Abstract
Intramuscular triacylglycerol is an important energy store and is also related to insulin resistance. The mobilization of fatty acids from this pool is probably regulated by hormone-sensitive lipase (HSL), which has recently been shown to exist in muscle and to be activated by both adrenaline and contractions. Adrenaline acts via cAMP-dependent protein kinase (PKA). The signalling mediating the effect of contractions is unknown and was explored in this study. Incubated soleus muscles from 70 g male rats were electrically stimulated to perform repeated tetanic contractions for 5 min. The contraction-induced activation of HSL was abolished by the protein kinase C (PKC) inhibitors bisindolylmaleimide I and calphostin C and reduced 50 % by the mitogen-activated protein kinase kinase (MEK) inhibitor U0126, which also completely blocked extracellular signal-regulated kinase (ERK) 1 and 2 phosphorylation. None of the inhibitors reduced adrenaline-induced HSL activation in soleus muscle. Both phorbol-12-myristate-13-acetate (PMA), which activates PKC and, in turn, ERK, and caffeine, which increases intracellular Ca2+ without eliciting contraction, increased HSL activity. Activated ERK increased HSL activity in supernatant from basal but not from electrically stimulated muscle. In conclusion, in muscle, PKC can stimulate HSL through ERK. Contractions and adrenaline enhance muscle HSL activity by different signalling mechanisms. The effect of contractions is mediated by PKC, at least partly via the ERK pathway.
Triacylglycerol contained in lipid droplets in the cytoplasm of skeletal muscle cells represents an important energy store, which can be mobilized by catecholamines and exercise (Carlson et al. 1971; Reitman et al. 1973; Froberg et al. 1975; Abumrad et al. 1980; Oscai et al. 1990; Schick et al. 1993; Van der Vusse & Reneman, 1996). However, the enzymatic regulation of muscle triacylglycerol breakdown has until recently been poorly understood. Then the presence of the neutral lipase hormone-sensitive lipase (HSL), which controls triacylglycerol breakdown in adipose tissue, was demonstrated by Western blotting in all muscle fibre types (Langfort et al. 1999). Furthermore, it was shown that analysed under conditions optimal for HSL, neutral lipase activity in muscle can be increased by adrenaline as well as by muscle contractions, and these increases were abolished by the presence of anti-HSL anti-body during analysis (Langfort et al. 1999, 2000). Moreover, immunoprecipitation with affinity-purified anti-HSL antibody caused similar reductions in muscle HSL protein concentration and in measured neutral lipase responses to contractions (Langfort et al. 2000).
Adrenaline has been shown to stimulate HSL in muscle via β-adrenergic activation of PKA (Langfort et al. 1999). From findings in adipocytes it is likely that PKA phosphorylates HSL at residues Ser563, Ser659 and Ser660, the latter two being the major activity controlling sites (Anthonsen et al. 1998). Contractions probably also enhance muscle-HSL activity by phosphorylation, because the contraction-induced increase in HSL activity is increased by the protein phosphatase inhibitor okadaic acid and reversed by alkaline phosphatase (Langfort et al. 2000). However, the signalling mechanisms mediating such an effect of contractions are not known. The fact that the effects of adrenaline and contractions on HSL activity in muscle are partially additive (Langfort et al. 2003) may suggest that the two stimuli activate different kinases, which, in turn, phosphorylate HSL at different sites. Because an increase in intracellular Ca2+ is characteristic of contractions, and because HSL activity increases at the onset of contractions (Langfort et al. 2000), it is tempting to speculate that contraction-induced HSL activation is mediated by a Ca2+-dependent protein kinase. Ca2+/calmodulin-dependent protein kinase II has been shown to inhibit rather than enhance HSL from adipose tissue (Garton et al. 1989), a fact that makes Ca2+-activated protein kinase C (PKC) a more likely mediator of contraction-induced HSL activation.
Because the regulation of HSL in skeletal muscle is probably of physiological as well as pathophysiological importance, we wanted to unravel the signalling mechanisms involved in contraction-induced HSL activation. While we were doing this by applying PKC inhibitors in incubated, electrically stimulated rat muscle, it was reported that, in adipocytes, PKC can stimulate lipolysis through a mitogen-activated protein kinase (MAPK)/ERK-mediated phosphorylation of HSL (Greenberg et al. 2001). Interestingly, this novel mechanism involves a new phosphorylation site, Ser600, located in close proximity to the already known phosphorylation sites within the regulatory module of HSL (Anthonsen et al. 1998; Greenberg et al. 2001). In the present study, we found that in muscle also, PKC can stimulate HSL through ERK. Furthermore, the contraction-induced HSL activation is mediated by PKC, at least partly via the ERK pathway. Finally, in adipocytes, β-adrenergic activation of HSL is in part mediated via ERK (Greenberg et al. 2001), while in muscle, activation of ERK does not contribute to adrenaline-induced stimulation of HSL.
METHODS
Muscle incubation and stimulation
The experiments were approved by the Animal Research Committee of the Danish Ministry of Justice. Male Wistar rats weighing about 70 g were obtained from Charles River Laboratories (Sulzfeld, Germany). Young rats were used to avoid adipocytes interlaced between muscle fibres (Langfort et al. 1999). The expression of the various PKC isoforms in muscle has been shown to be identical in young and old rats (Qu et al. 1999). To assure recovery from any stress due to transport from Germany, animals were housed for at least 4 days in our animal facilities at the Panum Institute before they were used for experiments. Rats were kept in a constant light-dark cycle (12 h each) and received a standard rat chow and water ad libitum. The rats were anaesthetized by intraperitoneal injection of sodium pentobarbital (Pharmacy of Rigshospitalet, Copenhagen, Denmark) (5 mg (100 g body weight)−1). The aorta was cannulated and the hindquarters were perfused for 1 min (25 ml min−1) with Krebs-Henseleit buffer gassed with 95 % O2 and 5 % CO2 and containing 8 mm glucose, 1 mm pyruvate and 0.2 % bovine serum albumin (BSA) at pH 7.4. Soleus muscles (about 29 mg) were gently dissected free with intact tendons and preincubated for 45 min in test tubes with 10 ml incubation medium identical to the perfusion medium and continuously gassed with 95 % O2 and 5 % CO2 at 29 °C.
The PKC inhibitors used were bisindolylmaleimide I (Calbiochem, La Jolla, CA, USA), final concentration 1 μm; and calphostin C (Alexis, San Diego, CA, USA), final concentration 2.5 μm. They block the ATP- and the diacylglycerol/phorbol ester binding site, respectively (Kobayashi et al. 1989; Toullec et al. 1991). The MEK inhibitor used was U0126 (Calbiochem), final concentration 10 μm (Favata et al. 1998). After preincubation, in experiments with the PKC inhibitors or the MEK inhibitor, soleus muscles were transferred to incubation medium containing inhibitor dissolved in dimethyl sulphoxide (DMSO; final concentration never exceeding 0.1 % w/v; Sigma Aldrich, St Louis, MO, USA) and incubated for 30 min before stimulation with muscle contractions or adrenaline. The inhibitors were also present during stimulation. In experiments with PKC and MEK inhibitors or phorbol-12-myristate-13-acetate (PMA) (see below), control soleus muscles were incubated with the same concentration of DMSO as used in media with inhibitors or PMA.
In experiments with muscle contractions and corresponding controls (Figs 1, 2, 3 and 7) the muscles were fixed in the vertical position at resting length by small clips attached to the tendons. The upper clip was connected to a strain gauge mounted on a steel rod to which the lower clip was also fixed. The rod could be lowered into a vial with incubation medium standing in a thermostat. The experimental set-up is shown in Langfort et al. (2003). Subsequently, muscles either remained resting or were stimulated electrically through electrodes in both ends (the lower clip and a platinum ring electrode (anode)) to perform repeated tetanic contractions (200 ms trains of 100 Hz, impulse duration 0.2 ms, delivered every second at 25 V) for 5 min. For each tetanus the tension-time area under the curve and above resting tension was determined by software adding 500 tension values (Ihlemann et al. 1999b). Total force development was the sum of force developed during all tetani in 5 min of stimulation. Calibration was performed with a 10 g weight (≈0.1 N) hanging from the force transducer in a 200 ms period. In experiments with activators and inhibitors and without contractions muscles were not fixed (Figs 4–6). Basal HSL activities were identical whether muscles were fixed (Figs 1, 2, 3 and 7) or not (Figs 4–6). In experiments with adrenaline (Pharmacy of Rigshospitalet, Copenhagen, Denmark) and corresponding control experiments, after 30 min incubation with or without inhibitor, the soleus muscles were moved to a new test tube with or without 5.5 μm adrenaline, still with or without inhibitor. In experiments with PMA, dissolved in DMSO (Calbiochem), final concentration 5 μm, or caffeine (Sigma Aldrich), final concentration 3 mm, soleus muscles were incubated for 30 min after the preincubation period.
Figure 1. Bisindolylmaleimide I selectively inhibits the contraction-induced increase in HSL (TG) activity.
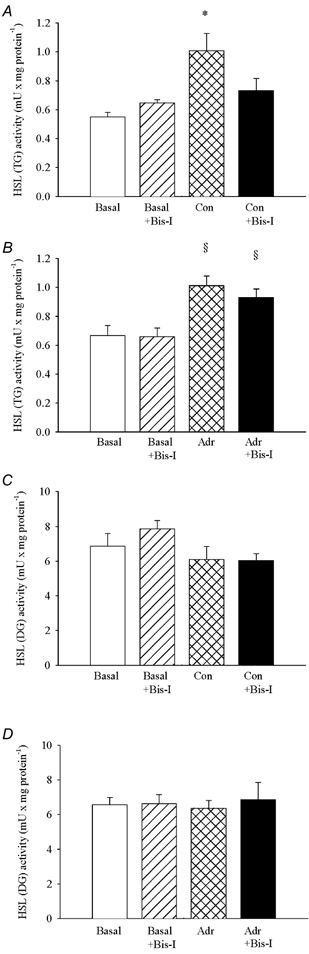
Soleus muscles were incubated in presence (Bis-I) or absence of 1 μm bisindolylmaleimide I for 35 min. Muscles either rested (Basal) or were stimulated electrically to perform repeated tetanic contractions (Con) or stimulated with 5.5 μm adrenaline (Adr) for the last 5 min during incubation. The two soleus muscles from a given rat were both stimulated or rested, and one was incubated with and the other without 1 μm bisindolylmaleimide. Muscles were freeze-clamped and analysed for HSL (TG) (A and B) and HSL (DG) (C and D) activities, which are measures of the activated form of HSL and of total enzyme concentration, respectively. Three muscles were used per analysis. Values are means ± s.e.m., n = 6 analyses in each group. * Effect of contractions (P < 0.05); § effect of adrenaline (P < 0.05).
Figure 2. Calphostin C selectively inhibits the contraction-induced increase in HSL (TG) activity.
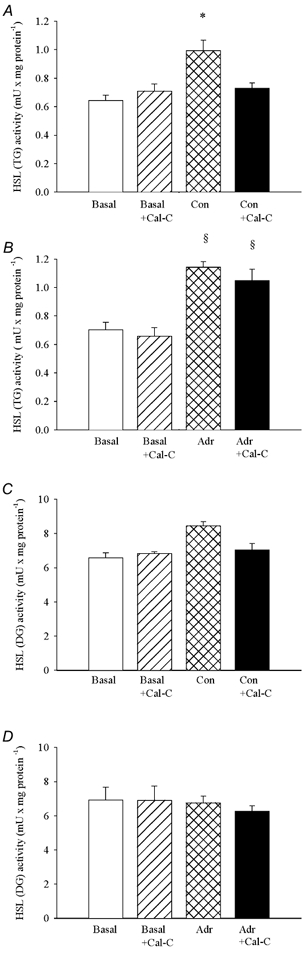
Soleus muscles were incubated in presence (Cal-C) or absence of 2.5 μm calphostin C for 35 min. Muscles either rested (Basal) or were stimulated electrically to perform repeated tetanic contractions (Con) or stimulated with 5.5 μm adrenaline (Adr) for the last 5 min during incubation. The two soleus muscles from a given rat were both stimulated or rested, and one was incubated with and the other without 2.5 μm calphostin C. Muscles were freeze-clamped and analysed for HSL (TG) (A and B) and HSL (DG) (C and D) activities, which are measures of the activated form of HSL and of total enzyme concentration, respectively. Three muscles were used per analysis. Values are means ± s.e.m., n = 3–9 analyses in each group. * Effect of contractions (P < 0.05); § effect of adrenaline (P < 0.05).
Figure 3. U0126 inhibits the contraction-induced increases in HSL (TG) activity and ERK phosphorylation.
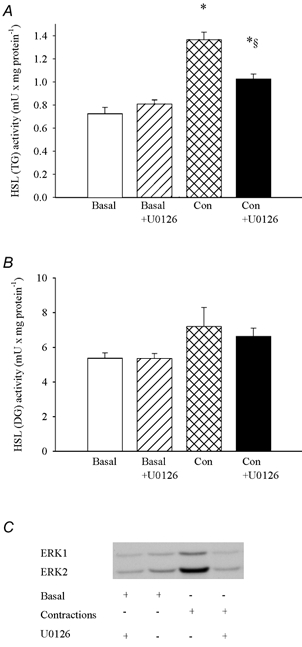
Soleus muscles were incubated in the presence or absence of 10 μm U0126 for 35 min. Muscles either rested (Basal) or were stimulated electrically to perform repeated tetanic contractions (Con) for the last 5 min during incubation. The two soleus muscles from a given rat were both stimulated electrically or rested, and one was incubated with and the other without U0126. Muscles were freeze-clamped and later analysed chemically for HSL (TG) (A) and HSL (DG) (B) activities and by Western blotting for phosphorylated ERK (C). Three muscles were used per analysis. Values are means ± s.e.m., n = 6–9 in each group. * Indicates value different from Basal and Basal + U0126 (P < 0.05); § indicates value different from Con (P < 0.05).
Figure 7. ERK activates HSL in crude supernatants from basal but not from electrically stimulated muscle.
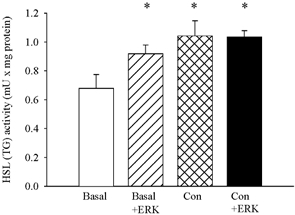
Crude supernatants from three rested (Basal) or three electrically stimulated (Con, 5 min of repeated tetanic contractions) soleus muscles were incubated with or without activated ERK. Values are means ± s.e.m. of 4–6 incubations. * Indicates value different from Basal (P < 0.05).
Figure 4. The effects of adrenaline and U0126 on HSL activity and ERK phosphorylation in muscle.
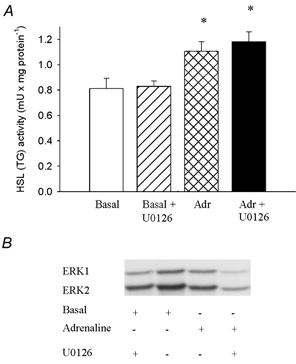
Soleus muscles were incubated in presence or absence of 10 μm U0126 for 35 min. Adrenaline (5.5 μm) (Adr) was either present or absent during the last 5 min of incubation. The two soleus muscles from a given rat were both either stimulated or not with adrenaline, and one was incubated with and the other without U0126. Muscles were freeze-clamped and later analysed chemically for HSL (TG) activity (A) and by Western blotting for phosphorylated ERK (B). Three muscles were used per analysis. Values are means ± s.e.m., n = 6–8 in each group. * Indicates value different from basal and basal + U0126 (P < 0.05).
Figure 6. Effect of caffeine on HSL activity and ERK phosphorylation.
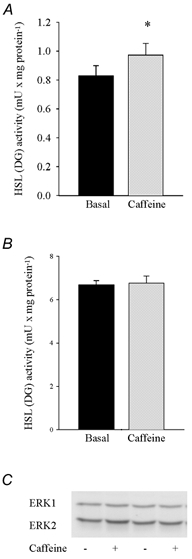
Rat soleus muscles were incubated with or without (Basal) 3 mm caffeine for 30 min. Three muscles were used per analysis. Values are means ± s.e.m. of 4 determinations. *Indicates value different from Basal (P < 0.05).
At the end of incubation, muscles were freeze-clamped with aluminium tongs cooled in liquid nitrogen and then trimmed of tendons while kept in liquid nitrogen. Muscles were stored at −80 °C. Within 4 weeks they were homogenized (Polytron PT 3100, maximum speed) on ice in 10 vol. 0.25 M sucrose, 1 mm dithiothreitol (Sigma Aldrich), 40 mm β-glycerophosphate (Sigma Aldrich), 10 mm sodium pyrophosphate (Sigma Aldrich), 0.31 μm okadaic acid (Roche, Mannheim, Germany), 20 μg ml−1 leupeptin (Sigma Aldrich), 20 μg ml−1 antipain (Sigma Aldrich) and 6.25 μg ml−1 pepstatin A (Sigma Aldrich). The crude homogenate was centrifuged at 15 800 g in an Eppendorf microcentrifuge tube at 4 °C for 45 s. The supernatant was recovered and stored at −80 °C until analysis within 4 weeks. Protein was determined with the BCA protein assay (Sigma Aldrich) using BSA as a standard.
Incubation of muscle crude supernatant with activated ERK
A 50 μl volume of supernatant from muscle which had been preincubated for 60 min and then either electrically stimulated or not for 5 min was mixed with 20 μl assay buffer (final concentrations: 25 mm Hepes, pH 7.5, 10 mm magnesium acetate, 0.5 μm ATP) and 0.1 μg of activated ERK (the phosphorylated form of ERK, Calbiochem). The mixture was incubated at 30 °C for 30 min during shaking. The reaction was stopped by adding 200 μl of an ice-cold stop solution containing 10 mm EDTA, 1 mm dithioerythritol and 0.02 % defatted BSA (Sigma Aldrich). Subsequently HSL (TG) activity (see below) was measured without delay.
HSL assays
HSL assays are based on measurements of release of [3H]oleic acid from 1(3)-mono[3H]oleoyl-2-oleylglycerol (a diacylglycerol analogue not hydrolysable at position 2), referred to as HSL (DG) activity, and from tri[3H]olein, referred to as HSL (TG) activity, under conditions optimal for HSL. In the basal state, the catalytic activity of adipose tissue HSL towards diacylglycerol (DG) substrates is about 10-fold higher than the activity against triacylglycerol (TG) substrates (Straalfors et al. 1987). Upon phosphorylation by protein kinase A the activity of adipose tissue and soleus muscle HSL towards TG increases markedly, whereas the HSL (DG) activity does not change significantly (Straalfors et al. 1987; Langfort et al. 1999, 2000; Holm et al. 2000). Furthermore, the increases in muscle HSL (TG) activity induced by adrenaline or contractions can be completely abolished by an anti-HSL antibody present during analysis (Langfort et al. 1999, 2000). Accordingly, HSL (TG) activity is a measure of the activated form of HSL and the assay of choice for monitoring changes in the activation state of HSL, while HSL (DG) activity has been taken as a measure of total enzyme concentration (Straalfors et al. 1987; Holm et al. 2000). Tri[3H]olein (TG) and 1(3)-mono[3H]oleoyl-2-oleylglycerol (DG) (Tornqvist et al. 1978) were synthesized by Lennart Krabisch (Department of Cell and Molecular Biology, Lund University, Lund, Sweden). Tri[3H]olein was also obtained from Amersham Pharmacia Biotech. The TG and DG substrates were emulsified with phospholipids by sonication (Osterlund et al. 1996). BSA was used as a fatty acid acceptor. For measurements of HSL (TG) activity and HSL (DG) activity, 14 μl and 7 μl, respectively, of muscle supernatant (protein concentration ≈2.5 mg ml−1) were incubated for 30 min at 37 °C with 100 μl of 5 mm (available acyl chains) TG (1.25 × 104 c.p.m.) or DG (0.4 × 106 c.p.m.) substrate and enzyme dilution buffer (20 mm potassium phosphate, pH 7.0, 1 mm EDTA, 1 mm dithioerythritol and 0.2 mg ml−1 BSA) in a total volume of 200 μl. For measurements of HSL (TG) activity in the experiment with activated ERK, 50 μl of the assay mix was used. Hydrolysis was stopped by addition of 3.25 ml of methanol-chloroform-heptane (10:9:7, by vol.) followed, after vortexing for 10 s, by 1.1 ml of 0.1 M potassium carbonate and 0.1 M boric acid (pH 10.5). The mixture was vortexed vigorously for 10 s and centrifuged at 1100 g for 20 min. Then 1 ml of the upper phase containing the released fatty acids (Osterlund et al. 1996) was mixed with 10 ml of scintillation liquid. Radioactivity was determined in a Tri-Carb 2200 CA (Packard) scintillation counter. One unit of enzyme activity is equivalent to the amount needed for 1 μmol of fatty acid to be released per minute at 37 °C.
Western blot analysis of ERK1 and ERK2
Muscle proteins from crude supernatants were separated by SDS-PAGE on 10 % Criterion gels (Bio-Rad, Hercules, CA, USA) and then by semi-dry blotting electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Hybond-P, Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK). Membranes were blocked in 5 % defatted milk (Irma, Denmark) in TS buffer (10 mm Tris, 150 mm NaCl, 0.05 % Tween 20, pH 7.4). Membranes were incubated with primary rabbit antibody recognizing the phosphorylated forms of ERK1 and ERK2 (Biosource International, CA, USA) overnight at 4 °C. Membranes were washed in TS buffer and incubated with secondary goat horseradish peroxidase-labelled anti-rabbit antibody (DAKO, Denmark), and then again washed in TS. Proteins were visualized with an ECL detection system (Amersham Pharmacia Biotech) and quantified by densitometry using a GS-710 densitometer (Bio-Rad).
Statistics
The computer program SigmaStat for Windows version 2.0 was used for statistical analysis. Data are presented as means ± s.e.m. Analysis of variance (ANOVA) was used to test differences in experiments comprising more than two conditions. The Student-Newman-Keuls test was used as a post hoc test. Student's paired t test was used to test differences in experiments with only two conditions, the two soleus muscles from a given rat making one pair. A significance level of 0.05 in two tailed testing was chosen a priori.
RESULTS
PKC inhibitors abolish the contraction-induced activation of HSL
In control experiments similar increases in HSL (TG) activity in soleus muscle were seen in response to contractions and adrenaline (Figs 1A and B, and 2A and B). However, in the presence of either bisindolylmaleimide I or calphostin C the contraction-induced activation of HSL was abolished (Fig. 1A and Fig. 2A), whereas the adrenaline-induced increase in HSL (TG) activity was not significantly reduced (Fig. 1B and Fig. 2B). HSL (DG) activity in soleus muscle did not change during contractions or adrenaline stimulation, and was not influenced by the two PKC inhibitors (Figs 1C and D, and 2C and D). The inhibitors also did not influence force output during contractions (Table 1).
Table 1.
Total force output (percentage of control) during electrically induced muscle contractions in the presence of MEK or PKC inhibitors
| Bisindolylmaleimide I (1 μm) | 109 ± 7 |
| Calphostin C (2.5 μm) | 99 ± 6 |
| U0126 (10 μm) | 93 ± 6 |
The two soleus muscles from a given rat were stimulated to produce maximum iosmatric tetanic constractions for 5 min, one in the presence and one in the absence (control) of inhibitor. Values are means of 17–27 paris of muscles. None of the three inhibitors significantly influenced force development.
The MAPK/ERK pathway is involved in the contraction-induced activation of HSL
Contractions doubled the phosphorylation of both ERK1 (2.1 ± 0.3 vs. 1.1 ± 0.1 (basal) arbitrary units (a.u.), n = 6–9, P < 0.05) and ERK2 (1.9 ± 0.2 vs. 0.9 ± 0.1 (basal) a.u., n = 6–9, P < 0.05) (Fig. 3). These increases were abolished by the specific MEK inhibitor U0126, ERK1 being 1.0 ± 0.2 a.u. and ERK2 being 0.9 ± 0.1 a.u. after contractions in the presence of U0126, n = 9, P > 0.05 vs. basal (Fig. 3). The effect of U0126 on ERK was accompanied by a 50 % reduction in the contraction-induced increase in HSL (TG) activity, while HSL (DG) activity was not changed (Fig. 3). Adrenaline did not change the phosphorylation of ERK1 and 2, and U0126 did not influence HSL (TG) activity in adrenaline-stimulated muscle (Fig. 4).
In order to clarify whether ERK and HSL can be activated by PKC in muscle, we used the PKC stimulating compound PMA. This substance more than doubled phosphorylation of both ERK1 (1.4 ± 0.1 vs. 0.6 ± 0.1 (basal) a.u., n = 8, P < 0.05) and ERK2 (1.6 ± 0.1 vs. 0.4 ± 0.1 (basal) a.u., n = 8, P < 0.05) and also caused a moderate increase (≈25 %, P < 0.05) in HSL (TG) activity, whereas HSL (DG) activity was not changed (Fig. 5A-C). Evidence, but more indirect, in favour of a role of PKC in HSL stimulation was also provided by incubation of soleus muscle with caffeine at a concentration (3 mm) known to increase sarcoplasmic Ca2+ concentration without eliciting contraction (Palade, 1987a,b; Youn et al. 1991). Caffeine caused a moderate increase (≈17 %, P < 0.05) in HSL (TG) activity, while HSL (DG) activity and ERK phosphorylation did not change (Fig. 6A-C).
Figure 5. Effect of PMA on HSL activity and ERK phosphorylation.
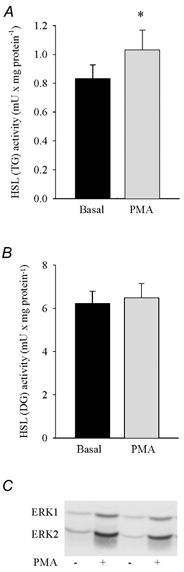
Rat soleus muscles were incubated with or without (Basal) 5 μm PMA for 30 min. Three muscles were used per analysis. Values are means ± s.e.m. of 8 determinations. * Indicates value different from Basal (P < 0.05).
In order to further substantiate the relationship between ERK and HSL activity, crude supernatants from soleus muscle were incubated with activated ERK. In supernatant from resting muscle, ERK increased HSL (TG) activity to the level seen in supernatant from contraction-stimulated muscle, whereas no effect of ERK was seen in supernatant from contraction-stimulated muscle (Fig. 7).
DISCUSSION
The major new findings of the present study are that PKC and ERK are involved in the stimulation of HSL by contractions, and that this stimulation probably at least partly reflects the existence of a PKC-MAPK-HSL pathway in muscle. Furthermore, in contrast to the previous finding in adipocytes that β-adrenergic activation of HSL is partially mediated via ERK, in muscle, activation of ERK does not contribute to stimulation of HSL by adrenaline. These conclusions were based on several lines of evidence. Thus, the phorbol ester PMA, which stimulates PKC and, in turn, ERK (Ryder et al. 2000), increased HSL activity in incubated soleus muscle (Fig. 5). This finding is in line with a recent study in which diacylglycerol stimulation of PKC in adipocytes increased ERK phosphorylation and lipolysis, while stimulation of ERK in transfected preadipocytes increased HSL activity (Greenberg et al. 2001). So the two studies indicate the existence of a novel pathway for the regulation of HSL in the two tissues with the highest rates of lipolysis. In line with the view that this pathway exists in muscle and is employed by contractions but not by adrenaline, two PKC inhibitors, bisindolylmaleimide I (Fig. 1) and calphostin C (Fig. 2) (Kobayashi et al. 1989; Toullec et al. 1991), and a specific inhibitor of ERK kinase (MEK), U0126 (Fig. 3) (Favata et al. 1998; Greenberg et al. 2001), fully and partly, respectively, blocked the contraction-induced stimulation of HSL, whereas the effect of adrenaline on HSL was not impaired. Further supporting that ERK is important in contraction-induced HSL activation, the effect of contractions could be accurately mimicked by incubating control muscle supernatant with activated ERK, while no effect of activated ERK was seen with supernatant from electrically stimulated muscle (Fig. 7).
While stimulation of PKC may fully account for activation of HSL during contractions, the effect of PKC may only partly be mediated through ERK. This is so because U0126 completely abolished the contraction-induced increases in ERK phosphorylation above normal basal levels but nevertheless only reduced the increase in HSL activity by 50 % (Fig. 3). Correspondingly, the increase in HSL activity in response to PMA in resting muscle was only about one-half of the increase in HSL activity during contractions, even though increases in ERK phosphorylation were similar in the two conditions (Fig. 3 and Fig. 5). It cannot be excluded that these findings reflect co-operation of allosteric enhancement of ERK activity during contractions. However, the finding that caffeine, in a concentration known to increase sarcoplasmic Ca2+ concentration without eliciting contraction (Palade, 1987a,b; Youn et al. 1991), caused a moderate increase in HSL activity without any change in ERK phosphorylation (Fig. 6) is compatible with stimulation of HSL by PKC via other routes than through ERK. Glucose transport in muscle is stimulated by caffeine (Youn et al. 1991), and the view that PKC can influence metabolism via other pathways than through ERK is illustrated by the fact that PKC enhances glucose transport in contracting muscle (Ihlemann et al. 1999a), whereas ERK does not (Hayashi et al. 1999; Wojtaszewski et al. 1999). Alternative mechanisms through which PKC could act on HSL might include direct phosphorylation of HSL by PKC, or phosphorylation through other signalling pathways. Our results with caffeine are also compatible with Ca2+ signalling leading to HSL activation without involvement of PKC. However, this possibility is unlikely, because no increase in HSL activity was elicited, when sarcoplasmic Ca2+ concentrations were increased by contractions in the presence of PKC blockers (Fig. 1 and Fig. 2).
We have previously shown in muscle that adrenaline can stimulate HSL via β-adrenergic activation of PKA (Langfort et al. 1999), as is the case in adipose tissue (Holm et al. 2000). So, in both muscle and adipose tissue, HSL may be regulated by PKA as well as PKC. However, the recruitment of and signalling induced by these kinases may differ between the two tissues. Thus, from experiments with MEK blockers it has been concluded that in adipocytes, activation of ERK accounts for 30 % of β-adrenergic stimulation of lipolysis (Greenberg et al. 2001). In contrast, the present study did not indicate any involvement of ERK in adrenergic stimulation of HSL in muscle (Fig. 4). These findings agree with the fact that adrenergic stimulation has been found to increase ERK phosphorylation in adipocytes (Greenberg et al. 2001), but not in muscle (Napoli et al. 1998). On the other hand, in adipose tissue the physiological stimuli causing HSL activation through PKC remain to be defined, whereas in muscle PKC seems crucial for stimulation of HSL by contractions (Fig. 1 and Fig. 2). Consistent with PKC playing a role are the findings that contractions induce translocation of conventional PKC from the cytosol to the particulate fraction in muscle (Richter et al. 1987) and that both Ca2+ and diacylglycerol levels increase in contracting muscle (Cleland et al. 1989). These signalling molecules activate conventional and both conventional and novel PKC isoforms, respectively, and these PKC subgroups can be inhibited by both of the PKC inhibitors which abolished contraction-induced HSL stimulation in the present study (Kobayashi et al. 1989; Toullec et al. 1991). In contrast, atypical PKCs are not inhibited by calphostin C (Kobayashi et al. 1989), ruling out that these isoforms activate HSL during contractions. Pharmacological inhibitors are important tools in physiological and biochemical research. However, results obtained by use of inhibitors should always be treated with caution. This may be of particular importance, when inhibitors are used to block small effects. In this context it is worth noting that while the apparent increases in HSL (TG) activity in response to stimulation by contractions or adrenaline were 50–90 % (Figs 1–3) as also found previously (Langfort et al. 1999, 2000), the actual increases were about twice as high. This is so, because studies with immunoinhibition and immunoprecipitation have shown that while increases in neutral lipase activity in response to contractions or adrenaline are fully accounted for by HSL, about half of the basal neutral lipase activity is due to other lipases (Langfort et al. 1999, 2000). It is also worth noting that in the present study, results obtained with inhibitors agreed with other lines of evidence. Furthermore, neither the PKC nor the MEK inhibitors applied in the present study influenced force production during contractions (Table 1). Accordingly, the observed inhibition of HSL responses in the presence of the inhibitors was not secondary to a decrease in force production, which might reduce metabolic requirements or reflect major muscle dysfunction.
The fact that in the present study HSL activity measured with a triacylglycerol substrate (HSL (TG)) changed during experimental interferences, whereas HSL activity measured with a diacylglycerol analogue as substrate (HSL (DG)) was not changed (Figs 1, 2, 3, 5 and 6), indicates that changes in HSL activity were due to changes in phosphorylation of the enzyme, while total enzyme concentration remained constant (Straalfors et al. 1987; Holm et al. 2000). PKA and ERK phosphorylate HSL at different sites (Anthonsen et al. 1998; Greenberg et al. 2001). The finding that these kinases are involved in adrenaline- and contraction-stimulated HSL activation, respectively, may explain that the effects of the two stimuli on HSL activity are partially additive (Langfort et al. 2003). HSL probably accounts for regulation of lipolysis in both adipose tissue and muscle, while glycogen phosphorylase regulates glycogenolysis in liver and muscle. It is interesting to note that apparently these two enzymes are activated in parallel by both adrenaline and contractions via PKA and Ca2+-dependent phosphorylation, respectively (Connett & Sahlin, 1996; Langfort et al. 1999, 2000). The finding of similar signalling mechanisms involved in activation of the rate limiting enzymes adds to the view that no selectivity exists in the primary setting of mobilization of the major extra- and intra-muscular energy stores in response to catecholamine stimulation and exercise (Galbo, 1995; Langfort et al. 1999, 2000;). Furthermore, both the PKA and the Ca2+-mediated signalling mechanism are compatible with the existence of a feed-forward control of fuel mobilization independent of metabolic needs (Galbo, 1995).
ERK has been proposed to be involved in exercise-induced regulation of gene expression in muscle (Widegren et al. 2001), but it is not considered to be important in acute regulation of metabolic processes (Napoli et al. 1998). In accordance with the latter view, it has been shown that ERK signalling is not necessary for contraction-induced increases in glucose and amino acid transport or glycogen synthase activity in muscle (Hayashi et al. 1999; Wojtaszewski et al. 1999). However, in line with recent findings in adipocytes (Greenberg et al. 2001), the present study points at a role for ERK in acute regulation of lipolysis in muscle. In addition to its physiological importance, the stimulation of HSL mediated by PKC through ERK, which has been demonstrated in muscle in the present study, is of pathophysiological and pharmacological interest, because the size and turnover of the intramyocellular triacylglycerol stores may play key roles in the pathogenesis of the insulin resistance syndrome (Kraegen et al. 2001; Virkamaki et al. 2001).
The increases in HSL (TG) activity seen in the present study in response to contractions or adrenaline were of the same magnitude as the increases previously found in muscle (Langfort et al. 1999, 2000) and adrenaline-stimulated adipose tissue (Osterlund et al. 1996). These increases measured in homogenates are at least 10-fold lower than maximal increases in lipolytic rates in the intact tissues or cells. Findings in fat cells have indicated that in these cells the discrepancy may be ascribed to the fact that β-adrenergic stimulation in addition to phosphorylation of HSL also causes translocation of HSL from the cytosol to the lipid storage droplets as well as phosphorylation of perilipin, a protein coating the lipid droplets and probably imposing an adjustable barrier to HSL action in vivo (Londos et al. 1999; Brasaemle et al. 2000). It is not yet known whether similar mechanisms may be at play in skeletal muscle.
We have no reason to believe that our muscle preparation was suffering, for example, from damage or hypoxia. This is so, because during electrical stimulation, tension did not decline faster than previously seen in perfused muscle (Han et al. 1995), and because the soleus preparation has previously been shown, after 2 h of incubation, to have basal values of glycogen, nucleotides and AMP-activated kinase (AMPK) identical to values in anaesthetized rats (Ihlemann et al. 1999b). Furthermore, in incubated muscle preparations, basal AMPK activity (Ihlemann et al. 2001) as well as basal and insulin-induced glucose transport (Ihlemann et al. 1999a) have previously been found to be identical to values seen in the perfused hindlimb (Wojtaszewski et al. 1998; Derave et al. 2000).
In conclusion, the present study demonstrates a novel signalling pathway in muscle by which HSL activity may be stimulated by PKC via ERK. Furthermore, contraction-induced HSL activation is mediated by PKC, at least partly via the ERK pathway. ERK is known to phosphorylate HSL at a different site from those phosphorylated by PKA, a fact which may explain that the effects of contractions and adrenaline on HSL activity are partially additive. Finally, in contrast to the previous finding in adipocytes that β-adrenergic activation of HSL is in part mediated via ERK, in muscle, activation of ERK does not contribute to stimulation of HSL by adrenaline.
Acknowledgments
The study was supported by grants from Novo Nordic Foundation, the Danish National Research Foundation (grant no. 504–14), the Danish Diabetes Foundation, the Swedish Research Council (grant no. 112 84) and the Swedish Diabetes Association.
REFERENCES
- Abumrad NA, Tepperman HM, Tepperman J. Control of endogenous triglyceride breakdown in the mouse diaphragm. J Lipid Res. 1980;21:149–155. [PubMed] [Google Scholar]
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- Brasaemle DL, Levin DM, Adler-Wailes DC, Londos C. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim Biophys Acta. 2000;1483:251–262. doi: 10.1016/s1388-1981(99)00179-1. [DOI] [PubMed] [Google Scholar]
- Carlson LA, Ekelund LG, Froberg SO. Concentration of triglycerides, phospholipids and glycogen in skeletal muscle and of free fatty acids and beta-hydroxybutyric acid in blood in man in response to exercise. Eur J Clin Invest. 1971;1:248–254. doi: 10.1111/eci.1971.1.4.248. [DOI] [PubMed] [Google Scholar]
- Cleland PJ, Appleby GJ, Rattigan S, Clark MG. Exercise-induced translocation of protein kinase C and production of diacylglycerol and phosphatidic acid in rat skeletal muscle in vivo. Relationship to changes in glucose transport. J Biol Chem. 1989;264:17704–17711. [PubMed] [Google Scholar]
- Connett R, Sahlin K. Control of glycolysis and glycogen metabolism. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 870–910. [Google Scholar]
- Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Froberg SO, Hultman E, Nilsson LH. Effect of noradrenaline on triglyceride and glycogen concentrations in liver and muscle from man. Metabolism. 1975;24:119–126. doi: 10.1016/0026-0495(75)90012-8. [DOI] [PubMed] [Google Scholar]
- Galbo H. Integrated endocrine responses and exercise. In: DeGroot LJ, editor. Endocrinology. Vol. 3. Philadelphia: W. B. Saunders; 1995. pp. 2692–2701. [Google Scholar]
- Garton AJ, Campbell DG, Carling D, Hardie DG, Colbran RJ, Yeaman SJ. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur J Biochem. 1989;179:249–254. doi: 10.1111/j.1432-1033.1989.tb14548.x. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Shen WJ, Muliro K, Patel S, Souza SC, Roth RA, Kraemer FB. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J Biol Chem. 2001;276:45456–45461. doi: 10.1074/jbc.M104436200. [DOI] [PubMed] [Google Scholar]
- Han X, Ploug T, Galbo H. Effect of diet on insulin- and contraction-mediated glucose transport and uptake in rat muscle. Am J Physiol. 1995;269:R544–551. doi: 10.1152/ajpregu.1995.269.3.R544. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Dufresne SD, Goodyear LJ. Skeletal muscle contractile activity in vitro stimulates mitogen-activated protein kinase signaling. Am J Physiol. 1999;277:C701–707. doi: 10.1152/ajpcell.1999.277.4.C701. [DOI] [PubMed] [Google Scholar]
- Holm C, Osterlund T, Laurell H, Contreras JA. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutr. 2000;20:365–393. doi: 10.1146/annurev.nutr.20.1.365. [DOI] [PubMed] [Google Scholar]
- Ihlemann J, Galbo H, Ploug T. Calphostin C is an inhibitor of contraction, but not insulin-stimulated glucose transport, in skeletal muscle. Acta Physiol Scand. 1999a;167:69–75. doi: 10.1046/j.1365-201x.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- Ihlemann J, Ploug T, Galbo H. Effect of force development on contraction induced glucose transport in fast twitch rat muscle. Acta Physiol Scand. 2001;171:439–444. doi: 10.1046/j.1365-201X.2001.00807.x. [DOI] [PubMed] [Google Scholar]
- Ihlemann J, Ploug T, Hellsten Y, Galbo H. Effect of tension on contraction-induced glucose transport in rat skeletal muscle. Am J Physiol. 1999b;277:E208–214. doi: 10.1152/ajpendo.1999.277.2.E208. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Kraegen EW, Cooney GJ, Ye JM, Thompson AL, Furler SM. The role of lipids in the pathogenesis of muscle insulin resistance and beta cell failure in type II diabetes and obesity. Exp Clin Endocrinol Diabetes. 2001;109:S189–S201. doi: 10.1055/s-2001-18581. [DOI] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Baranczuk E, Donsmark M, Górski J, Galbo H. Additivity of adrenaline and contractions on hormone-sensitive lipase, but not on glycogen phosphorylase, in rat muscle. Acta Physiol Scand. 2003;178:51–60. doi: 10.1046/j.1365-201X.2003.01111.x. [DOI] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Holm C, Galbo H. Stimulation of hormone-sensitive lipase activity by contractions in rat skeletal muscle. Biochem J. 2000;351:207–214. doi: 10.1042/0264-6021:3510207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfort J, Ploug T, Ihlemann J, Saldo M, Holm C, Galbo H. Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochem J. 1999;340:459–465. [PMC free article] [PubMed] [Google Scholar]
- Londos C, Brasaemle DL, Schultz CJ, Segrest JP, Kimmel AR. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin Cell Dev Biol. 1999;10:51–58. doi: 10.1006/scdb.1998.0275. [DOI] [PubMed] [Google Scholar]
- Napoli R, Gibson L, Hirshman MF, Boppart MD, Dufresne SD, Horton ES, Goodyear LJ. Epinephrine and insulin stimulate different mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Diabetes. 1998;47:1549–1554. doi: 10.2337/diabetes.47.10.1549. [DOI] [PubMed] [Google Scholar]
- Oscai LB, Essig DA, Palmer WK. Lipase regulation of muscle triglyceride hydrolysis. J Appl Physiol. 1990;69:1571–1577. doi: 10.1152/jappl.1990.69.5.1571. [DOI] [PubMed] [Google Scholar]
- Osterlund T, Danielsson B, Degerman E, Contreras JA, Edgren G, Davis RC, Schotz MC, Holm C. Domain-structure analysis of recombinant rat hormone-sensitive lipase. Biochem J. 1996;319:411–420. doi: 10.1042/bj3190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P. Drug-induced Ca2+ release from isolated sarcoplasmic reticulum. I. Use of pyrophosphate to study caffeine-induced Ca2+ release. J Biol Chem. 1987a;262:6135–6141. [PubMed] [Google Scholar]
- Palade P. Drug-induced Ca2+ release from isolated sarcoplasmic reticulum. II. Releases involving a Ca2+-induced Ca2+ release channel. J Biol Chem. 1987b;262:6142–6148. [PubMed] [Google Scholar]
- Qu X, Seale JP, Donnelly R. Tissue- and isoform-specific effects of aging in rats on protein kinase C in insulin-sensitive tissues. Clin Sci (Lond) 1999;97:355–361. doi: 10.1042/cs0970355. [DOI] [PubMed] [Google Scholar]
- Reitman J, Baldwin KM, Holloszy JO. Intramuscular triglyceride utilization by red, white, and intermediate skeletal muscle and heart during exhausting exercise. Proc Soc Exp Biol Med. 1973;142:628–631. doi: 10.3181/00379727-142-37081. [DOI] [PubMed] [Google Scholar]
- Richter EA, Cleland PJ, Rattigan S, Clark MG. Contraction-associated translocation of protein kinase C in rat skeletal muscle. FEBS Lett. 1987;217:232–236. doi: 10.1016/0014-5793(87)80669-5. [DOI] [PubMed] [Google Scholar]
- Ryder JW, Fahlman R, Wallberg-Henriksson H, Alessi DR, Krook A, Zierath JR. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle. Involvement Of the mitogen- and stress-activated protein kinase 1. J Biol Chem. 2000;275:1457–1462. doi: 10.1074/jbc.275.2.1457. [DOI] [PubMed] [Google Scholar]
- Schick F, Eismann B, Jung WI, Bongers H, Bunse M, Lutz O. Comparison of localized proton NMR signals of skeletal muscle and fat tissue in vivo: two lipid compartments in muscle tissue. Magn Reson Med. 1993;29:158–167. doi: 10.1002/mrm.1910290203. [DOI] [PubMed] [Google Scholar]
- Straalfors P, Olsson H, Belfrage P. Hormone-sensitive lipase. In: Boyer PD, Krebs EG, editors. The Enzymes. New York: Academic Press; 1987. pp. 147–177. [Google Scholar]
- Tornqvist H, Bjorgell P, Krabisch L, Belfrage P. Monoacylmonoalkylglycerol as a substrate for diacylglycerol hydrolase activity in adipose tissue. J Lipid Res. 1978;19:654–656. [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Dunhamel L, Charon D, Lirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Van der Vusse G, Reneman R. Lipid metabolism in muscle. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 952–994. [Google Scholar]
- Virkamaki A, Korsheninnikova E, Seppala-Lindroos A, Vehkavaara S, Goto T, Halavaara J, Hakkinen AM, Yki-Jarvinen H. Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes. 2001;50:2337–2343. doi: 10.2337/diabetes.50.10.2337. [DOI] [PubMed] [Google Scholar]
- Widegren U, Ryder JW, Zierath JR. Mitogen-activated protein kinase signal transduction in skeletal muscle: effects of exercise and muscle contraction. Acta Physiol Scand. 2001;172:227–238. doi: 10.1046/j.1365-201x.2001.00855.x. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Jakobsen AB, Ploug T, Richter EA. Perfused rat hindlimb is suitable for skeletal muscle glucose transport measurements. Am J Physiol. 1998;274:E184–191. doi: 10.1152/ajpendo.1998.274.1.E184. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Lynge J, Jakobsen AB, Goodyear LJ, Richter EA. Differential regulation of MAP kinase by contraction and insulin in skeletal muscle: metabolic implications. Am J Physiol. 1999;277:E724–732. doi: 10.1152/ajpendo.1999.277.4.E724. [DOI] [PubMed] [Google Scholar]
- Youn JH, Gulve EA, Holloszy JO. Calcium stimulates glucose transport in skeletal muscle by a pathway independent of contraction. Am J Physiol. 1991;260:C555–561. doi: 10.1152/ajpcell.1991.260.3.C555. [DOI] [PubMed] [Google Scholar]


