Abstract
Interleukin-6 (IL-6) is a cytokine involved in a number of immunological processes, but it is also linked to exercise and possibly energy status. During exercise, muscle IL-6 mRNA levels and plasma IL-6 levels are increased and further augmented when intramuscular glycogen levels are low. In contrast, the increase in plasma IL-6 is blunted if carbohydrate is administered, indicating a substrate-regulated induction of IL-6 in human skeletal muscle. Recent studies have demonstrated that IL-6 is also released from adipose tissue in response to an exercise bout. Furthermore, IL-6 has been demonstrated to have a lipolytic effect, thus possibly playing a role in mobilisation of energy as free fatty acids (FFA) in response to exercise. The purpose of the present study was to investigate the gene expression pattern of IL-6 in adipose tissue in response to exercise, and to determine whether gene expression was affected by the ingestion of carbohydrate. Eight male subjects performed 3 h of bicycling with ingestion of a carbohydrate drink or placebo. Fat biopsy samples and blood samples were obtained before, during and in the recovery phase of exercise. Both plasma IL-6 and adipose IL-6 mRNA levels increased in response to exercise. IL-6 gene expression was lower (P < 0.05) in the CHO trial (1.98-fold increase, confidence interval (CI) 1.16–3.83) compared with the control (6.49-fold increase, CI 3.57–13.91) at end of exercise. Furthermore, CHO ingestion blunted the increase in plasma IL-6 levels (P < 0.05) at end of exercise (26.0 ± 3.7 pg ml−1 in the control vs. 15.6 ± 2.4 pg ml−1 in the CHO trial). In conclusion, exercise results in an increase in IL-6 gene expression in adipose tissue in response to exercise, an effect that is significantly blunted by ingestion of carbohydrate.
IL-6 is a pleiotropic, abundantly expressed protein, involved in cell-to-cell signalling within the immune system. The major focus with respect to cytokine function has been on the immune response. However, research within the last few years has also demonstrated a role of a number of cytokines in response to exercise. Plasma IL-6 is especially markedly increased during exercise, peaking at the end of an exercise bout (Pedersen & Hoffman-Goetz, 2000; Febbraio & Pedersen, 2002). Measurements over the working limb have indicated that skeletal muscle is the major source of this IL-6 production in response to exercise (Steensberg et al. 2000). Also brain (Nybo et al. 2002), peritendon tissue (Langberg et al. 2002) and adipose tissue (Lyngso et al. 2002a) have been demonstrated to release IL-6 in response to exercise. Several studies have demonstrated a large increase in IL-6 gene expression in skeletal muscle as well as in plasma IL-6 protein levels in response to exercise (Ostrowski et al. 1998; Steensberg et al. 2001; Keller et al. 2001). Furthermore, the transcriptional activity of IL-6 in skeletal muscle is not only affected by exercise, but is also inversely related to glycogen content within the working muscle (Keller et al. 2001). Ingestion of carbohydrate during cycling exercise has been shown to result in decreased plasma IL-6 levels (Nieman et al. 1998; Starkie et al. 2001), but it does not affect the gene expression pattern in skeletal muscle (Starkie et al. 2001), suggesting a posttranslational or transport inhibitory mechanism of glucose. Thus, IL-6 is released from the working limb, and is regulated through substrate availability such as blood glucose and intramuscular glycogen levels. Our group (Steensberg et al. 2000) and others (Gleeson, 2000) have suggested a hormonal role of IL-6 with respect to its biological role during exercise. According to this hypothesis, IL-6 is released from the working muscle to signal to liver or adipose tissue to increase glycogenolysis or lipolysis, respectively. Previous studies have demonstrated a glucose generating effect of IL-6 on rat hepatocytes (Blumberg et al. 1995; Kanemaki et al. 1998) and a positive relation between IL-6 release and glucose uptake, suggesting that IL-6 may be linked to regulation of glucose homeostasis during exercise and/or that IL-6 may work as a sensor of carbohydrate availability (Helge et al. 2003). However, IL-6 infusion into resting subjects did not affect glucose metabolism (Steensberg et al. 2003). Recent studies from our group and others have demonstrated an increase in plasma free fatty acids (FFA), by both an increase in FFA rate of appearance and disappearance as well as an increased oxygen uptake in humans in response to rhIL-6 infusion (Lyngso et al. 2002b; van Hall et al. 2003). Thus, IL-6 increases FFA availability through increased lipolysis, indicating a hormonal role of IL-6 in response to exercise. In accordance with this, IL-6 knockout mice have been demonstrated to develop mature-onset obesity (Wallenius et al. 2002). IL-6 protein therefore seems important in the overall regulation of basal fat metabolism.
Peripheral tissue such as adipose tissue contributes to the secretion of IL-6. A study by Mohamed-Ali et al. (1997) has demonstrated that IL-6 is released from adipose tissue at rest, in an amount that can account for 15–35 % of the basal IL-6 level. In response to exercise, IL-6 is also released from adipose tissue (Lyngso et al. 2002a), suggesting an increased IL-6 gene expression. If IL-6 gene expression is increased in adipose tissue in response to exercise, this gene expression may be glucose-sensitive as has been observed in skeletal muscle. The release of IL-6 would thus affect lipolysis during exercise, possibly by increased local adipose IL-6 concentration in order to further enhance lipolysis in this area.
The purpose of the present study was firstly to examine whether adipose tissue IL-6 gene expression was affected by exercise, and secondly to determine whether carbohydrate ingestion would blunt this effect. We expected that carbohydrate ingestion would inhibit gene expression in the end of exercise at the time point where plasma glucose levels would be expected to display the greatest diversity between the two trials and where plasma IL-6 reaches its peak level. Thus, we hypothesised that IL-6 gene expression would increase in adipose tissue in response to exercise and that IL-6 gene expression would be inhibited by carbohydrate. To investigate the expression pattern of IL-6 and the effect of carbohydrate, healthy young subjects commenced 3 h of bicycling at two occasions with carbohydrate or placebo ingestion and retrieval of fat biopsy samples from the abdominal subcutaneous fat.
METHODS
Eight healthy, untrained male subjects (24 ± 1 years, 186 ± 1 cm, 81.9 ± 1.4 kg) participated in the study. The subjects were given both oral and written information about the experimental procedures before giving their written informed consent. The study was approved by the Copenhagen and Frederiksberg Ethics Committee, Denmark, and performed according to the Declaration of Helsinki.
Subjects underwent two experimental trials of 3 h of bicycling in a randomised order, separated by at least 1 week. Both trials were identical in design, and differed only by ingestion of either a carbohydrate drink (Gatorade, 6 % carbohydrate), in the CHO trial, or a sweetened placebo in the control trial, throughout the exercise bout.
One week prior to the experimental start, subjects performed a bicycling max-test on a cadence-independent cycle ergometer (Monark 839E, Monark Ltd, Varberg, Sweden). After an 8 min warm up at 100 W, workload was increased by 50 W every 3 min until a workload of 200 W was reached. Hereafter, the workload was increased 25 W every minute until a cadence of 60 r.p.m. could no longer be maintained. The highest workload the subject could maintain for 1 min was set as the maximal workload.
On the experimental day, subjects reported to the lab at 08.00 h after an overnight fast. A catheter was placed in the forearm brachial vein for blood sampling. Adipose tissue biopsy samples were taken from the abdominal subcutaneous fat by the percutaneous needle biopsy technique with suction, preceded by a subcutaneous injection of lidocaine (lignocaine). A pre-exercise biopsy sample and blood sample were obtained after 10 min of supine rest. Subjects commenced 3 h of bicycling at 60 % of their maximal workload with concomitant ingestion of 250 ml of either the carbohydrate drink or placebo every 15 min. Fat biopsy samples were obtained pre-exercise, at 3 h exercise, and in the recovery period (1.5 and 3 h), then cleaned of connective tissue and blood and quickly frozen in liquid nitrogen. Blood samples were obtained at pre-exercise, during exercise (1, 2 and 3 h) and post-exercise (1.5 and 3 h). Subjects were permitted to drink water ad libitum throughout the experiment. Subjects consumed an isocaloric diet the 2 days preceding the experimental days.
RNA extraction
Adipose tissue RNA extraction was performed with TRIzol (Life Technologies) according to the manufacturer's directions. In short, 50-100 mg of adipose tissue was dissolved in 1 ml of TRIzol and homogenised on a Brinkman Polytron (version PT 2100) on setting 26. Samples were allowed to sit for a few minutes in order for a triglyceride phase to form. The lower aqueous phase was transferred to a fresh tube and 100 μl of chloroform-isoamyl alcohol (24:1) was added and vigorously shaken. Samples were allowed to sit for 5 min and spun at 12 000 g for 15 min at 4 °C after which the upper aqueous phase was transferred to a new tube. The aqueous phase was mixed with 0.5 ml of isopropanol, and samples were placed in the freezer for 1 h. Samples were centrifuged at 12 000 g for 15 min at 4 °C, and the resulting pellet was washed with 0.5 ml of 75 % ethanol in diethylpyrocarbonate (DEPC)-treated water. After centrifugation at 7500 g for 10 min, pellets were re-dissolved in 15 μl of DEPC-treated water and allowed to dissolve on ice, after which samples were ready for reverse transcription.
Reverse transcription
One microgram of total RNA was reverse transcribed using the Applied Biosystems (Denmark) Taqman RT-Kit.
Real-time PCR
IL-6 gene expression was analysed using semi-quantitative real-time PCR with 18S mRNA as the internal reference gene. We used the pre-developed, primer-limited assay reagents for 18S mRNA determination. The IL-6 primers and probe sequences used were obtained from Starkie et al. (2001). All PCR reagents were obtained from Applied Biosystems.
An 81 bp fragment was amplified using: IL-6 forward primer: 5′-GGTACATCCTCGACGGCATCT-3′; IL-6 reverse primer: 5′-GT GCCTCTTTGCTGCTTTCAC-3′, IL-6 probe: 5′-FAM-TGTTAC TCTTGTTACATGTCTCCTTTCTCAGGGCT-TAMRA-3′.
A reagent mixture of 75 μl was made up for each sample with 1 × MasterMix, 900 nm IL-6 forward primer, 300 nm reverse primer, 100 nm IL-6 probe, 1 × 18S mix (primers and probe), 50–100 ng of sample and made up to a final volume of 75 μl with water. Each sample was run in triplicate in a reaction volume of 20 μl for 50 cycles using standard real-time PCR cycling conditions.
Determination of plasma IL-6 and blood metabolites
For protein determination, blood was immediately spun at 2200 g for 15 min at 4 °C and stored at −80 °C until analysis. To determine the amount of IL-6 protein in samples, high-sensitivity ELISA-kits were used (Quantikine, R&D Systems, Minneapolis, MN, USA). This assay does not distinguish between soluble and receptor-bound IL-6 and thus gives a measure of total IL-6 concentration. Blood glucose was determined immediately after collection of blood in EDTA-containing tubes, on an ABL 5 (Radiometer, Copenhagen). Blood FFA was determined by COBAS (Fara, Roche) analysis on plasma.
Statistics
All data are expressed as means ± s.e.m. and mRNA data were log-transformed prior to analysis. Two-way ANOVA for repeated measures was used to evaluate the effect of exercise (time). Differences between specific time points were determined by a Student-Newman-Keuls test. Student's t test was used to determine any difference between control and carbohydrate trials at end of exercise. Differences were considered significant at P < 0.05.
RESULTS
Blood glucose levels did not differ when comparing the two trials pre-exercise. Upon exercise and CHO ingestion, glucose levels were higher in the CHO trial compared with the control trial (P < 0, 05) throughout the experiment (Fig. 1).
Figure 1. Blood glucose before, during and after exercise presented as control and CHO trials.
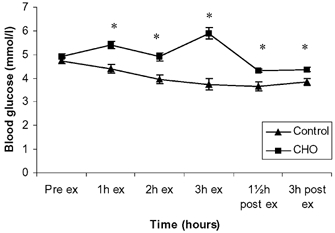
Data are presented as means ± s.e.m.; * significant difference between control and CHO trial.
There was no difference in plasma FFA concentration between the two trials at pre-exercise and 1 h of exercise (Fig. 2). However, thereafter, FFA levels increased throughout the exercise bout, but were attenuated in the CHO trial, and there was a significant difference between the two trials from 1 h of exercise until 1.5 h post-exercise after which FFA levels reached the same level in the two trials.
Figure 2. Plasma free fatty acids before, during and after exercise presented as control and CHO trials.
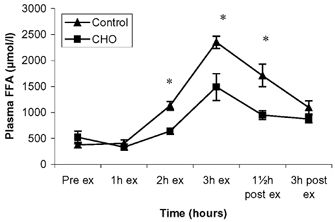
Data are presented as means ± s.e.m.; * significant difference between control and CHO trial.
IL-6 mRNA levels are expressed as fold induction relative to the pre-exercise level (Fig. 3). IL-6 gene expression was elevated in response to exercise (P < 0.05) and remained elevated throughout the recovery period. IL-6 gene expression was significantly higher (P < 0.05) in the control trial (6.49, confidence interval (CI) 3.57–13.91) compared with the CHO trial (1.98, CI 1.16–3.83) at end of exercise. The peak value in IL-6 expression was observed at 1.5 h post (7.41) in the control trial and at 3 h post exercise (5.64) in the CHO trial.
Figure 3. IL-6 gene expression before, during and after exercise presented as control and CHO trials.
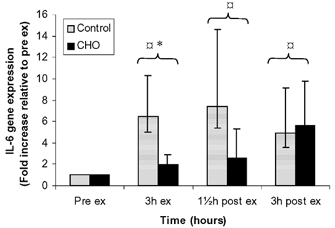
Data are presented as means ± s.e.m.;  significant difference from pre-exercise; * significant difference between control and CHO trial.
significant difference from pre-exercise; * significant difference between control and CHO trial.
IL-6 plasma levels increased throughout the exercise bout and decreased during the recovery period. There was no difference in plasma IL-6 levels between the two trials before exercise (Fig. 4). At end of exercise, IL-6 levels were significantly higher (P < 0.05) in the control trial compared with the CHO trial (26.0 ± 3.7 vs. 15.6 ± 2.4 pg ml−1 respectively).
Figure 4. Plasma IL-6 protein levels before, during and after exercise presented as control and CHO trials.
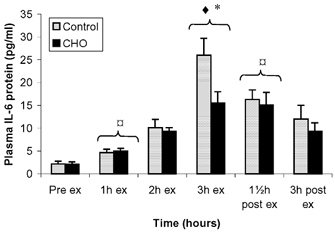
Data are presented as means ± s.e.m.;  significant difference from pre-exercise; * significant difference between control and CHO trial; ♦ significant difference from previous time points.
significant difference from pre-exercise; * significant difference between control and CHO trial; ♦ significant difference from previous time points.
DISCUSSION
The major finding from the present study was a gradual increase in adipose tissue IL-6 mRNA during and after exercise. The relative IL-6 gene expression in adipose tissue over time in response to an exercise bout differs from that of skeletal muscle. Thus, IL-6 in muscle is expressed almost only during the exercise bout and rapidly shut off again (Steensberg et al. 2001), whereas the IL-6 expression in adipose tissue is longer lasting as it extends over the recovery period, but does not reach relatively as high expression levels as observed in skeletal muscle (Ostrowski et al. 1998; Keller et al. 2001; Steensberg et al. 2001), where IL-6 mRNA levels can increase up to 100-fold during low glycogen conditions (Keller et al. 2001).
Another important finding from the present study was that the induction of IL-6 gene expression in adipose tissue at the end of an exercise bout was blunted by carbohydrate ingestion. However, whereas carbohydrate ingestion during exhaustive exercise decreased IL-6 mRNA levels in skeletal muscle (Nieman et al. 2003), two other studies (Starkie et al. 2001; Febbraio et al. 2003) with exercise modes comparable to the one used in the present study found that carbohydrate did not influence IL-6 mRNA in muscle. Thus, this indicates that the regulation of IL-6 in adipose tissue and in muscle may differ in that adipose tissue IL-6 mRNA is decreased during carbohydrate ingestion whereas muscle IL-6 mRNA is not. Of note, however, whereas carbohydrate in general does not influence muscle IL-6 mRNA, low muscle glycogen enhances IL-6 gene expression in response to exercise (Keller et al. 2001; Steensberg et al. 2001).
The stimulatory response for the increased IL-6 expression in adipose tissue has not yet been elucidated. Recent data from our group have demonstrated that infusion of IL-6 in human subjects can exert a positive feedback on IL-6 gene expression (P. Keller, C. Keller, A. Steensberg, C. Fischer & B. K. Pedersen, unpublished data). As adipocytes express the IL-6 receptor (Path et al. 2001), one factor for adipose tissue IL-6 gene induction could be the binding of IL-6 released from the contracting skeletal muscles, hereby enhancing the IL-6 production in adipose tissue. Recently, we demonstrated that although carbohydrate did not influence IL-6 gene expression in skeletal muscle, the release of IL-6 from contracting skeletal muscle was attenuated (Febbraio et al. 2003). Thus, the decreased muscle-derived IL-6 during carbohydrate ingestion is compatible with the suggestion that muscle-derived IL-6 induces adipose IL-6 production.
Furthermore, adipose tissue lipolysis is decreased in response to carbohydrate ingestion during exercise (De et al. 1998), which corresponds with decreased plasma IL-6 levels. As previously suggested (Steensberg et al. 2000), IL-6 may serve as an energy status hormone in muscle in response to exercise, signalling to liver or adipose tissue to increase substrate availability through glycogenolysis or lipolysis, respectively. The increased IL-6 production in adipose tissue in the late exercise phase may thus counteract the increased energy need in the working skeletal muscle and overall energy deficiency in the recovery period. The mechanism of production of IL-6 from adipose tissue could be an autocrine function, binding of circulating IL-6 to its receptor, which is expressed on adipocyte surfaces (Path et al. 2001), thus leading to increased lipolysis in this tissue.
In conclusion, we have demonstrated that exercise can induce IL-6 gene expression, not only in the muscle, but also in adipose tissue. Given that IL-6 induces an increased appearance and disappearance of FFA, the increased IL-6 production in adipose tissue may provide a link from contracting skeletal muscle to enhanced fat metabolism. The adipose tissue IL-6 production extends over the recovery period, reflecting the increased need of FFA as metabolism goes towards fat oxidation in the post-exercise period where glycogen stores are low.
Acknowledgments
We would like to thank the subjects for their participation. Ruth Rovsing, Hanne Willumsen and Carsten Nielsen are acknowledged for their excellent technical assistance. The study was supported by grants from the Danish National Research Foundation (no. 504–14), the Novo Nordisk Foundation, Lundbeckfonden, Rigshospitalet, H:S, and civil engineer Frode VNyegaard og Hustrus Fond, Danfoss. Sonya Marshall was a visiting fellow from the School of Exercise Science and Sport Management, Southern Cross University, Lismore, NSW, 2480, Australia.
REFERENCES
- Blumberg D, Hochwald S, Brennan MF, Burt M. Interleukin-6 stimulates gluconeogenesis in primary cultures of rat hepatocytes. Metabolism. 1995;44:145–146. doi: 10.1016/0026-0495(95)90255-4. [DOI] [PubMed] [Google Scholar]
- De G, Harant I, Crampes F, Trudeau F, Felez A, Cottet-Emard JM, Garrigues M, Riviere D. Effect of carbohydrate ingestion on adipose tissue lipolysis during long-lasting exercise in trained men. J Appl Physiol. 1998;84:1627–1632. doi: 10.1152/jappl.1998.84.5.1627. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Steensberg A, Keller C, Starkie RL, Nielsen HB, Krustrup P, Ott P, Secher NH, Pedersen BK. Glucose ingestion attenuates interleukin-6 release from contracting skeletal muscle in humans. J Physiol. 2003;549:607–612. doi: 10.1113/jphysiol.2003.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M. Interleukins and exercise. J Physiol. 2000;529:1. doi: 10.1111/j.1469-7793.2000.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helge JW, Stallknecht B, Pedersen BK, Galbo H, Kiens B, Richter EA. The effect of graded exercise on IL-6 release and glucose uptake in human skeletal muscle. J Physiol. 2003;546:299–305. doi: 10.1113/jphysiol.2002.030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemaki T, Kitade H, Kaibori M, Sakitani K, Hiramatsu Y, Kamiyama Y, Ito S, Okumura T. Interleukin 1beta and interleukin 6, but not tumor necrosis factor alpha, inhibit insulin-stimulated glycogen synthesis in rat hepatocytes. Hepatology. 1998;27:1296–1303. doi: 10.1002/hep.510270515. [DOI] [PubMed] [Google Scholar]
- Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD. Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J. 2001;15:2748–2750. doi: 10.1096/fj.01-0507fje. [DOI] [PubMed] [Google Scholar]
- Langberg H, Olesen J, Gemmer C, Kjaer M. Substantial elevation of interleukin-6 concentration in peritendinous tissue, in contrast to muscle, following prolonged exercise in humans. J Physiol. 2002;542:985–990. doi: 10.1113/jphysiol.2002.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngso D, Simonsen L, Bulow J. Interleukin-6 production in human subcutaneous abdominal adipose tissue: the effect of exercise. J Physiol. 2002a;543:373–378. doi: 10.1113/jphysiol.2002.019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngso D, Simonsen L, Bulow J. Metabolic effects of interleukin-6 in human splanchnic and adipose tissue. J Physiol. 2002b;543:379–386. doi: 10.1113/jphysiol.2002.021022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack C. Subcutaneous adipose tissue releases interleukin-6, but not tumour necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol. 2003;94:1917–1925. doi: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Henson DA, Utter A, Davis JM, Williams F, Butterworth DE. Influence of mode and carbohydrate on the cytokine response to heavy exertion. Med Sci Sports Exerc. 1998;30:671–678. doi: 10.1097/00005768-199805000-00005. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B, Pedersen BK, Moller K, Secher NH. Interleukin-6 release from the human brain during prolonged exercise. J Physiol. 2002;542:991–995. doi: 10.1113/jphysiol.2002.022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998;508:949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Path G, Bornstein SR, Gurniak M, Chrousos GP, Scherbaum WA, Hauner H. Human breast adipocytes express interleukin-6 (IL-6) and its receptor system: increased IL-6 production by beta-adrenergic activation and effects of IL-6 on adipocyte function. J Clin Endocrinol Metab. 2001;86:2281–2288. doi: 10.1210/jcem.86.5.7494. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: Regulation, integration and adaption. Physiol Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Starkie RL, Arkinstall MJ, Koukoulas I, Hawley JA, Febbraio MA. Carbohydrate ingestion attenuates the increase in plasma interleukin 6, but not skeletal muscle interleukin 6 mRNA, during exercise in humans. J Physiol. 2001;533:585–591. doi: 10.1111/j.1469-7793.2001.0585a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol. 2001;537:633–639. doi: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Fischer CP, Sacchetti M, Keller C, Osada T, Schjerling P, Hall GG, Febbraio MA, Pedersen BK. Acute interleukin-6 administration does not impair muscle glucose uptake or whole-body glucose disposal in healthy humans. J Physiol. 2003;548:631–638. doi: 10.1113/jphysiol.2002.032938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Pedersen BK. Production of IL-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma IL-6. J Physiol. 2000;529:237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Steensberg A, Sachetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Febbraio MA, Pedersen BK. Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003 doi: 10.1210/jc.2002-021687. in press. [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson J-O. Interleukin-6-deficient mice develop mature-onset obesity. Nature Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]


