Abstract
P2X receptors are ATP-gated cation channels composed of one or more of seven different subunits. P2X receptors participate in intestinal neurotransmission but the subunit composition of enteric P2X receptors is unknown. In this study, we used tissues from P2X3 wild-type (P2X3+/+) mice and mice in which the P2X3 subunit gene had been deleted (P2X3−/−) to investigate the role of this subunit in neurotransmission in the intestine. RT-PCR analysis of mRNA from intestinal tissues verified P2X3 gene deletion. Intracellular electrophysiological methods were used to record synaptic and drug-induced responses from myenteric neurons in vitro. Drug-induced longitudinal muscle contractions were studied in vitro. Intraluminal pressure-induced reflex contractions (peristalsis) of ileal segments were studied in vitro using a modified Trendelenburg preparation. Gastrointestinal transit was measured as the progression in 30 min of a liquid radioactive marker administered by gavage to fasted mice. Fast excitatory postsynaptic potentials recorded from S neurons (motoneurons and interneurons) were similar in tissues from P2X3+/+ and P2X3−/− mice. S neurons from P2X3+/+ and P2X3−/− mice were depolarized by application of ATP but not α,β-methylene ATP, an agonist of P2X3 subunit-containing receptors. ATP and α,β-methylene ATP induced depolarization of AH (sensory) neurons from P2X3+/+ mice. ATP, but not α,β-methylene ATP, caused depolarization of AH neurons from P2X3−/− mice. Peristalsis was inhibited in ileal segments from P2X3−/− mice but longitudinal muscle contractions caused by nicotine and bethanechol were similar in segments from P2X3+/+ and P2X3−/− mice. Gastrointestinal transit was similar in P2X3+/+ and P2X3−/− mice. It is concluded that P2X3 subunit-containing receptors participate in neural pathways underlying peristalsis in the mouse intestine in vitro. P2X3 subunits are localized to AH (sensory) but not S neurons. P2X3 receptors may contribute to detection of distention or intraluminal pressure increases and initiation of reflex contractions.
Myenteric neurons play a prominent role in controlling gastrointestinal motility (Kunze & Furness, 1999). Based on studies using tissues from the guinea-pig gastrointestinal tract it has been established that there are 14 different functional classes of myenteric neurons (Brookes, 2001). Although there are many functional classes of neurons, only two types of myenteric neuron have been identified using electrophysiological criteria: these have been labelled S and AH neurons (Hirst et al. 1974).
Action potentials in AH neurons are followed by a long-lasting (> 1 s) afterhyperpolarization. The afterhyperpolarization is mediated by a calcium-dependent potassium channel, which is activated by calcium entering the neuron during the action potential (Morita et al. 1982; Hirst et al. 1985). In the guinea-pig small intestine, AH neurons receive only slow excitatory synaptic input and are likely to be intrinsic intestinal sensory neurons (Furness et al. 1998; Kunze & Furness, 1999). P2X receptors are expressed by the mucosal endings and cell bodies of AH neurons (Bertrand & Bornstein, 2002) and it has been suggested that a P2X receptor may contribute to activation of sensory neurons caused by release of ATP in the mucosa in response to mechanical or chemical stimuli (Bertrand & Bornstein, 2002).
S neurons receive fast and slow excitatory synaptic input and are motoneurons and interneurons (Furness et al. 1998; Kunze & Furness, 1999). Fast excitatory postsynaptic potentials (fEPSPs) recorded from all S neurons in the guinea-pig intestine are mediated principally by acetylcholine acting at nicotinic acetylcholine receptors (nAChRs). However, ATP acting at P2X receptors contributes to fEPSPs in 60-70 % of S neurons (Galligan et al. 2000). Electrophysiological and functional studies have shown that non-cholinergic synaptic transmission predominates in descending excitatory and inhibitory motor pathways in the guinea-pig small intestine (Smith et al. 1990; Johnson et al. 1999; Le Pard & Galligan, 1999; Spencer et al. 2000; Bian et al. 2000; Monro et al. 2002). Therefore, neurotransmission mediated by P2X receptors could contribute to propulsive motor patterns in the small intestine.
P2X receptors are multimeric proteins that combine to form a ligand-gated cation channel (Khakh et al. 2001; North, 2002). There are seven P2X receptor subunit proteins (P2X1-P2X7) with each subunit having two membrane-spanning domains (Khakh et al. 2001; North, 2002). P2X subunits are able to assemble into functional homo- and hetero-oligomeric channels (Khakh et al. 2001). The subunit composition of P2X receptors determines their pharmacological and functional properties (North & Surprenant, 2000). For example, the stable ATP analogue α,β-methylene ATP causes rapidly desensitizing inward currents in cells expressing homomeric P2X3 receptors but does not activate homomeric P2X2 receptors (North & Surprenant, 2000). When P2X3 and P2X2 subunits are co-expressed as a heteromeric receptor, α,β-methylene ATP causes a biphasic response consisting of rapidly desensitizing and slowly desensitizing components (Lewis et al. 1995; North & Surprenant, 2000). The subunit composition of functional P2X receptors expressed by enteric neurons has not been established. However, in the guinea-pig intestine descending interneurons, motoneurons and sensory neurons express P2X2 subunits (Castelluci et al. 2002). Also in the guinea-pig intestine, P2X3 subunits are localized to motoneurons and ascending interneurons (Poole et al. 2002; Nassauw et al. 2002) while P2X7 subunits are localized to nerve endings in the myenteric plexus (Hu et al. 2001).
An alternative to the pharmacological approach for receptor characterization in tissues is to use tissues from genetically modified animals to probe the subunit composition of functional P2X receptors (Cockayne et al. 2000; Vlaskovska et al. 2001). In the present study, the role of P2X3 subunits in synaptic transmission in the myenteric plexus and in controlling intestinal motility in vitro and in vivo was evaluated using P2X3 wild-type (P2X3+/+) and P2X3 subunit gene knockout (P2X3−/−) mice (Cockayne et al. 2000). As there have been no previous electrophysiological studies of myenteric neurons in the mouse small intestine we have also characterized the basic membrane and synaptic properties of these neurons.
METHODS
Animals
All protocols for the use of animals were approved by the All University Committee on Animal Use and Care at Michigan State University.
P2X3−/− mice were generated as described previously (Cockayne et al. 2000). All mice analysed in this study have the genetic background 129Ola × C57BL/6 (Harlan), and were derived from homozygous F2 crosses maintained at Roche Palo Alto. Genotype confirmation of all animals was carried out by Southern blot analysis as previously described (Cockayne et al. 2000).
For in vitro motility and electrophysiological studies, mice were anaesthetized with halothane and then killed by cervical dislocation. A segment of ileum was removed from the animal and placed in Krebs solution of the following composition (mM): NaCl 117, KCl 4.7, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, NaHCO3 25 and glucose 11. The Krebs solution was oxygenated continuously with 95 % O2 : 5 % CO2 at room temperature.
Reverse transcriptase PCR analysis of RNA from mouse intestinal tissues
Total RNA was isolated from whole ileum, ileal myenteric plexus, whole colon and colonic myenteric plexus using a Trizol (Invitrogen, Carlsbad, CA, USA) isolation method, and RQ1 RNAse-free DNAse I (Promega, Keene, USA) digestion. To perform semi-quantitative RT-PCR analysis, cDNA was reverse transcribed from 1 μg of total RNA, in a reaction volume of 22 μl, using 1 μl (2.5 U) Superscript RNAseH reverse transcriptase (Invitrogen), 2 μl 10 × PCR buffer II (Perkin Elmer, Fremont, CA, USA), 2 μl 25 mM MgCl2 (Perkin Elmer), 1 μl 25 mM dNTP mix (Invitrogen), 2 μl 0.1 M DTT (Invitrogen), 1 μl RNAsin (Invitrogen) and 1 μl 15 μM random hexamers (Amersham Biosciences, New Jersey, USA). RT reactions were carried out at 42 °C for 50 min, followed by a 15 min elongation at 70 °C, and a 20 min RNAse H digestion (2 U, Invitrogen) at 37 °C. PCR analysis was performed in a 50 μl reaction volume using 230 ng reverse-transcribed cDNA, 1 μl each of 10 μM 5′or 3′ primers (Amersham Biosciences or Sigma Genosys, The Woodlands, TX, USA, respectively), 0.5 μl Failsafe PCR enzyme mix (1.25 U, Epicentre, Monterrey, Mexico) and 25 μl Premix D (Epicentre). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers (BD Biosciences, Palo Alto, CA, USA) were used as a control for all RNA samples, and amplified a 450 bp DNA fragment. P2X2 amplification was carried out using the following primers: sense primer 5′ ACG TTC ATG AAC AAA AAC AAG 3′, and antisense primer 5′ GGT TTC CAA ACC GGG TTG AAA CA 3′. These primers amplify a 360 bp cDNA fragment corresponding to the P2X2 carboxy terminus in exon 11. P2X3 amplification was carried out using the following primers: sense primer 5′ CTG TAT ATC AGA CTT CTT CAC CTA CGA 3′ and antisense primer 5′ GAT TGG AGT GGC TGT TCC TGT ATT 3′. These primers amplify a 596 bp cDNA fragment corresponding to exon 1 to exon 7 of P2X3. PCR products were electrophoresed through 1 % agarose, and the gels were transferred to Nytran N membrane (Schleicher and Schuell, Dassel, Germany), followed by formamide hybridization at 42 °C with probes specific for P2X3 and P2X2 subunits and for GAPDH. Blots were exposed to Kodak Biomax MR film (Kodak, New Haven, CT, USA).
Drug-induced longitudinal muscle contractions
Whole segments (3 cm) of ileum were suspended between stationary hooks and isometric force transducers (FT03C; Grass Instruments, Quincy, MA, USA) in jacketed baths (volume, 20 ml) containing Krebs solution at 37 °C. Tissues were placed under 10 mN of initial tension and were allowed to equilibrate for 30 min before starting the experimental protocols. The mechanical activity of the strips was recorded using a chart recorder (7DAE; Grass Instruments).
Drugs were added to the baths in volumes of 2-20 μl. Agonist concentration-response curves were constructed in a non-cumulative manner with 15 min intervals between successive doses. Four preparations were obtained from each animal. One preparation provided control agonist concentration-response curves. The remaining three preparations were treated continuously with either tetrodotoxin (TTX, 0.3 μM), the muscarinic receptor antagonist scopolamine (1 μM), or the nicotinic receptor antagonist mecamylamine (10 μM).
Assessment of peristalsis in vitro
Ileal segments (length ≈6 cm) were mounted in a plexiglass organ bath (volume, 30 ml) using a technique described previously (Huizinga et al. 1998; Abdu et al. 2002). Tissues were equilibrated in Krebs solution at 37 °C for 10 min before experiments were started. Intraluminal pressure was generated by raising the level of a buffer-containing reservoir connected to the ileal segment via a plastic tube. The filling tube was connected to a pressure transducer (TRN 050; Kent Scientific Co.) via a T-connector so that intraluminal pressure increases caused by raising the reservoir could be recorded. The pressure transducer was connected to a chart recorder (7DAE). Responses to pressure increases of 1.25, 2.5, 3.75 and 5 mmHg were evaluated. Ileal responses to each pressure increase were recorded for 30 s and then the intraluminal pressure was returned to the basal level. Intraluminal pressure increases caused a series of phasic contractions and these responses were quantified by measuring the peak contraction amplitude and the frequency of contractions occurring during the 30 s period of stimulation.
Measurement of gastrointestinal transit
Approximately 0.5 μCi of 51Cr as Na51CrO4 in 0.2 ml saline was administered to each mouse by gavage using a flexible plastic feeding tube. After 30 min, the mice were anaesthetized with halothane and then killed by cervical dislocation. The stomach, heart and small intestine were carefully removed. The small intestine was placed on a pre-measured template and then divided into 10 equal segments. The stomach, heart and each intestinal segment were placed in individual borosilicate glass tubes and the amount of radioactivity in each tube was counted directly using a gamma counter. No radioactivity was found in the heart of any mouse indicating that the 51Cr was not absorbed from the gastrointestinal tract. The amount of radioactivity found in the stomach and each intestinal segment was expressed as a fraction of the total radioactivity counted in each animal. Gastrointestinal transit was then measured as the geometric centre (Miller et al. 1981) of the distribution of radioactivity using the following relationship:
Geometric centre = Σ(fraction of counts × segment number),
where the stomach was taken as segment 1 and the 10 intestinal segments were numbered consecutively as segments 2 to 11. Percentage gastric emptying was determined by subtracting the fraction of counts remaining in the stomach from the total counts in the stomach and small intestinal and multiplying this by 100.
Intracellular recordings from myenteric neurons
A segment of ileum taken 5-10 cm proximal to the ileocaecal junction was placed in Krebs solution containing nifedipine (1 μM) and scopolamine (1 μM) to block longitudinal muscle contraction and muscarinic receptors, respectively, on myenteric nerves during intracellular recordings. A 1.5 cm segment of ileum was cut open along the mesenteric border and pinned out flat (mucosal surface up) in a Petri dish lined with silastic elastomer. A longitudinal muscle-myenteric plexus preparation was made by stripping away the mucosa, submucosa and circular muscle layer. A 5 mm × 5 mm piece of longitudinal muscle myenteric plexus was transferred to a small recording chamber (volume, 2 ml) lined with silastic elastomer. The preparation was stretched slightly and pinned to the chamber bottom using small stainless steel pins. The chamber was superfused with oxygenated Krebs solution at 36 °C, at a flow rate of 3.5 ml min−1.
Individual myenteric ganglia were viewed at 200 × magnification using an inverted microscope (Olympus CK-2) with differential interference contrast optics (Hoffman modulation). Intracellular recordings were obtained from single neurons using glass microelectrodes filled with 2 M KCl (tip resistance, 80-120 MΩ). An amplifier with an active bridge circuit (Axoclamp 2A, Axon Instruments, Foster City, CA, USA) was used for membrane potential recordings and for injection of current into neurons via the recording microelectrode. In most experiments, the membrane potential was adjusted to approximately -70 mV using constant DC current to avoid action potentials when evoking fast excitatory postsynaptic potentials (fEPSPs). Signals were filtered at 1 kHz using a 4-pole Bessel filter (Warner Instruments, New Haven, CT, USA), digitized at 5 kHz using a Digitdata 1200 analog/digital converter (Axon Instruments) and analysed with a personal computer.
Synaptic potentials were evoked using a glass pipette filled with Krebs solution (tip diameter, 40-60 μm) as a focal stimulating electrode. The stimulating electrode was positioned on an interganglionic nerve strand. Single stimuli (duration, 0.3 ms) applied to interganglionic connectives at a rate of 0.2 Hz were used to evoke fEPSPs. Slow EPSP (sEPSPs) were evoked using trains of stimuli (20 Hz for 0.5 s).
Neurons were classified as ‘S’ type neurons if the action potential was blocked completely by TTX (0.3 μM) and if a single electrical stimulus applied to an interganglionic connective elicited a fEPSP (Bornstein et al. 1994). Neurons were classified as ‘AH’ cells, if the action potential was only partly inhibited by TTX and if it was followed by an afterhyperpolarization that lasted longer than 1 s (Bornstein et al. 1994). All neurons encountered in this study fitted into one of these two classes of myenteric neuron.
Drugs
All drugs were obtained from Sigma Chemical Company, St Louis, MO, USA.
Statistical analysis
Data are expressed as means ± S.E.M. When describing electrophysiological data, n refers to the number of neurons. For studies of peristalsis or drug-induced longitudinal muscle contractions, n refers to the number of animals from which tissues were obtained for each type of experiment. In the gastrointestinal transit studies n refers to the number of animals from which the data were obtained. Statistical analysis was performed using Student's t test for unpaired data or analysis of variance when applicable. P < 0.05 was considered statistically significant.
RESULTS
The data described here were obtained from 40 P2X3+/+ and 35 P2X3−/− mice. There were no differences in body weight between P2X3+/+ (24 ± 0.5 g) and P2X3−/− (24 ± 0.4 g) mice when used in this study (8-16 weeks old).
RT-PCR analysis of P2X3 transcripts in intestinal tissues
In order to determine whether P2X3 and P2X2 receptor transcripts were present in myenteric neurons of the mouse intestine, we performed RT-PCR on total RNA from whole ileal segments (I), ileal longitudinal muscle myenteric plexus (IMP), whole colon segments (C) and colonic longitudinal muscle myenteric plexus (CMP) (Fig. 1). P2X3 transcripts were present in all tissues analysed from P2X3+/+ mice, but were not detected in tissues from P2X3−/− mice. P2X2 transcripts were also detected in intestinal tissues from both P2X3+/+ and P2X3−/− mice, as were transcripts for the internal control, GAPDH. None of the control reactions performed without reverse transcriptase showed amplification of DNA, indicating that the amplicons detected were derived from reverse-transcribed cDNA and not contaminating genomic DNA.
Figure 1. Representative RT-PCR data showing the presence of P2X3 subunit transcripts in P2X3+/+ tissues but not in P2X3−/− tissues.

RT-PCR was performed on whole ileum segments (I), ileal myenteric plexus (IMP), whole colon segments (C) and colonic myenteric plexus (CMP) from two P2X3+/+ and two P2X3−/− mice (data shown from one of each). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), P2X2 and P2X3 autoradiograms were exposed for 15 min, 15 min and 5 h, respectively.
Electrophysiological responses in myenteric neurons from P2X3+/+ and P2X3−/− mice
There are no data available that describe the electrophysiological properties of murine small intestinal myenteric neurons. Therefore, the electrical behaviour of murine myenteric neurons was studied prior to assessing changes that might be associated with P2X3 gene deletion.
Properties of murine myenteric AH neurons
AH type neurons (n = 12, five from P2X3+/+ and seven from P2X3−/− mice) were encountered infrequently and represented about 10 % of the total number of neurons impaled in this study. This observation is similar to that reported previously in a study of the properties of myenteric neurons in the mouse colon (Furukawa et al. 1986). Injection of an intracellular current pulse evoked an action potential that was only partly inhibited by TTX (0.3 μM)(Fig. 2A). Action potentials in AH neurons from P2X3+/+ and P2X3−/− mice were similar (Fig. 2B); the durations at half amplitude were 2.0 ± 0.2 and 2.4 ± 0.1 ms (P > 0.05) in P2X3+/+ and P2X3−/− mice, respectively. The amplitude of the action potential in AH neurons from P2X3+/+ mice was 49 ± 2 mV, whilst in those from P2X3−/− mice this value was 48 ± 2 mV (P < 0.05). A long-lasting afterhyperpolarization followed the action potential in all AH neurons studied (Fig. 2C). The durations of the afterhyperpolarization in neurons from P2X3+/+ and P2X3−/− mice were 6.3 ± 1.3 and 6.5 ± 0.7 s (P > 0.05), respectively. The amplitude of the afterhyperolarization in P2X3+/+ AH neurons was 5.2 ± 0.7 mV and in AH neurons from P2X3−/− mice this amplitude was 5.4 ± 0.6 mV (P > 0.05). In addition, the membrane potentials and input resistances of AH neurons from P2X3+/+ and P2X3−/− mice were not significantly different (Table 1). Single electrical stimuli applied to interganglionic connectives did not elicit a synaptic response in any AH cell. However, trains of stimuli elicited sEPSPs in all AH cells in tissues from P2X3+/+ and P2X3−/− mice (Fig. 3).
Figure 2. Recordings of action potentials from AH type neurons in tissues from P2X3+/+ and P2X3−/− mice.
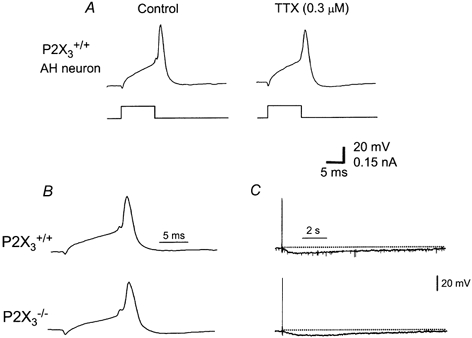
A, the action potential in AH neurons from P2X3+/+ mice is only partly blocked by tetrodotoxin (TTX). B, the action potential in AH neurons was similar in amplitude and duration in AH neurons from P2X3+/+ and P2X3−/− mice. C, afterhyperpolarization was also similar in amplitude and duration in AH neurons from P2X3+/+ and P2X3−/− mice.
Table 1.
Resting membrane potential (RMP) and input resistance (Rinput) of AH and S myenteric neurons from P2X3+/+ and P2X3−/− mice
| AH neurons | S neurons | ||||
|---|---|---|---|---|---|
| P2X3+/+ | P2X3−/ | P2X3+/+ | P2X3−/− | ||
| (n = 5) | (n = 7) | (n = 22) | (n = 22) | ||
| RMP (mV) | Mean | 63 ± 4 | 63 ± 2 | 62 ± 2 | 59 ± 2 |
| Range | 50–71 | 57–73 | 49–73 | 42–72 | |
| Rinput(MΩ) | Mean | 172 ± 35 | 153 ± 20 | 121 ± 14 | 127 ± 10 |
| Range | 93–285 | 79–212 | 57–277 | 65–204 | |
Figure 3. Short trains of stimuli (10 Hz, 0.5 s) elicit slow excitatory postsynaptic potentials in AH neurons from P2X3+/+ and P2X3−/− mice.

The amplitude and duration of the slow synaptic response was similar in neurons from both types of mice. NS indicates nerve stimulation.
ATP- and α,β-methylene ATP-induced responses in AH neurons
Local application of ATP (2 mM) from a pipette positioned near the neurons caused a depolarization that was similar in amplitude in neurons from P2X3+/+ and P2X3−/− mice (Fig. 4A and B). The ATP-induced response was blocked by pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) (not shown) and was associated with a decrease in membrane input resistance (Fig. 4A). PPADS blocks some P2X receptors and P2Y1 receptors for ATP so responses in murine neurons could be mediated by either P2X or P2Y1 receptors. To address this possibility, we tested the effect of α,β-methylene ATP, an agonist of P2X1 and P2X3 subunit-containing P2X receptors. It was found that α,β-methylene ATP caused depolarization in AH neurons in preparations from P2X3+/+ but not P2X3−/− mice (Fig. 4A and B).
Figure 4. Effect of ATP and α,β-mATP in AH neurons from P2X3+/+ and P2X3−/− mice.
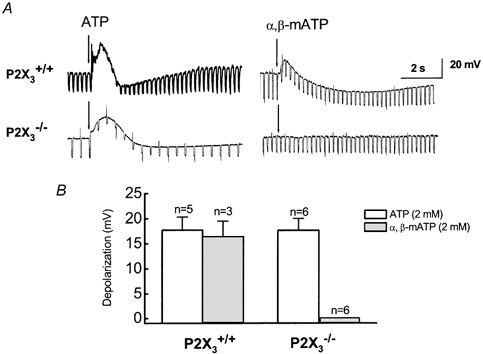
A, representative responses caused by ATP and α,β-mATP. ATP depolarized AH neurons from both types of mice. α,β-mATP caused depolarization of AH neurons in tissues from P2X3+/+ but not P2X3−/− mice. B, pooled data from experiments illustrated in A. The amplitude of ATP-induced depolarizations was similar in AH neurons from P2X3+/+ and P2X3−/− mice whilst α,β-mATP induced depolarization in AH neurons from P2X3+/+ but not P2X3−/− mice.
Properties of murine myenteric S neurons
The action potential in S neurons was completely blocked by TTX (Fig. 5A). The action potential duration at half amplitude in S neurons from both P2X3+/+ (n = 9) and P2X3−/− (n = 13) mice was 1.6 ± 0.1 ms (P > 0.05). The action potential in all S neurons was followed by a brief afterhyperpolarization (Fig. 5B). The amplitude of the action potential in S neurons from P2X3+/+ mice was 45 ± 2 mV and in those from P2X3−/− mice it was 41 ± 2 mV (P > 0.05). The resting membrane potential and input resistance of S neurons from P2X3+/+ and P2X3−/− mice were not significantly different (Table 1).
Figure 5. Action potentials in S neurons.
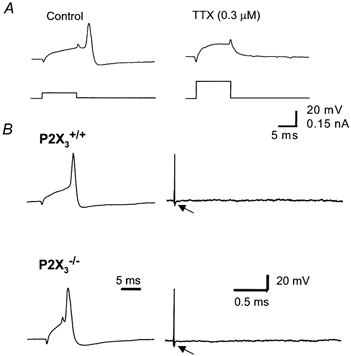
A, the action potential in S neurons from P2X3+/+ mice was blocked completely by tetrodotoxin (TTX), even when the amplitude of the depolarizing current pulse was increased. B, the action potential was similar in amplitude in S neurons from P2X3+/+ and P2X3−/− mice. The action potential in S neurons was followed by a brief afterhyperpolarization (arrow).
Single stimuli applied to internodal nerve strands evoked fEPSPs in S neurons from P2X3+/+ and P2X3−/− mice (Fig. 6A). The mean amplitude of fEPSPs recorded from neurons in P2X3+/+ and P2X3−/− tissues was not significantly different (Fig. 6B). All fEPSPs were inhibited, but not completely blocked, by the nicotinic receptor antagonist mecamylamine (10 μM)(Fig. 6A and B). Similarly, fEPSPs in tissues from P2X3+/+ and P2X3−/− mice were partly inhibited by PPADS (Fig. 7A and B). ATP caused a depolarization that was similar in amplitude in S neurons from P2X3+/+ and P2X3−/− mice (Fig. 8). However, α,β-methylene ATP did not cause depolarization in S neurons in tissues from either type of mouse (Fig. 8).
Figure 6. Fast excitatory postsynaptic potentials (fEPSPs) recorded from S neurons in P2X3+/+ and P2X3-/- mice.

A, fEPSPs are reduced in amplitude by mecamylamine (10 μM). B, pooled data from experiments shown in A. There was no significant difference in the amplitude of fEPSPs recorded from S neurons from P2X3+/+ and P2X3−/− mice. Mecamylamine reduced the amplitude of the fEPSP in all S neurons but it did not completely block the fEPSP. * Significantly different from fEPSP amplitude in the absence of mecamylamine.
Figure 7. fEPSPs recorded from S neurons in P2X3+/+ and P2X3−/− mice.
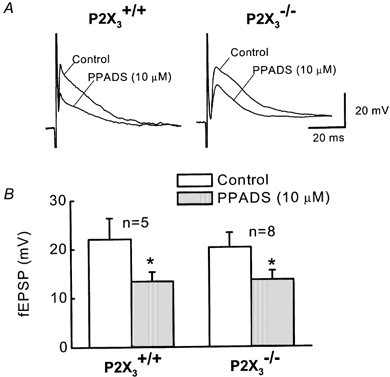
A, fEPSPs recorded from S neurons in tissues from P2X3+/+ and P2X3−/− mice are inhibited but not completely blocked by PPADS. B, pooled data from experiment illustrated in A. PPADS produces a significant inhibition of fEPSPs but it does not completely block the synaptic response. There was no significant difference in the amplitude of the fEPSPs recorded from S neurons in P2X3+/+ and P2X3−/− tissues.
Figure 8. ATP- and α,β-methylene ATP-induced depolarizations in S neurons.

A, ATP, but not α,β-methylene ATP, (α,β-mATP) causes a depolarization of S neurons. B, there were no significant differences in the amplitude of ATP-induced depolarizations in S neurons from P2X3+/+ and P2X3−/− mice. α,β-methylene ATP did not cause a depolarization in any S neuron tested.
Peristaltic activity
Raising intraluminal pressure (1.25-5 mmHg) increased the frequency and amplitude of phasic contractions in ileal segments from P2X3+/+ and P2X3−/− mice (Fig. 9). Visual inspection revealed that these contractions propagated in an oral to aboral direction and these propagated contractions were associated with fluid expulsion from the aboral end of the segment. This response is similar to that reported previously by others studying peristalsis in segments of mouse intestine maintained in vitro (Huizinga et al. 1998; Abdu et al. 2002).
Figure 9. Representative recordings of distention-induced phasic contractions in small intestinal segments from P2X3+/+ and P2X3−/− mice.
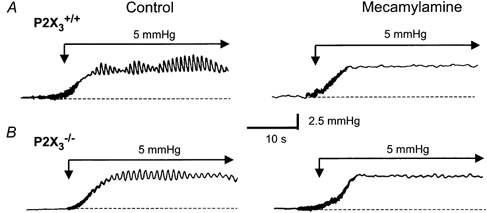
A, recordings of phasic contractions caused by a 5 mmHg increase in intraluminal pressure. The phasic contractions were blocked by the nicotinic receptor antagonist mecamylamine (10 μM). B, pressure-induced phasic contractions in an intestinal segment from a P2X3−/− mouse. Contraction frequency and amplitude were smaller than in the P2X3+/+ tissue but these contractions were also blocked by mecamylamine.
The propagated contractions required nicotinic cholinergic neurotransmission, as the nicotinic receptor antagonist mecamylamine (10 μM) reduced the frequency and amplitude of peristaltic contractions in tissues from P2X3+/+ and P2X3−/− mice (Figs 9, 10A and 10B). P2 receptor-dependent mechanisms contributed to peristalsis in tissues from P2X3+/+ mice as PPADS (10 μM) inhibited pressure-induced contractions in these tissues. The frequency and amplitude of peristaltic contractions were reduced in tissues from P2X3−/− mice compared to the values in tissues from P2X3+/+ mice (Fig. 9 and Fig. 10). PPADS did not produce an additional reduction in peristaltic contractions in tissues from P2X3−/− mice (Fig. 10C and D).
Figure 10. Pressure-response curves in ileal segments.

The curves were obtained from experiments illustrated in Fig. 9. A, pressure-related increases in the peak amplitude of contractions. The peak amplitude in tissues from P2X3−/− mice was smaller than that in P2X3+/+ mice (* P < 0.05). Mecamylamine (10 μM) reduced the peak contractions in tissues from P2X3+/+ and P2X3−/− mice (# P < 0.05). B, pressure-related increases in contraction frequency were reduced in tissues from P2X3−/− mice (* P < 0.05). Mecamylamine reduced contraction frequency in tissues from P2X3+/+ and P2X3−/− mice (# P < 0.05). C, peak amplitude of phasic contractions was smaller in tissues from P2X3−/− mice (* P < 0.05) but PPADS (10 μM) did not further reduce contraction amplitude in these tissues. PPADS reduced the amplitude of phasic contractions in tissues from P2X3+/+ mice (# P < 0.05). D, the frequency of pressure-induced phasic contractions was smaller in P2X3−/− tissues (* P < 0.05) but PPADS did not cause a decrease in contraction frequency. PPADS reduced contraction frequency in tissues from P2X3+/+ mice (# P < 0.05).
Drug-induced longitudinal muscle contractions
Disruption of peristalsis in tissues from P2X3−/− mice could be due to alterations in contractile mechanisms in intestinal smooth muscle. To test this possibility, longitudinal muscle contractions caused by the muscarinic receptor agonist, bethanechol (BeCh) and by nicotine were studied in vitro. BeCh activates muscarinic receptors on longitudinal smooth muscle to cause muscle contractions and BeCh concentration-response curves obtained in P2X3+/+ and P2X3−/− mice were similar (Fig. 11A). BeCh-induced contractions were completely blocked by scopolamine, verifying that this agonist was acting at muscarinic receptors (Fig. 11A). In order to assess the function of excitatory motoneuron input to the longitudinal muscle, nicotine was used to stimulate motor neurons. Nicotine caused concentration-dependent contractions of the muscle layer and there were no differences between curves obtained in P2X3+/+ and P2X3−/− tissues (Fig. 11B). The contractions caused by nicotine were partly reduced by TTX (Fig. 11B). Nicotine-induced contractions in P2X3+/+ and P2X3−/− tissues were completely blocked by both mecamylamine and scopolamine (data not shown). These data indicate that nicotine acted on cholinergic motoneurons to cause neurogenic contractions of the longitudinal muscle. ATP (0.01-1 mM) did not cause contractions of the longitudinal muscle in tissues from P2X3+/+ or P2X3−/− mice.
Figure 11. Bethanechol- and nicotine-induced longitudinal muscle contractions in ileal segments from P2X3+/+ and P2X3-/- mice.

A, BeCh caused concentration-dependent contractions of the longitudinal muscle layer that were completely blocked by the muscarinic antagonist scopolamine (* Significantly different from responses in the absence of scopolamine). There were no significant differences in curves obtained from P2X3+/+ and P2X3−/− mice. B, nicotine caused concentration-dependent longitudinal muscle contractions that were inhibited by tetrodotoxin. There were no significant differences in nicotine-induced responses obtained in tissues from P2X3+/+ and P2X3−/− tissues.
Gastrointestinal transit
The distribution of radioactive marker in the stomach and small intestine of P2X3+/+ and P2X3−/− mice is shown in Fig. 12. These data show that the leading edge of marker in the small intestine of P2X3+/+ mice reached the ninth intestinal segment whilst in P2X3−/− mice the marker progressed only as far as the seventh segment. However, when the geometric centre of the marker distribution was calculated there was no difference in gastrointestinal transit between P2X3+/+ and P2X3−/− mice. The geometric centre in P2X3+/+ mice was 4.6 ± 0.8 (n = 6) while in P2X3−/− mice this value was 4.4 ± 0.4 (n = 7; P > 0.05). There was no significant difference between the two groups of mice in gastric emptying of the liquid marker. Gastric emptying in P2X3+/+ mice was 68 ± 12 % whilst in P2X3−/− mice this value was 78 ± 8 % (P > 0.05)
Figure 12. Gastrointestinal transit of 51Cr.
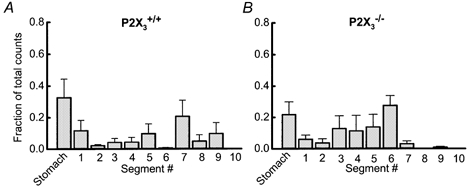
51Cr was administered by gavage to conscious P2X3+/+ (A) and P2X3−/− (B) mice. There were no significant differences in gastric emptying or gastrointesintal transit of the radioactive marker in the two groups of mice.
DISCUSSION
P2X receptors contribute to fast synaptic excitation in the enteric nervous system (Galligan & Bertrand, 1994; Le Pard et al. 1997; Johnson et al. 1999). However, studies of the contribution of different P2X receptor subunits to functional P2X receptors are hampered due to the lack of drugs of sufficient selectivity for P2X receptors composed of different receptor subunits. There are at least seven different P2X receptor subunits and homomeric or heteromeric P2X receptors have unique functional and pharmacological properties (Khakh et al. 2001; North & Surprenant, 2000). In the present study, we made use of mice in which the P2X3 subunit gene had been deleted. These studies were designed to determine whether P2X3 subunit-containing receptors were essential for fast excitatory synaptic transmission in the myenteric plexus and for normal intestinal motility. We used RT-PCR techniques to verify successful deletion of the P2X3 receptor subunit gene. These studies showed that whilst the P2X3 gene was expressed in the ileum and colon of P2X3+/+ mice, it was absent in tissues obtained from the P2X3−/− mice. Furthermore, P2X3 gene deletion did not alter expression of the P2X2 receptor subunit.
Electrophysiological properties of murine myenteric neurons
Most of what is known about the electrophysiological properties of enteric neurons has been derived from studies using guinea-pig tissues. There has been only one study describing the properties of enteric neurons in the mouse gastrointestinal tract and these studies used the myenteric plexus of the colon (Furukawa et al. 1986). Therefore, we characterized the basic properties of myenteric neurons in the small intestine of P2X3+/+ and P2X3−/− mice in order to determine whether P2X3 subunit gene deletion altered these properties.
In the mouse ileum, AH neurons had a broad action potential that was only partly reduced by TTX. The action potential has a prominent calcium component that accounts for the long duration and action potential shoulder in AH neurons from guinea-pig ileum and colon (Furness et al. 1998; Nuragli et al. 2003), rat colon (Browning & Lees, 1996), pig ileum (Cornelissen et al. 2001), mouse colon (Furakawa et al. 1986) and human colon (Brookes et al. 1987). Therefore, the calcium shoulder is a general property of AH neurons in the gastrointestinal tract of a number of species. Although not tested in this study, the long duration and TTX-resistance of mouse ileal myenteric AH neurons suggests that there is also a calcium component to the action potential. The action potential in mouse ileal myenteric AH neurons was followed by an afterhyperpolarization that lasted more than 2 s. This property is also similar to that in AH neurons in the myenteric plexus of the guinea-pig ileum and colon (Hirst et al. 1974; Furness et al. 1998;) rat colon (Browning & Lees, 1996), pig ileum (Cornelissen et al. 2001), mouse colon (Furakawa et al. 1986) and human colon (Brookes et al. 1987).
In guinea-pig and pig intestine, fEPSPs can be evoked in some AH neurons (Liu et al. 1997; Cornelissen et al. 2001) while sEPSPs can be evoked in nearly all AH neurons in the guinea-pig gastrointestinal tract (Furness et al. 1998). We found that single electrical stimuli did not elicit synaptic responses in mouse ileal AH neurons. As we recorded from only a small number of AH neurons, it is possible that we did not sample those neurons receiving fast synaptic input or that we did not stimulate the interganglionic connectives providing the fast synaptic inputs to the neurons that we did sample. However, we were able to elicit sEPSPs in all AH neurons studied. Slow synaptic excitation is the predominant synaptic mechanism in guinea-pig AH neurons (Kunze et al. 1993). Taken together, these data indicate that the properties of AH neurons in the mouse ileal myenteric plexus are similar to those identified in the gastrointestinal tract of other species.
S type enteric neurons have an action potential that is completely blocked by TTX and all S neurons exhibit fEPSPs following single stimuli applied to the interganglionic connectives in the myenteric plexus (Hirst et al. 1974; Bornstein et al. 1994; Galligan et al. 2000). In the mouse myenteric plexus, we identified neurons that received fast excitatory synaptic input and the action potential in these neurons was completely blocked by TTX. Therefore, we concluded that these were S neurons. We also found that the fEPSP in S neurons was only partly blocked by mecamylamine, a nicotinic cholinergic receptor antagonist, and that fEPSPs were also inhibited by PPADS. These data indicate that both acetylcholine and ATP are fast synaptic transmitters in the mouse small intestine as they are in the guinea-pig intestine (Le Pard et al. 1997; Johnson et al. 1999) but there may be additional fast neurotransmitters including 5-HT (Galligan et al. 2000).
P2X-mediated depolarization of AH neurons in P2X3+/+ and P2X3−/− tissues
ATP caused a rapidly developing depolarization in all AH neurons. This depolarization was associated with a decrease in input resistance and was blocked by PPADS. These data are consistent with this response being mediated by either a P2X or P2Y1 receptor. α,β-Methylene ATP is an agonist at homomeric P2X1, homomeric P2X3 or heteromeric P2X2/3 receptors, but does not activate P2Y receptors (North & Surprenant, 2000). We found that ATP caused a depolarization of AH neurons in P2X3+/+ and in P2X3−/− tissues, whilst α,β-methylene ATP caused a depolarization of AH neurons in P2X3+/+ but not in P2X3−/− tissues. These data indicate that P2X3 subunits contribute to the P2X receptor expressed by murine AH neurons. However, sensory neurons and some sympathetic neurons also express P2X2/3 heteromeric receptors (Lewis et al. 1995; Zhong et al. 2000), and the loss of response to α,β-methylene ATP in P2X3−/− mice could result from the loss of P2X2/3 heteromeric receptors in murine AH neurons. Other subunits must also be present in order for an ATP-induced depolarization to persist in AH neurons from P2X3−/− mice. P2X2 subunits remaining in P2X3−/− mice could account for this ATP sensitivity. Our suggestion that the P2X2 subunits are expressed in AH neurons is supported by recent immunohistochemical studies in guinea-pig gastrointestinal tissues. These studies showed that P2X2 subunits are localized to neurons that also contain the calcium binding protein calbindin (Castelucci et al. 2002). Calbindin is a protein marker for most AH neurons in the guinea-pig gastrointestinal tract (Furness et al. 1998). However, it has also been shown that P2X3 subunits are not found in calbindin-containing neurons in the guinea-pig gastrointestinal tract but that these subunits are localized to calretinin- and nitric oxide synthase-containing cells (Nassauw et al. 2002; Poole et al. 2002). Calretinin and nitric oxide synthase are contained in S type neurons and are markers for interneurons and motoneurons (Brookes, 2001). It is possible that receptor expression and subunit composition of P2X receptors in subsets of neurons are different in the mouse and guinea-pig small intestine. A detailed study of the neurochemical content and receptor expression of subsets of murine enteric neurons is needed to address this issue.
Fast synaptic transmission in the myenteric plexus is not altered in P2X3−/− mice
We hypothesized that a P2X receptor contributes to the fEPSP recorded from S neurons in the mouse small intestine. This hypothesis was based on the finding that fEPSPs were only partly inhibited by mecamylamine, indicating that a neurotransmitter in addition to acetylcholine mediates the fEPSP. As fEPSPs were also reduced by PPADS, the additional neurotransmitter in the mouse myenteric plexus is likely to be ATP. It is unlikely that P2X3 receptor subunits contribute to the P2X receptor expressed by S neurons as the properties of fEPSPs in tissues from P2X3+/+ and P2X3−/− mice were identical. Furthermore, S neurons in tissues from P2X3+/+ and P2X3−/− mice were depolarized by ATP but not by α,β-methylene ATP. α,β-Methylene ATP insensitivity suggests that the P2X receptor in S neurons is a P2X2 homomeric receptor (Zhou & Galligan, 1996).
Peristalsis is impaired in the small intestine of P2X3−/− mice
We used fluid distention-evoked peristalsis in segments of the mouse small intestine to assess the contributions of P2X3 subunit-containing receptors to neural pathways mediating a motor reflex in vitro. We found that instilling small volumes of saline into the lumen of an ileal segment evoked a series of phasic contractions and the frequency and amplitude of these contractions were pressure related. The distention-evoked contractions in all tissues were blocked by mecamylamine, indicating a requirement for synaptic transmission mediated at nicotinic cholinergic receptors in myenteric ganglia. These data are similar to those reported previously by others who have studied peristalsis in the mouse intestine (Huzinga et al. 1998; Abdu et al. 2002).
The new data reported here show that both the amplitude and frequency of distention-evoked contractions are reduced in preparations from P2X3−/− mice. This result suggests that P2X receptors containing the P2X3 subunit contribute to the neural mechanisms mediating peristalsis. This conclusion is supported by data obtained using PPADS to block P2X receptors in the peristalsis studies. PPADS inhibited peristalsis in tissues from P2X3+/+ mice to a level comparable to that recorded from P2X3−/− tissues so that pharmacological inhibition of P2X-mediated mechanisms mimics the effects of P2X3 subunit gene deletion. PPADS did not alter peristalsis in tissues from P2X3−/− mice as the target for PPADS blockade is missing.
Impairment of peristalsis in the intestine of P2X3−/− mice could be due to: (1) loss of sensory mechanisms activated by distention of the intestine; (2) disruption of synaptic transmission in the myenteric plexus; or (3) disruption of neuromuscular transmission. This latter issue was addressed in studies of drug-induced contractions of the longitudinal muscle in whole-segment preparations of the small intestine. Contractions caused by the muscarinic receptor agonist BeCh were identical in tissues from P2X3+/+ and P2X3−/− mice. We used BeCh to mimic the effects of endogenously released acetylcholine onto longitudinal muscle to cause contractions. The data show that P2X3 gene deletion did not disrupt signalling mechanisms coupled to muscarinic receptors in the longitudinal muscle layer. Furthermore, P2X3 gene deletion did not disrupt contractile mechanisms in the longitudinal muscle. Nicotine activates cholinergic excitatory motoneurons supplying the longitudinal muscle layer as nicotine-induced longitudinal muscle contractions were inhibited by TTX and by scopolamine, a muscarinic receptor antagonist. Nicotine-induced contractions of the longitudinal muscle were similar in P2X3+/+ and P2X3−/− mice indicating that P2X3 gene deletion did not cause a general disruption of excitatory neurotransmission to the longitudinal muscle layer in the ileum of P2X3−/− mice. While there does not appear to be a general disruption of excitatory contractile mechanisms in the longitudinal muscle layer of P2X3−/− mice, these data do not rule out the possibility that there is a disruption of excitatory contractile mechanisms in the circular muscle layer in the same mice.
Mecamylamine and PPADS inhibited peristalsis in tissues from P2X3+/+ mice and these drugs inhibit fast synaptic excitation in myenteric ganglia of the guinea-pig and mouse. Therefore, P2X3 gene deletion could inhibit fast synaptic transmission in the myenteric plexus and this effect could account for impaired peristalsis seen in tissues from P2X3−/− mice. However, it was found that the properties of fEPSPs recorded from myenteric neurons in P2X3+/+ and P2X3−/− mice were identical and that fast synaptic transmission was unaffected by P2X3 gene deletion. Alterations in fast synaptic transmission are unlikely to account for impaired peristalsis in P2X3−/− mice.
Studies in guinea-pig small intestine have shown that AH neurons are sensory neurons (Furness et al. 1998). In addition, ATP acting at an unidentified P2X receptor activates mucosal endings and cell bodies of AH neurons (Bertrand & Bornstein, 2002). ATP may be released in the mucosa or in myenteric ganglia to stimulate sensory neurons resulting in activation of neural pathways mediating motor reflexes (Bertrand & Bornstein, 2002). Inhibition of P2X receptors in sensory neurons from P2X3−/− mice could contribute to impairment of peristalsis in these animals. Murine AH neurons have properties similar to those in guinea-pig intestine and AH neurons in the mouse intestine may also be sensory neurons. ATP caused depolarization of AH neurons from P2X3+/+ and P2X3−/− mice. As discussed above, AH neurons in P2X3+/+ mice may express P2X2/3 heteromeric receptors while the receptor in AH neurons from P2X3−/− would be a P2X2 homomer. Although loss of the P2X3 subunit alters the pharmacological properties of the P2X receptor expressed by AH neurons, these cells still respond to ATP and therefore, peristalsis should be unaffected in the intestine of P2X3−/− mice. However, ATP is approximately 10-fold less potent at activating P2X2 homomeric receptors compared to P2X2/3 heteromeric receptors (North & Surprenant, 2000). The amount of ATP released during pressure-induced distention of the ileal segments may be insufficient to fully activate the P2X receptors on AH neurons in P2X3−/− mice and peristalsis would be impaired in these animals.
Changes in peristalsis studied in vitro are not reflected in alterations in gastrointestinal propulsion of saline in fasted mice. This conclusion is based on data from the transit studies where we showed that propulsion of Na51CrO4 in saline was similar in the P2X3+/+ and P2X3−/− mice. The gastrointestinal transit assay used in the present study may not be sensitive enough to detect in vivo motility alterations associated with P2X3 subunit gene deletion. Alternatively, it may be necessary to study gastrointestinal transit in the freely fed state using a solid marker for gastrointestinal propulsion in order to reveal motility changes associated with P2X3 gene deletion.
Summary and conclusions
Murine myenteric S and AH neurons exhibit the same general electrophysiological properties as S and AH neurons in the guinea-pig gastrointestinal tract. AH neurons do not receive a prominent fast synaptic input, the action potential is partly TTX resistant and is followed by a long-lasting afterhyperpolarization. P2X3 gene deletion alters the pharmacological properties (loss of α,β-methylene ATP sensitivity) of the P2X receptor expressed by AH neurons. In contrast, the action potential in S neurons is blocked by TTX and S neurons receive fast excitatory synaptic input mediated by acetylcholine and ATP. P2X3 gene deletion does not alter fEPSPs recorded from S neurons. Distention-evoked peristalsis is impaired in the ileum of P2X3−/− mice and this could be due to a decrease in the sensitivity of sensory (AH) neurons to ATP released from the intestine in response to fluid distention. The decrease in ATP sensitivity of sensory neurons may be attributed to a change in the subunit composition, or to a complete loss of, sensory neuron P2X receptors.
REFERENCES
- Abdu F, Hicks GA, Hennig G, Allen JP, Grundy D. Somatostatin sst(2) receptors inhibit peristalsis in the rat and mouse jejunum. Am J Physiol Gastrointest Liver Physiol. 2002;282:G624–633. doi: 10.1152/ajpgi.00354.2001. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Bornstein JC. ATP as a putative sensory mediator: activation of intrinsic sensory neurons of the myenteric plexus via P2X receptors. J Neurosci. 2002;22:4767–4775. doi: 10.1523/JNEUROSCI.22-12-04767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian X, Bertrand PP, Bornstein JC. Descending inhibitory reflexes involve P2X receptor-mediated transmission from interneurons to motor neurons in guinea-pig ileum. J Physiol. 2000;528:551–560. doi: 10.1111/j.1469-7793.2000.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Kunze WA. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? J Auton Nerv Syst. 1994;48:1–15. doi: 10.1016/0165-1838(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Ewart WR, Wingate DL. Intracellular recordings from myenteric neurones in the human colon. J Physiol. 1987;390:305–318. doi: 10.1113/jphysiol.1987.sp016702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Lees GM. Myenteric neurons of the rat descending colon: electrophysiological and correlated morphological properties. Neuroscience. 1996;73:1029–1047. doi: 10.1016/0306-4522(96)00118-2. [DOI] [PubMed] [Google Scholar]
- Castelucci P, Robbins HL, Poole DP, Furness JB. The distribution of purine P2X(2) receptors in the guinea-pig enteric nervous system. Histochem Cell Biol. 2002;117:415–422. doi: 10.1007/s00418-002-0404-4. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu Q-M, Dunn PM, Zhong Y, Novavic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford APDW. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Cornelissen W, De Laet A, Kroese AB, van Bogaert PP, Scheuermann DW, Timmermans JP. Excitatory synaptic inputs on myenteric Dogiel type II neurones of the pig ileum. J Comp Neurol. 2001;432:137–154. doi: 10.1002/cne.1093. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WAA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Taylor GS, Bywater RA. An intracellular study of myenteric neurons in the mouse colon. J Neurophysiol. 1986;55:1395–1406. doi: 10.1152/jn.1986.55.6.1395. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Bertrand PP. ATP mediates fast synaptic transmission in enteric nerves. J Neurosci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan JJ, Le Pard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000;81:97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- Hirst GD, Holman ME, Spence I. Two types of neurones in the myenteric plexus of duodenum in the guinea-pig. J Physiol. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GD, Johnson SM, Van Helden DF. The slow calcium-dependent potassium current in a myenteric neurone of the guinea-pig ileum. J Physiol. 1985;361:315–337. doi: 10.1113/jphysiol.1985.sp015648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HZ, Gao N, Lin Z, Gao C, Liu S, Ren J, Xia Y, Wood JD. P2X(7) receptors in the enteric nervous system of guinea-pig small intestine. J Comp Neurol. 2001;440:299–310. doi: 10.1002/cne.1387. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Ambrous K, Der-Silaphet T. Co-operation between neural and myogenic mechanisms in the control of distension-induced peristalsis in the mouse small intestine. J Physiol. 1998;506:843–856. doi: 10.1111/j.1469-7793.1998.843bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PJ, Shum OR, Thornton PD, Bornstein JC. Evidence that inhibitory motor neurons of the guinea-pig small intestine exhibit fast excitatory postsynaptic potentials mediated via P2X receptors. Neurosci Lett. 1999;266:169–172. doi: 10.1016/s0304-3940(99)00275-x. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PPA. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Ann Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Furness JB, Bornstein JC. Simultaneous intracellular recordings from enteric neurons reveal that myenteric AH neurons transmit via slow excitatory postsynaptic potentials. Neuroscience. 1993;55:685–694. doi: 10.1016/0306-4522(93)90434-h. [DOI] [PubMed] [Google Scholar]
- Le Pard KJ, Galligan JJ. Analysis of fast synaptic pathways in myenteric plexus of guinea-pig ileum. Am J Physiol. 1999;276:G529–538. doi: 10.1152/ajpgi.1999.276.2.G529. [DOI] [PubMed] [Google Scholar]
- Le Pard KJ, Messori E, Galligan JJ. Purinergic (P2X) fast excitatory postsynaptic potentials in myenteric neurons of guinea-pig: distribution and pharmacology. Gastroenterology. 1997;113:1522–1534. doi: 10.1053/gast.1997.v113.pm9352854. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Liu MT, Rothstein JD, Gershon MD, Kirchgessner AL. Glutamatergic enteric neurons. J Neurosci. 1997;17:4764–4784. doi: 10.1523/JNEUROSCI.17-12-04764.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods. 1981;6:211–217. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
- Monro RL, Bertrand PP, Bornstein JC. ATP and 5-HT are the principal neurotransmitters in the descending excitatory reflex pathway of the guinea-pig ileum. Neurogastroenterol Motil. 2002;14:255–264. doi: 10.1046/j.1365-2982.2002.00325.x. [DOI] [PubMed] [Google Scholar]
- Morita K, North RA, Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. J Physiol. 1982;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassauw LV, Brouns I, Adriaensen D, Burnstock G, Timmermans JP. Neurochemical identification of enteric neurons expressing P2X3 receptors in the guinea-pig ileum. Histochem Cell Biol. 2002;118:193–203. doi: 10.1007/s00418-002-0447-6. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Ann Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Nurgali K, Furness JB, Stebbing MJ. Correlation of electrophysiology, shape and synaptic properties of myenteric AH neurons of the guinea-pig distal colon. Auton Neurosci. 2003;103:50–64. doi: 10.1016/s1566-0702(02)00212-6. [DOI] [PubMed] [Google Scholar]
- Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB. The distribution of P2X3 purine receptor subunits in the guinea-pig enteric nervous system. Auton Neurosci. 2002;101:39–47. doi: 10.1016/s1566-0702(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Distension-evoked ascending and descending reflexes in the circular muscle of guinea-pig ileum: an intracellular study. J Auton Nerv Syst. 1990;29:203–217. doi: 10.1016/0165-1838(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Walsh M, Smith TK. Purinergic and cholinergic neuro-neuronal transmission underlying reflexes activated by mucosal stimulation in the isolated guinea-pig ileum. J Physiol. 2000;522:321–331. doi: 10.1111/j.1469-7793.2000.t01-1-00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Dunn PM, Burnstock G. Guinea-pig sympathetic neurons express varying proportions of two distinct P2X receptors. J Physiol. 2000;523:391–402. doi: 10.1111/j.1469-7793.2000.t01-1-00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Galligan JJ. P2X purinoceptors on cultured myenteric neurons of guinea-pig small intestine. J Physiol. 1996;496:719–729. doi: 10.1113/jphysiol.1996.sp021722. [DOI] [PMC free article] [PubMed] [Google Scholar]


