Abstract
Loss of K+ from active muscles, leading to increased [K+]o, has been proposed to cause muscle fatigue by reducing excitability. Since exercise increases muscle temperature, we investigated the influence of temperature on muscle [K+]o sensitivity. Intact rat soleus or extensor digitorum longus (EDL) muscles were mounted on force transducers and stimulated electrically to evoke short isometric tetani at regular intervals. In each experiment, control force at 4 mM K+ was initially determined at every temperature used. In soleus muscles at 20 °C, 9 mM K+ reduced force to 33 ± 5 % of control force. Increasing the temperature to 30 °C restored force to 89 ± 5 % of control force. Likewise, at 30 °C 11 mM K+ reduced force to 16 ± 4 % and increasing the temperature to 35 °C restored force to 35 ± 5 %. Similar results were obtained using EDL. The force recovery induced by elevating temperature, reflecting reduced [K+]o sensitivity, was associated with improved excitability assessed from compound action potentials. Force recovery induced by a temperature elevation from 20 to 30 °C was associated with hyperpolarization (5 mV), reduced [Na+]i and a 93 % increase in Na+-K+ pump activity. The force recovery was blocked by ouabain. Since intensive exercise leads to lactic acidosis and increased plasma catecholamines, the effect of these two factors was also investigated. At 11 mM K+, force was completely restored by combining temperature elevation (30 to 35 °C), L-lactic acid (10 mM) and the β2-agonist salbutamol (10−5 M). We suggest an exercise scenario where the depressing action of exercise-induced hyperkalaemia is counteracted by elevated muscle temperature, lactic acidosis and catecholamines.
During exercise contracting muscles lose K+, leading to increased [K+]o. During strenuous exercise a plasma concentration of 8 mM K+ has been reported (Hermansen, 1984; Hallen et al. 1994). Interstitial accumulation of K+ in active muscle may become even greater (Hnik, 1976; Juel et al. 2000; Green et al. 2000; Nielsen et al. 2003). Elevated [K+]o leads to a depolarization, which is known to increase the probability that voltage-dependent Na+ channels will enter a state of slow inactivation (Ruff, 1996). Hence, increased [K+]o can result in a loss of excitability and thereby potentially contribute to muscle fatigue (Sjøgaard, 1990; Ruff, 1996).
Intense muscle activity increases metabolism, heat production and muscle temperature. An increase in temperature of active muscles from 35 to 40 °C has been demonstrated in exercising humans working at submaximal intensities (Saltin et al. 1968). In the rat the temperature of active muscles was found to increase from 36 to 44 °C during exercise (Brooks et al. 1971). For two reasons it can be envisaged that the increase in muscle temperature associated with exercise will reduce the depressing effects of increased [K+]o on excitability and force. (1) Due to the temperature sensitivity of the Na+-K+ pump with a Q10 of around 2.3 (Clausen & Kohn, 1977), elevated muscle temperature is likely to cause an increase in Na+-K+ pump activity. Increased Na+-K+ pump activity has previously been shown to restore contractility and excitability in K+-depressed muscles (Clausen et al. 1993; Overgaard et al. 1999). (2) Muscle temperature elevation has also been shown to reduce the slow inactivation of voltage-dependent Na+ channels (Ruff, 1999).
The aim of the present study was to determine the possible relevance of muscle temperature for loss of excitability and force induced by elevated [K+]o. The working hypothesis was that elevation of muscle temperature reduces the depression in excitability and force resulting from high [K+]o, i.e. that temperature elevation reduces the [K+]o sensitivity of muscles.
Recently in vitro studies have shown that the depressing effects of high [K+]o on muscle excitability and force can be counteracted by lactic acidosis or β2-agonists (Clausen et al. 1993; Cairns et al. 1995; Nielsen et al. 2001). Strenuous exercise leading to lactic acidosis is usually associated with high catecholamine levels in the plasma (Galbo et al. 1976; Savard et al. 1987). In vitro studies performed at 30 °C have shown that lactic acidosis and adrenaline (β2-agonist) offer additive protection against loss of excitability and force in K+-depressed muscles (de Paoli et al. 2002). In the last part of this study we determined whether similar protective effects of L-lactic acid and a specific β2-agonist (salbutamol) were present in muscles at physiological temperature (35 °C) and whether such effects could be added to a possible effect of temperature elevation. The working hypothesis was that temperature elevation, lactic acidosis and catecholamines would offer additive protection against the loss of force induced by increasing [K+]o.
METHODS
Animal handling and muscle preparation
All experiments were carried out using 4-week-old male or female Wistar rats weighing 60-70 g. Animals were fed ad libitum and maintained under 12 h light-12 h dark conditions at a thermostatically controlled temperature of 21 °C. Rats were killed by cervical dislocation followed by decapitation and intact soleus or extensor digitorum longus (EDL) muscles weighing around 22 mg were dissected out. In experiments where muscles were stimulated via the nerve, muscles were dissected out with approximately 10 mm of intact nerve attached. All handling of animals was in accordance with Danish Animal Welfare Regulations.
Muscles were incubated in standard Krebs-Ringer bicarbonate buffer (pH 7.4 at 30 °C) containing (mM): 122 NaCl, 25 NaHCO3, 2.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.3 CaCl2 and 5.0 D-glucose. All buffers were equilibrated with a mixture of 95 % O2 and 5 % CO2. In buffers with high K+, an equivalent amount of Na+ was omitted to maintain iso-osmolarity. The pH of incubation buffers with and without 10 mM L-lactic acid was 7.12 and 7.4 at 30 °C, respectively. To avoid large fluctuations in pH, buffer containing L-lactic acid was equilibrated for 20 min with a mixture of 95 % O2 and 5 % CO2 before use.
Isometric force and electrical stimulation
Muscles were mounted on force transducers at optimal length and exposed to field stimulation across the central part of the muscle through platinum electrodes using 2 s trains of 0.2 ms pulses of 12 V every 10 min for soleus, and 0.5 s trains of 0.2 ms pulses of 12 V every 20 min for EDL. The choice of stimulation frequency (30 or 90 Hz) was based on a force-frequency analysis to make certain that full tetanic force was obtained at all temperatures. A stimulation frequency of 90 Hz was used in experiments with soleus muscles where temperature exceeded 30 °C and in all experiments with EDL muscles. Stimulation of muscles every 10 or 20 min was controlled by a timer. Force was recorded on a chart recorder and/or digitally on a computer. In experiments where force and compound action potentials (M-waves) were recorded simultaneously, contractions were evoked via nerve stimulation using fixed current pulses (5 mA, 0.2 ms, 30 Hz) applied through a suction electrode (Overgaard et al. 1999).
Recordings of unipolar M-waves and membrane potentials
In experiments where unipolar M-wave signals were measured, the muscles were placed in an experimental set-up previously described in detail (Overgaard & Nielsen, 2001). Briefly, contractions were evoked via the nerve using a stimulus isolator (ISU 165, Cibertec, Spain). M-waves were recorded from one or in some cases two circular silver electrodes with recording areas of 0.79 mm2 (Overgaard & Nielsen, 2001). In experiments where both electrodes detected signals, the conduction velocities of M-waves were calculated by dividing the distance between the electrodes (4 mm) by the time taken for the signal to travel between the electrodes. Resting membrane potential (Vm) was recorded using standard electrophysiological techniques essentially as described previously (Overgaard & Nielsen, 2001).
Na+-K+ pump activity and [Na+]i
The activity of the Na+-K+ pump was determined from the ouabain-suppressible 86Rb+ uptake in resting muscles as previously described (Buchanan et al. 2002). Muscles were dissected out, mounted at resting length on holders for isometric contractions and placed in Krebs-Ringer buffer containing 9 mM K+. After 70 min of equilibration muscles were preincubated for 15 min with or without 10−3 M ouabain at 20 °C, followed by another 10 min in buffer containing 9 mM K+ and 86Rb+ (0.1 μCi ml−1) with or without ouabain at 20 or 30 °C. Finally muscles were washed 4 × 15 min at 0 °C in Na+-free non-radioactive Tris-sucrose buffer (pH 7.4) to remove Na+ and 86Rb+ from the extracellular space, and they were then blotted, weighed and soaked for 24 h in 2 ml 0.3 M trichloroacetic acid (TCA) (Clausen et al. 1993). The quantity of 86Rb+ taken up by muscles was determined by Cerenkov radiation in a β-counter, whilst the muscles were soaking in 2 ml 0.3 M TCA in 4 ml counting vials. The Na+ content of the TCA extract was measured by flame photometry (FLM3, Radiometer, Copenhagen). Correction for the loss of intracellular Na+ during washout and calculations of [Na+]i were carried out as described in a previous paper (Buchanan et al. 2002). Intracellular water content measured by Buchanan et al. (2002) at 30 °C was used in the calculations and presumed to be the same for muscles at 20 and 30 °C.
Temperature regulation
All incubation media were thermostatically controlled (±0.1 °C). The temperature of the buffers was checked manually at regular intervals. The temperature could be changed by 10 °C within 7 min in all experimental set-ups. In experiments where Na+-K+ pump activity and [Na+]i were determined, temperature was elevated promptly by moving muscles from an incubation bath at 20 °C to a bath at 30 °C. Similar to other studies on both whole muscle and isolated fibres, muscles showed an irreversible loss of force when the temperature was elevated above 35 °C (Segal & Faulkner, 1985; Lännergren & Westerblad, 1987). For this reason effects of temperature were only examined in the range 20-35 °C. Changes in temperature in the range 20-35 °C did not cause any changes in resting tension, and control force remained stable throughout all experiments.
Chemicals and isotope
All chemicals were of analytical grade. Salbutamol and ouabain were from Sigma-Aldrich and 86RbCl (0.4 Ci mmol−1) was from Amersham International (Aylesbury, Bucks, UK).
Statistics
All data are expressed as means ± S.E.M. The statistical significance of any difference between groups was ascertained using Student's two-tailed t test for non-paired observations.
RESULTS
Effect of temperature elevation on tetanic force in soleus and EDL muscles incubated at high [K+]o
The first series of experiments was undertaken to clarify the role of temperature elevation (20 to 30 °C) on force in muscles exposed to high [K+]o. Soleus muscles were stimulated to contract using a 30 Hz pulse train of 2 s duration every 10 min throughout the experiments. Muscles were initially incubated at 4 mM K+ at 30 °C and when a steady level of force had been determined, the temperature was reduced to determine the steady force at 20 °C. The levels of steady force at 4 mM K+ were used as control levels for the force produced at high [K+]o at the corresponding temperatures. Muscles were first exposed to high [K+]o at 20 °C and after steady force had been reached, the temperature was elevated to 30 °C and a new steady force was allowed to develop. Figure 1A shows the time course of such experiments. Following the decrease in temperature from 30 to 20 °C at 4 mM K+, control force dropped from 0.37 ± 0.01 to 0.33 ± 0.01 N in 10 min and remained, thereafter, constant for several hours. When muscles at 20 °C were exposed to 9 mM K+, tetanic force production dropped to 0.11 ± 0.03 N. The effect of 9 mM K+ was very slow in onset and 180 min were required to obtain a steady level of force. When the temperature was subsequently elevated to 30 °C, force production returned rapidly to 0.33 ± 0.02 N. To further evaluate the effect of elevated [K+]o on force at 20 and 30 °C, the steady force indicated by a-d in Fig. 1A are shown in relation to the control force at 4 mM K+ at the corresponding temperatures in Fig. 1B. At 20 °C, 9 mM K+ reduced force to 33 ± 5 % of the force at 4 mM K+. At 30 °C, however, force was only reduced to 89 ± 5 % of the force produced at 4 mM K+.
Figure 1. Effect of temperature on tetanic force at 4 and 9 mM K+.
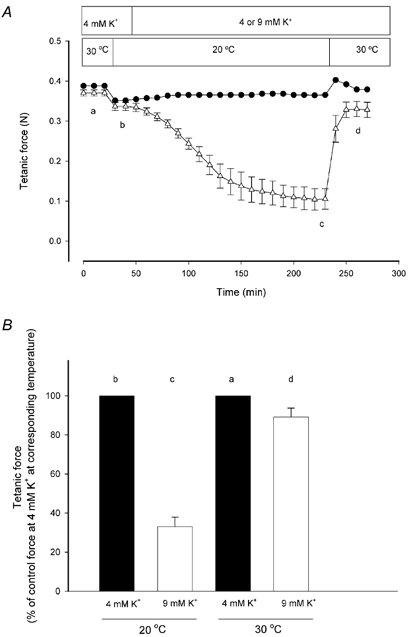
A, soleus muscles were stimulated every 10 min using 0.2 ms pulses of 12 V at 30 Hz for 2 s. Tetanic force is expressed in newtons (N) and changes in temperature and [K+]o are indicated by bars. •, 4 mM K+ throughout, n = 2. ▵, [K+]o changed from 4 to 9 mM K+ after 50 min, n = 6; error bars show S.E.M. B, influence of temperature on force depression induced by 9 mM K+. Steady tetanic force at points indicated by a-d in A is related to the control force at 4 mM K+ at the corresponding temperature; error bars show S.E.M.
These results indicated that increasing the temperature induced a reduction in the [K+]o sensitivity of the muscles. To clarify to what extent temperature elevation from 20 to 30 °C was able to reduce [K+]o sensitivity, the experiment in Fig. 1A was repeated in a series of experiments with varying [K+]o (8-13 mM). Figure 2A shows the relationship between steady tetanic force and [K+]o in muscles at 20 and 30 °C. The [K+]o needed to reduce force to 50 % (IC50) increased from 8.8 mM at 20 °C to 10.5 mM at 30 °C. To determine whether a further protection against the force-depressing effects of elevated [K+]o could be induced by increasing muscle temperature to above 30 °C, a series of experiments was performed in which the force-[K+]o relationship of muscles was determined at 30 and 35 °C (Fig. 2B). In concord with previous studies (Segal & Faulkner, 1985; Segal et al. 1986) pilot experiments demonstrated that at 35 °C, 30 Hz stimulation was insufficient to produce full tetanic force. Based on force-frequency relationships, showing that 90 Hz stimulation produced full tetanic force at both 30 and 35 °C (data not shown), 90 Hz stimulation was used to elicit contractions in all experiments at 30 and 35 °C. Except for the use of 90 Hz stimulation, the experiments were carried out in the same manner as those in Fig. 2A. A comparison of Fig. 2A and B shows that at 30 °C, the [K+]o sensitivity revealed when force was tested with 90 Hz stimulation was similar to the [K+]o sensitivity seen using 30 Hz stimulation. In addition, Fig. 2B demonstrates that an increase in the temperature from 30 to 35 °C led to a further reduction in the [K+]o sensitivity of the muscles. Thus, the IC50 increased from 10.1 mM at 30 °C to 10.7 mM at 35 °C. Together with Fig. 2A, this shows that in the range from 20 to 35 °C, the [K+]o sensitivity of soleus muscles was reduced with increasing temperature.
Figure 2. Effect of temperature on [K+]o sensitivity of soleus muscles incubated at high [K+]o.
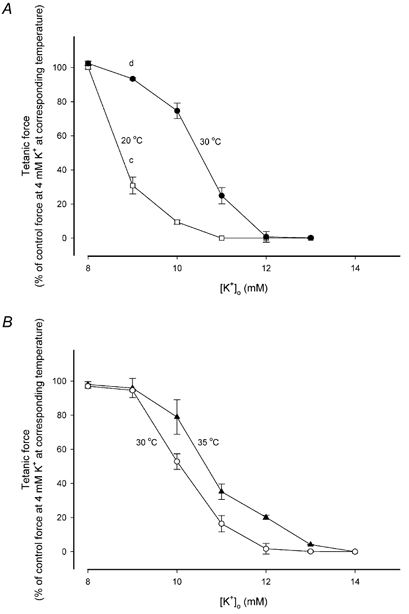
Data points represent steady force expressed relative to force at 4 mM K+ at the corsreponding temperatures. Each point is the mean of 6 muscles with error bars showing S.E.M. A, muscles incubated at 20 °C (□) and 30 °C (•) with a stimulation frequency of 30 Hz. Letters indicate data from the experiments shown in Fig. 1A. B, muscles incubated at 30 °C (○) and 35 °C (▴) with a stimulation frequency of 90 Hz.
To determine whether the protective effect of temperature elevation was a general phenomenon in skeletal muscles a series of experiments similar to those shown in Fig. 1A was conducted on EDL muscles, which in contrast to the slow twitch soleus are composed primarily of fast twitch fibres. In agreement with a study by Cairns et al. (1997) showing that fast twitch muscles are less sensitive to [K+]o than slow twitch muscles, it was necessary to incubate EDL muscles in a buffer containing 11 mM K+ to observe a marked reduction in force. Figure 3 shows that when [K+]o was changed from 4 to 11 mM at 20 °C, the force was reduced from 0.42 ± 0.02 to 0.09 ± 0.03 N corresponding to 24 ± 8 % of the control force. When the temperature was subsequently elevated to 30 °C, force was restored to 0.42 ± 0.02 N corresponding to 88 ± 2 % of control force at 30 °C and 4 mM K+. Hence temperature elevation induced a reduction in [K+]o sensitivity of EDL, comparable to the results obtained with soleus.
Figure 3. Effect of temperature on tetanic force at 4 and 11 mM K+ in rat EDL muscles.
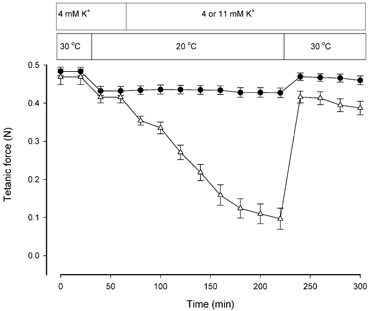
Muscles were stimulated every 20 min using 0.2 ms pulses of 12 V at 90 Hz for 0.5 s. •, 4 mM K+ throughout, n = 5; error bars show S.E.M. ▵, [K+]o changed from 4 to 11 mM K+ after 60 min, n = 6; error bars show S.E.M.
Effect of temperature on excitability in soleus muscles exposed to 4 and 10 mM K+
The reduction in the [K+]o sensitivity of contracting muscles by temperature elevation could potentially be explained by effects on excitability. To clarify whether excitability changed with temperature, compound action potentials were measured during experiments similar to those shown in Fig. 1A. Pilot experiments showed that muscles were less sensitive to [K+]o when stimulated through the nerve and therefore 10 mM K+ was used instead of 9 mM K+. As illustrated in Fig. 4 (top panel), an increase in [K+]o from 4 to 10 mM led to a broadening of the M-wave and to a reduction in its amplitude at both 20 and 30 °C. These changes were much more pronounced at 20 than at 30 °C. To further examine the relevance of changes in muscle excitability for the effect of temperature on force at high [K+]o, the changes in M-wave area and tetanic force induced by an increase in [K+]o from 4 to 10 mM were compared in muscles at 20 and 30 °C. Figure 4 (bottom panel) shows that at both temperatures, the relative reduction in force induced by increasing [K+]o to 10 mM was associated with a similar relative reduction in M-wave area. Again, the reduction was much more pronounced at 20 than at 30 °C. Thus, increasing [K+]o from 4 to 10 mM at 20 °C reduced tetanic force production to 24 ± 7 % and M-wave area to 21 ± 9 % of the control values obtained with 4 mM K+ at 20 °C. When the temperature was elevated to 30 °C, force and M-wave area were restored to 69 ± 7 and 63 ± 7 %, respectively, of the control values obtained at 4 mM K+ at 30 °C. In all experiments, the improvements of force and M-wave area showed similar time courses.
Figure 4. Effect of temperature on M-wave and force in soleus muscles incubated at 4 and 10 mM K+ at 20 and 30 °C.
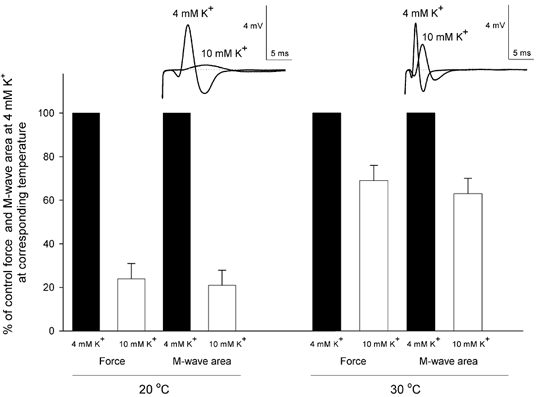
Muscles were stimulated through the nerve using a stimulation frequency of 30 Hz, while recording M-waves and force. The top panel shows recordings of M-waves at 4 and 10 mM K+ from the same muscle at 20 and 30 °C. The bottom panel shows steady force and M-wave area at 4 and 10 mM K+, related to control recordings at 4 mM K+ at the corresponding temperature, n = 6; error bars show S.E.M.
Temperature elevation also caused a marked increase in the conduction velocity of the M-wave. At 4 mM K+, the conduction velocity was 1.2 ± 0.09 m s−1 at 20 °C and it increased to 2.4 ± 0.1 m s−1 at 30 °C. At 10 mM K+, the conduction velocity was 0.5 ± 0.1 m s−1 at 20 °C and it increased to 1.1 ± 0.03 m s−1 at 30 °C.
Effect of Na+-K+ pump inhibition on force in soleus and EDL muscles exposed to 4 and 9 mM K+ at 20 and 30 °C
It has previously been shown in the rat soleus that the Na+-K+ pump is temperature dependent with a Q10 of around 2.3 (Clausen & Kohn, 1977) and that increased Na+-K+ pump activity can restore force in K+-depressed muscles (Clausen et al. 1993). To assess the role of the Na+-K+ pump in the effect of temperature on the [K+]o sensitivity of muscles, ouabain was added to muscles 15 min prior to temperature elevation in a series of experiments similar to those shown in Fig. 1A. In rat soleus muscles at 30 °C the ouabain concentration used (10−5 M) produced an inhibition of the Na+-K+ pump activity of around 80 % at 30 °C (Clausen & Everts, 1991). Figure 5 shows that at 4 mM K+, force was unaffected by ouabain whereas at 9 mM K+ force fell gradually to zero. At [K+]o of 9 mM, ouabain binding is reduced (Clausen & Hansen, 1974) but still ouabain completely suppressed the force recovery induced by increasing temperature. Similar observations were made using EDL muscles (data not shown). Hence, it appeared that muscles depend on an intact Na+-K+ pump activity to cope with elevated [K+]o and that the Na+-K+ pump could participate in the effect of elevated temperature on [K+]o sensitivity.
Figure 5. Effect of Na+-K+ pump inhibition on force at 4 and 9 mM K+ at 20 and 30 °C.
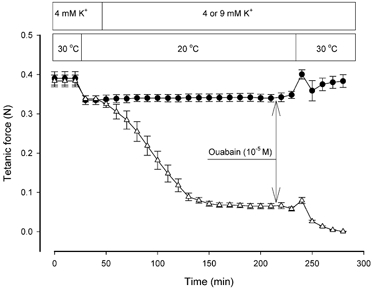
Experimental conditions as in Fig 1A. Ouabain (10−5 M) was added 15 min prior to temperature elevation as indicated by arrows. •, 4 mM K+ throughout. ▵, [K+]o changed from 4 to 9 mM after 50 min. Each point indicates the mean of observations from 6 muscles with error bars showing S.E.M.
Effect of temperature on Na+-K+ pump activity, [Na+]i and membrane potential at 9 mM K+
Measurements of ouabain-suppressible 86Rb+ uptake showed that in resting muscles at 9 mM K+, the Na+-K+ pump activity was augmented by 93 % when the temperature was elevated from 20 to 30 °C, reflecting an apparent Q10 of 1.93 (Table 1). The increased Na+-K+ pump activity was associated with a 5 mM reduction in [Na+]i and a hyperpolarization of 5 mV.
Table 1.
Effect of temperature on Na+-K+ pump activity, [Na+]i and membrane potential in resting rat soleus at 9 mm K+
| 20°C | 30°C | P | |
|---|---|---|---|
| Ouabain-suppressible 86Rb+ uptake (nmol (g wet weight)−1 min−1) | 177 ± 16 | 341 ± 39 | < 0.001 |
| [Na+]i (mm) | 31 ± 0.6 | 26 ± 0.6 | < 0.001 |
| Membrane potential (mV) | −62 ± 2 | −67 ± 1 | < 0.05 |
Muscles were mounted on holders at resting length and incubated in 9 mM K+ at 20°C. Fifteen minutes prior to temperature elevation, ouabain (10−3M) was added. Temperature elevation was accomplished by moving muscles into buffers containing 86Rb+ at 20 or 30°C and after 10 min incubation ouabain-suppressible 86Rb+ uptake was measured (n = 9). [Na+]i was determined by flame photometry (n = 9). Membrane potentials were determined with glass microelectrodes at 20 and 30°C. Six muscles rested at 9 mM K+ on holders and after 85 min at 20°C 10 impalements were performed in each muscle followed by elevation of the temperature to 30°C. Ten minutes after the temperature had reached 30°C, another 10 impalements were performed. Each result indicates the mean of 60 impalements (6 ± 10 = 60) at 20°C and 60 impalements at 30°C ±s.e.m.
Effects of temperature, L-lactic acid and salbutamol on force in soleus
Since reduced intracellular pH (pHi) has been shown to reduce the Ca2+ sensitivity of the contractile apparatus in muscles at subphysiological temperatures, it has been proposed that a reduction in pHi by lactic acidosis plays a major role in the development of muscle fatigue (Fitts, 1994). However, this role of reduced pHi has been questioned by recent studies showing that the force-depressing action of reduced pHi is small or non-existent at near-physiological temperatures (Westerblad et al. 1997; Posterino & Fryer, 2000). In addition, it has been demonstrated that at 30 °C, the depressing actions of high [K+]o on excitability and force can be counteracted by L-lactic acid and, furthermore, that this reduction in [K+]o sensitivity by L-lactic acid is additive to a similar protective effect of adrenaline (Nielsen et al. 2001; de Paoli et al. 2002). Thus, it appears that the effect of reduced pHi associated with lactic acidosis depends on temperature. Therefore, the last part of the study was undertaken to determine whether the protective effects against force depression of L-lactic acid and a β2-agonist were present at physiological temperatures (35 °C) and whether they were additive to the protective effect of elevated temperature.
Figure 6 shows that at 30 °C the tetanic force at 11 mM K+ was reduced to 0.07 ± 0.02 N, which corresponds to 16 ± 4 % of the control force at 4 mM K+ at 30 °C. When the temperature was elevated to 35 °C the tetanic force increased to 0.15 ± 0.02 N and thereby reached 35 ± 5 % of control force at 35 °C. Muscles were then exposed to 10 mM L-lactic acid, which increased force to 0.22 ± 0.01 N or 52 ± 3 % of control force. Finally, when the β2-agonist salbutamol was added at a supramaximal concentration (10−5 M), force was restored to 0.36 ± 0.01 N or 87 ± 2 % of the control force at 4 mM K+. If L-lactic acid and salbutamol were added simultaneously with temperature elevation, a rapid and complete force recovery was observed. Thus, in less than 10 min, force increased from 0.07 ± 0.02 N at 30 °C to 0.41 ± 0.01 N at 35 °C, corresponding to an increase in relative force from 16 ± 4 to 97 ± 1 % of the levels of control force recorded at 4 mM K+. Eighty minutes after temperature elevation, force was the same in muscles incubated at 11 mM K+ irrespective of whether all three factors were introduced simultaneously or in a stepwise manner (P > 0.43). To what extent the presence of L-lactic acid and salbutamol could reduce the sensitivity of the muscles exposed to high [K+]o at 35 °C was assessed by repeating the experiments shown in Fig. 6 at varying [K+]o (Fig. 7).
Figure 6. Effects of temperature, 10 mM L-lactic acid and 10−5 M salbutamol on tetanic force at 11 mM K+.
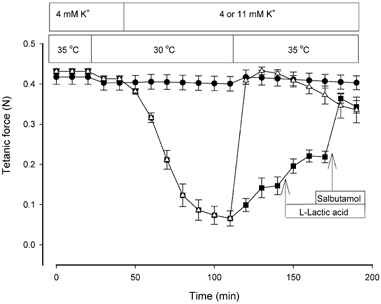
Soleus muscles were stimulated every 10 min using 0.2 ms pulses of 12 V at 90 Hz for 2 s. •, 4 mM K+ throughout; ▪, 11 mM K+ with stepwise addition of 10 mM L-lactic acid and 10−5 M salbutamol after temperature elevation; ▵, 11 mM K+ with addition of L-lactic acid and salbutamol occurring simultaneously with temperature elevation. Each point indicates the mean of 6-12 muscles with error bars showing S.E.M.
Figure 7. Effects of combined addition of 10 mM L-lactic acid and 10−5 M salbutamol on the [K+]o sensitivity of contracting soleus muscles at 35 °C.
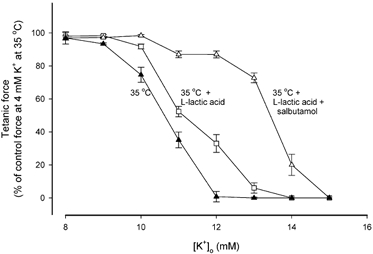
Data points represent steady force at the indicated [K+]o expressed relative to force at 4 mM K+ at 35 °C. ▴, 35 °C; □, 35 °C with 10 mM L-lactic acid; ▵, 35 °C with 10 mM L-lactic acid and 10−5 M salbutamol. Each point is the mean of 6 muscles with error bars showing S.E.M.
The curves in Fig. 7 illustrate the force-[K+]o relationships recorded at 35 °C with L-lactic acid and with L-lactic acid in combination with salbutamol. When L-lactic acid was added, the IC50 increased from 10.7 to 11.2 mM. When L-lactic acid and salbutamol were added together, IC50 reached 13.5 mM. For the sake of comparison with Fig. 2B, the results are shown as relative force.
DISCUSSION
The present study suggests that elevation of muscle temperature during exercise protects active muscles against the depressing actions of exercise-induced hyperkalaemia. Furthermore, this effect of temperature elevation can be added to similar protective effects of lactic acidosis and β2-agonist stimulation. Hence it appears that during exercise a number of factors are involved in protecting active muscle against the potential loss of excitability and force elicited by high [K+]o.
Effects of temperature on force and excitability in muscle incubated at 4 mM K+ and at high [K+]o
Since the level of force at 4 mM K+ depended on temperature, relative force was used to assess the effect of temperature on the loss of force induced by high [K+]o (Fig. 1B). Figure 2 shows that the loss of tetanic force induced by elevated [K+]o was most pronounced at 20 °C and least pronounced at 35 °C. Thus, increasing muscle temperature decreases the [K+]o sensitivity of muscles in the temperature range 20-35 °C. Since increasing the temperature also reduced the [K+]o sensitivity of EDL muscles, it appears that reduced [K+]o sensitivity with elevated muscle temperature is a general phenomenon in skeletal muscles. Figure 4 shows that at a given temperature, the relative depression in force induced by 10 mM K+ was closely associated with the relative depression in M-wave area. Hence, it appeared that the reduction in [K+]o sensitivity observed by elevating muscle temperature was mediated by improved excitability. This conclusion is strengthened by a study showing that the Ca2+ sensitivity of the contractile apparatus of rat soleus muscles is temperature insensitive (Stephenson & Williams, 1981). The M-wave recordings also showed that at 20 °C the conduction velocities at 4 and 10 mM K+ were about half those observed at 30 °C. This difference in conduction velocity at the two temperatures is believed to reflect the temperature dependency of gating kinetics and open channel conductance of the ion channels involved in generating M-waves (Hille, 2001). Temperature elevation did not counteract the effect of elevated [K+]o on the conduction velocity, which would have been expected because temperature elevation caused a hyperpolarization (Table 1). The reason for this discrepancy remains unanswered.
Several mechanisms may contribute to the restoration of force and excitability by temperature elevation in K+-depressed muscles
Firstly, the improvement of excitability was associated with hyperpolarization and a reduction in [Na+]i (Table 1). In muscles at 9 mM K+, an increase in temperature from 20 to 30 °C reduced [Na+]i by 5 mM, augmenting the Nernst reversal potential for Na+ (ENa) by +6 mV under the assumption of constant [Na+]o. Together with a concomitant 5 mV hyperpolarization of the membrane potential, this increases the driving force for the electrodiffusion of Na+ (Vm - ENa) by 11 mV. Since enhanced driving force for Na+ is known to improve excitability (Hodgkin & Katz, 1949), these changes in membrane potential and [Na+]i are likely to contribute to the improved excitability. Because the activity of the Na+-K+ pump is electrogenic and since [Na+]i depends on the rate of active Na+ extrusion, both the hyperpolarization and the reduction in [Na+]i could possibly be explained by an increase in Na+-K+ pump activity induced by temperature elevation (Table 1). Together with the finding that the temperature-induced recovery of force was suppressed by inhibition of the Na+-K+ pump (Fig. 5), this indicates that the Na+-K+ pump plays a central role in the improved excitability induced by temperature elevation in K+-depressed muscles. Another potential mechanism for the temperature-induced hyperpolarization is an increase in the K+ permeability of the muscle fibres, bringing the membrane potential closer to the Nernst reversal potential for K+ (EK). However, if the permeability of other ions that contribute to the membrane potential, such as Na+, shows similar increases with temperature, this would cancel out the effect of increased K+ permeability on the membrane potential.
Secondly, the loss of force induced by increased [K+]o is probably related to slow inactivation of Na+ channels, causing a reduction in the maximal Na+ current during action potentials (Ruff, 1996) and the ensuing action potential failure and reduction in action potential amplitude. Studies on rat omohyoid muscles have demonstrated that an increase in temperature shifts the steady-state voltage dependence of slow inactivation towards less negative potentials (Ruff, 1999). In depolarized muscles, such a shift would increase the number of functional Na+ channels and, thus, contribute to the improved excitability at high temperatures.
Thirdly, reduced muscle pH has been shown to induce a marked recovery of excitability and force in K+-depressed muscles (Nielsen et al. 2001). In this context, the increase in metabolism with elevated temperature may contribute to the recovery of excitability because of the development of hypoxia and the ensuing accumulation of lactic acid in the muscle. In all experiments at 20 and 30 °C, however, both resting tension and active force were maintained throughout the experiments. Moreover, in isolated soleus muscles incubated at 30 °C, pH has been shown to be well maintained at normal resting levels for at least 70 min (Nielsen et al. 2001). Thus, although the possibility cannot be excluded that hypoxia and the accumulation of lactic acid played a role in the recovery of excitability in muscles at 35 °C, it is unlikely that they contributed to the recovery induced by elevating the temperature to 30 °C (Fig. 1), indicating that the accumulation of lactic acid is not a prerequisite for the improved excitability at increased temperature.
Effects of combining temperature elevation with lactic acidosis and salbutamol
The present study suggests that the depressing actions of exercise-induced hyperkalaemia on excitability and force are counteracted by elevated muscle temperature during exercise. Therefore, a warm-up before engaging in vigorous exercise could improve the resistance of muscles to fatigue resulting from elevated [K+]o. This conclusion is in agreement with the results of Asmussen & Bøje (1945) who showed that during intensive exercise of short duration the performance was greatly improved if muscle temperature was elevated prior to exercise either by preliminary exercise or by passively heating muscles. Conversely, exposure of muscles to low temperatures while exercising can be expected to reduce the ability of muscles to cope with elevated [K+]o, i.e. augment the development of muscle fatigue.
In contrast to studies performed at subphysiological temperatures the results shown in Fig. 6 demonstrate that instead of causing fatigue, lactic acid protects muscles against the development of fatigue induced by hyperkalaemia at physiological temperature (35 °C). These results are in agreement with studies performed at near-physiological temperature (30 °C), where L-lactic acidosis in K+-depressed muscles was associated with a marked recovery in excitability and force (Nielsen et al. 2001). Therefore, because exercise is associated with a loss of K+ from active muscles leading to an increase in [K+]o, the role of lactic acid in the development of muscle fatigue during intensive exercise must be considered to be a protecting rather than a fatigue-inducing factor. Figure 6 shows that in addition to the protective effect of L-lactic acid there was a further restoration of force when salbutamol was added. Salbutamol has recently been shown to increase the affinity of the Na+-K+ pump for [Na+]i, leading to an increased Na+ efflux and an elevated electrochemical gradient for Na+ (Buchanan et al. 2002), which will improve excitability in muscles exposed to high [K+]o (Overgaard et al. 1999). Figure 7 shows that the combined actions of L-lactic acid and salbutamol caused the IC50 to increase from 10.7 to 13.5 mM, suggesting a large reduction in [K+]o sensitivity by L-lactic acid and salbutamol. It is therefore proposed that elevation of muscle temperature combined with lactic acidosis and high levels of circulating catecholamines counteract the depressing actions of high [K+]o on muscle performance during exercise.
In conclusion, the present study suggests an exercise scenario in which the loss of excitability and force by increased [K+]o is counteracted by the simultaneous elevation of muscle temperature, lactic acidosis and the presence of catecholamines.
Acknowledgments
We thank Marianne Stürup Johansen, Tove Lindahl Andersen, Vibeke Uhre and Ann-Charlotte Andersen for skilled technical assistance. The study was supported by grants from the Danish Medical Research Council (no. 22-01-0189), the Danish Biomembrane Research Centre, Aarhus Universitets Forskningsfond and The Carlsberg Foundation.
REFERENCES
- Asmussen E, Bøje O. Body temperature and capacity for work. Acta Physiol Scand. 1945;10:1–22. [Google Scholar]
- Brooks GA, Hittelman KJ, Faulkner JA, Beyer RE. Temperature, skeletal muscle mitochondrial functions, and oxygen debt. Am J Physiol. 1971;220:1053–1059. doi: 10.1152/ajplegacy.1971.220.4.1053. [DOI] [PubMed] [Google Scholar]
- Buchanan R, Nielsen OB, Clausen T. Excitation- and β2-agonist-induced activation of the Na+-K+ pump in rat soleus muscle. J Physiol. 2002;545:229–240. doi: 10.1113/jphysiol.2002.023325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns SP, Flatman JA, Clausen T. Relation between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+-K+ pump. Pflugers Arch. 1995;430:909–915. doi: 10.1007/BF01837404. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Hing WA, Slack JR, Mills RG, Loiselle DS. Different effects of raised [K+]o on membrane potential and contraction in mouse fast- and slow-twitch muscle. Am J Physiol. 1997;273:C598–611. doi: 10.1152/ajpcell.1997.273.2.C598. [DOI] [PubMed] [Google Scholar]
- Clausen T, Andersen SL, Flatman JA. Na+-K+ pump stimulation elicits recovery of contractility in K+-paralysed rat muscle. J Physiol. 1993;472:521–536. doi: 10.1113/jphysiol.1993.sp019960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen T, Everts ME. K+-induced inhibition of contractile force in rat skeletal muscle: role of active Na+-K+ transport. Am J Physiol. 1991;261:C799–807. doi: 10.1152/ajpcell.1991.261.5.C799. [DOI] [PubMed] [Google Scholar]
- Clausen T, Hansen O. Ouabain binding and Na+-K+ transport in rat muscle cells and adipocytes. Biochim Biophys Acta. 1974;345:387–404. [Google Scholar]
- Clausen T, Kohn PG. The effect of insulin on the transport of sodium and potassium in rat soleus muscle. J Physiol. 1977;265:19–42. doi: 10.1113/jphysiol.1977.sp011703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paoli F, Overgaard K, Nielsen OB. Protective effects of acidosis and Na+,K+-pump activation on force in K+-depressed skeletal muscle. J Physiol. 2002;544.P:82P. [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Galbo H, Holst JJ, Christensen NJ, Hilsted J. Glucagon and plasma catecholamines during beta-receptor blockade in exercising man. J Appl Physiol. 1976;40:855–863. doi: 10.1152/jappl.1976.40.6.855. [DOI] [PubMed] [Google Scholar]
- Green S, Langberg H, Skovgaard D, Bülow J, Kjaer M. Interstitial and arterial-venous [K+] in human calf muscle during dynamic exercise: effect of ischaemia and relation to muscle pain. J Physiol. 2000;529:849–861. doi: 10.1111/j.1469-7793.2000.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallen J, Gullestad L, Sejersted OM. K+ shifts of skeletal muscle during stepwise bicycle exercise with and without β-adrenoceptor blockade. J Physiol. 1994;477:149–159. doi: 10.1113/jphysiol.1994.sp020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansen L, Orheim A, Sejersted OM. Metabolic acidosis and changes in water and electrolyte balance in relation to fatigue during maximal exercise of short duration. Int J Sports Med. 1984;5:110–115. [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 3. Sunderland, MA, USA: Sinauer Associates; 2001. [Google Scholar]
- Hnik P, Hollas M, Krekule I, Kuriz N, Mejsnar J, Smiesko V, Ujec E, Vyskocil F. Work-induced changes in skeletal muscle and effluent venous blood assessed by liquid ion-exchanger microelectrodes. Pflugers Arch. 1976;362:85–94. doi: 10.1007/BF00588685. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R400–406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. The temperature dependence of isometric contractions of single, intact fibres dissected from a mouse foot muscle. J Physiol. 1987;390:285–293. doi: 10.1113/jphysiol.1987.sp016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JJ, Kristensen M, Hellsten Y, Bangsbo J, Juel C. Localization and function of ATP-sensitive potassium channels in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2003;284:R558–563. doi: 10.1152/ajpregu.00303.2002. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, De Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol. 2001;536:161–166. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard K, Nielsen OB. Activity induced recovery of excitability in K+-depressed rat soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2001;279:R1–8. doi: 10.1152/ajpregu.2001.280.1.R48. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Nielsen OB, Flatman JA, Clausen T. Relations between excitability and contractility in rat soleus muscle: role of the Na+-K+ pump and Na+/K+ gradients. J Physiol. 1999;518:215–225. doi: 10.1111/j.1469-7793.1999.0215r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino GS, Fryer MW. Effects of myoplasmic L-lactate concentration on E-C coupling in mammalian skeletal muscle. J Appl Physiol. 2000;89:517–528. doi: 10.1152/jappl.2000.89.2.517. [DOI] [PubMed] [Google Scholar]
- Ruff RL. Sodium channel slow inactivation and the distribution of sodium channels on skeletal muscle fibres enable the performance properties of different skeletal muscle fibre types. Acta Physiol Scand. 1996;156:159–168. doi: 10.1046/j.1365-201X.1996.189000.x. [DOI] [PubMed] [Google Scholar]
- Ruff RL. Effects of temperature on slow and fast inactivation of rat skeletal muscle Na+ channels. Am J Physiol. 1999;277:C937–947. doi: 10.1152/ajpcell.1999.277.5.C937. [DOI] [PubMed] [Google Scholar]
- Saltin B, Gagge AP, Stolwijk JA. Muscle temperature during submaximal exercise in man. J Appl Physiol. 1968;25:679–688. doi: 10.1152/jappl.1968.25.6.679. [DOI] [PubMed] [Google Scholar]
- Savard G, Strange S, Kiens B, Richter EA, Christensen NJ, Saltin B. Noradrenaline spillover during exercise in active versus resting skeletal muscle in man. Acta Physiol Scand. 1987;131:507–515. doi: 10.1111/j.1748-1716.1987.tb08270.x. [DOI] [PubMed] [Google Scholar]
- Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol. 1985;248:C265–270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Segal SS, Faulkner JA, White TP. Skeletal muscle fatigue in vitro is temperature dependent. J Appl Physiol. 1986;61:660–665. doi: 10.1152/jappl.1986.61.2.660. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G. Exercise-induced muscle fatigue: the significance of potassium. Acta Physiol Scand. 1990;(suppl. 593):1–63. [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lannergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]


