Abstract
Oesophageal peristalsis is controlled by vagal motor neurones, and intrinsic neurones have been identified in the striated muscle oesophagus. However, the effect(s) of intrinsic neurones on vagally mediated contractions of oesophageal striated muscles has not been defined. The present study was designed to investigate the role of intrinsic neurones on vagally evoked contractions of oesophageal striated muscles, using hamster oesophageal strips maintained in an organ bath. Stimulation (30 μs, 20 V) of the vagus nerve trunk produced twitch contractions. Piperine inhibited vagally evoked contractions, while capsaicin and NG-nitro-L-arginine methyl ester (L-NAME) abolished the inhibitory effect of piperine. The effect of L-NAME was reversed by subsequent addition of L-arginine, but not by D-arginine. L-NAME did not have any effect on the vagally mediated contractions and presumed 3H-ACh release. NONOate, a nitric oxide donor, and dibutyryl cyclic GMP inhibited twitch contractions. Inhibition of vagally evoked contractions by piperine and NONOate was fully reversed by ODQ, an inhibitor of guanylate cyclase. Immunohistochemical staining showed immunoreactivity for nitric oxide synthase (NOS) in nerve cell bodies and fibres in the myenteric plexus and the presence of choline acetyltransferase and NOS in the motor endplates. Only a few NOS-immunoreactive portions in the myenteric plexus showed vanilloid receptor 1 (VR1) immunoreactivity. Our results suggest that there is a local neural reflex that involves capsaicin-sensitive neurones, nitrergic myenteric neurones and vagal motor neurones.
Disturbances of the enteric nervous system are implicated in disorders of oesophageal peristalsis, such as megaoesophagus in dog striated muscle oesophagus. However, to date, our understanding of the enteric nervous system that controls oesophageal peristalsis is incomplete. In the striated muscle segment of the oesophagus, chemical and mechanical stimulation of the mucosa can initiate a peristaltic reflex in vivo (Broussard et al. 1998; Jean, 2001). It has been demonstrated that oesophageal peristalsis is controlled by vagal motor neurones (Andrew, 1956; Mukhopadhyay & Weisbrodt, 1975; Park & Conklin, 1999), and intrinsic neurones have been identified in the striated muscle segment of the dog (Hudson & Cummings, 1985) and rat (Neuhuber et al. 1994; Wörl et al. 1998; Kuramoto et al. 1999) oesophagus. However, the possible effect(s) of intrinsic neurones on vagally mediated contractions of oesophageal striated muscles has not been defined.
Inhibitory non-adrenergic, non-cholinergic innervation, including nitrergic neurones, has been observed within the intrinsic nervous system of the rat oesophagus (Neuhuber et al. 1994; Wörl et al. 1994). However, the role of nitrergic neurones in vagally evoked oesophageal striated muscle contraction is not fully characterized. The distribution of sensory neurones has been mapped using capsaicin and piperine, a polypeptide component of black and long peppers (Izzo et al. 2001). Capsaicin- and piperine-sensitive sensory neurones can modulate intestinal motility as they convey signals and may simultaneously release transmitters that can influence the activity of intrinsic neurones (Holzer et al. 1987).
The purpose of the present study was to investigate the role of intrinsic nitrergic neurones on vagally evoked contractions of the striated muscle segment of the hamster oesophagus.
METHODS
Animals
Male Syrian hamsters (110-130 g) were maintained in temperature-controlled rooms and the experiments were performed in accordance with the Gifu University Animal Care and Use Committee and Japanese Department of Agriculture guidelines.
Tissue preparation
Animals were anaesthetized with sodium pentobarbital (60 mg kg−1, I.P.). Following exsanguination through the axillary arteries, the whole oesophagus of the hamster was dissected out and excised from the larynx to the level of the diaphragm, together with the two attached vagi. The 4-5 cm segment of the oesophagus was immediately immersed in physiological salt solution (PSS; see below) at room temperature and the contents of the excised segment were flushed by a small cannula containing PSS.
Recording of mechanical activity
The whole segment was transferred to a 2 ml thermostatically controlled (35 °C) organ bath containing PSS bubbled with 95 %O2-5 %CO2 gas mixture, maintained at pH 7.0. The tissue was mounted between two L-shaped hooks by means of silk ligatures. One was connected to an isometric force transducer for the measurement of isometric tension; the other was fixed to the hook to allow adjustment of tension. The mechanical responses were recorded isometrically on a pen recorder chart through an AC amplifier (AS1202, NEC, Tokyo, Japan). During the 90 min equilibration period, the resting tension was adjusted to about 1 g.
Electrical stimulation
In experiments using vagal stimulation, the end of the nerve trunk was drawn into a bipolar suction electrode and the electrode was immersed together with the oesophagus preparation in the bath. Transmural stimulation could also be applied through a pair of platinum electrodes placed one on either side of the tissue. The vagus nerve and tissue were stimulated using an electronic stimulator (model SEN-3301, Nihon Kohden, Tokyo, Japan) connected to the electrodes. Trains of square-wave pulses of 30 μs duration for stimulation of vagus nerves and of 10 ms duration for stimulation of muscle were applied at 1 min intervals and supramaximal intensity. The vagus nerve was stimulated before and after incubation with the test drug.
Tissue preparation and collection of superfusate for acetylcholine release
Tissues were excised and suspended in the organ bath as described above. They were incubated in 3H-choline (see below for optimal conditions) and then rinsed in choline-free PSS before suspension in the stimulus apparatus as follows. Using a peristaltic pump, tissues were superfused at an initial rate of 3 ml min−1 for the first 20 min. This removed any extracellular 3H-choline or tritiated derivatives of 3H-choline released. The superfusate flow rate was then reduced to 1 ml min−1 for the remainder of the experiment.
Superfusion samples were collected in tubes in a fraction collector (Iwaki, Tokyo, Japan) at 4 min intervals. Samples were vortexed and 0.5 ml aliquots were placed in scintillation vials containing 4.5 ml of Ready Safe liquid scintillation cocktail (Beckman Coulter, CA, USA). 3H levels were determined by counting samples for 10 min in a scintillation counter at 53 % efficiency.
Experimental conditions for acetylcholine release
Optimal incubation conditions were determined by evaluating 3H release during vagal stimulation at 50 Hz, 30 μs, supramaximal voltage after incubation in 3H-choline (1.98, 7.94 or 18.86 μCi ml−1) for 30 min. The optimal 3H-choline concentration was 7.94 μCi ml−1 because vagal stimulation produced maximal release of 3H at that concentration. The baseline level of released 3H-acetylcholine (ACh) and 3H-choline derivatives became stable within 40 min of superfusing the tissue segment. More than 20 min were required between consecutive vagus nerve stimulations to re-establish the basal release of 3H. This concentration of 3H-choline (7.94 μCi ml−1) was also used to incubate tissues for 30, 60 and 90 min at 35 °C while they were continually bubbled with 95 % O2-5 % CO2 gas mixture. The length of the incubation period did not alter the establishment of a steady baseline by between 30 and 60 min and did not affect the tissue responses. Thus, 45 min was selected as the experimental incubation time.
Immunohistochemistry
For immunohistochemical studies, hamsters were intracardially perfused through the ascending aorta with 10 mM phosphate-buffered saline (PBS; pH 7.3). After removal of the oesophagus, one end of the oesophagus was ligated with a silk thread, and the lumen was distended with a fixative solution of 4 % paraformaldehyde in 0.1 M phosphate buffer (PB) injected through a needle inserted into the other end of the oesophagus. The tissue was immersed for 2 h in the same fixative at room temperature and then opened longitudinally. It was washed out in three changes of PBS (pH 7.3), then the mucosa and the outer and inner muscle layers were removed with fine forceps under a dissection microscope for whole-mount preparation. Whole-mount specimens were immunostained for choline acetyltransferase (ChAT) and nitric oxide synthase (NOS). In brief, they were first soaked overnight in PBS containing 0.5 % Triton X-100. After washing with PBS, the specimens were treated with 0.3 % H2O2 in PBS for 30 min and exposed for 1 h to 1 % normal goat (for NOS) or 1 % normal donkey (for ChAT) serum and incubated with rabbit anti-NOS (1:10 000, 24287, Diasorin) or goat anti-ChAT (1:500, AB1449, Chemicon) for 6 days at 4 °C. The specimens were then washed in three changes of PBS and incubated with biotin-conjugated goat anti-rabbit IgG solution (1:400, BA1000, Vector) (for NOS) or donkey anti-goat IgG solution (1:400, 705-065-147, Jackson ImmunoResearch Laboratories) (for ChAT) for 1 h, followed by incubation with avidin-biotin peroxidase complex (Elite ABCkit; Vector) for l h at room temperature. Immunoreaction sites were visualized by treatment with 0.05 M Tris-HCl buffer (pH 7.4) containing 0.01 % 3,3′-diaminobenzidine and H2O2. The reaction was stopped by the addition of an excess amount of PBS and the whole-mounts were stretched on glass slides. The specimens were dehydrated through a graded series of ethanols and cleared with xylene. They were then sealed with coverslips.
For double immunostaining of NOS and vanilloid receptor 1 (VR1), whole-mount specimens, after 1 h exposure to 1 % normal donkey serum, were incubated with a mixture of the rabbit anti-NOS serum (1:1000, 24287, Diasorin) and goat anti-VR1 serum (1:200, SC-8670, Santa Cruz) for 6 days at 4 °C. The specimens were then applied for 3 h with mixed antisera of fluorescein isothiocyanate (FITC)-labelled anti-rabbit IgG solution (1:100, 711-095-152, Jackson ImmunoResearch Laboratories) and tetramethylrhodamine isothiocyanate (TRITC)-labelled anti-goat IgG solution (1:100, 705-025-147, Jackson Immunoresearch Laboratories), both of which were raised in donkey. The specimens were examined using a Nikon fluorescence microscope equipped with G and B filter sets for FITC and TRITC, respectively.
Solutions and drugs
During functional experiments, tissues were maintained in PSS solution containing (mM): NaCl 137, KCl 4.0, CaCl2 2.0, MgCl2 1.0, glucose 5.6 and Hepes 10. NG-nitro-L-arginine methyl ester (L-NAME), L-arginine hydrochloride, dibutyryl cGMP, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), D-tubocurarine, hexamethonium, tetrodotoxin, capsaicin, piperine, atropine and 3H-choline were obtained from Sigma Chemical Co. (St Louis, MO, USA). [Z]-1-[2-aminoethyl]-N-[2-aminoethyl]diazen-1-ium-1,2-diolate (NONOate) was obtained from RBI (Natick, MA, USA).
Capsaicin was dissolved in ethanol. ODQ and piperine were dissolved in DMSO. Other drugs were dissolved in distilled water. Stock solutions were at 100 or more times higher concentrations than those used for experiments, and further dilutions were made in PSS. Final concentrations of ethanol and DMSO in the bathing solution were less than 0.01 % and had no effect on the tone of the tissues. The drug concentrations given in the text are final concentrations in the bathing solution.
Data processing and statistical analysis
All results are presented as means ± S.E.M. The significance of differences between mean values was determined by one-way analysis of variance followed by a Dunnett test for multiple group comparisons or Student's t test for comparison of two groups. A P value less than 0.05 denotes the presence of a statistically significant difference.
RESULTS
Characteristics of twitch contraction induced by vagal stimulation
No spontaneous mechanical activity was recorded at rest from the in vitro oesophageal segment. Stimulation (30 μs, 20 V) of the vagus nerve trunk with single pulses, delivered automatically at 1 min intervals, produced twitch-like contractile responses (Fig. 1) that were stable in magnitude for over 3 h. The contractile responses were abolished by D-tubocurarine (10−5 M) and tetrodotoxin (10−6 M) but were unaffected by atropine (10−6 M). Exogenously applied ACh- (10−4 M) induced contraction was antagonized by D-tubocurarine (3 × 10−5 M). Thus, these responses were confirmed as being mediated by ACh, which was released from motor neurones and bound to nicotinic receptors. However, tetrodotoxin did not significantly affect electrical field stimulation- (EFS; 10 ms, 20 V) evoked contractions, indicating that EFS directly produces the contraction of oesophageal striated muscle.
Figure 1. Effects of piperine (10−4 M) on twitch contractions elicited by electrical stimulation (30 μs, 20 V) of the vagus nerve.
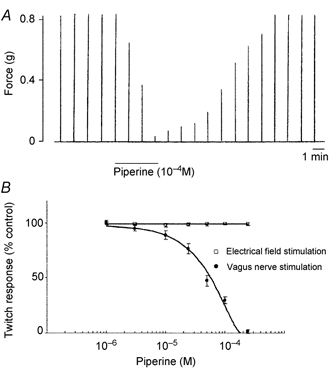
A, representative traces showing twitch contractions evoked by vagal stimulation delivered every minute, and the effect of piperine (10−4 M for 3 min) on twitch contractions elicited by vagal stimulation. After the removal of piperine, the twitch contractions recovered to the control level. B, summary plot of the inhibitory effect of piperine on twitch contractions elicited by either electrical field stimulation or vagus nerve stimulation. Each data point represents the mean ± S.E.M. of five experiments.
Hexamethonium (10−4 M), a blocker of nicotinic receptors on postganglionic neurones, did not affect the vagus nerve-mediated twitch contractions, indicating that the hamster oesophageal striated muscle may receive vagal preganglionic input.
Effects of capsaicin on piperine-induced inhibition of twitch contractions elicited by vagal nerve stimulation
To investigate the role of intrinsic neurones in the oesophageal motility, we examined the effect of piperine on the twitch contractions elicited by vagal nerve stimulation. Addition of piperine (10−4 M for 3 min), a VR1 agonist, did not significantly modify the basal tone. Piperine inhibited the vagally mediated twitch contractions. After the removal of piperine, the twitch contractions recovered to the control level (Fig. 1A). The inhibitory effect of piperine on the twitch contractions was reproducible within three applications. However, piperine did not inhibit EFS-evoked contractions (n = 5).
In another series of experiments, we used capsaicin, a VR1 agonist, to investigate whether the inhibitory effect of piperine was mediated by activation of capsaicin-sensitive neurones. Twitch contractions induced by vagal nerve stimulation were inhibited by capsaicin (3 × 10−7 to 3 × 10−4 M for 10 min) and returned to the control level after washing out of capsaicin by PSS (Fig. 2). After pretreatment with capsaicin, piperine (10−4 M) did not induce inhibition of twitch contractions (n = 5) (Fig. 3B and C). EFS-induced contractions were unaffected by capsaicin (10−4 M). Neither capsaicin nor piperine altered the contractions of the striated muscle to exogenously applied ACh (10−4 M) (data not shown).
Figure 2. Effects of capsaicin on twitch contractions elicited by electrical stimulation (30 μs, 20 V) of the vagus nerve.
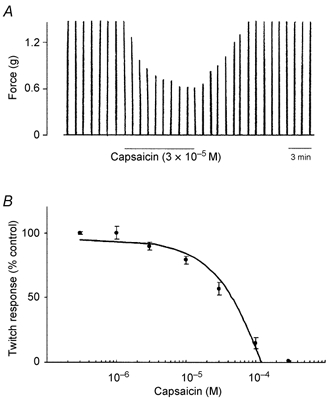
A, representative traces showing twitch contractions mediated by vagal stimulation applied every minute, and the effect of capsaicin (3 × 10−5 M for 10 min) on the twitch contractions. The tissue was then washed with PSS for 3 min. After the removal of capsaicin, the twitch contractions recovered to the control level. B, summary plot of the inhibitory effect of capsaicin (3 × 10−7 to 3 × 10−4 M) on twitch contractions. Each data point represents the mean ± S.E.M. of four experiments.
Figure 3. Effect of capsaicin on piperine-induced inhibition of twitch contractions.
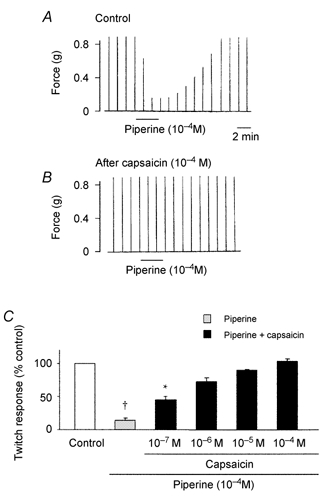
A, twitch contractions in response to vagal stimulations (30 μs, 20 V), before and after the addition of piperine (10−4 M). After the application of piperine, the tissue was washed with PSS for 3 min. Vagus nerve stimulation was applied every 1 min. B, capsaicin (10−4 M) was added 25 min before the addition of piperine. C, summary plot of the effect of capsaicin pretreatment on piperine-induced inhibition of twitch contractions. Each column represents the mean ± S.E.M. of 5- 7 preparations. † P < 0.01, * P < 0.05, compared to the response before addition of piperine and capsaicin.
These results suggest that piperine-induced inhibition of twitch contractions may be mediated by capsaicin-sensitive neurones.
Effects of L-NAME and ODQ on piperine-induced inhibition of twitch contractions
To investigate whether the inhibitory effect of piperine was mediated by activation of nitrergic nerves or guanylate cyclase, we examined the effects of L-NAME and ODQ on piperine-induced inhibition of twitch contractions. Neither L-NAME (2 × 10−4 M) nor ODQ (10−5 M) had an effect on the basal tone and vagally mediated twitch contractions. However, in the presence of L-NAME or ODQ, piperine did not cause inhibition of twitch contractions. The inhibitory action of L-NAME was reversed by L-arginine (5 × 10−3 M), but not by D-arginine (5 × 10−3 M) (n = 6-8) (Fig. 4).
Figure 4. Effects of L-NAME (2 × 10−4 M), L-arginine (5 × 10−3 M) and ODQ (10−5 M) on the inhibitory effects of piperine (10−4 M) on twitch contractions.
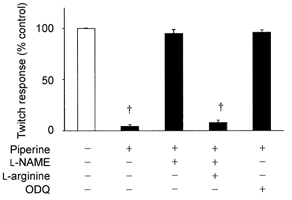
Each bar represents the mean ± S.E.M. of 6- 8 preparations. † P < 0.01, compared with the response before addition of piperine, L-NAME, L-arginine and ODQ.
Effects of NONOate and dibutyryl cGMP on twitch contractions
In order to ascertain that the effects of L-NAME and L-arginine were due to production of NO, we checked the influences of exogenously applied NO on the twitch contractions.
Exogenous application of an NO-donor, NONOate (10−4-10−3 M), and a membrane permeant analogue of cGMP, dibutyryl cGMP (10−3 to 3 × 10−2 M), caused a concentration-dependent increase in inhibition of twitch contractions (n = 4; Fig. 5). This inhibition of twitch contractions induced by NONOate occurred within 2-4 min, and reached a maximum within 10-30 min. After removal of NONOate, 1-2 min were required for full restoration of twitch contractions (n = 4). The inhibitory effect of dibutyryl cGMP was observed within 2 min and reached a peak within 10-15 min. Restoration of twitch contractions after removal of dibutyryl cGMP was observed before 10-15 min (n = 4).
Figure 5. Effects of NONOate and dibutyryl cGMP on twitch contractions elicited by electrical stimulation (30 μs, 20 V) of the vagus nerve.
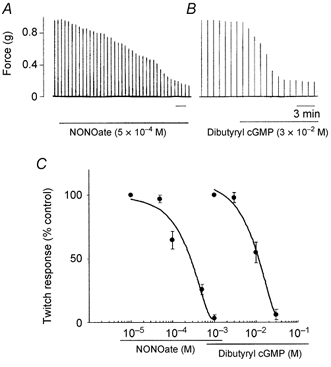
A and B, representative traces showing twitch contractions mediated by vagal stimulation applied every 1 min before and in the presence of NONOate (left trace) or dibutyryl cGMP (right trace). Scale bars indicate 3 min. C, summary plot of the inhibitory effects of NONOate and dibutyryl cGMP on twitch contractions. Each data point represents the mean ± S.E.M. of four experiments.
Effects of piperine, L-NAME, ODQ and NONOate on ACh release
Because piperine inhibited the contractions elicited by vagal nerve stimulation, we tested the effect of piperine, L-NAME, ODQ and NONOate on presumed 3H-ACh releases.
The level of 3H-labelled derivatives released from tissue strips was initially elevated during superfusion, but it reached a steady level within 30 to 40 min. The basal level decreased at a constant rate throughout the experiment. Vagal stimulation increased the release of 3H from tissue strips above basal levels (n = 8; Fig. 6A). Application of piperine (10−4 M) for 5 min significantly reduced presumed 3H-ACh release induced by vagal stimulation, compared with the control (n = 8; Fig. 6B). Addition of L-NAME (2 × 10−4 M) or ODQ (10−5 M) significantly reversed the effects of piperine, keeping presumed 3H-ACh release at the level induced by vagal stimulation. The blocking effect of L-NAME on piperine-induced inhibition of presumed 3H-ACh release was selectively reversed by subsequent addition of L-arginine (5 × 10−3 M), but not by D-arginine (5 × 10−3 M) (n = 5). L-NAME (2 × 10−4 M) had no effect on presumed 3H-ACh release (Fig. 6C). Exogenously applied NONOate (10−3 M) diminished presumed 3H-ACh release induced by vagal stimulation (n = 6; Fig. 6C).
Figure 6. Effects of L-NAME (2 × 10−4 M), ODQ (10−5 M) and NONOate (10−3 M) on 3H-ACh release in response to vagal stimulation (20 Hz, 30 μs for 2 min).
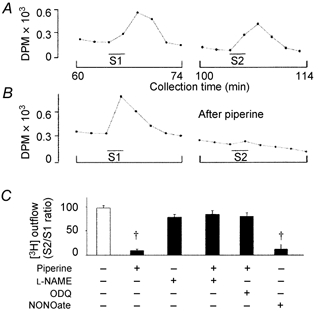
Typical profile of presumed 3H-ACh release in the absence (A) or presence (B) of piperine (10−4 M). Presumed 3H-ACh release was elicited by two periods of vagal stimulation at the indicated times (S1 and S2). Tritium outflow was measured in samples collected every 2 min. DPM, disintegrations min−1. C, summary plot of the effects of L-NAME, ODQ and NONOate on the inhibitory effect of piperine (10−4 M) on vagal stimulation-evoked presumed 3H-ACh release. The S2/S1 ratio was calculated after subtraction of the basal release. L-NAME, ODQ and NONOate were applied 25 min before S2 and remained in the bath until the end of the experiments. Each bar represents the mean ± S.E.M. of 6- 8 preparations. † P < 0.01, compared with the response before addition of piperine, L-NAME, ODQ and NONOate.
Distribution of ChAT, VR1 AND NOS immunoreactivity
ChAT immunoreactivity was found in nerve fibres that terminated on oesophageal striated muscles. NOS-immunoreactive (IR) nerve cell bodies and nerve fibres were detected in the myenteric plexus. In addition, the presence of some NOS immunoreactivity was confirmed at motor endplates (Fig. 7). Only a few NOS-IR portions in the myenteric plexus showed VR1 immunoreactivity (Fig. 8).
Figure 7. Whole-mount preparations of the hamster oesophagus showing the myenteric plexus stained for ChAT and NOS.
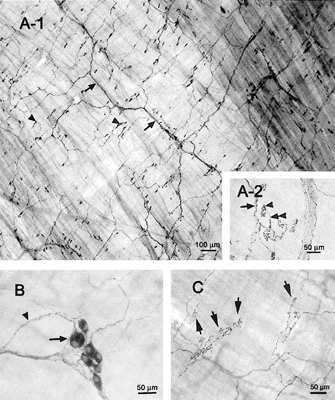
A1, ChAT immunoreactivity is found in nerve fibres (arrows) and motor endplates (arrowheads). A2, high magnification view of motor endplates immunostained with ChAT. Motor nerve fibres that have no varicosities (arrow) are divided into short and thin branches (double arrowhead) whose terminals have a patch-like feature like a grape (arrowhead). B, NOS-like immunoreactivity is present in nerve cell bodies (arrow) and fibres (arrowhead). C, NOS immunoreactivity is present in motor endplates (arrows).
Figure 8. Whole-mount preparations of the hamster oesophagus showing the myenteric plexus stained with NOS and VR1.
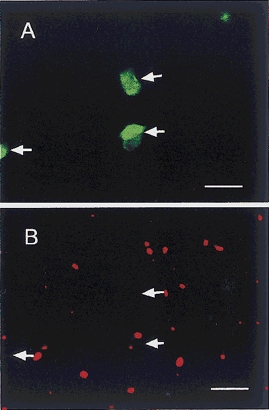
A, NOS immunoreactivity is found in myenteric nerve cells (arrows). B, VR1 immunoreactivity is not in accordance with NOS immunoreactivity. Arrows indicate NOS-IR sites in A. Scale bars = 50 μm.
DISCUSSION
The major finding of the present study is the existence of a local neural reflex that involves capsaicin-sensitive neurones, nitrergic myenteric neurones and vagal motor neurones in the hamster oesophagus. Evidence supporting this conclusion includes: (1) piperine inhibited the vagally mediated twitch contractions; (2) capsaicin and L-NAME abolished the inhibitory effect of piperine. L-NAME had no effect on vagally evoked twitch contractions and presumed 3H-ACh release. The inhibitory effect of L-NAME was reversed by subsequent application of L-arginine, but not by D-arginine; (3) NONOate and dibutyryl cGMP inhibited vagally evoked twitch contractions. Inhibition of twitch contractions by piperine was fully reversed by ODQ; (4) NOS-IR nerve cell bodies and fibres were observed in the myenteric plexus. Both ChAT- and NOS-IR motor endplates were observed in the striated muscles. Furthermore, a few NOS-IR portions showed VR1 immunoreactivity in the myenteric plexus.
Vagal stimulation resulted in a rapid phasic contractile response of the hamster oesophageal striated muscle. These responses were selectively blocked by tetrodotoxin and D-tubocurarine, but not by hexamethonium, which blocks nicotinic receptors on postganglionic neurones with relative selectivity, indicating that the twitch response was mediated by vagal pre-fibres projecting into striated muscle in the oesophagus. Exogenously applied ACh evoked oesophageal striated muscle contraction. However, there is a possibility that myenteric neurones, which project into other tissues than striated muscle, may receive vagal preganglionic input in the hamster oesophagus. In addition, it has been found that vagal stimulation increased 3H-choline release from the oesophagus, and ChAT, the synthesizing enzyme for acetylcholine, is present in the myenteric plexus along with ChAT-IR motor endplates and nerve fibres. These results further support the notion that the peristaltic contraction in oesophageal striated muscles is controlled by vagal excitation (Park & Conklin, 1999; Jean, 2001).
To our knowledge, this is the first report to shed light on the role of intrinsic neurones in the inhibition of vagus nerve-mediated twitch contractions by piperine in the oesophageal striated muscle of the hamster. Capsaicin, which activates and desensitizes C-type sensory neurones (Marsh, 1987; Yeats et al. 1991), prevented inhibition of twitch contractions via activation of nitrergic neurones by piperine. This suggests that the inhibitory effect exerted upon the twitch response may be mediated predominantly by capsaicin-sensitive sensory C-fibres. NOS-IR nerve cell bodies and fibres are observed in the myenteric plexus of the hamster oesophagus and only a few NOS-IR portions in the myenteric plexus have shown VR1 immunoreactivity. This result suggests that the source of NO, which is released by piperine, may be the neurones of the myenteric plexus in the hamster oesophagus. This is further supported by immunoreactivity of motor endplates in the myenteric plexus of the hamster oesophagus to NOS. It has been suggested that afferent and efferent limbs of an intrinsic reflex pathway in the oesophageal wall could be formed by interstitial cells of Cajal (ICC) (Thuneberg, 1999; Rumessen et al. 2001) and inhibitory nerve fibres (Kuramoto et al. 1999). Considering all the evidence, we conclude that nitrergic neurones may be involved in the piperine-induced inhibition of the vagally stimulated contractions of oesophageal striated muscle. Storr et al. (2001) proposed that NO may not inhibit oesophageal striated muscle contraction in response to vagal nerve stimulation, based on the evidence that addition of an NO donor, DEA-NO, had no substantial effect on vagally mediated contractions. A possible explanation for this discrepancy may be the use of the different NO donors.
Our results indicate that piperine does not act directly on the motor nerves in the hamster oesophagus but that it acts on nitrergic neurones that mediate capsaicin-sensitive neurones. This conclusion has been reached from our findings that L-NAME completely abolished the inhibitory effects of piperine on twitch contractions and ACh release. Further support was derived from inhibition by capsaicin of piperine-induced inhibitory response as well as the identification of neuronal NOS in the myenteric plexus and motor endplates of the hamster oesophagus, and incompatibility NOS-IR with VR1-IR. Additional confirmation may be given by previously reported observations that the effects of piperine involve capsaicin-sensitive neurones and that these neurones are subsets of either B- or C-type sensory neurones in mammals (Marsh, 1987; Maggi & Meli, 1988; Wood et al. 1988; Holzer, 1991; Akerman & Gronblad, 1992). However, further studies are necessary to clarify the mechanism(s) of piperine-induced activation of nitrergic neurones.
Previous studies proposed that peristaltic contractions of oesophageal striated muscles are controlled by vagal motor neurones arising in the brain stem (Andrew, 1956; Park & Conklin, 1999; Jean, 2001). Because this innervation is purely excitatory, the lack of contractile activity in the striated muscle oesophagus at rest reflects inactivity of these neurones at rest (Roman, 1966). In the present study, L-NAME did not have any significant effect on vagally evoked twitch contractions and presumed 3H-ACh release. Thus, the entire oesophageal peristalsis was believed to be controlled by the central nervous system. However, applications of capsaicin and piperine resulted in the inhibition of vagally evoked twitch contractions in the hamster oesophagus, suggesting that sensory afferent or other cells (ICC and inhibitory myenteric neurones) in the oesophagus can modulate the central vagal output that controls peristaltic contraction of the striated muscle. The inhibitory nerve fibres, which modulate the activity of the motor endplates (Wörl et al. 1994; Kuramoto et al. 1999) could form the efferent limb of such reflex pathway. Further studies are necessary to investigate whether this inhibitory circuit might be mediated by nitrergic nerves with a regulatory role in peristalsis of the striated muscle oesophagus.
Morphological and functional evidence has demonstrated the presence of extrinsic neural elements in the guinea-pig ileum and colon (Holzer & Holzer-Petsche, 1997), bile duct (Patacchini et al. 1999), dog heart (Foreman et al. 2000), cat and human urinary tract (Blok, 2002) and mouse respiratory tract (Gaytán et al. 2002). On the other hand, capsaicin inhibited the human bronchospasm (Calignano et al. 2000), caused bradycardia in the guinea-pig (Chang et al. 2000) and enhanced the spontaneous ureteric action potentials (Exintaris & Lang, 1999) via activation of intrinsic neurones. In the present study, L-NAME did not have any significant effect on vagally evoked twitch contractions and presumed 3H-ACh release. This might suggest that piperine acts on capsaicin-sensitive neurones or other cells (ICC and inhibitory myenteric neurones), which regulate vagus nerve activities.
In conclusion, the present study indicates that there is a local neural reflex which involves capsaicin-sensitive neurones, nitrergic myenteric neurones and vagal motor neurones. Our results also suggest that nitrergic neurones are involved in regulating vagally stimulated contractions of oesophageal striated muscles.
Acknowledgments
This study was supported by ASR grant number 13660297.
REFERENCES
- Akerman KE, Gronblad M. Intracellular free [Ca2+] and [Na+] in response to capsaicin in cultured dorsal root ganglion-cells. Neurosci Lett. 1992;147:13–15. doi: 10.1016/0304-3940(92)90763-w. [DOI] [PubMed] [Google Scholar]
- Andrew BL. The nervous control of the cervical oesophagus of the rat during swallowing. J Physiol. 1956;134:729–740. doi: 10.1113/jphysiol.1956.sp005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok BFM. Central pathways controlling micturition and urinary continence. Urology. 2002;59(suppl. 5A):13–17. doi: 10.1016/s0090-4295(01)01633-8. [DOI] [PubMed] [Google Scholar]
- Broussard DL, Lynn RB, Wiedner EB, Altschuler SM. Solitarial premotor neuron projections to the rat esophagus and pharynx: implications for control of swallowing. Gastroenterology. 1998;114:1268–1275. doi: 10.1016/s0016-5085(98)70433-0. [DOI] [PubMed] [Google Scholar]
- Calignano A, Katona I, Desarnaud F, Giuffrida A, La Rana G, Mackie K, Freund TF, Piomelli D. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature. 2000;408:96–101. doi: 10.1038/35040576. [DOI] [PubMed] [Google Scholar]
- Chang Y, Hoover DB, Hancock JC. Endogenous tachykinins cause bradycardia by stimulating cholinergic neurons in the isolated guinea pig heart. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1483–1489. doi: 10.1152/ajpregu.2000.278.6.R1483. [DOI] [PubMed] [Google Scholar]
- Exintaris B, Lang RJ. Effects of nerve stimulation on spontaneously active preparations of the guinea pig ureter. Urol Res. 1999;27:328–335. doi: 10.1007/s002400050159. [DOI] [PubMed] [Google Scholar]
- Foreman RD, Linderoth B, Ardell JL, Barron KW, Chandler MJ, Hull SS, Jr, Terhorst GJ, Dejongste MJL, Armour JA. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for its therapeutic use in angina pectoris. Cardiovasc Res. 2000;47:367–375. doi: 10.1016/s0008-6363(00)00095-x. [DOI] [PubMed] [Google Scholar]
- Gaytán SP, Pásaro R, Coulon P, Bevengut M, Hilaire G. Identification of central nervous system neurons innervating the respiratory muscles of the mouse: a transneuronal tracing study. Brain Res Bull. 2002;57:335–339. doi: 10.1016/s0361-9230(01)00674-8. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanism of action, and selectivity of thin sensory neurones. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Holzer P, Holzer-Petsche U. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol Ther. 1997;73:173–217. doi: 10.1016/s0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- Holzer P, Schluet W, Lippe IT, Sametz W. Involvement of capsaicin-sensitive sensory neurones in gastrointestinal function. Acta Physiol Hung. 1987;69:403–411. [PubMed] [Google Scholar]
- Hudson LC, Cummings JF. The origins of innervation of the esophagus of the dog. Brain Res. 1985;326:125–136. doi: 10.1016/0006-8993(85)91391-5. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Capasso R, Pinto L, Di Carlo G, Mascolo N, Capasso F. Effect of vanilloid drugs on gastrointestinal transit in mice. Br J Pharmacol. 2001;132:1411–1416. doi: 10.1038/sj.bjp.0703975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A. Brain stem control of swallowing: neuronal control and cellular mechanisms. Physiol Rev. 2001;81:929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- Kuramoto H, Kawano H, Sakamoto H, Furness JB. Motor innervation by enteric nerve fibers containing both nitric oxide synthase and galanin immunoreactivities in the striated muscle of the rat esophagus. Cell Tissue Res. 1999;295:241–245. doi: 10.1007/s004410051230. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurones. Gen Pharmacol. 1988;19:1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Marsh SJ. The mechanism of action of capsaicin on sensory C-type neurones and their axons in vitro. Neuroscience. 1987;23:275–289. doi: 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay AK, Weisbrodt NW. Neural organization of esophageal peristalsis: role of the vagus nerve. Gastroenterology. 1975;58:444–447. [PubMed] [Google Scholar]
- Neuhuber WL, Wörl J, Berthoud HR, Conte B. NADPH-diaphorase-positive nerve fibers associated with motor endplates in the rat esophagus: new evidence for co-innervation of striated muscle by enteric neurons. Cell Tissue Res. 1994;276:23–30. doi: 10.1007/BF00354780. [DOI] [PubMed] [Google Scholar]
- Park H, Conklin JL. Neuromuscular control of esophageal peristalsis. Curr Gastroenterol Rep. 1999;1:186–197. doi: 10.1007/s11894-999-0033-3. [DOI] [PubMed] [Google Scholar]
- Patacchini R, Barthó L, Giorgio RD, Lénárd L, JR, Stanghellini V, Barbara G, Lecci A, Maggi CA. Involvement of endogenous tachykinins and CGRP in the motor responses produced by capsaicin in the guinea-pig common bile duct. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:344–353. doi: 10.1007/s002109900048. [DOI] [PubMed] [Google Scholar]
- Roman C. Nervous control of peristalsis in the esophagus. J Physiol. 1966;58:79–108. [PubMed] [Google Scholar]
- Rumessen JJ. Identification of interstitial cells of cajal. Significance for studies of human small intestine and colon. Dan Med Bull. 2001;41:275–293. [PubMed] [Google Scholar]
- Storr M, Geisler F, Neuhuber WL, Schusdziarra V, Allescher H. Characterization of vagal input to the rat esophageal muscle. Auton Neurosci. 2001;91:1–9. doi: 10.1016/S1566-0702(01)00290-9. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. One hundred years of interstitial cells of Cajal. Microsc Res Tech. 1999;4:223–238. doi: 10.1002/(SICI)1097-0029(19991115)47:4<223::AID-JEMT2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wood JN, Winter J, James IF, Rang HP, Yeats J, Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988;8:3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wörl J, Fischer J, Neuhuber WL. Nonvagal origin of galanin-containing nerve terminals innervating striated muscle fibers of the rat esophagus. Cell Tissue Res. 1998;292:453–461. doi: 10.1007/s004410051074. [DOI] [PubMed] [Google Scholar]
- Wörl J, Mayer B, Neuhuber WL. Nitrergic innervation of the rat esophagus: focus on motor endplates. J Auton Nerv Syst. 1994;49:227–233. doi: 10.1016/0165-1838(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Yeats JC, Docherty RJ, Bevan S. Calcium dependent and independent desensitization of capsaicin-evoked responses in voltage clamped adult rat dorsal root ganglion (DRG) neurones in culture. J Physiol. 1991;446:390P. [Google Scholar]


