Abstract
We have examined the distribution of the sensory neuron-specific Na+ channel Nav1.8 (SNS/PN3) in nociceptive and non-nociceptive dorsal root ganglion (DRG) neurons and whether its distribution is related to neuronal membrane properties. Nav1.8-like immunoreactivity (Nav1.8-LI) was examined with an affinity purified polyclonal antiserum (SNS11) in rat DRG neurons that were classified according to sensory receptive properties and by conduction velocity (CV) as C-, Aδ- or Aα/β. A significantly higher proportion of nociceptive than low threshold mechanoreceptive (LTM) neurons showed Nav1.8-LI, and nociceptive neurons had significantly more intense immunoreactivity in their somata than LTM neurons. Results showed that 89, 93 and 60 % of C-, Aδ- and Aα/β-fibre nociceptive units respectively and 88 % of C-unresponsive units were positive. C-unresponsive units had electrical membrane properties similar to C-nociceptors and were considered to be nociceptive-type neurons. Weak positive Nav1.8-LI was also present in some LTM units including a C LTM, all Aδ LTM units (D hair), about 10 % of cutaneous LTM Aα/β-units, but no muscle spindle afferent units. Nav1.8-LI intensity was negatively correlated with soma size (all neurons) and with dorsal root CVs in A- but not C-fibre neurons. Nav1.8-LI intensity was positively correlated with action potential (AP) duration (both rise and fall time) in A-fibre neurons and with AP rise time only in positive C-fibre neurons. It was also positively correlated with AP overshoot in positive neurons. Thus high levels of Nav1.8 protein may contribute to the longer AP durations (especially in A-fibre neurons) and larger AP overshoots that are typical of nociceptors.
Somata of DRG neurons with nociceptive properties differ from those with low threshold mechanoreceptive (LTM) properties in their electrical membrane properties including broader/inflected action potentials (APs) (Koerber & Mendell, 1992; Ritter & Mendell, 1992; Djouhri et al. 1998; Gee et al. 1999) and larger AP overshoots (Djouhri & Lawson, 2001a). Ion channel subunits that are preferentially expressed in nociceptive neurons and may be responsible for these differences could be useful targets for novel therapeutic drugs for chronic pain treatment.
Initiation and propagation of APs depends on inward Na+ currents. In adult DRG neurons, Na+ currents include: TTX-sensitive fast-inactivating (TTX-SF), TTX-resistant fast-inactivating (TTX-RF), TTX-R slowly inactivating (TTX-RS) and TTX-R persistent (TTX-RP) currents (see Baker & Wood, 2001; Chung et al. 2003). Because TTX-SF currents are in all types of DRG neurons (Kostyuk et al. 1981; Caffrey et al. 1992; Roy & Narahashi, 1992), and TTX-RF is detectable in only ≈3 % of small DRG neurons of adult rat (Renganathan et al. 2002), neither is likely to be associated selectively with most nociceptive neurons. On the other hand, TTX-RS (Roy & Narahashi, 1992; Elliott & Elliott, 1993; Rush et al. 1998) and TTX-RP currents (Dib-Hajj et al. 2002; Tate et al. 1998) are primarily in smaller DRG neurons, making them candidates for expression in nociceptive neurons. Two α-subunits, Nav1.8 and Nav1.9, that have been cloned, are associated with these TTX-R currents. Nav1.9 has a low (hyperpolarized) activation threshold and ultraslow inactivation, and gives rise to the TTX-RP current (Waxman, 1999; Cummins et al. 1999; Chung, 2003), while Nav1.8, also known as SNS, PN3 or ScN10A (Akopian et al. 1996; Sangameswaran et al. 1996; Oaklander & Belzberg, 1997; Goldin et al. 2000), has a relatively depolarized (high) threshold of activation (−30 mV), and is thought to give rise to the TTX-RS inward Na+ current and contribute to the inward current in TTX-R APs (Akopian et al. 1996; Renganathan et al. 2001; Chung et al. 2003). Although we have previously found that expression of Nav1.9 was limited to nociceptive neurons in rat DRGs (Fang et al. 2002), the sensory properties of DRG neurons expressing Nav1.8 have not been studied.
The properties of Nav1.8 and its expression (mRNA and protein) mainly in small-medium sized DRG neurons (Akopian et al. 1996; Sangameswaran et al. 1996; Tate et al. 1998; Novakovic et al. 1998; Amaya et al. 2000) are consistent with the known distribution of TTX-RS current and with its contribution to the high threshold TTX-R broad APs in nociceptive DRG neurons (Waddell & Lawson, 1990; Ritter & Mendell, 1992; Djouhri et al. 1998).
So far, the evidence linking this channel with nociceptors is indirect, being based on cell size and membrane properties mainly of isolated DRG neurons in vitro. However, the assumption that all small-medium sized neurons are nociceptive may be misleading, because there is overlap in cell size between DRG neurons with C-, Aδ- and Aα/β-fibres (Harper & Lawson, 1985) and ample evidence for nociceptive and LTM neurons with all these types of fibre (see review, Lawson, 2002). We have therefore examined directly whether Nav1.8 is indeed expressed selectively or preferentially in nociceptive primary afferent neurons, and whether it is associated with electrophysiological properties in such neurons in vivo. Preliminary results of this study were reported in abstract form (Fang et al. 2001).
METHODS
Meticulous
Young female Wistar rats (weight 160–180 g) were anaesthetized with an initial dose of 65–75 mg kg−1 I.P. of sodium pentobarbitone (25 mg ml−1 in distilled water with 10 % ethanol). This anaesthetic dose produced deep anaesthesia with areflexia (i.e. total absence of limb withdrawal reflex). A tracheotomy was performed to allow artificial ventilation and end-tidal CO2 monitoring. To enable regular intravenous (I.V.) injections of the additional doses of the anaesthetic (10 mg kg−1) that were required to maintain this deep level of anaesthesia, the right carotid vein was cannulated. The left carotid artery was also cannulated to allow monitoring of blood pressure. The temperature in the paraffin pool near the DRG was maintained throughout at 28–32 °C. Experimental procedures complied throughout with UK Home Office Guidelines. Full details of the animal preparation were as reported previously in guinea-pig (Lawson et al. 1997; Djouhri & Lawson, 1999) and rat (Fang et al. 2002).
Just prior to electrophysiological recording all animals were given a muscle relaxant to improve stability during recording. This was pancuronium (0.5 mg kg−1 I.V.) accompanied always by an additional dose (10 mg kg−1, I.V.) of the anaesthetic. The same doses of muscle relaxant and anaesthetic (always given together) were administered at approximately hourly intervals. These anaesthetic doses were the same as those that induced deep anaesthesia during the period (2–3 h) of animal preparation. Blood pressure measurements indicated that using these same doses of muscle relaxant and anaesthetic, the blood pressure remained stable showing no indication of any reduction in the depth of anaesthesia at any stage in the experiment.
Intracellular recordings
Intracellular recordings from DRG somata were made using glass microelectrodes filled with a fluorescent dye. The fluorescent dyes were Lucifer yellow CH (LY) (5 mg ml−1) in 0.1 M LiCl, ethidium bromide (EB) (6 mm) in 1 M KCl or Cascade blue (CB) as a 3 % solution in 0.1 M LiCl. The microelectrode was advanced until a membrane potential was seen and an action potential (AP) could be evoked by dorsal root stimulation with single rectangular pulses (0.03 ms duration for A-fibre units or 0.3 ms for C-fibre units). The stimulus intensity was usually adjusted to twice threshold (for A-fibre units) and between one and two times threshold (for C-fibre units). The somatic APs were recorded online with a CED (Cambridge Electronic Design) 1401 plus interface and the SIGAV program from CED, and they were subsequently analysed offline with a script running in the Spike II program (CED). For each unit, a number of AP variables were measured as described previously (see Djouhri & Lawson, 1999). These included the AP duration at base (AP duration), AP rise time (time from inception of the AP to the peak) and AP fall time (time from the peak to the point where the potential crosses or reaches the resting membrane potential (Vm)). AP overshoots were examined as previously described (Djouhri & Lawson, 2001a); however, unlike that study, we were limited to cells recorded with dye-filled electrodes, some of which had high resistances (> 400 MΩ). While this may have affected the height of some APs, there was no correlation in the study of Djouhri & Lawson (2001a) between AP height and electrode resistance. Even if there was an effect, this would introduce non-systematic scatter into the data rather than affecting patterns related to Nav1.8-like immunoreactivity (Nav1.8-LI) intensity.
The conduction velocity (CV) of each unit was estimated from the latency to onset of the dorsal root evoked somatic AP and the conduction distance. The latter was measured at the end of the experiment from the cathode of the stimulating electrode pair on the dorsal root to the approximate (±0.25 mm) location of the injected neuron in the DRG and was between 4.5 and 13 mm. Utilization time was not taken into account. Neurons were classified as C-, Aδ- and Aα/β-units with C cells conducting in the dorsal root at < 0.8 m s−1, C/Aδ at 0.8–1.4 m s−1, Aδ at 1.4–6.5 m s−1 and Aα/β at > 6.5 m s−1. This classification was based on compound AP recordings from L5 and L6 dorsal roots in rats of the same sex and weight, and paraffin pool temperatures as in this experimental series (see Fang et al. 2002) using methods as described by Djouhri & Lawson (2001b).
Sensory receptive properties
The sensory receptive properties of DRG neurons were identified and classified as described previously (Lawson et al. 1997; Djouhri et al. 1998). Briefly, A-fibre low threshold mechanoreceptive (LTM) units were those that responded to non-noxious mechanical stimuli including light brush of the limb fur, light skin contact and light pressure with blunt objects, light tap, tuning forks vibrating at 100 or 250 Hz and light pressure with calibrated von Frey hairs. Most Aα/β LTM units in this study were cutaneous, with superficial or dermal receptive fields (see Lawson et al. 1997), but a group of non-cutaneous Aα/β-fibre LTM units that responded to light pressure against muscle tissue and that followed vibration of 100 or 250 Hz applied with a tuning fork and which often showed ongoing discharges were classified as muscle spindle (MS) afferent units. This group included group I and II muscle afferents and probably also Golgi tendon organ afferents. Aδ LTM down hair (D hair) units were those with Aδ-fibres that were extremely sensitive to slow movement of hairs, to stretch and to cooling stimuli. Cooling of the skin was usually achieved with a very brief spray with ethyl chloride. C LTM units (also known as C mechanoreceptors) were those units that responded preferentially to very gentle contact moving across the skin at < 1 mm s−1 and sometimes to cooling as previously reported in several species (e.g. Light & Perl, 1993; Djouhri et al. 1998).
Units not responding to the above low intensity stimuli but responding either to noxious mechanical stimuli applied with a needle, fine forceps or coarse toothed forceps, or to both noxious mechanical and noxious heat (hot water at > 50 °C) stimuli were classified as nociceptive neurons. The approximate depth of the receptive field was determined from the minimum amount (depth) of tissue that was stimulated in order to evoke a response, and thus response to a needle prick or stimulation of very superficial tissue with the finest (no. 5) forceps was interpreted as a superficial cutaneous (epidermal or superficial dermal) receptive field. If squeeze of tissue including the dermis was required, this was interpreted as a deep cutaneous (dermal) field, and if squeeze of deeper tissues was required the field depth was designated as ‘deep’. A-fibre nociceptive units in this study included: (a) Aδ- and Aα/β-fibre high threshold mechanoreceptive (A HTM) units responding to noxious mechanical stimuli, (b) A mechano-cooling (A MC) responding to both noxious mechanical stimuli and cooling. No A-mechano-heat units were encountered in these experiments, although we have found small numbers of these in other experiments in guinea-pig (Lawson et al. 1997) and rat (L. Djouhri, X. Fang & S. N. Lawson, unpublished observations). C-fibre nociceptive units in this study include units that responded vigorously to both noxious heat and noxious mechanical stimuli; these we called C polymodal units (C PM) if their receptive fields were in the superficial cutaneous tissue or mechano-heat (C MH) units if their receptive fields were in the deep cutaneous tissues. They also include cutaneous C HTM units that required strong mechanical stimulation but lacked prompt responses to noxious heat and C HTM units with deep receptive fields that responded to strong mechanical stimulation but were not tested with thermal noxious stimuli. Specific C-fibre heat and cooling units were not found in this study, although we found C cooling units in similar studies in guinea-pig (Lawson et al. 1997) and rat (L. Djouhri, X. Fang & S. N. Lawson, unpublished observations).
Units that showed no response to the above non-noxious and noxious mechanical and thermal stimuli are called unresponsive. These unresponsive neurons have been described in several species (Meyer et al. 1991; Handwerker et al. 1991; Schmidt et al. 1995; Gee et al. 1996; Djouhri et al. 1998). They may be nociceptive units with extremely high thresholds, so-called ‘silent nociceptors’, or units for which the appropriate stimuli (e.g. inflammatory mediators) were not applied or which had inaccessible receptive fields. We found that C-fibre unresponsive units had long AP and AHP durations and large AP overshoots (values not shown) typical of nociceptive C-fibre units both in this study and in the guinea-pig (see Djouhri et al. (1998) and Djouhri & Lawson (2001a)). The C-unresponsive units were therefore called ‘nociceptive-type’ neurons in contrast to C LTM units (Djouhri et al. 1998) that had much lower values for AP and AHP durations and AP overshoots. The term ‘nociceptive-type’ is therefore used to encompass C- and A-fibre nociceptive neurons and C-unresponsive neurons. In contrast A-fibre unresponsive units were not included as nociceptor-type units for the following reasons. There were only two Aδ-fibre unresponsive units, again with properties similar to nociceptive units, but too few to form a separate group. Many Aα/β-fibre unresponsive neurons had AP and AHP durations closer to those of the Aα/β LTM units and may therefore have included LTM units with inaccessible receptive fields. Because of small numbers and their mixed properties, A-fibre unresponsive units were not included as a separate group in the analyses to follow.
Fluorescent dye injection
Following characterization of each cell, a dye (LY, EB or CB) was ejected into the soma electrophoretically by rectangular current pulses. These were usually 1 nA, maximum 1.3 nA, for 500 ms at 1 Hz for periods of up to 10–15 min for A-fibre neurons and 6–10 min for C-fibre neurons. Membrane potential and receptive field response were monitored throughout this time. The currents passed were negative for LY and CB and positive for EB. L5 DRGs were about 2 mm long. The usual procedure was to label three neurons in L5 DRGs with LY (two at opposite ends of the ganglion and one in the middle), two further neurons with EB, between the first and second, and between the second and third LY-labelled neurons, respectively. In addition, sometimes neurons were labelled with CB at locations lateral to the tracks made with LY electrodes. The problems associated with identifying the dye-injected cells have been discussed (Lawson et al. 1997) and all precautions outlined in that report were taken and are now routine procedure.
Under deep anaesthesia, each animal was terminally perfused through the heart with 0.9 % saline followed by Zamboni's fixative (Stefanini et al. 1967) at the end of the experiment. The DRGs were dissected out, post-fixed for about 1 h and left overnight in 30 % sucrose in 0.1 M phosphate buffer at 4 °C. Then serial 7 μm cryostat sections were cut and mounted on 20 slides so that on each slide there was a series made up of every 20th section. Each section was examined with fluorescence microscopy and fluorescently labelled neurons were recorded with camera lucida drawings and images captured with a high resolution CCD camera (Optronix Dei-470).
Polyclonal antibody production and Western blotting
Antisera were raised following methods outlined in Harlow & Lane (1988) by immunizing rabbits to an epitope in Nav1.8 (residues 1892–1956), which has no homology to other known Na+ channels.
Antibodies were affinity purified on peptide columns before use. Western blot tests were carried out as follows. Tissues from 3-week-old Sprague-Dawley rats were homogenized in 10 mm phosphate buffer (pH 7.4) and centrifuged at 2000 g for 10 min. The supernatant was centrifuged again at 200 000 g for 60 min. The pellet was solubilized in 10 mm phosphate buffer containing 2 % Triton X-100 and 4 % SDS. This plasma membrane fraction was electrophoresed on SDS-5 % PAGE (0.2 mg lane−1) and transferred to a Hybond ECL nitrocellulose filter (Amersham Pharmacia Biotech) using a wet electrophoretic transfer system. The membrane was blocked for 1 h at room temperature in 5 % non-fat dry milk in PBS-T (0.1 % Tween-20 in PBS, pH 7.4). The blot was probed with polyclonal antibody to NaV1.8 (called SNS11) at 1:10 000 dilution for 1 h at room temperature and visualized with horseradish peroxidase-conjugated sheep anti-rabbit IgG (Amersham Pharmacia Biotech) in 1:2000 dilution using the ECL Western Blotting Detection Reagents (Amersham Pharmacia Biotech) and the blot was exposed to BioMax film (Kodak).
Anti-NaV1.8 antibody SNS11 specifically recognizes NaV1.8
Western blot analysis of the subtype specificity of anti-Nav1.8 polyclonal antibody SNS11 showed a single band of strong immunoreactivity at approximately 240 kDa in the membrane fraction of rat DRG (Fig. 1A) which is consistent with the result obtained with the anti-Nav1.8 monoclonal antibody 9D11C8 (Okuse et al. 1997). Membrane fractions of rat whole brain, cerebellum, skeletal muscle, heart and superior cervical ganglia (SCG), which are known to express other types of voltage-gated Na+ channel such as Nav1.1, 1.2, 1.4, 1.5, 1.6 and 1.7, and membrane fractions from the liver and the kidney all showed no detectable immunoreactivity. The membrane extract from CHO-SNS22, a Chinese hamster ovary cell line stably transfected with rat Nav1.8 cDNA (Okuse et al. 2002), showed a strong band of immunoreactivity at a similar size to the DRG band, while non-transfected CHO cells did not show any bands. The finding of immunoreactive bands only in the membrane extracts from rat DRG and CHO-SNS22 cells suggest that the anti-Nav1.8 polyclonal antibody SNS11 specifically recognizes Nav1.8.
Figure 1. Western blot analysis and relationship of subjective score to relative intensity of Nav1.8-LI.
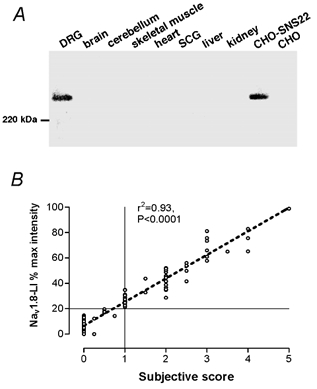
A, Western blot analysis. A single band of strong immunoreactivity at approximately 240 kDa in the membrane fraction of rat DRG is shown. Note that membrane fractions of rat whole brain, cerebellum, skeletal muscle, heart, superior cervical ganglia, liver and kidney showed no detectable immunoreactivity. Membrane extract from CHO-SNS22 (a cell line stably transfected with rat NaV1.8 cDNA) showed strong immunoreactivity at the same size with DRG. Non-transfected CHO cells did not show any bands. B, relationship of subjective score to relative intensity of Nav1.8-LI. The x-axis shows the subjective score (0 for the most negative, 1 for just positive and 5 for the most intense Nav1.8-LI). The y-axis shows the percentage of relative intensity of Nav1.8-LI from 0 to 100 %, ranging from the most negative (0 %) to the most intense Nav1.8-LI (100 %).
Immunocytochemistry
Details of the methods of assessing relative intensity of an immunocytochemical reaction product in DRG neurons have been reported previously (Lawson et al. 1997, 2002; Fang et al. 2002). Briefly, after block of endogenous peroxidase with H2O2, and blocking of endogenous biotin-like activity using an Avidin-Biotin kit (Vector laboratories, Burlingame, CA, USA), sections were first incubated for 2 days at 4 °C in primary antibody (SNS11) against the Nav1.8α-subunit (1.7 × 10−3 μg ml−1) in 0.3 % Triton X-100 in Tris buffer with 1 % normal goat serum and they were then incubated for 30 min at room temperature with biotinylated secondary antibody (anti-rabbit Ig, 1:200) (Vector Laboratories). Diaminobenzidine (DAB) was used to form a coloured reaction product. The sections were dehydrated and coverslips were placed on the slides. Non-specific staining with secondary antibody was not found in the absence of primary antibody.
Cell size
The cross-sectional area of the largest section through each dye-injected neuron was used an indication of its size. In addition, for comparison with the dye-injected experimental neurons a general size distribution of DRG neurons was generated. For this, cross-sectional areas were measured of all neuronal profiles containing a nucleus in transverse DRG sections taken at 420 μm intervals through one L5 DRG from each of three female rats with weights near the mid-range used in these experiments.
Assessment of relative intensity of Nav1.8-LI
For each dye-injected neuron, the intensity of Nav1.8-LI was scored subjectively from 0 to 5, and then, using image analysis techniques, the intensity relative to other cells in that section was calculated as a percentage of maximum staining intensity seen in that section, as previously described (Fang et al. 2002). There was a highly significant linear correlation between objective scores of relative intensity and subjective scores of Nav1.8-LI with r2 = 0.93 (P < 0.0001), suggesting a close agreement between subjective and objective scores (Fig. 1B). All neurons categorized subjectively as positive (≥ 1) had a relative intensity ≥ 20 % and vice versa. Accordingly, a relative intensity of 20 % was used as the borderline between negative and positive neurons in this study, although we cannot exclude the possibility that some cells with absorbance values of < 20 % may express low levels of the protein.
As a comparison the relative intensities of neurons from three L5 DRGs were calculated as follows: the minimum (0 %) was the average overall pixel intensity in the cytoplasm for the 10 least intensely labelled neurons and the maximum (100 %) was the average for the 10 most intensely labelled cells; each cell was given a percentage value on a linear scale scaled between these two values (see Fang et al. 2002).
Acceptance criteria
Data were included only if the temperature at the DRG was 28–32 °C. For the analysis of the AP characteristics, data were included only if the somatic AP was overshooting, and neurons had membrane potentials more negative than −50 mV; the exception was a single C LTM unit with membrane potential of −48 mV. Units with a marked inflection on the AP rising phase were excluded from the AP analysis (n = 2, both C-fibre units) as these were atypical.
RESULTS
A total of 104 neurons with identified sensory receptive properties, labelled with a fluorescent dye and successfully recovered from histology, were examined for Nav1.8-LI. Of these neurons, 47 were labelled with Lucifer yellow (LY), 45 with ethidium bromide (EB) and 12 with Cascade blue (CB). The DRGs were L5 for 97 neurons, L4 for one neuron and L6 for six neurons. Of the total sample, 38 were nociceptive units (9 C-, 1 C/Aδ-, 13 Aδ- and 15 Aα/β-fibres), 18 were unresponsive (8 C-, 1 C/Aδ-, 1 Aδ- and 8 Aα/β-fibres) and 48 were LTMs (1 C-, 4 Aδ- and 43 Aα/β-fibres). The Aα/β LTM units included 16 field or guard hair (F/G) neurons, 12 rapidly adapting (RA), 3 slowly adapting (SA) and 12 muscle spindle (MS) afferent neurons. Unless otherwise stated the C/Aδ-neurons are included with the Aδ-neurons in the following analyses.
Cell size
The relationship between cell size and Nav1.8-LI is shown in Fig. 2, in A and C for all cells in the DRG and in B and D for the 72 dye-injected neurons for which the largest section was available for measurement. The overall size distribution in Fig. 2A was used to divide neurons into small (< 400 μm2, 23 μm diameter, within the small cell peak), medium-sized (400–800 μm2, 23–32 μm diameter) and large (above 800 μm2 or 32 μm) including only the right hand side (larger cells) of the ‘light cell’ distribution (Lawson et al. 1984). Although many groups use 30 μm diameter (≈700 μm2 cross-sectional area) as the upper limit for ‘small’ DRG neurons, for the animals and tissue processing in the present study, this range would include most medium-sized neurons and is therefore not appropriate.
Figure 2. Cell area and Nav1.8-LI relative intensity.
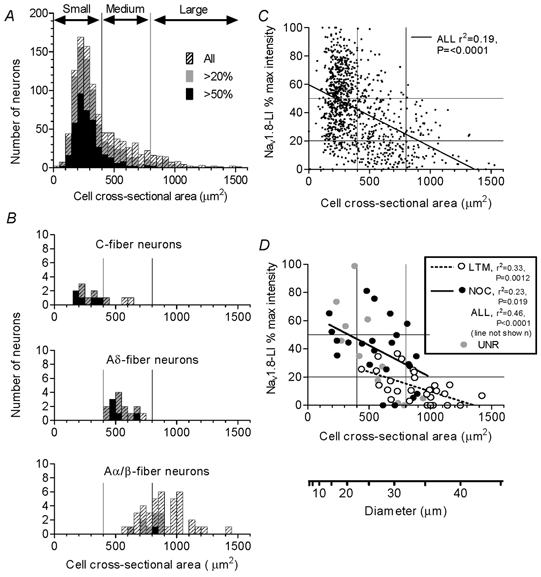
The cross-sectional areas (x-axis) through each neuronal profile containing a nucleus (A) and through the largest section of each identified dye-injected neuron (B) are plotted against the relative intensity (percentage maximum intensity). In both cases, all neurons are shown as hatched histograms. Superimposed in grey are neurons with Nav1.8-LI relative intensity > 20 % and superimposed in black are those with > 50 % maximum intensity. B, histograms for neurons with dorsal root C-, Aδ- and Aα/β-fibres are displayed separately. C and D, relative intensity is plotted against cell size. The lines (y-axis) show 20 and 50 % relative intensity, and those on the x-axis show the borderlines between small, medium and large neurons. C and D, regression lines, r2 and P values are given where a significant correlation exists (P < 0.05). LTM, low threshold mechanoreceptors; NOC, nociceptors; UNR, unresponsive units.
Most small neurons, both in the whole DRG (Fig. 2A) and in the group of dye-injected neurons (Fig. 2B), were Nav1.8-positive and the strongest immunoreactivity was in the smallest neurons in both cases (Fig. 2C and D). In addition, a few medium-sized neurons showed strong immunoreactivity, and even some large neurons also showed clear immunoreactivity (Fig. 2A). In Fig. 2B, C-fibre neurons were mainly small and most were positive, all Aδ-fibre neurons were medium-sized and most were positive, while Aα/β-fibre neurons were medium and large, including both medium and large positive neurons, but none of the very largest neurons (> 900 μm2) were positive. Lower percentages of Aα/β-fibre neurons compared with Aδ- or C-fibre neurons were positive. These percentages were, respectively, for Aα/β-, Aδ- and C-fibre neurons: for positive neurons (> 20 %) 25, 93 and 83 %, and for strongly positive neurons (> 50 %) 3, 33 and 42 %. Note that the percentage classed as positive depends on the borderline chosen (here 20 or 50 % maximum intensity) between positive and negative. Figure 2C shows a highly significant negative correlation between cell size and relative intensity, albeit with a high variability of intensity at any given cell size, leading to a low r2 value. There is a negative correlation between cell size and intensity for all cells and for nociceptive and LTM neurons separately (Fig. 2D).
The similarity in pattern (Fig. 2D) of the relationship between cell size and intensity of immunoreactivity in non-experimental and dye-injected neurons indicates no detectable effect of recording and dye injection on the level of immunoreactivity.
Conduction velocity
The relative intensity of Nav1.8-LI was negatively correlated with dorsal root CV in all neurons (n = 104), as well as in nociceptive and LTM neurons separately, with the majority of strongly (> 50 % intensity) labelled units being in the C- and Aδ-fibre range (Fig. 3A). Similar correlations were seen for A-fibre units alone (Fig. 3C) but there was no correlation for C-fibre units alone (Fig. 3B), in direct contrast to the findings for Nav1.9 (Fang et al. 2002). There was a highly significant difference between the elevations but not the slopes (Prism 3) of regression lines for nociceptive and LTM data shown in Fig. 3A (all units) and C (A-fibre units) (P < 0.001 in both cases). Only one of the fastest (> 15m s−1) conducting units was positive and that was only weakly positive (Fig. 3A).
Figure 3. Nav1.8-LI intensity and conduction velocity.
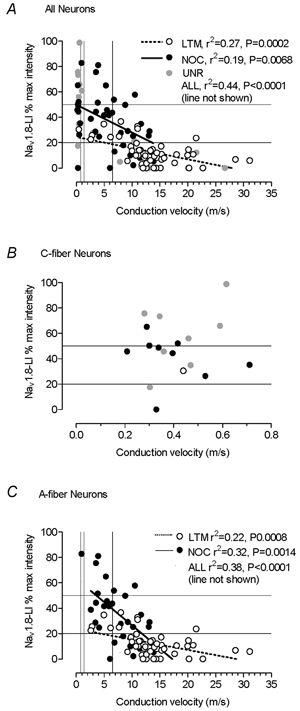
Nav1.8-LI intensity plotted against dorsal root CVs for all DRG neurons examined (A) and for all A-fibre neurons (B). The P and r2 values are given only if the correlation is significant (P < 0.05). Both the borderline between Nav1.8-LI-positive and -negative neurons (20 % maximum intensity) and 50 % maximum intensity are shown by lines on the y-axis. The lines from the x-axes show the C (0.8 m s−1), C/Aδ (1.4 m s−1) and the Aδ/Aα/β (6.5 m s−1) borderlines. NOC, nociceptive neurons; LTM, low threshold mechanoreceptive neurons; UNR, unresponsive neurons.
Nociceptive versus LTM properties
Nociceptive neurons grouped together regardless of CV had a greater (highly significant) median relative intensity than similarly grouped LTM neurons; none of the LTM units had a relative intensity > 40 %, whereas half the nociceptive units had values of > 40 % (Fig. 4A). A significantly greater proportion of nociceptive than LTM neurons was positive (≥ 20 % relative intensity) (Fig. 4B). These differences are probably underestimated due to the under-representation in the identified sample of the C-fibre neurons many of which are nociceptive and show relatively strong immunoreactivity.
Figure 4. Nav1.8-LI intensity in different neuronal types.
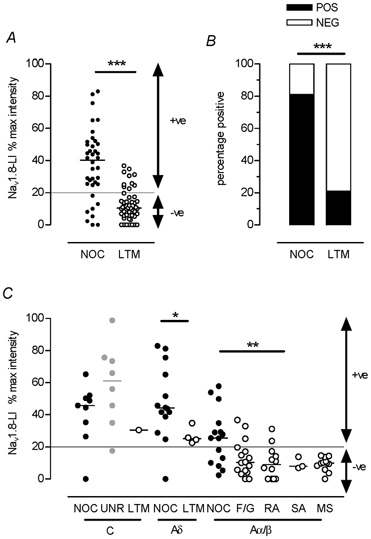
A, scattergraph of the relative intensities of nociceptive and LTM units in all CV groups together shows a much greater median relative intensity in nociceptors (Mann-Whitney test, P < 0.0001). B, the proportions of positive (< 20 %, black) and negative (white) nociceptive and LTM units from all CV groups together are compared (χ2 test, P < 0001). C, neurons are subdivided by sensory modality within C, Aδ and Aα/β CV groups. The borderline between negative (< 20 %) and positive (≥ 20 %) neurons is indicated by the line at 20 % relative intensity. Abbreviations: NOCI, nociceptive neurons; LTM, low threshold mechanoreceptive neurons; UNR, unresponsive neurons; F/G, field or guard hair LTM neurons; RA, rapidly adapting LTM neurons; SA, slowly adapting LTM neurons; MS, muscle spindle neurons; +ve, Nav1.8-LI-positive (≥ 20 %) neurons; -ve, Nav1.8-LI-negative (< 20 %) neurons.
Nociceptive and non-nociceptive neurons with C- and A-fibres
Figure 4C shows the relative intensity of Nav1.8-LI in different subgroups of identified neurons. The most intensely labelled neurons were nociceptive (C, Aδ and Aα/β) and C-unresponsive neurons; only these neurons had relative intensities of > 40 %. Examples of Nav1.8-LI in C- and A-fibre neurons with identified sensory properties are shown in Fig. 5.
Figure 5. Examples of Nav1.8-LI in identified neurons.
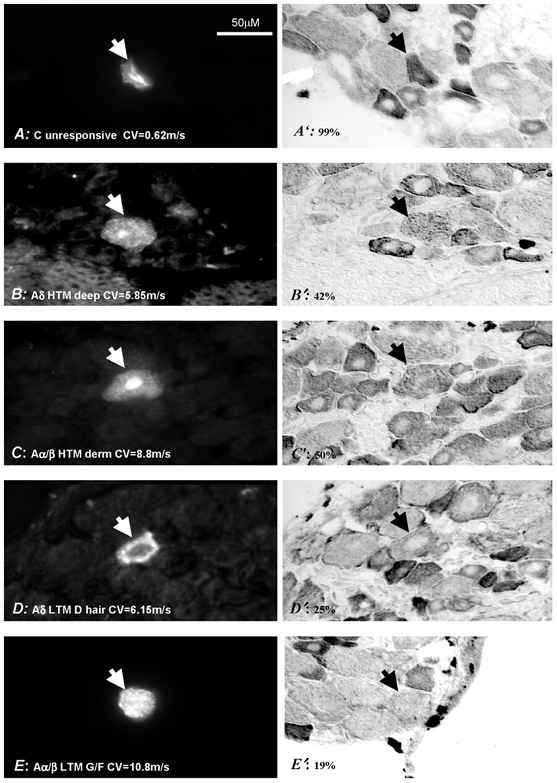
Shown on the left are 5 DRG neurons injected with a fluorescent dye (marked with arrowheads) and on the right are the same neurons stained for Nav1.8-LI. Their sensory properties, dorsal root CV (bottom left) and Nav1.8-LI relative intensity (bottom right) are given. Neurons were considered positive if their Nav1.8-LI was ≥ 20 % and negative if it was < 20 %. HTM, high threshold mechanoreceptive neurons; CV, conduction velocity; LY, Lucifer yellow; CB, Cascade blue.
C-fibre units
Most (8/9, 89 %) C-fibre nociceptive units were clearly positive. Positive units included six HTMs, two HTM-cooling (MC) units and one unit responsive to noxious heat (C PM). The only Nav1.8-LI negative C-fibre nociceptive neuron was a C HTM unit. Interestingly, most (7/8, 87 %) C-fibre unresponsive units were clearly positive with relatively high intensity of labelling (for example see Fig. 5). The only C-fibre LTM unit was weakly positive and was among the few C-fibre units with Nav1.8-LI intensity of < 40 %.
Aδ-fibre units
Almost all Aδ fibre nociceptive units (13/14, 93 %) were clearly positive (including the only C/Aδ-fibre nociceptive unit which was strongly positive at 83 %), see Fig. 4C, Table 1. These positive neurons included 11 HTM and two mechano-cooling (MC) units; the negative unit was an HTM with a deep receptive field. A positive HTM unit is shown in Fig. 5. The only two unresponsive Aδ-fibre units were positive (one with a CV of 3 m s−1 and intensity of 27 % and the other 1 m s−1 and intensity of 61 %). Strong Nav1.8-LI (≥ 50 %) was only in nociceptive neurons, although weak but clear positive immunoreactivity was also seen in all four Aδ LTM (D hair) units (23–35 %), for example see Fig. 5.
Table 1.
Nav 1.8-LI relative intensity and action potential duration
| Intensity vs. AP | All | All C positive | All A positive | All A | A LTM | A Nociceptive | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | r2 | n | P | r2 | n | P | r2 | n | P | r2 | n | P | r2 | n | P | r2 | n | |
| Duration at base | **** | 0.4 | 41 | ns | — | 8 | * | 0.49 | 12 | **** | 0.61 | 32 | ns | — | 21 | ** | 0.58 | 11 |
| Slope+7.3 ± 1.4 | — | Slope+23 ± 7.4 | Slope+26.6 ± 3.8 | — | Slope+35.6 ± 10.1 | |||||||||||||
| Rise time | **** | 0.51 | 41 | ** | 0.85 | 8 | * | 0.42 | 12 | *** | 0.64 | 32 | ** | 0.37 | 21 | * | 0.42 | 11 |
| Slope+24.5 ± 3.8 | Slope+16.3 ± 2.8 | Slope+44.8 ± 16.5 | Slope+60.8 ± 8.4 | Slope+44.7 ± 13.3 | Slope+59.4 ± 23 | |||||||||||||
| Fall time | *** | 0.31 | 41 | ns | — | 8 | * | 0.44 | 12 | **** | 0.51 | 32 | ns | — | 21 | ** | 0.59 | 11 |
| Slope+8.9 ± 3.1 | — | Slope+40 ± 13 | Slope+40.6 ± 7.2 | — | Slope+65.3 ± 18 | |||||||||||||
Linear regression analysis was carried out on the groups indicated to examine the correlation of Nav 1.8-like immunoreactivity (Nav 1.8-LI) relative intensity with AP duration at base, AP rise time and AP fall time. A-fibre unresponsive units were excluded. All neurons had membrane potentials (Em) equal to or more negative than −50mV, except for the only C LTM (−48mV). All correlations were positive. In each case where P < 0.05, on the first line the P, r2 and n values are given, and on the second line the slope is shown, ns, Not significant.
P < 0.05
P < 0.01
P < 0.001
P < 0.0001. Plots of these correlations are shown in Fig. 6.
Aα/β-fibre units
Only 21 % (14/66) of the total Aα/β-fibre neurons were Nav1.8 positive (Fig. 4C). Of those that were positive, most (9/14, 64 %) were nociceptive. Of the nociceptive neurons, 60 % (9/15) were positive; an example of a moderately labelled Aα/β-fibre nociceptive unit can be seen in Fig. 5. The only neurons with Nav1.8 intensity of > 40 % were nociceptive. All unresponsive Aα/β-fibre units (n = 8) were negative (not shown). Since most of these unresponsive Aα/β-fibre units had electrophysiological properties typical of LTM units (short AP and AHP durations), they were likely to include LTMs with inaccessible receptive fields; they were therefore not included in subsequent analyses. About 10 % (5/43) of LTM Aα/β-fibre units exhibited weak positive Nav1.8-LI. These included three (F/G) field or guard hair (intensity 25–37 %) and two rapidly adapting (RA) neurons (intensities 24–31 %). An example of a borderline Aα/β LTM (G hair/field) unit can be seen in Fig. 5. None of the MS and SA neurons was positive for Nav1.8-LI.
Nav1.8-LI and action potential duration
In some DRG neurons TTX-R currents contribute to the AP inward current and may therefore influence the AP configuration. Correlation between Nav1.8-LI intensity and AP duration at base, AP rise time and AP fall time were therefore examined. Only neurons with membrane potentials equal to or more negative than −50 mV plus a C LTM with a membrane potential of −48 mV were included. Similar patterns and correlations were seen when neurons with membrane potentials between −40 and −50 mV were also included (not shown). All units included had overshooting APs. Positive correlations for all A-fibre neurons and for A-fibre nociceptive neurons were very clear, especially with AP fall time; however, for A-fibre LTMs a significant correlation was only found for AP rise time (Fig. 6). C-fibre neurons showed no correlation between Nav1.8-LI intensity and AP duration at base (Fig. 6A) or AP rise time in contrast to A-fibre neurons. There was, however, a correlation with AP rise time both for C-nociceptors (Fig. 6) and for all positive C-fibre units (nociceptors, unresponsive and an LTM) (Fig. 6 and Table 1).
Figure 6. Nav1.8-LI intensity and action potential shape.
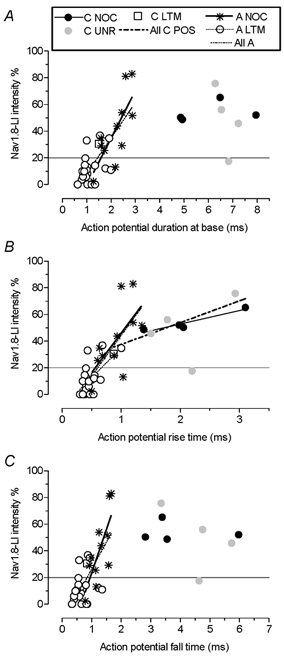
Only units with membrane potentials of at least −50 mV except the sole C-LTM (−48 mV) were included. Plots for AP duration at base (A), AP rise time (B) and AP fall time (C) are shown. Linear regressions were calculated for A-fibre nociceptors, A-fibre LTMs and all A-fibres together. Where regressions reached significance (P < 0.05) regression lines are shown, except for all units together (omitted for clarity). P and r2 values for these and for other correlations are given in Table 1. In addition, there was a significant correlation for C-fibre nociceptor AP rise times (P < 0.05, r2 = 0.91, n = 4). For abbreviations see legend to Fig. 4.
Membrane potential
There was no correlation of Nav1.8-LI with membrane potential for A-fibre neurons, for all nociceptive or for all LTM neurons. However, there was a significant negative correlation between membrane potential and Nav1.8-LI in C-fibre nociceptors but not C-fibre unresponsive units (Fig. 7) such that more intense immunoreactivity was seen in C-nociceptors with more negative membrane potentials. We have no explanation for this unexpected finding, since no difference was seen in membrane potentials in Nav1.8-/- DRG neurons compared with wild-type (Renganathan et al. 2001). While it is possible that the expression of Nav1.8 might be influenced by membrane potential in C-fibre nociceptors, we know of no other evidence for this. In addition, since real membrane potential is notoriously difficult to assess accurately, and may vary during recording, it may be misleading to emphasise this observation. There was no significant correlation between membrane potential and AP rise time or AP fall time for C-fibre nociceptive neurons, making it unlikely that the correlation of Nav1.8-LI with membrane potential led indirectly to the correlation in C-fibre nociceptors between Nav1.8 and AP fall time.
Figure 7. Nav1.8-LI intensity and membrane potential.
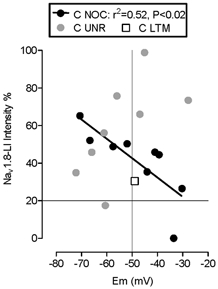
Membrane potentials of C-fibre neurons are plotted against Nav1.8-LI intensity. A significant correlation was found for C-fibre nociceptive neurons but not for C-fibre unresponsive neurons. For abbreviations see legend to Fig. 4.
Action potential overshoot
Recently it was shown (Renganathan et al. 2001) that the peak height of the AP was reduced in small DRG neurons in -/- neurons from Nav1.8 knockout mice, compared with those from wild-type animals, indicating an important influence of Nav1.8 on AP height. We therefore examined whether AP overshoot was related to Nav1.8-LI relative intensity in neurons with membrane potentials equal to or more negative than −50 mV, with AP peaks that were at least −20 mV. There were indeed positive correlations between Nav1.8-LI intensity and AP overshoot for all units together, for all nociceptive units, and for all positive units (Fig. 8).
Figure 8. Nav1.8-LI relative intensity versus action potential overshoot.
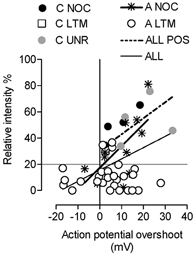
Only units with membrane potentials of at least −50 mV except the sole C-LTM (−48 mV) and somatic APs with peaks that reached at least −20 mV at peak were included. Regression lines are shown where there was a significant (P < 0.05) linear regression. P and r2 values for these correlations were: for all A-fibre units, P < 0.0001, r2 = 0.56; for all positive units, P < 0.0001, r2 = 0.56; for A-fibre nociceptive units P < 0.0001, r2 = 0.42. For abbreviations see legend to Fig. 4.
DISCUSSION
This is the first study to examine in vivo the properties of sensory neurons that express Nav1.8. Moderately to strongly immunoreactive neurons were nociceptive with C-, Aδ- or Aα/β-fibres, and tended to have broad APs with large overshoots. Units with low, but clearly positive, immunoreactivity included both nociceptive and some LTM neurons with C-, Aδ or Aα/β-fibres.
There was clear cytoplasmic but not clear membrane Nav1.8-LI in DRG neurons as previously described (Novakovic et al. 1998). This is not surprising as the density of Na+ channels in the membrane required for the peak Na+ current densities in DRG neurons are considerably below immunocytochemically detectable levels as previously discussed (Djouhri et al. 2003). As previously discussed (see Djouhri et al. 2003), there is evidence that the amount of channel in the cytoplasmic pool is much greater than that inserted into the membrane. The correlations between cytoplasmic immunoreactivity and membrane electrical properties (which presumably relate to the protein in the membrane) in this study appear to indicate some quantitative relationship between these two pools.
Neuronal size
Our finding of Nav1.8 protein mainly in small-medium sized and but also in some large DRG neurons agrees with previous studies of mRNA and protein and with the presence of TTX-Rs Na+ current in mainly small-medium sized DRG neurons (see Introduction) and also in some large neurons (Sangameswaran et al. 1996; Tate et al. 1998; Novakovic et al. 1998; Coward et al. 2000; Renganathan et al. 2000).
Conduction velocity
The expression of Nav1.8 protein in C-fibre neurons was expected. However, even the expression in A-fibre neurons is consistent with: (a) the medium to large size of some positive neurons; (b) TTX-RS current in large diameter afferents (Renganathan et al. 2000) which is likely to have A-fibres (Harper & Lawson, 1985); and (c) with Nav1.8 expression in neurofilament-rich somata (Amaya et al. 2000) again indicating A-fibre CV (Lawson et al. 1984).
The correlations between CV and Nav1.8-LI may imply a functional link between the two. Although not detected in normal rat peripheral nerve fibres (Novakovic et al. 1998), the amount required for AP conduction may not be immunocytochemically detectable (see above). The evidence that this channel is transported along fibres includes: (a) its detection in fibres in human normal peripheral nerve (Coward et al. 2000); (b) its presence in dorsal horn (Novakovic et al. 1998; Amaya et al. 2000); and (c) TTX-R Na+ currents (presumably Nav1.8 related) in peripheral terminals of afferent nerve fibres (Brock et al. 1998). In addition, TTX-R currents (presumably also Nav1.8 related) can contribute to AP conduction, because some C-fibre conduction is TTX resistant (Jeftinija, 1994; Quasthoff et al. 1995; Kobayashi et al. 1996; Buchanan et al. 1996; Steffens et al. 2001). The absence of a specific blocker for TTX-RS currents means that an important contribution to conduction by these currents, where present, cannot be ruled out even where conduction is blocked by TTX.
Although CV was not correlated with Nav1.8-LI intensity in C-fibre somata, this does not exclude a role of Nav1.8 in conduction along C-fibres (see above). It does, however, appear from the correlation between Nav1.9 immunoreactivity and C-fibre CV, that Nav1.9 may have an influence on CV in these unmyelinated fibres (Fang et al. 2002). In contrast to the C-fibres, the clear correlation between Nav1.8 and CV in A-fibres may result from the presence of TTX-SF currents in A-fibre neurons with much faster kinetics than the TTX-RS current associated with Nav1.8. If more available Nav1.8 is accompanied by less TTX-SF current, this could account for units with more available Nav1.8 having slower AP rise times, leading to slower CVs.
C- and A-fibre nociceptive neurons
We found Nav1.8 in nociceptive neurons with C-fibres as expected (Introduction). The presence of Nav1.8 in nociceptive A-fibre neurons was also consistent with previous descriptions of TTX-RS Na+ currents in these neurons (Ritter & Mendell, 1992).
The Nav1.8-LI in nociceptors and its even higher intensity in C-unresponsive neurons are interesting in view of the suggestion that the high activation threshold of Nav1.8 (Sangameswaran et al. 1996; Waxman, 1999) may contribute to the high thresholds of nociceptive neurons (Khasar et al. 1998; Akopian et al. 1999). Since C-unresponsive neurons probably include very high threshold ‘silent’ nociceptive neurons (see Methods), their high levels of Nav1.8 may contribute to this high threshold.
Nociceptors versus low threshold mechanoreceptive units
The labelling of both nociceptive and some LTM neurons in our study accords with Nav1.8-LI distribution in superficial (I and II) and deeper (III-V) laminae of the dorsal horn (Amaya et al. 2000). The TTX-RS Na+ current described in half the large cutaneous afferent neurons (Renganathan et al. 2000) could be explained by Nav1.8 being in 60 % of Aα/β-fibre nociceptive and in 15–20 % of LTM Aα/β-fibre cutaneous afferent neurons (this study). Nonetheless, the intensity of Nav1.8 was much greater in nociceptive than LTM neurons; indeed unlike nociceptors, LTMs were never more than weakly positive. The proportion of A LTM neurons that was positive was low, and Nav1.8-LI was absent in muscle spindle afferent neurons consistent with their absence of TTX-R currents (Honmou et al. 1994). Thus despite its detectable presence in some LTMs, its low levels in these neurons may indicate a smaller influence of this channel on LTM than on nociceptive membrane properties.
Nav1.8-LI and action potential duration
Estimates of the contribution of Nav1.8-related TTX-RS current to APs in small DRG neurons include ≈45 % of total peak Na+ current density (Akopian et al. 1999), 60 % of total Na+ channel conductance (Novakovic et al. 1998) and about 60 % (Blair & Bean, 2002) or up to 80–90 % of the inward Na+ current during the rising phase of the AP (Renganathan et al. 2001). Compared with TTX-S Na+ currents, TTX-Rs currents in sensory neurons have slower rates of activation and much slower rates of inactivation (e.g. Kostyuk et al. 1981; Elliott & Elliott, 1993; Schild & Kunze, 1997). Therefore greater availability of Nav1.8 should slow the AP kinetics. This effect may be more evident across a group of neurons many of which have APs generated largely by the faster TTX-S current, namely the larger diameter faster conducting neurons, which could account for the stronger correlation of Nav1.8 with AP duration in A- than C-fibre neurons.
In positive C-fibre neurons the correlation between Nav1.8-LI intensity and AP rise time but not fall time or overall duration is similar to the finding for Nav1.9-LI (Fang et al. 2002). Interestingly in A-fibre neurons the correlation with rise time was also stronger than with AP fall time. This may indicate a greater influence of Nav1.8 on rise than fall time, perhaps because the rising phase of the AP must depend almost entirely on the availability and kinetics of Na+ channels, whereas the fall time is probably also influenced by other types of current such as Ca2+ current (e.g. see Blair & Bean, 2002) and K+ currents.
Broad/inflected APs are characteristic of nociceptive DRG neurons in vivo (see Introduction). Thus, if greater Nav1.8 in the soma results in greater TTX-RS AP inward current, the presence of Nav1.8 in nociceptors could contribute to their broader APs. However, other Na+ channels such as Nav1.9 (see above) and Nav1.7-LI (Djouhri et al. 2003), also found preferentially in smaller DRG neurons, have immunoreactivity correlated with AP duration. Thus all three may contribute to the characteristic broad AP of nociceptive neurons either directly (e.g. Nav1.8 because of its slow kinetics) or indirectly (perhaps via the postulated depolarising effect of Nav1.9 persistent current (Dib-Hajj et al. 2002) inactivating low threshold TTX-S Na+ channels (e.g. Nav1.7), and thus slowing AP rise time (Djouhri et al. 2003). The strong correlation of Nav1.9 with AP rise time and CV (Fang et al. 2002) may indicate that this channel has a dominant influence on these in C-fibre neurons, while the stronger correlation of Nav1.8 with AP duration and CV in A-fibre neurons may result from the probably smaller influence of Nav1.9, allowing the slow kinetics of Nav1.8 to have a greater influence.
The correlation between AP overshoot and Nav1.8-LI was consistent with the findings of Renganathan et al. (2001) indicating an influence of Nav1.8 on AP height. This may result from the slow kinetics of TTX-Rs current associated with Nav1.8, which may enable the membrane potential to approach more closely the Na+ equilibrium potential than channels with faster kinetics. It may therefore be the presence of Nav1.8 that leads to the consistently greater overshoot in nociceptive than LTM DRG neurons of all CV ranges (Djouhri & Lawson, 2001a).
Conclusions
Nav1.8 was present in most C- and A-fibre nociceptive neurons and C-fibre unresponsive (nociceptor type) neurons. In contrast detectable immunoreactivity was not found in muscle spindle, nor in most cutaneous Aα/β LTM units and only weak positive labelling was found in other LTM units. The finding that Nav1.8-LI intensity was correlated with properties associated with nociceptive function, namely long AP durations (especially AP rise time) and large AP overshoots (Lawson, 2002), supports the previous suggestion (Akopian et al. 1996) that Nav1.8 may be important in influencing the membrane properties of nociceptors
Acknowledgments
This work was supported by Wellcome Trust UK grants to S.N.L., J.W. and K.O., by a Bristol University Studentship to X.F. and an MRC grant to J.W. Thanks to B. Carruthers for technical assistance.
REFERENCES
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, Costigan M, Woolf CJ. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15:331–342. doi: 10.1006/mcne.1999.0828. [DOI] [PubMed] [Google Scholar]
- Baker MD, Wood JN. Involvement of Na+ channels in pain pathways. Trends Pharmacol Sci. 2001;22:27–31. doi: 10.1016/s0165-6147(00)01585-6. [DOI] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci. 2002;22:10277–10290. doi: 10.1523/JNEUROSCI.22-23-10277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock JA, McLachlan EM, Belmonte C. Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea. J Physiol. 1998;512:211–217. doi: 10.1111/j.1469-7793.1998.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan S, Harper AA, Elliott JR. Differential effects of tetrodotoxin (TTX) and high external K+ on A and C fibre compound action potential peaks in frog sciatic nerve. Neurosci Lett. 1996;219:131–134. doi: 10.1016/s0304-3940(96)13189-x. [DOI] [PubMed] [Google Scholar]
- Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- Chung JM, Dib-Hajj SD, Lawson SN. Sodium channel subtypes and neuropathic pain. In: Dostrovsky JO, Carr DB, Koltzenburg M, editors. Progress in Pain Research and Management Proceeding of the 10th World Congress of Pain. Seattle: IASP Press; 2003. pp. 99–114. [Google Scholar]
- Coward K, Plumpton C, Facer P, Birch R, Carlstedt T, Tate S, Bountra C, Anand P. Immunolocalization of SNS/PN3 and NaN/SNS2 sodium channels in human pain states. Pain. 2000;85:41–50. doi: 10.1016/s0304-3959(99)00251-1. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj S, Black JA, Akopian AN, Wood JN, Waxman SG. A novel persistent tetrodotoxin-resistenat sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci. 1999;19:1–6. doi: 10.1523/JNEUROSCI.19-24-j0001.1999. RC43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj S, Black JA, Cummins TR, Waxman SG. NaN/Nav1. 9: a sodium channel with unique properties. Trends Neurosci. 2002;25:253–259. doi: 10.1016/s0166-2236(02)02150-1. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Bleazard L, Lawson SN. Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurons. J Physiol. 1998;513:857–872. doi: 10.1111/j.1469-7793.1998.857ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Changes in somatic action potential shape in guinea-pig nociceptive primary afferent neurons during inflammation in vivo. J Physiol. 1999;520:565–576. doi: 10.1111/j.1469-7793.1999.t01-1-00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Differences in the size of the somatic action potential overshoot between nociceptive and non-nociceptive dorsal root ganglion neurons in the guinea-pig. Neurosci. 2001a;108:479–491. doi: 10.1016/s0306-4522(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Increased conduction velocity of nociceptive primary afferent neurons during unilateral hindlimb inflammation in the anesthetised guinea-pig. Neurosci. 2001b;102:669–679. doi: 10.1016/s0306-4522(00)00503-0. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Newton R, Levinson SR, Lawson SN. Sensory and electrophysiological properties of guinea-pig sensory neurons expressing Nav1. 7 (PN1). Na+ channel α subunit protein. J Physiol. 2003;546:565–576. doi: 10.1113/jphysiol.2002.026559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AA, Elliott JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol. 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, Black JA, Dib-Hajj SD, Waxman SG, Lawson SN. The presence and role of the tetrodotoxin-resistant sodium channel Nav 1. 9 (NaN) in nociceptive primary afferent neurons. J Neurosci. 2002;22:7425–7433. doi: 10.1523/JNEUROSCI.22-17-07425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Djouhri L, Okuse K, Wood JN, Lawson SN. Sensory and electrophysiological properties of DRG neurones with SNS-like immunoreactivity (SNS-LI) in rats. Soc Neurosci Abstr. 2001;27:819–815. [Google Scholar]
- Gee MD, Lynn B, Basile S, Pierau FK, Cotsell B. The relationship between axonal spike shape and functional modality in cutaneous C-fibres in the pig and rat. Neurosci. 1999;90:509–518. doi: 10.1016/s0306-4522(98)00454-0. [DOI] [PubMed] [Google Scholar]
- Gee MD, Lynn B, Cotsell B. Activity-dependent slowing of conduction velocity provides a method for identifying different functional classes of C-fibre in the rat saphenous nerve. Neurosci. 1996;73:667–675. doi: 10.1016/0306-4522(96)00070-x. [DOI] [PubMed] [Google Scholar]
- Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–368. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Handwerker HO, Kilo S, Reeh PW. Unresponsive afferent nerve fibres in the sural nerve of the rat. J Physiol. 1991;435:229–242. doi: 10.1113/jphysiol.1991.sp018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press; 1988. pp. 72–123. [Google Scholar]
- Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglia. J Physiol. 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Utzschneider DA, Rizzo MA, Bowe CM, Waxman SG, Kocsis JD. Delayed depolarization and slow sodium currents in cutaneous afferents. J Neurophysiol. 1994;71:1627–1637. doi: 10.1152/jn.1994.71.5.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeftinija S. The role of tetrodotoxin-resistant sodium channels of small primary afferent fibres. Brain Res. 1994;639:125–134. doi: 10.1016/0006-8993(94)91772-8. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Gold MS, Levine JD. A tetrodotoxin-resistant sodium current mediates inflammatory pain in the rat. Neurosci Lett. 1998;256:17–20. doi: 10.1016/s0304-3940(98)00738-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Ohta M, Terada Y. Tetrodotoxin-resistant Na+ spikes of C fibres have at least two subtypes in the isolated bullfrog sciatic nerve. Neurosci Lett. 1996;221:9–12. doi: 10.1016/s0304-3940(96)13269-9. [DOI] [PubMed] [Google Scholar]
- Koerber HR, Mendell LM. Functional heterogeneity of dorsal root ganglion cells. In: Scott SA, editor. Sensory Neurons: Diversity, Development and Plasticity. Oxford: Oxford University Press; 1992. pp. 77–96. [Google Scholar]
- Kostyuk PG, Veselovsky NS, Tsyndrenko AY. Ionic currents in the somatic membrane of rat dorsal root ganglion neurons: I. sodium currents. Neurosci. 1981;6:2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurons with C-, Adelta or Aalpha/beta-fibres. Exp Physiol. 2002;87:239–244. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Crepps BA, Perl ER. Relationship of substance P to afferent characteristics of dorsal root ganglion neurons in guinea-pig. J Physiol. 1997;505:177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Crepps B, Perl ER. CGRP immunoreactivity and afferent receptive properties of dorsal root ganglion neurons in guinea-pigs. J Physiol. 2002;540:989–1002. doi: 10.1113/jphysiol.2001.013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson SN, Harper AA, Harper EI, Garson JA, Anderton BH. A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurons. J Comp Neurol. 1984;228:263–272. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Peripheral sensory systems. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF, editors. Peripheral Neuropathy. Philadelphia: W. B. Saunders Co.; 1993. pp. 149–165. [Google Scholar]
- Meyer RA, Davis KD, Cohen RH, Treede RD, Campbell JN. Mechanically insensitive afferents (MIAs) in cutaneous nerves of monkey. Brain Res. 1991;561:252–261. doi: 10.1016/0006-8993(91)91601-v. [DOI] [PubMed] [Google Scholar]
- Novakovic SD, Tzoumaka E, McGivern JG, Haraguchi M, Sangameswaran L, Gogas KR, Eglen RM, Hunter JC. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J Neurosci. 1998;18:2174–2187. doi: 10.1523/JNEUROSCI.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaklander AL, Belzberg AJ. Unilateral nerve injury down-regulates mRNA for Na+ channel SCN10A bilaterally in rat dorsal root ganglia. Brain Res Mol Brain Res. 1997;52:162–165. doi: 10.1016/s0169-328x(97)00239-8. [DOI] [PubMed] [Google Scholar]
- Okuse K, Chaplan SR, McMahon SB, Luo ZD, Calcutt NA, Scott BP, Akopian AN, Wood JN. Regulation of expression of the sensory neuron-specific sodium channel SNS in inflammatory and neuropathic pain. Mol Cell Neurosci. 1997;10:196–207. doi: 10.1006/mcne.1997.0657. [DOI] [PubMed] [Google Scholar]
- Okuse K, Malik-Hall M, Baker MD, Poon WY, Kong H, Chao MV, Wood JN. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature. 2002;417:653–656. doi: 10.1038/nature00781. [DOI] [PubMed] [Google Scholar]
- Quasthoff S, Grosskreutz J, Schroder JM, Schneider U, Grafe P. Calcium potentials and tetrodotoxn-resistant sodium potentials in unmyelinated C fibres of biopsied human sural nerve. Neurosci. 1995;69:955–965. doi: 10.1016/0306-4522(95)00307-5. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Hormuzdiar WN, Waxman SG. Alpha-SNS produces the slow TTX-resistant sodium current in large cutaneous afferent DRG neurons. J Neurophysiol. 2000;84:710–718. doi: 10.1152/jn.2000.84.2.710. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1. 8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–640. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Dib-Hajj S, Waxman SG. Na(v)1. 5 underlies the ‘third TTX-R sodium current’ in rat small DRG neurons. Brain Res Mol Brain Res. 2002;106:70–82. doi: 10.1016/s0169-328x(02)00411-4. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: Effects of nerve growth factor. J Neurophysiol. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- Roy ML, Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AM, Brau ME, Elliott AA, Elliott JR. Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia. J Physiol. 1998;511:771–789. doi: 10.1111/j.1469-7793.1998.771bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J Biol Chem. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- Schild JH, Kunze DL. Experimental and modeling study of Na+ current heterogeneity in rat nodose neurons and its impact on neuronal discharge. J Neurophysiol. 1997;78:3198–3209. doi: 10.1152/jn.1997.78.6.3198. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjork E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15:333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanini M, De Martino C, Zamboni L. Fixation of ejaculated spermatozoa for electron microscopy. Nature. 1967;216:173–175. doi: 10.1038/216173a0. [DOI] [PubMed] [Google Scholar]
- Steffens H, Hoheisel U, Eek B, Mense S. Tetrodotoxin-resistant conductivity and spinal effects of cutaneous C- fibre afferents in the rat. Neurosci Res. 2001;39:413–419. doi: 10.1016/s0168-0102(01)00198-5. [DOI] [PubMed] [Google Scholar]
- Tate S, Benn S, Hick C, Trezise D, John V, Mannion RJ, Costigan M, Plumpton C, Grose D, Gladwell Z, Kendall G, Dale K, Bountra C, Woolf CJ. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nat Neurosci. 1998;1:653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- Waddell PJ, Lawson SN. Electrophysiological properties of subpopulations of rat dorsal root ganglion neurons in vitro. Neurosci. 1990;36:811–822. doi: 10.1016/0306-4522(90)90024-x. [DOI] [PubMed] [Google Scholar]
- Waxman SG. The molecular pathophysiology of pain: abnormal expression of sodium channel genes and its contributions to hyperexcitability of primary sensory neurons. Pain Suppl. 1999;6:S133–S140. doi: 10.1016/S0304-3959(99)00147-5. [DOI] [PubMed] [Google Scholar]


