Abstract
Paired-pulse transcranial magnetic stimulation (TMS) was used to assess the effectiveness of intracortical inhibition (ICI) acting on corticospinal neurons controlling three intrinsic hand muscles in humans. We hypothesised that the suppression of ICI with selective activation of a muscle would be restricted to corticospinal neurons controlling the muscle targeted for activation. Surface EMG was recorded from abductor pollicis brevis (APB), first dorsal interosseous (FDI) and abductor digiti minimi (ADM) muscles of the left hand. Subjects were tested at rest and during weak selective activation of APB or ADM, while they attempted to keep the other muscles relaxed using visual feedback. Paired-pulse TMS was applied with a circular coil oriented to produce antero-posterior (AP) current flow in the right motor cortex (to preferentially evoke I3 waves in corticospinal neurons) and with postero-anterior (PA) currents (to preferentially evoke I1 waves). Paired-pulse TMS was less effective in suppressing the muscle evoked potential (MEP) when the muscle was targeted for selective activation, with both AP and PA stimulation. The mechanism for this includes effects on late I waves, as it was evident with a weak AP test TMS pulse that elicited negligible I1 waves in corticospinal neurons. ICI circuits activated by TMS, which exert their effects on late I waves but do not affect I1 waves, are strongly implicated in this modulation. With AP stimulation, paired-pulse inhibition was not significantly altered for corticospinal neurons controlling other muscles of the same hand which were required to be inactive during the selective activation task. This differential modulation was not seen with PA stimulation, which preferentially activates I1 waves and evokes a MEP that is less influenced by ICI. The observations with AP stimulation suggest that selective activation of a hand muscle is accompanied by a selective suppression of ICI effects on the corticospinal neurons controlling that muscle. The pattern of differential modulation of ICI effectiveness with voluntary activation suggests that the ICI circuits assist the corticospinal system in producing fractionated activity of intrinsic hand muscles.
Conditioning transcranial magnetic stimulation (TMS) which is below active motor threshold intensity activates interneuronal circuits within human motor cortex that influence the excitability of corticospinal neurons to suprathreshold TMS delivered up to 20 ms later (Kujirai et al. 1993; Di Lazzaro et al. 1998b; Hanajima et al. 1998). Suppression of the muscle evoked potential (MEP) with interstimulus intervals (ISIs) between 1–5 ms is due to activation of intracortical GABAergic inhibitory interneurons (Ziemann et al. 1996a, 1998a; Chen et al. 1998; Hanajima et al. 1998; Di Lazzaro et al. 2000), and is termed intracortical inhibition (ICI).
Since the initial report by Kujirai et al. (1993), there have been a number of studies using paired-pulse TMS to investigate ICI in patient populations and normal subjects. ICI is less effective in movement disorders such as Parkinson’ s disease (Ridding et al. 1995a) and focal task-specific dystonia (Ridding et al. 1995b), and in patients with a lesion of the putamen or globus pallidus (Hanajima et al. 1996). While the reduced ICI in these conditions presumably represents an imbalance in GABAergic inhibition in motor cortex, there is little to guide us on the mechanisms for altered effectiveness of ICI circuits, and what, if any, relationship exists between the operation of ICI circuits and motor disability.
It has been suggested that in normal subjects ICI may contribute to the ability to selectively activate different muscles (fractionation) which is a feature of the corticospinal system (Ridding et al. 1995c). This was based on the observation that ICI circuits activated by subthreshold conditioning TMS are less effective in suppressing the MEP when the muscle is activated by voluntary commands. This suppression is somewhat selective, as activation of a proximal muscle (biceps) did not influence effectiveness of ICI for the resting FDI muscle (Ridding et al. 1995c). If the GABAergic ICI circuits contribute to fractionation, this might explain the overflow of muscle activation seen in some conditions (e.g. focal task-specific dystonia) in which ICI is less effective (Ridding et al. 1995b), or when GABAergic inhibition is blocked pharmacologically in motor cortex of the monkey (Matsumura et al. 1991). Several other lines of evidence in normal subjects suggests a role for ICI in selective muscle activation. ICI is selectively suppressed for wrist extensor muscles, prior to, and during wrist extension, with no change for the wrist flexors (Reynolds & Ashby, 1999). ICI tested at rest before, and after a phasic task requiring selective activation of hand muscles shows differential plastic changes in ICI for corticospinal neurons controlling muscles required to be active and inactive during the task (Liepert et al. 1998). If ICI does contribute to fractionation, however, it is curious that ICI of corticospinal neurons controlling a hand muscle is reduced in musicians (Nordstrom & Butler, 2002).
None of the aforementioned studies using TMS have specifically addressed the issue of differential modulation of ICI for active and inactive synergist muscles during a voluntary task requiring their selective activation. If ICI does contribute to fractionation, the suppression of ICI should be greater for the active muscle than the muscles required to be inactive during the task. In the present study we have examined this issue for differential activation of three intrinsic hand muscles, including two muscles acting on thumb and index finger which are commonly activated together to manipulate objects with the digits. We considered this to be a stringent test of the role of ICI in the modulation of the corticospinal commands to produce fractionation, as the motor pools of intrinsic hand muscles receive the strongest direct projection from the motor cortex (Clough et al. 1968), and in primates single corticomotoneuronal (CM) cells provide branched-axon inputs to motoneuron pools of several muscles (Buys et al. 1986). We reasoned that ICI may promote selective activation of one muscle of a pair when the task requires it by suppressing activity in the subset of CM cells facilitating both muscles of a pair.
We assessed the modulation of ICI during selective muscle activation using paired-pulse TMS with current flow in the ‘preferred’ and ‘non-preferred’ direction for activation of corticospinal neurons (Day et al. 1989). This allowed us to investigate the role of neural elements responsible for later I-waves in the suppression of ICI with voluntary activation, and in the differential modulation of ICI for corticospinal neurons controlling the three muscles. It has been suggested that the reduction in ICI effectiveness observed when a muscle is activated could be due to increased contribution of I1-waves to the MEP (Hanajima et al. 1998) rather than changes in ICI circuits. This could arise because the I1-wave is not influenced by the ICI circuit (Nakamura et al. 1997; Di Lazzaro et al. 1998b; Hanajima et al. 1998). We observed a suppression of ICI for the active muscle with both coil orientations, even with minimal I1-wave contribution to the test MEP. Conditioning TMS is therefore less effective at suppressing later I-waves in corticospinal neurons controlling the active muscle. The suppression of ICI was restricted to corticospinal neurons controlling the muscle targeted for activation when tested with ‘non-preferred’ coil orientation favouring a greater I3-wave contribution to the test MEP (i.e. when the MEP was most susceptible to effects of ICI). Our evidence suggests that ICI circuits in motor cortex are differentially modulated to produce selective disinhibition of corticospinal neurons controlling the hand muscle engaged in the task. By this action, ICI assists the corticospinal system to produce fractionated activity in human hand muscles.
METHODS
Twenty-five (13 M, 12 F) neurologically normal adult volunteers participated in this study. All were right-handed by self report. Subjects completed the Adult Safety Screen Questionnaire (Keel et al. 2000) prior to the experiment to ensure that there was no contraindication for using TMS. The subjects gave informed written consent to participate in the study. All procedures used conformed with the Declaration of Helsinki, and the protocol was approved by the Human Research Ethics Committee of the University of Adelaide.
Subjects were seated in a comfortable dental chair with a headrest to support the head and neck. The left shoulder was abducted and left forearm supported on a side table equipped with the manipulandum. The manipulandum consisted of a thermoplastic hand and forearm splint and restraining Velcro straps attached to a metal frame. A rod connected to a load cell at one end and a metal ring at the other was attached to the frame. The metal ring contained a removable nylon insert customised for each subject to ensure a secure and comfortable fit with the proximal phalanx of the thumb. The left forearm and hand were placed in the manipulandum and the position of forearm, wrist and hand were fixed and held with the fingers and thumb comfortably at rest. Velcro straps secured the limb at two points (mid-forearm and proximal to the wrist). The thumb was inserted into the ring and the position and orientation of the rod adjusted so that the load cell measured thumb abduction force.
Surface EMG activity was recorded from three intrinsic muscles of the left hand with self-adhesive pre-gelled disposable surface electrodes (3M Red Dot, Ontario, Canada). These muscles were abductor pollicis brevis (APB), first dorsal interosseous (FDI) and abductor digiti minimi (ADM). EMG signals were amplified (×1000–2000), filtered (20–500 Hz or 1 kHz) and recorded along with the force signal from the load cell in separate channels of a pulse code modulator, data recorder sampling at 22 kHz per channel (Vetter 400, A.R. Vetter Co., PA, USA). Force and EMG signals were digitised on-line (1 or 2 kHz sampling rate) using a Macintosh computer and customised LabView software that controlled stimulus delivery and performed on-line stimulus-triggered averaging of force and rectified EMG signals.
Two oscilloscope screens were positioned in front of the subject, on which were displayed the thumb abduction force and EMG signals from each muscle at high amplification. Using visual feedback subjects practised the selective activation of APB muscle during isometric thumb abduction or ADM muscle during isometric fifth finger abduction to various force targets while attempting to maintain electrical silence in the other two muscles. Subjects practised the task until they, and the experimenters were satisfied that their performance was optimal. This was normally achieved after several minutes of familiarity with the task.
TMS was delivered with two Magstim 200 stimulators (Magstim Company Limited, UK) connected with a Bistim module (Magstim Company Limited, UK) to allow the output of both stimulators to be discharged through the same circular coil (90 mm diameter). The coil was positioned near the vertex at a scalp site optimal for evoking a muscle evoked potential (MEP) at rest in the three monitored muscles of the left hand. This site was marked on the scalp as a reference. The coil was held flat on the head with both hands and with the handle directed backwards. Coil position and orientation were constantly checked during the experiment to ensure that no changes occurred.
Experimental protocol 1: ICI tested with ‘preferred’ coil orientation
In the first series of experiments, TMS was applied with the coil oriented with side B up so that the current in the coil flowed in a clockwise direction when viewed from above (inducing an anti-clockwise current in the brain). This coil orientation induces current flow in a posterior to anterior direction in the right motor cortex, and will be referred to as PA stimulation. It is the conventional approach used for most studies with TMS. Weak PA stimulation with a circular coil preferentially produces I1 and/or D waves in corticospinal neurons (Day et al. 1989; Di Lazzaro et al. 2002).
Fifteen subjects (10 M, 5 F) with a mean (±S.D.) age of 30 ± 7 years were tested with paired-pulse TMS and PA stimulation. Subjects were tested at rest with all three intrinsic hand muscles relaxed, and with APB active during a 3 N isometric thumb abduction while they attempted to keep the FDI and ADM muscles relaxed. Subjects performed each task with visual feedback of thumb abduction force and EMG of all three muscles. TMS thresholds were assessed for APB at rest and active during the 3 N thumb abduction. MEP threshold was tested in steps of 2 % maximum stimulator output, and defined as the lowest intensity for which three of five successive MEPs exceed 50 μV (rest) or 100 μV (active) peak-to-peak amplitude. Test TMS intensity was adjusted to produce a test MEP in APB at rest of 0.5–1 mV amplitude, and consistent responses in the other two muscles. Conditioning TMS intensity was below active motor threshold (T) and adjusted to produce ≈60 % suppression of the MEP in APB at rest with a 3 ms interstimulus interval (ISI). Conditioning intensities ranged between 0.65–0.9 T for different subjects.
Single or paired-pulse TMS was delivered randomly in a block of 25 trials (< 0.2 s−1) with subjects at rest or with APB active. ISIs between 1–5 ms were tested in separate blocks for each task, making 10 combinations of task and ISI. The sequence of testing combinations of task and ISI was randomised for each subject.
In five experiments we included two additional blocks of trials with APB active using ISIs of 1 and 3 ms and test TMS intensity reduced to match the size of the APB MEP under rest and active conditions. Conditioning intensity was unchanged. This condition is denoted APB activeRI. These trials served as a control for the effect of test MEP size on the effectiveness of conditioning at rest and with APB active.
Experimental protocol 2: ICI tested with ‘non-preferred’ coil orientation and different conditioning intensities
To determine whether neural elements responsible for production of late I waves in corticospinal neurons are involved in the modulation of ICI effectiveness, we conducted a second series of experiments using the circular coil to activate the right motor cortex with current flow in the preferred (PA stimulation) direction, and with the coil reversed to produce current flow in the non-preferred (AP stimulation) direction. The direction of current flow does not affect the ability to activate the ICI circuit by conditioning TMS (Ziemann et al. 1996b; Hanajima et al. 1998), but does affect the pattern of I waves produced in corticospinal neurons by suprathreshold TMS. PA stimulation preferentially activates I1 and/or D waves at low intensities, whereas AP stimulation preferentially activates I3 waves at lowest intensities (Day et al. 1989). Weak AP stimulation with a circular coil activates corticospinal neurons without producing I1 waves (Day et al. 1989). One explanation proposed for the reduced effectiveness of ICI during voluntary activation is that in the relaxed state temporal summation of multiple I waves is required to produce the test MEP, whereas in the active state the increased motoneuron excitability means that the I1 wave alone may bring the motoneurons to threshold, and thus I1 waves become more important in producing the MEP when the muscle is active (Hanajima et al. 1998). The increased size of all I waves with voluntary activation (Di Lazzaro et al. 1998a) would further favour motoneurons being brought to firing threshold by earlier I waves. ICI circuits do not act on neural elements responsible for I1 waves (Nakamura et al. 1997; Di Lazzaro, et al. 1998b; Hanajima et al. 1998; Trompetto et al. 1999), so a greater contribution of I1 waves to the MEP would be accompanied by reduced suppression of the MEP with paired-pulse TMS, in the absence of any change in ICI. In this second series of experiments we used AP stimulation to activate corticospinal neurons with weak or negligible I1-wave activation, to determine whether modulation of ICI could still be observed with voluntary activation, and to assess the selectivity of this effect for the three muscles.
Eight subjects (6 M, 2 F) with a mean (±S.D.) age of 28 ± 5 years were tested in this series. Four of these subjects also participated in the experiments conducted with protocol 1. Subjects were tested at rest and with APB active during a 3 N isometric thumb abduction while they attempted to keep FDI and ADM muscles relaxed, as described for protocol 1. TMS thresholds were assessed for APB at rest and active during the 3 N thumb abduction with both AP and PA stimulation. Test TMS intensity for each coil orientation was adjusted to produce a test MEP in APB at rest of 0.5–1 mV amplitude, and consistent responses in the other two muscles. A second, weaker test intensity was determined for both AP and PA stimulation with APB active. This produced a test MEP in active APB that was matched in size with the APB test MEP obtained at rest with the stronger test pulse. By reducing the test TMS intensity in trials with APB active (APB activeRI condition), we aimed to minimise or eliminate the I1 wave evoked in the descending corticospinal volleys produced by AP stimulation. With the weaker test intensity in this series, however, reliable MEPs were not obtained in FDI and ADM.
Once the threshold and test intensities had been established, test MEPs were averaged (n = 8) for the rest, APB active and APB activeRI conditions, with both AP and PA stimulation. Onset latencies of the APB test MEPs produced under these conditions were examined for evidence of I1 -wave activation.
ICI was tested using AP stimulation for both conditioning and test pulses. An ISI of 3 ms was used with a range of conditioning intensities below active motor threshold for APB with AP stimulation (0.5, 0.6, 0.7, 0.8 and 0.9 T). Single or paired-pulse TMS was delivered randomly in a block of 20 trials (< 0.2 s−1) with subjects at rest or with APB active. The strong test pulse was used for rest and APB active trials, and the weaker test pulse used for APB activeRI trials. Thus there were three combinations of task/test TMS intensity tested with five conditioning intensities, making 15 blocks of trials performed in random order.
Experimental protocol 3: ICI tested with ‘non-preferred’ coil orientation and different ISIs
We conducted another series of experiments using paired-pulse AP stimulation to examine the effect of ISI on the effectiveness of ICI under rest and APB active conditions. Seven subjects (5M, 2F) aged 30 ± 7 years participated in this series. The protocol was similar to that used in protocol 2, except that a conditioning intensity of 0.8 T was used throughout, and ISIs of 1, 2, 3, 4, and 5 ms used in separate trials. Single or paired-pulse TMS was delivered randomly in a block of 20 trials (< 0.2 s−1) with subjects at rest or with APB active. The strong test pulse was used for rest and APB active trials, and a weaker test pulse used for APB activeRI trials. In this series, the weak test pulse was adjusted to match the MEP size in FDI and ADM at rest and with APB active. This was stronger than the weak test pulse in protocol 2, and probably still contained some I1 waves, but was necessary to produce a MEP from FDI and ADM and allow an assessment of differential modulation of ICI for the three muscles at the two test intensities.
Experimental protocol 4: ICI tested with ‘non-preferred’ coil orientation to compare the effects of selective activation of APB or ADM
To confirm that the differential modulation of ICI could be observed with a different hand muscle targeted for activation, we conducted a fourth series of experiments with six subjects (1 M, 5 F). A second aim of these experiments was to compare the effectiveness of ICI during weak selective vs. non-selective activation of a muscle to the same EMG level. Single or paired-pulse TMS (AP stimulation; conditioning at 0.8 or 0.9 T with 3 ms ISI) were delivered randomly in a block of 20 trials (< 0.2 s−1) while subjects performed four tasks with the aid of visual feedback of EMG and force. These were: (1) complete relaxation of all muscles (rest); (2) isometric thumb abduction of 3 N while attempting to keep FDI and ADM relaxed (APB active [3N]); (3) weak, selective activation of ADM during isometric fifth digit abduction to match the level of ADM EMG seen during task 2 when it was unintentionally co-activated with APB (ADM active [weak vol.]) and (4) activation of ADM during fifth digit abduction of 0.5 N, while attempting to keep APB and FDI relaxed (ADM active [0.5N]). Thus there were eight blocks of trials with different combination of task/conditioning intensity which were performed in pseudo-random order (with the constraint that task 3 followed task 2).
Data analysis
Mean prestimulus EMG (11–50 ms preceding the test pulse) and MEP area were quantified off-line from the digitised averages of rectified EMG for conditioned and unconditioned trials in each block. The size of the conditioned MEP was expressed as a percentage of the unconditioned test MEP to assess the effectiveness of ICI. For protocol 1 data, repeated measures ANOVA was used to assess the effect of muscle (APB, FDI, ADM) and activation (rest, APB active) on the size of the test MEP and the prestimulus EMG. Repeated measures ANOVA was used to assess the effect of activation, and ISI (1–5 ms) on the relative size of the conditioned MEP for each muscle. For protocol 2 and 3 data, repeated measures ANOVA were used to assess the effect of muscle and activation state (rest, APB active, APB activeRI) on the size of the test MEP and the prestimulus EMG. Three-way repeated measures ANOVA was used to assess the effect of muscle, activation, and conditioning intensity (protocol 2) or ISI (protocol 3) on the relative size of the conditioned MEP. For protocol 4 data, two-way repeated measures ANOVA were used to assess the effect of conditioning intensity and task (rest, APB active [3 N], ADM active [weak vol.], ADM active [0.5 N]) on the size of the prestimulus EMG and conditioned MEP for each muscle. A significance level of P < 0.05 was adopted for all comparisons. Post-hoc tests (Tukey's test and Student's t tests with Bonferroni correction) were performed where indicated. Means are reported ±s.e.m. unless otherwise stated.
RESULTS
Protocol 1: PA stimulation
Mean (±s.e.m) TMS thresholds for APB were 55 ± 1 % of maximum stimulator output at rest and 44 ± 1 % with APB active. Test MEP size was similar for all three muscles in the rest condition (Fig. 1A; F2, 28 = 0.5, P > 0.05). With APB active, test MEP size increased significantly compared to rest in all three muscles (Fig. 1A), but the increase was much larger for APB than FDI and ADM.
Figure 1.
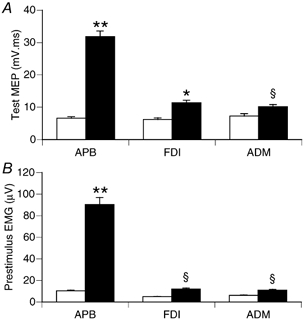
Mean (±s.e.m.) size of the unconditioned test MEP and pre-stimulus EMG in three muscles at rest (□) and with APB active during 3 N thumb abduction (▪). Data from 15 subjects pooled across all ISIs for PA stimulation. A, the test MEP was significantly larger in each muscle when APB was active compared to resting state. B, average rectified EMG levels in the pre-stimulus period were significantly larger for each muscle when APB was active compared to resting state. ANOVA: ** P < 0.0001, *P < 0.001, §P < 0.05.
Prestimulus EMG increased about 9-fold for APB muscle when it was active during the 3 N thumb abduction, compared to rest (Fig. 1B). Despite the instruction to keep FDI and ADM inactive during thumb abduction, there was a small but significant increase in EMG from those muscles when APB was active (Fig. 1B).
A representative example of the effects of conditioning TMS on the MEPs from all three muscles in a single subject is shown in Fig. 2. At rest, conditioning TMS using a 3 ms ISI suppressed the MEP in all three muscles. With APB active, conditioning TMS was clearly less effective at suppressing the APB MEP. Conditioning also had less effect on the MEP in FDI and ADM when APB was active compared to rest.
Figure 2.
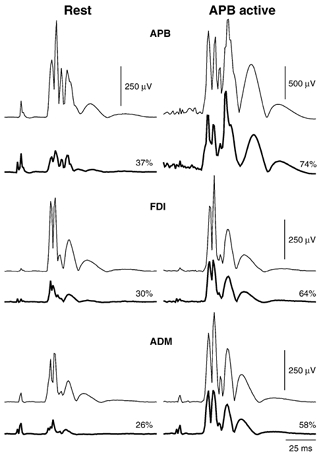
Averaged MEPs in three muscles of one subject following PA TMS at rest (left) and with APB active during 3 N thumb abduction (right). Conditioned (thick lines) and unconditioned test MEPs (thin lines) are shown. At rest, conditioning TMS (3 ms ISI) suppressed the MEP in all three muscles. With APB active, conditioning TMS was less effective at suppressing the MEP in all three muscles. Numbers indicate the size of the conditioned MEP as a percentage of the test response. Note the different amplitude scale for the APB data in the active condition.
Figure 3 summarises the effects of conditioning TMS using PA stimulation for all 15 subjects. There was significantly less suppression of the APB MEP with APB active vs. rest (F1,14 = 33.5, P < 0.0001) (Fig. 3A). The interaction of activation state with ISI was significant (F4, 56 = 8.7, P < 0.0001). Post-hoc tests revealed significantly less suppression of the APB MEP with APB active vs. rest for ISIs of 1 and 3 ms (Bonferroni t test; P < 0.001). Activation of APB reduced the effectiveness of conditioning TMS in both FDI (F1,14 = 5.5, P < 0.05) and ADM (F1,14 = 4.7, P < 0.05), although the effects were weaker than for APB. The interaction with ISI was significant for FDI (F4,56 = 2.6, P < 0.05) but not ADM (F4,6 = 2.3, P > 0.05). For FDI, differences between rest and APB active conditions were significant with an ISI of 3 ms (Bonferroni t test, P < 0.01).
Figure 3.
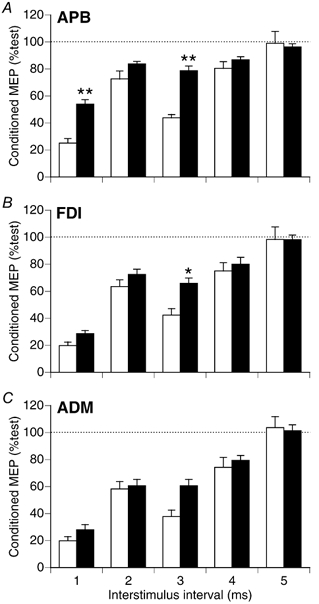
Mean (±s.e.m.) relative size of conditioned MEPs at rest (□)and with APB active (▪) for the three muscles. Data from 15 subjects tested with PA stimulation. Conditioning TMS produced less suppression of the APB MEP with APB active vs. rest (A). **Significantly different, rest vs. APB active (Bonferroni t test; P < 0.0001). Conditioning was also less effective for the FDI (B) and ADM (C) muscles with APB active (ANOVA, P < 0.05). Differences were significant for FDI with an ISI of 3 ms. *Significantly different, rest vs. APB active (Bonferroni t test; P < 0.01).
For five subjects we included two additional trials with APB active in which the test TMS intensity was reduced in order to match the size of the test MEP in active APB with that obtained at rest. Conditioning intensity was unchanged compared to rest, and we used ISIs of 1 and 3 ms. The results with 3 ms ISI are summarised in Fig. 4. Similar results were obtained with ISI of 1 ms. Test TMS intensity was 70 ± 10 % of maximum stimulator output for rest and APB active conditions, and 49 ± 9 % for the APB activeRI condition. With APB active, the test MEP increased fourfold (Fig. 4A; Tukey's test, P < 0.01). With the reduced TMS intensity the test MEP in active APB was similar to that obtained at rest with the stronger test TMS (Tukey's test, P > 0.05). Conditioning TMS was not equally effective for the three conditions (Fig. 4B; F2,8 = 5.4, P < 0.05). Conditioning was less effective with APB active, although differences from rest in this smaller sample were only significant for the Active APBRI condition (Tukey's test, P < 0.05). These data indicate that the effectiveness of conditioning TMS is a function of activation state and test TMS intensity, but not test MEP size per se. This is probably due to a relatively greater contribution of I1 waves to the MEP with weak test TMS and PA stimulation (see Discussion).
Figure 4.
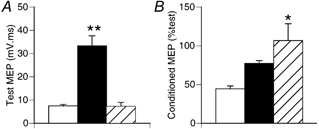
Effect of TMS test intensity on effectiveness of conditioning TMS in active APB muscle. Data from 5 subjects tested with PA stimulation. A, APB test MEP size increased when APB was active (▪), but was not different from rest (□)with the muscle active and reduced test intensity ( , APB activeRI). **Significantly different from rest (Tukey’ s test, P < 0.01). B, the conditioned MEP was larger in the APB activeRI condition compared to rest (* Tukey’ s test, P < 0.05). Effectiveness of conditioning TMS is a function of activation state and test TMS intensity, but not test MEP size, per se.
, APB activeRI). **Significantly different from rest (Tukey’ s test, P < 0.01). B, the conditioned MEP was larger in the APB activeRI condition compared to rest (* Tukey’ s test, P < 0.05). Effectiveness of conditioning TMS is a function of activation state and test TMS intensity, but not test MEP size, per se.
To determine whether the reduced effectiveness of ICI seen in all three muscles with APB active was dependent on neural elements responsible for production of later I waves in corticospinal neurons, we conducted a second series of experiments in eight subjects using a circular coil with the direction of current flow reversed so as to preferentially activate elements producing I3 waves in corticospinal neurons (see Methods).
Assessment of I1 wave contribution to MEPs with different coil orientations
To provide evidence on the presence or absence of I1 waves in the descending corticospinal volleys, for each subject we compared the APB MEP onset latency with PA and AP stimulation, under the three conditions (rest, APB active, APB activeRI). TMS thresholds in APB were higher with AP vs. PA stimulation both at rest (65 ± 4 vs. 52 ± 3 %) and with APB active (54 ± 4 vs. 41 ± 2 %). The strong and weak test intensities adopted were lower with PA stimulation (69 ± 4 and 53 ± 4 %) than AP stimulation (84 ± 5 and 65 ± 4 %). Test MEP size did not differ significantly between rest and APB activeRI conditions for both PA (9.1 ± 1.6 vs. 15.7 ± 2.0 mV ms−1) and AP stimulation (10 ± 0.9 vs. 12 ± 0.9 mV ms−1; Tukey’ s tests, P > 0.05).
The MEP onset latencies for APB are summarised in Table 1. APB test MEP latency varied with coil orientation (F1,14 = 140, P < 0.001) and the combination of muscle activation/TMS intensity (F2,14 = 36.3, P < 0.0001). At rest, MEP onset latency was ≈1.3 ms longer with AP stimulation. This is consistent with the motoneurons being brought to threshold by a later I-wave volley with AP stimulation. With APB active, MEP latencies declined with both AP and PA stimulation (1.4 - 2.2 ms), consistent with the increased alpha motoneuron pool excitability resulting in motoneurons reaching threshold on earlier I-wave volleys. With APB active using AP stimulation there was a large (> 2 ms) slowing of MEP onset latency when the reduced test intensity was used. The mean MEP latency in this active state was 2.6 ms longer than the shortest latency in Table 1 obtained under the optimal conditions for I1 wave influence on MEP latency (i.e. APB active with stronger TMS and PA stimulation). We conclude that I1 waves were weak or negligible with AP stimulation using the reduced test TMS intensity.
Table 1.
Latency of APB test MEP (ms)
| PA stimulation | AP stimulation | |
|---|---|---|
| Rest | 21.9 ± 0.3 | 23.2 ± 0.3§# |
| APB active | 20.5 ± 0.4* | 21.0 ± 0.4** |
| APB activeRT | 21.0 ± 0.5 | 23.1 ± 0.4§§# |
Data are mean ± s.e.m. (n = 8). Significant difference PA vs. AP stimulation (paired t test
P < 0.001
P < 0.02). Significant difference from rest (paired t test
P < 0.001
P < 0.01). Significant difference from APB active with PA stimulation (paired t test
P < 0.001).
Assessment of ICI using AP stimulation
ICI was assessed for the three muscles at rest and with APB active during a 3 N thumb abduction using AP stimulation for both conditioning and test TMS. The test MEP and prestimulus EMG data from the protocol 2 series of experiments with AP stimulation are summarised in Fig. 5. The APB test MEP was ×2.5 larger when APB was active compared to rest when the same TMS intensity was used for both (Fig. 5A; Tukey's test, P < 0.01). The size of the APB test MEP was not significantly different from rest in the active muscle with weaker test TMS (Fig. 5A; Tukey's test, P > 0.05). MEPs were significantly larger in FDI when APB was active (Fig. 5A; F1, 28 = 13.0, P < 0.01), and the increase in ADM just failed to reach significance (Fig. 5A; F1,28 = 5.3, P > 0.05). EMG increased significantly for all three muscles when APB was active (Fig. 5B).
Figure 5.

Mean (±s.e.m) size of the unconditioned test MEP and pre-stimulus EMG in three muscles at rest (□) and with APB active (▪). Data from eight subjects tested with AP stimulation, pooled across blocks of trials with different conditioning intensities. A, test MEP size increased in APB and FDI with APB active vs. rest. ** Tukey's test, P < 0.01; *ANOVA, P < 0.01. Test MEP size in APB was well matched for APB activeRI ( )and rest states. B, Pre-stimulus EMG increased in all three muscles with APB active. ** Tukey’ s test, P < 0.01; * ANOVA, P < 0.02).
)and rest states. B, Pre-stimulus EMG increased in all three muscles with APB active. ** Tukey’ s test, P < 0.01; * ANOVA, P < 0.02).
An example of the effect of conditioning TMS on MEPs from the three muscles is shown for AP stimulation in Fig. 6. ISI was 3 ms and conditioning intensity 0.9 T. When APB was active, conditioning was less effective in suppressing the APB MEP with both the strong and weak test TMS (Fig. 6A). In contrast, conditioning was equally effective in FDI and ADM at rest and with APB active (Fig. 6B and C). Modulation of the effectiveness of conditioning TMS was restricted to corticospinal neurons controlling the muscle targeted for selective activation.
Figure 6.
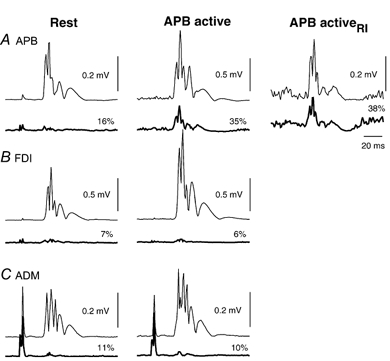
Averaged MEPs from three muscles of one subject at rest (left) and with APB active (middle and right), obtained with AP stimulation. Test TMS intensity was the same for MEPs in left and middle panels, and reduced on the right. Conditioned (thick lines) and unconditioned (thin lines) test MEPs are shown. Numbers indicate the size of the conditioned MEP as a percentage of the test response. At rest, conditioning TMS (0.9 T, 3 ms ISI) suppressed the MEP to a similar extent in all three muscles. With APB active (middle column), conditioning TMS was less effective at suppressing the APB MEP, but MEP suppression in FDI and ADM was similar to that seen at rest. The reduced inhibition of the MEP in active APB was not a function of test MEP size, as conditioning was equally effective in active APB with reduced test intensity (right panel). Note amplitude calibration scale differs for APB data in the middle column.
The results obtained using paired pulse AP stimulation over a range of conditioning intensities and a 3 ms ISI (protocol 2) are summarised for the eight subjects in Fig. 7. Three-way ANOVA revealed a significant effect of muscle (F2,14 = 12.0, P < 0.001), conditioning intensity (F4,28 = 100.0, P < 0.0001), and activation (F1,7 = 22.2, P < 0.01) on the size of the conditioned MEP. Conditioning intensities of 0.7–0.9 T were effective in suppressing the MEP, and remaining statistical analyses were performed on data obtained using these conditioning intensities.
Figure 7.
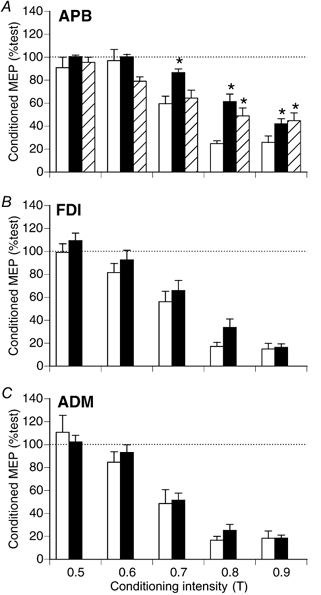
Effect of conditioning intensity on relative size of conditioned MEPs using paired-pulse AP stimulation at rest (□) and with APB active (▪). Test TMS intensity was reduced for the APB activeRI ( ) trials to match the rest and active APB MEP. At this intensity, reliable MEPs were not produced in FDI and ADM, and their data are not shown. Data pooled from eight subjects, ISI 3 ms. There was less suppression of the APB MEP (A) in the active states vs. rest (* Tukey's test, P < 0.01). With APB active, the size of the conditioned MEP did not differ significantly from the resting state in FDI (B) or ADM (D) (ANOVA, P > 0.05).
) trials to match the rest and active APB MEP. At this intensity, reliable MEPs were not produced in FDI and ADM, and their data are not shown. Data pooled from eight subjects, ISI 3 ms. There was less suppression of the APB MEP (A) in the active states vs. rest (* Tukey's test, P < 0.01). With APB active, the size of the conditioned MEP did not differ significantly from the resting state in FDI (B) or ADM (D) (ANOVA, P > 0.05).
For APB (Fig. 7A), there was significantly less suppression of the MEP with APB active compared to rest (F2,14 = 32.9, P < 0.0001), and this effect varied with conditioning intensity (F4,28 = 4.0, P < 0.02). With 0.8 and 0.9 T conditioning, there was less suppression of the APB MEP in the active state, when tested with either strong or weak test intensity (Fig. 7A). With 0.7 T conditioning, differences from rest were significant for the active APB only with the stronger test TMS (Fig. 7A). For FDI (Fig. 7B), there was no significant difference in size of the conditioned MEP between rest and APB active conditions (F1,7 = 4.3, P > 0.05). This was also the case for ADM (Fig. 7C; F1,7 = 0.9, P > 0.05).
At rest, conditioning TMS was equally effective in suppressing MEPs from the three muscles (F2,14 = 3.0, P > 0.05). With APB active, a comparison of the effectiveness of conditioning for the three muscles was performed using the trials with stronger test TMS. With the weaker test pulse reliable MEPs were not obtained in FDI and ADM. With APB active, there was less suppression of the MEP in APB than in FDI (Tukey's test, P < 0.01) and ADM (Tukey's test, P < 0.01).
The results obtained using paired-pulse AP stimulation and different ISIs (protocol 3) are summarised in Fig. 8 for another group of seven subjects. At rest, 0.8 T conditioning was equally effective at suppressing the MEP in all three muscles (F2,12 = 1.06, P > 0.05), however the ISIs were not equally effective (F4,24 = 10.7, P < 0.0001). The 2 ms ISI was less effective than 1, 3 and 4 ms intervals (Tukey's test, P < 0.05). The effect of APB activation and the different test intensities were examined for all three muscles. For APB (Fig. 8A), there was a significant effect of activation/ test intensity (F2,12 = 12.2, P < 0.01), and ISI (F4,24 = 15.0, P < 0.0001), but no significant interaction between them (F8,48 = 1.5, P > 0.05). Post-hoc tests revealed significantly less suppression of the APB MEP with APB active vs. rest with either strong (Tukey's test, P < 0.01) or weak test TMS (Tukey's test, P < 0.05). For FDI (Fig. 8B), there was no significant difference in the amount of MEP suppression by conditioning TMS between rest and the APB active states (Tukey's test, P > 0.05). With APB active, conditioning was more effective in suppressing the FDI MEP with weak test TMS than strong test TMS (Tukey's test, P < 0.05). For ADM (Fig. 8C), there was no significant difference in MEP suppression at rest or with APB active (F2,12 = 1.6, P > 0.05). With APB active and both weak and strong test TMS, there was significantly less suppression of the MEP in APB than in FDI and ADM (Tukey's tests, P < 0.05). Mean (±S.D.) conditioning intensity for this series of experiments was 46 ± 8 % of maximum stimulator output, with test intensities of 87 ± 12 % (strong) and 80 ± 11 % (weak). Test MEP size in FDI and ADM did not differ for rest vs. APB activeRI trials (Tukey's tests, P > 0.05). To summarise the results using ISIs of 1–5 ms, activation of APB reduced the effectiveness of conditioning TMS on the APB MEP, but not for the other two muscles which the subjects attempted to keep relaxed (i.e. the same result observed with AP stimulation in Fig. 7). This effect was less evident with the 2 ms ISI, which was less effective at rest than ISIs of 1, 3 and 4 ms. With ISIs of 4 and 5 ms, there was a tendency for less effective MEP suppression in FDI and ADM with APB active and strong test TMS, which was not seen with APB active and weak test TMS.
Figure 8.
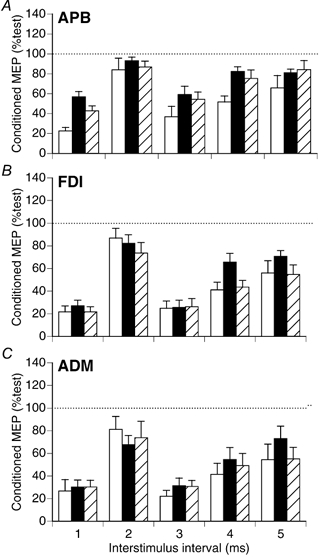
Effect of ISI on relative size of conditioned MEPs using paired-pulse AP stimulation at rest (□) and with APB active (▪). Test TMS intensity was reduced for the APB activeRI trials ( ) to match the FDI and ADM MEPs at rest and with APB active. Data pooled from seven subjects, with conditioning intensity 0.8 T. There was less suppression of the APB MEP in the active states vs. rest (Tukey's tests; APB active, P < 0.01; APB activeRI, P < 0.05). With APB active, the size of the conditioned MEP did not differ significantly from the resting state in FDI (B) or ADM (C) (ANOVA, P > 0.05).
) to match the FDI and ADM MEPs at rest and with APB active. Data pooled from seven subjects, with conditioning intensity 0.8 T. There was less suppression of the APB MEP in the active states vs. rest (Tukey's tests; APB active, P < 0.01; APB activeRI, P < 0.05). With APB active, the size of the conditioned MEP did not differ significantly from the resting state in FDI (B) or ADM (C) (ANOVA, P > 0.05).
Assessment of ICI with selective activation of APB or ADM
In protocol 4 (see Methods), ICI was assessed using AP stimulation for the three muscles at rest and while APB or ADM were targeted for selective activation. The prestimulus EMG (pooled data from six subjects) for the three muscles during the various tasks is summarised in Fig. 9A. Prestimulus EMG increased significantly in APB during 3 N thumb abduction, but did not change significantly in APB when ADM was targeted for activation. FDI EMG did not change significantly with activation of APB or ADM. ADM EMG increased when the 0.5 N fifth digit abduction was performed (Fig. 8A). When APB was activated, there was a small, non-significant increase in ADM EMG, despite the instruction to keep ADM relaxed. Note that the EMG level in ADM when it was unintentionally co-activated with APB (Fig. 8A; APB active [3 N]) was well matched during the weak selective activation trial of ADM (ADM active [weak vol.]). We were thus able to compare effectiveness of ICI for ADM when it was activated to a similar extent during selective voluntary activation vs. non-selective unintentional co-activation.
Figure 9.
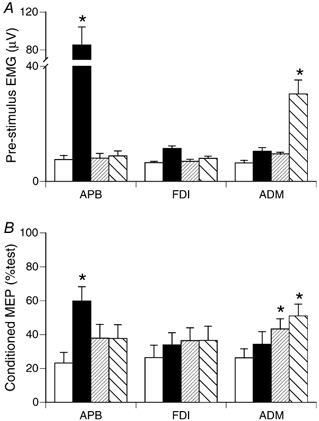
Effect of target muscle and task on the effectiveness of ICI. A, mean pre-stimulus EMG for three muscles under four task conditions (rest (□), APB active [3 N](▪), ADM active [weak vol.]( ), ADM active [0.5 N](▪)). * Significantly different from rest for that muscle (Tukey's test, P < 0.01). ADM EMG did not differ significantly for trials in which it was unintentionally active (APB active [3 N]) and with matching weak selective activation (ADM active [weak vol.]). B, size of conditioned MEPs during these four tasks. * Significantly different from rest (Tukey's test, P < 0.01). Voluntary selective activation significantly reduced the effectiveness of ICI for the muscle targeted for activation, but not for muscles required to be inactive during the task. For ADM, ICI was more effectively suppressed when it was activated selectively, compared with unintentional non-selective activation to the same EMG level.
), ADM active [0.5 N](▪)). * Significantly different from rest for that muscle (Tukey's test, P < 0.01). ADM EMG did not differ significantly for trials in which it was unintentionally active (APB active [3 N]) and with matching weak selective activation (ADM active [weak vol.]). B, size of conditioned MEPs during these four tasks. * Significantly different from rest (Tukey's test, P < 0.01). Voluntary selective activation significantly reduced the effectiveness of ICI for the muscle targeted for activation, but not for muscles required to be inactive during the task. For ADM, ICI was more effectively suppressed when it was activated selectively, compared with unintentional non-selective activation to the same EMG level.
The effectiveness of ICI for the three muscles during these tasks is summarised in Fig. 8B. Data are pooled for both 0.8 and 0.9 T conditioning intensities, as a similar pattern was seen with each, and none of the interactions of conditioning intensity with task were significant when tested with ANOVA. Activation of APB reduced the effectiveness of ICI for APB, but did not significantly suppress ICI for FDI or ADM; i.e. the same result obtained in protocols 2 and 3 (cf. Fig. 7 and Fig. 8). Activation of ADM during 0.5 N digit five abduction significantly suppressed ICI for ADM, but not APB or FDI (Fig. 8B). These data show that differential modulation of ICI can be observed with either APB or ADM as the muscle targeted for activation. ICI effectiveness for ADM was significantly reduced from resting level during weak selective activation of ADM (Fig. 8B; ADM active [weak vol.]), but not with non-selective unintentional co-activation of ADM to the same level of EMG during the 3 N thumb abduction (Fig. 8B; APB active [3 N]). Suppression of ICI is therefore influenced by the task and not simply by the presence of background EMG in a muscle. ICI suppression is more effective when the muscle is targeted for selective activation by voluntary commands.
DISCUSSION
There are two key findings in the present study. First, the reduced ability of paired-pulse TMS to suppress the MEP in an active muscle is due, at least in part, to effects on late I waves. It was evident with a weak AP test TMS pulse that elicited negligible I1 waves in corticospinal neurons. ICI circuits activated by TMS, which exert their effects on late I waves but do not affect I1 waves (Nakamura et al. 1997; Di Lazzaro et al. 1998b; Hanajima et al. 1998), are strongly implicated in this effect. Second, with AP stimulation, paired-pulse inhibition was not significantly altered for corticospinal neurons controlling other muscles of the same hand which were required to be inactive during the selective activation task. This differential modulation of the effectiveness of paired-pulse inhibition during selective activation was not seen with PA stimulation. We consider that a reason for the disparate results with PA and AP stimulation is that the MEP produced by PA stimulation (which preferentially produces I1 waves) is less susceptible to ICI than the MEP produced by AP stimulation (which preferentially produces I3 waves). The observations with AP stimulation suggest that selective activation of a hand muscle is accompanied by a selective suppression of ICI effects on the corticospinal neurons controlling that muscle. The pattern of differential modulation of ICI effectiveness with voluntary activation suggests that the ICI circuits assist the corticospinal system in producing fractionated activity of intrinsic hand muscles.
Mechanisms for the reduced effectiveness of ICI with voluntary activation
The weak conditioning TMS used in the present study does not produce descending volleys in the corticospinal tract (Di Lazzaro et al. 1998b) or alter spinal H reflexes (Kujirai et al. 1993), so the effects of conditioning are mediated at the cortical level. ICI neurons do not inhibit corticospinal neurons directly, but rather act indirectly by inhibiting interneurons responsible for the later I waves of excitation in corticospinal cells (Nakamura et al. 1997; Di Lazzaro et al. 1998b; Hanajima et al. 1998; Trompetto et al. 1999). Direct recordings of corticospinal volleys from the epidural space in man (Nakamura et al. 1997; Di Lazzaro et al. 1998b) and single motor unit recordings (Hanajima et al. 1998) show that the I1 wave is not influenced by the ICI circuit.
With PA stimulation, I1 and D waves are preferentially activated at low intensities and later I waves recruited at higher intensities (Day et al. 1989; Di Lazzaro et al. 2002). Because the I1 wave is not affected by ICI, it has been suggested that the reduction in ICI effectiveness seen when a muscle is activated (Ridding et al. 1995c) could be due to an increased contribution of I1 waves to the MEP in the active state (Hanajima et al. 1998). Our data are consistent with this. With PA stimulation ICI effectiveness was reduced with APB active (Fig. 3), and reduced still further when tested in the active state with weaker test TMS (Fig. 4). The weaker test stimulus with PA stimulation favours a relatively greater contribution of I1 waves to the descending volley due to their preferential activation with this direction of current flow. All I waves are facilitated with voluntary activation (Di Lazzaro et al. 1998a), and this combined with the increased motoneuron excitability means that temporal summation of EPSPs from multiple I waves is no longer required to bring motoneurons to threshold. The result is that under active conditions the (larger) I1 volley has a relatively greater influence on the MEP than the later I waves. As the relative contribution of I1 waves to the descending volleys increases (with weaker PA stimulation) and the influence of I1 waves on the production of the MEP increases with excitation of the motoneuron pool, there is a reduction in the ability of ICI to suppress the MEP in the active state.
To determine whether factors associated with I1 waves were sufficient to explain the reduced effectiveness of ICI during muscle activation we conducted the second series of experiments in which we varied the intensity of the test TMS, and compared results obtained with PA and AP stimulation. These manipulations altered the balance of the various I waves in the descending volleys elicited by the test stimulus. AP stimulation was used to test ICI because with voluntary activation of a hand muscle, weak suprathreshold AP TMS reliably produces descending volleys with I3 waves but lacking earlier waves (Day et al. 1989; Sakai et al. 1997). We did not record single motor unit responses to TMS, as was done in these earlier studies to unambiguously detect the I waves in the descending volleys. However, comparisons of test MEP latencies (Table 1) support the view that negligible I1 waves were elicited in descending volleys to APB motoneurons when the muscle was active and the weak AP test pulse was used in protocol 2. The mean MEP latency in this condition was 2.6 ms longer than that obtained with optimal conditions for I1 wave influence on MEP latency (APB active, strong PA stimulation). This corresponds with the ≈3 ms difference between I1 and I3 waves in human corticospinal volleys (Nakamura et al. 1997). It is unlikely to reflect the difference between D and I2 waves, as it is difficult to elicit I2 waves with AP stimulation (Day et al. 1989; Sakai et al. 1997). In contrast, MEP latencies were only slightly longer with AP vs. PA stimulation when APB was active and the stronger test intensity was used (Table 1). This suggests that some I1 waves were evoked in the descending corticospinal volleys with AP stimulation at the stronger intensities.
ICI can be demonstrated with paired-pulse AP stimulation with a circular coil (Trompetto et al. 1999) and by varying the direction of conditioning and test TMS several studies have concluded that ICI circuits are insensitive to the direction of current flow (Ziemann et al. 1996b; Hanajima et al. 1998). With paired-pulse AP stimulation there was a clearly reduced effectiveness of ICI in the active APB compared to rest for conditioning intensities of 0.8 and 0.9 T, for both weak and strong test TMS (Fig. 7A). Note that with AP stimulation, weaker test TMS resulted generally in more effective conditioning in active APB than that seen with the stronger test stimulus (Fig. 7). With PA stimulation, weaker test TMS resulted in less effective conditioning (Fig. 4). The divergent effects of test intensity on ICI effectiveness with AP and PA stimulation are consistent with the preferential activation of I3 waves (which can be suppressed by ICI) with weak AP stimulation, and preferential activation of I1 waves (which are insensitive to ICI) with weak PA stimulation. With the weak AP test pulse (APB activeRI condition in Fig. 7) the reduced effectiveness of paired-pulse inhibition in the active APB cannot be attributed to I1 waves, but must be due to some other mechanism. ICI circuits in motor cortex are strongly implicated in this.
Differential modulation of ICI for corticospinal neurons controlling the muscle targeted for activation
The influence of ICI circuits on the size of the MEP increases as the test pulse favours I3 waves at the expense of I1 waves. It follows that weak AP stimulation provides the most sensitive assessment of changes in ICI with voluntary activation. With AP stimulation, conditioning suppressed the MEP with equal effectiveness in all three muscles at rest (Fig. 7 and Fig. 8). Even with the stronger AP test intensity, which may have produced I1 waves with APB active (Table 1), there was still a clear suppression of ICI effectiveness with APB active compared to rest that was restricted to APB. ICI effectiveness was virtually unchanged in ADM at rest and with APB active (Fig. 7 and Fig. 8). FDI showed a trend for reduced effectiveness of ICI with APB active in some trials with stronger test TMS, but this was not significant (Fig. 7 and Fig. 8). The slight unintentional activation of FDI and ADM when APB was active may partly explain this effect via an increased I1 wave contribution to the MEP. This would have a greater impact with the larger I1 wave component of PA stimulation, and probably explains why paired-pulse inhibition was also suppressed for FDI and ADM muscles when APB was active and PA stimulation was used (Fig. 3).
Differential modulation of ICI effects were seen with APB activation across a range of ISIs with AP stimulation (Fig. 8). The clearest differential effects on ICI with selective activation of APB were seen with ISIs of 1, 3 and 4 ms (Fig. 8). Conditioning with each of these ISIs suppresses later I waves, with variable effects on earlier I waves depending on ISI (Di Lazzaro et al. 1998b). In this series, with 4 and 5 ms ISIs the differential modulation was more evident with weaker test TMS (favouring larger I3 than I1 waves in the descending volley), whereas similar results were obtained with weak and strong test TMS for ISIs 1–3 ms. The explanation for this is not clear. The weak AP test stimulus in this series was stronger than that used in protocol 2, and probably still contained some I1 waves based on test MEP latency comparisons (data not shown). This was unavoidable, in order to obtain a MEP in FDI and ADM for comparison with APB when APB was active.
Conditioning with a 2 ms ISI was less effective for MEP suppression at rest than ISIs of 1 and 3 ms, with both PA and AP paired-pulse stimulation. Similar trends have been observed in other studies with PA (Kujirai et al. 1993; Ridding et al. 1995; Fischer et al. 2002) and AP (Hanajima et al. 1998; Trompetto et al. 1999) paired-pulse TMS. Fischer et al. (2002) used a threshold-tracking technique to study ICI and found optimal ISIs for inhibition were 1 ms and 2.5 ms, with a much weaker inhibition at 1.6 ms ISI. The reason for the reduced inhibition between 1 and 2.5 ms is not clear, but may involve a complex interaction between intracortical inhibition and facilitation (Fischer et al. 2002). They concluded that inhibition with ISI of 1 ms is due in part to refractoriness of cortical axons activated by the first stimulus, whereas inhibition with 2.5 ms ISI appears to reflect the GABAergic inhibitory system better in motor cortex. Our observation that with a 1 ms ISI and PA stimulation the conditioning TMS became less effective with a weaker test stimulus and APB active argues against axonal refractoriness of cortical elements as a principal cause of the MEP suppression. If this were the case, it should be overcome with a stronger, not weaker, test stimulus. Because the 2 ms ISI produced little MEP suppression at rest with 0.8 T conditioning and AP stimulation, it was not suitable for studying the modulation of ICI with voluntary activation in the present study. It is possible, however, that with a stronger conditioning stimulus we may have seen a differential modulation of ICI with a 2 ms ISI as well.
Two additional conclusions can be drawn from the fourth series of experiments (Fig. 9). First, ICI is differentially suppressed for corticospinal neurons of the muscle targeted for activation when either APB or ADM is the target muscle. The effect is therefore not unique for corticospinal control of thumb muscles. Second, Fig. 9 shows that ICI effectiveness is different when ADM is activated to the same EMG level under different task conditions. ICI effectiveness was significantly reduced from resting level during selective activation of ADM, but not with activation of ADM to the same EMG level when the subject was actively trying to prevent its co-activation with APB. This observation suggests that the changes in ICI are not simply related to altered afferent feedback from the contracting muscle, although the extent of a contribution from this source could not be established in the present study. ICI is known to be influenced by peripheral afferents (Ridding & Rothwell, 1999), but it can be modified without changes in afferent feedback, for example before a movement begins (Reynolds & Ashby, 1999) and by motor imagery of sequential finger movements (Abbruzzese et al. 1999). Modulation of ICI therefore seems to be incorporated in the plan for movement. Our ADM data are consistent with this. Suppression of ICI is influenced by the voluntary nature of the task, and is more effective when the muscle is targeted for selective activation by voluntary commands.
Mechanism for the differential modulation of ICI
Possible mechanisms for a differential modulation of ICI effects include reduced excitability of ICI neurons, increased presynaptic inhibition of axon terminals of ICI neurons (cf. Werhahn et al. 1999; Sanger et al. 2001), and increased excitability of a facilitatory process that could counteract the ICI effects on corticospinal neurons. An interaction of two processes seems to be responsible for the selective suppression of ICI with APB active. The process responsible for the differential modulation of ICI during voluntary activation does not appear to involve altered excitability of ICI neurons to TMS, as differences between APB activeRI and rest were not seen with 0.7 T conditioning (Fig. 7). The emergence of differences at higher conditioning intensities of 0.8 and 0.9 T suggest a second process is involved in the modulation of ICI with voluntary activation, which has a higher threshold for activation by TMS.
With ISIs between 1.0–1.5, 2.3–3.0 and 4.1–5.0 ms, MEP facilitation is seen when a supra-threshold first stimulus (S1) is followed by a sub-threshold to threshold second stimulus (S2) (Tokimura et al. 1996; Ziemann et al. 1998b). This phenomenon has been termed I-wave facilitation. This facilitation represents an interaction in the cortex of waves of synaptic excitation which are normally involved in I-wave generation (Tokimura et al. 1996; Di Lazzaro et al. 1999). I-wave facilitation does not seem likely to explain the present results across a range of ISIs because sub-threshold conditioning and suprathreshold test TMS as used in the present study does not favour an important contribution from I-wave facilitation to the size of MEPs (cf. Di Lazzaro et al. 1999), and I-wave facilitation is negligible when tested with a 3 ms ISI in active muscles (Ziemann et al. 1998b).
A sub-threshold conditioning stimulus can activate an intracortical facilitatory (ICF) circuit using glutamate as a neurotransmitter (Liepert et al. 1997), and distinct from the circuits producing ICI and I-wave facilitation (Ziemann et al. 1996b; Chen et al. 1998; Hanajima et al. 1998; Chen & Garg, 2000). ICF opposes the effects of ICI at rest, however ICF is reduced with voluntary activation (Ridding et al. 1995c; Ziemann et al. 1996b) and thus cannot contribute to the reduced effectiveness of ICI with activation of APB.
There is another intracortical inhibitory circuit with a higher threshold for activation and longer acting inhibition which may explain our findings as it suppresses effects of the short duration ICI tested with ISIs of 1–5 ms. The TMS threshold for long duration (up to 150 ms) ICI (which is mediated by GABAB receptors; Nakamura et al. 1997; Werhahn et al. 1999) is higher than that for short duration (up to 20 ms) ICI which is mediated by GABAA receptors (Ziemann et al. 1996a,b, 1998a; Hanajima et al. 1998). The neurons mediating the two forms of ICI appear to be distinct (Sanger et al. 2001). Axon terminals of neurons mediating short duration ICI are subject to GABAB presynaptic inhibition (Werhahn et al. 1999) by the higher threshold neurons mediating long duration ICI (Sanger et al. 2001). It is possible that higher-threshold neurons mediating long duration ICI produce selective suppression of short duration ICI with voluntary activation by increasing presynaptic inhibition at terminals of neurons producing short duration ICI. A subset of these higher threshold inhibitory neurons could be engaged in the task by the premotor commands, to allow selective suppression of ICI onto excitatory interneurons synapsing with corticospinal neurons controlling the muscle(s) targeted for activation. A task-specific, selective increase in excitability of the higher threshold inhibitory neurons would allow them to be more strongly activated by sub-threshold (0.8–0.9 T) conditioning TMS with APB active, thus reducing the effectiveness of ICI in APB from that seen at rest. The activation of higher threshold neurons mediating presynaptic inhibition of ICI terminals could also explain the observation that in all three muscles at rest, ICI effectiveness did not increase with 0.9 T conditioning compared to 0.8 T (Fig. 7).
Functional significance of ICI circuits for fractionation of muscle activity
The present study provides the first evidence that ICI is differentially modulated during the selective activation of one muscle of a pair of close functional synergists. The findings extend the previous observations of Ridding et al. (1995c) who showed that ICI of relaxed FDI was not influenced by contraction of a remote muscle (biceps). These authors did not demonstrate a differential modulation of ICI with selective activation of one muscle of the pair as they did not present ICI data for the active biceps. Indirect brainstem pathways may play a prominent role in activation of biceps, which has weak direct corticospinal input. Different mechanisms may be important for selective activation of biceps and/or FDI than when the task calls for selective activation of intrinsic hand muscles which have strong direct corticospinal input, including branched CM axons to synergist muscles.
While changes in the contribution of I1 waves to the production of the MEP in the active state may be important for the reduced effectiveness of ICI when tested with PA stimulation, the results with AP stimulation show that mechanisms other than the I1 inputs contribute to the reduction of ICI effectiveness with voluntary activation. Our results suggest that GABAergic inhibitory neurons assist in the selective activation of muscles by focussing the premotor excitatory commands to corticospinal neurons controlling muscles required to be active in the task, and maintaining inhibition of excitatory interneurons acting on corticospinal neurons controlling muscles required to be inactive during the task. This is consistent with observations that application of drugs which alter GABA neurotransmission to the motor cortex in the monkey degrades the independence of finger movements (Schieber & Poliakov, 1998), disrupts task-specificity of pyramidal neurons (Matsumura et al. 1992), and produces excessive co-activation of agonist and antagonist muscles (Matsumura et al. 1991). Such a role for ICI neurons in normal motor control may explain the involuntary overflow of excitation to inappropriate muscles in patients with focal, task-specific dystonia, in whom the ICI circuits are less effective (Ridding et al. 1995b) and do not show task-specific modulation (Bütefisch et al. 2000). We found no evidence for an increase in ICI during the task for corticospinal neurons controlling muscles required to be inactive (Fig. 7 and Fig. 8). This is in agreement with findings of Reynolds & Ashby (1999), but not with those of Liepert et al. (1998) who tested ICI with hand muscles at rest after a selective muscle activation task. It is unlikely that we failed to see such an effect due to saturation of the inhibitory responses, as we tested over a wide range of conditioning intensities.
Previous studies have investigated task-dependent modulation of ICI using PA stimulation. AP stimulation has some advantages for further investigating the mechanism for the selective modulation of ICI effectiveness with voluntary commands, and abnormalities in the operation of ICI circuits in patients with neurological disorders. This is because a test MEP produced with minimal I1-wave involvement is most sensitive to the effects of ICI circuits. It is possible, for example, that in some neurological conditions the reduced effectiveness of paired-pulse TMS is due to alterations in the I1 wave contribution to the MEP, rather than an alteration in the ICI circuit per se. This could be addressed by comparing paired-pulse inhibition with both PA and AP TMS.
Acknowledgments
M. Z. is a postgraduate student supported by Adelaide Scholarship International (ASI), and the work described herein forms part of her PhD studies. The authors acknowledge the valuable statistical advice of Dr Michael Garry, and his helpful comments on the manuscript. This project was funded by a grant (157975) from the NHMRC of Australia.
REFERENCES
- Abbruzzese G, Assini A, Buccolieri A, Marchese R, Trompetto C. Changes of intracortical inhibition during motor imagery in human subjects. Neurosci Lett. 1999;263:113–116. doi: 10.1016/s0304-3940(99)00120-2. [DOI] [PubMed] [Google Scholar]
- Bütefisch CM, Boroojerdi B, Battaglia F, Chen R, Hallett M. Task dependent intracortical inhibition is impaired in patients with task specific dystonia. Mov Disord. 2000;15(Suppl. 3):153. doi: 10.1002/mds.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys EJ, Lemon RN, Mantel GWH, Muir RB. Selective facilitation of different hand muscles by single corticospinal neurones in the conscious monkey. J Physiol. 1986;381:529–549. doi: 10.1113/jphysiol.1986.sp016342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Garg R. Facilitatory I wave interaction in proximal arm and lower limb muscle representations of the human motor cortex. J Neurophysiol. 2000;83:1426–1434. doi: 10.1152/jn.2000.83.3.1426. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Bütefisch CM, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Clough JFM, Kernell D, Phillips CG. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968;198:145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Dressler D, Marten S, de Noordhout A, Marsden CD, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Insola A, Mazzone P, Tonali P, Rothwell JC. Descending volleys evoked by transcranial magnetic stimulation of the brain in conscious humans: effects of coil shape. Clin Neurophysiol. 2002;113:114–119. doi: 10.1016/s1388-2457(01)00696-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 1998a;508:625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonalli P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998b;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P. Intracortical origin of the short latency facilitation produced by pairs of threshold magnetic stimuli applied to the human motor cortex. Exp Brain Res. 1999;129:494–499. doi: 10.1007/s002210050919. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Ogata K, Kanazawa I. Ipsilateral cortico-cortical inhibition of the motor cortex in various neurological disorders. J Neurol Sci. 1996;140:109–116. doi: 10.1016/0022-510x(96)00100-1. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel JC, Smith MJ, Wassermann EM. Letter to the editor: A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2000;112:720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Classen J, Cohen LG, Hallett M. Task-dependent changes of intracortical inhibition. Exp Brain Res. 1998;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- Liepert J, Schwenkreis P, Tegenthoff M, Malin JP. The glutamate antagonist riluzole suppresses intracortical facilitation. J Neural Transm. 1997;104:1207–1214. doi: 10.1007/BF01294721. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Sawaguchi T, Kubota K. GABAergic inhibition of neuronal activity in the primate motor and premotor cortex during voluntary movement. J Neurophysiol. 1992;68:692–702. doi: 10.1152/jn.1992.68.3.692. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Sawaguchi T, Oishi K, Ueki K, Kubota K. Behavioral deficits induced by local injection of bicuculline and muscimol into the primate motor cortex. J Neurophysiol. 1991;65:1542–1553. doi: 10.1152/jn.1991.65.6.1542. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom MA, Butler SL. Reduced intracortical inhibition and facilitation of corticospinal neurons in musicians. Exp Brain Res. 2002;144:336–342. doi: 10.1007/s00221-002-1051-7. [DOI] [PubMed] [Google Scholar]
- Reynolds C, Ashby P. Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology. 1999;53:730–735. doi: 10.1212/wnl.53.4.730. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Inzelberg R, Rothwell JC. Changes in excitability of motor cortical circuitry in patients with Parkinson's disease. Ann Neurol. 1995a;37:181–188. doi: 10.1002/ana.410370208. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Afferent input and cortical organisation: a study with magnetic stimulation. Exp Brain Res. 1999;126:536–544. doi: 10.1007/s002210050762. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Sheean G, Rothwell JC, Inzelberg R, Kujirai T. Changes in the balance between motor cortical excitation and inhibition in focal, task-specific dystonia. J Neurol Neurosurg Psychiatry. 1995b;59:493–498. doi: 10.1136/jnnp.59.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Taylor JL, Rothwell JC. The effect of voluntary contraction on cortico-cortical inhibition in human motor cortex. J Physiol. 1995c;487:541–548. doi: 10.1113/jphysiol.1995.sp020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res. 1997;113:24–32. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Poliakov AV. Partial inactivation of the primary motor cortex hand area: effects on individuated finger movements. J Neurosci. 1998;18:9038–9054. doi: 10.1523/JNEUROSCI.18-21-09038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol. 1996;101:263–272. doi: 10.1016/0924-980x(96)95664-7. [DOI] [PubMed] [Google Scholar]
- Trompetto C, Assini A, Buccolieri A, Marchese R, Abbruzzese G. Intracortical inhibition after paired transcranial magnetic stimulation depends on the current flow direction. Clin Neurophysiol. 1999;110:1106–1110. doi: 10.1016/s1388-2457(99)00043-7. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998a;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996a;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996b;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998b;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


