Abstract
The basis of rhythmic activity observed at the dorsal column nuclei (DCN) is still open to debate. This study has investigated the electrophysiological properties of isolated DCN neurones deprived of any synaptic influence, using the perforated-patch technique. About half of the DCN neurones (64/130) were spontaneously active. More than half of the spontaneous neurones (36/64) showed a low threshold membrane oscillation (LTO) with a mean frequency of 11.4 Hz (range: 4.3–22.1 Hz, n = 20; I = 0). Cells showing LTOs also invariably showed a rhythmic 1.2 Hz clustering activity (groups of 2–5 action potentials separated by silent LTO periods). Also, more than one-third of the silent neurones presented clustering activity, always accompanied by LTOs, when slightly depolarised. The frequency of LTOs was voltage dependent and could be abolished by TTX (0.5 μM) and riluzole (30 μM), suggesting the participation of a sodium current. LTOs were also abolished by TEA (15 mM), which transformed clustering into tonic activity. In voltage clamp, most DCN neurones (85 %) showed a TTX-/riluzole-sensitive persistent sodium current (INa,p), which activated at about -60 mV and had a half-maximum activation at −49.8 mV. An M-like, non-inactivating outward current was present in 95 % of DCN neurones, and TEA (15 mM) inhibited this current by 73.7 %. The non-inactivating outward current was also inhibited by barium (1 mM) and linopirdine (10 μM), which suggests its M-like nature; both drugs failed to block the LTOs, but induced a reduction in their frequency by 56 and 20 %, respectively. These results demonstrate for the first time that DCN neurones have a complex and intrinsically driven clustering discharge pattern, accompanied by subthreshold membrane oscillations. Subthreshold oscillations rely on the interplay of a persistent sodium current and a non-inactivating TEA-sensitive outward current.
It has long been known that dorsal column nuclei (DCN) neurones display resting discharge in anaesthetised animals. Spontaneous and evoked activity of DCN cells has been proved to be complex, with bursting behaviour being a common characteristic (Galindo et al. 1968; Pubols et al. 1989). Determining the mechanisms of the rhythmic activity has been a major concern in the field for years. However, the general use of anaesthetised animals with complex synaptic circuits and/or extracellular recording precluded an unambiguous answer. In 1968, Galindo et al. hypothesised that the characteristic discharge of DCN neurones in vivo might be partially determined by their intrinsic tendency to repetitive firing (but see Amassian & Giblin, 1974).
Recent work in anaesthetised cats from Canedo's group also pointed out the possible importance of intrinsic properties to explain the complex behaviour of cuneate nucleus neurones, particularly the 1-4 Hz delta-like rhythm (Mariño et al. 1996, 2000; Canedo, 1997). In anaesthetised cats, both presumed interneurones and cuneothalamic cells showed spontaneous activity, and membrane depolarisation often turned oscillatory (rhythmic bursts of 2-3 action potentials) into tonic (rhythmic single action potentials) activity (Canedo et al. 1998; Mariño et al. 2000). Since activity at certain frequencies was still present in DCN neurones when the fronto-parietal cortex was removed or when the pyramidal tract was sectioned, it was concluded that these rhythms were generated inside the DCN (Mariño et al. 2000; see also Panetsos et al. 1998). Whether this activity was due to intrinsic membrane properties or to a property of the internal DCN circuitry remains to be determined.
Rat DCN neurones are classified into two types based on the presence or absence of a repolarising rebound in response to the injection of negative currents (Núñez & Buño, 1999). It is therefore tempting to speculate that cells showing a sag project to the thalamus, while cells presenting no sag are interneurones (Núñez & Buño, 1999; see also Canedo et al. 1998; Deuchars et al. 2000).
Thalamic pacemaker activity has been shown to depend on a hyperpolarising-activated current (Ih) and on a low threshold Ca2+ current (IT) (McCormick & Pape, 1990; Soltesz et al. 1991). Accordingly, it has been suggested that the delta-like rhythm observed in cat DCN cells could also be generated intrinsically (Canedo et al. 1998). However, experimental evidence is still lacking.
The aim of this study was to determine the characteristics and mechanisms of oscillations at the level of the DCN using neurones deprived of any synaptic influence. About half of rat DCN neurones presented clustering rhythmic activity in culture. Also a low amplitude oscillation of the membrane potential was consistently found, which has been termed low threshold oscillation (LTO; see Alonso & Llinás, 1989; Alonso & Klink, 1993; see also Pape et al. 1998; Pape & Driesang, 1998). These results show that DCN neurones have complex intrinsic membrane properties, which could explain many of the in vivo properties of these nuclei. A preliminary report has been presented in abstract form (Reboreda et al. 2002).
METHODS
Cell culture
Because a stereotaxic atlas of young rat brainstem was not available, the brainstems of three terminally anaesthetised (see below) animals were removed and fixed in 4 % paraformaldehyde. Frozen coronal sections (50 μm) were obtained, serially mounted and stained using the Nissl technique. Cuneate and gracile nuclei were easily located in these series by comparison against an atlas of adult animals (e.g. Paxinos & Watson, 1998; and see Fig. 1A, upper panel).
Figure 1. Localisation and identification of dorsal column nuclei (DCN) neurones.

A, cross sections at the level of the DCN in 10-day-old rats. A section (50 μm thick) processed with the Nissl technique (upper panel) showing the gracile and cuneate nuclei. Lower panel shows a transilluminated 300 μm thick fresh section showing the tissue extracted with the micropunch technique to be cultured. CC, central canal; Cu, cuneate nucleus; Gr, gracile nucleus; SpT, spinal trigeminal nucleus; XII, hypoglossus nucleus. B, fluorescence photomicrographs obtained from a 2-day-old culture of dissociated DCN cells. Fast blue was injected into the ventroposterolateral thalamus 3 days before the cells were cultured. A retrograde labelled cell (arrowed) projecting to the thalamus is shown under epifluorescence illumination (above) and under phase-contrast visible light (below).
Sprague-Dawley rats, 10- to 15-days-old, were terminally anaesthetised with ether (stabilised diethyl ether anaesthetic, Panreac Química SA, Barcelona, Spain) and decapitated. The head was fixed with needles onto a Sylgard-coated Petri dish and the skull and remaining cervical spinal bones were carefully removed. The brain, including the rostral part of the cervical spinal cord, was bathed in ice-cold dissection solution (for the composition of solutions see Solutions and drugs). For slicing, the brain was placed on the stage of an electrically driven McIllwain tissue chopper (Mickle Laboratory Engineering Co. Ltd, Gomshall, UK); coronal slices of 300-500 μm were obtained in less than 1 min and immediately immersed in ice-cold dissection solution. Under a stereoscopic microscope (Nikon SMZ645; Nikon, Tokyo, Japan), provided with a cold light source (Intralux 4000-1; Intralux, Schlieren, Switzerland), the one or two rostral slices clearly showing the central canal were chosen and all other slices discarded. As white and grey matter are easily differentiated when slices are transilluminated (see Fig. 1A, lower panel), hollow punching needles (0.5-0.7 mm inner diameter, Stoelting Co., Wood Dale, IL, USA) were aimed at the dorsal tip of the spinal trigeminal tract where the cuneate nucleus is located (Fig. 1A, lower panel). Punches were always taken as dorsal as possible to avoid the nucleus of the solitary tract and the dorsal motor nucleus of the vagus. However, since gracile-cuneate boundaries were unclear in unstained slices, no effort was made to distinguish between these DCN.
Punched samples were pushed into the enzymatic solution (see Solutions and drugs), using the self-contained micropunch spring-loaded expeller, washed twice and incubated in the solution for 15 min at 37 °C and 5 % CO2. After the enzymatic treatment, the tissue was washed with dissociation solution and gently disrupted using a flame-polished Pasteur pipette with an enlarged tip diameter. The supernatant was transferred to a 10 ml tube and centrifuged at 1000 r.p.m. (178 g) for 10 min; cells were then resuspended in culture medium, plated onto laminin-coated 35 mm dishes and stored at 37 °C and 5 % CO2. Neurones were used for recording 2 days after plating, most of them having no processes at that time.
Retrograde labelling
As the major projection of the DCN is to the ventroposterolateral (VPL) thalamic nucleus, 0.5-1 μl fast blue (1 %) was stereotaxically injected into the VPL (bilaterally) using a Hamilton syringe with a 0.3 mm internal diameter needle. The tracer was injected in 10-day-old rats anaesthetised (I.P.) with a mixture of ketamine (35 mg kg−1) and xilazine (5 mg kg−1). Animals were monitored during their recovery from anaesthesia and were returned to their cage for 3-5 days, during which time they suckled and showed no signs of distress. Animals were killed and neuronal cultures were prepared as described in the previous section (Cell culture). VPL coordinates were taken from the Sherwood & Timiras (1970) atlas for 10-day-old rats, using bregma as a reference point. Experimental procedures conformed to the Spanish and European directives for the protection of animals used for experimental purposes (R.D. 223/1988; 86/609/EEC) and they were approved by the Spanish Council and the University of Vigo Scientific Committees.
Labelled neurones were found in approximately 90 % of the cultures (filter: Nikon UV-2A, 330-380 nm; Fig. 1B). Unfortunately, although labelled cells fired action potentials, they could not be recorded successfully for more than a few seconds, perhaps due to the phototoxicity of fast blue when exposed to epifluorescence illumination (see Christian et al. 1993). The presence of labelled cells indicated that cells projecting to the thalamus were present and that the micropunch was correctly aimed at DCN.
Recording and data analysis
Cultured cells were placed onto the stage of a Nikon TE300 inverted microscope equipped with Hoffman modulation contrast, phase contrast and an epifluorescence attachment. Cells were continuously perfused by gravity with a standard bath solution (10 ml min−1) at room temperature (21-25 °C), the bath solution being bubbled with synthetic air (21 % oxygen : 79 % nitrogen; Carburos Metálicos SA, Barcelona, Spain).
Electrodes of 6-10 MΩ resistance after polishing (Narishige MF-830; Narishige, Tokyo, Japan) were pulled, in a two-step vertical puller (Narishige P-10), and filled with a standard pipette solution containing amphotericin B (50-75 μg ml−1) to perforate the patch. Series resistances lower than 30 MΩ were consistently obtained within 15 min of seal formation (Rae et al. 1991), and compensated by 40-60 % using the Axopatch 200B clamp circuitry (Axon Instruments Inc., Foster City, CA, USA).
For current-clamp experiments, the ‘clamp-fast’ mode of an Axopatch 200B amplifier (Magistretti et al. 1998; Bennet et al. 2000), also used for voltage-clamp experiments, or the ‘bridge’ mode of an Axoclamp 2B (Axon Instruments) was employed. Liquid junction potentials measured using a 3 M KCl reference electrode ranged from 4 to 6 mV and traces were corrected accordingly (Neher, 1992). Recordings were digitised through a Digidata 1200 interface (Axon Instruments) and stored in a PC-compatible hard disk. Data were captured, analysed and plotted using the pCLAMP 8 (Axon Instruments) and Origin 5 (OriginLab Corp., Northampton, MA, USA) software. Sampling frequency was 10 KHz filtered at 5 KHz for signals containing action potentials, and 1 KHz filtered at 0.5 KHz for voltage clamp. For clarity, experimental current- and voltage-clamp protocols are described in the appropriate sections of Results. Averages are given as means ± S.E.M. and statistical significance was assessed using the Student's t test at P < 0.01.
Short segments of data (1-3 s) were used to calculate the power spectrum using the clampfit (Axon Instruments) decimation-in-time fast Fourier transform (FFT) algorithm:
where, for samples x = x0 to xN-1, ai is the kth spectral component or harmonic, j is a complex number and N is the number of sample values in each period of the signal. Before digitisation, recordings were low-pass filtered at 2 KHz (4-pole Bessel filter) and sampled at 10 KHz, so that sampling rate was substantially higher than the Nyquist critical sampling rate. A rectangular window type was used.
TTX (tetrodotoxin)-sensitive Na+ current amplitude (measured at the end of 2 s voltage steps) was transformed into conductance using the Ohm law in the form: G = I/(Vm - VE), where Vm is the test potential and VE is the Na+ equilibrium potential calculated using the Nernst equation. Conductance was normalised, plotted against Vm and fitted with a Boltzmann function in the form: y = 1/(1 + exp((Vm - V1/2)/k)), where V1/2 is the half-activation voltage and k is the slope factor.
Solutions and drugs
Culture
The dissection solution was composed of GBSS (Gey's balanced salt solution) containing 10 mM MgCl2 and 0.6 % glucose. The enzymatic solution contained HBSS (Hanks’ balanced salt solution) with 10 mM Hepes (N-2-hydroxyethylpiperazine-N‘-2-ethanesulfonic acid) and 0.3 mg ml−1 trypsin. The dissociation solution contained HBSS with 10 % FCS (fetal calf serum), 10 mM MgCl2 and 0.01 mg ml−1 DNase I. The culture medium was neurobasal A medium containing: 2 % B-27, 100 ng ml−1 NGF (nerve growth factor), 1 mM L-glutamine, 50 i.u. ml−1 penicillin, 0.5 mg ml−1 streptomycin and 1 % glutamax I.
Recording
Standard bath solution contained (mM): NaCl 140, KCl 3, MgCl2 1, CaCl2 2.5, D-glucose 10 and Hepes 10, pH adjusted to 7.2 with Tris (Tris(hydroxymethyl)-aminomethane). Standard pipette solution contained (mM): potassium acetate 90, KCl 20, MgCl2 3, CaCl2 1, EGTA (ethyleneglycol-bis-(β-aminoethylether)-N,N,N′,N′-tetraacetic acid) 3 and Hepes 40, pH adjusted to 7.2 with NaOH (20 mM). The bath solution for the study of Na+ currents contained (mM): NaCl 100, TEA-Cl 40 (tetraethylammonium chloride), KCl 3, MgCl2 1, CaCl2 1, D-glucose 10, Hepes 10, BaCl2 1, CsCl 1, 4-AP (4-aminopyridine) 2 and CdCl2 0.1; the pipette solution contained (mM): CsCl 110, NaCl 5, MgCl2 3, CaCl2 1, EGTA 3 and Hepes 40, both adjusted with Tris. Drugs were added to the bath reservoir to reach the desired concentration.
Fast blue, ketamine, xylazine, amphotericin B, GBSS, glucose, trypsin, DNase I, NGF, L-glutamine, penicillin, streptomycin, EGTA, TEA-Cl, BaCl2, CsCl, 4-AP, CdCl2, DMSO (dimethyl sulfoxide), riluzole and linopirdine were obtained from Sigma (Sigma-Aldrich Chemie, Steinheim, Germany). Neurobasal A medium, B-27, glutamax I and FCS were obtained from Invitrogen (Invitrogen Corporation, Paisley, UK). TTX was obtained from Tocris (Tocris Cookson Ltd, Bristol, UK). All other chemicals were obtained from Merck (Merck, Darmstadt, Germany). Stock solutions were prepared as follows: riluzole (100 mM) in DMSO and linopirdine (10 mM) in 0.1 N HCl.
RESULTS
General behaviour of DCN neurones in culture
Current clamp-recorded DCN neurones can be divided into two main groups attending to their resting properties in culture. From a sample of 130 cells recorded, 66 were silent and 64 spontaneously active in the absence of direct current injection (I = 0). Most spontaneously active neurones were observed to fire when the electrode was approaching the membrane (‘action currents’), before ‘gigaseal’ formation, indicating that spontaneous activity was not induced by the pipette solution. They continued to fire until patch-perforation allowed clamping the membrane at -60 mV. In cells where the spontaneous activity allowed it (spontaneous tonic cells were not included), the resting membrane potential was measured, giving values of -53 ± 1.6 mV (n = 66) for silent and -46.2 ± 1.8 mV (n = 47) for spontaneously active cells; these means were statistically different at P < 0.01. In cells showing spontaneous clustering activity (see below), the resting potential was obtained at the silent intercluster period as the mean of membrane potentials reached during a period of 0.5-1 s. A striking characteristic of DCN neurones was a very high input resistance, often well above 1 GΩ, which was in accordance with the high resistance recently observed for DCN neurones in a brain stem-spinal cord preparation (Deuchars et al. 2000). The general observation should be borne in mind that patch-clamped neurones show input resistances about 10-fold greater than those that are intracellularly recorded (e.g. Raman et al. 2000).
A hyperpolarising-activated inward current (Ih) was present in 61 of 98 cells investigated. The presence of this current was confirmed by the existence of a depolarising rebound (sag) in response to negative current injections in current clamp (see Fig. 6C), or an inward rectification at negative voltages in voltage clamp (not shown). Both the ‘sag’ (n = 3) and the inward rectification (n = 11) were caesium- (1 mM) but not barium- (2 mM) sensitive (not shown; see Lamas, 1998). Ih was not specific to cells showing particular firing patterns (see below); it was observed in all types of silent and spontaneously active cells.
Figure 6. LTOs and persistent Na+ current are sensitive to riluzole.
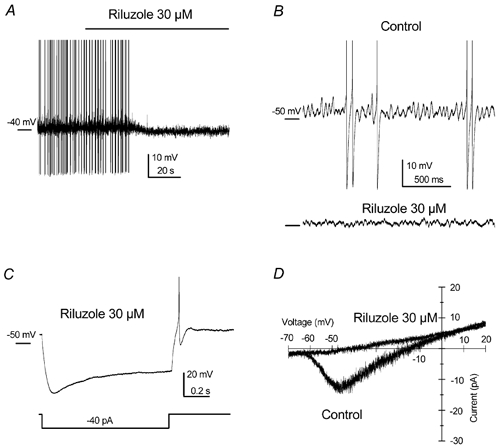
A, long-lasting recording showing the abolition of the clustering mode accompanied by a slight hyperpolarisation in a spontaneously clustering cell. B, expanded traces showing the effect of riluzole over the LTO in a silent clustering cell with 30 pA injection. C, although riluzole abolished spontaneous firing, under this treatment cells were still able to fire action potentials, as can be seen in the rebound at the end of a hyperpolarising current injection. Note the prominent depolarising rebound (sag) indicating the presence of hyperpolarising-activated current (Ih). D, as seen in Fig. 5B using TTX, the persistent Na+ current evoked in response to slow voltage ramps was also completely blocked by riluzole. Recordings in A and C are from the same cell.
Spontaneously active cells
In order to determine the characteristics of the firing pattern, DCN cells were recorded for 3-5 min in current-clamp mode and without current injection (I = 0). More than half of the 64 spontaneously active neurones presented rhythmic clustering activity (n = 36, Fig. 2A), 17 were tonically active (Fig. 2B) and 11 fired randomly (Fig. 2C). Based on their firing pattern they were classified as ‘clustering’, ‘tonic’ and ‘random’ cells. Cells were considered tonic when single action potentials were fired at a very regular pace, a preferred frequency thus being easily detected (Fig. 2B); the mean firing frequency was 14.3 ± 2.7 Hz (n = 17). Cells were considered to be ‘random’ when a single frequency could not be assigned to the firing of action potentials.
Figure 2. Firing pattern of three different spontaneously active neurones.

A, clustering activity of a spontaneously active DCN neurone in culture: at rest (control) and under continuous 10 pA (middle trace) and 50 pA (lower trace) intracellular current injection. Intracluster frequencies were 9.75, 12.2 and 14.65 Hz, respectively. Note the presence of subthreshold membrane potential oscillations with frequencies of 6, 7.5 and 9.5 Hz in upper, middle and lower traces, respectively, and the increase in the number of action potentials per cluster when the membrane was depolarised. B, spontaneously active DCN cell firing tonically. C, DCN neurone firing randomly at rest.
Clustering cells, during the control period (Fig. 2A, upper trace), fired clusters of two to five action potentials repetitively at frequencies around 1 Hz, doublets were very characteristic of DCN neurones with low activity (see Galindo et al. 1968). Note that subthreshold oscillations (LTO) can be observed in the silent periods, a similar behaviour called ‘spike clustering’ having been reported in entorhinal cortex stellate cells in slices (Alonso & Klink, 1993). Upon artificial depolarisation, instead of going into tonic firing, the number and frequency of the spikes inside the cluster and the frequency of the subthreshold oscillations increased. The frequency of the clusters (intercluster), however, did not change greatly (Fig. 2A, middle and lower traces).
Silent cells
Silent cells fired action potentials when depolarising currents were injected (less than 50 pA). When a continuous, low-amplitude positive current was applied to the 66 silent DCN neurones, 25 of them became clustering (Fig. 3A), 23 fired a few action potentials upon depolarisation and then adapted (Fig. 3C), 10 fired tonically (Fig. 3B) and only eight cells fired in an apparently unpredictable (random) way (Fig. 3D), which became tonic when higher currents were injected.
Figure 3. Responses of silent DCN neurones to depolarisation.
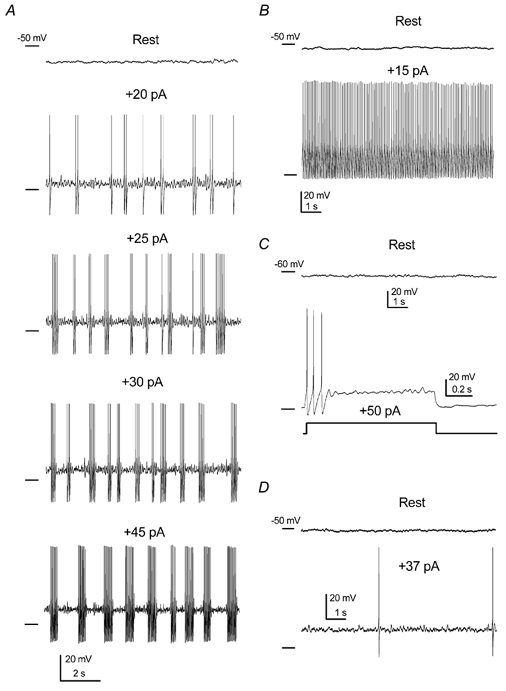
A, silent cells often became clustering when depolarised. The number and frequency of action potentials increased inside the cluster when increasing the injection current from 20 to 45 pA. Intercluster frequencies were 0.9, 1, 1.2 and 0.9 Hz. Action potential frequencies inside the clusters were 10, 12, 15 and 22 Hz for current injections of 20, 25, 30 and 45 pA, respectively. Note the presence of subthreshold oscillations upon depolarisation, with increasing frequencies of 9, 12, 15 and 19 Hz, respectively. B, some silent cells behaved tonically when low depolarising current injection was applied. C, example of a silent cell showing strong spike frequency adaptation. D, a small group of silent cells fired randomly upon depolarisation.
Note that, upon depolarisation, clustering cells showed a clear subthreshold oscillation (Fig. 3A), indistinguishable from that observed in spontaneously clustering cells (see Fig. 2A).
LTO properties
All cells showing clustering behaviour, either spontaneous (n = 36) or silent after a small depolarisation (n = 25), presented subthreshold oscillations with a maximum peak-to-peak amplitude of 5 to 10 mV (Fig. 4A). Figure 4B shows the power density spectrum for the low threshold oscillation shown in Fig. 4A, which had a dominant peak at 10 Hz.
Figure 4. Properties of low threshold oscillations (LTOs).

A, example of LTO in a cell displaying clustering discharge during the control period. B, power spectrum for the oscillation marked in A, showing a dominant frequency at 10 Hz. C, correlation between the frequency of LTOs and the frequency of action potentials inside clusters for 20 spontaneous DCN neurones. D, voltage dependency of the frequency of LTOs, intracluster action potentials and clusters (intercluster) in three spontaneously active DCN neurones.
In order to systematically analyse and compare them in similar conditions, all rhythmic events were analysed in a subgroup of 20 cells belonging to the group of 36 neurones showing spontaneous clustering activity. These 20 cells, which had a mean resting potential of -44 ± 1.6 mV, were chosen because the frequency of all those rhythmic events could be clearly resolved at the resting membrane potential (without injecting current; I = 0). The analysis gave mean frequencies of 1.2 ± 0.1, 12.5 ± 1.3 and 11.4 ± 1.2 Hz for intercluster, intracluster and subthreshold oscillations, respectively. For the same spontaneously clustering cells, the frequency of LTOs and the frequency of action potentials (intracluster) were strongly related (R = 0.86; P < 0.0001, n = 20) when measured without current injection (Fig. 4C).
In the 20 spontaneously clustering cells, no correlation could be found between the resting potential (I = 0) and the frequency of any rhythmic event (not shown). Notwithstanding this, when individual cells were manually clamped at different membrane voltages (e.g. Fig. 2A and Fig. 3A), the LTO and intracluster frequency showed a linear voltage dependency, with a rate of 1 ± 0.1 and 0.9 ± 0.2 Hz mV−1, respectively (n = 6); Fig. 4D shows individual data points for three of the six cells for clarity. Intercluster frequency was clearly voltage independent in the same six cells (see Fig. 4D). Also in silent cells, artificial membrane depolarisation induced a change both in the number of action potentials per cluster and in the frequency of action potentials inside the cluster, but no significant changes in the frequency of clusters. Much like in spontaneously clustering cells, the voltage dependence for LTO and intracluster frequency was linear, showing an increase upon depolarisation of 1.1 ± 0.3 and 1.6 ± 0.2 Hz mV−1, respectively (n = 6, not shown). Intercluster frequency was clearly voltage independent in the same six silent cells.
Persistent Na+ current and LTOs
In all cells tested (n = 6) subthreshold oscillations were reversibly abolished by TTX (0.5 μM, Fig. 5A), suggesting the contribution of a persistent Na+ current (INa,p) as previously described for similar oscillations in other cell types (Llinás et al. 1991; Klink & Alonso, 1993; Pape & Driesang, 1998; Boehmer et al. 2000).
Figure 5. Properties of the persistent Na+ current in cultured DCN neurones.

A, application of TTX (middle trace) reversibly abolished both action potentials and low threshold oscillations. B, the inward current carried through persistent Na+ channels (obtained in response to slow voltage ramps: 10 mV s−1) was completely blocked by TTX. C, persistent inward currents evoked by long lasting voltage steps were also abolished by TTX (upper traces). TTX-sensitive current (lower trace) was composed of a rapidly inactivating inward current (truncated), a slowly inactivating current with a time constant of 590 ms (best single exponential fit superimposed) and a persistent current which remained at the end of the step. D, current-voltage (I-V) relationships for the persistent Na+ current obtained from voltage ramps (○) and from voltage steps (•). E, voltage dependence of persistent Na+ current activation obtained from voltage steps (from I-V relationships of cells represented in D) gave a half-maximum activation at -49.8 mV.
Voltage-clamp experiments confirmed the presence of an INa,p-like current in most DCN neurones tested (39 out of 46). In order to block K+ and Ca2+ currents, slow voltage ramps (from -70 to +20 mV; 10 mV s−1) were applied to cells bathed in a solution containing: TEA-Cl (40 mM), Ba2+ (1 mM), Cs+ (1 mM), 4-AP (2 mM) and Cd2+ (100 μM). For these experiments, 110 mM Cs+ was substituted for the same concentration of K+ in the pipette, to further block K+ currents from inside. Figure 5B shows a low threshold (-54.9 ± 1.0 mV) inward current peaking at -37.3 ± 1.2 mV, which is strongly blocked by 0.5 μM TTX (n = 20), these characteristics matching those of persistent Na+ currents (see Crill, 1996). Figure 5C shows Na+ currents in response to a long-lasting depolarising step (from -80 mV to -30 mV, 2 s) before and after TTX application (upper traces). TTX-sensitive current was composed of an initial rapidly inactivating transient current (truncated) and a smaller sustained current that persisted throughout the depolarising step (Fig. 5C, lower trace). Persistent TTX-sensitive Na+ currents decayed very slowly, the time constant for the best single exponential fit being 570 ± 35.7 ms (n = 12). To avoid the transient current, the starting point for fitting was fixed 40 ms after the step initiation.
Current-voltage relationships (I-V) were constructed for TTX-sensitive currents from voltage ramps (Fig. 5D, ○, n = 10) and from voltage steps (Fig. 5D, •, n = 5). To construct I-V relationships, the current amplitude was measured every 5 mV when using continuous voltage ramps. When long-lasting voltage steps were used, the amplitude was obtained from the current average over the last 50 ms. The differences in current amplitude using both methods (Fig. 5D) indicate that voltage ramps, although slow, did not allow the characteristic partial inactivation of the persistent Na+ current to occur (Fig. 5C; see also Magistretti & Alonso, 1999). Steady-state normalised conductance was obtained from voltage steps (n = 5) and it is shown in Fig. 5E. TTX-sensitive current activated at -60 mV and the conductance reached the maximum at about -30 mV. The best Boltzmann fit gave a half-activation voltage of -49.8 ± 0.7 mV and a slope of 4.4 ± 0.7 mV, these values being comparable to those obtained in entorhinal cortex layer II neurones (Magistretti & Alonso, 1999).
Riluzole, a neuroprotective agent used for the treatment of amyotrophic lateral sclerosis, has been shown to inhibit the persistent Na+ current in rat cortical neurones in slices (Urbani & Belluzzi, 2000). Accordingly, riluzole (30 μM) slightly hyperpolarised clustering cells, strongly reduced the subthreshold oscillations and abolished the clustering mode in cultured DCN neurones (n = 3). Figure 6A shows the effect of riluzole on the membrane potential and the abolishment of the spontaneous clustering activity. The subthreshold oscillation of a different cell and the reduction of the oscillation after riluzole treatment are shown in Fig. 6B. Although the clustering activity disappeared in the presence of riluzole, the capacity of the cell to fire action potentials remained intact as shown by its response to a hyperpolarising current injection (Fig. 6C). Riluzole also blocked the persistent Na+ current evoked by slow voltage ramps (n = 8; Fig. 6D, see Urbani & Belluzzi, 2000).
Outward currents and LTOs
The M-current has been suggested to participate in the LTO repolarising mechanism (e.g. Pape & Driesang, 1998); Ba2+, a widely used M-current blocker, abolished LTOs in entorhinal cortex neurones (Klink & Alonso, 1993). Recently, theta-resonance has been partially ascribed to the presence of an M-current combined with a persistent Na+ current in rat hippocampal pyramidal cells (Hu et al. 2002). Also, TEA-sensitive currents have been proposed to be the countervailing influence to the persistent Na+ current for the generation of low threshold oscillations in several cell types (Klink & Alonso, 1993; Gutfreund et al. 1995; Boehmer et al. 2000). With this evidence in mind, the effect on the LTOs of several current blockers was investigated (see Table 1).
Table 1.
Effect of channel blockers on the membrane potential, LTO frequency and M-like current
| Drug | Depolarisation (mV) | LTO frequency reduction (%) | IM-like inhibition (%) |
|---|---|---|---|
| TEA (15 mm) | — | LTO abolished (4) | 73.7 ± 5.2(9)* |
| Ba2+ (1 mm) | 8.1 ± 1.9(6)* | 56.4 ± 44.4(6)* | 55.8 ± 4.9(7)* |
| Linopirdine (10 μm) | 7.3 ± 1.2(8)* | 20.4 ± 4.7(8)* | 60.2 ± 5.8(6)* |
| 4-AP(2mm) | 0.59 ± 0.25(7) | 18.5 ± 8.5(7) | 0.8 ± 3.3(11) |
| Apamin (100 nm) | 0.1 ± 1.1(4) | 2.3 ± 6(4) | — |
| Cd2+ (100 μm) | 1.4 ± 1.1(4) | 18 ± 3.7(4) | 6.6 ± 5.1(5) |
| Cs+ (1 mm) | 0.8 ± 0.5(5) | 6.2 ± 5.7(5) | 1.8 ± 2.7(10) |
LTO, low threshold oscillation; IM-like, M-like current; 4-aminopyridine.
Statistically significant at P < 0.05. Number of cells tested given in parentheses.
Ba2+ (1 mM) induced a membrane depolarisation of 8.1 ± 1.9 mV and eventually transformed clustering activity into tonic activity (n = 6, Fig. 7A; compare upper with middle trace). In the same cells Ba2+ reduced the frequency of the oscillation in a 56.4 ± 4.4 % (n = 6, P < 0.05), as seen when the Ba2+-induced depolarisation was overcame by current injection (Fig. 7A, lower panel). These results are in accordance with those obtained by Dickson et al. (2000) in entorhinal cortex neurones in slices.
Figure 7. Effect of the modulation of K+ currents on LTOs and the clustering mode.

A, application of barium to clustering cells (control) induced a depolarisation and the cell became tonic with time (middle trace); LTOs could still be observed when the cell was manually hyperpolarised (lower trace). B, TEA also induced depolarisation and tonicity (middle); when manually hyperpolarised the LTOs were absent (lower trace). C, application of 4-AP had no effect on the level of the resting potential and LTOs could still be observed, but the clustering mode was disorganised. D, linopirdine also failed to block LTOs, but reduced their frequency (lower panel).
The more specific M-current blocker linopirdine (see Lamas et al. 1997) was tested in eight clustering cells. Linopirdine (10 μM) induced a membrane potential depolarisation of 7.3 ± 1.2 mV (n = 8; see Fig. 7D, upper and middle panels). When the voltage was manually returned to the control (Fig. 7D, lower panel), a statistically significant reduction of the LTO frequency was observed (20.4 ± 4.7 %, n = 8, P < 0.05).
TEA (15 mM) also induced a depolarisation in DCN neurones (n = 15). As application of TEA generally provoked high-frequency tonic firing (Fig. 7B, middle panel), the effect on the subthreshold oscillations could not be clearly ascertained in every cell. However, in four cells where approximately the same membrane voltage was manually attained, the presence of TEA (15 mM) almost completely abolished the LTO. Even in these four cells, the frequency of LTOs in the presence of TEA could not be measured because a dominant frequency was absent (e.g. Fig. 7B, lower panel).
Cells tested with extracellular 4-AP (2 mM) showed a clear broadening of the action potential and a strong reduction of the hyperpolarising phase. A striking characteristic of the effect of 4-AP was an increase in the number of action potentials and the disruption of the clustering mode (Fig. 7C). The frequency of the low threshold oscillations was slightly reduced (18.5 ± 8.5 %, n = 7; not statistically significant at P < 0.05).
Most DCN cells presented a hyperpolarisation-activated cationic current (Ih), which has been involved in several oscillatory phenomena (see Pape, 1996 for a review). Application of 1 mM CsCl to block Ih did not affect the low threshold oscillations in DCN cells (n = 5; see Table 1). Subthreshold oscillations were also unaffected by the application of either 100 μM Cd2+ (n = 4) or 100 nM apamin (n = 4), suggesting that calcium-activated K+ currents are not involved. Although Cd2+ slightly reduced the frequency of LTOs (18 ± 3.7 %, n = 4), this effect was not statistically significant at P < 0.05 (see Table 1).
In summary, current-clamp experiments using K+ channel blockers suggest the involvement of a TEA-sensitive K+ current in the genesis of low threshold oscillations (see Boehmer et al. 2000).
Voltage clamping outward currents
LTOs are clearly detected in the -55 to -30 mV voltage range; if a TEA-sensitive K+ current is involved, it should be active in the same voltage range. To test this hypothesis, voltage-clamp experiments were carried out in which the membrane was held at -30 mV and a slow voltage ramp (10 mV s−1) to -100 mV was applied. Using voltage ramps, a non-inactivating M-like outward current of 110.8 ± 7.9 pA, after leak subtraction, was observed in 38 of 40 cells investigated (Fig. 8).
Figure 8. A non-inactivating M-like current was strongly blocked by TEA.
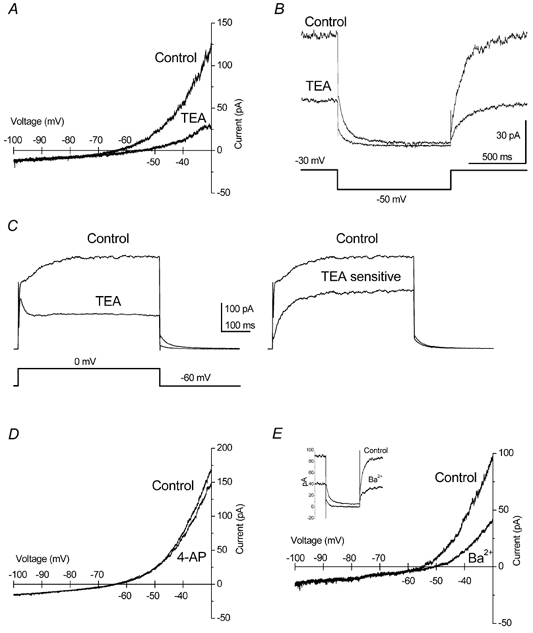
A, in voltage clamp, TEA (15 mM) strongly reduced the persistent outward current. Current recordings were obtained using slow voltage ramps (-30 to -100 mV, 10 mV s−1) and the cell was held at -30 mV between ramps. B, an M-like decay was observed when the channels were closed by a negative voltage step; sensitivity to TEA was similar to that obtained from ramps. C, TEA also inhibited a high threshold outward current (left traces); the TEA-sensitive current showed a very slow activation time constant (right trace). D, the non-inactivating current at -30 mV was not affected by 4-AP (2 mM). E, barium (1 mM) also inhibited the persistent M-like current at -30 mV; this can be observed using voltage ramps or negative voltage steps (inset).
Consistent with the effect over the LTOs, TEA (15 mM) potently reduced the M-like outward current in voltage-clamp experiments (Fig. 8A). In these voltage-clamp experiments, the bath contained 1 mM Cs+ to block the hyperpolarisation-activated Ih and 0.5 μM TTX to inhibit Na+ currents. TEA inhibited the non-inactivating outward current by 73.7 ± 5.2 % (n = 9). The M-like quality of this current is better seen using the most classical ‘M-current protocol’, which consists of holding the membrane at -30 mV to open M-channels, and then applying a 1 s step to -50 mV to close them (see Lamas, 1999). Figure 8B shows that the kinetics of current closure are indistinguishable from those of M-currents and that TEA is also a very effective inhibitor using this protocol (n = 6). When outward currents were activated using depolarising steps to 0 mV (from a holding potential of -60 mV), TEA (15 mM) inhibited a very slowly activating current (Fig. 8C). In the presence of TTX (0.5 μM), Cd2+ (100 μM) and Cs+ (1 mM), more than 50 % of the total current measured at the end of a 500 ms step was TEA-sensitive, as shown in Fig. 8C (n = 4, right panel).
Other current blockers were less effective than TEA (15 mM) at inhibiting the non-inactivating outward current, as assessed using voltage ramps (see Table 1). The addition of 4-AP (2 mM) did not affect the M-like current (Fig. 8D); the inhibition obtained was 0.8 ± 3.3 % (n = 11), with 4 of the 11 cells showing a slight increase in current. Ba2+ (1 mM) blocked the current by 55.8 ± 4.9 % (n = 7, Fig. 8E); the same inhibition can be observed using a voltage step (Fig. 8E, inset). The non-inactivating current was also blocked by linopirdine 10 μM (60.2 ± 5.8 %, n = 6; not shown). The inhibition caused by cadmium was inconsistent and amounted 6.6 ± 5.1 % (n = 5). It should be noted that Ba2+ and linopirdine, although effective, were significantly less potent at inhibiting the M-like current than TEA.
DISCUSSION
This is the first report of electrophysiological recordings of cultured neurones from the dorsal column nuclei. To our knowledge, this is also the first report showing the presence of TTX-sensitive LTOs in cultured neurones. About half of the recorded DCN neurones were spontaneously active (49.2 %) and a 1 Hz intrinsically driven rhythmic clustering activity was also observed in approximately half of the cells (61/130, 46.9 %). A voltage-dependent, TTX-sensitive subthreshold oscillation was found in all clustering cells. The mechanism subserving the low threshold oscillation was investigated and found to rely on the countervailing influence of a persistent Na+ current and an M-like, non-inactivating, TEA-sensitive outward current.
Interestingly, low threshold oscillations were clearly found only in cells showing cluster firing, and all clustering cells showed LTOs. The conductances responsible for the LTOs may therefore be related to the clustering mode. In general, a persistent Na+ conductance seems to strongly contribute to low threshold oscillations (Alonso & Llinás, 1989; Klink & Alonso, 1993; Amitai, 1994; Pape & Driesang 1998; Boehmer et al. 2000). The same applies in cultured DCN neurones: in this study most cells tested in voltage clamp showed a persistent inward Na+ current sensitive to TTX and to riluzole, another persistent Na+ current blocker (Urbani & Belluzzi, 2000).
While the depolarising phase of LTOs is currently ascribed to a TTX-sensitive Na+ current in most cell types, the nature of the countervailing outward current responsible for the repolarising phase is controversial. Klink & Alonso (1993) suggested a low-threshold delayed rectifier K+ current and discarded the participation of the M-current, while Pape and Driesang (1998) suggested the participation of the M-current. The present results confirm the sensitivity of LTOs to TEA (see Boehmer et al. 2000). Additionally, TEA (15 mM) strongly blocks the M-like, non-inactivating outward current present at -30 mV, suggesting that this current may contribute to the low threshold oscillation. This hypothesis explains the lack of effect of 4-AP and Cd2+ upon LTOs and the reduction of their frequency provoked by Ba2+; 4-AP and Cd2+ did not affect the M-like current and Ba2+ inhibited the current significantly less potently than TEA. The same line of argument could be applied for linopirdine, although the low efficacy of this drug, when compared with TEA, upon the LTOs can also be explained by the fact that it is a voltage-dependent inhibitor of M-currents, so that inhibition of the current at resting potential is expected to be small (Romero et al. 2002).
The complex circuitry underlying the workings of the DCN, including inputs from the sensorimotor cortex and primary afferents, but also an intricate internal circuitry (see Canedo et al. 2000), could account for the natural tendency of these neurones to show apparently spontaneous bursting activity in vivo. Results presented here demonstrate that this tendency can be based on intrinsic membrane properties of DCN cells that, however, can sometimes only be revealed by changes in membrane potential induced by influences external to the cell (e.g. silent cells becoming clustering upon slight depolarisation). It is tempting to speculate that a rhythmic input is not necessary to obtain a continuous bursting behaviour from DCN neurones, and that some DCN frequencies, presumed to be imposed, are indeed generated intrinsically by the DCN neurones. The frequency of the LTOs is voltage dependent and hence it may be modulated by afferent inputs to the DCN. Cortical and somatosensory information, therefore, must be capital in fine-tuning the oscillatory dynamics of DCN neurones. Repetitive bursting activity observed in vivo when the somatosensory afferents are stimulated could be explained by a simple sustained depolarisation of otherwise silent DCN neurones. However, it could also be explained by disinhibition of the spontaneously active cells shown here, if a tonic inhibitory influence coming from the cortex exists at DCN (see Canedo, 1997). The additional contribution of a possible rhythmic input to the nuclei and/or internal reverberating circuitry to the in vivo DCN behaviour cannot be ascertained using isolated cells in culture. Transmission of sensory information requires the participation of groups of time-locked neurones to enhance spatial summations in their target cells (see Canedo et al. 2000). Clustering activity is an intrinsic property of DCN neurones (as shown in this study); however, the external information from the cortex (Martínez et al. 1995) and/or somatosensory afferents should recruit the group of DCN neurones to transmit the information desired. The internal circuitry (e.g. recurrent collaterals, lateral inhibition and interneurones in general) could serve to synchronise the recruited projecting cells as suggested by Canedo et al. (1998).
In culture, spontaneous DCN neurones fired clusters of action potentials in the lower range of delta-like activity (1-4 Hz), which confirms the idea that this activity is generated inside the DCN (Mariño et al. 2000). The main point addressed here is that such activity can be generated intrinsically in individual DCN neurones and is not necessarily due to the internal DCN circuitry. It is worth noting that, in anaesthetised cats, cuneolemniscal cells discharged preferentially within the delta-like range, while non-cuneolemniscal cells tended to fire at higher frequencies and presented a more tonic behaviour (Canedo et al. 1998). If this is true, the data on clustering cells presented here could roughly correspond to projecting DCN neurones, while tonic cells could be interneurones.
Equivalent low threshold oscillations seem to be a rather common characteristic of different neuronal types; they have been found, for example, in entorhinal (Alonso & Llinás, 1989; Alonso & Klink, 1993; Klink & Alonso, 1997), hippocampal (Leung & Yim, 1991), cortical (Amitai, 1994; Gutfreund et al. 1995), amygdaloid (Pape et al. 1998) and supraoptic (Boehmer et al. 2000) neurones. A striking difference from these studies is that we have used isolated cells and hence the intrinsic nature of this activity is not in doubt. This apparently widespread phenomenon has been proposed to act as a functional synchronising device of synaptic input to promote population activity at a preferred frequency, and often has been related to the electroencephalographic theta-rhythm (Alonso & Llinás, 1989; Lampl & Yarom, 1993; Pape et al. 1998).
In summary, DCN neurones have intrinsic membrane properties which must be important for the integration and transmission of somatosensory information. This study demonstrates that these properties rely on the presence of two voltage-dependent currents, a persistent Na+ current and a TEA-sensitive M-like current.
Acknowledgments
This work and E.S. were supported by a DGESIC (PB98-1087) grant. M.R. and A.R. are PhD students under the Spanish FPU and FPI programmes. We wish to thank Professor Antonio Canedo for his support and criticism along the course of this work, Drs Luis Martínez and Alvaro Villarroel for critically reading the manuscript, J. López for general technical assistance and A. Senra for her assistance with the histology.
REFERENCES
- Alonso A, Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol. 1993;70:128–143. doi: 10.1152/jn.1993.70.1.128. [DOI] [PubMed] [Google Scholar]
- Alonso A, Llinás RR. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989;342:175–177. doi: 10.1038/342175a0. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Giblin D. Periodic components in steady-state activity of cuneate neurones and their possible role in sensory coding. J Physiol. 1974;243:353–385. doi: 10.1113/jphysiol.1974.sp010758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai Y. Membrane potential oscillations underlying firing patterns in neocortical neurons. Neuroscience. 1994;63:151–161. doi: 10.1016/0306-4522(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer G, Greffrath W, Martin E, Hermann S. Subthreshold oscillation of the membrane potential in magnocellular neurones of the rat supraoptic nucleus. J Physiol. 2000;526:115–128. doi: 10.1111/j.1469-7793.2000.t01-1-00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canedo A. Primary motor cortex influences on the descending and ascending systems. Prog Neurobiol. 1997;51:287–335. doi: 10.1016/s0301-0082(96)00058-5. [DOI] [PubMed] [Google Scholar]
- Canedo A, Mariño J, Aguilar J. Lemniscal recurrent and transcortical influences on cuneate neurons. Neuroscience. 2000;97:317–334. doi: 10.1016/s0306-4522(00)00063-4. [DOI] [PubMed] [Google Scholar]
- Canedo A, Martínez L, Mariño J. Tonic and bursting activity in the cuneate nucleus of the chloralose-anesthetized cat. Neuroscience. 1998;84:603–617. doi: 10.1016/s0306-4522(97)00554-x. [DOI] [PubMed] [Google Scholar]
- Christian EP, Togo JA, Naper KE, Koschorke G, Taylor GA, Weinreich D. A retrograde labeling technique for the functional study of airway-specific visceral afferent neurons. J Neurosci Methods. 1993;47:147–160. doi: 10.1016/0165-0270(93)90031-l. [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Deuchars SA, Trippenbach T, Spyer KM. Dorsal column nuclei neurons recorded in a brain stem-spinal cord preparation: characteristics and their responses to dorsal root stimulation. J Neurophysiol. 2000;84:1361–1368. doi: 10.1152/jn.2000.84.3.1361. [DOI] [PubMed] [Google Scholar]
- Dickson CT, Magistretti J, Shalinsky MH, Fransen E, Hasselmo ME, Alonso A. Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J Neurophysiol. 2000;83:2562–2579. doi: 10.1152/jn.2000.83.5.2562. [DOI] [PubMed] [Google Scholar]
- Galindo A, Krnjevic K, Schwartz S. Patterns of firing in cuneate neurones and some effects of Flaxedil. Exp Brain Res. 1968;5:87–101. doi: 10.1007/BF00238699. [DOI] [PubMed] [Google Scholar]
- Gutfreund Y, Yarom Y, Segev I. Subthreshold oscillations and resonant frequency in guinea-pig cortical neurons: physiology and modelling. J Physiol. 1995;483:621–640. doi: 10.1113/jphysiol.1995.sp020611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na+ current in rat hippocampal pyramidal cells. J Physiol. 2002;545:783–805. doi: 10.1113/jphysiol.2002.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, Alonso A. Ionic mechanisms for the subthreshold oscillations and differential electroresponsiveness of medial entorhinal cortex layer II neurons. J Neurophysiol. 1993;70:144–157. doi: 10.1152/jn.1993.70.1.144. [DOI] [PubMed] [Google Scholar]
- Klink R, Alonso A. Muscarinic modulation of the oscillatory and repetitive firing properties of entorhinal cortex layer II neurons. J Neurophysiol. 1997;77:1813–1828. doi: 10.1152/jn.1997.77.4.1813. [DOI] [PubMed] [Google Scholar]
- Lamas JA. A hyperpolarization-activated cation current (Ih) contributes to resting membrane potential in rat superior cervical sympathetic neurones. Pflugers Arch. 1998;436:429–435. doi: 10.1007/s004240050653. [DOI] [PubMed] [Google Scholar]
- Lamas JA. The role of calcium in M-current inhibition by muscarinic agonists in rat sympathetic neurons. Neuroreport. 1999;10:2395–2400. doi: 10.1097/00001756-199908020-00032. [DOI] [PubMed] [Google Scholar]
- Lamas JA, Selyanko AA, Brown DA. Effects of a cognition-enhancer, linopirdine (DuP 996), on M-type potassium currents (IK(M)) and some other voltage- and ligand-gated membrane currents in rat sympathetic neurons. Eur J Neurosci. 1997;9:605–616. doi: 10.1111/j.1460-9568.1997.tb01637.x. [DOI] [PubMed] [Google Scholar]
- Lampl I, Yarom Y. Subthreshold oscillations of the membrane potential: a functional synchronizing and timing device. J Neurophysiol. 1993;70:2181–2186. doi: 10.1152/jn.1993.70.5.2181. [DOI] [PubMed] [Google Scholar]
- Leung LW, Yim CY. Intrinsic membrane potential oscillations in hippocampal neurons in vitro. Brain Res. 1991;553:261–274. doi: 10.1016/0006-8993(91)90834-i. [DOI] [PubMed] [Google Scholar]
- Llinás RR, Grace AA, Yarom Y. In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc Natl Acad Sci U S A. 1991;88:897–901. doi: 10.1073/pnas.88.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti J, Alonso A. Biophysical properties and slow voltage-dependent inactivation of a sustained sodium current in entorhinal cortex layer-II principal neurons: a whole-cell and single-channel study. J Gen Physiol. 1999;114:491–509. doi: 10.1085/jgp.114.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti J, Mantegazza M de C, Wanke E. Modalities of distortion of physiological voltage signals by patch-clamp amplifiers: a modeling study. Biophys J. 1998;74:831–842. doi: 10.1016/S0006-3495(98)74007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariño J, Canedo A, Aguilar J. Sensorimotor cortical influences on cuneate nucleus rhythmic activity in the anesthetized cat. Neuroscience. 2000;95:657–673. doi: 10.1016/s0306-4522(99)00414-5. [DOI] [PubMed] [Google Scholar]
- Mariño J, Martínez L, Canedo A. Coupled slow and delta oscillations between cuneothalamic and thalamocortical neurons in the chloralose anesthetized cat. Neurosci Lett. 1996;219:107–110. doi: 10.1016/s0304-3940(96)13184-0. [DOI] [PubMed] [Google Scholar]
- Martínez L, Lamas JA, Canedo A. Pyramidal tract and corticospinal neurons with branching axons to the dorsal column nuclei of the cat. Neuroscience. 1995;68:195–206. doi: 10.1016/0306-4522(95)00133-4. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Núñez A, Buño W. In vitro electrophysiological properties of rat dorsal column nuclei neurons. Eur J Neurosci. 1999;11:1865–1876. doi: 10.1046/j.1460-9568.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- Panetsos F, Núñez A, Avendaño C. Sensory information processing in the dorsal column nuclei by neuronal oscillators. Neuroscience. 1998;84:635–639. doi: 10.1016/s0306-4522(97)00694-5. [DOI] [PubMed] [Google Scholar]
- Pape HC. Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol. 1996;58:299–327. doi: 10.1146/annurev.ph.58.030196.001503. [DOI] [PubMed] [Google Scholar]
- Pape HC, Driesang RB. Ionic mechanisms of intrinsic oscillations in neurons of the basolateral amygdaloid complex. J Neurophysiol. 1998;79:217–226. doi: 10.1152/jn.1998.79.1.217. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D, Driesang RB. Two types of intrinsic oscillations in neurons of the lateral and basolateral nuclei of the amygdala. J Neurophysiol. 1998;79:205–216. doi: 10.1152/jn.1998.79.1.205. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA, USA: Academic Press; 1998. [Google Scholar]
- Pubols BHJ, Haring JH, Rowinski MJ. Patterns of resting discharge in neurons of the raccoon main cuneate nucleus. J Neurophysiol. 1989;61:1131–1141. doi: 10.1152/jn.1989.61.6.1131. [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci. 2000;20:9004–9016. doi: 10.1523/JNEUROSCI.20-24-09004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboreda A, Sánchez E, Romero M, Lamas JA. Spontaneous firing and subthreshold oscillation in neurones of the rat dorsal column nucleus in culture. FENS Forum Abstracts. 2002:p.544. [Google Scholar]
- Romero M, Reboreda A, Sánchez E, Lamas JA. Effects of classical and novel M-current inhibitors on the adaptation of mouse sympathetic neurones in culture. FENS Forum Abstracts. 2002:p.544. [Google Scholar]
- Sherwood NM, Timiras PS. A Stereotaxic Atlas of the Developing Rat Brain. Berkeley, Los Angeles, CA, USA: University of California Press; 1970. [Google Scholar]
- Soltesz I, Lightowler S, Leresche N, Jassik G, Pollard CE, Crunelli V. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. J Physiol. 1991;441:175–197. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]


