Abstract
Vasoconstrictor responsiveness to acute sympathetic stimulation declines with advancing age in resting skeletal muscle. The purpose of the present study was to determine if age-related reductions in sympathetic vasoconstrictor responsiveness also occur in exercising skeletal muscle. Thirteen younger (20–30 years) and seven older (62–74 years) healthy non-endurance-trained men performed cycle ergometer exercise at ∼60 % of peak oxygen uptake while leg blood flow (femoral vein thermodilution), mean arterial blood pressure (radial artery catheter), and plasma adrenaline and noradrenaline concentrations were measured. After steady state was reached (i.e. ∼4 min), acute sympathetic stimulation was achieved by immersing a hand in ice water for 2–4 min (cold pressor test, CPT). CPT tended to cause a larger increase in mean arterial blood pressure in older men (older (O): 16 ± 3 mmHg; younger (Y): 10 ± 2 mmHg) during exercise, but increases in arterial noradrenaline were similar (O: 2.56 ± 0.96 nM; Y: 1.98 ± 0.40 nM). However, the older men demonstrated a larger percentage reduction in exercising leg vascular conductance (leg blood flow/mean arterial pressure) during CPT compared to younger men (O: -13.6 ± 3.1%; Y: -1.5 ± 4.3%; P = 0.04). Leg blood flow tended to increase in the younger men, but not in the older men (P = 0.10). These results suggest, in contrast to what has been observed in resting skeletal muscle, that vasoconstrictor responsiveness to sympathetic stimulation is not reduced, but may be augmented in exercising muscle of healthy older humans. This could reflect a reduced ability of local substances (e.g. nitric oxide) to impair vasoconstriction in response to sympathetic stimulation during exercise in older humans.
Under resting conditions, sympathetic vasoconstrictor outflow to skeletal muscle increases progressively with advancing age in humans, as evidenced by elevated basal systemic noradrenaline spillover rates and muscle sympathetic nerve activity (MSNA) (Seals & Esler, 2000). However, there appears to be a corresponding desensitization of arterial α-adrenergic receptors, such that blunted vasoconstrictor responses to acute sympathetic stimulation are observed. Although in vitro studies of human arterial α-adrenergic responsiveness have given mixed results, with some showing no change (Scott & Reid, 1982; Docherty, 1990) and others showing decreased sensitivity in arteries isolated from older individuals (Hatake et al. 1992; Nielsen et al. 1992), studies using in vivo approaches generally support an age-related decrease in responsiveness (Elliott et al. 1982; Hogikyan & Supiano, 1994; Sugiyama et al. 1996; Davy et al. 1998). Recent evidence in humans (Dinneno et al. 2002) suggests that a major component of this age-associated decrease in vascular responsiveness involves blunted α1, but not α2, adrenergic receptor responsiveness.
During dynamic exercise involving a large muscle mass, the sympathetic nervous system mediates vasoconstriction in non-active muscle and in visceral regions, which contributes to an increase in arterial perfusion pressure and facilitates redistribution of blood flow to exercising muscles (Rowell, 1993). However, sympathetic vasoconstrictor outflow is also directed to active skeletal muscle to balance active muscle vasodilatation with the rise in cardiac output so that systemic arterial pressure can be maintained. Although the vasculature of exercising muscle displays a reduced sensitivity to α-adrenergic stimuli, a phenomenon referred to as ‘functional sympatholysis’, sympathetic restraint of active muscle blood flow is still a quantitatively important contributor to systemic blood pressure maintenance during large-muscle exercise in humans (Buckwalter & Clifford, 2001).
In this context, most healthy older (> 60 years) adults demonstrate a reduced absolute cardiac output response during submaximal and especially during maximal exercise (Fagard et al. 1993), but a relatively well-preserved skeletal muscle vasodilator capacity (Jasperse et al. 1994; Martin et al. 1995). These results suggest that sympathetic restraint of active muscle vasodilatation during exercise may become even more important for older adults. Thus the need for increased sympathetic restraint of active muscle blood flow in older adults vs. the blunted vasoconstrictor responses seen in aged limbs is a potential paradox that has not previously been explored.
To gain insight into this issue, we measured changes in leg blood flow and vascular conductance during cycle ergometer exercise at a similar relative intensity (60 % peak oxygen consumption (V̇O2peak)) in non-endurance-trained younger and older men before, during, and after immersion of one hand in ice water (cold pressor test, CPT). Local cold stimulation was chosen because it is a robust sympathetic stimulus capable of doubling MSNA within 1-2 min (Victor et al. 1987; Seals, 1990), and its effects appear to be independent of age (Ng et al. 1994). We hypothesized that during moderate intensity exercise, the leg vasculature of older men would exhibit less vasoconstriction in response to acute sympathetic stimulation in comparison with younger men, as has been observed under resting conditions.
METHODS
Subject screening and preliminary tests
Thirteen younger (20-30 years) and seven older (62-74 years) men from State College, PA, USA and surrounding communities completed all phases of this study. Each subject was informed of potential risks and discomforts and signed an informed consent form approved by the Institutional Review Board of the Pennsylvania State University and the General Clinical Research Center (GCRC) at the University Park campus. All studies were performed in accordance with the Declaration of Helsinki. All subjects were recreationally active, but none had participated in moderate- or high-intensity aerobic exercise > 3 days week−1 during the previous 12 months or had a treadmill V̇O2max > 80th percentile according to age group norms (American College of Sports Medicine, 2000). Additionally, lower body-strength-trained subjects (> 1 day week during the previous 12 months) were excluded from participation.
All subjects were non-obese (<30 % body fat and body mass index < 30), non-smokers, and had clinically normal haemoglobin concentrations (12.5-16.6 g dl−1) and resting supine ankle-brachial index ratings (ABI < 0.90; American College of Sports Medicine, 2000). No subject had a history or symptoms of cardiac, vascular, pulmonary, metabolic, or neurological disease. Hypertensive individuals (resting blood pressure > 140/90 mmHg) were also excluded because their central (Fagard et al. 1995) and peripheral (Tanaka et al. 1998) haemodynamic responses to exercise differ compared with normotensive age-matched controls. No subjects were taking medications having significant haemodynamic effects, but four older men did take aspirin on a regular basis. Subjects underwent a treadmill test to maximal exertion to rule out exercise-induced electrocardiograph or blood pressure abnormalities.
After screening, subjects returned to the laboratory on two separate days, once for a preliminary cycle ergometer exercise session and once for the invasive leg blood flow study. All exercise testing for both sessions was performed in the upright posture using a Lode electronically braked cycle ergometer with toe clips. A padded forearm rest was attached above the handlebars to prevent the subject from leaning forward and to facilitate blood sampling from the radial artery catheter. Pulmonary gas exchange (V̇O2, V̇CO2, and minute ventilation, VE) was measured on both days using the TrueMax 2400 metabolic system (Parvomedics, Salt Lake City, UT, USA; Bassett et al. 2001). Heart rate was recorded from an electrocardiograph, and ratings of perceived exertion were assessed using the Borg 6 to 20-point scale. Room temperature was maintained between 19 and 22 °C, and subjects were encouraged to drink water between exercise bouts to remain well hydrated.
The purpose of the preliminary cycle ergometer exercise session was to familiarize the subject with the cycle ergometer and pulmonary gas exchange apparatus (i.e. mouthpiece, nose clip). Subjects completed two incremental tests to establish submaximal and peak V̇O2, heart rate, and rating of perceived exertion responses.
Subject preparation for invasive leg blood flow study
Subjects were instructed to abstain from products containing caffeine or aspirin for 12 h prior to testing. Subjects were provided a standardized dinner the evening before (≈18.00 h) and a breakfast on the morning of (06.00 h) the study. Therefore, all subjects were tested in the post-absorptive state. Subjects were also encouraged to drink 6-8 glasses of water the day before the study.
At the beginning of the study day, indwelling catheters were placed in the femoral vein and the radial artery for direct measurement of leg blood flow, mean arterial pressure (MAP), blood lactate and O2 content, and plasma adrenaline and noradrenaline concentrations. Preparation for catheter placements typically began between 07.00 and 10.00 h. Subjects shaved their right groin region and applied a topical anaesthetic (Emla crème). Catheters were placed by a physician using aseptic procedures and local anaesthetic (2 % lignocaine (lidocaine)). A thermister wire (IT-18, Physitemp Instruments, Clifton, NJ, USA) and an 18-gauge infusion catheter with 10 side ports (Cook royal flush II 4.0 Fr) were placed approximately 15 cm apart in the right femoral vein (anterograde and retrograde, respectively) for leg blood flow measurement and blood sampling. A 20-gauge Teflon catheter (Arrow arterial catheterization set FA-04020) was inserted into the radial artery for MAP measurements and blood sampling.
Experimental protocol
At the beginning of the protocol, subjects performed two submaximal exercise bouts to collect data for another study (Proctor et al. 2003). Briefly, subjects began with an incremental protocol with workloads ranging from 20-100 W. Next, subjects rested in the supine position for an hour to allow sympathetic and haemodynamic variables to return to baseline. At the end of the resting period, subjects were placed back on the cycle ergometer and exercised at a moderate intensity eliciting a V̇O2 of approximately 1.1 l min−1 (60-70 W) for 6 min, followed by 10 min of active recovery at a very light workload (20 W). Subjects resumed exercise at a workload eliciting 60 % V̇O2peak until steady-state heart rate and V̇O2 were reached (≈4 min). Next, subjects were asked to perform a cold pressor test (CPT), which consisted of placing the left hand in ice water (0-1 °C) for 2-4 min while continuing to exercise at the same workload. Subjects continued pedalling at the same workload for 4 min after removing their hand from the ice water (post-CPT). Leg blood flow, MAP, lactate, and catecholamines were measured before, during, and after the CPT. Specifically, leg blood flow and MAP were measured 2-3 times before the CPT (during the 3rd and 4th minute or 2nd, 3rd, and 4th minutes), 2-3 times during the CPT (within the first 30 s and then approximately every minute thereafter) and 2-3 times following the CPT (during the 1st and 3rd minute or during the 1st, 2nd, and 3rd minutes). Arterial and venous blood samples were drawn for measurement of lactate and catecholamines during the 30 s immediately before the CPT (pre-CPT), during the last 30 s of the CPT (CPT), and during the last 30 s of the exercise bout (post-CPT). Following an hour of rest, subjects performed a maximal graded exercise test on the cycle ergometer, and peak plasma adrenaline and noradrenaline responses were measured.
Measurements
Measurement of leg blood flow and arterial pressure
Whole-leg blood flow was measured during exercise by using the constant infusion femoral vein thermodilution technique as described previously (Proctor et al. 2003). Leg blood flow was calculated by using the thermal balance equation detailed by Andersen & Saltin (1985) and doubled to estimate two-leg blood flow (l min−1). Simultaneous recordings from the radial artery pressure transducer (Baxter PX-MK099) were displayed, recorded, and analysed using WinDaq software. The transducer was zeroed at the aortic arch (intercostal space 4) for each subject. Leg vascular conductance was calculated as leg blood flow × 2/MAP.
Measurement of catecholamines and lactate
Arterial and venous plasma catecholamine concentrations (5 ml each) were measured using high-performance liquid chromatography with electrochemical detection (Weicker, 1988). Arterial and venous lactate concentrations were measured using a commercially available analyser (Yellow Springs Instruments 2300 stat-plus).
Body composition
Total body fat, fat-free mass, and leg tissue composition were estimated on a separate laboratory visit using dual-energy X-ray absorptiometry (DXA; Hologic QDR 4500-W, software version 9.80D, Waltham, MA, USA). Weekly calibrations were performed on the DXA scanner to ensure accuracy.
Data analysis
For haemodynamic variables (i.e. leg blood flow, MAP, and leg conductance) the values reported for steady-state cycling exercise (pre-CPT) represent the average of the last two measurements before the hand was immersed in ice water. Responses reported during CPT represent the highest MAP measurement and its corresponding leg blood flow measurement. Age group comparisons of subject characteristics (Table 1) and various haemodynamic and blood variables (Tables 2 and 3) during exercise and CPT were analysed using two-tailed two-sample t tests assuming unequal variances (Mini-tab version 13.1). All data are presented as means ± S.E.M. Statistical significance was accepted when P ≤ 0.05.
Table 1.
Subject characteristics
| Variable | Younger men | Older men | P Value |
|---|---|---|---|
| Age (years) | 23 ± 1 | 67 ± 2 | 0.00 |
| Height (cm) | 176.8 ± 1.8 | 180.2 ± 2.5 | 0.30 |
| Weight (kg) | 78.5 ± 3.1 | 85.3 ± 3.1 | 0.14 |
| Body fat (%) | 19.2 ± 1.6 | 26.6 ± 1.4 | 0.00 |
| Arterial haemoglobin (g dl−1) | 15.1 ± 0.3 | 14.0 ± 0.3 | 0.02 |
| Total haemoglobin (g dl−1) | 169 ± 5 | 182 ± 13 | 0.37 |
| Resting systolic BP (mmHg) | 123 ± 3(12) | 126 ± 5(6) | 0.61 |
| Resting diastolic BP (mmHg) | 76 ± 2(12) | 80 ± 2(6) | 0.33 |
| Treadmill V̇O2,max (ml kg−1 min−1) | 44.9 ± 1.4(12) | 31.5 ± 1.9 | 0.00 |
| Resting arterial noradrenaline (nM) | 1.43 ± 0.3(12) | 2.49 ± 0.6 | 0.15 |
| Resting venous noradrenaline (nM) | 0.35 ± 0.1(11) | 0.56 ± 0.1 | 0.18 |
| Resting arterial adrealine (nM) | 0.35 ± 0.1(11) | 0.56 ± 0.1 | 0.16 |
| Resting venous adrenaline (nM) | 0.34 ± 0.1(11) | 0.39 ± 0.1(6) | 0.70 |
Values are mean ± s.e.m. for 13 younger and 7 older except where noted inparentheses. Percentahe of body fat was estimated by DXA as described in Methods. Resting BP indicates seated blood pressure (average of 2–3 visits) measured by auscultation.
Table 2.
Responses to submaximal leg cycling prior to local cold stimulation
| Variable | Younger men | Older men | P Value |
|---|---|---|---|
| Workload (W) | 120 ± 7 | 86 ± 6 | 0.00 |
| Systemic V̇O2 (1 min−1) | 1.70 ± 0.1 | 1.34 ± 0.1 | 0.02 |
| Systemic V̇O2(% peak) | 60.6 ± 1.6 | 36.3 ± 3.0 | 0.44 |
| Heart rate (beats min −1) | 143.2 ± 4.1 | 110.6 ± 3.3 | 0.00 |
| Leg O2 extreaction (%) | 66.4 ± 1.6(12) | 71.8 ± 1.9 | 0.05 |
| Arterial lactate (mM) | 2.76 ± 0.2(12) | 1.74 ± 0.2 | 0.01 |
| Venous lactate (mM) | 3.23 ± 0.3 | 1.88 ± 0.2 | 0.00 |
| Arterial noradrenaline (nM) | 4.44 ± 0.8(12) | 6060 ± 1.3 | 0.18 |
| Venous nordrenaline (nM) | 4.43 ± 0.9 | 7.63 ± 1.8(6) | 0.15 |
| Arterial adrenaline(nM) | 0.89 ± 0.1(11) | 1.05 ± 0.2 | 0.57 |
| Venous adrealine (nM) | 0.75 ± 0.1 | 0.87 ± 0.1(6) | 0.48 |
Values are mean ± s.e.m. for 13 younger and 7 older mean except where noted in parentheses.
Table 3.
Responses to local cold stimulation during submaximal cycling
| Variable | Younger men | Older men | P Value |
|---|---|---|---|
| Δ Systemic V̇O2 (1 min−1) | 0.16 ± 0.1 | 0.08 ± 0.1 | 0.40 |
| Δ Systemic V̇O2 (% peak) | 5.7 ± 0.8 | 4.0 ± 0.9 | 0.18 |
| Δ Heart rate (beats min −1) | 7.3 ± 1.5(12) | 6.6 ± 1.8(5) | 0.79 |
| Δ O2 extraction (%) | 3.0 ± 1.5(11) | 1.0 ± 1.3 | 0.32 |
| Δ Arterial lactate (mM) | 0.59 ± 0.1(12) | 0.30 ± 0.1 | 0.01 |
| Δ Venous lactate (mM) | 0.61 ± 0.1 | 0.36 ± 0.1 | 0.06 |
| Δ Arterial noradrenaline (nM) | 1.97 ± 0.4(12) | 2.56 ± 1.0 | 0.59 |
| Δ Venous noradrenaline(nM) | 2.09 ± 0.4 | 1.38 ± 1.3(5) | 0.63 |
| Δ Arterial adrenaline (nM) | 0.06 ± 0.1(11) | 0.20 ± 0.2 | 0.54 |
| Δ Venous adrealine(nM) | 0.21 ± 0.1 | 0.24 ± 0.3(5) | 0.92 |
Values are mean changes (Δ) ± s.e.m. for 13 younger and 7 older men except where noted in parentheses.
RESULTS
Subject characteristics
Table 1 presents subject characteristics of both the older and younger (control) men. Older men had a higher percentage of body fat and a lower arterial haemoglobin concentration and treadmill V̇O2max (P < 0.05). Height, weight, and plasma cholesterol did not differ between age groups, although the older men tended to be heavier (P = 0.14).
Baseline resting measurements
At rest, there were no age group differences observed for blood pressure (Table 1). Although arterial noradrenaline concentrations were ≈75 % higher in the older men, these differences were not significant (Table 1).
Responses to submaximal leg cycling
Absolute responses to steady-state cycle ergometer exercise are shown in Table 2. Although younger and older men exercised at similar relative workloads eliciting 61 ± 2 % and 63 ± 3 % of peak oxygen uptake, respectively (P > 0.05), the older men cycled at a significantly lower absolute workload and systemic V̇O2 due to their lower peak oxygen uptake. Heart rate and lactate concentrations were also significantly lower in the older men. There was a strong trend toward lower leg blood flow responses in the older men (younger (Y): 9.2 ± 0.5; older (O): 7.2 ± 0.4 l min−1; P = 0.07) (Fig. 1), but MAP responses were identical (Y: 116 ± 3; O: 116 ± 6 mmHg). Therefore, leg vascular conductance was lower in the older men (Y: 80 ± 5; O: 63 ± 9 ml min−1 mmHg−1), although this did not reach statistical significance (P = 0.14). It should be noted that the absolute MAP values measured for one of our older subjects were much higher than expected (Fig. 1B). These data were retained in this analysis because the primary focus of this study was on the change in MAP during CPT rather than absolute values and because no systematic drift of the blood pressure transducer was noted during this subject's study. Also, this subject's resting MAP was normotensive.
Figure 1.
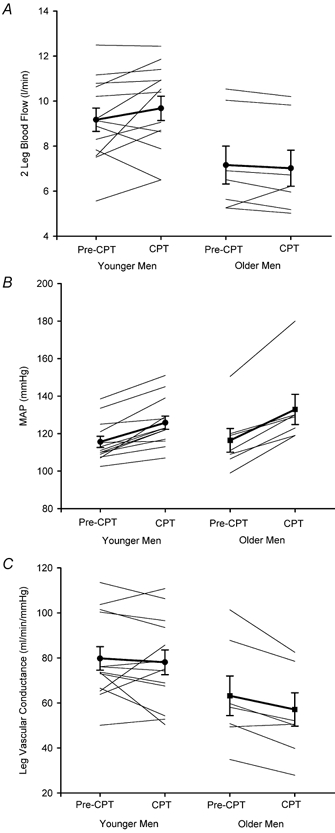
Leg blood flow (A), mean arterial pressure (B), and leg vascular conductance (C) before (Pre-CPT) and during (CPT) cold pressor test in younger and older men. Thin lines represent individual subject responses, and thick lines represent group means ± S.E.M. for 13 younger and 7 older men.
Increases in MAP from baseline during submaximal cycling exercise averaged 20-25 mmHg in both age groups, while heart rate increased to a lesser extent in older men. Arterial noradrenaline increased by 3.0 ± 0.5 nM and 4.1 ± 0.9 nM in younger and older men, respectively (P > 0.05).
Responses to local cold stimulation
The sympathetic responses to local cold stimulation are shown in Table 3. When subjects immersed their hand in ice water, arterial noradrenaline rose to a similar degree in both age groups. Systemic V̇O2 increased slightly in both age groups. Arterial lactate increased significantly more in the younger men, while venous lactate showed a trend in the same direction (Table 3).
The haemodynamic responses to local cold stimulation are highlighted in Fig. 1. Leg vascular conductance showed almost no change in the younger men (-1.5 ± 4 %), whereas it decreased by ≈14 % in the older men (P = 0.04 between age groups). In the younger men, there was little change in leg vascular conductance because MAP increased by 10 ± 2 mmHg, but two leg blood flow also increased by 0.5 ± 0.3 l min−1. In contrast, MAP tended to increase to a larger degree in older men than in young men (P = 0.08), but there was no corresponding increment in leg blood flow. Finally, the percentage change in leg vascular conductance per unit change in arterial noradrenaline concentration was much more pronounced in the older men, whether expressed in absolute terms (Fig. 2A) or expressed as a percentage of the peak noradrenaline response found during maximal exercise (Fig. 2B).
Figure 2.
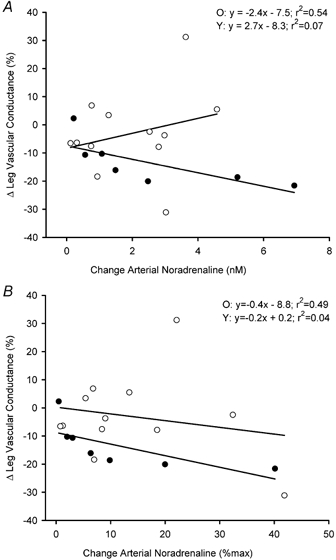
Change (Δ) in leg vascular conductance with addition of cold pressor stimulation to steady-state leg cycling exercise at 60 % V̇O2peak, as a function of the absolute change in arterial noradrenaline (A) and the change in arterial noradrenaline expressed as a percentage of peak (B) in 13 younger (○) and 7 older (•) men.
DISCUSSION
There were three major new findings in the present study. First, cold pressor stimulation applied during submaximal dynamic leg exercise evoked a rapid and marked increase in arterial blood pressure in healthy older men and younger men. Increases in mean arterial pressure (≈10-15 mmHg) were physiologically significant, but consistently less than those previously reported under resting conditions (≈20 mmHg; Victor et al. 1987; Ng et al. 1994). Second, increases in sympathetic outflow to the exercising legs during local cold stimulation, as estimated by arterial noradrenaline concentrations, were similar in the younger and older men. Our third, and most significant finding was that the older men demonstrated a larger percentage reduction in leg vascular conductance in response to local cold stimulation applied during exercise. These results suggest that vasoconstrictor responsiveness to acute sympathetic stimulation is not reduced, but may be augmented in the exercising legs of healthy non-endurance-trained older men. These findings have important implications for the regulation of active limb vasomotor tone and systemic blood pressure regulation during dynamic exercise in older humans.
Age and vasoconstrictor responsiveness in exercising muscle
Previous studies have attempted to alter vascular tone in exercising muscles through acute sympathetic stimulation. Strange (1999) found a reduction in leg vascular conductance in young men during one leg knee extensor exercise at both light and moderate intensities when the sympathetic nervous system was stimulated by ischaemic handgrip. Leg blood flow was not affected by the sympathetic stimulus, but MAP was significantly elevated (Strange, 1999). Pawelczyk et al. (1992) reported a decrease in leg vascular conductance during baroreflex unloading achieved through β1-adrenoreceptor blockade. To the best of our knowledge, however, the present study is the first to test for possible age-associated differences in responsiveness to sympathetic stimulation in active skeletal muscle. Our major new finding was that older men demonstrated a larger percentage reduction in exercising leg vascular conductance during cold pressor stimulation than was observed in younger men. The percentage change in vascular conductance was used because it is the most appropriate index for comparing changes in vascular tone when differences in baseline blood flow exist (e.g. pre-cold stimulation) (Buckwalter & Clifford, 2001). When evaluated in relation to individual changes in arterial noradrenaline concentrations (Table 3), the augmented vasoconstrictor responsiveness to sympathetic stimulation in the older men was even more evident. Collectively, these results provide the first evidence of an age-related augmentation of vascular responsiveness to sympathoexcitation in active limbs in healthy humans.
The decrease in leg vascular conductance in response to local cold stimulation in our older subjects relative to their younger counterparts could be due to age differences in the sensitivity, density, or distribution of various adrenergic receptor subtypes. β2-adrenoreceptors cause local vasodilatation in vascular smooth muscle through both cAMP and nitric oxide mechanisms (Dawes et al. 1997). Because adrenaline readily binds to β2-adrenoreceptors, a larger increase in circulating adrenaline in younger men could offset a potential α-mediated vasoconstriction. However, increases in both arterial and venous adrenaline concentration with cold stimulation did not significantly differ between younger and older men in this study (Table 3). If anything, increases in circulating adrenaline during CPT were larger in older men (Table 3). We cannot exclude the possibility that an age-related decline in vascular β2 responsiveness could explain our results (van Brummelen et al. 1981; Pan et al. 1986).
Another possible explanation for the reduced leg vascular conductance seen in our older men during dynamic leg exercise would be an increase in the density or sensitivity of α-adrenoreceptors relative to younger men. In this context, Rudner et al. (1999) found an increased α1-adrenoreceptor density in mammary arteries isolated from older patients. However, Dinenno et al. (2002) reported a decrease in α1 responsiveness in the forearms of older men at rest, evidenced by a smaller decrement in forearm blood flow in response to phenylephrine (a specific α1 agonist). If these results can be extrapolated to the legs, this would argue against an increase in density or sensitivity of α-adrenoreceptors in our older men.
Our results could also be due to age group differences in myogenic responsiveness. Although an age-related increase in myogenic responsiveness to a large change in MAP could explain the augmented vasoconstriction in older men, there is recent evidence suggesting that myogenic responsiveness is diminished, rather than augmented, in rat soleus and gastrocnemius arterioles (Mueller-Delp et al. 2002).
Although this was not directly tested in the current investigation, an age-related decrease in metabolic modulation of sympathetic vasoconstriction could also explain the decrease in exercising leg vascular conductance in older men during cold stimulation. Recently, Chavoshan et al. (2002) provided evidence that nitric oxide plays a major role in modulating vasoconstrictor responsiveness to acute sympathetic stimulation in the microcirculation of exercising forearm muscle. In the context of ageing, Taddei et al. (2000) found that healthy untrained older humans demonstrate reduced nitric oxide-mediated vasodilatation in the forearm. If there is a similar age-related reduction in nitric oxide availability in exercising leg muscles, this could impair the metabolic modulation of sympathetic activity and increase the vasoconstrictor responsiveness of exercising leg muscles to an acute sympathetic stimulus.
Implications for blood pressure regulation during exercise in older adults
Why would the leg vasculature of older non-endurance-trained men demonstrate an augmented responsiveness to sympathetic stimulation during dynamic exercise? As mentioned in the Introduction, vasoconstriction in active skeletal muscle is necessary for maintaining arterial blood pressure during large-muscle dynamic exercise in humans. Cardiac output measured in these subjects at an intensity of 60 % of V̇O2peak averaged 3.7 l min−1 lower in the older than in the younger men (authors’ unpublished data). Therefore, to achieve the same arterial blood pressure response, the older men would need to maintain a higher level of systemic vascular resistance than the younger men. Taylor et al. (1992) speculated that inactive muscle (i.e. forearm muscle) might be an important contributor to the augmented systemic vascular resistance seen in older men during moderate intensity leg exercise. The results of the present study suggest that active leg muscle is likely to be another major target for sympathetic vasoconstriction to generate a pressor response of this magnitude during moderate-intensity exercise involving a large muscle mass.
Experimental considerations
One alternative explanation for the augmented vasoconstrictor responses seen in our older subjects is that the sympathoexcitatory stimulus was greater than that of the younger subjects. This possibility cannot be excluded because calculations of leg noradrenaline spillover (Savard et al. 1987) were negative in several subjects due to the limitations associated with our plasma adrenaline measurements (i.e. venous adrenaline concentrations higher than arterial). We also decided against the use of deep venous noradrenaline concentrations as a measure of the sympathetic stimulus because two of these samples from our older men could not be obtained, and another showed a questionable 2.5 nM reduction during cold pressor stimulation. For these reasons, we opted to estimate the strength of the sympathetic stimulus using arterial noradrenaline concentrations. It should be noted that this provided similar estimates to what would have been obtained using venous noradrenaline concentrations after missing and questionable measurements were excluded.
Although arterial noradrenaline concentrations were used to estimate sympathetic outflow, we believe that local cold stimulation evoked a similar degree of sympathetic outflow in our younger and older subjects for the following reasons: first, although there were trends toward higher arterial noradrenaline concentrations in the older men pre-stimulation (i.e. 60 % V̇O2peak), the absolute increases in both adrenaline and noradrenaline concentrations with cold stimulation were similar between groups (Table 3). Secondly, under resting conditions, Ng et al. (1994) reported similar absolute increases in MSNA in similar groups of younger and older men during 2.5 min of hand immersion in ice water. Together, these findings suggest that the increase in sympathetic outflow to the legs under the conditions of the present study was equivalent between the two age groups. Our results are also in agreement with accumulating evidence indicating that sympathoadrenal responsiveness to several types of acute laboratory stress is not exaggerated with age in healthy humans (Ng et al. 1994; Mazzeo et al. 1997).
It is also possible that our results could be explained by differences in the amount of metabolites available to interfere with sympathetic vasoconstriction. In an attempt to match the degree of sympathetic activation between age groups prior to the application of local cold stress, we had subjects exercise at the same relative oxygen consumption (≈60 % V̇O2peak) (Fleg et al. 1985; Lehmann & Keul, 1986; Mazzeo & Grantham, 1989). This resulted in our older subjects exercising at a lower absolute workload (Table 2). Presumably, then, the active muscle mass was smaller in the older men, which would account for the tendency toward lower absolute limb blood flows in the older men during exercise at 60 % V̇O2peak. The lower arterial and venous lactate concentrations observed in the older men during exercise at 60 % V̇O2peak both before (Table 2) and during cold stimulation (data not shown) suggest that the amount of metabolites available to interfere with sympathetic vasoconstriction may have been lower in the older men. Whether similar age group differences in leg vascular conductance would be observed if subjects were tested at the same absolute workload cannot be determined.
Despite the acknowledged limitations of this investigation, we believe the key consideration is that the cold pressor test produced a similar increase in sympathetic vasoconstrictor neural outflow (i.e. increase in arterial noradrenaline concentrations, Table 3) without increasing the metabolic demand appreciably in either subject group (i.e. systemic V̇O2, Table 3). Because metabolic demand showed a similar small increase in both subject groups, while conductance declined only in the older men, it is reasonable to conclude that the larger percentage reduction in leg vascular conductance seen in the older men compared to the younger men in this study reflects an age-associated increase in vascular responsiveness to sympathetic stimulation in exercising skeletal muscle.
Conclusions
In summary, the present study demonstrated an augmented vasoconstrictor response to sympathoexcitation in the arterial vasculature of exercising skeletal muscle of healthy older men compared to younger men. These findings add to the accumulating evidence in the literature suggesting that active limb vasomotor tone is actively regulated by the sympathetic nervous system during exercise. Moreover, these findings have important implications for blood pressure regulation in older humans and support the need for further study of the underlying mechanisms.
Acknowledgments
The authors thank the subjects for participating. We also thank Sean Newcomer, Khoi Le, Kristin Shay, Benjamin Tu and Jaime Platts for assistance with data collection, Sandy Smithmyer for recruiting and scheduling subjects, Fred Weyandt, Don Fink and Doug Johnson for technical assistance, and the GCRC nursing staff for their assistance during in-patient studies. This work was supported by National Institute of Health Grants R01-AG-18246 (to D. N. Proctor), M01-RR-10732 (GCRC), and T32-GM-08619 (to D. W. Koch).
REFERENCES
- American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 6. Philadelphia: Lippincott; 2000. pp. 64–209. [Google Scholar]
- Anderson P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DR, Jr, Howley ET, Thompson DL, King GA, Strath SJ, McLaughlin JE, Parr BB. Validity of inspiratory and expiratory methods of measuring gas exchange with a computerized system. J Appl Physiol. 2001;91:218–224. doi: 10.1152/jappl.2001.91.1.218. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sport Sci Rev. 2001;29:159–163. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension. 1998;32:298–304. doi: 10.1161/01.hyp.32.2.298. [DOI] [PubMed] [Google Scholar]
- Dawes M, Chowienczyk PJ, Ritter JM. Effects of inhibition of the L-arginine/nitric oxide pathway on vasodilation caused by β-adrenergic agonists in human forearm. Circulation. 1997;95:2293–2297. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Docherty JR. Cardiovascular responses to ageing: a review. Pharmacol Rev. 1990;42:103–125. [PubMed] [Google Scholar]
- Elliott HL, Sumner DJ, McLean K, Reid JL. Effect of age on the responsiveness of alpha-adrenoceptors in man. J Cardiovasc Pharmacol. 1982;4:388–392. doi: 10.1097/00005344-198205000-00008. [DOI] [PubMed] [Google Scholar]
- Fagard RH, Thijs LB, Amery AK. Age and the hemodynamic response to posture and exercise. Am J Geriatr Cardiol. 1993;2:23–40. [PubMed] [Google Scholar]
- Fagard RH, Thijs LB, Amery AK. The effect of gender on aerobic power and exercise hemodynamics in hypertensive adults. Med Sci Sports Exerc. 1995;27:29–34. [PubMed] [Google Scholar]
- Fleg JL, Tzankoff SP, Lakatta EG. Age-related augmentation of plasma catecholamines during dynamic exercise in healthy males. J Appl Physiol. 1985;59:1033–1039. doi: 10.1152/jappl.1985.59.4.1033. [DOI] [PubMed] [Google Scholar]
- Hatake K, Wakabayashi I, Kakishita E, Hishida S. Effect of aging on contractile response to KCl, norepinephrine and 5-hydroxytryptamine in isolated human basilar artery. Gen Pharmacol. 1992;23:417–420. doi: 10.1016/0306-3623(92)90104-r. [DOI] [PubMed] [Google Scholar]
- Hogikyan RV, Supiano MA. Arterial alpha-adrenergic responsiveness is decreased and SNS activity is increased in older humans. Am J Physiol. 1994;266:717–724. doi: 10.1152/ajpendo.1994.266.5.E717. [DOI] [PubMed] [Google Scholar]
- Jasperse JL, Seals DR, Callister R. Active forearm blood flow adjustments to handgrip exercise in young and older healthy men. J Physiol. 1994;474:353–360. doi: 10.1113/jphysiol.1994.sp020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman M, Keul J. Age-associated changes of exercise-induced plasma catecholamine responses. Eur J Appl Physiol Occup Physiol. 1986;55:302–306. doi: 10.1007/BF02343803. [DOI] [PubMed] [Google Scholar]
- Martin HL, Loomis JL, Kenney WL. Maximal skin vascular conductance in subjects aged 5–85 yr. J Appl Physiol. 1995;79:297–301. doi: 10.1152/jappl.1995.79.1.297. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, Grantham PA. Sympathetic response to exercise in various tissues with advancing age. J Appl Physiol. 1989;66:1506–1508. doi: 10.1152/jappl.1989.66.3.1506. [DOI] [PubMed] [Google Scholar]
- Mazzeo RS, Rajkumar C, Jennings G, Esler M. Norepinephrine spillover at rest and during submaximal exercise in young and old subjects. J Appl Physiol. 1997;82:1869–1874. doi: 10.1152/jappl.1997.82.6.1869. [DOI] [PubMed] [Google Scholar]
- Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;282:H1843–1854. doi: 10.1152/ajpheart.00666.2001. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am J Physiol. 1994;267:344–353. doi: 10.1152/ajpheart.1994.267.1.H344. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Hasenkam JM, Pilegaard HK, Aalkjaer C, Mortensen FV. Age-dependent changes in alpha-adrenoceptor-mediated contractility of isolated human resistance arteries. Am J Physiol. 1992;263:1190–1196. doi: 10.1152/ajpheart.1992.263.4.H1190. [DOI] [PubMed] [Google Scholar]
- Pan HY, Hoffman BB, Pershe RA, Blaschke TF. Decline in beta adrenergic receptor-mediated vascular relaxation with aging in man. J Pharmacol Exp Ther. 1986;239:802–807. [PubMed] [Google Scholar]
- Pawelczyk JA, Hanel B, Pawelczyk RA, Warberg J, Secher NH. Leg vasoconstriction during dynamic exercise with reduced cardiac output. J Appl Physiol. 1992;73:1838–1846. doi: 10.1152/jappl.1992.73.5.1838. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA, Leuenberger UA. Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J Appl Physiol. 2003;94:1859–1869. doi: 10.1152/japplphysiol.00898.2002. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. pp. 204–254. [Google Scholar]
- Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D'Amico EB, El-Moalem H, Page SO, Richardson CD, Winters B, Marucci L, Schwinn DA. Subtype specific regulation of human vascular alpha-adrenergic receptors by vessel bed and age. Circulation. 1999;100:2336–2343. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- Savard G, Strange S, Kiens B, Saltin B, Richter EA, Christensen NJ. Norepinephrine spillover during exercise in active versus resting skeletal muscle in man. Acta Physiol Scand. 1987;131:507–516. doi: 10.1111/j.1748-1716.1987.tb08270.x. [DOI] [PubMed] [Google Scholar]
- Scott PJW, Reid JL. The effect of age on the responses of human isolated arteries to noradrenaline. Br J Clin Pharmacol. 1982;13:237–239. doi: 10.1111/j.1365-2125.1982.tb01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DR. Sympathetic activation during the cold pressor test: influence of stimulus area. Clin Physiol. 1990;10:123–129. doi: 10.1111/j.1475-097x.1990.tb00246.x. [DOI] [PubMed] [Google Scholar]
- Seals DR, Esler MD. Human ageing and the sympathadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange S. Cardiovascular control during concomitant dynamic leg exercise and static arm exercise in humans. J Physiol. 1999;514:283–291. doi: 10.1111/j.1469-7793.1999.283af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Matsukawa T, Shamsuzzaman ASM, Okada H, Watanabe T, Mano T. Delayed and diminished pressor response to muscle sympathetic nerve activity in the elderly. J Appl Physiol. 1996;80:869–875. doi: 10.1152/jappl.1996.80.3.869. [DOI] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Reiling MJ, Seals DR. Regular walking increases peak limb vasodilatory capacity of older hypertensive humans: implications for arterial structure. J Hypertens. 1998;16:423–428. doi: 10.1097/00004872-199816040-00003. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Hand GA, Johnson DG, Seals DR. Augmented forearm vasoconstriction during dynamic exercise in healthy older men. Circulation. 1992;86:1789–1799. doi: 10.1161/01.cir.86.6.1789. [DOI] [PubMed] [Google Scholar]
- van Brummelen P, Bühler FR, Kiowski W, Amann FW. Age-related decrease in cardiac and peripheral vascular responsiveness to isoprenaline: studies in normal subjects. Clinical Science. 1981;60:571–577. doi: 10.1042/cs0600571. [DOI] [PubMed] [Google Scholar]
- Victor RG, Leimbach WN, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9:429–436. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- Weicker H. Determination of free and sulfoconjugated catecholamines in plasma and urine by high-performance liquid chromatography. Int J Sports Med. 1988;9(suppl. 2):68–74. doi: 10.1055/s-2008-1025619. [DOI] [PubMed] [Google Scholar]


