Abstract
In this second study, we have combined two-photon calcium imaging with whole-cell recording and anatomic reconstructions to directly characterize synaptically evoked calcium signals in three types of mouse V1 supragranular interneurones: parvalbumin-positive fast spikers (FS), calretinin-positive irregular spikers (IS), and adapting cells (AD). We observed that subthreshold synaptic activation evoked calcium signals locally restricted to individual dendritic compartments. These signals were mediated by NMDA receptors (NMDARs) in AD and IS cells, whereas in FS cells, calcium-permeable AMPA receptors (CP-AMPARs) provided an additional and kinetically distinct influx. Furthermore, even a single, subthreshold synaptic activation evoked a larger dendritic calcium influx than backpropagating action potentials. Our results demonstrate that NMDARs dominate subthreshold calcium dynamics in interneurones and reveal the functional contribution of CP-AMPARs to a specific subclass of cortical interneurone. These data highlight different strategies in dendritic signal processing by distinct classes of interneurones.
GABAergic interneurones comprise an extremely diverse subset of neurones in the neocortex. Although this diversity is poorly understood, interneurones are essential to cortical processing. Pharmacological blockade of their fast output at GABAA receptors induces epileptic seizures. (Wood, 1975; Prince & Connors, 1984). Moreover, interneurones appear to play essential roles in regulating the timing of action potential (AP) discharge in pyramids (Pouille & Scanziani, 2001), and are capable of generating oscillations (Buzsaki & Chrobak, 1995; Cobb et al. 1995). Dendritic excitability in these cells is thus crucial to the temporal organization of information flow through the cortical circuit. However, since these dendrites are narrow and take unpredictable routes through the cortex, direct examination of their synaptic excitability has proved difficult.
Thus, much of our knowledge of dendritic excitability in interneurones comes from studies of paired recordings in brain slices wherein presynaptic pyramidal cells generate unitary EPSP/Cs in postsynaptic interneurones. Even though separate studies carried out in zero magnesium and during suprathreshold stimulation have shown that interneurones prominently express NMDA receptors (NMDARs) (Jones & Buhl, 1993; Koh et al. 1995), one common finding from the dual-cell recording experiments was that subthreshold, unitary EPSPs were largely resistant to NMDAR blockade (Geiger et al. 1997; Angulo et al. 1999). This has called into question the relevance of NMDAR activation at subthreshold potentials and has led some groups to focus on AMPA receptors (AMPARs) as the primary determinant of interneuronal subthreshold synaptic activity (Fuchs et al. 2001).
An additional characteristic of interneurone excitability is that distinct subclasses can express distinct glutamatergic conductances (Angulo et al. 1999; Rozov et al. 2001). For example, in rat somatosensory neocortex, parvalbumin-positive fast-spiking, multipolar interneurones differ from bipolar interneurones in that they express calcium-permeable AMPA receptors (CP-AMPARs) with extremely fast kinetics (Rozov et al. 2001). A variety of potentially homologue parvalbumin-positive FS cells throughout the cortex have been shown to express CP-AMPARs, and the fast kinetics of these receptors have been proposed to subserve tight coincidence detection in these cells (Geiger et al. 1997).
After establishing the basis of AP backpropagation in different types of supragranular interneurones (Goldberg et al. 2003), in our second study, our goal was to elucidate the mechanisms of synaptic excitation and associated dendritic calcium influx during subthreshold activation of multiple synapses converging onto a single dendritic compartment. We have found, first, that localized subthreshold synaptic activation caused calcium signals restricted to individual dendritic domains. Second, NMDAR activation dominated synaptic calcium entry in all interneuronal classes examined, and AMPAR-driven depolarization was necessary for NMDAR recruitment. Third, we have characterized specifically in FS cells the functional contribution of CP-AMPARs to dendritic calcium dynamics. Lastly, we found that synaptic excitation dominates over backpropagating APs as a source of dendritic calcium. Together these data force a re-evaluation of the prominence of NMDAR activation in interneurones and emphasize the diversity across distinct interneurone classes.
METHODS
Slice preparation and electrophysiology
Experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH publication no. 86-23, revised 1987) and with the Society for Neuroscience 1995 Statement (http://www.jneurosci.org/misc/itoa.shtml). Coronal slices of primary visual cortex were made from P13-17 C57BL/6mice. Animals were anaesthetized with ketamine-xylazine (50 and 10 mg kg−1). After decapitation, brains were rapidly removed and transferred into ice-cold cutting solution containing (mM): 222 sucrose, 27 NaHCO3, 2.5 KCl, 1.5 NaH2PO4, bubbled with 95 % O2-5 % CO2 to pH 7.4. Brains were cooled for at least 2 min and 300-μm-thick slices were prepared with a Vibratome (Leica VT1000S, Leitz, Germany). Slices were then transferred to a heated solution (35 °C) containing (mM): 126 NaCl, 3 KCl, 1.1 NaH2PO4, 26 NaHCO3, 1 CaCl2, 3 MgSO4, bubbled with 95 % O2-5 % CO2 to pH 7.4, which cooled down in the next half hour to room temperature. Slices were transferred to the imaging chamber 1-6 h after cutting. Artificial cerebral spinal fluid (ACSF) during experiments contained (mM): 126 NaCl, 3 KCl, 1.1 NaH2PO4, 26 NaHCO3, 3 CaCl2, 1 MgSO4, bubbled with 95 % O2-5 % CO2 to pH 7.4. All experiments were performed at 37 °C. Whole-cell recordings from non-pyramidal cells in layer 2/3 were obtained with a patch-clamp amplifier (Axoclamp 2B, Axon Instruments, Foster City, CA, USA; or BVC-700, Dagan Corp., Minneapolis, MN, USA). Mechanisms of synaptic excitation were explored with several drugs (Sigma), including cyclopiazonic acid (CPA) (50 μM), which depletes internal calcium stores by blocking the smooth endoplasmic reticulum calcium (SERCA)-ATPase; 6,7-dinitroquinoxaline-2,3-dione (DNQX) (20 μM), an AMPAR antagonist; Dl-APV (100-200 μm), an NMDAR antagonist; and philanthotoxin (5 μM, RBI, Natick, MA, USA), an antagonist of calcium-permeable AMPARs. Trolox (100 μM, Aldrich) was used in some Fluo-4 experiments to reduce phototoxicity. Synaptic inputs to neurones were stimulated electrically using an extracellular pipette filled with 200 μM Alexa-488 dextran (Molecular Probes, Eugene, OR, USA) in ACSF. Tips of stimulation pipettes were bent by about 70 deg with a microforge (Narishige, Japan). This allowed positioning of the stimulation pipette perpendicular to the slice surface. In order to achieve local subthreshold stimulation with monophasic EPSPs it was necessary to place glass electrodes in the immediate vicinity (< 15 μm) of the dendrite of interest, and use small-amplitude (20 μA or 1 V), and short-duration (100 μs) single shocks.
Two-photon imaging
Cells were filled via patch pipette with 200 μM CaGreen-1 or 100 or 400 μM Fluo-4 (Molecular Probes). Pipette solution contained (mM): 130 KMeSO4, 5 KCl, 5 NaCl, 10 Hepes, 2.5 Mg-ATP, 0.3 GTP, calcium indicator, and 0.3 % biocytin and was titrated to pH 7.3. Following break-in, we waited for 20-30 min before imaging to ensure that dendrites filled with indicator. Imaging was done using a custom-made two-photon laser scanning microscope, consisting of a modified Fluoview (Olympus, Melville, NY, USA) confocal microscope with a Ti:sapphire laser providing 130 fs pulses at 75 MHz (Mira, Coherent, Santa Clara, CA, USA), and pumped by a solid-state source (Verdi, Coherent). A 60 ×, 0.9 NA water immersion objective (IR1, Olympus) was used. Fluorescence was detected with photo-multiplier tubes (HC125-02, Hamamatsu, Hamamatsu City, Japan) in external whole-area detection mode, and images were acquired and analysed with Fluoview (Olympus) software. Images of dendrites were acquired at 10 × digital zoom, resulting in a nominal spatial resolution of 30 pixels μm−1 and at a time resolution of 12.64 ms per point (79 Hz) in line-scan mode.
Analysis
Fluorescence levels of calcium measurements were analysed using Fluoview (Olympus) and ImageJ (NIH, Bethesda, MD, USA). Time courses were analysed using Igor (Wavemetrics, Lake Oswego, OR, USA). Signals were corrected for background fluorescence by measuring a non-fluorescent area close to the dendrite. The relative change of fluorescence of baseline (from 400 ms prior to AP generation) (ΔF/F) was used as indicator for the change in calcium. Between 3 and 10 line scans were typically averaged to generate ΔF/F transients during synaptic stimulation and APs. Decay kinetics were fitted using single exponential fitting algorithms in Igor (Wavemetrics). Membrane time constants were obtained from mono-exponential fits to hyperpolarizing voltage deflections during -100 pA injections through the somatic recording electrode. EPSP time to peak was calculated as the time from synaptic stimulation to the peak depolarization, and slope was the amplitude of the EPSP divided by the time-to-peak. The time-to-peak in the calcium signal was calculated as the time from the synaptic stimulation to the peak amplitude. We also quantified calcium influx rate as maximal percentage of peak amplitude reached in one time point (12.64 ms). Dendrite diameters were measured using ImageJ (NIH) as the half-widths of Gaussian fits to fluorescence versus distance plots obtained from lines placed orthogonal to the dendrite of interest. For intergroup comparisions, two-tailed Student's t tests were used, and data are presented as means ± standard deviation (S.D.). One-tailed Student's t tests were used to test the significance of percentage changes within a group. In some cells, kinetic analysis of EPSPs was not performed due to stimulation artifacts which obstructed the EPSP rise.
Histology
Visualization of biocytin was performed as described (Buhl et al. 1994; Tamas et al. 1997). Three-dimensional light microscopic reconstructions were carried out using Neurolucida and Neuro Explorer (MicroBrightfield, Colchester, VT, USA) with an 100 × oil objective.
RESULTS
The mechanisms of postsynaptic excitation and calcium influx in interneurone dendrites were compared in three types of supragranular non-pyramidal cells: parvalbumin-positive fast spikers (FS) (n = 29) (Simons, 1978), calretinin-positive irregular spikers (IS) (n = 31) (Cauli et al. 1997) and adapting (AD) (n = 14) cells (Dantzker & Callaway, 2000). FS cells had multipolar dendritic morphologies, IS cells were bipolar, and AD cells were anatomically a heterogeneous group (see Fig. 1 in accompanying paper, Goldberg et al. 2003). Two-photon calcium imaging was performed on cells from P13-17 mouse visual cortical slices which were patched in whole-cell mode and filled with fluorescent calcium indicator (see Methods and accompanying paper, Goldberg et al. 2003). Fluorescently labelled glass stimulation electrodes were placed via visual guidance next to dendrites of interest. Calcium signals were imaged in space- (xyt) or line- (xt) scan mode while stimulation-evoked EPSPs were recorded at the soma.
Figure 1. Subthreshold synaptic activation produced localized Ca2+ signals in dendrites of interneurones.
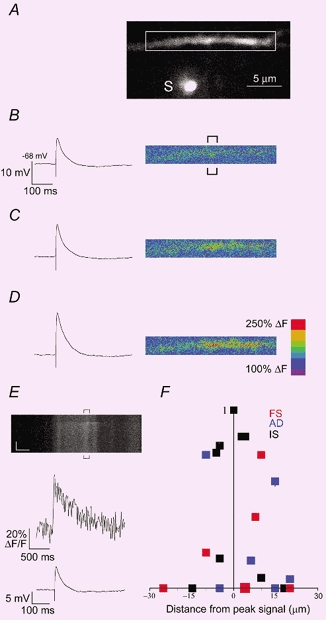
A, two-photon image of a basal dendritic branch of an FS cell filled with 400 μM Fluo-4. White box indicates the dendritic compartment analysed during synaptic stimulation in B-E. The stimulation electrode, S, was filled with 200 μM Alexa and placed via visual guidance 5 μm from the dendrite. B-D, EPSP response, left, and pseudocolour images of fluorescence change (%ΔF), right, during the first 200 ms after single shock synaptic stimulations of progressively increasing intensity (top to bottom). E, top, line scan (xt mode) was placed horizontally through the dendrite and the signal corresponds to the region of interest indicated by brackets in B. Synaptic activation was stimulated 400 ms into the xt scan. Vertical scale, 0.4 s; horizonal scale, 2 μm. Middle, average calcium signal (ΔF/F), from the position indicated in brackets above. Bottom, EPSP response recorded at the soma. F, xt scans were performed at multiple dendritic sites to confirm the limited spatial extent of calcium signals obtained in xyt mode. Amplitudes were normalized to peak (at position x = 0) and are plotted versus distance from peak signal (data are from 4 AD (blue), 8 FS (red) and 7 IS (black) cells.
Compartmentalized calcium signals during single subthreshold synaptic stimulations
The computational power of dendritic arbors can be enhanced by functionally segregating geographically distinct inputs (Poirazi & Mel, 2001). We reliably observed calcium signals localized to individual dendritic branches and domains during single subthreshold synaptic activation (see Methods). The typical experimental regime is demonstrated in Fig. 1. The stimulation electrode, S, was positioned beneath a basal dendritic branch of an FS cell. In Fig. 1B-D, increasing stimulation intensities within the range used in this study caused progressively larger subthreshold EPSPs and calcium signals which were highly localized to about 10 μm of dendritic space (Fig. 1D). Given a density of excitatory terminals on interneurone dendrites ranging from 150-500 (100 μm)−1 (Gulyas et al. 1999), we estimate that there were probably tens of activated synapses surrounding this calcium domain. Consistent with this, somatically recorded EPSPs (Fig. 1B-D) ranged from 10.1 to 22.3 mV, suggesting simultaneous summation of at least 10-20 synapses, assuming average unitary EPSP size of ≈1-2 mV (Ali et al. 1998; Tamas et al. 1998). Subthreshold EPSPs included in this study were monophasic and amplitudes were (19.6 ± 7.5 mV n = 53 cells), and were not significantly different among cell groups (see Table 1).
Table 1.
| FS | IS | AD | |
|---|---|---|---|
| Calcium signal kinetics | |||
| Peak amplitude (ΔF/F) 200 μM Ca Green** | 151 ± 62(n = 10) | 91 ± 36(n = 13)** | 121 ± 59(n = 8) |
| Peak amplitude (ΔF/F) 400 μM Fluo-4 | 208 ± 121 (n = 7) | 156 ± 83 (n = 4) | 390 ± 304 (n = 2) |
| Peak amplitude (ΔF/F) 100 μM Fluo-4* | 343.7 ± 116(n = 4) | 164 ± 85(n = 6)* | 155 ± 7(n = 2) |
| Offset τ (ms) 200 μM Ca Green | 1575 ± 1089 (n = 10) | 1753 ± 1213 (n = 13) | 1323 ± 678 (n = 8) |
| Offset τ (ms) 400 μM Fluo-4 | 696 ± 384 (n = 7) | 892 ± 332 (n = 4) | 470 ± 59 (n = 2) |
| Offset τ (ms) 100 μM Fluo-4 | 160 ± 87 (n = 4) | 312 ± 152(n = 6) | 358 ± 214(n = 2) |
| Influx rate (%amplitude (12 ms)−1)** | 73.4 ± 18.3 (n = 22) | 44.2 ± 18.0 (n = 13)*** | 47.0 ± 12.4 (n = 9)*** |
| Time to peak (ms)*** | 24.0 ± 18.2 (n = 22) | 80.4 ± 43.6 (n = 13)*** | 49.7 ± 24.8 (n = 9)** |
| EPSP kinetics† | |||
| Amplitude (mV) | 19.5 ± 7.2 | 19.2 ± 5.4 | 18.5 ± 7.1 |
| Time to peak (ms)*** | 7.4 ± 5.6 | 15.9 ± 11.0* | 24.1 ± 12.7*** |
| Slope (mV ms−1)** | 4.3 ± 2.7 | 1.7 ± 0.9*** | 1.1 ± 0.7** |
| Half-width (ms)*** | 15.2 ± 7.3 | 54.7 ± 30.2*** | 47.7 ± 19.1*** |
| Membrane time constant τ (ms)†† | 8.2 ± 4.2†† | 11.7 ± 6.9† | 21.4 ± 8.0 |
| AP latency on suprathreshold trials (ms)* | 2.0 ± 0.6 | 26 ± 32* | N/A |
| Distance from soma of signal (μm) | 66 ± 31 | 74 ± 42 | 50 ± 14 |
| Dendrite diameter (μm) | 0.96 ± 0.34 | 1.05 ± 0.35 | 0.91 ± 0.31 |
Two-tailed paired t test
P < 0.05
P < 0.01
P < 0.001 relative to FS group.
P < 0.01
P < 0.001 relative to AD group.
‡For all FS values, n = 27; for all IS values, n = 18; for all AD values, n = 10. N/A, data not available.
In order to more quantitatively evaluate the magnitude and kinetics of calcium influxes, for the rest of the study transients were acquired with higher temporal resolution (79 Hz) in line-scan mode (Fig. 1E). We confirmed that we were imaging at the site of the synaptic activation and mapped out the spatial extent of calcium accumulations by placing the line scan at multiple dendritic sites (Fig. 1F). Further, to ensure that we were not imaging calcium diffusion from nearby activated synapses, line scans for kinetic and pharmacological analysis were positioned at dendritic locations exhibiting the peak calcium responses (Fig. 1B and E).
We conclude that calcium signals were restricted to small dendritic compartments (< 15 μm).
NMDA receptor activation dominated calcium influx in all interneurone types
The recruitment of NMDAR activation in interneurones at subthreshold potentials is controversial, and NMDARs are not significantly recruited during unitary EPSPs (Geiger et al. 1997; Angulo et al. 1999). To test the contribution of NMDAR activation during the subthreshold activation of multiple synapses, we compared EPSPs and calcium signals before and after addition of the NMDAR antagonist APV. In all cell classes, APV had a profound effect on both the calcium transient and the EPSP (Fig. 2). APV alone eliminated calcium accumulations in 4/5 AD and 8/11 IS cells. However, in FS cells, APV eliminated calcium accumulations in only 2/17 cells, and significantly reduced it in the remaining 13/15. This APV-resistant calcium accumulation in FS cells was extremely variable (range 6-95 % of control, mean ± S.D. 26 ± 22 %, n = 15) and was blocked by subsequent addition of the AMPAR antagonist DNQX (10 μM) (Fig. 2D, n = 5/5) or philanthotoxin, a specific antagonist for calcium-permeable AMPARs (philanthotoxin, 5 μM, Fig. 2D, n = 2/2).
Figure 2. NMDA dominated subthreshold synaptic calcium influx.

Dendritic calcium influxes captured in line-scan mode,(top) and somatically recorded synaptic potentials (bottom) from an FS cell (A), and an IS cell (B). Results are shown for control synaptic stimulations (Aa, Ba) and hyperpolarization (Ab, Bb), single action potential (Ac, Bc), and APV (50 μm) (Ad, Bd). In the IS cell, APV fully blocked the calcium signal (Bd), while in the FS cell, APV caused a partial reduction (Ad). Complete blockade of this signal was accomplished by the subsequent addition of DNQX (10 μM) (Ae). In both cells, recovery of the physiology and the calcium signal was achieved after drug washout (Af, Be). C, percentage of control peak calcium signal, EPSP area, and EPSP peak amplitude during hyperpolarization (23.7 ± 3.7 mV, n = 15) in IS (▪) FS (□) and AD ( ) cell groups. D, pooled data showing the % peak calcium signal remaining after APV addition. Note that in 8/11 IS, 2/17 FS and 4/5 AD cells, APV achieved full blockade. + and # signs above bars in the FS group indicate that subsequent addition of DNQX (n = 5) or philanthotoxin (n = 2), respectively, achieved total blockade. * P < 0.01; ** P < 0.005; *** P < 0.001.
) cell groups. D, pooled data showing the % peak calcium signal remaining after APV addition. Note that in 8/11 IS, 2/17 FS and 4/5 AD cells, APV achieved full blockade. + and # signs above bars in the FS group indicate that subsequent addition of DNQX (n = 5) or philanthotoxin (n = 2), respectively, achieved total blockade. * P < 0.01; ** P < 0.005; *** P < 0.001.
EPSP area and peak amplitude were also significantly reduced by APV in all cell classes, whereas EPSP time-to-peak was not (EPSP area reduction (%): IS, 41 ± 27, n = 8, P = 0.008; FS, 50 ± 32, n = 10, P = 0.003; AD, 75 ± 10, n = 4, P = 0.006; EPSP peak reduction (%): IS, 41 ± 27, P = 0.009; FS, 29 ± 23, P = 0.005; AD, 67 ± 12, P = 0.01; EPSP time-to peak: FS, 78 ± 31 %, n = 10, P = 0.09; IS, 95 ± 20 %, n = 9, P = 0.54; AD, 72 ± 21 %, n = 4, P = 0.15), but these reductions were not significantly different between cell classes. On the other hand, while APV also significantly reduced half-width in all cell classes (EPSP half-width reduction (%): IS, 34 ± 16, P = 0.0002; FS, 17 ± 17, P = 0.02; AD, 33 ± 12, P = 0.01), this effect was significantly less profound in FS cells (P = 0.008 relative to IS cells, two-tailed Student's t test).
These data indicated that NMDAR activation was the primary synaptic source of dendritic calcium in AD and IS cells, and acted jointly with CP-AMPA in FS cells, and that NMDARs contributed significantly in all cell classes to both peak amplitude and duration of synaptically evoked EPSPs.
FS cells had fast calcium influx and fast EPSP influx kinetics
CP-AMPARs underlie the fast synaptic kinetics of FS cells (Geiger et al. 1997; McBain et al. 1999) and can mediate plasticity in interneurones (Mahanty & Sah, 1998; Lei & McBain, 2002). We explored the functional contribution of CP-AMPA to dendritic calcium dynamics and found that in all three buffer conditions, FS cells had faster calcium influx kinetics (see Methods) than the other cell groups (Fig. 3, Table 1).
Figure 3. FS cells had faster EPSP and calcium influx kinetics.

A-D, left panels, overlaid calcium transients imaged with 200 μM calcium green (A), 400 μM Fluo-4 (B) and 100 μM Fluo-4 (C). D, overlaid EPSPs from IS (black), FS (red) and BT (blue) cell groups. Right panels, traces are normalized to peak amplitudes. E, reprentative suprathreshold traces from FS cell (left) and IS cell (right). Note the shorter latency to peak of the AP in the FS cell.
One complicating issue related to this finding is that distinct endogenous buffers can differentially alter the binding reaction between the exogenous indicator and free calcium, and thus may bias our assessment of calcium influx. For example, parvalbumin, which was expressed in FS cells (Fig. 1 in accompanying paper, Goldberg et al. 2003), is a slow buffer which preferentially impacts calcium decays (Lee et al. 2000b). Consistent with this, we have found that in all three buffer conditions, FS cells had faster decays than IS cells, although this was only statistically significant in the least perturbed (100 μM Fluo-4) buffer environment (Table 1).
Calretinin, expressed in the IS cell group, is an especially fast buffer which could compete with the BAPTA-based indicators used in this study during binding (Roberts, 1993; Edmonds et al. 2000). However, in separate experiments involving the same set of neurones included in this study, calcium influx during single backpropagating APs was not different across the cell groups, indicating that resident buffers did not differentially alter influx kinetics (see accompanying paper, Goldberg et al. 2003). Thus, we conclude that the fast calcium influx measured in the FS cell group was not due to cell-specific buffering but was due to fast influx through CP-AMPARs.
Consistent with a role of CP-AMPA, we found that FS cells had significantly faster EPSP kinetics than IS or AD cells, as indicated by three measures: time-to-peak, slope of the rising phase, and half-width, (Fig. 3, Table 1). The dendrite diameters and the distances between the activated dendrite compartment and the soma were not significantly different between cell types (Table 1). Thus we estimated that the electrotonic distance was not significantly different across cell groups. However, AD cells had significantly slower membrane time constants (see Methods) than FS (P < 0.001) and IS (P < 0.01) cells (Table 1), indicating that their longer EPSP half-widths may have been due to passive filtering of EPSPs. However, FS and IS cells did not have significantly different membrane time constants, suggesting that the significantly different EPSP kinetics were due to distinct synaptic conductances. Importantly, these faster EPSP kinetics translated to distinct spike-generating behaviour, since during suprathreshold stimulations IS cells discharged with longer latencies and greater variability than FS cells (Table 1, Fig. 3E).
AMPARs controlled NMDAR activation
The large effect that APV had on synaptically evoked calcium influxes led us to question how NMDARs were recruited during our single synaptic shock stimulations. We found that DNQX (10 μM) alone blocked both calcium influx and synaptically evoked depolarization in all interneurone types (Fig. 4). Subsequent washout of magnesium in the continued presence of DNQX returned the calcium signal, confirming the prominent role of NMDARs in synaptic calcium flux (Fig. 4), and indicating that in all interneurone classes examined, NMDARs were blocked by magnesium at rest, but needed background depolarization provided by AMPARs.
Figure 4. AMPAR activation was necessary for NMDAR recruitment.

A, subthreshold EPSP (bottom) and a time-locked calcium influx (top). B, DNQX (10 μM) blocked both the calcium signal and the EPSP. C, subsequent washout of magnesium yielded a putative pure equal-amplititude NMDAR-mediated EPSP which was associated with a significantly larger calcium influx (note different vertical scale bar from A). D, the AMPAR-dependent fast influxes of both the calcium signal and the EPSP are clearly visualized by the superimposition of the control (dark) and NMDA (light) signals. E, peak calcium signals, left, and EPSP amplitudes, right, in DNQX or DNQX/0Mg conditions, plotted as a percentage of control signal. * P << 0.01.
If NMDAR recruitment is wholly dependent on depolarization provided by AMPARs, then hyperpolarization should reduce calcium signals in IS and AD cells. In FS cells, the impact of hyperpolarization on the total calcium signal will be more complicated: calcium influx through CP-AMPARs may increase due to an increased driving force, but NMDAR activation may be reduced. We explored the importance of membrane potential on synaptic calcium influx by stimulating at resting membrane potential (Vm; -62.3 ± 2.4 mV) and after hyperpolarization (-84.8 ± 3.8 mV; -23 ± 3 mV difference, n = 17; Fig. 2). We observed that hyperpolarization reduced the peak calcium influx in IS and AD cell groups by 50 ± 29 % (P < 0.005, n = 8; Fig. 2). In FS cells, hyperpolarization also significantly reduced peak calcium signals (60 ± 23 %, P < 0.005, n = 9), suggesting that the effect of decreased NMDAR activation dominated over increased drive through resident CP-AMPARs. In one FS cell, however, hyperpolarization had no effect (not shown), suggesting that the relative contributions of CP-AMPARs and NMDARs is variable. This is consistent with the variable extent of APV blockade we observed (Fig. 2).
We confirmed that the distinctly fast calcium influx and EPSP kinetics in fast spikers were mediated by calcium-permeable AMPARs by comparing EPSPs and calcium signals in putatively pure NMDAR (DNQX/0 Mg2+) and control conditions (Fig. 4C). While the purely NMDAR-mediated EPSPs were significantly slower than control in both FS and IS cells (EPSP time-to-peak increased in FS, 700 ± 250 %, n = 4, P < 0.001; IS, 412 ± 51 %, n = 5, P < 0.005), calcium influx was slowed exclusively in FS cells (calcium signal time-to-peak (ms) increased in FS, 375 ± 97 %, n = 4, P = 0.01; IS, 158 ± 69 %, n = 5, P = 0.13). Thus calcium influx in IS cells in control conditions depended on recruitment of NMDARs by AMPARs, and in FS cells CP-AMPA both provided a fast calcium influx and and facilitated an additional, slower calcium signal via NMDA activation. The finding that FS cells have an extra source of synaptic calcium may explain their significantly larger peak signals during synaptic activation (Table 1).
In conclusion, our observations that AMPAR activation was necessary to recruit NMDARs, and that hyperpolarization significantly reduced calcium influxes, indicated that synaptic calcium influx in interneurones was extremely sensitive to membrane potential due to its heavy reliance on NMDAR activation.
Release from internal stores did not significantly contribute to calcium influx during single shock stimulation
In addition to evoking calcium influx through ionotropic glutamate receptors, synaptic activity can mobilize calcium release from intracellular stores (Emptage et al. 1999; Kapur et al. 2001). To test if internal release contributed to calcium influx during our subthreshold stimulations, we measured peak responses before and after depleting internal stores with CPA (50 μM), a SERCA pump blocker. We found that CPA did not significantly reduce the peak calcium signal in any of the cell classes examined (80 ± 20 %, P = 0.1, n = 4 IS; n = 2 FS; n = 2 AD; Fig. 5). However, CPA dramatically increased the calcium decays (214 ± 60 %, P < 0.001), confirming drug wash-in and suggesting that ER uptake mechanisms were involved in calcium clearance.
Figure 5. Internal release did not significantly contribute to calcium signals during single shock stimulation.

A, two-photon image of an FS cell filled with 200 μM calcium green. Inset shows position of stimulating electrode, S, and position of line scan through two dendrites of interest. B, calcium responses from dendrite 2 (top) and dendrite 1 (bottom) during control stimulation (black) and after addition of 50 μM CPA (grey). Note that the calcium signal was localized to dendrite 1. Decays of calcium transients were fitted to single exponentials as indicated (τcontrol = 0.98 s and τCPA = 1.48 s). C, calcium transient from B viewed at an expanded time scale, and EPSP (bottom) at same time scale. Control and CPA traces are plotted as in B. D, percentage control peak calcium signal and decay time constants from IS (4, n = 4, FS (□, n = 2)and BT ( , n = 2) cell groups. CPA did not significantly reduce peak Ca2+ signal but prolonged decays.
, n = 2) cell groups. CPA did not significantly reduce peak Ca2+ signal but prolonged decays.
Synaptic receptor activation dominated over backpropagating action potentials as source of dendritic calcium
Somatically generated action potentials actively invaded the dendrites of FS, IS and AD cells and evoked calcium accumulations (see accompanying paper, Goldberg et al. 2003). In order to compare the relative contributions to dendritic calcium by trains of APs and synaptic stimulation, we imaged calcium signals during single backpropagating APs, backpropagating AP trains (10 at 40 Hz), and during single shock synaptic stimulation at identical dendritic sites.
We found that in all cell types and all buffer conditions, the peak calcium signal during synaptic stimulation was overwhelmingly larger than during single APs (Fig. 2) (ratio (peak signal during 1AP)/(peak signal during single synaptic shock): 200 μM calcium green = 0.04 ± 0.15, P < 0.001, n = 17; 400 μM fluo-4 = 0.18 ± 0.21, P < 0.001, n = 17; 100 μM fluo-4: 0.29 ± 0.22, P < 0.001, n = 11). Peak signal amplitudes during synaptic stimulation over those during AP propagation were even dominant when compared to trains of 10 backpropagating APs (40 Hz) (Fig. 6). However, the dominance was only statistically significant in the 200 μM calcium green and 400 μM fluo-4 conditions: (200 μM calcium green = 0.33 ± 0.22, P < 0.001, n = 17; 400 μM Fluo-4 = 0.68 ± 0.58, P < 0.05, n = 16; 100 μM Fluo-4: 1.14 ± 0.78, P > 0.5, n = 11). This suggested that during experiments with higher exogenous buffer capacities synaptically evoked signals were exaggerated relative to AP-mediated signals. Indeed, we found that for both the syn-1 AP and syn-10 AP comparisons, the least perturbed buffer environment (100 μM Fluo-4) was the least biased towards the synaptic excitation (Fig. 6). As more exogenous buffer is introduced (and the total buffer capacity is increased), the absolute characteristics of the calcium signal are distorted: the peaks of calcium transients are reduced and the decays are prolonged (Helmchen, 1999). In addition, compared to a fast AP-mediated signal, a slow NMDA-mediated calcium influx may be relatively overrepresented by increasing buffer capacities. Since Fluo-4 and calcium green are fast indicators which bind virtually every calcium ion entering the cell at concentrations necessary to perform calcium imaging, they sequester free calcium and inhibit extrusion. Thus, during prolonged influxes, such as during NMDAR activation, efflux is effectively prevented and the peak %ΔF/F increasingly represents the integrated calcium signal rather than the true peak signal (Sabatini et al. 2002). Thus, our results indicated that the total calcium influx during synaptic activation was larger than during backpropagating APs.
Figure 6. Subthreshold synaptic calcium influx dominated over backpropagating action potentials.
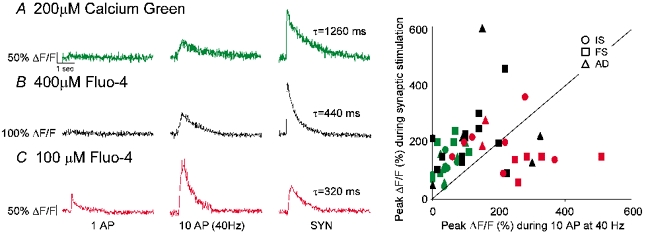
Typical calcium transient during 1 AP (left), 10 APs (40 Hz; middle) and synaptic stimulation (right) in 200 μM calcium green (A), 400 μM Fluo-4 (B) and 100 μM Fluo-4 (C). D, peak calcium transient amplitude (%ΔF/F) during synaptic stimulation plotted versus peak during 10 APs (40 Hz). Line is slope = 1. Notice that in the least perturbed buffer environment (100 μM Fluo-4), there is an increased sensitivity to AP-mediated signals. Colour scheme throughout the figure: green, 200 μM calcium green; black, 400 μM Fluo-4; red, 100 μM Fluo-4.
DISCUSSION
We examined the mechanisms and kinetics of calcium influx during subthreshold synaptic stimulations in three classes of supragranular interneurone in the primary visual cortex of P13-17 mice. The main findings of this study were (1) localized subthreshold synaptic activation caused calcium signals to be restricted to individual dendritic compartments (2) NMDAR activation dominated subthreshold synaptic calcium influx while AMPAR activation was necessary for NMDAR recruitment, (3) FS cells had unique synaptic calcium and EPSP dynamics due to the expression of calcium-permeable AMPARs and (4) calcium influx triggered by synaptic activity dominated over that mediated by backpropagating action potentials.
Subthreshold synaptic stimulation of dendritic compartments
The localized calcium signals we reliably triggered in this study with subthreshold synaptic stimulations indicated that interneurone dendrites can functionally compartmentalize inputs at distinct locations. This feature of dendritic integration has been demonstrated in pyramidal neurones, and can increase dendritic computational power (Schiller et al. 2000; Poirazi & Mel, 2001; Wei et al. 2001; Goldberg et al. 2002). In interneurones, functional compartmentalization of inputs may be especially important since distinct sources of excitation selectively target specific coordinate spaces along the somatodendritic axis (Freund et al. 1985).
In order to achieve these local synaptic signals, we found it necessary to place stimulation electrodes in the immediate vicinity of dendritic compartments of interest. Our goal was to avoid significant recurrent excitation in order to directly examine mechanisms of local dendritic calcium influx during stimulation of small numbers of clustered synapses. Since interneurones are densely interconnected with neighbouring pyramids (Tamas et al. 1998; Kozloski et al. 2001) with highly reliable synapses (Geiger et al. 1997; Csicsvari et al. 1998), even single extracellular shocks can recruit significant recurrent activation, wherein interneurones receive biphasic excitation from, first, axons directly triggered by stimulation and, second, by the suprathreshold drive of pyramidal neurones whose axons target the interneurone of interest (Maccaferri & McBain, 1996). The monophasic EPSPs observed in this study, and the spatial restriction of the calcium signals, strongly suggest that we avoided significant recurrent excitation and were able to directly examine the mechanisms of local calcium influx. Since calcium influx and EPSPs were sensitive to synaptic transmission blockers, we believe that direct activation of the imaged dendrite by the shock of the stimulation electrode did not interfere with our signals.
NMDAR drives synaptic calcium influx at subthreshold potentials
Although the expression of NMDARs is well established in a variety of interneuronal subtypes (Perouansky & Yaari, 1993; Jones & Buhl, 1993; Porter et al. 1998), several studies have suggested that NMDAR activation plays a minimal role at subthreshold potentials (Geiger et al. 1997; Angulo et al. 1999). Unitary EPSPs are largely insensitive to the NMDAR antagonist APV, and the kinetics of purely AMPAR-mediated synaptic excitation have been proposed to subserve tight coincidence detection as well as gamma oscillations across large distances (McBain et al. 1999; Fuchs et al. 2001). From the perspective of calcium influx during synaptic activation of dendritic compartments, however, NMDAR activation was crucial. Calcium was not significantly mobilized from internal stores (Fig. 5), and our observations that (1) somatic hyperpolarization reduced calcium influx in all cell types and (2) APV alone blocked calcium influx in IS and AD cells, and significantly reduced it in FS cells, support this prominent role of NMDARs (Fig. 2).
It is important to note that there is increasing evidence in pyramidal cells that NMDAR activation can cooperate with voltage-gated calcium channels (VGCCs) to jointly mediate calcium influx (Schiller et al. 2000; Golding et al. 2002), and thus VGCC activation cannot be ruled out in our experiments. But since regenerative VGCC recruitment can cause plateau potentials and a ‘shoulder’ in the recorded EPSP (K. Holthoff, Y. Kovalchuk, R. Yuste & A. Konnerth, unpublished observations; Larkum & Zhu, 2002), and since we did not observe such complexities in our monophasic EPSPs, we do not believe that we evoked regenerative VGCC or NMDA spikes. Consistent with this, we did not observe non-linearities in calcium response as a function of stimulation strength (Fig. 1). Nonetheless, we cannot rule out the non-regenerative recruitment of VGCCs.
We were surprised, particularly in the FS cell group, by the large contribution of NDMARs to subthreshold calcium signals and synaptic excitation. Cells of the FS phenotype, which mediate feedforward inhibition in layer 4 (Gibson et al. 1999) and in CA1 (Pouille & Scanziani, 2001), have fast kinetics which ideally suit them to detect coincident activity in populations of pyramids (Konig et al. 1996; Geiger et al. 1997). It thus seems puzzling, that NMDARs prominently contributed to synaptic excitation and calcium influx at subthreshold potentials (see Geiger et al. 1997; Angulo et al. 1999). One reason for this new finding may be that previous assessments of NMDAR contribution at subthreshold potentials were obtained from dual cell recordings, where AMPAR-mediated excitation from single presynaptic pyramidal neurones may have been insufficient to relieve the Mg2+ block of NMDARs. Consistent with this, we found that NMDAR recruitment was tightly controlled by AMPAR activation: the AMPAR antagonist DNQX alone prevented calcium influx and synaptic depolarization (Fig. 4).
We consider it important, however, that APV did not significantly alter the time-to-peak in FS cells, and that APV reduced the EPSP half-width of IS cells significantly more than of FS cells. These observations suggest that while NMDARs and AMPARs jointly contributed to calcium influx in FS cells, the fast kinetics of FS AMPARs determined the influx time course and, by reducing the duration of the depolarization required to unblock Mg2+, tightly controlled NMDAR recruitment. In support of this, we found that in the FS cell group the first spike discharged during suprathreshold stimulations emerged from the initial rise to peak, while initial spikes discharged by IS cells emerged more variably and later in the EPSP (Fig. 3E, Table 1). These results extend to the neocortex a recent finding from hippocampal interneurones in which NMDARs were differentially involved in regulating the timing of spike discharge in two interneuronal populations (Maccaferri & Dingledine, 2002). Our results emphasize the diversity among interneurones, and suggest that the fast kinetics and precision timing frequently ascribed to interneurones applies only to specific subclasses.
Functional contribution of calcium-permeable AMPARs to synaptic calcium dynamics in FS cells
Parvalbumin-positive FS cells constituted a unique group of INs in this study. First, they had faster EPSP and calcium kinetics. Second, they prominently expressed an APV-resistant calcium influx, which was blocked by 10 μM DNQX or 5 μM philanthotoxin. Together, these data demonstrate a role of calcium-permeable AMPARs. While a variety of studies have explored the fast kinetics and current-voltage relationships of CP-AMPARs on neocortical FS and hippocampal basket cells (Koh et al. 1995; Geiger et al. 1997; Rozov et al. 2001), here we show that in addition to contributing to faster EPSP kinetics and spike initiation, CP-AMPARs endowed FS cell dendrites with uniquely fast synaptic calcium kinetics. The differential dependence on NMDARs versus AMPARs in distinct interneuroneal subclasses could translate into distinct spatial dynamics of synaptic calcium influx. In AD and IS cells, where synaptic calcium is wholly triggered by NMDARs, significant calcium influx may demand the simultaneous activation of multiple synapses converging onto a single dendritic region. FS cells, on the other hand, owing to their calcium influx associated with CP-AMPAR activation, may not need background depolarization for synaptic calcium entry and thus may sustain several spatially isolated calcium micro-compartments during simultaneous activation of spatially segregated synapses (J. Goldberg, unpublished observations).
Roles of calcium in interneurone dendrites
Our observation that the total calcium influx during synaptic excitation dominated over that during AP backpropagation (Fig. 6) suggests that calcium-dependent processes can discriminate between distinct mechanisms of calcium entry (Alkon & Rasmussen, 1988; Yuste et al. 2000). Importantly, our detection of the distinct calcium influxes caused by synaptic stimulation versus AP backpropagation was extremely sensitive to the exogenous indicator conditions we employed to perform calcium imaging. Fig. 6 provides a case-in-point for how buffer conditions can drastically affect the relative amplitudes of kinetically distinct calcium influxes. This point is not merely academic since it illustrates how distinct calcium influxes and a neurone's control of its endogenous buffer environment can jointly act to read calcium signals. For example, the ostensible paradox in the calcium dependence of both long term depression and long term potentiation could involve a neurone's capacity to initiate distinct signalling pathways during slow, low-amplitude calcium signals versus faster, high-amplitude ones (Yang et al. 1999).
For years, traditional conditioning paradigms failed to elicit long-term synaptic modifications in inteneurones and it appeared as though calcium-dependent plasticity was not prominent in interneurones (Bi & Poo, 1998; McBain et al. 1999). Calcium handling in interneurones is very different from that in their pyramidal cell counterparts, with respect to endogenous buffer capacity, expression of class-specific buffers, and absence of CamKII expression (Baimbridge et al. 1992; Sik et al. 1998; Lee et al. 2000a). However, recent experiments in a variety of interneuronal subclasses have demonstrated that extremely precise dendritic calcium signals can alter synaptic strength or facilitate transynaptic communication (Mahanty & Sah, 1998; Zilberter, 2000; Perez et al. 2001; Lei & McBain, 2002). In all of these studies, plasticity induction depended exquisitely on the class of interneurone and even type of synapse examined, reinforcing the notion that the many classes of interneurone subserve a variety of circuit functions (Klausberger et al. 2003).
Our finding that distinct IN types possessed kinetically distinct calcium influxes during synaptic excitation resonates with the many interneurone-specific functions of dendritic calcium. In addition, the observation that NMDAR activation dominated at subthreshold potentials during activation of dendritic compartments suggested that calcium signalling in interneurone dendrites is very sensitive to the spatial arrangements of active inputs. Future experiments are needed to determine if the robust calcium signals we observed during convergent activation of dendritic domains translate into plastic processes.
Acknowledgments
We thank Gloster Aaron, Rosa Cossart and Jason MacLean for comments. The study was funded by the NEI (EY11787), NINDS (NS40726), the New York STAR Center for High Resolution Imaging of Functional Neural Circuits and the John Merck Fund.
REFERENCES
- Ali AB, Deuchars J, Pawelzik H, Thomson AM. CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. J Physiol. 1998;507:201–217. doi: 10.1111/j.1469-7793.1998.201bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkon DL, Rasmussen H. A spatial-temporal model of cell activation. Science. 1988;239:998–1005. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Rossier J, Audinat E. Postsynaptic glutamate receptors and integrative properties of fast- spiking interneurons in the rat neocortex. J Neurophysiol. 1999;82:1295–1302. doi: 10.1152/jn.1999.82.3.1295. [DOI] [PubMed] [Google Scholar]
- Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. doi: 10.1016/0959-4388(95)80012-3. [DOI] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Buzsaki G. Reliability and state dependence of pyramidal cell-interneuron synapses in the hippocampus: an ensemble approach in the behaving rat. Neuron. 1998;21:179–189. doi: 10.1016/s0896-6273(00)80525-5. [DOI] [PubMed] [Google Scholar]
- Dantzker JL, Callaway EM. Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci. 2000;3:701–707. doi: 10.1038/76656. [DOI] [PubMed] [Google Scholar]
- Edmonds B, Reyes R, Schwaller B, Roberts WM. Calretinin modifies presynaptic calcium signaling in frog saccular hair cells. Nat Neurosci. 2000;3:786–790. doi: 10.1038/77687. [DOI] [PubMed] [Google Scholar]
- Emptage N, Bliss TV, Fine A. Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron. 1999;22:115–124. doi: 10.1016/s0896-6273(00)80683-2. [DOI] [PubMed] [Google Scholar]
- Freund TF, Martin KA, Somogyi P, Whitteridge D. Innervation of cat visual areas 17 and 18 by physiologically identified X- and Y–type thalamic afferents. II. Identification of postsynaptic targets by GABA immunocytochemistry and Golgi impregnation. J Comp Neurol. 1985;242:275–291. doi: 10.1002/cne.902420209. [DOI] [PubMed] [Google Scholar]
- Fuchs EC, Doheny H, Faulkner H, Caputi A, Traub RD, Bibbig A, Kopell N, Whittington MA, Monyer H. Genetically altered AMPA-type glutamate receptor kinetics in interneurons disrupt long-range synchrony of gamma oscillation. Proc Natl Acad Sci U S A. 2001;98:3571–3576. doi: 10.1073/pnas.051631898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JR, Lubke J, Roth A, Frotscher M, Jonas P. Submillisecond AMPA receptor-mediated signaling at a principal neuron- interneuron synapse. Neuron. 1997;18:1009–1023. doi: 10.1016/s0896-6273(00)80339-6. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Goldberg J, Holthoff K, Yuste R. A problem with Hebb and local spikes. Trends Neurosci. 2002;25:433–435. doi: 10.1016/s0166-2236(02)02200-2. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Tamas G, Yuste R. Ca2+ imaging of mouse neocortical interneurone dendrites: Ia-type K+ channels control action potential backpropagation. J Physiol. 2003;551:49–65. doi: 10.1113/jphysiol.2003.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F. Dendrites as biochemical compartments. In: Stuart G, Spruston N, Hausser M, editors. Dendrites. Oxford: Oxford University Press; 1999. pp. 161–192. [Google Scholar]
- Jones RS, Buhl EH. Basket-like interneurones in layer II of the entorhinal cortex exhibit a powerful NMDA-mediated synaptic excitation. Neurosci Lett. 1993;149:35–39. doi: 10.1016/0304-3940(93)90341-h. [DOI] [PubMed] [Google Scholar]
- Kapur A, Yeckel M, Johnston D. Hippocampal mossy fiber activity evokes Ca2+ release in CA3 pyramidal neurons via a metabotropic glutamate receptor pathway. Neuroscience. 2001;107:59–69. doi: 10.1016/s0306-4522(01)00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsaki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- Koh DS, Geiger JR, Jonas P, Sakmann B. Ca(2+)-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J Physiol. 1995;485:383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig P, Engel AK, Singer W. Integrator or coincidence detector? The role of the cortical neuron revisited. Trends Neurosci. 1996;19:130–137. doi: 10.1016/s0166-2236(96)80019-1. [DOI] [PubMed] [Google Scholar]
- Kozloski J, Hamzei-Sichani F, Yuste R. Stereotyped position of local synaptic targets in neocortex. Science. 2001;293:868–872. doi: 10.1126/science.293.5531.868. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ. Signaling of layer 1 and whisker-evoked Ca2+ and Na+ action potentials in distal and terminal dendrites of rat neocortical pyramidal neurons in vitro and in vivo. J Neurosci. 2002;22:6991–7005. doi: 10.1523/JNEUROSCI.22-16-06991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Rosenmund C, Schwaller B, Neher E. Differences in Ca2+ buffering properties between excitatory and inhibitory hippocampal neurons from the rat. J Physiol. 2000a;525:405–418. doi: 10.1111/j.1469-7793.2000.t01-3-00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Schwaller B, Neher E. Kinetics of Ca2+ binding to parvalbumin in bovine chromaffin cells: implications for [Ca2+] transients of neuronal dendrites. J Physiol. 2000b;525:419–432. doi: 10.1111/j.1469-7793.2000.t01-2-00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron. 2002;33:921–933. doi: 10.1016/s0896-6273(02)00608-6. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Freund TF, Mody I. Glutamatergic synapses onto hippocampal interneurons: precision timing without lasting plasticity. Trends Neurosci. 1999;22:228–235. doi: 10.1016/s0166-2236(98)01347-2. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Dingledine R. Control of feedforward dendritic inhibition by NMDA receptor-dependent spike timing in hippocampal interneurons. J Neurosci. 2002;22:5462–5472. doi: 10.1523/JNEUROSCI.22-13-05462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G, McBain CJ. Long-term potentiation in distinct subtypes of hippocampal nonpyramidal neurons. J Neurosci. 1996;16:5334–5343. doi: 10.1523/JNEUROSCI.16-17-05334.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Lacaille JC. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci U S A. 2001;98:9401–9406. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perouansky M, Yaari Y. Kinetic properties of NMDA receptor-mediated synaptic currents in rat hippocampal pyramidal cells versus interneurones. J Physiol. 1993;465:223–244. doi: 10.1113/jphysiol.1993.sp019674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirazi P, Mel BW. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 2001;29:779–796. doi: 10.1016/s0896-6273(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Staiger JF, Lambolez B, Rossier J, Audinat E. Properties of bipolar VIPergic interneurons and their excitation by pyramidal neurons in the rat neocortex. Eur J Neurosci. 1998;10:3617–3628. doi: 10.1046/j.1460-9568.1998.00367.x. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed- forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Prince DA, Connors BW. Mechanisms of epileptogenesis in cortical structures. Ann Neurol. 1984;16:S59–64. doi: 10.1002/ana.410160710. [DOI] [PubMed] [Google Scholar]
- Roberts WM. Spatial calcium buffering in saccular hair cells. Nature. 1993;363:74–76. doi: 10.1038/363074a0. [DOI] [PubMed] [Google Scholar]
- Rozov A, Jerecic J, Sakmann B, Burnashev N. AMPA receptor channels with long-lasting desensitization in bipolar interneurons contribute to synaptic depression in a novel feedback circuit in layer 2/3 of rat neocortex. J Neurosci. 2001;21:8062–8071. doi: 10.1523/JNEUROSCI.21-20-08062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca(2+) ions in dendritic spines. Neuron. 2002;33:439–452. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature. 2000;404:285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- Sik A, Hajos N, Gulacsi A, Mody I, Freund TF. The absence of a major Ca2+ signaling pathway in GABAergic neurons of the hippocampus. Proc Natl Acad Sci U S A. 1998;95:3245–3250. doi: 10.1073/pnas.95.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons DJ. Response properties of vibrissa units in rat SI somatosensory neocortex. J Neurophysiol. 1978;41:798–820. doi: 10.1152/jn.1978.41.3.798. [DOI] [PubMed] [Google Scholar]
- Tamas G, Buhl EH, Somogyi P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J Neurosci. 1997;17:6352–6364. doi: 10.1523/JNEUROSCI.17-16-06352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamas G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei DS, Mei YA, Bagal A, Kao JP, Thompson SM, Tang CM. Compartmentalized and binary behavior of terminal dendrites in hippocampal pyramidal neurons. Science. 2001;293:2272–2275. doi: 10.1126/science.1061198. [DOI] [PubMed] [Google Scholar]
- Wood JD. The role of gamma-aminobutyric acid in the mechanism of seizures. Prog Neurobiol. 1975;5:77–95. doi: 10.1016/0301-0082(75)90008-8. [DOI] [PubMed] [Google Scholar]
- Yang SN, Tang YG, Zucker RS. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J Neurophysiol. 1999;81:781–787. doi: 10.1152/jn.1999.81.2.781. [DOI] [PubMed] [Google Scholar]
- Yuste R, Majewska A, Holthoff K. From form to function: calcium compartmentalization in dendritic spines. Nat Neurosci. 2000;3:653–659. doi: 10.1038/76609. [DOI] [PubMed] [Google Scholar]
- Zilberter Y. Dendritic release of glutamate suppresses synaptic inhibition of pyramidal neurons in rat neocortex. J Physiol. 2000;528:489–496. doi: 10.1111/j.1469-7793.2000.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


