Abstract
In most mammalian species, an increase in stimulation frequency (ISF) produces an increase in contractility (treppe phenomenon), which results from larger Ca2+ transients at higher frequencies, due to an increase in sarcoplasmic reticulum Ca2+ load and release. The present study attempts to elucidate the contribution of the Na+–Ca2+ exchanger (NCX) to this phenomenon. Isolated cat ventricular myocytes, loaded with [Ca2+]i- and [Na+]i-sensitive probes, were used to determine whether the contribution of the NCX to the positive inotropic effect of ISF is due to an increase in Ca2+ influx (reverse mode) and/or a decrease in Ca2+ efflux (forward mode) via the NCX, due to frequency-induced [Na+]i elevation, or whether it was due to the reduced time for the NCX to extrude Ca2+. The results showed that the positive intropic effect produced by ISF was temporally dissociated from the increase in [Na+]i and was not modified by KB-R7943 (1 or 5 μm), a specific blocker of the reverse mode of the NCX. Whereas the ISF from 10 to 30 beats min−1 (bpm) did not affect the forward mode of the NCX (assessed by the time to half-relaxation of the caffeine-induced Ca2+ transient), the ISF to 50 bpm produced a significant reduction of the activity of the forward mode of the NCX, which occurred in association with an increase in [Na+]i (from 4.33 ± 0.40 to 7.25 ± 0.50 mm). However, both changes became significant well after the maximal positive inotropic effect had been reached. In contrast, the positive inotropic effect produced by ISF from 10 to 50 bpm was associated with an increase in diastolic [Ca2+]i, which occurred in spite of a significant increase in the relaxation rate and at a time at which no increases in [Na+]i were detected. The contribution of the NCX to stimulus frequency inotropy would therefore depend on a decrease in NCX-mediated Ca2+ efflux due to the reduced diastolic interval between beats and not on [Na+]i-dependent mechanisms.
The Na+–Ca2+ exchanger (NCX) is a sarcolemmal Ca2+ transport system present in the plasma membrane of almost every cell type. The exchanger is an electrogenic counter ion transporter in which three Na+ are exchanged for each Ca2+. Thus, the function of this exchanger is controlled by the membrane potential as well as by the gradients of Na+ and Ca2+ across the cell membrane. In cardiac myocytes, the NCX usually operates in the forward mode, in which Na+ enters the cell and Ca2+ is extruded, playing a central role in the control of muscle relaxation and diastolic calcium (Bers et al. 1989; Bassani et al. 1994). By these actions, the NCX indirectly regulates the Ca2+ stored in the sarcoplasmic reticulum (SR). During the action potential, the NCX can also operate in the reverse mode (Blaustein & Lederer, 1999; Bers, 2001; Pieske et al. 2002), in which Ca2+ enters the cell and Na+ is extruded. This could increase the SR Ca2+ content and also modulate SR Ca2+ release, working in synergism with L-type Ca2+ channels (Litwin et al. 1998). Under conditions in which [Na+]i is increased, the activity of the forward mode of the NCX would be limited and that of the reverse mode would be favoured. Thus, an increase in [Na+]i may increase [Ca2+]i because either Ca2+ efflux via the forward mode of the NCX is reduced (failing to match Ca2+ influx), or Ca2+ influx via the reverse mode of the exchanger is increased.
A typical example of how the NCX can influence myocardial contractility is provided by cardiac glycosides. Experimental evidence indicates that the positive inotropy produced by these agents is due to Na+-K+ pump blockade, increases in [Na+]i and increases in [Ca2+]i. Recent experiments in rat myocytes have shown that the positive inotropic effect of strophanthidin is due to inhibition of Ca2+ efflux via the forward mode of the exchanger. The Ca2+ influx mode of the NCX seems to be only responsible for the toxic effects of these drugs and not for the inotropic effects (Satoh et al. 2000).
The NCX has been also associated with the mechanisms involved in the increase in contractility produced by increasing stimulation frequency (treppe phenomenon; Langer et al. 1971; Cohen et al. 1982; Wang et al. 1988; Bers, 2001). The positive staircase results from an increase in the intracellular Ca2+ transient (CaiT), mainly due to an increased SR Ca2+ content at higher stimulation frequencies. The increase in SR Ca2+ content would mainly result from an increase in Ca2+ influx per unit time and a reduced Ca2+ efflux between beats (reviewed in Bers, 2001). Moreover, additional mechanisms, like an increase in the activity of Ca2+-calmodulin-dependent protein kinase II (CaMKII) produced by higher average [Ca2+]i (Bassani et al. 1995; Li et al. 1997) or the frequency-dependent facilitation of L-type Ca2+-current described at higher frequencies (Zygmunt & Maylie, 1990) would further enhance SR Ca2+ uptake and release. In this scenario, one of the mechanisms by which the NCX could contribute to the positive staircase is through the reduced time available for the exchanger to extrude Ca2+, which would favour the increase in SR Ca2+ content. But in addition, and as in the case of cardiac glycosides, the increase in contraction frequency is also associated with an increase in [Na+]i produced by the larger number of depolarizations per unit time (Cohen et al. 1982; Wang et al. 1988; Despa et al. 2002). The increase in [Na+]i may either reduce the activity of the forward (Ca2+-efflux) mode of the NCX limiting Ca2+ extrusion, which would increase SR Ca2+ content, or favour the reverse (Ca2+-influx) mode, which would increase SR Ca2+ content and enhance SR Ca2+ release (Litwin et al. 1998; Bers 2001). Indeed, previous studies have suggested that an increase in [Na+]i is a significant mediator of the positive force- frequency staircase (Cohen et al. 1981; Boyett et al. 1987; Lee et al. 1987). Whether the NCX contributes to stimulus frequency inotropy by either increasing Ca2+ influx or decreasing Ca2+ efflux due to the frequency-induced elevation in [Na+]i, or whether the NCX-dependent increase in [Ca2+]i is simply due to the reduced time between beats to extrude Ca2+ via the forward mode of the exchanger, has never been explored. The present experiments were undertaken with the aim of answering these questions.
METHODS
Myocyte isolation
All experiments were performed in accordance with the guidelines for Animal Care of the Scientific Committee of the University of La Plata School of Medicine. Cats were anaesthetized by intra-abdominal injection of sodium pentobarbitone (35 mg (kg body weight)−1). Cat myocytes were isolated according to the technique previously described (Vila Petroff et al. 2000) with some modifications. Briefly, the hearts were attached via the aorta to a cannula, excised and mounted in a Langendorff apparatus. They were then retrogradly perfused at 37 °C at a constant perfusion pressure of 70–80 mmHg with Krebs-Henseleit solution (K-H) of the following composition (mm): 146.2 NaCl, 4.7 KCl, 1.35 CaCl2, 10.0 Hepes, 0.35 NaH2PO4, 1.05 MgSO4, 10.0 glucose (pH adjusted to 7.4 with NaOH). The solution was continuously bubbled with 100 % O2. After a stabilization period of 4 min, the perfusion was switched to a nominally Ca2+-free K-H for 6 min. Hearts were then recirculated with collagenase (118 u ml−1), 0.1 mg ml−1 pronase and 1 % bovine serum albumin (BSA), in K-H containing 50 μm CaCl2. Perfusion continued until hearts became flaccid (15–25 min). Hearts were then removed from the perfusion apparatus by cutting at the atrio-ventricular junction. The isolated myocytes were separated from the undigested tissue and rinsed several times with a K-H solution containing 1 % BSA and 500 μm CaCl2. After each wash, myocytes were left for sedimentation for 10 min. Myocytes were kept in K-H solution at room temperature (20–22 ° C) until use. Rod-shaped myocytes with clear and distinct striations and obvious marked shortening and relaxation on stimulation were used. Cells that were unstable at low frequencies, that did not show an obvious positive inotropic effect in response to an increase in stimulation frequency, or that showed early signs of deterioration, such as large decreases in resting cell length at low stimulation frequencies, were discarded. Experiments were performed at room temperature.
Indo-1 fluorescence and cell-shortening measurements
The isolated myocytes were loaded at room temperature with the cell-permeant acetomethyl ester form of indo-1 (17 μm for 9 min) according to the bulk method described by Spurgeon et al. (1990) and left for de-esterification for 45 min. Cells were then placed on the stage of an inverted microscope (Nikon Diaphot 200) adapted for epifluorescence. Myocytes were continuously superfused with K-H (pH 7.4) at a constant flow of 1 ml min−1 and field-stimulated via two platinum electrodes on either side of the bath (square waves, 2 ms duration and 20 % above threshold) at the frequencies indicated in the text and figures. The excitation light was centred at 350 nm through the ×40 objective and the fluorescence emitted by the cell passed a barrier filter of 400 nm, a 450 nm dichroic mirror, and was recorded at 410 and 490 nm. Fluorescence signals were sampled at rate of 103 Hz and averaged. Background fluorescence was subtracted from each signal before obtaining the 410 nm/490 nm fluorescence ratio. The diastolic fluorescence ratio was measured as the mean value over a 100 ms period after the twitch was completed. The systolic fluorescence ratio was determined directly from the peak of the recorded ratio. The ratio of the indo-1 emission at the two wavelengths was taken as an index of the [Ca2+]i. The indo-1 fluorescence was used to measure systolic, diastolic and amplitude of the CaiT. The rate of CaiT decline was assessed by the time to half-relaxation, t1/2, and by the time constant of CaiT decline, τ.
The stage of the microscope was illuminated with red light (640–750 nm) through its normal bright-field illumination optics to allow simultaneous measurement of fluorescence and shortening. Resting cell length and cell shortening were measured by a video-based motion detector (Crescent electronics, UT, USA) and stored by software (PowerLab/400 ADInstruments) for off-line analysis. Relaxation was assessed by time to half-relaxation, t1/2. Cell shortening was continuously monitored on-line and stored by software (PowerLab/400 ADInstruments).
SBFI fluorescence measurements
For [Na+]i measurements, the isolated myocytes were loaded with the cell-permeant acetomethyl ester form of sodium-binding benzofuran isophthalate (SBFI AM). Myocytes were incubated for 120 min at 37 °C under regular gentle shaking with 10 μm SBFI AM and 0.01 % (w/v) pluronic acid. Myocytes were washed and resuspended in 5 ml Hepes solution and kept for 15 min to ensure complete de-esterification of all residual intracellular SBFI AM. SBFI-loaded myocytes were used in the emission ratio mode, according to the technique previously described (Baartscheer et al. 1997). Briefly, fluorescence was excited (Omega optical XF1093 340AF15) at 340 nm through the × 40 objective. Emitted light passed a barrier filter of 400 nm, a 450 nm dichroic mirror and two narrow band interference filters of 410 and 590 nm. Fluorescence signals were sampled at a rate of 103 Hz and averaged. Background fluorescence was subtracted from each signal before obtaining the 410 nm/590 nm fluorescence ratio.
Fluorescence signals of SBFI-loaded myocytes were calibrated at the end of each experimental day by superfusion with 2.0 μm gramicidin, 5 μm monensin and 0.05 mm ouabain, according to Harootunian et al. (1989). Calibration media containing various Na+ concentrations were made from appropriate mixtures of 150 mm Na+ and 0 mm Na+ solutions. The relationship between the SBFI fluorescence ratio and Na+ activity was linear in the physiological range (4–16 mm). The values of [Na+]i were estimated for each cell from this in vivo calibration.
Papillary muscles
Papillary muscles were dissected from the right ventricle and mounted vertically in a chamber to contract isometrically. Mean cross-sectional area of the muscles were calculated from the muscles’ length and weight, assuming a uniform cross-section and a density of 1.0. The mural end of the muscle was firmly fixed to the bottom of the chamber by a small clamp and the tendinous end to a force transducer (Statham G1-4-250 or Hewlett-Packard FTA-10-1) via a stainless steel wire. Developed tension was monitored on-line and stored by software (PowerLab/400 ADInstruments). The muscles were paced to contract at the frequencies indicated in the text and figures, at constant temperature (30 °C), and maintained with the same solution used for isolated myocytes experiments. Contractility was assessed by the developed tension (DT) and relaxation was assessed by time to half-relaxation (t1/2).
After the papillary muscles were mounted, they were stretched until they reached the length at which maximal DT occurred and then allowed to stabilize for 1 h.
Materials
Collagenase type B was purchased from Worthington Biochemical Corp. (Lakewood, NJ, USA); pronase from Boerhinger Mannheim Corp. (GmbH, Mannheim, Germany); BSA essentially fatty acid free, thapsigargin, ryanodine, caffeine and ouabain from Sigma Chemical Co. (St Louis, MO, USA); indo-1 AM and SBFI AM from Molecular Probes Inc. (Eugene, OR, USA); and KB-R7943 2-[2-[4-(4-nitrobenzyloxy) phenyl]ethyl]isothiourea methanesulphonate from Tocris Cookson Inc. (Ballwin, MO, USA). All other chemicals were of the purest reagent grade available.
Statistics
All data are presented as means ± s.e.m. Comparisons within groups were assessed by Student's t test, either paired or unpaired as appropriate. A value of P < 0.05 was taken to indicate statistical significance.
RESULTS
Effect of increasing stimulation frequency on myocyte contraction and intracellular calcium transients
Using indo-1-loaded cardiac myocytes we examined the effects of increasing stimulation frequency from 10 beats min−1 (bpm) to 50 bpm, in steps of 10 bpm, on contraction and the associated CaiT. Figure 1A and B shows a representative example of this type of experiment. The increase in frequency produced a rate-dependent increase in the amplitude of contraction and CaiT (positive inotropic effect) that was associated with a parallel increase in the rate of CaiT decline and of twitch relaxation (relaxant effect). Increasing stimulation frequency also resulted in a modest but gradual increase in diastolic [Ca2+], as well as a reduction in diastolic cell length. Figure 1C shows the overall results of these experiments (n = 8), indicating the parallel increase in contraction and CaiT amplitude produced upon an increase in stimulation frequency. Additionally, the average results of increasing frequency on contraction, CaiT and their kinetics are provided in Table 1. The increase in contraction frequency produced a significant positive inotropic and relaxant effect from 20 to 50 bpm. The increase in diastolic [Ca2+] and the decrease in resting cell length, although present in most of the experiments from 30 bpm and beyond, only attained significant levels at 50 bpm. In a parallel group of experiments performed in the presence of 1 μm ryanodine (Ry) plus 1 μm thapsigargin (Tg) to functionally abolish SR function (Vila Petroff et al. 2000), the positive inotropic effect of increasing stimulation frequency was still observed. However, the increase in diastolic [Ca2+] and the decrease in resting cell length attained significant levels at lower frequencies of stimulation than in the absence of these drugs. In the absence of a functional SR, a significant increase in diastolic [Ca2+] (8.9 ± 2.3 %) and a significant decrease in resting cell length (6.2 ± 1.4 %) were already observed at 30 bpm.
Figure 1. Effect of increasing stimulation frequency on myocyte contraction and CaiT.
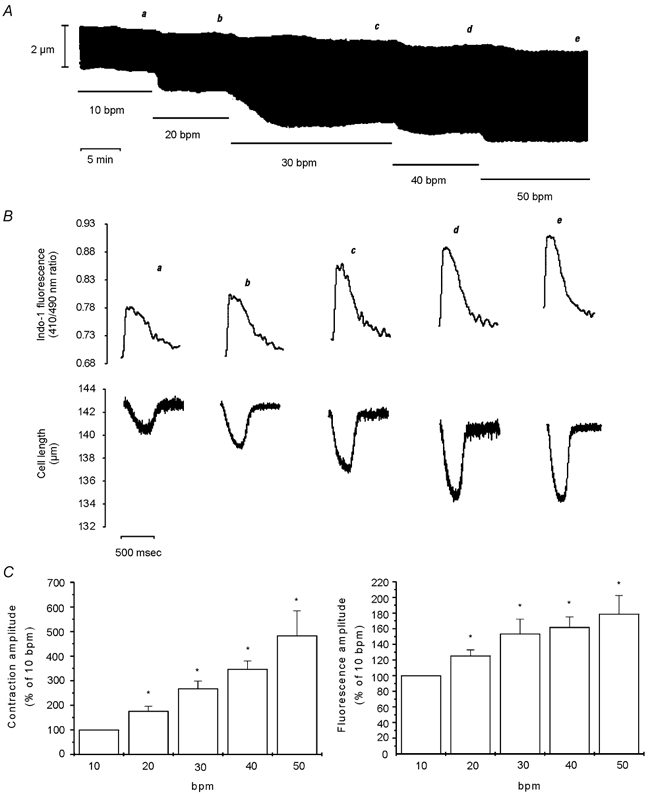
A, a typical continuous recording of cell length at the stimulation frequencies indicated in the figure (bpm, beats min−1). B, traces of the individual twitch contractions and the associated indo-1 fluorescence transients at the moment indicated by the letters a-e on A. The traces show, from a to e, a gradual increase in diastolic [Ca2+]i, rate of CaiT decline and rate of twitch relaxation, and reduction in diastolic cell length. C, bar graphs depicting the overall results of the effect of increasing stimulation frequency on myocyte contraction amplitude and indo-1 transient amplitude, expressed as a percentage of the value at 10 bpm. Data are means ± s.e.m. of 8 cells, * P < 0.05 with respect to 10 bpm. Increasing stimulation frequency from 10 to 50 bpm resulted in parallel increases in contraction and CaiT amplitude.
Table 1.
Effect of increasing stimulation frequency on contraction and Ca2+ transient parameters of single cat myocytes
| Contractile parameters | |||||
| Frequency (bpm) | 10 | 20 | 30 | 40 | 50 |
| L0 (μm) | 138.16 ± 7.78 | 137.95 ± 7.72 | 137.69 ± 7.77 | 137.27 ± 7.72 | 136.67 ± 7.70* |
| TA(%of L0) | 1.35 ± 0.23 | 2.21 ± 0.38* | 3.25 ± 0.50* | 4.14 ± 0.47* | 5.05 ± 0.59* |
| t1/2 contraction (ms) | 177.94 ± 20.39 | 155.19 ± 23.60* | 105.53 ± 13.43* | 82.95 ± 10.2* | 79.85 ± 9.45* |
| Indo-1 fluorescence ratio (410 nm/490 nm) | |||||
| Diastolic | 0.71 ± 0.04 | 0.71 ± 0.04 | 0.72 ± 0.04 | 0.76 ± 0.03 | 0.87 ± 0.05* |
| Systolic | 0.81 ± 0.04 | 0.83 ± 0.03* | 0.86 ± 0.04* | 0.91 ± 0.04* | 1.04 ± 0.09* |
| Amplitude | 0.09 ± 0.01 | 0.12 ± 0.02* | 0.14 ± 0.02* | 0.15 ± 0.03* | 0.17 ± 0.04* |
| τ | 495.45 ± 41.43 | 383.41 ± 25.71* | 271.58 ± 26.76* | 213.19 ± 14.81* | 198.80 ± 16.39* |
| t1/2 transient (ms) | 319.44 ± 40.32 | 275.68 ± 17.64* | 229.55 ± 14.54* | 196.26 ± 15.20* | 195.49 ± 12.32* |
| n | 8 | 8 | 8 | 8 | 8 |
bpm, beats per minute; L0, resting cell length; TA, twitch amplitude; t1/2 contraction, half-relaxation time of contraction; τ, time constant of Ca2+ transient decline; t1/2 transient, half-relaxation time of indo-1 fluorescence transient. Values are means ± s.e.m..
Significant vs. the corresponding value at 10 bpm value (P < 0.05).
Effect of increasing stimulation frequency on myocyte contraction and cytosolic [Na+]
In SBFI-loaded cells, we examined the time course of the changes in cytosolic [Na+] and contraction during an increase in stimulation frequency. Figure 2A shows that increasing stimulation frequency from 10 to 30 bpm resulted in a rapid increase in contraction amplitude that reached its maximum after approximately 2.5 min, whereas the [Na+]i increase developed slowly, reaching statistical significance only after 10 min of the change in frequency. Similarly, an increase in frequency from 10 to 50 bmp resulted in a faster increase in contraction amplitude than in [Na+]i (Fig. 2B). Figure 3A shows a representative example of the effect of increasing frequency in one step from 10 to 50 bpm on myocyte contraction. The increase in frequency induced an initial increase in contraction amplitude that reached its maximum after 1 min and was followed by a slow decay until a new steady state was reached. The initial increase in contraction was followed by a decrease in diastolic cell length, which then continued to decrease while contraction amplitude declined. The gaps in the continuous chart recording correspond to the intervals during which [Na+]i was measured. The values obtained are represented below the recording. Note that 1 min after the increase in stimulation frequency, the positive inotropic effect reached its maximum, whereas [Na+]i was not affected (vertical dotted line). Figure 3B shows the overall results of these experiments, indicating the time course of the effect of increasing frequency from 10 to 50 bpm on resting cell length and [Na+]i. The increase in frequency produced a significant decrease in resting cell length within 1 min, whereas the increase in [Na+]i became significant only after 5 min of the change in frequency. Furthermore, similar experiments in which [Ca2+]i instead of [Na+]i was measured during the increase in stimulation frequency from 10 to 50 bpm, showed that both diastolic and CaiT amplitude were significantly increased within 1 min of the change in stimulation frequency by 7 ± 0.9 and 46 ± 19 %, respectively (n = 4). The increase in diastolic [Ca2+] and the decrease in resting cell length occurred in association with the typical relaxant effect of the increase in stimulation frequency (decrease in the time to half-relaxation, t1/2, and in the time constant of CaiT decline, τ). The striking dissociation between the changes at the level of contraction (resting cell length and contraction amplitude as well as diastolic and CaiT amplitude) and the increase in [Na+]i produced by increasing stimulation frequency, may be taken as evidence that the NCX does not participate through a Na+-dependent mechanism in the positive inotropic effect of increasing stimulation frequency. However, subsarcolemmal [Na+]i gradients, not detectable by the method of SBFI fluorescence used herein, could significantly modulate the function of the NCX, either by slowing the forward mode or favouring the Ca2+ influx mode of the exchanger (Leblanc & Hume, 1990; Lipp & Niggli, 1994; Litwin et al. 1998; Blaustein & Lederer, 1999). In either case, there would be an increase in [Ca2+]i, which would contribute to the increase in myocardial contractility produced by increasing contraction frequency. In an attempt to clarify this possibility, three different strategies were followed: (1) we studied the effects of increasing stimulation frequency after specific inhibition of the reverse mode of the NCX; (2) we studied the rate of CaiT decline mediated by the forward mode of the exchanger on caffeine-induced contractures, after an increase in contraction frequency; (3) we compared the time course of [Na+]i and contractility changes produced by ouabain, a typical positive inotropic intervention mediated by the NCX (Satoh et al. 2000), with that of increasing stimulation frequency.
Figure 2. Effect of increasing stimulation frequency on contraction and [Na+]i.
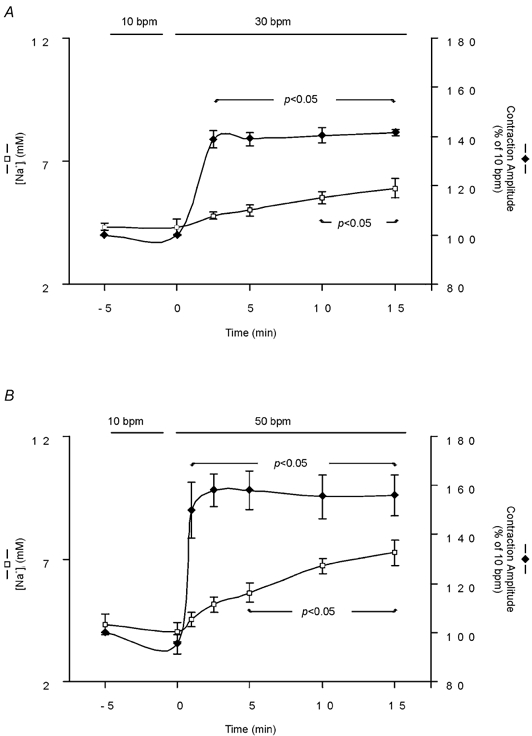
The graphs show the time course of simultaneous [Na+]i and contraction amplitude measurements, during an increase in stimulation frequency from 10 to 30 bpm (A, n = 6) and from 10 to 50 bpm (B, n = 5). Data are means ± s.e.m. Increasing stimulation frequency either from 10 to 30 bpm or from 10 to 50 bpm resulted in a temporal dissociation between the frequency-dependent positive inotropic effect and the increment in [Na+]i.
Figure 3. Effect of increasing stimulation frequency on contraction amplitude, resting cell length and [Na+]i.
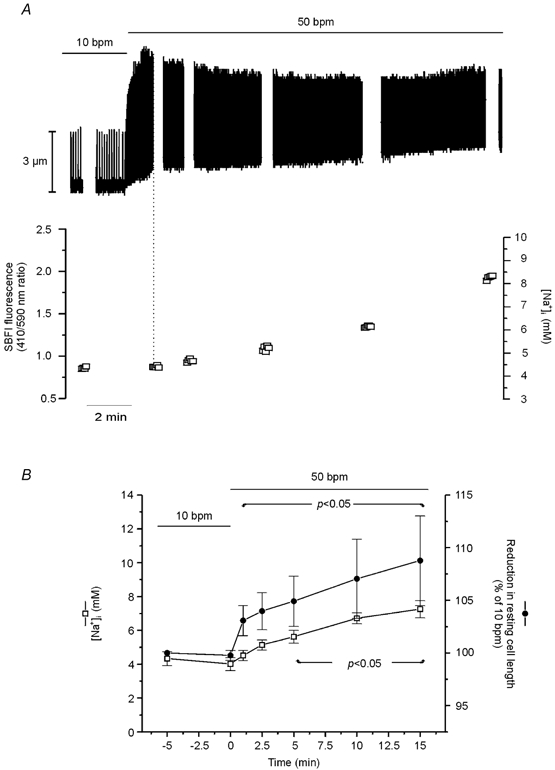
A, continuous recording of cell length when frequency was increased from 10 to 50 bpm. The gaps in the continuous chart recording correspond to the measurements of [Na+]i, plotted below. The increase in stimulation frequency induced a slow increase in [Na+]i that was temporally dissociated from both the positive inotropic effect and the increase in resting length evoked by the increase in contraction frequency. B, overall results indicating the temporal dissociation between the reduction in resting cell length and the increase in [Na+]i. Data are means ± s.e.m. of 5 cells. Two of the five cells studied had a rather high increase in resting cell length after the first 5 min of the increase in stimulation frequency and are responsible for the large error bars observed. Repeated mean [Na+]i values from Fig. 2 are placed here for visual comparison.
Lack of contribution of the reverse mode NCX to the frequency-induced positive inotropic effect
To examine the possible contribution of the reverse mode of the NCX, we performed experiments in the presence and absence of a specific blocker of the reverse mode NCX, KB-R7943 (KBR) (Iwamoto et al. 1996). Figure 4A shows a representative trace from a group of control experiments where the ability of 1 μm KBR to inhibit the reverse mode of the NCX was examined. This concentration of KBR did not affect basal contractility levels (Table 2). For these experiments, SR function was completely blocked by pretreatment of cells for 30 min with 1 μm Ry + 1 μm Tg. Caffeine-induced CaiTs in the presence and absence of Ry + Tg were used to assess the ability of these drugs to effectively inhibit SR function. The top traces in Fig. 4A show that application of 25 mm caffeine for 10 s induced a CaiT that was not reproducible when caffeine was applied after incubation with Ry + Tg. These results indicate the failure of the SR to accumulate and release Ca2+ in the presence of Ry + Tg. Similar results have previously been published (Vila Petroff et al. 2000). Figure 4A shows that, when extracellular Na+ was abruptly removed (replaced with choline chloride), which changes the thermodynamic driving force of the NCX to favour Ca2+ influx, the indo-1 fluorescence ratio rose, as expected for Ca2+ influx via the NCX. A similar method of challenging the cell with a Na+ gradient to force the NCX to work in the reverse mode was previously used by us (Vila Petroff et al. 2000), and others (Ladilov et al. 1999, Satoh et al. 2000). The reproducibility of the Ca2+ increase produced by two cycles of Na+ removal was established in additional experiments (results not shown). KBR at 0.15 and 1 μm partially and completely prevented, respectively, the increase in the fluorescence ratio produced by extracellular Na+ removal, which would indicate that these concentrations of KBR were able to inhibit the reverse mode of the NCX. Similar results were obtained in four other cells. Additionally, in a further attempt to assess whether the reverse mode NCX was fully inhibited by 1 μm KBR, we performed experiments where the exchanger was forced to work in the reverse mode in the presence of 10 mm Ni2+, a concentration capable of fully blocking the NCX (Kimura et al. 1987). The results depicted in Fig. 4A indicate that, similar to 1 μm KBR, in the presence of Ni2+ there was no detectable increase in indo-1 fluorescence. The bar graph on the right of Fig. 4A shows the overall results of these experiments. The results indicate that the inhibition produced by 1 μm KBR was not significantly different from that produced by 10 mm Ni2+.
Figure 4. Effect of KBR on the reverse and forward mode NCX.
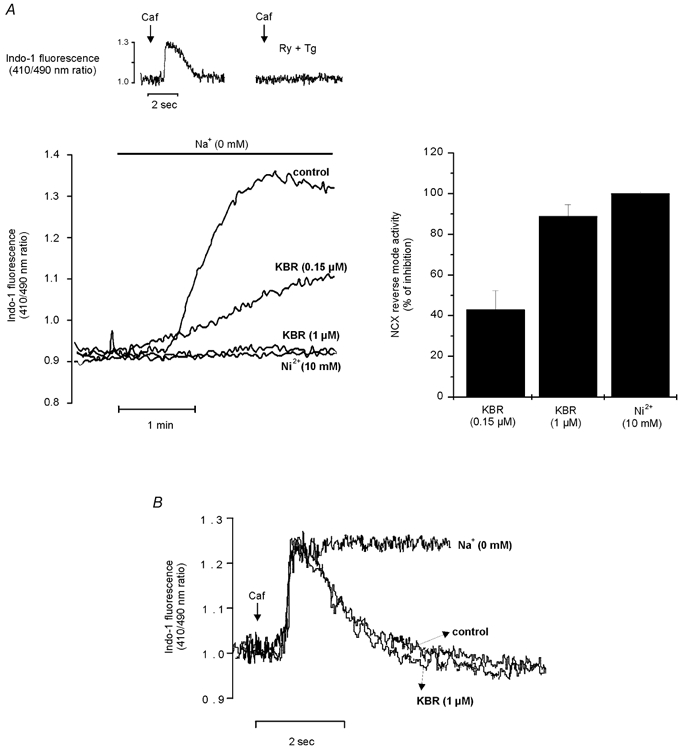
A, the top traces show the ability of 1 μm Ry + 1 μm Tg to completely prevent the caffeine (caf)-induced CaiT. The traces below depict the Ca2+ measurements in the absence and presence of KBR. In the absence of a functional SR, 1 μm KBR and 10 mm Ni2+ were able to completely prevent the increase in Ca2+ entry produced by the activation of the reverse mode of the NCX due to external Na+ removal (control). Application of 0.15 μm KBR, in contrast, only partially prevented this increase. The bar graph on the right depicts the overall results of these experiments expressed as percentage inhibition of NCX reverse mode activity. B, the caffeine-induced CaiT does not relax in zero [Na+] solution. The absence of the declining phase shows that (1) caffeine is capable of completely inhibiting SR Ca2+ uptake and (2) the declining phase of the CaiT of the caffeine contracture is mostly due to Ca2+ extrusion via the NCX. Application of 1 μm KBR did not alter the [Ca2+]i decline of the caffeine-induced CaiT, indicating the lack of effect of the drug on the forward mode of the NCX.
Table 2.
Effect KB-R7943 on contraction and Ca2+ transient parameters of single cat myocytes at 10 bpm
| Indo-1 ratio (410 nm/490 nm) | ||||||
|---|---|---|---|---|---|---|
| L0 (μm) | TA (%of L0) | t1/2 contraction (ms) | Amplitude | t1/2 transient (ms) | n | |
| Control | 137 ± 6 | 1.02 ± 0.20 | 211 ± 20 | 0.16 ± 0.05 | 340 ± 21 | 6 |
| KB-R7943 (1 μm) | 137 ± 6 | 1.14 ± 0.25 | 212 ± 24 | 0.15 ± 0.04 | 320 ± 13 | 6 |
L0, resting cell length; TA, twitch amplitude; t1/2 contraction, half-relaxation time of contraction; t1/2 transient, half-relaxation of indo-1 fluorescence transient. Values are means ± s.e.m.
To assess the effect of KBR on Ca2+ efflux via the NCX, the rate of Ca2+ decline from a caffeine-induced CaiT, produced by a fast (10 s) application of 25 mm caffeine, was used. During the caffeine-induced contracture, the declining phase of the CaiT in the cat is almost entirely due to Ca2+ extrusion from the cell via the NCX (Puglisi et al. 1996). Consistent with the findings of Puglisi et al. (1996), Fig. 4B shows that the caffeine-induced CaiT does not relax when caffeine is applied in a zero [Na+] solution, to fully inhibit NCX function. The absence of this declining phase indicates that, in the presence of caffeine, SR uptake is completely inhibited. Figure 4B also shows that KBR failed to affect the rate of Ca2+ decline of the caffeine-induced CaiT, indicating that, at the concentration used, the blocker does not interfere with forward mode activity of the exchanger.
Figure 5 depicts a representative example of the positive inotropic response to increments in contraction frequency in the absence and presence of 1 μm KBR. Figure 5A shows a continuous chart recording of cell length and Fig. 5B, on an expanded time scale, shows the CaiT acquired at the steady state of each stimulation frequency. The continued presence of 1 μm KBR did not affect the increase in contraction and CaiT amplitude associated with the increase in stimulation frequency. The overall results of these experiments, expressed as a percentage of the value at 10 bpm, are depicted in Fig. 6A. Although in the presence of KBR the increase in contractility produced by increasing stimulation frequency to 50 bpm tended to be lower than in the absence of the NCX inhibitor, this difference did not attain significant levels. This tendency results from the fact that in five out of eight experiments, the presence of KBR produced a modest decrease in the enhancement of CaiT amplitude and shortening evoked by increasing stimulation frequency to 50 bpm.
Figure 5. Failure of KBR to prevent the frequency-induced positive inotropic effect.
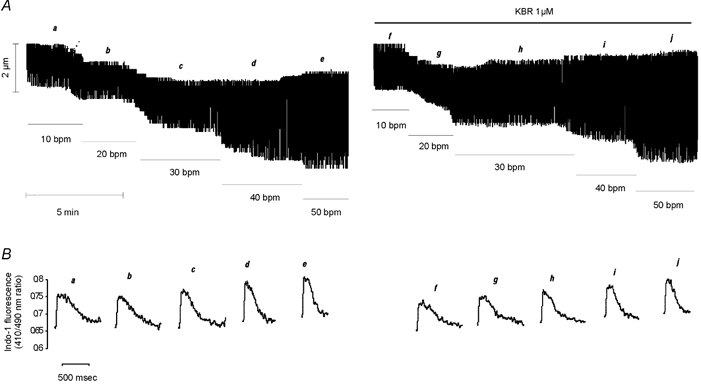
A, typical continuous recording of cell length showing a similar increase in cell shortening induced by increasing stimulation frequency either in the absence or in the continued presence of the specific blocker of the reverse mode of the NCX. B, traces of the individual CaiT at the steady state of each frequency indicated by letters a–j in A.
Figure 6. Lack of contribution of the reverse mode of the NCX to the stimulation frequency inotropy.
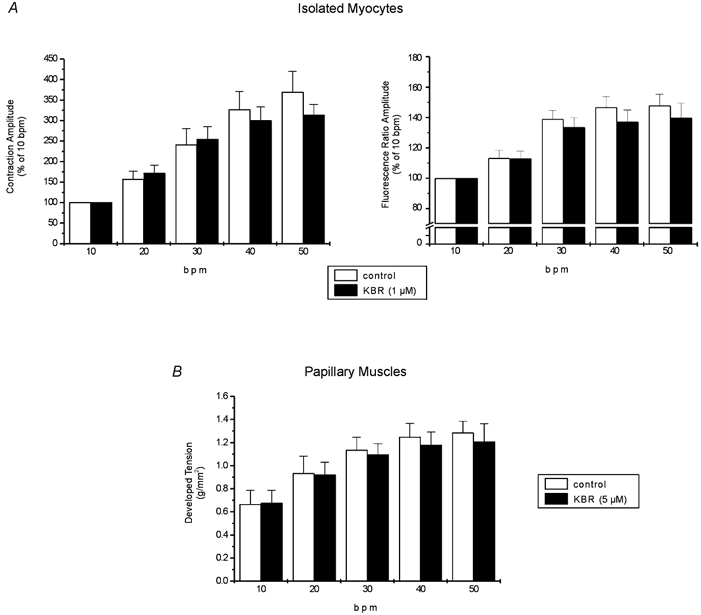
A, overall results of increasing stimulation frequency on contraction and indo-1 fluorescence amplitude in isolated myocytes under control conditions and after administration of 1 μm KBR. Data are means ± s.e.m. of 10 cells expressed as a percentage of the value at 10 bpm. The increase in contraction and CaiT amplitude had a similar magnitude in the absence and in the presence of drug. B, overall results of force-frequency relationship on papillary muscles with (n = 9) and without (n = 5) 5 μm KBR. Data are means ± s.e.m. The increase in developed tension was not statistically different between treated and non-treated muscles. These results exclude a contribution of the reverse mode of the NCX in the frequency-induced positive inotropic effect.
Because higher concentrations of KBR seriously depress basal cardiac myocyte contraction and CaiT, we performed a parallel group of experiments in papillary muscles contracting isometrically, incubated for 1 h with a fivefold higher concentration of the blocker. In this preparation, 5 μm KBR did not significantly reduce the basal developed tension (5 ± 3 %). Figure 6B shows the overall results of these experiments. These results are in agreement with those obtained in the isolated myocytes, indicating that KBR fails to significantly affect the typical force- frequency relationship. Taken together, the results failed to demonstrate a significant contribution of the reverse mode of the NCX to the increment in contractility induced by increasing stimulation frequency, in cat ventricle, in the range of frequencies explored.
Role of forward mode NCX in the frequency dependence of contraction
Figure 7A shows representative traces of the caffeine-induced CaiT recorded after the rapid application of caffeine (25 mm) at the steady state of 10 and 30 bpm (upper panel) and at the maximum positive inotropic effect (1 min) and the steady state of 50 bpm (lower panel). The amplitude of the caffeine-induced CaiT was larger when caffeine was applied after the train of stimuli at either 30 or 50 bpm than after that at 10 bpm. In contrast, the amplitudes of the caffeine-induced CaiTs recorded at stimulation frequencies of 30 and 50 bpm were of similar magnitude. These results are consistent with an increase in SR Ca2+ load at the higher frequencies (Frampton et al. 1991) and also indicate that at a stimulation frequency of 30 bpm the SR is already maximally loaded. The rate of CaiT decline of the caffeine-induced CaiT was not significantly affected when stimulation frequency was increased from 10 to 30 bpm, whereas it was significantly prolonged at 50 bpm at the time at which a significant increase in [Na+]i was observed (beyond 5 min of increasing stimulation frequency, trace d). When the caffeine pulse was applied only 1 min after the stimulation frequency was changed to 50 bpm (peak of the positive inotropic effect), the rate of CaiT decline was not affected, while the amplitude of the caffeine-induced CaiT was already increased (trace c). These findings on the kinetics of the caffeine-induced CaiT, can be better observed in the superimposed traces of the individual caffeine-induced CaiT at the frequencies studied, normalized to a similar peak of the CaiT. Because the rate of twitch CaiT decline depends on the peak [Ca2+]i (Bers & Berlin 1995), an increase in the rate of CaiT decline produced by this mechanism could mask a plausible decrease in NCX forward mode activity at the stimulation frequency of 30 bpm or after stimulating at 50 bpm for 1 min. To rule out this possibility, the rate of the caffeine-induced CaiT decline at a given [Ca2+]i was investigated according to the method described by Hulme & Orchard (1998). The exponential curve fitted to the declining phase of the caffeine-induced CaiT was differentiated to give the rate of change of [Ca2+]i, (d[Ca2+]i/dt), and plotted against the undifferentiated [Ca2+]i (Fig. 7B). This method allows analysis of the instantaneous rate of [Ca2+]i decline as a function of [Ca2+]i, independently of peak [Ca2+]i. The slopes were similar for the linear regressions obtained from the caffeine-induced CaiTs recorded at 10 or 30 bpm or after 1 min of the increase in frequency to 50 bmp. These results indicate that for any given change in [Ca2+]i, the rate of CaiT decline at 30 bpm and after 1 min of 50 bpm were not different from that obtained at 10 bpm. In contrast, when the frequency of stimulation was maintained at 50 bpm, the rate of CaiT decline was decreased for any given change in [Ca2+]i.
Figure 7. Contribution of the forward mode of the NCX to the contraction frequency inotropy.
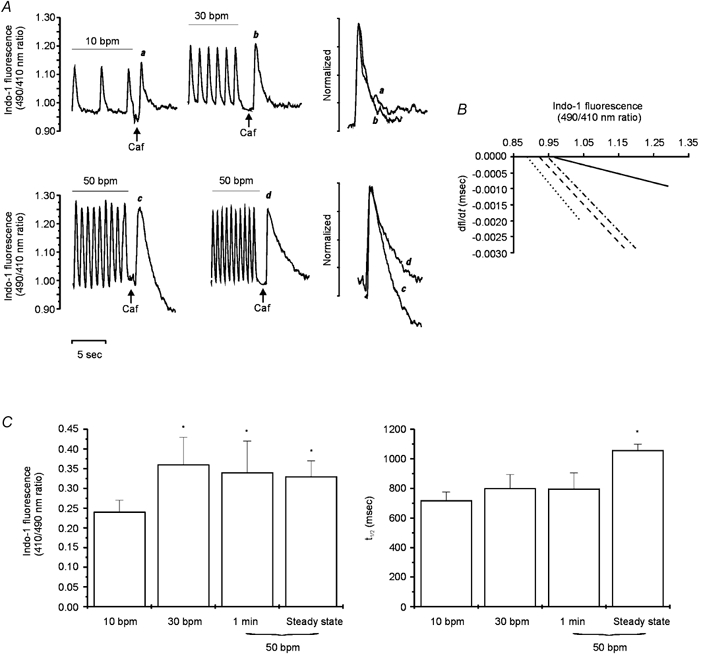
A, caffeine-induced CaiT after a train of stimuli at 10 (a), 30 (b) and 50 bpm, either after 1 min of the change in stimulation frequency (c) or after having reached the steady state (d). Increasing stimulation frequency from 10 to 30 or 50 bpm resulted in similar increases in the amplitude of the caffeine-induced CaiT. The superimposed and normalized traces show the caffeine-induced CaiTs under the conditions mentioned (a and b above and c and d below) for a better comparison of the rate of CaiT decline. The declining phases of the caffeine-induced CaiTs were not different at 10 or 30 bpm or after 1 min at 50 bpm. However, a prolongation of the rate of CaiT decline was observed at the steady state of 50 bpm. B, plots of the rates of the caffeine-induced CaiT decline (dfl/dt) against [Ca2+]i (indo-1 fluorescence) at stimulation frequencies of 10 (dotted line), 30 (dashed line) and 50 bpm for 1 min (dotted-dashed line) and 50 bpm at the stage where [Na+]i was significantly increased (continuous line). C, the bar graphs show the overall results of the caffeine-induced CaiT at 10 (n = 17), 30 (n = 13) and 50 bpm either after 1 min of the change in stimulation frequency (n = 9) or at the steady state (n = 11), on indo-1 fluorescence amplitude and the time to half-relaxation (t1/2). Data are means ± s.e.m. These results indicate that the activity of forward mode of the NCX was reduced well after the maximal positive inotropic effect of increasing frequency at 50 bpm was reached.
The bar graphs in Fig. 7C represent the overall data from these experiments. The left panel shows the similar increase in the caffeine-induced CaiT amplitude produced by increasing frequency from 10 to 30 or 50 bpm after either 1 min of stimulation or at the steady state. The right panel shows the failure of the increase in contraction frequency to 30 or to 50 bpm for 1 min to affect the time course of CaiT decline (assessed by the time to half-relaxation of the caffeine-induced CaiT). This finding is in contrast to the significant prolongation observed in the rate CaiT decline when it was measured after the increase in [Na+]i (produced by increasing contraction frequency to 50 bpm) had reached significant levels (see Fig. 2). The results obtained indicate that forward mode NCX activity is diminished at the highest stimulation frequency used (50 bpm). However, the reduced activity observed became evident after the maximal positive inotropic effect occurred and was not associated with any further increase of the inotropic effect of increasing stimulation frequency.
Effect of ouabain on myocyte contraction and cytosolic [Na+]
The time course of the changes in cytosolic [Na+] and contraction after the administration of 10 μm ouabain was examined in SBFI-loaded myocytes. The overall results of these experiments, shown in Fig. 8A, demonstrate that, in contrast to the results obtained with the increase in contraction frequency (Fig. 2), the positive inotropic effect of cardiac glycosides is tightly associated to the increase in [Na+]i. In an additional group of experiments, the effect of ouabain at 10 bpm and of increasing contraction frequency from 10 to 50 bpm was studied in an attempt to make a more accurate comparison of both inotropic interventions. In Fig. 8B, the [Na+]i level measured at the peak of the positive inotropic effect was compared with that associated with the same positive inotropy evoked by ouabain. Whereas the positive inotropic effect of ouabain was associated with a large and significant increase in [Na+]i from 4.2 ± 0.2 to 12.39 ± 1.5 mm, there was no detectable increase in [Na+]i associated with the maximal positive inotropic effect at the stimulation frequency of 50 bpm, in agreement with the results shown in Fig. 2B. It is important to point out that, whereas the increase in contractility produced by the change in stimulation frequency, as shown in other experiments (see Fig. 3), was associated with a significant reduction in resting cell length (5 ± 1.2 %), the administration of ouabain, for a similar inotropic effect, did not significantly affect resting cell length.
Figure 8. Effect of ouabain vs. frequency on contraction and [Na+]i.
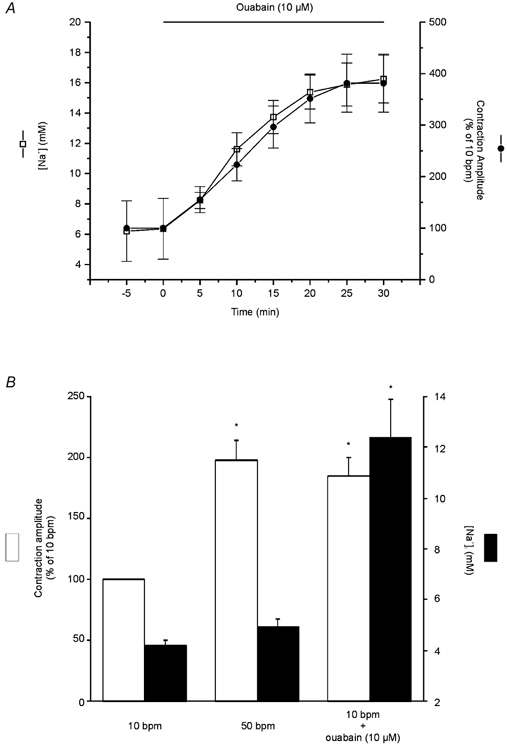
A, time course of ouabain-induced positive inotropic effect and the increase in [Na+]i. The increase in contraction and [Na+]i induced by ouabain are tightly associated. B, overall results of the effect of 10 μm ouabain at 10 bpm and of increasing stimulation frequency from 10 to 50 bpm on contraction amplitude (expressed as percentage of 10 bpm control) and [Na+]i (expressed in mm). Data are means ± s.e.m. of 8 cells, * P < 0.05. The contractility was matched by ouabain to the maximal increase in contractility evoked by increasing stimulation frequency. For a similar increase in contraction, the positive inotropic effect of ouabain was associated with a large and significant increase in [Na+]i, whereas that of contraction frequency occurred in the absence of any detectable increase in [Na+]i.
DISCUSSION
Frequency potentiation of contractility represents a potent inotropic mechanism in the myocardium of most species, including human non-failing hearts (Cohen et al. 1982; Wang et al. 1988; Schillinger et al. 1998; Alpert et al. 1998). The increase in contractility is the consequence of an increase in intracellular Ca2+ transients, produced by an enhanced SR Ca2+ load and release at the higher frequencies (reviewed in Bers, 2001). The NCX has been largely associated with the subcellular mechanisms that converge to produce this enhancement. Although direct experimental evidence is still lacking, the participation of the NCX in the positive staircase has mainly been related to the increase in [Na+]i that occurs with the increase in the frequency of stimulation (Langer 1971; Cohen et al. 1982; Lee et al. 1987; Boyett et al. 1987; Wang et al. 1988), and/or to the reduced time between contractions for Ca2+ extrusion via the forward mode of the exchanger (Cohen et al. 1982; Bers 2001; Antoons et al. 2002). In this study, a dissection of the possible mechanisms by which the NCX may be involved in the stimulation frequency-induced positive inotropic effect was performed.
The results showed that in the cat ventricle (1) the positive inotropic effect produced by increasing contraction frequency was temporally dissociated from the increase in [Na+]i; (2) the positive inotropic effect of increasing stimulation frequency was not affected by inhibiting the reverse mode of the NCX; (3) the increase in stimulation frequency from 10 to 30 bpm did not produce any significant inhibition of the forward mode of the NCX; (4) the increase in stimulation frequency from 10 to 50 bpm produced a significant reduction in the activity of the forward mode of the NCX, which occurred in association with a significant increase in [Na+]i. However, these changes became significant well after the maximal positive inotropic effect was reached and did not contribute further to the positive inotropic effect of increasing contraction frequency; (5) the increase in stimulation frequency from 10 to 50 bpm produced an increase in diastolic [Ca2+] associated with a decrease in resting cell length, which occurred in parallel with the increase in contractility and before the detection of any significant increase in [Na+]i. Taken together, the results indicate that neither the reverse nor the forward mode of the NCX contributes significantly to the increase in contractility produced by increasing frequency via a Na+-dependent mechanism, in the frequency range explored. The contribution of the NCX to the positive inotropism of increasing contraction frequency would be indirect and dependent on the reduced time between beats. The reduced diastolic interval would preclude the NCX from compensating for the increase in Ca2+ influx. This failure of the NCX to match Ca2+ influx became evident by the significant increase in diastolic [Ca2+] that occurred either when the SR was disabled in the presence of Ry and Tg or at the higher contraction frequency explored, i.e. 50 bpm, a condition in which the SR is fully saturated.
Ca2+ influx via the NCX and stimulation frequency-induced increase in contractility
The role of the reverse mode of the NCX in cardiac excitation-contraction coupling is still a matter of debate. Previous studies suggest that Na+ entry via INa could cause a rise in local submembrane [Na+]i and local elevation of [Ca2+]i via the NCX and consequent triggering of SR Ca2+ release (Leblanc & Hume, 1990; Lipp & Niggli, 1994; Litwin et al. 1998). Although there is experimental evidence indicating that the Ca2+-influx mode of the NCX cannot trigger Ca2+ sparks directly (Lopez-Lopez et al. 1995), recent experiments have suggested that the reverse mode of the NCX may locally regulate resting Ca2+ concentration in the restricted space of the diadic cleft, and modulate the threshold for triggering Ca2+ sparks (Litwin et al. 1998; Goldhaber et al. 1999). Litwin et al. (1998) further proposed that the net Ca2+ influx by the ICa and the reverse NCX may not sum linearly, but rather act in synergism in the modulation of Ca2+ release. In line with this concept, the results of Viatchenko-Karpinski & Györke (2001), indicated that the reverse mode of the NCX may play a role in the β-adrenergic enhancement of Ca2+-induced Ca2+ release by effectively contributing as a Ca2+ trigger. A similar role for the reverse mode of the NCX might be expected in the positive staircase phenomenon. In the present experiments, KBR tended to decrease the enhancement of both CaiT amplitude and shortening produced by increasing stimulation frequency, mainly at 50 bpm. However, this effect was modest and did not reach significant levels. Moreover, stimulation frequencies higher than 50 bpm failed to further increase contractility and CaiT in cat myocytes (results not shown). Thus, the present results do not support the view of a significant contribution of the reverse mode of the NCX in the positive inotropic effect of increasing contraction frequency in the cat ventricle.
A limitation of the above conclusion is that it relies on the ability of KBR to preferentially block the Ca2+ influx (reverse) mode of the NCX rather than the extrusion (forward) mode (Iwamoto et al. 1996). This reported property of KBR has recently been used to examine the possible contribution of the reverse mode of the exchanger to the influx of Ca2+, under different physiological or pathological conditions (Ladilov et al. 1999; Satoh et al. 2000; Perez et al. 2001; Vittone et al. 2002). However, several reports claim that KBR is not specific in blocking the reverse mode of the exchanger, and that several other currents may be blocked as well (Satoh et al. 2000; Lu et al. 2002; Reuter et al. 2002). The results shown in Fig. 4A indicate that the concentration of KBR used in the present experiments in cat isolated myocytes (1 μm) was able to selectively block the Ca2+ influx mode of the NCX, without affecting the Ca2+ efflux mode (Fig. 4B). Figure 4A shows, in addition, that 1 μm KBR is a concentration sufficiently high to produce a virtually complete inhibition of the reverse mode of the NCX, when compared with the inhibition produced by 10 mm Ni2+ (Fig. 4A). Moreover, this concentration of KBR failed to significantly affect basal contractility and relaxation parameters (Table 2). This latter finding would further indicate that the contribution of the Ca2+ influx mode of the NCX to the excitation-contraction coupling in the cat ventricle is minimal and barely significant, under basal conditions, in agreement with what has been found in other species (Satoh et al. 2000). More important for the conclusion of this study, the lack of effect of KBR on basal contractility would also suggest that the L-type Ca2+ current is not affected by this concentration of the drug. Supporting our own data, previous experiments, also performed in isolated cat myocytes under the same conditions as in the present experiments, have shown that concentrations of KBR up to 5 μm failed to affect the inward NCX current (Aiello et al. 2002) and that 1 μm of the inhibitor does not affect the L-type Ca2+ current (E. A. Aiello & H. E. Cingolani, personal communication). Similar results were obtained by Watano et al. (1996), in guinea-pig ventricular myocytes.
Thus, although KBR may be an imperfect agent, our results seem to indicate that in our experimental conditions, KBR behaved as a specific blocker of the reverse mode NCX, and support the conclusion that this mode of the NCX does not significantly participate in the positive staircase in the cat ventricle.
Ca2+ efflux via NCX and stimulation frequency-induced increase in contractility
The NCX plays an important role in the decrease in cytosolic Ca2+ that induces relaxation in several species. In isolated cat myocytes, it has been shown that the NCX is responsible for approximately 30 and 50 % of cytosolic Ca2+ decline, at 25 and 35 °C, respectively, and a stimulation frequency of 0.5 Hz (Puglisi et al. 1996). A decrease in Ca2+ extrusion by this mechanism would therefore favour SR Ca2+ loading due to an increase in diastolic [Ca2+]. As a consequence, myocardial contractility would increase. The present results are not consistent with a reduction in the activity of the Ca2+ efflux mode of the NCX, when stimulation frequency was increased from 10 to 30 bpm. Although the increase in contraction frequency did produce an increase in SR Ca2+ content, the rate of Ca2+ decline of the caffeine-induced contracture was similar at the two different frequencies. This was so, even after correction for the higher amplitude of the caffeine-induced CaiT at 30 bpm, which, by increasing by itself the rate of Ca2+ decline, could have masked a possible reduction of Ca2+ efflux via the NCX (Bers & Berlin, 1995). At the higher contraction frequency explored (50 bpm), there was a reduction in the activity of the forward mode of the NCX (Fig. 7), which could contribute to the increase in diastolic [Ca2+]i and to the decrease in diastolic cell length produced by increasing stimulation frequency. However, there was a temporal dissociation between the increase in [Na+]i that mediates the reduction in NCX forward mode activity, and the increase in contractility produced by increasing stimulation frequency. Whereas the increase in contractility and SR Ca2+ load fully developed within 1 min, the increase in [Na+]i and the reduction in the activity of the forward mode of the NCX reached significant levels only several minutes after stimulation frequency was increased to 50 bpm and the maximal positive inotropic effect was reached. At 50 bpm, the development of the maximal positive inotropic effect occurred in parallel with an initial increase in diastolic [Ca2+] and with a decrease in resting cell length. In the absence of any significant decrease in the Ca2+ efflux mode of the NCX, this increase in diastolic [Ca2+] and decrease in resting cell length would reflect either a decrease in the rate of Ca2+ removal by the SR or an insufficient time between beats for Ca2+ extrusion via the NCX. Since the increase in diastolic [Ca2+] occurred in association with the typical relaxant effect of increasing frequency, which seems to be dependent on the SR (Bassani et al. 1995; DeSantiago et al. 2002), the rate of SR Ca2+ removal must be increased rather than decreased at higher stimulation frequencies, which would exclude the first of the two possibilities considered above. Thus our results argue in favour of an indirect, Na+-independent contribution of the NCX to the stimulation frequency-induced positive inotropic effect. This action might also be present at lower stimulation frequencies, i.e. 30 bpm, but would be undetectable in the presence of a functional and unsaturated SR. However, it became evident at 50 bpm, due to the fact that the SR was maximally loaded (Fig. 7), or when the SR was disabled in the presence of Ry and Tg. Furthermore, the increase in [Na+]i, and the associated reduced activity of the NCX, that occurred later than the maximal positive inotropic effect was reached, did not further modify the positive staircase. The results therefore exclude the participation of a Na+-dependent reduction in the activity of the forward mode of the NCX in the positive inotropic effect induced by the increase in stimulation frequency. The reduced activity of the NCX is more likely to be responsible for the slow increase in diastolic [Ca2+] and decrease in resting cell length associated with the increase in [Na+]i. A lack of contribution of a [Na+]i-dependent reduction in NCX forward mode activity to contraction frequency inotropy can be also drawn from the experiments performed using cardiac glycosides, the typical NCX-dependent inotropic intervention (Satoh et al. 2000). In contrast to what was observed with the increase in stimulation frequency, ouabain produced an increase in contractility that occurred in close association with the increase in [Na+]i (Fig. 8A). Moreover, for an increase in contractility similar to the maximal effect evoked by increasing contraction frequency, ouabain produced a large and significant increase in [Na+]i whereas the maximal positive inotropic effect of the increase in contraction frequency occurred in the absence of any detectable increase in [Na+]i (Fig. 8B).
Although the positive inotropic effect of increasing contraction frequency has been largely associated with an increase in [Na+]i (Cohen et al. 1982; Wang et al. 1988; Despa et al. 2002), there is also evidence indicating that this association is not so straightforward and should be supported by parallel increases in contractility and [Na+]i. In this scenario, our results indicate that frequency-dependent increases in [Na+]i are not necessary for a positive force- frequency relationship. Similarly, Antoons et al. (2002) observed, in mouse myocytes, a stimulation frequency-induced increase in [Na+]i that was rather slow to explain the increase in contraction. Furthermore, careful examination of the experiments presented by Cohen et al. (1982) reveals that, when stimulation frequency is increased from 0.1 to 3 Hz, there is an immediate, large increase in contraction followed by a small progressive secondary increase. The increase in [Na+]i observed in these experiments was closely associated with the secondary increase in contraction. A dissociation between simulation-frequency inotropy and the increase in [Na+]i has also been observed in the rat and the failing human heart, where a negative force-frequency relation has been shown to occur in parallel with a frequency-dependent increase in [Na+]i (Maier et al. 1997; Pieske et al. 2002). It has been shown that the relationship between [Na+]i and force is highly non-linear (Eisner et al. 1984). A similar non-linear relationship must be expected between [Na+]i and shortening of unloaded cells. Thus, it might be argued that small elevations in [Na+]i could lead to a significant increase in shortening, which then saturates due to cell stiffness, while [Na+]i continues to rise. This possibility seems unlikely, however, because (1) as described above, neither the activity of the forward mode nor that of the reverse mode of the NCX, was affected at the moment of the initial maximal inotropic effect produced by increasing stimulation frequency; (2) the lack of further increase in unloaded shortening observed when the elevation in [Na+]i reached significant levels was associated with a lack of increase in the amplitude of CaiT, which cannot be attributed to limitations due to cell stiffness. This lack of contractile and CaiT amplitude response may be more likely to be associated with a saturated SR at 50 bpm, as shown in the caffeine-induced contractures (Fig. 7). Furthermore, slower CaiT decline and elevated diastolic [Ca2+] may also slow the recovery of Ca2+ channels between beats, and this could further limit the extent of enhancement of systolic function at higher heart rates (Sipido et al. 1998).
The results presented herein would also indicate that subsarcolemmal [Na+]i gradients, that might have occurred during the increase in stimulation frequency, are not sufficient to modulate NCX activity. If this were the case, a dissociation between the increase in [Na+]i and the activity of the NCX should have been detected. However, this was not the case in the present experiments.
In summary, the present results indicate that the NCX does not participate directly in the positive inotropic effect of increasing stimulation frequency in the cat ventricle. The reduced diastolic interval produced by the increase in frequency, by precluding the exchanger from extruding the Ca2+ that entered the cell in each beat, would constitute the only mechanism by which the NCX contributes to the increase in SR Ca2+ load and positive inotropism of the increase in stimulation frequency. Since the NCX is the main mechanism of Ca2+ extrusion from the cell in the cat myocardium, the increase in diastolic [Ca2+]i that occurred immediately after the stimulation frequency was increased, in the absence of significant changes in [Na+]i, and tightly associated with an increase in contractility, gives strong support to this conclusion.
Acknowledgments
This study was supported by a grant (PICT 05-08592) from the Agencia Nacional de Promoción Científica y Tecnológica, Argentina. The technical assistance of Mrs Mónica Rando is gratefully acknowledged. M. G. Vila Petroff and A. R. Mattiazzi are established Investigators of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
REFERENCES
- Aiello EA, Villa-Abrille MC, Cingolani HE. Autocrine stimulation of cardiac Na+–Ca2+ exchanger currents by endogenous endothelin released by angiotensin II. Circ Res. 2002;90:374–376. doi: 10.1161/hh0402.105373. [DOI] [PubMed] [Google Scholar]
- Alpert NR, Leavitt BJ, Ittleman FP, Hasenfuss G, Pieske B, Mulieri LA. A mechanistic analysis of the force-frequency relation in non-failing and progressively failing human myocardium. Basic Res Cardiol. 1998;93:23–32. doi: 10.1007/s003950050200. [DOI] [PubMed] [Google Scholar]
- Antoons G, Mubagwa K, Nevelsteen I, Sipido KR. Mechanisms underlying the frequency dependence of contraction and [Ca2+]i transients in mouse ventricular myocytes. J Physiol. 2002;543:889–898. doi: 10.1113/jphysiol.2002.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baartscheer A, Schumacher CA, Fiolet JWT. Small changes of cytosolic sodium in rat ventricular myocytes measured with SBFI in emission ratio mode. J Mol Cell Cardiol. 1997;29:3375–3383. doi: 10.1006/jmcc.1997.0567. [DOI] [PubMed] [Google Scholar]
- Bassani JWM, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani RA, Mattiazzi A, Bers DM. CaMKII is responsible for activity-dependent acceleration of relaxation in rat ventricular myocytes. Am J Physiol. 1995;268:H703–712. doi: 10.1152/ajpheart.1995.268.2.H703. [DOI] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2. Kluwer Academic Publishers; 2001. [Google Scholar]
- Bers DM, Bridge JHB, Spitzer KW. Intracellular Ca2+ transients during rapid cooling contractures in guinea-pig ventricular myocytes. J Physiol. 1989;417:537–553. doi: 10.1113/jphysiol.1989.sp017817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Boyett MR, Hart G, Levi AJ, Roberts A. Effects of repetitive activity on developed force and intracellular sodium in isolated sheep and dog Purkinje fibers. J Physiol. 1987;388:295–322. doi: 10.1113/jphysiol.1987.sp016616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen CJ, Fozzard HA, Sheu SS. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res. 1982;50:651–662. doi: 10.1161/01.res.50.5.651. [DOI] [PubMed] [Google Scholar]
- Cohen I, Falk R, Kline R. Membrane currents following activity in canine cardiac purkinje fibers. Biophys J. 1981;33:281–288. doi: 10.1016/S0006-3495(81)84890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantiago J, Maier LS, Bers DM. Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban. J Mol Cell Cardiol. 2002;34:975–984. doi: 10.1006/jmcc.2002.2034. [DOI] [PubMed] [Google Scholar]
- Despa S, Islam MA, Pogwizd S, Bers DM. Intracellular [Na+] and Na+ pump rate in rat and rabbit ventricular myocytes. J Physiol. 2002;539:133–143. doi: 10.1113/jphysiol.2001.012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner DA, Lederer WJ, Vaughan-Jones RD. The quantitative relationship between twitch tension and intracellular sodium activity in sheep cardiac Purkinje fibres. J Physiol. 1984;355:251–266. doi: 10.1113/jphysiol.1984.sp015417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton JE, Harrison MR, Orchard CH. Ca2+ and Na+ in rat myocytes showing different force-frequency relationships. Am J Physiol. 1991;30:C739–750. doi: 10.1152/ajpcell.1991.261.5.C739. [DOI] [PubMed] [Google Scholar]
- Goldhaber JI, Lamp ST, Walter DO, Garfinkel A, Fucumoto GH, Weiss JN. Local regulation of the threshold for calcium sparks in rat ventricular myocytes: role of sodium-calcium exchange. J Physiol. 1999;520:431–438. doi: 10.1111/j.1469-7793.1999.00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harootunian AT, Kao JPY, Eckert BK, Tsien RY. Fluorescence ratio imaging of cytoplasmic free Na+ in individual fibroblast and lymphocytes. J Biol Chem. 1989;264:19458–19467. [PubMed] [Google Scholar]
- Hulme JT, Orchard CH. Effect of acidosis on Ca2+ uptake and release by sarcoplasmic reticulum of intact rat ventricular myocytes. Am J Physiol. 1998;275:H977–987. doi: 10.1152/ajpheart.1998.275.3.H977. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Watano T, Shigekawa M. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J Biol Chem. 1996;271:1121–1125. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- Kimura J, Miyamae S, Soma A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol. 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladilov Y, Haffner S, Balser-Schafer C, Maxeiner H, Piper HM. Cardioprotective effects of KB-R7943: a novel inhibitor of the reverse mode of Na+/Ca2+ exchanger. Am J Physiol. 1999;279:H1868–1876. doi: 10.1152/ajpheart.1999.276.6.H1868. [DOI] [PubMed] [Google Scholar]
- Langer GA. The intrinsic control of myocardial contraction ionic factors. N Engl J Med. 1971;285:1065–1071. doi: 10.1056/NEJM197111042851910. [DOI] [PubMed] [Google Scholar]
- Leblanc N, Hume JR. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990;248:372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Lee CO, Im WB, Sonn JK. Intracellular sodium ion activity: reliable measurement and stimulation-induced changes in cardiac Purkinje fibers. J Physiol Pharmacol. 1987;65:954–962. doi: 10.1139/y87-152. [DOI] [PubMed] [Google Scholar]
- Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca2+-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J Physiol. 1997;501:17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Niggli E. Sodium current-induced calcium signals in isolated guinea-pig ventricular myocytes. J Physiol. 1994;474:439–446. doi: 10.1113/jphysiol.1994.sp020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin S, Li J, Bridge JHB. Na+/Ca2+-exchange, and the trigger for sarcoplasmic reticulum Ca2+ release: studies in adult rabbit ventricular myocytes. Biophys J. 1998;75:359–371. doi: 10.1016/S0006-3495(98)77520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Lopez JR, Shacklock PS, Balke GW, Wier WG. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995;268:1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- Lu J, Liang Y, Wang X. Amiloride and KB-R7943 in outward Na+/Ca2+ exchange current in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 2002;40:106–111. doi: 10.1097/00005344-200207000-00013. [DOI] [PubMed] [Google Scholar]
- Maier LS, Pieske B, Allen DG. Influence of stimulation frequency on [Na+]i and contractile function in Langendorff-perfused rat heart. Am J Physiol. 1997;273:H1246–1254. doi: 10.1152/ajpheart.1997.273.3.H1246. [DOI] [PubMed] [Google Scholar]
- Perez NG, Camilión de Hurtado MC, Cingolani HE. Reverse mode of the Na+–Ca2+ exchange after myocardial stretch. Underlying mechanisms of the slow force response. Circ Res. 2001;88:376–382. doi: 10.1161/01.res.88.4.376. [DOI] [PubMed] [Google Scholar]
- Pieske B, Maier LS, Piacentino V, III, Weisser J, Hasenfuss G, Houser S. Rate dependence of [Na+]i and contractility in nonfailing and failing human myocardium. Circulation. 2002;106:447–453. doi: 10.1161/01.cir.0000023042.50192.f4. [DOI] [PubMed] [Google Scholar]
- Puglisi JL, Bassani RA, Bassani JWM, Amin JN, Bers DM. Temperature and relative contributions of Ca2+ transport systems in cardiac myocyte relaxation. Am J Physiol. 1996;270:H1772–1778. doi: 10.1152/ajpheart.1996.270.5.H1772. [DOI] [PubMed] [Google Scholar]
- Reuter H, Henderson SA, Han T, Matsuda T, Baba A, Ross RS, Goldhaber JI, Philipson KD. Knockout mice for pharmacological screening. Testing the specificity of Na+–Ca2+ excange inhibitors. Circ Res. 2002;91:90–92. doi: 10.1161/01.res.0000027529.37429.38. [DOI] [PubMed] [Google Scholar]
- Satoh H, Ginsburg KS, Qing K, Terada H, Hayashi H, Bers DM. KB-R7943 block of Ca2+ influx via Na+/Ca2+ exchange does not alter twitches or glycoside inotropy but prevents Ca2+ overload in rat ventricular myocytes. Circulation. 2000;101:1441–1446. doi: 10.1161/01.cir.101.12.1441. [DOI] [PubMed] [Google Scholar]
- Schillinger W, Lehnsrt SE, Prestle J, Preuss M, Pieske B, Maier LS, Meyer M, Just H, Hasenfuss G. Influence of SR Ca2+-ATPase and Na+–Ca2+ exchanger on the force-frecuency relation. Basic Res Cardiol. 1998;93:38–45. doi: 10.1007/s003950050208. [DOI] [PubMed] [Google Scholar]
- Sipido KR, Stankovicova T, Flameng W, Vanhaecke J, Verdonck F. Frequency dependence of Ca2+ release from the sarcoplasmic reticulum in human ventricular myocytes from end-stage heart failure. Cardiovasc Res. 1998;37:478–488. doi: 10.1016/s0008-6363(97)00280-0. [DOI] [PubMed] [Google Scholar]
- Spurgeon HA, Stern MD, Baartz G, Raffaeli S, Hansford RG, Talo A, Lakatta EG, Capogrossi MC. Simultaneous measurements of Ca2+, contraction, and potential in cardiac myocytes. Am J Physiol. 1990;258:H574–586. doi: 10.1152/ajpheart.1990.258.2.H574. [DOI] [PubMed] [Google Scholar]
- Viatchenko-Karpinski, Györke S. Modulation of the Ca2+-induced Ca2+ release cascade by β-adrenergic stimulation in rat ventricular myocytes. J Physiol. 2001;533:837–848. doi: 10.1111/j.1469-7793.2001.t01-1-00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila Petroff MG, Aiello A, Palomeque J, Salas MA, Mattiazzi A. Subcellular mechanisms of the positive inotropic effect of angiotensin II in cat myocardium. J Physiol. 2000;529:189–203. doi: 10.1111/j.1469-7793.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittone L, Mundiña-Weilenmann C, Said M, Ferrero P, Mattiazzi A. Time course and mechanisms of phospholamban residues in ischemia-reperfused rat hearts. Dissociation of phospholamban phosphorylation pathways. J Mol Cell Cardiol. 2002;34:39–50. doi: 10.1006/jmcc.2001.1488. [DOI] [PubMed] [Google Scholar]
- Wang DY, Chae SW, Gong QY, Lee CO. Role of in positive force-frequency staircase in guinea pig papillary muscle. Am J Physiol. 1988;255:C798–807. doi: 10.1152/ajpcell.1988.255.6.C798. [DOI] [PubMed] [Google Scholar]
- Watano T, Kimura J, Morita T, Nakanishi H. A novel antagonist, No. 7943, of the Na+/Ca2+ exchange current in guinea-pig cardiac ventricular cells. Br J Pharmacol. 1996;119:555–563. doi: 10.1111/j.1476-5381.1996.tb15708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt AC, Maylie J. Stimulation-dependent facilitation of the high threshold calcium current in guinea-pig ventricular myocytes. J Physiol. 1990;428:653–671. doi: 10.1113/jphysiol.1990.sp018233. [DOI] [PMC free article] [PubMed] [Google Scholar]


