Abstract
The tetrodotoxin-resistant sodium channel α subunit, Nav1.8, is exclusively expressed in primary sensory neurons and is suggested to play a role in the generation of ectopic action potentials after axonal injury and thereby contribute to neuropathic pain. Here we investigated the involvement of Nav1.8 in ectopic impulse generation in damaged axons by examining spontaneous activity and mechanosensitivity in neuromas formed by section of the saphenous nerve in Nav1.8 null mice and in their wild-type littermates. We recorded 522 identified units from 24 neuromas in vitro at two time points, 8–11 days (median 10 days) and 19–29 days (median 22 days) post-operatively. At ≈10 days, neither genotype showed spontaneous activity, but a significantly higher proportion of fibres were mechanosensitive in wild-type (54 %) compared to Nav1.8 null neuromas (18 %). At ≈22 days, 19 % of fibres recorded in wild-type neuromas showed spontaneous activity, whereas only one fibre of the 238 (0.4 %) recorded in neuromas taken from null mice showed ongoing activity. In recordings at ≈22 days, a similar proportion of fibres were mechanosensitive in wild-type and Nav1.8 null neuromas (51 and 46 %, respectively). We conclude that Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons, and may also contribute to the development of ectopic mechanosensitivity.
Chronic axonal damage of sensory neurons often results in painful and dysaesthetic sensations. These positive sensory symptoms of peripheral nerve injury are produced by ectopic nerve impulses in the damaged neurons generated at the site of injury by sprouting axons (known as a neuroma) and also at the soma. The molecular mechanisms underlying spontaneous electrogenesis in neuromas after axonal damage are unknown, but there is evidence for the involvement of voltage-gated sodium channels, including those containing the tetrodotoxin-resistant α subunit Nav1.8.
Sodium channels accumulate at the sites of sprouting after axonal damage (Devor et al. 1993) and pharmacological experiments reveal an important role for voltage-gated sodium channels in spontaneous electrogenesis in neuromas (Matzner & Devor, 1994). A selective accumulation of Nav1.8 in injured nerve fibres in the rat (Novakovic et al. 1998) and in damaged nerves and skin from patients with painful neuropathy (Coward et al. 2000; Yiangou et al. 2000) has been shown using subtype-specific antibodies.
All these data suggest that Nav1.8 may play a role in the generation of spontaneous activity after axonal injury and therefore contribute to neuropathic pain. This hypothesis is reinforced by the observation that inhibiting the expression of Nav1.8 protein using antisense oligonucleotides reverses thermal and mechanical hyperalgesia produced by spinal nerve ligation in rats (Porreca et al. 1999; Lai et al. 2002). In the present study we investigated the involvement of Nav1.8 in ectopic impulse generation by examining spontaneous activity and ectopic mechanosensitivity in neuromas formed by section of the saphenous nerve in wild-type and Nav1.8 null mice. For these experiments we developed a preparation for recording single unit activity from mouse saphenous nerve neuromas in vitro.
METHODS
Adult mice of either sex (n = 19, body weight 20–35 g) were used. Mice homozygous for the disrupted allele (-/- or null) were compared with littermate wild-type (+/+) mice (Akopian et al. 1999). European Union and Spanish State legislation regulating animal experiments was followed and the Animal Care Committee of the University of Alcala (Madrid, Spain) approved the experimental protocols. Statistical analyses were performed using ANOVA with post hoc tests, or Mann-Whitney U tests or Student's t tests, as appropriate. The level of statistical significance was set at P < 0.05.
Neuroma formation
These methods were adapted from those described in rats by Rivera et al. (2000). Under deep anaesthesia with halothane (2–4 % in pure O2) and with sterile precautions, the saphenous nerve was exposed at the level of the mid-thigh, dissected free and tightly ligated with 8–0 silk. The nerve was cut distal to the ligature and the cut end inserted into a 5 mm long silicone tube (0.45 cm internal diameter) to prevent lateral innervation of surrounding tissue. The tube was tied in place with the same piece of 8–0 silk so that the cut end of the nerve was ≈2 mm from the distal end of the tube. The distal end of the tube was left open and ≈5 mm of the distal nerve stump was excised to prevent reinnervation. The incision was closed in layers. The animals were housed in groups of two to four and inspected daily for infections or abnormal behaviour. The mice had access to water and food ad libitum. In nine mice (3 wild-type and 6 Nav1.8 null) neuromas were made in either one or both saphenous nerves. In the remaining animals (5 wild-type and 5 Nav1.8 null), one neuroma were formed in the saphenous nerve and another was formed in the sciatic nerve of the same limb. Only the saphenous nerve neuromas of all animals were used for the electrophysiological experiments. None of the animals showed any signs of autotomy of the denervated tissue.
Electrophysiological procedures
The mice were humanely killed by cervical dislocation, the saphenous nerve with the neuroma was dissected and the silicon tube around the neuroma was carefully removed. The nerve trunk and the neuroma were excised and placed in a chamber and superfused with oxygenated synthetic interstitial fluid (SIF) with the following composition (mm): 108 NaCl, 3.48 KCl, 0.7 MgSO4, 26 NaHCO3, 1.7 NaH2PO4, 1.53 CaCl2, 9.6 sodium gluconate, 5.55 glucose, 7.6 sucrose (Cervero & Sann, 1989). Temperature was monitored with a thermocouple and maintained at 35 ± 1 °C by means of a Peltier device. The proximal end of the nerve was electrically isolated in a second chamber filled with paraffin oil and positioned on top of a splitting platform where the distal stump was teased into small filaments suitable for recording activity from identified single fibres. When a second neuroma from the same animal was used, it was stored in oxygenated SIF at 4 °C until required. There were no detectable differences in results between the first and second neuromas studied.
Once a filament was teased it was left for a minimum of 2 min in order to record spontaneous activity (defined as a discharge rate ≥ 1 spikes min−1). Then the neuroma was gently probed with a smooth-tipped glass rod (diameter = 1 mm) to search for fibres with mechanosensitivity. ‘Trains’ of 10 tap stimuli of > 1 s at ≈1 s intervals (measured using a metronome) were also applied. Finally, controlled electrical pulses of variable strength and intensity (0.2–0.5 ms pulse width, maximum strength 10 V) were delivered to the neuroma via a tungsten bipolar stimulating electrode, in order to identify the number of A- and C-fibres present in the filament and to establish the identity of the mechanical and spontaneous units. The electrical activity was recorded with a low-noise AC-coupled amplifier and a monopolar platinum wire electrode. The signals were monitored on an oscilloscope and the recordings were digitized and stored using a digital tape-recorder. The data was analysed off-line on a computer (running Spike 2 software, CED Ltd, UK). The spike shape of a spontaneous fibre was averaged (at least three action potentials) and then the shape was superimposed on the recording obtained with the electrical stimulation to identify spontaneous fibres. An experimenter unaware of genotype reviewed the data analysis independently.
The electrophysiological experiments were conducted over two different time periods after nerve section. A first group of animals was studied between 8–11 days (median 10 days) after nerve section, referred to as 10 days post-operatively. A second group of mice was studied after 19–29 days (median 22 days), referred to as 22 days post-operatively.
RESULTS
A total of 522 identified axotomized fibres were recorded, in 24 neuromas, eight from wild-type and 16 from Nav1.8 null mice. No differences between neuromas from male and female mice were noted. In neuromas from both wild-type and Nav1.8 null mice the fibres were classified off-line according to their conduction velocity as A-units (CV (conduction velocity) > 1 m s−1) or C-units (CV < 1 m s−1). There were no statistically significant differences in the conduction properties of the fibres overall between the two groups of mice (see Table 1). However, in both groups of neuromas a proportion of the action potentials recorded (52 of 234 or 22 % in wild-type and 40 of 288 or 14 % in Nav1.8 null mice) were not identified by electrical stimulation but by their spontaneous activity or their responsiveness to mechanical stimulation. In our preparation the conduction distance was ≈10 mm (range = 9.5–11 mm), and the stimulus artefact obscured the first 1–1.5 ms of the record. Therefore, it would have been impossible to record the electrically evoked action potential of any fibre conducting faster than 10 m s−1. Thus it is likely that the population of fibres not identified electrically were either Aβ-fibres or the faster Aδ-fibres (Cain et al. 2001).
Table 1.
Mean conduction velocities (m s−1) of the different populations of fibres recorded from neuromas taken from wild-type and Nav1.8 null mice
| Fibre properties | Wild-type | Nav1.8 null |
|---|---|---|
| All Units | 2.56 ± 0.15 | 2.05 ± 0.14 |
| Mechanical | 3.26 ± 0.27* | 2.96 ± 0.24* |
| Non-mechanical | 2.16 ± 0.19 | 1.59 ± 0.15 |
| Spontaneous | 2.27 ± 0.48 | — |
| Non-Spontaneous | 2.64 ± 0.16 | — |
Data are shown as mean ± s.e.m.
Statistically significant difference between mechanosenstive fibres (P <0.005).
Spontaneous activity develops over time in wild-type mice
In wild-type neuromas studied 10 days post-operatively, none of the filaments recorded showed any signs of spontaneous firing. In these experiments, 42 fibres were identified. In contrast, neuromas from wild-type mice examined 22 days post-operatively showed a substantial incidence of repetitive spontaneous firing. Of the 192 fibres identified, 38 showed spontaneous activity. These 38 units included 10 C-fibres (mean CV 0.45 ± 0.03 m s−1) and 16 A-fibres (mean CV 2.4 ± 0.9 m s−1). The remaining 12 fibres were not electrically identified. Two distinct patterns of repetitive firing were found; 30 fibres showed irregular ongoing discharges throughout the recording period and eight fibres fired in bursts. Examples of these two patterns are shown in Fig. 1. When the group of spontaneous fibres with ongoing discharges was analysed according to their conduction velocity, C-units had significantly greater firing rates than the A-units and the unidentified units (Fig. 2). Within the group of fibres with a known conduction velocity, there was an inverse correlation between the discharge rate and conduction velocity.
Figure 1. Examples of original recordings of fibres with spontaneous activity recorded from neuromas taken from wild-type mice 22 days post-operatively.
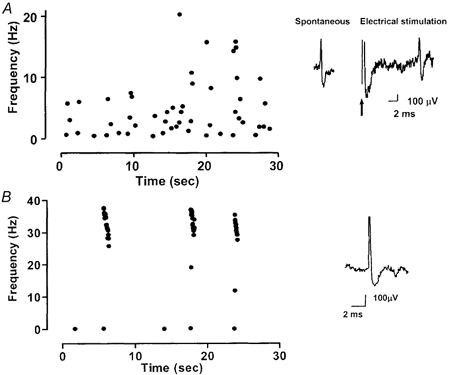
The discharge rate is represented as instantaneous frequency. A, an example of regular ongoing discharge in a C-fibre. The inset shows the spike shape averaged from the spontaneous firing and the response to electrical stimulation of the neuroma. The arrow indicates the stimulus artefact. The recording distance was 9.5 mm. B, an example of bursting discharge in an Aδ-fibre. The inset shows the spike shape averaged from the spontaneous firing.
Figure 2. Firing rate of fibres from the neuroma.
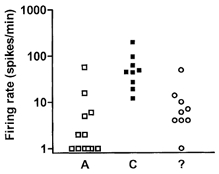
Firing rate of the fibres with regular (non-bursty) spontaneous activity recorded from neuromas taken from wild-type mice 22 days post-operatively shown according to fibre class. A, A-fibres; C, C-fibres; ?, electrically unidentified fibres. The C-fibres showed significantly higher rates of firing than A δ-units and electrically unidentified fibres.
Nav1.8 is essential for the development of spontaneous activity
When the presence of spontaneous firing was compared in wild-type and Nav1.8 null mice, we found that the incidence of spontaneous activity in Nav1.8 null mice was almost zero, in sharp contrast to neuromas taken from wild-type mice (Fig. 3A). None of the 50 fibres studied at 10 days post-operatively in Nav1.8 null neuromas showed any kind of spontaneous firing. Only one fibre of the 238 fibres identified in neuromas from Nav1.8 null mice studied 22 days post-operatively showed ongoing spontaneous activity. This fibre was classified as a C-unit and fired at a rate of 0.6 Hz.
Figure 3. Spontaneous activity and mechanosensitivity of neuroma fibres.
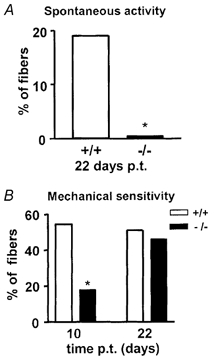
Percentage of spontaneously active (A) and mechanoresponsive (B) fibres recorded from neuromas at 10 and 22 days post-operatively in wild-type and Nav1.8 null mice. p.t., post trauma. * P < 0.05.
Nav 1.8 has a role in the expression of ectopic mechanical responsiveness at early time points
In neuromas taken from wild-type mice studied 10 days post-operatively, more than half the identified fibres were excited by mechanical stimulation of the neuroma (Fig. 3). These fibres included 20 that were not electrically identified, along with two Aδ-fibres and one C-fibre. Neuromas taken from Nav1.8 null mice 10 days post-operatively showed a significantly lower incidence of mechanoresponsive fibres; less than 20 % of units responded to mechanical stimulation of the neuroma (Fig. 3B). Of these, three units were Aδ-fibres, three were C-fibres and the remaining three units were not identified electrically. However, in neuromas studied at 22 days post-operatively from both wild-type and null mice, there was a similar incidence of mechanosensitive fibres in both groups of mice (Fig 3). The mechanosensitive fibres had significantly faster conduction velocities than the non-mechanically sensitive fibres in both groups of mice (see Table 1). A proportion of the spontaneously active fibres recorded in wild-type mice at 22 days post-operatively were also mechanosensitive (20 of the 38 spontaneously active fibres). These fibres that showed both mechanosensitivity and ongoing activity included 13 Aδ-fibres, three C-fibres and four fibres that were not identified electrically. However, the single spontaneously active C-fibre recorded in a Nav1.8 null neuroma was not affected by mechanical stimulation.
Nav 1.8 is not required for the expression of afterdischarges
In uninjured nerves, afferent fibres almost never show more than one action potential to a single electrical stimulus. Similarly, repetitive firing after application of a brief mechanical stimulus is very rare. However, after axotomy, afterdischarges are present in 2–5 % of A-fibre cell bodies recorded in the dorsal root ganglion (see Amir et al. 2002). In the present study a small number of fibres with afterdischarges were recorded in neuromas taken from both wild-type and Nav1.8 null mice. In wild-type neuromas studied 10 days post-operatively, one fibre of the 42 recorded showed signs of afterdischarges. This fibre was an A-fibre that showed variable bursting afterdischarges after electrical stimulation. In wild-type neuromas recorded at 22 days post-operatively, two A-fibres showed occasional afterdischarges during the recording period related to either mechanical or electrical stimulation. Similarly, in Nav1.8 null neuromas recorded at 22 days post-operatively, two A-fibres showed afterdischarges after electrical stimulation.
DISCUSSION
In the present study we found that 19 % of the axotomized fibres ending in a neuroma in wild-type mice ≈22 days after surgery showed spontaneous firing. In contrast, in neuromas taken from Nav1.8 null mice ≈22 days post-operatively, only one fibre showed low grade spontaneous firing. The spontaneous activity developed over time in the wild-type animals, since no activity was seen 10 days post-operatively. At 10 days, a significantly higher proportion of fibres was mechanosensitive in wild-type (54 %) compared to Nav1.8 null neuromas (18 %). However by 22 days, a similar proportion of fibres was mechanosensitive in wild-type and Nav1.8 null neuromas.
Nav 1.8 is essential for the development of spontaneous activity
Only one fibre in Nav1.8 null neuromas showed ongoing firing, giving an extremely low incidence of spontaneous activity of 0.4 %. This fibre was a C-fibre that discharged at a rate of 0.6 Hz. In normal animals without nerve lesions, occasional C-fibres are encountered with low frequency ongoing activity. These fibres usually respond to cooling. For example Cain et al. (2001) found one such fibre in a study of 225 fibres recorded in the normal mouse tibial nerve. Thus the incidence of spontaneous activity in the Nav1.8 null neuromas was in the range found in a normal, undamaged nerve innervating the skin. In contrast, neuromas from wild-type mice showed a much higher incidence of spontaneous activity (19 %), as expected from previous work (for review see Devor & Seltzer, 1999).
The present observations strongly suggest that the accumulation of Nav1.8 protein in neuromas revealed using subtype-selective antibodies (Novakovic et al. 1998; Coward et al. 2000; Yiangou et al. 2000) is required for the spontaneous activity. The expression of tetrodotoxin-sensitive sodium channels in the dorsal root ganglion cell body is increased in Nav1.8 null mice (Akopian et al. 1999), which in principle could contribute to the absence of spontaneous firing in neuromas in these mice. However, pharmacological evidence supports the hypothesis that sodium channels in damaged axonal endings are the substrate for spontaneous firing (Matzner & Devor, 1994; Omana-Zapata et al. 1997), thus it seems unlikely that the compensatory increase in tetrodotoxin-sensitive sodium channel expression prevents ectopic firing. Furthermore, the intensity of Nav1.8 expression in normal dorsal root ganglion neurons is inversely correlated with the conduction velocity of the axon (Fang et al. 2001). In the present study, we also found that the rate of spontaneous activity in wild-type mice was greater in fibres with slower conduction velocities, suggesting that dense accumulations of Nav1.8 channels may increase the probability of higher rates of spontaneous firing. It is striking that the accumulation of Nav1.8 protein is unrelated to the lower levels of mRNA encoding the Nav1.8 protein in DRG somata that occur in a variety of neuropathic pain models (Okuse et al. 1997).
Nav1.8 mRNA is expressed in 69 % of cell bodies in normal rat dorsal root ganglion (Novakovic et al. 1998). However, here only 19 % of all fibres showed spontaneous activity 22 days after injury. Two factors may account for this apparent mismatch. Firstly, Nav1.8 appears to be necessary but may not be sufficient to provoke spontaneous electrogenesis in a damaged axon. Secondly, the proportions of fibres developing spontaneous activity after complete nerve section varies widely depending on the species, the nerve and the time post-injury examined (for review see Devor & Seltzer, 1999). The expression of Nav1.8 and/or the subcellular redistribution of Nav1.8 that occurs after axonal injury may also vary depending on the species, peripheral nerve, time after injury and type of nerve injury (Dib-Hajj et al. 1996; Novakovic et al. 1998).
Nav 1.8 contributes to the expression of ectopic mechanosensitivity at early time points
Ectopic mechanosensitivity is proposed to be due to the incorporation in the axonal terminal of transducer proteins destined for the normal receptor ending of the fibre (Devor & Goran-Lippman, 1983; Korschorke et al. 1991; Michaelis et al. 1999). This is consistent with our observation that the conduction velocity of the mechanosensitive population of fibres was greater than those that did not exhibit mechanosensitivity, because in normal, undamaged nerves, mechanically sensitive afferent fibres tend to have more rapidly conducting axons. Mechanosensitivity of sectioned axons can be detected only a few hours after injury, before spontaneous firing develops (Michaelis et al. 1999). Our data show that mechanosensitive terminals do occur in the absence of Nav1.8, so Nav1.8 is not essential. However, voltage-gated sodium channels control membrane excitability and action potential generation. The accumulation of Nav1.8 in the terminals may increase excitability and reduce firing thresholds, enabling the activation of the fibre by mechanotransducers. The mechanosensitive sites within a fibre are located very close to the sites of spontaneous electrogenesis (Chen & Devor, 1998), supporting this possibility. In the present study, the absence of Nav1.8 reduced mechanosensitivity at 10 days after injury but not thereafter, suggesting that with time mechanotransducers accumulate or other sodium channel subtypes compensate for the lack of Nav1.8. In human neuromas, a positive and painful Tinel's sign (sensation produced by tapping the injured nerve) is associated with intense staining for Nav1.8 (Coward et al. 2000; Yiangou et al. 2000).
Potential role of Nav1.8 in neuropathic pain
Spontaneous impulse discharge from axonal sprouts clearly produces positive sensory symptoms including pain in patients with chronic sensory axonal damage (see Rizzo et al. 1996; Waxman et al. 1999) and our data strongly suggest that Nav1.8 is essential for this spontaneous electrogenesis. Several sodium channel blockers are effective analgesics in the clinic, but they lack specificity and so their effects on other excitable membranes are dose-limiting. Nav1.8 is exclusively expressed in primary afferent neurons and in the light of our data is thus an extremely attractive target for drug development.
The most direct sensory consequence of spontaneous impulse generation in the neuroma is likely to be continuous dysaesthetic and paraesthetic sensation (Rizzo et al. 1996), although this discharge may also maintain hyperalgesia and allodynia (e.g. Gracely et al. 1992). Experiments testing the participation of Nav1.8 in spontaneous pain-related behaviour after nerve injury have not been reported. Nonetheless, mechanical and thermal hyperalgesia in rats after spinal nerve ligation is reversed by treatment with Nav1.8 antisense (Porreca et al. 1999; Lai et al. 2001), strongly supporting a role for Nav1.8 in hyperalgesia of neuropathic origin. A very recent report (Gold et al. 2003) describes redistribution of Nav1.8 in uninjured axons following nerve injury and suggests a prominent role of Nav1.8 in neuropathic pain.
However, Nav1.8 null mice showed normal hyperalgesia in a different model of peripheral nerve injury, partial ligation of the sciatic nerve (Kerr et al. 2001). The behavioural experiments were performed 3–14 days after nerve injury (Kerr et al. 2001), when no spontaneous activity is observed in the neuroma in wild-type animals (present data). This suggests that at these early time points, hyperalgesia is maintained by other mechanisms, for example, spontaneous discharge from the soma of damaged axons (Devor & Seltzer, 1999), or from adjacent, undamaged axons (e.g. Wu et al. 2001). Spontaneous activity from these alternative sources has an earlier onset than spontaneous activity in the neuroma (see Devor & Seltzer, 1999; Wu et al. 2001). Nav1.8 is not the strongest candidate for mediating spontaneous activity arising from the soma, since after some (although not all) types of axonal injury its expression in the soma is markedly reduced (Dib-Hajj et al. 1996; Okuse et al. 1997; Novakovic et al. 1998). However, it is also possible that Nav1.8 plays a partial role in the expression of neuropathic hyperalgesia, but that compensatory over-expression of tetrodotoxin-sensitive sodium channels in the null mutant mice masks the full Nav1.8 null phenotype (Akopian et al. 1999). This type of phenotype masking occludes a deficit in thermal hyperalgesia due to intraplantar carrageenan in these mice (Akopian et al. 1999).
Acknowledgments
This study was supported by the Madrid Regional Government (Contrato Programa), the Ministry of Science & Technology, Spain (SAF-2000-0199), the Medical Research Council and the Wellcome Trust, UK. The authors are grateful to María-José García for expert technical assistance and to Fleur Geoghegan for help with genotyping.
REFERENCES
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill RG, Stanfa L, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M. Burst discharge in primary sensory neurons: triggered by subthreshold oscillations, maintained by depolarizing afterpotentials. J Neurosci. 2002;22:1187–1198. doi: 10.1523/JNEUROSCI.22-03-01187.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol. 2001;85:1561–1574. doi: 10.1152/jn.2001.85.4.1561. [DOI] [PubMed] [Google Scholar]
- Cervero F, Sann H. Mechanically evoked responses of afferent fibres innervating the guinea-pig's ureter: An in vitro study. J Physiol. 1989;412:245–266. doi: 10.1113/jphysiol.1989.sp017613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Devor M. Ectopic mechanosensitivity in injured sensory axons arises from the site of spontaneous electrogenesis. Eur J Pain. 1998;2:165–178. doi: 10.1016/s1090-3801(98)90009-x. [DOI] [PubMed] [Google Scholar]
- Coward K, Plumpton C, Facer P, Birch R, Carlstedt T, Tate S, Bountra C, Anand P. Immunolocalization of SNS/PN3 and NaN/SNS2 sodium channels in human pain states. Pain. 2000;85:41–50. doi: 10.1016/s0304-3959(99)00251-1. [DOI] [PubMed] [Google Scholar]
- Devor M, Govrin-Lippmann R, Angelides K. Na+ channel immunolocalization in peripheral mammalian axons and changes following nerve injury and neuroma formation. J Neurosci. 1993;13:1976–1992. doi: 10.1523/JNEUROSCI.13-05-01976.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M, Seltzer Z. The pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R, editors. Textbook of Pain. Edinburgh: Churchill Livingstone; 1999. pp. 129–164. [Google Scholar]
- Dib-Hajj S, Black JA, Felts P, Waxman SG. Down-regulation of transcripts for Na channel alpha-SNS in spinal sensory neurons following axotomy. Proc Natl Acad Sci U S A. 1996;93:14950–14954. doi: 10.1073/pnas.93.25.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, Okuse K, Wood JN, Lawson SJ. Sensory and electrophysiological properties of DRG neurones with SNS-like immunoreactivity (SNS-Li) in rats. Soc Neurosci Abst. 2001;27:819. [Google Scholar]
- Gold MS, Weinreich D, Kim CS, Wang R, Treanor J, Porreca F, Lai J. Redistribution of Na(V)1. 8 in uninjured axons enables neuropathic pain. J Neurosci. 2003;23:158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracely RH, Lynch SA, Bennett GJ. Painful neuropathy: Altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194. doi: 10.1016/0304-3959(92)90259-E. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Souslova V, McMahon SB, Wood JN. A role for the TTX-resistant sodium channel Nav1. 8 in NGF-induced hyperalgesia but not neuropathic pain. Neuroreport. 2001;12:3077–3080. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- Koschorke GM, Meyer RA, Tillman DB, Campbell JN. Ectopic excitability of injured nerves in monkey: entrained responses to vibratory stimuli. J Neurophysiol. 1991;65:693–701. doi: 10.1152/jn.1991.65.3.693. [DOI] [PubMed] [Google Scholar]
- Lai J, Gold MS, Kim CS, Biana D, Ossipov MH, Hunter JC, Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1. 8. Pain. 2002;95:143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Matzner O, Devor M. Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol. 1994;72:349–359. doi: 10.1152/jn.1994.72.1.349. [DOI] [PubMed] [Google Scholar]
- Michaelis M, Blenk KH, Vogel C, Janig W. Distribution of sensory properties among axotomized cutaneous C-fibres in adult rats. Neuroscience. 1999;94:7–10. doi: 10.1016/s0306-4522(99)00325-5. [DOI] [PubMed] [Google Scholar]
- Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci. 1998;18:2174–2187. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuse K, Chaplan SR, McMahon S, Luo ZD, Calcutt NA, Scott BP, Akopian AN, Wood JN. Regulation of expression of the sensory neuron specific sodium channel SNS in inflammatory and neuropathic pain. Mol Cell Neurosci. 1997;10:196–207. doi: 10.1006/mcne.1997.0657. [DOI] [PubMed] [Google Scholar]
- Omana-Zapata I, Khabbaz MA, Hunter JC, Bley KR. QX-314 inhibits ectopic nerve activity associated with neuropathic pain. Brain Res. 1997;771:228–237. doi: 10.1016/s0006-8993(97)00770-1. [DOI] [PubMed] [Google Scholar]
- Porreca F, Lai J, Bian D, Wegert S, Ossipov MH, Eglen RM, Kassotakis L, Novakovic S, Rabert DK, Sangameswaran L, Hunter JC. A comparison of the potential role of the tetrodotoxin-insensitive sodium channels PN3/SNS and NaN/SNS2, in rat models of chronic pain. Proc Natl Acad Sci U S A. 1999;96:7640–7644. doi: 10.1073/pnas.96.14.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera L, Gallar J, Pozo MA, Belmonte C. Responses of nerve fibres of the rat saphenous nerve neuroma to mechanical and chemical stimulation: an in vitro study. J Physiol. 2000;527:305–313. doi: 10.1111/j.1469-7793.2000.t01-1-00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo MA, Kocsis JD, Waxman SG. Mechanisms of paresthesiae, dysesthesiae, and hyperesthesiae: Role of Na+ channel heterogeneity. Eur Neurol. 1996;36:3–12. doi: 10.1159/000117192. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Cummins TR, Dib-Hajj SD, Fjell J, Black JA. Sodium channels, excitability of primary sensory neurons and the molecular basis of pain. Muscle Nerve. 1999;22:1177–1187. doi: 10.1002/(sici)1097-4598(199909)22:9<1177::aid-mus3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fibre nociceptors after injury to neighboring nerve fibres. J Neurosci. 2001;21:RC6–RC10. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiangou Y, Birch R, Sangameswaran L, Eglen R, Anand P. SNS/PN3 and SNS2/NaN sodium channel-like immunoreactivity in human adult and neonate injured sensory nerves. FEBS Lett. 2000;467:249–252. doi: 10.1016/s0014-5793(00)01166-2. [DOI] [PubMed] [Google Scholar]


