rac1 modulates secretory responses from intact chromaffin cells
The effect of expression of recombinant Rac1 on secretion from intact cells was examined as a further test of physiological relevance. Figure 5A demonstrates that expression of dominant active Rac1-V12 or Rac1-L61 significantly enhanced DMPP-induced (20 μM, 2 min) hGH secretion compared to the empty plasmid control. The averaged increase in DMPP-induced secretion mediated by the constitutively active Rac1 constructs (approximately 20–40 %) was somewhat less than that observed from permeabilized chromaffin cells (approx. 55 %). The Rac1-V12 effect was reduced by lengthening the period of DMPP stimulation to 15 min (average of 13.7 % increase, n = 6). Expression of Rac1-N17 produced no significant change from control in the DMPP-induced hGH secretory response. The enhancement of secretion by Rac1-V12 in intact cells is consistent with a rapid activation of Rac1 observed in response to DMPP stimulation in intact cells and of active Rac1 facilitating Ca2+-dependent secretion from permeabilized cells. In addition, expression of RhoGDI in the intact cells inhibited DMPP-induced secretion by approximately 50 % (Fig. 5B), an effect that paralleled the inhibition observed on Ca2+-stimulated secretion from permeabilized cells. Co-expression of Rac1-V12 with RhoGDI resulted in an amelioration of the inhibitory effect of RhoGDI on DMPP-induced secretion in intact chromaffin cells. These data show that Rac1-V12 remains capable of facilitating secretion in the presence of RhoGDI overexpression.
Figure 5. Rac1 mutants and RhoGDI alter secretion in intact chromaffin cells.
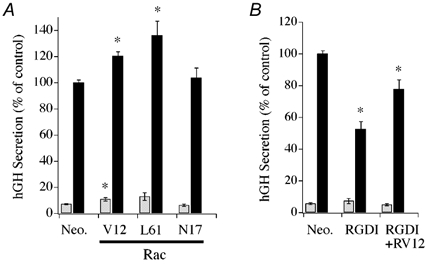
A, effect of constitutively active (V12 and L61) or dominant negative (N17) Rac1 mutants on basal (open bars) and evoked (filled bars) hGH secretion. Control (Neo.) cells were transfected with empty parent plasmid. Secretion was evoked by addition of the nACh receptor agonist DMPP (20 μM, 2 min). B, effect of recombinant RhoGDI expression alone and with Rac1-V12 on basal (open bars) and DMPP-induced (20 μM, 2 min; filled bars) hGH secretion. Asterisks represent statistically significant difference (P < 0.05) from control (Neo.). A, Neo, n = 15; Rac1-V12, n = 15; Rac1-L61, n = 3; Rac1-N17, n = 9; B, Neo, n = 21; RhoGDI, n = 17; RhoGDI+Rac1-V12, n = 18.
Our findings using digitonin-permeabilized cells demonstrated that constitutively active Rac1 exerts enhancing effects on secretion downstream of Ca2+. To determine whether Rac1 expression in intact chromaffin cells exerted effects on secretion by altering calcium influx we compared changes in cytosolic calcium induced by brief localized application of DMPP (20 μM, 10 s) to control and transfected cells. No significant difference was found in the averaged peak amplitude (control, 230 ± 47 nM, n = 7; Rac1-V12, 256 ± 26 nM, n = 10; Rac1-N17, 374 ± 76 nM, n = 9; mean ± s.e.m.) or shape of the elicited calcium responses monitored by the fluorescent calcium indicator fura-2 (Molecular Probes, Eugene, OR, USA). These data are in agreement with a previous report on chromaffin cells demonstrating no effect of Rac1 inactivation by Clostridium sordellii lethal toxin on nicotine-evoked Ca2+ responses (Gasman et al. 1999).
To evaluate the effect of Rac1 on the calcium influx-secretion relationship in single chromaffin cells, we recorded evoked changes in membrane capacitance elicited by depolarization mediated calcium influx. Figure 6A shows the stimulus protocol used and a representative evoked ICa and corresponding ΔCm recorded from a control chromaffin cell expressing the ANP–EmGFP protein. Repetitive depolarizing stimuli resulted in a repeating cycle of induced ICa (i.e. Ca2+ influx) and associated increases in Cm. Figure 6B presents averaged data that demonstrate the relationship between Ca2+ influx and ΔCm for control (ANP–EmGFP) cells with those expressing recombinant Rac1-V12, and RhoGDI protein. Rac1-V12 expression resulted in a consistent enhancement in secretory responsiveness with respect to control while RhoGDI expression decreased responsiveness. No differences were found in charge density between the different treatments. Differences in the Ca2+-secretory releationships were most apparent following the initial few step-depolarizations, as the level of Ca2+ influx rose. These data suggest that the primary effect of Rac1 signalling may not be on an immediate release component. This interpretation would be consistent with predominant enhancing effects of Rac1-V12 on secretion being observed in the slower kinetic assays of hGH secretion from cell populations.
Figure 6. Effect of Rac1-V12 and Rho-GDI on the relationship of Ca2+ influx to ΔCm.
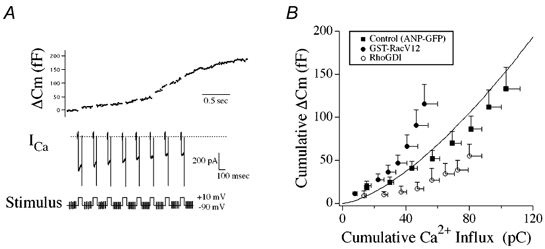
A, whole-cell patch clamp recording from a control ANP–EmGFP transfected cell illustrating evoked ICa and ΔCm responses to a train of repetitive step depolarizations (-90 to +10 mV, 50 ms duration). B, comparison of relationships between cumulative Ca2+ influx and cumulative ΔCm for control (ANP–EmGFP, filled squares, n = 9), Rac1-V12 (filled circles, n = 9) and recombinant RhoGDI (open circles, n = 4) chromaffin cells. Continuous line represents the standard Ca2+-exocytosis relationship previously described for cultured bovine chromaffin cells (Engisch et al. 1997).
Stimulus-induced changes in cortical cytoskeleton
As Rac1 is a central regulator of cytoskeletal organization the effects of constitutively active Rac1 on secretion may be associated with localized cytoskeletal reorganization triggered by the rise in intracellular [Ca2+]. Figure 7A compares representative examples of the cortical actin cytoskeleton of control and stimulated (20 μM DMPP, 2 min) chromaffin cells as visualized by Alexa568-phalloidin labelling. Chromaffin cells were identified by immunoreactivity to dopamine-β-hydroxylase, an enzyme essential for noradrenaline synthesis, or by localization of fluorescence to secretory granules in ANP–EmGFP transfected cells. In control cells an intense and nearly continuous band of cortical F-actin staining was readily apparent adjacent to the membrane. Nicotinic acetylcholine receptor stimulation resulted in only limited disruption of perimeter actin staining. The most prominent observed change to stimulation was an increase in cytosolic staining for F-actin. Figure 7B shows representative examples of the F-actin cytoskeleton in Rac1-V12-transfected cells prior to and following DMPP stimulation (20 μM, 2 min). Although Rac1-V12 is a constitutively active GTPase, the cortical actin cytoskeleton appeared of nearly identical morphology to that of the non-transfected controls, with only a slight increase in cytosolic F-actin labelling. Treatment of the Rac1-V12 transfected cells with DMPP also resulted in F-actin changes that reflected those of the stimulated, non-transfected controls, but with an augmentation of punctate F-actin labelling in the cytosol. Therefore, transfection with active Rac1-V12 alone did not produce dramatic alterations in the cortical cytoskeleton, but increased DMPP-stimulated F-actin reorganization with respect to control.
Figure 7. Effect of nACh receptor activation on cortical F-actin in bovine chromaffin cells.
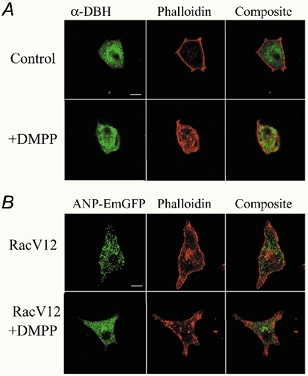
A, chromaffin cells were identified by dopamine-β-hydroxylase immunoreactivity and the cortical cytoskeleton visualized by Alexa568-phalloidin staining. A representative example of a control and DMPP-stimulated (20 μM, 2 min) cell is shown. B, visualization of the cortical actin cytoskeleton by Alexa568-phalloidin in Rac1-V12-transfected chromaffin cells under control conditions and following DMPP stimulation (20 μM, 2 min). Chromaffin cells were identified by cotransfection and expression of ANP–EmGFP directed to secretory granules. Scale bar in A and B = 10 μm.
Effects of pharmacological modulators of F-actin on regulated secretion
If Rac1 mediates its effects on secretion by alteration of cytoskeletal organization then drugs that alter cortical F-actin should modulate the actions of Rac1. Figure 8A and B shows that phalloidin (40 μM) or jasplakinolide (100 nM) treatment of chromaffin cells resulted in an enhancement of Ca2+-induced secretory or DMPP-induced (20 μM, 2 min) responses, respectively, with respect to control. Expression of Rac1-V12 strongly facilitated Ca2+-dependent secretion in these same cell preparations. However, combination of phalloidin or jasplkinolide with Rac1-V12 expression resulted in Ca2+-induced secretory responses that were not significantly different from either the F-actin modifying drug or Rac1-V12 alone. In complimentary investigations, treatment of permeabilized chromaffin cells with latrunculin-A, which avidly binds G-actin to promote loss of F-actin, resulted in a strong inhibition of Ca2+-stimulated hGH secretion. Although Rac1-V12 alone strongly enhanced secretion, latrunculin-A treatment completely eliminated the ability of Rac1-V12 expression to facilitate hGh secretion.
Figure 8. Effects of pharmacological modifiers of F-actin on secretion in transfected chromaffin cells.
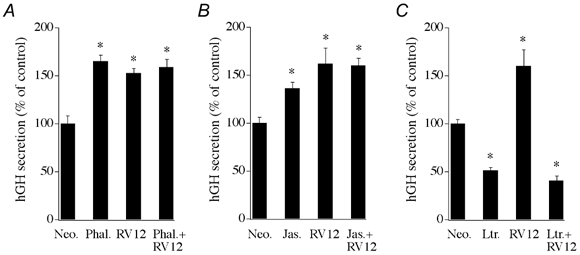
A, effect of phalloidin on Ca2+-evoked hGH secretion from digitonin-permeabilized chromaffin cells (n = 6). Phalloidin (40 μM) was present during the 2 min permeabilization period and the subsequent 15 min Ca2+ (30 μM) stimulation period. B, representative effect of the membrane-permeant F-actin stabilizing drug jasplakinolide (100 nM) on hGH secretion from intact chromaffin cells stimulated with DMPP (20 μM, 2 min, n = 3). Effect was replicated in 3 separate cell preparations. C, effect of latrunculin-A on Ca2+-evoked hGH secretion from digitonin-permeabilized chromaffin cells (n = 3). Latrunculin-A (10 μM) was present during both the permeabilization period and the 10 min Ca2+ stimulation period. Chromaffin cells were transfected with the constitutively active Rac1 construct (Rac1-V12) or an empty parent plasmid control (Neo.), along with the reporter gene hGH, 3 days prior to initiation of secretion assays.
Action of Rac1 effectors and Rac1 effector domain mutants on secretion
A primary effector of activated Rac1 is the serine/ threonine protein kinase Pak1. Therefore, we examined if expression of human Pak1 could mimic the secretory effect of active Rac1-V12 in response to DMPP stimulation. Figure 9A shows that overexpression of Pak1 significantly enhanced DMPP (20 μM) evoked secretion from intact chromaffin cells. A potential mechanism for Rac1-Pak1-mediated enhancement of secretion is via actions on cytoskeletal reorganization. ADF acts as an F-actin depolymerization factor that is negatively regulated by Rac1-Pak1 activation via Lim kinase. Overexpression of ADF significantly promoted DMPP-induced secretion relative to control (Fig. 9A). The effects of Pak1 and ADF on secretion are consistent with an effect of F-actin reorganization to modulate secretion. No effect was observed on basal secretion in response to overexpression of Pak1 or ADF.
Figure 9. Effects of Rac1 effectors and Rac1-V12 effector domain mutants on secretion in transfected chromaffin cells.
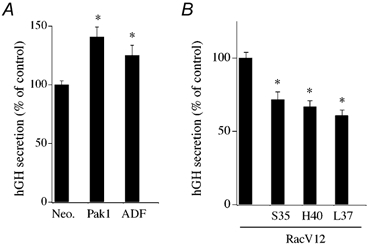
A, effect of Pak1 and ADF overexpression on DMPP-induced (20 μM, 2 min) hGH secretion from intact chromaffin cells (n = 6). B, effect of expression of Rac1-V12 and Rac1-V12 effector domain mutants on Ca2+-induced (30 μM; 15 min) hGH secretion from digitonin-permeabilized chromaffin cells (n = 6).
Amino acid mutations in the Rac1 effector domain decrease or eliminate the ability of Rac1 to activate specific downstream effectors (Self et al. 1993; Joneson et al. 1996; Lamarche et al. 1996; Tapon et al. 1998). Figure 8B shows the effects of known effector domain mutations in Rac1-V12 to alter Ca2+-evoked hGH secretion. Expression of the T35S and Y40H mutants, which decrease Rac1 signalling through Pak1, as well as JNK/SAPK, resulted in lowered secretion compared to control Rac1-V12. Use of a F37L mutant, which reduces signalling through POR1 and STAT3, also lowered Ca2+-evoked secretion. As Rac1 effects are limited to evoked versus basal secretion, these data are consistent with a rapid Ca2+-dependent effect being mediated through cytoskeletal regulators such as Pak1 or POR1.
DISCUSSION
A role of Rho family GTPases on regulated exocytosis in chromaffin cells had been previously proposed based on altered catecholamine secretion following differential inhibition of Rho family members by clostridial toxins (Gasman et al. 1999). In this study we provide further insight on the activation and physiological relevance of Rac1 in controlling regulated secretion in chromaffin cells. We demonstrate that: (1) Rac1, but not Cdc42, is rapidly activated in cells exposed to a nicotinic acetylcholine receptor agonist or to membrane depolarization induced by elevated K+; (2) expression of constitutively active constructs of Rac1 led to enhancement of hGH secretion in both digitonin-permeabilized and intact chromaffin cells while recombinant RhoGDI expression inhibited induced secretion; (3) Rac1 regulation of secretion occurs downstream of Ca2+ influx; (4) expression of a primary Rac1 effector, Pak1, also enhanced secretion suggesting that Rac1 may act via effects on actin cytoskeletal pathways; and (5) phalloidin and jasplakinolide enhanced evoked secretion, while latrunculin-A inhibited secretion and eliminated the enhancing effect of Rac1-V12. Taken together, the data suggest that Rac1 is activated by secretory stimuli and acts as a positive physiological regulator of Ca2+-dependent secretion involving regulation of the cytoskeleton.
Ca2+-dependent activation of Rac1 to secretory stimuli
To date, although effects on secretion of endogenous Rho-family GTPases have been reported from various cell types, no one has yet examined if the activity of theses GTPases is regulated by a secretory stimulus. We found that endogenous Rac1, but not Cdc42, was subject to rapid activation by secretory stimuli in our highly purified preparation of cultured chromaffin cells. Furthermore, activation of Rac1 in response to secretagogue stimulation was dependent upon Ca2+ influx. The specific GEF involved in Ca2+-dependent Rac1 activation in chromaffin cells remains unknown, although a large number of Rho family GEFs that catalyse exchange of bound GDP for GTP have been identified (Boguski & McCormick, 1993; Mackay & Hall, 1998). Support for Ca2+-dependent activation of Rac1 exists, however, as the GEF Tiam 1 has been reported to be activated in response to an ionophore-mediated rise in cytosolic calcium in NIH 3T3 fibroblasts, perhaps through a pathway involving calcium/calmodulin kinase II (Fleming et al. 1999; Buchanan et al. 2000). Our findings show that activation of endogenous Rac1 in response to sustained secretagogue stimulation of chromaffin cells was transient. Prolonged fMet-Leu-Phe (fMLP) or phorbol-12-myristate-13-acetate (PMA) stimulation of HL-60 cells has also been reported to elicit only transient activation of Rac2 (Benard et al. 1999).
Activated Rac1 promotes secretion
Our results demonstrate that expression of constitutively active constructs of Rac1 significantly enhanced stimulus-induced, Ca2+-dependent secretion. The facilitating effect on secretion was observed in both early and late phases of hGH secretion and occurred in both intact and permeabilized chromaffin cells. In comparison, Rac1-V12 had little effect on basal secretion. The ability of Rac1-V12 to enhance evoked secretion was also observed in Cm measurements of single chromaffin cells. Indeed, the electrophysiological results closely correlate to the effects of Rac1-V12 seen in permeabilized cells, where for a given calcium concentration a greater secretory response was obtained. Differences in the Ca2+ influx-ΔCm relationships between Rac1-V12 and control were most apparent following the initial step-depolarizations, suggesting that the primary effect of Rac1 signalling may be downstream of an immediate release vesicle pool. The enhancement of secretion by expression of constitutively active Rac1 mutants extends and promotes analysis of prior reported results which demonstrated a strong inhibition of catecholamine secretion following treatment with C. sordellii lethal toxin, which inactivates endogenous Rac, but may also target Ras, Ral and Rap1 (Gasman et al. 1999). Our results demonstrate consistently greater effects of Rac1-V12 on secretion in permeabilized over intact cells. The mechanism underlying this difference is not understood. A time-dependent loss of RhoGDI from permeabilized chromaffin cells may partially account for the enhanced responsiveness. RhoGDI proteins interact with GDP-bound Rho family GTPases to depress nucleotide dissociation and, in general, to direct their removal from membrane compartments. RhoGDI thus acts as negative regulator of Rho-family GTPase activity. Although loss of RhoGDI is unlikely to directly influence Rac1-V12, this loss may reduce inhibition on endogenous Rac1 and on other Rho family GTPases that may exert a permissive or modulatory role on the secretory pathway. We observed that expression of RhoGDI led to strong inhibition of evoked secretion in both permeabilized and intact chromaffin cells. Co-expression of Rac1-V12 with RhoGDI partially offset the inhibitory effect of RhoGDI on secretion. These data support an important role of Rac1 in secretory regulation, but as co-expression did not result in a full facilitatory effect they also indicate that other Rho family GTPases may modulate the secretory pathway.
In comparison to Rac1-V12, expression of Rac1-N17 had no effect on evoked secretion relative to control. One explanation is that the level of expression of Rac1-N17 was insufficient to fully inhibit Rac1 GEF activity. We believe that this is unlikely, as cells were transfected with plasmid encoding Rac1-N17 at 10 times the plasmid concentration used to obtain significant enhancement by Rac1-V12, and evoked secretory responses were still similar to control. In addition, it is possible that Rac1-N17 in chromaffin cells exists as a GDP-bound protein (rather than nucleotide free) and is tightly associated with RhoGDI. Since it is unlikely that the Rac1 GTPase can interact with a GEF while bound to RhoGDI, this may explain why it fails to negatively regulate endogenous Rac1. Consistent with our findings, Rac1-V12 has been reported to enhance calcium-induced secretion in permeabilized mast cells, and this secretory response was found to be relatively resistant to inhibition by Rac1-N17 (Price et al. 1995). However, in mast cells secretion is under regulatory control by multiple GTPases, including Rac2 and Cdc42, which may also explain a lack of effect of Rac1-N17 on secretion (Brown et al. 1998).
Cdc42 has previously been proposed in chromaffin cells as a key regulator coordinating Ca2+-dependent exocytosis with reorganization of the cytoskeleton (Gasman et al. 1999). This was based largely on the effects of clostridial toxin inhibition of members of the Rho-GTPase family. We did not observe an increase of GTP-bound Cdc42 in response to nicotinic acetylcholine receptor activation. Therefore, although Cdc42 activity may be necessary for secretion it may not dynamically regulate Ca2+-dependent secretion. The relative contribution of the different Rho family GTPases to regulate secretion may also be cell type specific as expression of constitutively active and inactive Rac and Cdc42 mutants in PC-12 cells was reported to inhibit and enhance secretion for each GTPase, respectively (Frantz et al. 2002).
Mechanisms of Rac1 action in the secretory pathway
Consistent with the Rho GTPase family as central mediators of cytoskeletal reorganization in cells, the facilitating actions of Rac1 on regulated secretion reported here may operate in part via cytoskeletal regulation. The sub-plasma-membrane cytoskeleton has often been proposed to exert a regulatory role in the recruitment, docking and fusion of secretory granules in chromaffin cells (Cheek & Burgoyne, 1986; Nakata & Hirokawa, 1992; Trifaro & Vitale, 1993; Vitale et al. 1995; Plattner et al. 1997; Tchakarov et al. 1998; Lang et al. 2000). For example, phorbol ester-mediated disruption of the cortical F-actin network increased the number of chromaffin granules within 50 nm of the plasma membrane and the rate of stimulated catecholamine release (Vitale et al. 1995). The disassembly of the actin cytoskeleton with Clostridium spiroforme toxin treatment also enhanced the rate of secretory activity and frequency of unitary exocytic events measured electrophysiologically in rat melanotrophs (Chowdhury et al. 1999, 2002). Furthermore, using the appearance of dopamine-β-hydroxylase on the surface membrane of chromaffin cells as a marker of exocytotic activity, DBH became exposed preferentially on plasma membrane areas devoid of rhodamine-phalloidin staining in response to stimulation (Nakata & Hirokawa, 1992). In light of these reports, Rac1 provides a possible molecular link between secretagogue activation and cytoskeletal reorganization. A primary Rac1 effector pathway to cytoskeletal remodelling in fibroblasts involves p21-activated kinases (PAKs), which are serine/threonine kinases. Interaction of GTP-Rac1 with Pak1 elicits a conformational change in Pak1 that results in Pak1 autophosphorylation and activation. Recently, Pak1 has also been shown to be an important downstream effector of Rac1 in neurons (Hayashi et al. 2002). Rac1 activation of Pak leads to phosphorylation of LIM kinase, which negatively regulates ADF/cofilin, thereby altering the level of F-actin assembly. In the present study, transfection of chromaffin cells with Pak1 and ADF expression constructs significantly enhanced secretion in response to secretagogue stimulation. Moreover, expression of effector domain mutants of Rac1-V12 that exhibit reduced activation of the cytoskeletal regulators Pak and POR1 resulted in a loss of Rac1-V12-mediated enhancement of evoked secretion. Pak and POR1 thus comprise two mediators which may act in chromaffin cells to link secretagogue-induced activation of Rac1 to cytoskeletal reorganization.
The prevalent hypothesis about the role of cortical actin cytoskeleton in adrenal chromaffin cells is that it forms a physical barrier for secretory granule movement to release sites and that stimulation of secretion induces cortical actin disruption. However, the extent to which cortical actin changes on stimulation differs considerably among published reports, from minimal alterations (e.g. Nakata & Hirokawa, 1992) to substantial reorganization (e.g. Cheek & Burgoyne, 1986; Vitale et al. 1991). It also remains unclear if cortical actin reorganization precedes or follows exocytotic activity, as exocytotic addition of vesicle membrane may be visualized as focal actin reorganization. Moreover, block of nicotine-evoked secretion with botulinum and tetanus toxins blocks cortical actin disassembly (Vitale et al. 1991). In the present study, resolution of cortical F-actin by phalloidin staining suggested that actin disassembly in response to brief secretagogue stimulation is of limited extent. This raises the possibility of effects being very transient and localized, and has made difficult a definitive assessment of effects of the cortical cytoskeleton on secretion. Pharmacological modulators of actin have also been used to provide evidence that the cytoskeleton may modulate evoked secretion in chromaffin cells. In general, agents that stabilize F-actin, such as phalloidin (Lelkes et al. 1986) and phosphatidylinositol 3-kinase inhibitors (Cheek, 1991; Chasserot-Golaz et al. 1998) have been reported to inhibit secretion, while agents that disrupt F-actin such as cytochalasin-D and DNase 1 (Lelkes et al. 1986; Sontag et al. 1988), latrunculin A (Gil et al. 2000) and gelsolin or scinderin (Trifaro et al. 1992a) enhance secretion. In the present study, phalloidin and jasplakinolide, which bind F-actin to prevent dissociation and to promote actin monomer association, enhanced secretion and limited further secretory enhancement by Rac1-V12. An opposite effect was observed with latrunculin-A, which binds G-actin to result in F-actin disassembly. The discrepancy between the present results and those of prior investigations may be reflective of differences in the protocols utilized. In the present study, latrunculin A effects were studied on permeabilized cells rather than on intact cells (Gil et al. 2000) and in the case of phalloidin treatment, the cell permeabilization time was limited to 2 min versus 20 min in the prior report (Lelkes et al. 1986). In addition, treatment of chromaffin cells with clostridial toxins that block activation of Rho family GTPases greatly inhibited exocytosis and at the same time disrupted the peripheral actin cytoskeleton (Gasman et al. 1999). These results, together with those of the present study suggest involvement of the cortical cytoskelton in regulation of secretory responsiveness is likely to be more complicated than a simple vesicle barrier model.
Recently, the quantification of granule movements in PC-12 cells has demonstrated that cortical actin limits as well as mediates secretory granule movement (Lang et al. 2000). Local rather than global reorganization of F-actin would allow sustained physiological responsiveness while simultaneously reducing the F-actin barrier to permit granule fusion with the membrane. Cortical actin may be required to designate release sites, to tether molecular mediators, or to serve as a scaffold to provide directional cues that facilitate supply of vesicles to available release sites and the readily releasable pool. Notably, a recent report on the calyx of Held synapse demonstrated that latrunculin A treatment retards replenishment of releasable vesicle pools, while phalloidin had no effect (Sakaba & Neher, 2003). These data are consistent with F-actin being important to maintenance of sustained secretion.
Rac1 may enhance secretion through pathways in addition to those involving the actin cytoskeleton. Indeed, Rac and Cdc42 have been reported to act at multiple sites to regulate antigen-stimulated degranulation of RBL-2H3 cells (Hong-Geller & Cerione, 2000). Additional targets of Rac1 include mixed lineage kinases (MLKs) and the mitogen activated protein kinases, JNK and p38 (Hall, 1998; Mackay & Hall, 1998; Kaibuchi et al. 1999), which may have specific roles in regulating gene transcription. Moreover, Rac and Cdc42 have been reported to directly interact with specific phospholipases (e.g. PLCβ2, PLCγ1 and PLD1) and mediate GTP-dependent phospholipase activation (Hammond et al. 1997; Illenberger et al. 1997, 1998; Hong-Geller & Cerione, 2000; Illenberger et al. 2000), which may further affect the vesicle and/or plasma membrane configuration at release sites (Humeau et al. 2001). In addition to potential effects of these phospholipases on PIP2 hydrolysis and second messenger generation, secretion from chromaffin cells requires the synthesis of polyphosphoinositides, with the generation of PIP2 considered as particularly important (Eberhard et al. 1990; Eberhard & Holz, 1991; Hay & Martin, 1993; Holz et al. 2000). The polyphosphoinositol phosphate synthetic reactions comprise, in part, the ATP-dependent priming steps of the secretory cycle and require activity of phosphatidylinositol 4-phosphate 5-kinase (PIP5K) (Hay et al. 1995). Of specific interest, Rac and Rho have been reported to interact and specifically activate the type I PIP5K (Tolias et al. 1995; Ren & Schwartz, 1998; Carpenter et al. 1999). Only the type I PIP5K supports PIP2 synthesis that is essential for ATP-dependent vesicle priming. Consistent with a role late in the exocytotic pathway, Rac activity has recently been proposed to be essential following docking of synaptic vesicles in Aplysia neurons (Humeau et al. 2002). Clearly, the elucidation of the downstream signalling pathway(s) by which Rac1 regulates secretion is an important challenge for future investigations.
Acknowledgments
We thank Dr Mary Bittner (University of Michigan) for many helpful suggestions and comments throughout this study. This work was supported by grants from the U.S. Public Health Service NIH NS39914 (to E.L.S.), NIH GM44428 (to G.B.) and NIH DK50127 (to R.W.H.).
REFERENCES
- Benard V, Bohl BP, Bokoch GM. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Reorganization of actin in depolarized synaptosomes. J Neurosci. 1985;5:2565–2569. doi: 10.1523/JNEUROSCI.05-10-02565.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- Brown AM, O'Sullivan AJ, Gomperts BD. Induction of exocytosis from permeabilized mast cells by the guanosine triphosphatases Rac and Cdc42. Mol Biol Cell. 1998;9:1053–1063. doi: 10.1091/mbc.9.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan FG, Elliot CM, Gibbs M, Exton JH. Translocation of the Rac1 guanine nucleotide exchange factor Tiam1 induced by platelet-derived growth factor and lysophosphatidic acid. J Biol Chem. 2000;275:9742–9748. doi: 10.1074/jbc.275.13.9742. [DOI] [PubMed] [Google Scholar]
- Carpenter CL, Tolias KF, Van Vugt A, Hartwig J. Lipid kinases are novel effectors of the GTPase Rac1. Adv Enzyme Regul. 1999;39:299–312. doi: 10.1016/s0065-2571(98)00009-0. [DOI] [PubMed] [Google Scholar]
- Chasserot-Golaz S, Hubert P, Thierse D, Dirrig S, Vlahos CJ, Aunis D, Bader MF. Possible involvement of phosphatidylinositol 3-kinase in regulated exocytosis: studies in chromaffin cells with inhibitor LY294002. J Neurochem. 1998;70:2347–2356. doi: 10.1046/j.1471-4159.1998.70062347.x. [DOI] [PubMed] [Google Scholar]
- Cheek TR. Calcium signalling and the triggering of secretion in adrenal chromaffin cells. Pharmacol Ther. 1991;52:173–189. doi: 10.1016/0163-7258(91)90007-9. [DOI] [PubMed] [Google Scholar]
- Cheek TR, Burgoyne RD. Nicotine-evoked disassembly of cortical actin filamanets in adrenal chromaffin cells. FEBS Lett. 1986;207:110–114. doi: 10.1016/0014-5793(86)80022-9. [DOI] [PubMed] [Google Scholar]
- Chowdhury HH, Kreft M, Zorec R. Distinct effect of actin cytoskeleton disassembly on exo- and endocytic events in a membrane patch of rat melanotrophs. J Physiol. 2002;545:879–886. doi: 10.1113/jphysiol.2002.028043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury HH, Popoff MR, Zorec R. Actin cytoskeleton depolymerization with Clostridium spiroforme toxin enhances the secretory activity of rat melanotrophs. J Physiol. 1999;521:389–395. doi: 10.1111/j.1469-7793.1999.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doussau F, Gasman S, Humeau Y, Vitiello F, Popoff M, Boquet P, Bader M-F, Poulain B. A Rho-related GTPase is involved in Ca2+-dependent neurotransmitter exocytosis. J Biol Chem. 2000;275:7764–7720. doi: 10.1074/jbc.275.11.7764. [DOI] [PubMed] [Google Scholar]
- Eberhard DA, Cooper CL, Low MG, Holz RW. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem J. 1990;268:15–25. doi: 10.1042/bj2680015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard DA, Holz RW. Regulation of the formation of inositol phosphates by calcium, guanine nucleotides and ATP in digitonin-permeabilized bovine adrenal chromaffin cells. Biochem J. 1991;279:447–453. doi: 10.1042/bj2790447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engisch KL, Chernevskaya NI, Nowycky MC. Short-term changes in the Ca2+-exocytosis relationship during repetitive pulse protocols in bovine adrenal chromaffin cells. J Neurosci. 1997;17:9010–9025. doi: 10.1523/JNEUROSCI.17-23-09010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming IN, Elliott CM, Buchanan FG, Downes CP, Exton JH. Ca2+/calmodulin-dependent protein kinase II regulates Tiam1 by reversible protein phosphorylation. J Biol Chem. 1999;274:12753–12758. doi: 10.1074/jbc.274.18.12753. [DOI] [PubMed] [Google Scholar]
- Frantz C, Coppola T, Regazzi R. Involvement of Rho GTPases and their effectors in the secretory process of PC12 cells. Exp Cell Res. 2002;273:119–126. doi: 10.1006/excr.2001.5432. [DOI] [PubMed] [Google Scholar]
- Gasman S, Chasserot-Golaz S, Popoff MR, Aunis D, Bader M-F. Involvement of Rho GTPases in calcium-regulated exocytosis from adrenal chromaffin cells. J Cell Sci. 1999;112:4763–4771. doi: 10.1242/jcs.112.24.4763. [DOI] [PubMed] [Google Scholar]
- Gil A, Rueda J, Viniegra S, Gutierrez LM. The F-actin cytoskeleton modulates slow secretory components rather than readily releasable vesicle pools in bovine chromaffin cells. Neuroscience. 2000;98:605–614. doi: 10.1016/s0306-4522(00)00132-9. [DOI] [PubMed] [Google Scholar]
- Guillemot JP, Montcourrier P, Vivier E, Davoust J, Chavrier P. Selective control of membrane ruffling and actin plaque assembly by the Rho GTPases Rac1 and Cdc42 in Fc e RI-activated rat basophilic leukemia (RBL-2H3) cells. J Cell Sci. 1997;110:2215–2225. doi: 10.1242/jcs.110.18.2215. [DOI] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hammond SM, Jenco JM, Nakashima S, Cadwallader K, Gu Q, Cook S, Nozawa Y, Prestwich GD, Frohman MA, Morris AJ. Characterization of two alternately spliced forms of phospholipase D1. Activation of the purified enzymes by phosphatidylinositol 4, 5-bisphosphate, ADP-ribosylation factor, and Rho family monomeric GTP-binding proteins and protein kinase C-alpha. J Biol Chem. 1997;272:3860–3868. doi: 10.1074/jbc.272.6.3860. [DOI] [PubMed] [Google Scholar]
- Hay JC, Fisette PL, Jenkins GH, Fukami K, Takenawa T, Anderson RA, Martin TF. ATP-dependent inositide phosphorylation required for Ca2+-activated secretion. Nature. 1995;374:173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Hay JC, Martin TF. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca2+-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ohshima T, Mikoshiba K. Pak1 is involved in dendrite initiation as a downstream effector of Rac1 in cortical neurons. Mol Cell Neurosci. 2002;20:579–594. doi: 10.1006/mcne.2002.1144. [DOI] [PubMed] [Google Scholar]
- Holz RW, Brondyk WH, Senter RA, Kuizon L, Macara IG. Evidence for the involvement of Rab3A in Ca2+-dependent exocytosis from adrenal chromaffin cells. J Biol Chem. 1994;269:10229–10234. [PubMed] [Google Scholar]
- Holz RW, Hlubek MD, Sorensen SD, Fisher SK, Balla T, Ozaki S, Prestwich GD, Stuenkel EL, Bittner MA. A pleckstrin homology domain specific for phosphatidylinositol 4, 5-bisphosphate (PtdIns-4, 5-P2) and fused to green fluorescent protein identifies plasma membrane PtdIns-4, 5-P2 as being important in exocytosis. J Biol Chem. 2000;275:17878–17885. doi: 10.1074/jbc.M000925200. [DOI] [PubMed] [Google Scholar]
- Hong-Geller E, Cerione RA. Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP3/calcium pathway in RBL-2H3 mast cells. J Cell Biol. 2000;148:481–493. doi: 10.1083/jcb.148.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Popoff MR, Kojima H, Doussau F, Poulain B. Rac GTPase plays an essential role in exocytosis by controlling the fusion competence of release sites. J Neurosci. 2002;22:7968–7981. doi: 10.1523/JNEUROSCI.22-18-07968.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeau Y, Vitale N, Chasserot-Golaz S, Dupont JL, Du G, Frohman MA, Bader MF, Poulain B. A role for phospholipase D1 in neurotransmitter release. Proc Natl Acad Sci U S A. 2001;98:15300–15305. doi: 10.1073/pnas.261358698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illenberger D, Schwald F, Gierschik P. Characterization and purification from bovine neutrophils of a soluble guanine-nucleotide-binding protein that mediates isozyme-specific stimulation of phospholipase C beta2. Eur J Biochem. 1997;246:71–77. doi: 10.1111/j.1432-1033.1997.t01-1-00071.x. [DOI] [PubMed] [Google Scholar]
- Illenberger D, Schwald F, Pimmer D, Binder W, Maier G, Dietrich A, Gierschik P. Stimulation of phospholipase C-beta2 by the Rho GTPases Cdc42Hs and Rac1. EMBO J. 1998;17:6241–6249. doi: 10.1093/emboj/17.21.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illenberger D, Stephan I, Gierschik P, Schwald F. Stimulation of phospholipase C-beta 2 by Rho GTPases. Methods Enzymol. 2000;325:167–177. doi: 10.1016/s0076-6879(00)25441-4. [DOI] [PubMed] [Google Scholar]
- Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- Jungermann J, Lerch MM, Weidenbach H, Lutz MP, Kruger B, Adler G. Disassembly of rat pancreatic acinar cell cytoskeleton during supramaximal secretagogue stimulation. Am J Physiol. 1995;268:G328–338. doi: 10.1152/ajpgi.1995.268.2.G328. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Kowluru A, Li G, Rabaglia ME, Segu VB, Hofmann F, Aktories K, Metz SA. Evidence for differential roles of the Rho subfamily of GTP-binding proteins in glucose- and calcium-induced insulin secretion from pancreatic β cells. Biochem Pharmacol. 1997;54:1097–1108. doi: 10.1016/s0006-2952(97)00314-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamarche N, Tapon N, Stowers L, Burbelo PD, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Lang T, Wacker I, Wunderlich I, Rohrbach A, Giese G, Soldati T, Almers W. Role of actin cortex in the subplasmalemmal transport of secretory granules in PC-12 cells. Biophys J. 2000;78:2863–2877. doi: 10.1016/S0006-3495(00)76828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelkes PI, Friedman JE, Rosenheck K, Oplatka A. Destabilization of actin filaments as a requirement for the secretion of catecholamines from permeabilized chromaffin cells. FEBS Lett. 1986;208:357–363. doi: 10.1016/0014-5793(86)81049-3. [DOI] [PubMed] [Google Scholar]
- Mackay DJ, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J Cell Biol. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Hirokawa N. Organization of cortical cytoskeleton of cultured chromaffin cells and involvement in secretion as revealed by quick-freeze, deep-etching, and double-label immunoelectron microscopy. J Neurosci. 1992;12:2186–2197. doi: 10.1523/JNEUROSCI.12-06-02186.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Norman JC, Price LS, Ridley AJ, Hall A, Koffer A. Actin filament organization in activated mast cells is regulated by heterotrimeric and small GTP-binding proteins. J Cell Biol. 1994;126:1005–1015. doi: 10.1083/jcb.126.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman JC, Price LS, Ridley AJ, Koffer A. The small GTP-binding proteins Rac and Rho, regulate cytoskeletal organizaation and exocytosis in mast cells by parallel pathways. Mol Biol Cell. 1996;7:1429–1442. doi: 10.1091/mbc.7.9.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan AJ, Brown AM, Freeman HN, Gomperts BD. Purification and identification of FOAD-II, a cytosolic protein that regulates secretion in streptolysin-O permeabilized mast cells, as a rac/rhoGDI complex. Mol Biol Cell. 1996;7:397–408. doi: 10.1091/mbc.7.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinxteren JA, O'Sullivan AJ, Larbi KY, Tatham PE, Gomperts BD. Thirty years of stimulus-secretion coupling: from Ca2+ to GTP in the regulation of exocytosis. Biochimie. 2000;82:385–393. doi: 10.1016/s0300-9084(00)00197-8. [DOI] [PubMed] [Google Scholar]
- Plattner H, Artalejo AR, Neher E. Ultrastructural organization of bovine chromaffin cell cortex-analysis by cryofixation and morphometry of aspects pertinent to exocytosis [published erratum appears in J Cell Biol (1998) 140, 973] J Cell Biol. 1997;139:1709–1717. doi: 10.1083/jcb.139.7.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prepens U, Just I, Von Eichel-Streiber C, Aktories K. Inhibition of Fc e RI-mediated activation of rat basophilic leukemia cells by Clostridium difficile toxin B (monoglucosyltransferase) J Biol Chem. 1996;271:7324–7329. doi: 10.1074/jbc.271.13.7324. [DOI] [PubMed] [Google Scholar]
- Price LS, Norman JC, Ridley AC, Koffer A. The small GTPases Rac and Rho as regulators of secretion in mast cells. Curr Biol. 1995;5:68–73. doi: 10.1016/s0960-9822(95)00018-2. [DOI] [PubMed] [Google Scholar]
- Ren XD, Schwartz MA. Regulation of inositol lipid kinases by Rho and Rac. Curr Opin Genet Dev. 1998;8:63–67. doi: 10.1016/s0959-437x(98)80063-4. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. Distinct patterns of actin organization regulated by the small GTP- binding proteins Rac and Rho. Cold Spring Harb Symp Quant Biol. 1992;57:661–671. doi: 10.1101/sqb.1992.057.01.072. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Involvement of actin polymerization in vesicle recruitment at the calyx of held synapse. J Neurosci. 2003;23:837–846. doi: 10.1523/JNEUROSCI.23-03-00837.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self AJ, Paterson HF, Hall A. Different structural organization of Ras and Rho effector domains. Oncogene. 1993;8:655–661. [PubMed] [Google Scholar]
- Shuang R, Zhang L, Fletcher A, Groblewski GE, Pevsner J, Stuenkel EL. Regulation of Munc-18/syntaxin 1A interaction by cyclin-dependent kinase 5 in nerve endings. J Biol Chem. 1998;273:4957–4966. doi: 10.1074/jbc.273.9.4957. [DOI] [PubMed] [Google Scholar]
- Sontag JM, Aunis D, Bader MF. Peripheral actin filaments control calcium-mediated catecholamine release from streptolysin-O-permeabilized chromaffin cells. Eur J Cell Biol. 1988;46:316–326. [PubMed] [Google Scholar]
- Tapon N, Nagata K, Lamarche N, Hall A. A new rac target POSH is an SH3-containing scaffold protein involved in the JNK and NF-kappaB signalling pathways. EMBO J. 1998;17:1395–1404. doi: 10.1093/emboj/17.5.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchakarov LE, Zhang L, Rose SD, Tang R, Trifaro JM. Light and electron microscopic study of changes in the organization of the cortical actin cytoskeleton during chromaffin cell secretion. J Histochem Cytochem. 1998;46:193–203. doi: 10.1177/002215549804600208. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Cantley LC, Carpenter CL. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- Trifaro JM, Rodriguez Del, Castillo A, Vitale ML. Dynamic changes in chromaffin cell cytoskeleton as prelude to exocytosis. Mol Neurobiol. 1992a;6:339–358. doi: 10.1007/BF02757940. [DOI] [PubMed] [Google Scholar]
- Trifaro JM, Vitale ML. Cytoskeleton dynamics during neurotransmitter release. Trends Neurosci. 1993;16:466–472. doi: 10.1016/0166-2236(93)90079-2. [DOI] [PubMed] [Google Scholar]
- Trifaro JM, Vitale ML, Rodriguez Del Castillo A. Cytoskeleton and molecular mechanisms in neurotransmitter release by neurosecretory cells. Eur J Pharmacol. 1992b;225:83–104. doi: 10.1016/0922-4106(92)90088-d. [DOI] [PubMed] [Google Scholar]
- Vitale ML, Rodriguez Del, Castillo A, Tchakarov L, Trifaro JM. Cortical filamentous actin disassembly and scinderin redistribution during chromaffin cell stimulation precede exocytosis, a phenomenon not exhibited by gelsolin. J Cell Biol. 1991;113:1057–1067. doi: 10.1083/jcb.113.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale ML, Seward EP, Trifaro JM. Chromaffin cell cortical actin network dynamics control the size of the release-ready vesicle pool and the initial rate of exocytosis. Neuron. 1995;14:353–363. doi: 10.1016/0896-6273(95)90291-0. [DOI] [PubMed] [Google Scholar]
- Wick PF, Senter RA, Parsels LA, Uhler MD, Holz RW. Transient transfection studies of secretion in bovine chromaffin cells and PC12 cells. Generation of kainate-sensitive chromaffin cells. J Biol Chem. 1993;268:10983–10989. [PubMed] [Google Scholar]


