Abstract
Whether odorants are transduced by only one or more than one second messenger has been a long-standing question in olfactory research. In a previous study we started to address this question mainly by using calcium imaging in the olfactory bulb. Here, we present direct evidence for our earlier conclusions using the calcium imaging technique in the mucosa slice. The above question can now unambiguously be answered. We show that some olfactory receptor neurons (ORNs) respond to stimulation with amino acids with an increase of the intracellular calcium concentration [Ca2+]i. In order to see whether or not these responses were mediated by the cAMP transduction pathway we applied forskolin or the membrane-permeant cAMP analogue pCPT-cAMP to the olfactory epithelium. The ensemble of ORNs that was activated by amino acids markedly differed from the ensemble of neurons activated by forskolin or pCPT-cAMP. Less than 6 % of the responding ORNs showed a response to both amino acids and the pharmacological agents activating the cAMP transduction pathway. We conclude that ORNs of Xenopus laevis tadpoles have both cAMP-dependent and cAMP-independent olfactory transduction pathways and that most amino acids are transduced in a cAMP-independent way.
Odorants are the natural stimuli of olfactory receptor neurons (ORNs). Upon entering the nose they bind to olfactory receptors, and this signal is then amplified by a second messenger cascade. In many systems cAMP has been shown to be the second messenger (Gold, 1999). Knowledge of the molecular basis of other second messenger cascades in ORNs is still very heterogeneous and incomplete (for a review see Schild & Restrepo, 1998). While in ORNs of the lobster (Michel & Ache, 1994) and in the vomeronasal organs of rat (Inamura et al. 1997) and mouse (Holy et al. 2000; Stowers et al. 2002; Leypold et al. 2002) the existence of transduction mechanisms other than the cAMP-mediated one has been clearly shown, there are no studies, besides our recent work (Manzini et al. 2002b), that give clear and unambiguous evidence for cAMP-independent transduction in ORNs in the main olfactory epithelium of a vertebrate. With this paper we give direct evidence for our earlier conclusions and show, by imaging 1001 ORNs, that the transduction of most amino acids in the main olfactory epithelium of Xenopus laevis tadpoles is cAMP-independent.
In the past, studying odorant-dependent signal processing on individual ORNs, whether cAMP-dependent or not, using electrophysiological techniques has been inherently difficult owing to one major problem. Whenever recordings were taken from an ORN, its specificity was unknown, and finding an adequate high affinity stimulus for this ORN took longer than the cell could be held in the experiment.
We therefore employed calcium imaging of the Xenopus laevis tadpole olfactory epithelium slice. In this model the number of olfactory receptors (Mezler et al. 1999, 2001) and presumably also the number of potential stimuli, are smaller than in higher vertebrates. Furthermore, it is feasible to image many ORNs simultaneously, thereby increasing the response probability considerably. To determine whether amino acids, known to be potent stimuli in fish and amphibia (Caprio & Byrd, 1984; Restrepo et al. 1990; Friedrich & Korsching, 1997; Vogler & Schild, 1999; Iida & Kashiwayanagi, 1999; Sato & Suzuki, 2001; Lipschitz & Michel, 2002), are transduced by cAMP or not, we measured responses to amino acids, to forskolin and to pCPT-cAMP. Forskolin is known to activate adenylate cyclase thereby increasing the concentration of cAMP. pCPT-cAMP is a membrane-permeant analogue of cAMP.
Individual ORNs responding to both an amino acid and an activator of the cAMP transduction cascade could either transduce the amino acid stimulus through the cAMP cascade or there could be two transduction cascades as shown in the lobster (Michel & Ache, 1994). In the opposite case, in which an ORN responds to an amino acid but neither to forskolin nor to pCPT-cAMP, the amino acid must be transduced in a cAMP-independent way. The hypothesis of this study was to demonstrate such cAMP-independent olfactory transduction in Xenopus laevis tadpoles.
METHODS
Slice preparation for calcium imaging and patch-clamp recordings
Tadpoles of Xenopus laevis (stages 51 to 54; Nieuwkoop & Faber, 1956) were chilled in a mixture of ice and water and decapitated, as approved by the Göttingen University Committee for Ethics in Animal Experimentation. A block of tissue containing the olfactory mucosae, the olfactory nerves and the anterior two thirds of the brain was cut out and kept in bath solution (see below). The tissue was glued on to the stage of a vibroslicer (VT 1000S, Leica, Bensheim, Germany) and cut horizontally into 120-130 μm thick slices. Figure 1A shows a mucosa slice stained with biocytin-avidin by backfilling the receptor axons from the glomerular layer of the olfactory bulb. The slice was counterstained with propidium iodide (for staining procedures see Manzini et al. 2002a). For patch-clamp experiments the slices were placed under a grid in a recording chamber (Edwards et al. 1989) and viewed using Nomarski optics (Axioskop 2, Zeiss, Göttingen, Germany). For calcium imaging experiments the tissue slices were transferred to a recording chamber, and 200 μl of bath solution (see below) containing 50 μM fluo-4 AM (Molecular Probes, Leiden, The Netherlands) and 50 μM MK571 (Alexis Biochemicals, Grünberg, Germany) was added. The fluorescence of fluo-4 increases with increasing intracellular calcium concentration. Fluo-4 AM was dissolved in DMSO (Sigma, Deisenhofen, Germany) and Pluronic F-127 (Molecular Probes). ORNs of Xenopus laevis tadpoles express multidrug resistance transporters (Manzini & Schild, 2003) with a wide substrate spectrum, including calcium-indicator dyes. To avoid transporter mediated destaining of the slices, MK571, a specific inhibitor of the multidrug resistance-associated proteins (MRP, Gekeler et al. 1995; Abrahamse & Rechkemmer, 2001) was added to the incubation solution. After incubation on a shaker at room temperature for 1 h, the tissue slices were placed between two grids in a recording chamber to allow diffusion from both sides and placed on the microscope stage of an Axiovert 100M (Zeiss, Jena, Germany) to which a laser scanning unit (LSM 510, Zeiss, Jena, Germany) was attached. Before starting the calcium imaging experiments, the slices were rinsed with bath solution for at least 20 min.
Figure 1. Slice of the olfactory epithelium of a Xenopus laevis tadpole and amino acid-induced [Ca2+]i increases in individual ORNs in a mucosa slice.
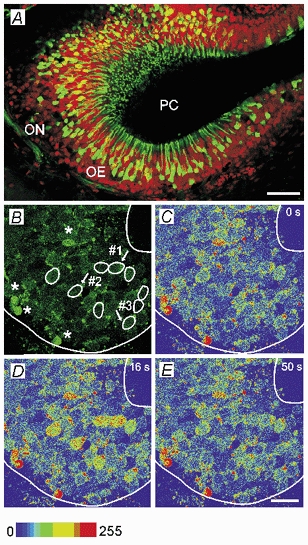
A, overview of a horizontal slice of the olfactory epithelium of a Xenopus laevis tadpole (stage 52, PC, principal cavity, OE, olfactory epithelium and ON, olfactory nerve). The neurons were backfilled through the nerve using biocytin-avidin staining (green fluorescence), and then the slice was counterstained with propidium iodide (red fluorescence). B, fluorescence image of a mucosa slice (stage 52, image acquired at rest) stained with fluo-4. Amino acid-sensitive ORNs are encircled. * ORNs showing high basal fluorescence levels at rest. The responses to amino acids of the ORNs indicated by arrows are shown in Fig. 2. C-E, sequence of three pseudocoloured images of the slice showing that stimulation with a mixture of amino acids (200 μM, each) transiently increases calcium-dependent fluorescence in the ORNs encircled in B. C, before the application of the amino acid mixture (time (t) 0 s). D, at the peak of the response (t, 16 s) and E, after return to the basal fluorescence level (t, 50 s). Scale bars: 50 μM in A and 20 μM in B-E.
Calcium imaging of odour responses
Intracellular calcium was monitored using a laser-scanning confocal microscope (Zeiss LSM 510/Axiovert 100M, Jena, Germany). The confocal pinhole was about 80 μm and excluded fluorescence detection from more than one cell layer. Fluorescence images (excitation at 488 nm; emission > 505 nm) of the olfactory mucosa were acquired at 0.25-1.27 Hz and 786, 4 ms exposure time per image with 3 to 5 images taken as control images before the onset of odour delivery. The fluorescence changes ΔF/F were calculated for individual ORNs as ΔF/F = (F1 - F2)/F2, where F1 was the fluorescence averaged over the pixels of an ORN, while F2 was the average fluorescence of that ORN prior to stimulus application, averaged over three images. Background intensity was zero.
Patch-clamp recordings
Patch electrodes with a tip diameter of 1-2 μm and approximately 7-10 MΩ resistance were fabricated from borosilicate glass with 1.8 mm outer diameter (Hilgenberg, Malsfeld, Germany) using a two-stage electrode puller (Narishige, Tokyo, Japan) and fire polished. Pulse protocols, data acquisition and evaluation programs were written in ‘C’. Pulses were delivered from a microcontroller (Schild et al. 1996) to a D/A converter and to the patch-clamp amplifier (EPC7; List, Darmstadt, Germany). Holding voltage in the on-cell configuration was 0 mV, while in whole-cell recordings, it was set to -80 mV. Currents and voltages were recorded on video tape using a PCM unit (Instrutech, Elmont, NY, USA). The data were digitized off-line using an 8-pole Bessel filter, an A/D converter and a PC. Further data analyses were performed on a PC under the LINUX operating system.
Solutions and stimulus application
The composition of the bath solution was (mM): 98 NaCl, 2 KCl, 1 CaCl2, 2 MgCl2, 5 glucose, 5 sodium pyruvate, 10 Hepes. The pipette solution used for whole-cell recordings contained (mM): 2 NaCl, 2 MgCl2, 44 CsMeSO3, 40 CsCl, 10 Hepes, 0.2 EGTA, 2 Na2-ATP, 0.1 Na2-GTP. The pipette solution used for on-cell recordings contained (mM): 2 NaCl, 47 KCl, 2 MgCl2, 43 potassium gluconate, 10 Hepes, 0.2 EGTA, 2 Na2-ATP, 0.1 Na2-GTP. The pH of all solutions was adjusted to 7.8. This is the physiological pH in this poikilothermal species (Howell et al. 1970). Osmolarities of the bath and pipette solutions were 230 and 190 mosmol (l solution)−1, respectively. As odorants, we used the amino acids (Sigma, listed in Table 1) applied either as a mixture of 19 amino acids (AA), as submixtures, or as single amino acids. The amino acids were dissolved in bath solution (10 mM stock, each) and used at a final concentration of 200 μM in all the experiments. Forskolin (Sigma) was dissolved in DMSO (stock of 10 mM) and used at a final concentration of 50 μM. 8-(4-Chlorophenylthio) adenosine 3′, 5′-cyclic monophosphate (pCPT-cAMP, Sigma) was dissolved in bath solution (stock of 10 mM) and used at a final concentration of 2.5 mM. Tetrodotoxin (TTX, 2 μM, Molecular Probes) was dissolved in the bath solution where indicated. Stimulus solutions were prepared immediately before use by dissolving the respective stock solution in bath solution. The bath solution was applied by gravity feed from a storage syringe through a funnel drug applicator (Schild, 1985) to the recording chamber. The flow was 350 μl min−1. Odorants were pipetted directly into the funnel without stopping the flow. Outflow was through a syringe needle placed close to the mucosa to ensure that odorant molecules were removed rapidly. The minimum interstimulus interval between odorant applications was at least 2 min.
Table 1.
Water-soluble mixtures of l-amino acids
| Mixture | Amino acids |
|---|---|
| LCN | proline, valine, leucine, isoleucine, methionine |
| SCN | glycine, alanine, serine, threonine, cysteine, asparagine, glutamine |
| BAS | arginine, lysine, histidine |
| ACID | glutamate, aspartate |
| AROM | tryptophan, phenylalanine |
| AA | LCN, SCN, BAS, ACID and AROM |
Mixtures of L-amino acids following Caprio & byrd, (1984): LCN, long chain neutral amino acids; SCN, short chain neutral amino acids; BAS, basic amino acids; ACID, acidic amino acids and AROM, aromatic amino acids.
The dilution of the stimulus within the funnel was less than 1 %. In the mucosa the dilution of the stimulus was determined by putting a confocal volume (approximately 1 fl (1 × 10−15 l) of a laser-scanning confocal microscope (Zeiss LSM 510 and/or Axiovert 100, Jena, Germany), firstly, in front of the funnel outlet and, secondly, in front of the epithelial surface and measuring the respective fluorescences. For this control measurement we used the fluorescent probe tetramethylrhodamine (TMR 500 nM, Sigma) as a ‘dummy stimulus’. The dilution factor was 0.91 ± 0.02 (mean ± S.D., n = 7). The delay between TMR leaving the funnel outlet and reaching the mucosal surface was less than 1 s and after the end of stimulation, TMR was completely rinsed from the mucosa within 15 s.
RESULTS
We performed calcium imaging experiments on 52 slices of the Xenopus laevis tadpole olfactory mucosa (Fig. 1A). Figure 1B shows cells of the olfactory epithelium stained with the calcium-indicator dye fluo-4. Some of the cells had a high fluorescence from the beginning (asterisks) presumably because they did not survive the tissue slicing. Such cells were discarded from further evaluation. Apart from these cells, all ORNs in a slice responded upon application of KCl (100 mM) with a transient increase of fluorescence. On the other hand, upon application of bath solution as a negative control stimulus no response was ever detected (data not shown). The encircled ORNs were responsive to a mixture of 19 amino acids (AA) as seen from the increase of the intracellular calcium concentration [Ca2+]i in Fig. 1C-E. After an interstimulus interval of 2 min we applied the five submixtures of amino acids (LCN, SCN, BAS, ACID and AROM, see Table 1 for explanation of these) and subsequently the 19 single amino acids, one after another. All the responses shown throughout this paper were repeatable for any given ORN. In most preparations, the reproducibility of responses was ascertained by applying some stimuli at least twice.
Figure 2 shows the selectivities of the three ORNs marked with an arrow in Fig. 1B. ORN 1 (Fig. 2A) responded only to L-glycine and the corresponding submixture of short chain neutral amino acids. ORN 2 (Fig. 2B) responded to L-methionine and, though slightly weaker, to L-isoleucine, as well as to the corresponding long chain neutral submixture. It also responded to L-alanine and to L-arginine, as well as to the corresponding short chain neutral and basic submixtures. The third ORN (Fig. 2C) responded to the eight amino acids L-leucine, L-methionine, L-cysteine, L-alanine, L-threonine, L-arginine, L-histidine and L-tryptophan, whereby the response to L-histidine was small but clearly correlated to the stimulus onset. We did not try to assess any concentration dependence of the responses, nor did we attempt a thorough classification of amino acid responses. Instead we concentrated on the questions (i) how the stimulus-induced increases in [Ca2+]i were related to activity, and (ii) whether the responses were mediated by the cAMP-mediated transduction pathway. The odorant-induced [Ca 2+]i increase could be brought about either by the spiking activity of the ORN and concomitant activation of high-voltage activated calcium channels (Schild & Restrepo, 1998), or by calcium influx through calcium-permeable ion channels, directly or indirectly activated by the odorants, but independent from spiking. Obviously, both effects could overlap.
Figure 2. Amino acid-induced changes in calcium-dependent fluorescence of three individual ORNs in a mucosa slice.

A, time course of [Ca2+]i transients of ORN 1 (see Fig. 1B) evoked by the application of amino acids. The traces show responses to the
mixture of 19 amino acids (AA), to the mixture of short chain neutral amino acids (SCN) and to L-glycine. No response to the mixtures of the long chain neutral (LCN), the basic (BAS), the aromatic (AROM) and the acidic (ACID) amino acids. No response to the remaining single amino acids of the SCN mixture. B, ORN 2 (see Fig. 1B) responded to the mixture of AA, the mixture of LCN, to L-methionine and, though slightly weaker, to L-isoleucine, the mixture of SCN, to L-alanine, the mixture of BAS and to L-arginine. No response to the mixtures AROM or ACID, nor to the remaining single amino acids of the responsive groups. C, ORN 3 (see Fig. 1B) responded to the mixtures of AA and LCN; to L-leucine and L-methionine the mixture of SCN; to L-cysteine, L-alanine and L-threonine the mixture of BAS; to L-arginine and, though slightly weaker, to L-histidine the mixture of AROM and to L-tryptophan. No response to the ACID mixture, nor to the remaining single amino acids of the responsive mixtures. All amino acids were applied at a concentration of 200 μM.
Figure 3A-C show that the odorant-induced [Ca2+]i increase in an ORN upon stimulation with an amino acid is reduced but not completely blocked by 2 μM TTX. This evidence was taken in one mucosa slice and confirmed in 59 ORNs in other mucosa slices (Fig. 3D). The reduction of odorant-induced [Ca 2+]i increase under TTX varied between approximately 18.6 and 93.8 % (mean 59.7 %). On the other hand, the same concentration of TTX completely blocked spike-associated currents measured in ORNs of the mucosa slice using the on-cell configuration of the patch-clamp technique (Fig. 3E) and the voltage-gated Na+ current in the whole-cell configuration of the patch-clamp technique (Fig. 3F and G). The evidence shown in Fig. 3E, F and G was confirmed in six and seven ORNs, respectively. Taken together, these experiments indicate that odorant-induced spiking on the one hand and transduction channels on the other contribute in a varying manner to the overall [Ca2+]i increase of the ORNs after stimulation with amino acids.
Figure 3. Influence of TTX on odorant-induced [Ca2+]i transients, spike-associated currents and sodium currents of Xenopus laevis tadpole ORNs.
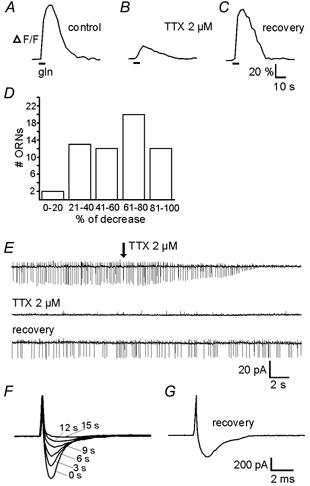
A, L-glutamine (200 μM) -induced [Ca2+]i transient of an individual ORN (stage 53) of a mucosa slice. B, 5 min after the addition of 2 μM TTX to the bath solution the L-glutamine-induced [Ca2+]i transient was clearly smaller but still present. With TTX the slope of the transient was smaller. C, after a wash-out time of 12 min the L-glutamine-induced [Ca2+]i transient recovered completely. D, relative decrease of odorant-induced [Ca2+]i transients after addition of TTX (2 μM) to the bath solution plotted as a histogram (n = 59 ORNs). E, current traces showing spike-associated currents of an ORN (stage 54) of a mucosa slice recorded in the on-cell configuration of the patch-clamp technique. Less than 15 s after the addition of TTX (2 μM) to the bath solution (see arrow in the upper trace) the spike-associated currents are completely blocked. As long as TTX was present in the bath solution the spike-associated currents did not recover (middle trace). Thirteen minutes after the beginning of wash-out the spike-associated currents are almost completely recovered (bottom trace). F, voltage-activated sodium currents of an ORN in the slice preparation recorded in the voltage-clamp configuration of the patch-clamp technique. To block potassium currents, an intracellular solution containing caesium instead of potassium was used (see solutions in Methods). The holding potential was -80 mV. The current responses were evoked by depolarizing voltage steps to -30 mV given every 3 s. After the first depolarizing step TTX (2 μM) was added to the bath solution. 15 s after the beginning of TTX application the current was completely blocked. G, recovery of the current after 9 min of wash-out.
The main result of this study is demonstrated in Fig. 4. In order to stimulate all ORNs in a slice that would, under appropriate natural conditions, respond to a cAMP-mediated odour, we applied forskolin as a pseudostimulus to the olfactory mucosa. Imaging of the mucosa revealed the ORNs that responded with an increase in [Ca2+]i (sequence of images shown in Fig. 4A-C). Responsive ORNs are encircled in Fig. 4B. To confirm this result, and to rule out the possibility of forskolin acting through some other cAMP-independent mechanism, in five slices we also applied the membrane-permeant cAMP analogue pCPT-cAMP in addition to forskolin, one of them being the slice shown in Fig. 4. The set of ORNs responding to pCPT-cAMP (sequence of images shown in Fig. 4D-F, responsive ORNs are encircled in Fig. 4E) was virtually identical to that responding to forskolin. In the five slices tested for both forskolin and pCPT-cAMP, all the 56 ORNs responsive to forkolin also responded to pCPT-cAMP. Seven additional ORNs were responsive only to pCPT-cAMP. In order to stimulate all ORNs in the same slice that responded to one or more amino acids, we applied the mixture of 19 amino acids (sequence of images shown in Fig. 4G-I). Responsive ORNs are encircled in Fig. 4H. Figure 4J summarizes these results by giving the forskolin and pCPT-cAMP-sensitive ORNs in green and the amino acid-sensitive ORNs in red. In this slice, there was just one ORN that responded to all three of the stimuli (shown in yellow). We repeated the sequential application of amino acids and forskolin in 44 slices. The two kinds of stimuli always activated different sets of neurons, with little overlap. In all the slices tested, 1001 ORNs responded either to amino acids or to forskolin (Fig. 4K). Five hundred and three ORNs responded to the mixture of amino acids and 498 to forskolin. Fifty-four ORNs (less than 6 %) responded to both stimuli.
Figure 4. Comparison of changes of calcium-dependent fluorescence in olfactory receptor neurons of a mucosa slice in response to stimulation with forskolin, pCPT-cAMP and amino acids.
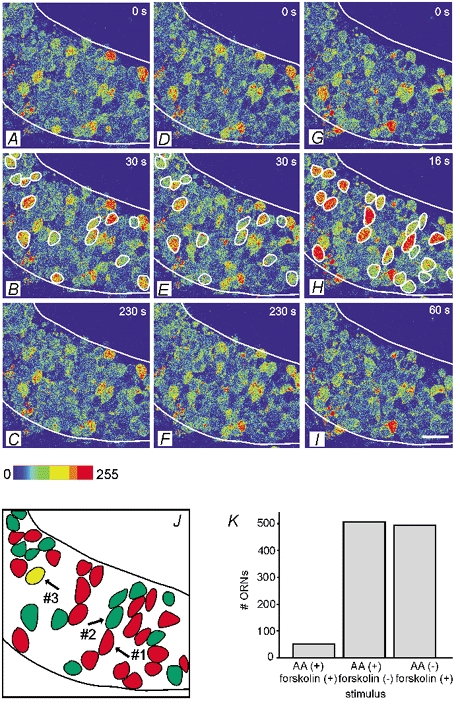
Sequences of pseudocoloured images of a mucosa slice (stage 53) showing that stimulation with forskolin (50 μM, A-C), pCPT-cAMP (2.5 mM, D -F) and the mixture of amino acids (200 μM, each, G-I) transiently elevates calcium-dependent fluorescence in two different ensembles of ORNs (encircled in B, E and H). The upper images show the fluorescence images before application (t 0 s), the images in the middle at the peak of the response (t 30 s in B and E, t 16 s in H) and the bottom images after return to the basal fluorescence levels (t 230 s in C and F, t 60 s in I). J, schematic diagram showing superimposition of the forskolin and pCPT-cAMP-sensitive ORNs and the amino acid-sensitive ORNs (encircled in B, E and H). ORNs sensitive to forskolin and pCPT-cAMP (green), to amino acids (red) and both (yellow). Only one ORN showed a response to both stimuli. The responses of the ORNs indicated with arrows are shown in Fig. 5. K, occurences of correlated and uncorrelated responses to forskolin and amino acids plotted as a histogram (n = 44 slices). Scale bar 20 μM.
The two classes of stimuli used, i.e. amino acids on the one hand and forskolin or pCPT-cAMP on the other, also differed in the time course of their respective responses. ORNs responding to the amino acid mixture always had a fast time course (e.g. ORN 1 marked with an arrow in Fig. 4J) and responded neither to forskolin nor to pCPT-cAMP (Fig. 5A). ORNs responding to forskolin and/or pCPT-cAMP (e.g. ORN 2 marked with an arrow in Fig. 4J) typically showed a much slower time course (Fig. 5B), but no response to amino acids. Only in a few cases could an increase in [Ca2+]i be observed upon stimulation with all three kinds of stimuli (e.g. ORN 3 marked with an arrow in Fig. 4J; see Fig. 5C). The average time to peak of the [Ca2+]i transients was 10.1, 19.3 and 19.4 s for responses to amino acids, forskolin and pCPT-cAMP, respectively (n = 25 ORNs), while the average duration of the [Ca2+]i transients was 39.2 s for responses to amino acids, and 174.2 and 128.7 s for those to forskolin and pCPT-cAMP (n = 50 ORNs).
Figure 5. Time courses of calcium-dependent fluorescence changes in ORNs upon stimulation with amino acids, forskolin and pCPT-cAMP.
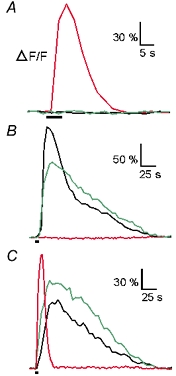
A, ORN 1 (see Fig. 4J) was responsive to L-asparagine (red trace) but insensitive to forskolin (green trace) and pCPT-cAMP (black trace). B, ORN 2 (see Fig. 4J) was responsive to forskolin and pCPT-cAMP (green and black trace, respectively) but insensitive to the mixture of amino acids (red trace). C, ORN 3 (see Fig. 4J) responded to all three stimuli applied (L-alanine, red trace; forskolin, green trace and pCPT-cAMP, black trace).Amino acids, forskolin and pCPT-cAMP were applied at a concentration of 200, 50 and 2.5 mM, respectively.
DISCUSSION
One of the first papers on olfactory transduction (in frog and rat, Sklar et al. 1986) showed that some odorants increased the concentration of cAMP in cilia of ORNs while others did not. Several reports in the rat have shown that odours sometimes also cause formation of inositol-1, 4,5-trisphosphate (IP3) (Boekhoff et al. 1990; Breer & Boekhoff 1991; Ronnett et al. 1993). Other studies, however, were unable to confirm a significant contribution of IP3 to the odour response in ORNs (bullfrog, Lowe et al. 1989; mouse, Brunet et al. 1996; Belluscio et al. 1998; tiger salamander, Chen et al. 2000). This long-standing controversy on whether olfactory transduction is mediated by one second messenger, cAMP, or by more than one has been going on until today. Over the last decade many investigators have reported pieces of evidence indicating an involvement of second messengers other than cAMP in olfactory transduction, yet there is no species in which all elements of a non-cAMP transduction cascade have been found. Taken together, the cAMP pathway in ORNs is very well documented, while the evidence for alternative pathways is still heterogeneous and not definitive. The interpretation of the currently available data ranges from an exclusive role for cAMP in olfactory transduction to a mediatory role for cAMP and a modulatory role for other second messengers, to a multiple mediation of olfactory transduction by different second messengers even within the same ORN (see reviews by Schild & Restrepo, 1998; Zufall & Munger, 2001 and references therein).
In our study we did not attempt to characterize any cAMP-independent transduction mechanism. We rather answered the simpler question, whether or not a particular ORN sensitive to a certain known odorant is also sensitive to pharmacological agents activating the cAMP-mediated tranduction pathway. We imaged odorant responses in a slice of the olfactory epithelium using the calcium-indicator dye fluo-4. In this way it could easily be established which ORNs of a slice responded to a particular stimulus. We then applied forskolin, an activator of the cAMP-dependent pathway. We chose forskolin because it had been successfully used to the same end in a number of previous studies (Frings & Lindemann, 1991; Kashiwayanagi et al. 1996) clearly demonstrating its efficacy to stimulate the adenylate cyclase in ORNs. To rule out the possibility that, in addition to or instead of its effect upon the adenylate cyclase, forskolin might act as an odorant or through some other mechanism, in some slices we also applied pCPT-cAMP. The efficacy of pCPT-cAMP in ORNs has also been shown previously (Kashiwayanagi & Kurihara, 1995; Reisert & Matthews, 2001).
In the mucosa slices tested for both drugs the ORNs activated by forskolin were also activated by pCPT-cAMP. Thus, both drugs activated the cAMP-dependent transduction pathway equally well. Moreover, pCPT-cAMP activated a few ORNs that were not activated by forskolin. In these cases pCPT-cAMP might have acted, in addition to its effect as a second messenger, as an odorant. This is in line with a previous report showing that nucleotides are important olfactory stimuli in fish (Kang & Caprio, 1995). Furthermore, it has been shown that a subset of ORNs in the olfactory epithelium of rat express no adenylate cyclase, but do express a guanylate cyclase together with a cyclic nucleotide-gated channel (Meyer et al. 2000). If a subset of ORNs in Xenopus laevis had these properties this could be a further explanation for the fact that there were slightly more responses to pCPT-cAMP than to forskolin.
The main observation of this paper is that amino acids in most cases failed to activate forskolin- or pCPT-cAMP-sensitive ORNs. Our overall conclusion is therefore that most amino acids are not transduced via a cAMP-dependent pathway in ORNs of Xenopus laevis tadpoles. This results seem to be in conflict with other studies which have shown that blocking fundamental steps of the cAMP-mediated transduction pathway eliminates olfactory transduction (tiger salamander, Chen et al. 2000; mouse, Brunet et al. 1996; Belluscio et al. 1998; Wong et al. 2000).
One possible explanation of these diverging results could be that we used a different species. Furthermore, we used different stimuli. It has been shown in zebra fish that the transduction of amino acid odorants does not rely primarily on cyclic nucleotide-gated channels (Michel, 1999). In a recent study Michel's group has shown that amino acids stimulate microvillar but not ciliated ORNs in the olfactory epithelium of zebra fish (Lipschitz & Michel, 2002). In the olfactory epithelium of Xenopus laevis both ciliated and microvillar ORNs have been shown (Hansen et al. 1998). Here the correlation with certain stimuli is not yet clear, but microvillar ORNs are certainly good candidates for amino acid-sensitive cells. This would parallel studies in ORNs of the vomeronasal organ where it has been shown that microvillar ORNs possess cAMP-independent transduction pathways (Wekesa & Anholt, 1997; Liman et al. 1999).
Our results are related to a number of points, some of which we have begun to investigate. First, given the two almost disjunct sets of cAMP- and non-cAMP-dependent ORNs, how do the respective sets of ORNs project to the olfactory bulb? We have addressed this question in a parallel study finding quite different projection areas in the olfactory bulb (Manzini et al. 2002b). Second, what are the second messenger pathways through which amino acids are transduced? Third, are amino acids transduced in microvilli rather than in cilia? These questions were beyond the scope of this study and we have, as yet, no answer to them. There is a strong probability that combining patch-clamp and imaging experiments will give some insight here.
REFERENCES
- Abrahamse SL, Rechkemmer G. Identification of an organic anion transport system in the human colon carcinoma cell line HT29 clone 19A. Pflugers Arch. 2001;441:529–537. doi: 10.1007/s004240000437. [DOI] [PubMed] [Google Scholar]
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- Boekhoff I, Tareilus E, Strotmann J, Breer H. Rapid activation of alternative second messenger pathways in olfactory cilia from rats by different odorants. EMBO J. 1990;9:2453–2458. doi: 10.1002/j.1460-2075.1990.tb07422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breer H, Boekhoff I. Odorants of the same odor class activate different second messenger pathways. Chem Senses. 1991;16:19–29. [Google Scholar]
- Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–693. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- Caprio J, Byrd RP. Electrophysiological evidence for acidic, basic, and neutral amino acid olfactory sites in the catfish. J Gen Physiol. 1984;84:403–422. doi: 10.1085/jgp.84.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Lane AP, Bock R, Leinders-Zufall T, Zufall F. Blocking adenylyl cyclase inhibits olfactory generator currents induced by ‘IP(3)-odors’. J Neurophysiol. 2000;84:575–580. doi: 10.1152/jn.2000.84.1.575. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch-clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Frings S, Lindemann B. Current recording from sensory cilia of olfactory receptor cells in situ. I. The neuronal response to cyclic nucleotides. J Gen Physiol. 1991;97:1–16. doi: 10.1085/jgp.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekeler V, Ise W, Sanders KH, Ulrich WR, Beck J. The leukotriene LTD4 antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem Biophys Res Commun. 1995;208:345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- Gold GH. Controversial issues in vertebrate olfactory transduction. Annu Rev Physiol. 1999;61:857–871. doi: 10.1146/annurev.physiol.61.1.857. [DOI] [PubMed] [Google Scholar]
- Hansen A, Reiss JO, Gentry CL, Burd GD. Ultrastructure of the olfactory organ in the clawed frog, Xenopus laevis, during larval development and metamorphosis. J Comp Neurol. 1998;398:273–288. doi: 10.1002/(sici)1096-9861(19980824)398:2<273::aid-cne8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Holy TE, Dulac C, Meister M. Responses of vomeronasal neurons to natural stimuli. Science. 2000;289:1569–1572. doi: 10.1126/science.289.5484.1569. [DOI] [PubMed] [Google Scholar]
- Howell BJ, Baumgardner FW, Bondi K, Rahn H. Acid-base balance in cold-blooded vertebrates as a function of body temperature. Am J Physiol. 1970;218:600–606. doi: 10.1152/ajplegacy.1970.218.2.600. [DOI] [PubMed] [Google Scholar]
- Inamura K, Kashiwayanagi M, Kurihara K. Blockage of urinary responses by inhibitors for IP3 -mediated pathway in rat vomeronasal neurons. Neurosci Lett. 1997;233:129–132. doi: 10.1016/s0304-3940(97)00655-1. [DOI] [PubMed] [Google Scholar]
- Iida A, Kashiwayanagi M. Responses of Xenopus laevis water nose to water-soluble and volatile odorants. J Gen Physiol. 1999;114:85–92. doi: 10.1085/jgp.114.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Caprio J. In vivo responses of single olfactory receptor neurons in the channel catfish, Ictalurus punctatus. J Neurophysiol. 1995;73:172–177. doi: 10.1152/jn.1995.73.1.172. [DOI] [PubMed] [Google Scholar]
- Kashiwayanagi M, Kawahara H, Kanaki K, Nagasawa F, Kurihara K. Ca2+ and Cl(-)-dependence of the turtle olfactory response to odorants and forskolin. Comp Biochem Physiol A Physiol. 1996;115:43–52. doi: 10.1016/0300-9629(95)02139-6. [DOI] [PubMed] [Google Scholar]
- Kashiwayanagi M, Kurihara K. Odor responses after complete desensitation of the cAMP-dependent pathway in turtle olfactory cells. Neurosci Lett. 1995;193:61–64. doi: 10.1016/0304-3940(95)11667-l. [DOI] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Corey DP, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci U S A. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipschitz DL, Michel WC. Amino acids odorants stimulate microvillar sensory neurons. Chem Senses. 2002;27:277–286. doi: 10.1093/chemse/27.3.277. [DOI] [PubMed] [Google Scholar]
- Lowe G, Nakamura T, Gold GH. Adenylate cyclase mediates olfactory transduction for a wide variety of odorants. Proc Natl Acad Sci U S A. 1989;86:5641–5645. doi: 10.1073/pnas.86.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini I, Peters F, Schild D. Odorant responses of Xenopus laevis tadpole olfactory neurons: a comparison between preparations. J Neurosci Methods. 2002a;121:159–167. doi: 10.1016/s0165-0270(02)00248-0. [DOI] [PubMed] [Google Scholar]
- Manzini I, Rössler W, Schild D. cAMP-independent responses of olfactory neurons in Xenopus laevis tadpoles and their projection onto olfactory bulb neurons. J Physiol. 2002b;545:475–484. doi: 10.1113/jphysiol.2002.031914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzini I, Schild D. Multidrug resistance transporters in the olfactory receptor neurons of Xenopus laevis tadpoles. J Physiol. 2003;546:375–385. doi: 10.1113/jphysiol.2002.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezler M, Fleischer J, Breer H. Characteristic features and ligand specificity of the two olfactory receptor classes from Xenopus laevis. J Exp Biol. 2001;204:2987–2997. doi: 10.1242/jeb.204.17.2987. [DOI] [PubMed] [Google Scholar]
- Mezler M, Konzelmann S, Freitag J, Rössler P, Breer H. Expression of olfactory receptors during development in Xenopus Laevis. J Exp Biol. 1999;202:365–376. doi: 10.1242/jeb.202.4.365. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Angele A, Kremmer E, Kaupp UB, Muller F. A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc Natl Acad Sci U S A. 2000;97:10595–10600. doi: 10.1073/pnas.97.19.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel WC. Cyclic nucleotide-gated channel activation is not required for activity-dependent labeling of zebrafish olfactory receptor neurons by amino acids. Biol Signals Recept. 1999;8:338–347. doi: 10.1159/000014607. [DOI] [PubMed] [Google Scholar]
- Michel WC, Ache BW. Odor-evoked inhibition in primary olfactory receptor neurons. Chem Senses. 1994;19:11–24. doi: 10.1093/chemse/19.1.11. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Amsterdam: North Holland; 1956. Normal Table of Xenopus laevis (Daudin) [Google Scholar]
- Reisert J, Matthews HR. Responses to prolonged odour stimulation in frog olfactory receptor cells. J Physiol. 2001;534:179–191. doi: 10.1111/j.1469-7793.2001.t01-1-00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo D, Miyamoto T, Bryant BP, Teeter JH. Odor stimuli trigger influx of calcium into olfactory neurons of the channel catfish. Science. 1990;249:1166–1168. doi: 10.1126/science.2168580. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Cho H, Hester LD, Wood SF, Snyder SH. Odorants differentially enhance phosphoinositide turnover and adenylyl cyclase in olfactory receptor neuronal cultures. J Neurosci. 1993;13:1751–1758. doi: 10.1523/JNEUROSCI.13-04-01751.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Suzuki N. Whole-cell response characteristics of ciliated and microvillous olfactory receptor neurons to amino acids, pheromone candidates and urin in rainbow trout. Chem Senses. 2001;26:1145–1156. doi: 10.1093/chemse/26.9.1145. [DOI] [PubMed] [Google Scholar]
- Schild D. A computer-controlled device for the application of odours to aquatic animals. J Electrophysiol Techn. 1985;12:71–79. [Google Scholar]
- Schild D, Gennerich A, Schultens HA. Microcontrollers as inexpensive pulse generators and parallel processors in electrophysiological experiments. Med Biol Eng Comput. 1996;34:305–307. doi: 10.1007/BF02511243. [DOI] [PubMed] [Google Scholar]
- Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev. 1998;78:429–466. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- Sklar PB, Anholt RR, Snyder SH. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986;261:15538–15543. [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Vogler C, Schild D. Inhibitory and excitatory responses of olfactory receptor neurons of Xenopus laevis tadpoles to stimulation with amino acids. J Exp Biol. 1999;202:997–1003. doi: 10.1242/jeb.202.8.997. [DOI] [PubMed] [Google Scholar]
- Wekesa KS, Anholt RR. Pheromone regulated production of inositol-(1, 4, 5)-trisphosphate in the mammalian vomeronasal organ. Endocrinology. 1997;138:3497–3504. doi: 10.1210/endo.138.8.5338. [DOI] [PubMed] [Google Scholar]
- Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Zufall F, Munger SD. From odor and pheromone transduction to the organization of the sense of smell. Trends Neurosci. 2001;24:191–193. doi: 10.1016/s0166-2236(00)01765-3. [DOI] [PubMed] [Google Scholar]


