Abstract
Fetal lung development is dependent upon secretion of liquid into the future airways which must be cleared at birth to establish air-breathing. Aquaporins (AQP) 1, 3, 4 and 5 are membranous water channel proteins that are present in the lung after birth in rodents, with little expression before birth. Our aim was to describe the changes in AQP1, 3, 4 and 5 expression and protein levels in the fetal lung of a long-gestation species (sheep) and in response to physiological factors known to alter fetal lung liquid dynamics. Both mRNA and high protein levels were detected for AQP1, 3, 4 and 5 by day 100 (term is ≈150 days in ovine fetuses). A cortisol infusion (120–131 days) significantly (P < 0.05) increased AQP1 (0.9 ± 0.2 (n = 4) vs.1.8 ± 0.3 (n = 5)) and AQP5 (8.8 ± 0.6 vs. 14.1 ± 1.2) mRNA levels in fetal lung (measured by real-time PCR). Ten days of tracheal obstruction significantly (P < 0.05) decreased AQP5 mRNA levels (6.1 ± 0.9 (n = 5) vs. 2.7 ± 0.3 (n = 5)). Immunohistochemistry was used to show that protein levels changed in parallel with the mRNA changes. These findings suggest that AQPs could be involved in lung liquid production and reabsorption during fetal development in long-gestation species.
During fetal life, the future airways of the lung are filled with a liquid that plays a crucial role in the growth and development of the lungs by maintaining them in an expanded state. Lung liquid is secreted across the pulmonary epithelium into the lung lumen due to the osmotic gradient established by the net movement of Cl− in the same direction. It is not known exactly when lung liquid secretion begins, but fluid is present by mid-gestation in fetal sheep and is secreted at 2–4 ml kg−1 h−1 between 120 days of gestation and term (≈150 days). Fetal lung liquid exits the lungs via the trachea, whereby it is either swallowed (approximately 50 %) or passes directly into the amniotic sac, where it contributes to amniotic fluid volume (Harding & Hooper, 1999).
If the fetal trachea is obstructed, which prevents the outward flow of lung liquid, the fetal lung expands with accumulated liquid. This is a potent stimulus for fetal lung growth and also greatly reduces the proportion of type-II alveolar epithelial cells (AECs). Lung liquid drainage on the other hand, deflates the lung, causes lung growth to cease, but increases the proportion of type-II AECs, possibly via type-I to type-II cell differentiation (Flecknoe et al. 2002). As a result it is now widely recognized that the degree to which the fetal lungs are expanded by lung liquid determines the growth and structural development of the lung, as well as the differentiated state of type-I and type-II AECs (Harding & Hooper, 1999). Despite the importance of lung liquid in the development of the lung, the factors controlling the movement of liquid across the pulmonary epithelium have not been fully explored. Furthermore, the effective clearance of lung liquid at birth is vital to allow the entry of air into the lungs with the onset of respiratory gas exchange. This process is largely dependent on the capability of the epithelium to reabsorb large quantities of water.
Aquaporins (AQPs) are specific water channels that allow the rapid transcellular movement of water in response to osmotic/hydrostatic pressure gradients (King & Yasui, 2002). At least four AQPs (AQP1, 3, 4 and 5) are expressed in the lungs of various species, including humans, rats, mice and rabbits, although some discrepancies exist in the specific sites of distribution of these proteins. In all species described so far (human, rat, mouse), AQP1 is expressed in the apical and basolateral membrane of the microvascular endothelium and decreased pulmonary vascular permeability has been shown in AQP1-null humans (King et al. 2002). AQP3 is expressed in the basolateral membrane of basal cells of the tracheal epithelium and in submucosal gland cell membranes in rodents, but is also found in bronchioles (apical membrane) and type-II alveolar epithelial cells of adult humans (Kreda et al. 2001). AQP4 is present in the basolateral membrane of columnar cells in the bronchi and trachea of rats but is also found in type-I AECs in humans. AQP5 is expressed in the apical membrane of type-I AECs and the apical plasma membranes of the secretory epithelium in upper airway and salivary glands (King et al. 2002); it has also been detected in type-II AECs in mice (Krane et al. 2001). In mice very low levels of AQP5 mRNA were detected before birth (Liu et al. 2002; Torday et al. 2002). The ontogeny of the AQPs has also been described throughout development in rats, but only AQP1 and a small amount of AQP4 were detected before birth (Umenishi et al. 1996; King et al. 1997; Yasui et al. 1997; Ruddy et al. 1998). Furthermore, little is known of the physiological factors controlling AQP1 mRNA expression before birth, although its expression (and protein levels) is increased in the lungs of fetal and neonatal rats following treatment with synthetic glucocorticoids (King et al. 1997; Yasui et al. 1997). In one study (Yasui et al. 1997), but not in another (King et al. 1997), AQP4 was increased by corticosteroids. In the same study (Yasui et al. 1997), β-adrenergic agents also increased AQP4.
Studies of mice, in which one or more AQP genes have been deleted, suggest that AQPs are not essential for neonatal survival (Verkman et al. 2000), although substantial defects in osmotically driven water movement can be detected in these mice. However, what is true in mice may not be true for all species, including humans (King & Yasui, 2002). Because the distributions of various AQPs in the lungs of different species are not the same as that observed in mice, it is difficult to extrapolate from ‘knockout’ studies in mice and make conclusions regarding the physiological importance of AQPs in fetal lung development and the transition to extra-uterine life at birth. This is particularly true in long-gestation species such as humans. Fetal sheep have been used as an important animal model for lung developmental studies, particularly of factors regulating the physiological development of the fetal lung (Lipsett et al. 1998; Harding & Hooper, 1999). These studies have conclusively shown that the factors controlling liquid movement into and out of the future airways are not only essential for the growth and development of the lung but also for the successful transition to extra-uterine life at birth. Similarly, corticosteroids are well known to markedly alter the structure and biomechanical properties of the lung as well as increase its ability to secrete and re-absorb liquid (Harding & Hooper, 1999). We have previously reported the cDNA sequence for ovine AQPs 1 and 3 (Wintour et al. 1998; Johnston et al. 2000). In this paper we report the sequence of ovine AQPs 4 and 5 and we show that AQPs 1, 3, 4, and 5 are expressed well before birth in the fetal sheep lung. In addition, we show that alterations in the expression of some AQPs occur when the secretion rate of fetal lung liquid is increased (by cortisol infusion) or decreased (by tracheal occlusion).
METHODS
Animals
Lung tissue was collected from either unoperated fetal sheep at different gestational ages or from chronically catheterized fetal sheep. All experimental procedures were approved by the Animal Ethics Committees of either the Howard Florey Institute or Monash University in accordance with the regulations of the National health and Medical Research Council of Australia.
Two experimental procedures, which have been described previously (Wallace et al. 1995; Nardo et al. 1998), were performed to examine, separately, the effect of increased lung expansion and cortisol infusion on AQP expression in the fetal lung. The effect of increased lung expansion was examined using two groups of chronically catheterized fetal sheep. In one group, the fetal trachea was obstructed for 10 days by occluding an exteriorized tracheal loop (118–128 days, n = 5), whereas in the second group (controls), liquid was allowed to flow through the tracheal loop unimpeded for 10 days (118–128 days, n = 5); all fetuses in this study were killed at 128 days of gestation. In a different study, two groups of fetuses were infused, intravenously, with either cortisol (n = 4) or saline (n = 5), with both infusions commencing on 120–121 days of gestation and continuing for 11 days; the cortisol infusion increased from 1.5 mg day−1 during the first 3 days to 2.5 mg day−1 for the next 5 days and 3.5 mg day−1 for the last 3 days; fetuses from this study were killed at 132 days of gestation. In addition, lungs were collected from one group of sheep, at 135 days of gestation, in which lung liquid had been allowed to flow normally through a tracheal loop from 105–135 days of gestation.
All animals were killed by an overdose of sodium pentobarbitone (Lethabarb, Arnolds, Boronia, Australia; 100 mg (kg body weight)−1). Lung tissue samples were fixed using 4 % paraformaldehyde in 0.1 M phosphate buffer, either by immersion in fixative for 4 h at room temperature or by instillation of the fixative into the lung lumen at a pressure of 20 cmH2O. Other sections of lung tissue, usually the entire left lung, were rapidly frozen in liquid nitrogen and stored at −80 °C until further use.
cDNA cloning of AQP4 and AQP5
Isolation of total RNA and RT-PCR
Total RNA was isolated from ovine brain and lung tissue using the guanidine isothiocyanate method (Chomczynski & Sacchi, 1987) and the integrity of the total RNA was verified by denaturing formaldehyde agarose gel electrophoresis (Lehrach et al. 1977). Oligonucleotide primers were synthesized by Oswel DNA service (UK) based on the aquaporin (4 and 5) sequences of rat (U14007 and RNU16245) and human (NM001650 and U46569), respectively. The sequences for the sense and antisense primers were 5′-GGGGAA GGC ATG AGTGACAGACC-3′ and 5′-ATGGGT GGA AGGAAA TCTGAG GAC-3′, respectively, for AQP4 and 5′-CCCCGC GGCCAC CAT GAAGAAGGA-3′ and 5′-ACCAGT CAGTGT GCCGTC AGCTC-3′, respectively, for AQP5.
Total RNA (3 μg) was reverse transcribed using 10 units of MuMLV reverse transcriptase, 40 ng of antisense primer, 2 μl of reverse transcription buffer (100 mm Tris-HCl, pH 8.4; 250 mm KCl; 12.5 mm MgCl2 and 0.5 mg ml−1 BSA) in a total volume of 10 μl at 42 °C for 1 h. All the reverse transcribed products (10 μl) were used for the polymerase chain reaction (PCR). Hot start (94 °C for 1 min) PCR amplifications were carried out on a Perkin Elmer, Cetus, Thermal Cycler Model 480 for 30 cycles with each cycle consisting of a denaturing step at 94 °C for 1 min, an annealing step at 55 °C for 2 min and an elongation step at 68 °C for 2 min followed by a final extension step of 10 min at 68 °C.
Cloning and sequencing of cDNAs
The PCR products were cloned into pT7Blue (R) vector (Novagen, USA) using the procedures described by the supplier. The ligated products were then transformed into the Escherichia coli, DH5α: supE44 ΔlacU169 (Φ80lacZΔm15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 (Wintour et al. 1998) and the recombinants were selected on an LB-ampicillin (50 μg ml−1) plate supplemented with IPTG and X-Gal. The plasmids isolated from the recombinant clones were then subjected to Sanger di-deoxy DNA sequencing (Sanger et al. 1977), using M13/pUC forward and reverse primers on an automated DNA sequencer (Applied Biosystems, Model 373A).
Real-time PCR
Total RNA was isolated from frozen tissue (Chomczynski & Sacchi, 1987) and each sample (20 μg of total RNA) was DNase treated (Johnston et al. 2000), before a portion (0.1 μg) was reverse transcribed.
AQP mRNA levels were quantified by real-time PCR using an ABI PRISM 7700 Sequence Detector (PE Applied Biosystems); endogenous 18S ribosomal RNA was used as an internal reference (Johnston et al. 2000). A multiplex comparative CT method was employed, where CT values reflect the cycle number at which DNA amplification was first detected. In the multiplex reaction, the target gene and 18S gene were detected in each well, where primers were limited for 18S. We demonstrated no effect on CT values when we compared reactions in which the 18S primer was either limited or non-limited. For the comparative CT method, a validation experiment was performed where we demonstrated approximately equal efficiencies of target gene and 18S gene amplification in the one reaction mix, over different initial template concentrations.
The AQP primers and probes used for real-time PCR were designed using Primer Express (PE Applied Biosystems); the accession numbers (GenBank/EMBL) for each of the AQP sequences are as follows: AQP1: AF00937; AQP3: AF123316; AQP4: AY177612; AQP5: AY177613. All AQP primers were generated by Pacific Oligos, whereas all TaqMan probes and 18S primers were supplied by PE Applied Biosystems (Table 1). FAM (6-carboxy fluorescein) was attached at the 5′ end of each TaqMan probe whereas TAMRA (6-carboxy-tetramethylrhodamine) was attached at the 3′ end. VIC was used as the fluorescent tag for the 18S probe.
Table 1.
Primer and probe sequences and concenrations used in real time PCR
| Genes | Sequence(5′–3′) | Nucleotide position | Final concentraion (nm) |
|---|---|---|---|
| 18S | |||
| Forward primer | 5′-CGGCTACCACATCCAAGGAA-3′ | N/A | 40 |
| Reverse primer | 5′-GCTGGAATTACGCGGCT-3′ | N/A | 20 |
| Taqman probe | 5′TGCTGGCACCAGACTTGCCCTC-3′ | N/A | 50 |
| AQP 1 | |||
| Forward primer | 5′CCATCGTCGCCACTGTCAT-3′ | 234–342 | 300 |
| Reverse primer | 5′-GAGGCCAAGCGAGTTGTCA-3′ | 388-370 | 300 |
| Taqman probe | 5′-TCTCGGGCATCACTCCTCTCTGC-3′ | 345–368 | 75 |
| AQP 3 | |||
| Forward primer | 5′-GGGTGCCCATTGTCTCTCC-3′ | 500–518 | 900 |
| Reverse primer | 5′-AGCCCGATCATGAGCTGGTACA-3′ | 568-547 | 900 |
| Taqman probe | 5′-TCCTGGTTCCATCGCGGGTG-3′ | 521–540 | 75 |
| AQP 4 | |||
| Forward primer | 5′-AGAATTTCTGGCCATGCTTATTTT-3′ | 135–158 | 300 |
| Reverse primer | 5′-TCTGCTCCACCCCAGTTGA-3′ | 203–185 | 900 |
| Taqman probe | 5′-TCCTGCTCAGCCTGGATCCACC-3′ | 161–183 | 75 |
| AQP 5 | |||
| Forward primer | 5′-CGCCGCAATCCTCTATGG-3′ | 315–332 | 300 |
| Reverse primer | 5′-GCTGGAATTACCGCGGCT-3′ | 396–376 | 900 |
| Taqman probe | 5′-CAATGCCCGAAGCAATCTGGCTG-3′ | 345–367 | 100 |
For each AQP TaqMan probe, FAM (6-carboxy fluorescein) was attached at the 5′ end and TAMRA (6-carboxy-tetramethyIrhodamine) was attached at the 3′ end. Accession numbers (GenBank/EMBL) are as follows: AQP1-AF00937, AQP3-AF123316. VIC was used for 18S.
Real-time PCR was carried out in a final volume of 25 μl, containing TaqMan Universal PCR Master Mix (including passive reference), 18S probe, 18S forward primer, 18S reverse primer, and 5 ng cDNA sample. The concentrations of each AQP primer and probes are shown in Table 1. The initial amplification conditions were 50 °C for 2 min and 95 °C for 10 min, which was followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. When comparing AQP mRNA concentrations from fetal and adult lung tissue, the mean ΔCT derived from adult lung tissue was used as the calibrator. Similarly, to determine the effect of cortisol infusions and tracheal obstruction on AQP mRNA levels in fetal lung tissue, the mean ΔCT of the adult was used as the calibrator. The equation used was
where
and
The intra-assay coefficients of variation were 8 %, 14 %, 4 % and 7 %, respectively, for AQPs 1, 3, 4 and 5. All assays were performed at least four times.
Antibody production for AQP5
AQP5 peptide synthesis
The AQP5 peptide (amino acids 244–265, plus a terminal cysteine) was assembled on an automated MilliGen 9050 synthesizer using standard Fmoc chemistry and Fmoc-amino acid pentafluorophenyl esters, as previously described (Wade et al. 1994). The peptide was cleaved from the resin and simultaneously deprotected using 82.5 % TFA, 5 % phenol, 5 % dH2O, 5 % thioanisole, 2.5 % ethanedithiol (Reagent K) plus four drops of triethylsilane for 3 h at room temperature. The crude product was extracted into 0.05 M ammonium acetate, pH8.0, lyophilized, and then purified by reverse-phase HPLC on a C18 Vydac column using a 0.1 % aqueous TFA-acetonitrile buffer system. The purified peptide was characterized by reverse-phase HPLC and by matrix-assisted laser desorption ionization (MALDI) mass spectrometry.
The AQP5 peptide (CEPDEDWEESQREERKKTMELTA) was coupled to keyhole limpet haemocyanin (KLH) (Sigma H2133) at the N-terminal cysteine using m-maleimidobenzolyl-N-hydroxysuccinimide. Rabbits (New Zealand White) were injected subcutaneously with 100 μg of KLH-AQP5 peptide conjugate in 1 ml Freund's complete adjuvant (Difco 0639-60-6) and were given subsequent booster injections monthly. Antibodies to AQP1 and AQP3 were as described before (Johnston et al. 2000), whereas the antibody for AQP4 was purchased from Alpha Diagnostic (San Antonio, TX, USA); we used the rabbit anti-rat affinity-purified antibody.
Western blot analysis for the AQPs
Lung and kidney tissue (from adult lung and kidney and fetal lung at 100 days and 130 days) was homogenized and centrifuged, and the protein concentration of the supernatant determined by the Bradford assay. Between 10 and 40 μg of protein was electrophoresed on a 12 % SDS-polyacrylamide gel and transferred onto nitrocellulose membrane filters; the successful transfer was checked by staining the membrane with Ponceaus S. For any given AQP, equal quantities of protein were loaded onto each lane of the gel. The filters were blocked with 5 % non-fat dry milk powder in Tris-buffered saline at room temperature, before exposure to the AQP antibodies at a dilution of either 1:500 (AQP1 and AQP5) or 1:250 (AQP3 and AQP4) in 1 % non-fat dry milk powder in Tris-buffered saline with 0.05 % tween-20. Filters were washed in Tris-buffered saline and incubated for 1 h with affinity-purified peroxidase conjugated goat anti-rabbit IgG (Bio-Rad, USA) diluted 1:3000 with 3 % non-fat dry milk powder, the filters were washed and incubated for 1 min in enhanced chemiluminescence detection reagents (Amersham, International, Little Chalfont, UK), before exposure to Fujifilm intelligent dark box II for 2–5 min. Images were captured by Fujifilm image gauge version and image Las 1000 version software.
Quantitative analysis of AQP5 levels was only performed on lung tissue from fetuses exposed to 10 days of tracheal obstruction (plus their controls) as tissue was not available for cortisol-infused fetuses. The density of each band was measured from the digitized image using image analysis software (ImageQuant). To demonstrate the specificity of the AQP5 antibody, the antibody was pre-absorbed with the AQP5 peptide (20 μg ml−1) before it was incubated with the nitrocellulose membrane (see Fig. 5).
Figure 5. Western blots of aquaporin proteins.
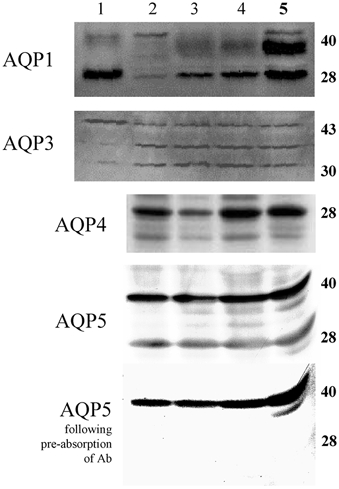
Results are for lane 1, adult sheep kidney; lane 2, fetal lung tissue at 100 days gestation; lanes 3 and 4, fetal lung tissue at 130 days of gestation; lane 5, adult sheep lung. Note that pre-absorption of the AQP5 antibody (Ab) with AQP5 peptide caused the 28 kDa band to disappear.
In situ hybridization histochemistry
Riboprobes
Riboprobes for all four aquaporins were prepared as described previously (Johnston et al. 2000). In brief, recombinant plasmids were linearized, and antisense and sense (negative controls) riboprobes were prepared by in vitro transcription using the Promega riboprobe kit (Promega, Madison, WI, USA) using [α-35S]UTP (100 Ci mmol−1). The riboprobes were hydrolysed, precipitated and resuspended in 10 mm DTT prior to hybridization histochemistry.
All riboprobes were used at a final concentration of 0.02 ng μl−1, as described previously (Johnston et al. 2000). All slides were hybridized in duplicate and sense probes were used as negative controls. For positive controls, sections of adult kidney or parotid gland were included to show that the probes labelled the correct cells (kidney AQP1, AQP3, AQP4) and parotid (AQP5); 4 μm paraffin sections were used.
Immunohistochemistry
This was performed as previously described (Johnston et al. 2000) on 4 μm paraffin sections. Antibodies were used at a concentration of either 1:250 (AQP4) or 1:1000 (AQP1, 3, 5). Images were acquired on a Nikon Microphot microscope, linked to a Sony 930P video camera (Sony, Australia), and the resulting images were digitized and processed using the microcomputer imaging device (MCID) software (Imaging Research Inc, St Catherines, Canada). Images were printed on a Fujix BAS HG-printer (Berthold, Australia, Pty Ltd, Victoria, Australia).
Statistical analyses
All data are reported as means ± s.e.m. unless otherwise stated. The level of significance for all tests was set at P < 0.05. Statistical comparisons between groups were made using one-way analyses of variance (ANOVA) with all pairwise multiple comparison procedures.
RESULTS
Sequence of ovine AQP4 and 5
Total RNA prepared from sheep lung and brain was used in the RT-PCR. Two independent RT-PCRs were carried out using AQP4A and AQP5 gene-specific primers, which yielded PCR products of 1046 bp and 815 bp for AQP4A and AQP5, respectively. The PCR products were subcloned and putative positive clones were subjected to DNA sequencing on both strands of each cDNA and analysed. The AQP4A and AQP5 cDNAs were found to encode 323 and 265 amino acids of the respective native proteins.
Comparisons of the ovine AQP4A protein sequence with the AQP4 of the human, mouse and rat shows that the ovine AQP4A shows 86–87 % similarity to AQP4 sequences in other species (Fig. 1A). However, a higher sequence similarity was observed in AQP5 between species. Ovine AQP5 from lung shows 93–96 % similarity to that of the human, mouse and rat (Fig. 1B). The GenBank accession numbers are: AQP4A, AY177612; AQP5, AY177613.
Figure 1. Comparative amino acid sequences for ovine AQP4 (A) and ovine AQP5 (B) with that of human, rat and mouse.
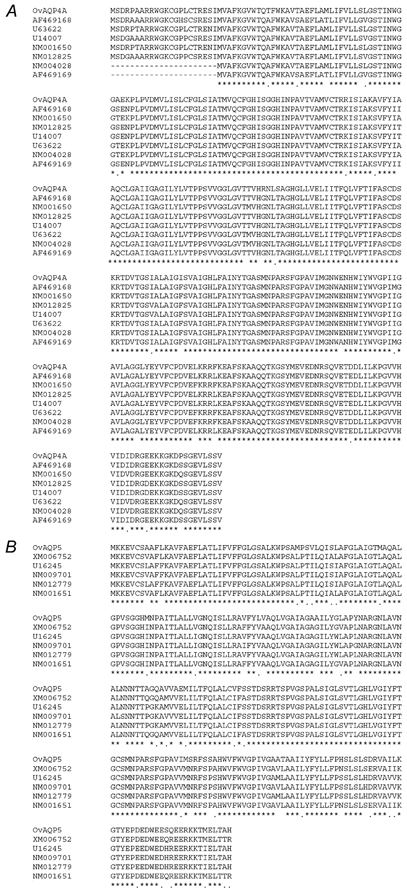
Asterisks indicate identity among species; dots indicate where differences in the ovine sequence exists.
Localisation of expression of aquaporins in adult sheep lung
The specificities of the riboprobes and antibodies used for AQP1 and AQP3 have been shown previously using kidney and placental tissue (Johnston et al. 2000). Similarly, we have used adult lung tissue to demonstrate that the riboprobes for AQP4 and AQP5 as well as the newly generated antibody for AQP5 show similar localizations to that previously reported (Fig. 2). In adult lung, AQP5 mRNA was present in the epithelial cells of the bronchus and in the glands of the submucosa (Fig. 2B); the protein was located at the apex of the ciliated columnar epithelial cells (Fig. 2D) and in the membranes of the bronchial glands (acinar cells, Fig. 2F). A very strong signal for AQP4 mRNA was found in the columnar epithelial cells of the bronchioles (Fig. 2H), although labelling of the basolateral membranes of some of these cells was less than expected, based on the protein levels (data not shown). Tissue localization of the AQPs was, in general, similar to that observed in the human lung. Both mRNA and protein were present for AQP1 in the endothelial cells of the blood vessels and in the vascular smooth muscle of the larger blood vessels. AQP3 was expressed in the basal cell of bronchus and bronchioles, but not in the alveoli. Similarly, AQP4 was found in the bronchus and bronchioles but not in the alveoli. In addition to the expression shown above in the bronchus, AQP5 was predominantly localized to type-I cells in alveolar tissue, with little or no staining in type-II alveolar epithelial cells (Fig. 3).
Figure 2. Localization of AQP4,5 in adult lung.
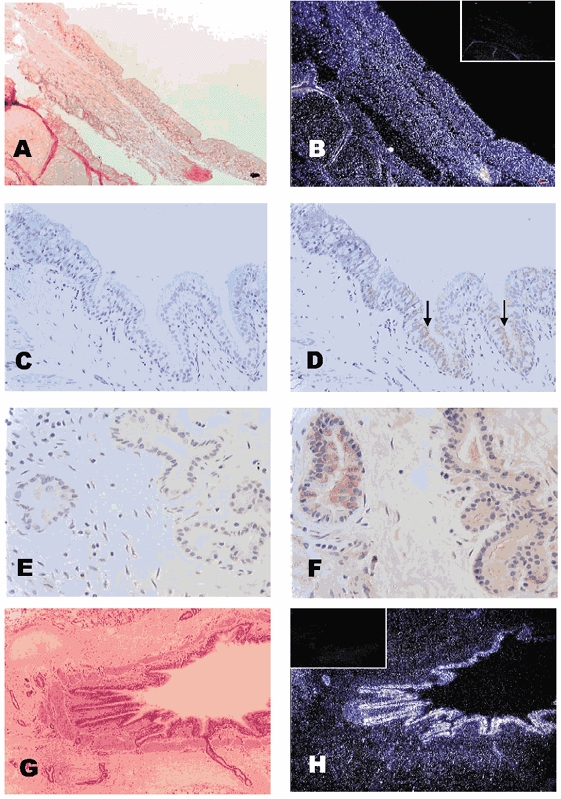
Light (A) and dark (B) field pictures of adult sheep lung, probed with a riboprobe to AQP5. In the adult lung, AQP5 mRNA (white dots) was present in the epithelial cells of the bronchus and in the glands of the submucosa (B). Similarly, the AQP5 protein was located at the apex of the ciliated columnar epithelial cells (D) and in the membranes of the bronchial glands (acinar cells, F) in adult lung tissue; C and E are negative controls for AQP5 immunohistochemistry. Light (G) and darkfield (H) pictures of an adult lung bronchiole demonstrate the distribution of mRNA for AQP4, with a strong signal found in the columnar epithelial cells (H); inserts in B and H are negative controls, using the sense probe. Magnification: A and B (× 100); C and D (× 200); E and F (× 400); G and H (× 40).
Figure 3. A histological section showing the distribution of AQP5 within the alveolar region of lung tissue from a control ovine fetus.
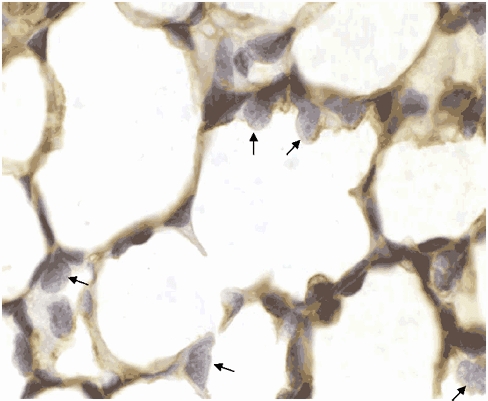
Note that AQP5 was not localized in type-II AEC which are indicated by arrows; magnification × 200.
Ontogeny of gene expression: mRNA
The mRNA for all four AQPs examined (AQP1, 3, 4 and 5) was present within fetal lung tissue from at least 100 days of gestation (Fig. 4). Compared to the expression level detected in adult lung tissue, the mRNA levels were significantly higher in fetal lung tissue for both AQP3 and AQP5 (2–6-fold), whereas the mRNA levels for AQP1 and AQP4 were similar. There was no significant difference between values at 100 and 135 days for AQPs 1, 3 and 4, but AQP5 mRNA levels were significantly higher (P < 0.05) at 135 days compared with 100 days of gestation.
Figure 4. Aquaporin mRNA levels, relative to that in adult lung, in ovine fetal lung tissue at four gestational ages.
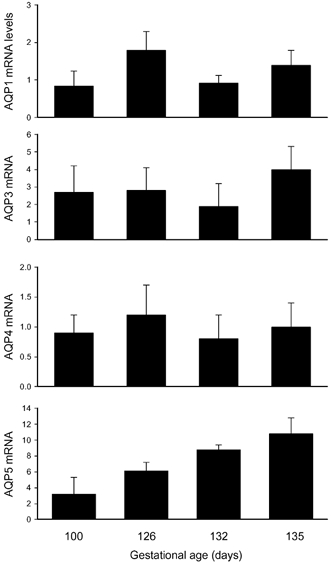
Values are means ± s.e.m.; *P < 0.05.
Ontogeny of gene expression: protein
Protein levels for all four AQPs (1, 3, 4 and 5) examined could be detected in fetal lung tissue by Western blot analysis from day 100 of gestation, the earliest age studied (Fig. 5). For AQP1, a strong band at 28 kDa was detected, as in the kidney (lane 1), but an even stronger band was detected at ≈35 kDa, as in the adult lung (lane 5), which possibly represents a more glycosylated form of the protein. For AQP3, three prominent bands were detected in the lung at ≈32, 35 and 45 kDa, with the largest molecular mass band corresponding to the predominant form in the kidney. For AQP4, there were major bands found at 28 and 36 kDa in adult lung tissue. The lower molecular mass band was more prominent in the fetal lung. The major specific band for AQP5 was detected at ≈28 kDa, with minor bands detected at much larger molecular masses. Following, pre-absorption of the antibody with the AQP5 peptide, the major band at 28 kDa as well as most of the higher molecular mass bands (Fig. 5) disappeared; a dense band at 36 kDa was not specific. No AQP5 was detected in the kidney (data not shown).
Effect of physiological perturbations on AQP mRNA levels and protein expression
Obstruction of the fetal trachea for 10 days, caused a significant decrease (P < 0.01) in the mRNA levels for AQP5, which decreased from 6.1 ± 0.9 to 2.7 ± 0.3, (Fig. 6). This decrease in AQP5 expression was paralleled by a decrease in the staining for this protein in histological sections within the alveolar region (data not shown). However, a decrease in whole lung AQP5 levels could not be detected by Western blot analysis (Fig. 7). The mRNA levels for the other AQPs examined (1, 3 and 4) were not altered by fetal tracheal obstruction.
Figure 6. Concentrations of aquaporin mRNA, relative to adult lung levels, in lung tissue from ovine fetuses.
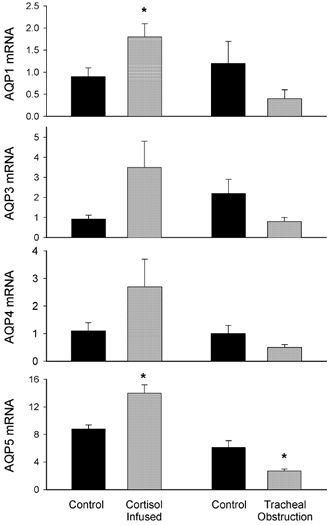
AQP mRNA levels in lung tissue from cortisol-infused fetuses (grey bars) and their saline-infused controls (black bars) are displayed in the left panel; fetuses were ≈132 days of gestation at tissue collection. AQP mRNA levels in lung tissue from fetuses exposed to 10 days of tracheal obstruction (grey bars) and their unobstructed controls (black bars) are displayed in the right panel; fetuses were 128 days at tissue collection. Values are means ± s.e.m.; *P < 0.05.
Figure 7.
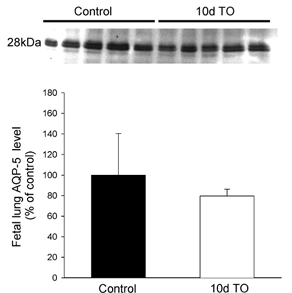
Western blot analysis for AQP5 in lung tissue from control fetuses and fetuses exposed to 10 days of tracheal obstruction (10d TO)
Cortisol infusions for 11 days caused a significant increase (P < 0.05) in the mRNA levels for both AQP1 and AQP5 (Fig. 6). AQP1mRNA levels increased from 0.9 ± 0.2 (n = 4) to 1.8 ± 0.3 (n = 5) whereas AQP5 mRNA levels increased from 8.8 ± 0.6 (n = 5) to 14.1 ± 1.2 (n = 5). These changes were paralleled by similar changes in the staining for these proteins in the alveolar region of lung tissue (data not shown). However, the mRNA levels for AQPs 4 and 3 were not altered by the infusion of cortisol. There was a tendency for the values in the cortisol-treated animals to be higher than those in the controls, but it did not reach significance, probably due to the large variability in control fetuses. As AQP3 and AQP4 are found in the airways, rather than in the alveoli, this could have been due to uneven representation of these tissues (bronchus, submucosal glands and bronchioles) in the extracted samples.
DISCUSSION
Previous studies in fetal rats and mice have indicated that, except for AQP1 and AQP4, the AQPs are not expressed in the lung before birth (Umenishi et al. 1996; King et al. 1997; Yasui et al. 1997; Ruddy et al. 1998; Verkman et al. 2000; Liu et al. 2002; Torday et al. 2002) and, therefore, may not play an important part in regulating transepithelial water flux before and during birth (Verkman et al. 2000). However, in the current study we have shown that the mRNAs for at least four AQPs (1, 3, 4 and 5), as well as their respective proteins, are present in the ovine fetal lung well before birth. For AQP1 and AQP5, the level of mRNA expression in the fetal lung exceeded that of the adult lung. Furthermore, we have shown that cortisol infusions significantly upregulated the expression of AQPs 1 and 5, whereas increases in fetal lung expansion, induced by tracheal obstruction, significantly decreased AQP5 mRNA levels in fetal lung tissue. Although AQP5 protein levels did not appear to decrease with tracheal obstruction (TO), measurable changes in AQP5 levels in whole lung tissue are likely to be complicated by the localization of this protein to multiple cell types within the lung. These findings indicate that factors known to regulate fetal lung growth and maturation as well as fluid secretion also regulate the expression of AQPs 1 and 5. This suggests that there are physiological roles for some lung aquaporins before birth.
We have cloned and now report the sequence for the ovine genes AQP4 and 5. Two transcripts for AQP4 have been previously reported in cerebral tissue from mice (M1 and M23), which encode proteins that share almost 100 % homology, except for an additional 22 amino acid residues at the N-terminal end of the protein encoded by M1. A third transcript for AQP4 (AQP4.M23X) has been reported (Zelenin et al. 2000), which contains an additional 206 nucleotides at the 5′ end of the coding sequence. Nevertheless, the translated product is identical to the translated product of AQP.M23 and AQP4.M23X is the major transcript expressed in adult brain and is thought to be developmentally regulated (Zelenin et al. 2000). The AQP4A we cloned from sheep brain is similar to that of the AQP4-M1 transcript in mice.
The ontogeny of AQP expression and protein accumulation in the lung has, so far, only been studied in rats and mice. In rats, AQP3 mRNA is undetectable in peripheral lung tissue by Northern blot analysis and no AQP5 mRNA was detected in the lung before birth (Yasui et al. 1997). AQP5 mRNA appeared in the lung around the time of birth and increased until postnatal day (P) 40. Similarly, AQP4 mRNA could only be detected in fetal lung tissue by RT-PCR whereas immediately after birth, levels increased sharply (≈8-fold) on days P1–2 before declining again (Yasui et al. 1997). However we now report that both AQP4 and AQP5 are expressed in fetal lung tissue as early as 100 days of gestation (66 %) in a long-gestation species such as sheep. In contrast to AQP4 and AQP5, low levels of AQP1 mRNA were detected by embryonic day (E) 18, they increased gradually on days E19 and E20, but then increased more than threefold between days E21 and P2. As a variety of studies have shown that AQP1 mRNA and protein are upregulated by both β-adrenergic agonists and corticosteroids (King et al. 1997; Yasui et al. 1997), the increase in AQP1 expression at birth probably results from the preparturient/birth-related increases in these endocrine factors.
Although AQP5 protein was almost undetectable in lung tissue homogenates at E21 and P1, a strong signal was detected at P2 (King et al. 1997), indicating that the accumulation of AQP5 protein in the rat lung is predominantly postnatal. Indeed, AQP5 protein levels in lung tissue increased 20-fold to P14 and then increased a further 10-fold from P14 to adult. In contrast to AQP1, AQP5 is not influenced by corticosteroids in rats (King et al. 1997), which is consistent with the finding that AQP5 protein predominantly accumulates in the lung postnatally. Similarly, AQP3 protein levels were undetectable in fetal lung tissue and then were only detected in the trachea of postnatal animals well after the time of birth. AQP4 protein seemed to be present transiently at P2 in peripheral lung membranes and only appeared by P12 in the trachea of rats (King et al. 1997). Thus, our findings in sheep are quite different from those reported in rodents.
In cultured fetal lung distal epithelial cells, AQP4 and the subunits of the amiloride-inhibitable sodium transporter, ENaC, were both downregulated when the cells were cultured on a matrix from day 17 fetal rat, whereas increasing the oxygen tension from 3 % to 21 % increased AQP4 expression (Ruddy et al. 1998). Thus, there is some support for the hypothesis that, in rats, AQP4 may be involved in the clearance of lung liquid after birth. However, AQP4 is found in the bronchi and bronchioles, whereas most liquid secretion and reabsorption is thought to occur across the epithleium of the terminal air sacs which have 100 times the surface area of the rest of the lung (Verkman et al. 2000).
In contrast to rats, AQP5 mRNA was present by E15.5 in mice and expression was found to decrease markedly at birth (2.6-fold) in mice with a null-mutant for the parathyroid hormone (PTH)/parathyroid hormone-related protein (PTHrP) receptor (Ramirez et al. 2000). We consider this to be an interesting observation because the PTH/PTHrP receptor appears to be localized to the lipofibroblasts within the lung. These cells secrete leptin, which is then thought to mediate the effect of PTH/PTHrP on the alveolar type-II cell, via the leptin receptor (Torday et al. 2002). Thus, deficiencies in leptin, or its receptor, could also alter AQP5 expression in the developing lung.
AQP expression has not been studied in the fetal lung of humans, but there are some differences in the sites of expression of various AQPs in adult human and rat lung. AQP3 is thought to be expressed by alveolar type-II cells in the human lung, whereas AQP4 is reportedly expressed by alveolar type-I cells (Kreda et al. 2001). The major differences between these species and adult sheep are that no AQP other than AQP5 was seen in alveolar tissue in sheep and AQP4 was found in the bronchioles of sheep but not in humans. It seems reasonable to predict, based on other similarities between lung development in humans and sheep, that AQP expression in the fetal lungs of humans would more closely resemble that in sheep than in rodents, particularly with regard to the factors that regulate its expression. Thus, in view of our findings, the conclusions drawn from single or multiple AQP ‘knockout’ mice, with respect to their putative roles in lung liquid secretion and clearance, may not apply to the human lung. This is not surprising, as terminal sac development starts well before birth in the human and sheep, whereas it starts only a day or two before birth in rats (Harding & Hooper, 1999).
It is not known how tracheal obstruction could induce a decrease in AQP5 mRNA in fetal lung tissue, although a direct effect of stretch on the cells expressing AQP5 is a likely possibility. Previous studies have suggested a role for TNFα in the inflammation-induced downregulation of AQP5 (Towne et al. 2000, 2001), which may be mediated by the pro-inflammatory transcription factor, NFκβ (Towne et al. 2001). However, it is unlikely that tracheal obstruction induces an inflammatory response within fetal lung tissue, which is supported by our inability to detect an increase in NFκβ activity in response to tracheal obstruction in fetal sheep (M. E. Probyn & S. B. Hooper, unpublished observations). Acute lung injury in rats, induced by lipopolysaccharide instillation into the alveolar compartment, resulted in the downregulation of both mRNA and protein of AQP1 and AQP5 (Jiao et al. 2002). The finding that keratinocyte growth factor (KGF) decreases AQP5 expression in association with type-I to type-II cell trans-differentiation in culture (Borok et al. 1998) has led to the suggestion that AQP5 expression may be indicative of the type-I phenotype in the alveolar epithelium. Indeed, we found that AQP5 was expressed in both alveolar type-I cells and bronchus epithelial cells and was not apparent in type-II alveolar epithelial cells (Fig. 3). However, we have previously shown that tracheal obstruction induces type-II to type-I cell differentiation, resulting in most epithelial cells (> 90 %) differentiating into the type-I cell phenotype within 10 days (Flecknoe et al. 2002). Thus, a decrease in AQP5 expression at a time when the proportion of type-I cells is markedly elevated indicates that, at the very least, expression of the AQP5 gene is not necessarily a good marker of the type-I cell phenotype in alveolar epithelial cells. Consequently, it would appear that AQP5 expression is decreased specifically, rather than there being fewer type-I cells expressing the gene.
The upregulation of AQP5 and AQP1 expression by physiological doses of cortisol has not been reported before. Whatever the mechanism, this finding is consistent with the previous findings that cortisol increases the secretion rate of fetal lung liquid as well as the ability of the lung to reabsorb liquid in response to adrenaline (Wallace et al. 1995). Previous studies have suggested that this results from an associated increase in Na+,K+-ATPase expression, as well as an increase in the amiloride-inhibitable Na+ transporter ENaC. Cortisol increases lung liquid reabsoption in response to exogenous arginine vasopressin (AVP) infusion (Cassin et al. 1994). However, it is possible that the increase in AQP expression plays an important role in mediating the cortisol-induced increase in fetal lung secretion as well as the lung's increased ability to reabsorb liquid in response to adrenaline/AVP. This clearance of lung liquid at term is essential for the successful transition to extra-uterine life. Infants born preterm, without adequate exposure to endogenous cortisol, suffer from an inability to clear their lungs of liquid, which greatly compromises their gas exchange ability (Wallace et al. 1995). It is also interesting to note that tracheal obstruction causes a marked reduction in fetal lung liquid secretion rates. Although this reduction was attributed to an increase in the intraluminal hydrostatic pressure, which opposes the osmotic pressure driving lung liquid secretion, a reduction in AQP5 expression may be involved. Indeed we have shown previously (Flecknoe et al. 2002) that net lung liquid production rates, as determined by lung liquid drainage, is substantially reduced following 10 days of tracheal obstruction (2.1 ± 0.2 ml h−1 kg−1) compared with age-matched lung liquid drained fetuses (3.1 ± 0.1 ml h−1 kg−1) (Nardo et al. 1995). On the other hand, adrenaline-induced lung liquid reabsorption is also reduced (1.8 ± 0.3 vs. 2.6 ± 0.2 ml h−1 kg−1) following a prolonged period of tracheal obstruction (Davey et al. 2002), indicating that liquid movement across the epithelium in both directions is reduced by TO.
Factors known to increase AQP5 gene expression include interferon α (IFNα), in salivary gland cells (Smith et al. 1999), and hypertonicity (Hoffert et al. 2000). Hypertonicity, even a relatively small increase in osmolality (25 mosmol (kg water)−1), can increase AQP5 expression in rats, in vivo, and in mouse distal lung epithelial cells, in culture (Herrlich et al. 2002). The most recent evidence suggests that hypertonicity can increase heregulin, which acts on the receptor HER3, to activate the ERK/ MAPkinase pathway, and increase AQP5 expression in both mouse and human airway epithelial cells (Calu-3) (Herrlich et al. 2002). However, the application of transcellular pressure (30 cm water) to differentiated bronchial epithelial cells also increases ERK phosphorylation, uniquely of the MAP kinases, and would therefore be expected to increase AQP5 expression (Tschumperlin et al. 2002). Cortisol infusion can increase fetal blood pressure (Tangalakis et al. 1992) but whether it has an effect on bronchoconstriction is not known, although an increase in intraluminal pressure would not be expected, as this is dependent on the upper airway resistance. In fact an increase in intraluminal pressure results, normally, in increased lung liquid efflux through the trachea (Harding & Hooper, 1999).
In conclusion, we have shown that the lung of a long-gestation species, such as sheep, expresses both the mRNA and protein of the four typical lung AQPs, beginning well before the expected time of birth. Furthermore, we found that the expression of some, particularly AQP5, are altered by factors known to regulate fetal lung growth and development and parallel changes in fetal lung liquid secretion rates in different animal models. Our findings suggest that gene knockout studies in mice, in which there is little lung expression of AQPs in fetal life, might not give a realistic picture of the role of AQPs during fetal life in long-gestation species. We predict that these AQPs are also expressed well before birth in the human fetal lung and are also differentially regulated by factors known to influence fetal lung development.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council (Australia) to the Howard Florey Institute (983001) and to Stuart Hooper. The Applied Biosystems PRISM sequence detector system was purchased with donations from the Philip Bushell Foundation, the Harold and Cora Brennen Benevolent Trust, the Viertel Foundation and the Clive and Vera Ramaciotti Foundation.
REFERENCES
- Borok Z, Lubman RL, Danto SI, Zhang XL, Zabski SM, King LS, Lee DM, Agre P, Crandall ED. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol. 1998;18:554–561. doi: 10.1165/ajrcmb.18.4.2838. [DOI] [PubMed] [Google Scholar]
- Cassin S, Demarco V, Perks AM, Kuck H, Ellis TM. Regulation of lung liquid secretion in immature fetal sheep: hormonal interaction. J Appl Physiol. 1994;77:1445–1450. doi: 10.1152/jappl.1994.77.3.1445. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Davey MG, Hedrick HL, Mendoza JM, Kanai M, Adzick NS, Flake AW. Pulmonary epithelial liquid absorption, expressed in relation to alveolar surface area, is reduced in fetal lambs following in utero tracheal occlusion. Pediatr Pulmonol. 2002;34:278–286. doi: 10.1002/ppul.10160. [DOI] [PubMed] [Google Scholar]
- Flecknoe SJ, Wallace MJ, Harding R, Hooper SB. Determination of alveolar epithelial cell phenotypes in fetal sheep: evidence for the involvement of basal lung expansion. J Physiol. 2002;542:245–253. doi: 10.1113/jphysiol.2001.014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R, Hooper SB. Lung development and maturation. In: Rodeck CH, Whittle MJ, editors. Fetal Medicine: Basic Science and Clinical Practice. London: Churchill Livingstone; 1999. pp. 181–196. [Google Scholar]
- Herrlich AM, Leitch V, King LS. Heregulin, a necessary intermediate in osmotic stress-induced AQP5 expression. FASEB J. 2002;16:A56. [Google Scholar]
- Hoffert JD, Leitch V, Agre P, King LS. Hypertonic induction of aquaporin-5 expression through an ERK-dependent pathway. J Biol Chem. 2000;275:9070–9077. doi: 10.1074/jbc.275.12.9070. [DOI] [PubMed] [Google Scholar]
- Jiao G, Li E, Yu R. Decreased expression of AQP1 and AQP5 in acute injured lungs in rats. Chin Med J. 2002;115:963–967. [PubMed] [Google Scholar]
- Johnston H, Koukoulas I, Jeyaseelan K, Armugam A, Earnest L, Baird R, Dawson N, Ferraro T, Wintour EM. Ontogeny of aquaporins 1 and 3 in ovine placenta and fetal membranes. Placenta. 2000;21:88–99. doi: 10.1053/plac.1999.0445. [DOI] [PubMed] [Google Scholar]
- King LS, Nielsen S, Agre P. Aquaporins in complex tissues. I. Developmental patterns in respiratory and glandular tissues of rat. Am J Physiol. 1997;273:C1541–1548. doi: 10.1152/ajpcell.1997.273.5.C1541. [DOI] [PubMed] [Google Scholar]
- King LS, Nielsen S, Agre P, Brown RH. Decreased pulmonary vascular permeability in aquaporin-1-null humans. Proc Natl Acad Sci U S A. 2002;99:1059–1063. doi: 10.1073/pnas.022626499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LS, Yasui M. Aquaporins and disease: lessons from mice to humans. Trends Endocrinol Metab. 2002;13:355–360. doi: 10.1016/s1043-2760(02)00665-3. [DOI] [PubMed] [Google Scholar]
- Krane CM, Fortner CN, Hand AR, McGraw DW, Lorenz JN, Wert SE, Towne JE, Paul RJ, Whitsett JA, Menon AG. Aquaporin 5-deficient mouse lungs are hyperresponsive to cholinergic stimulation. Proc Natl Acad Sci U S A. 2001;98:14114–14119. doi: 10.1073/pnas.231273398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol. 2001;24:224–234. doi: 10.1165/ajrcmb.24.3.4367. [DOI] [PubMed] [Google Scholar]
- Lehrach H, Diamond D, Wozney JM, Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochem. 1977;16:4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lipsett J, Cool JC, Runciman SC, Ford WDA, Kennedy JD, Martin AJ, Parsons DW. Effect of antenatal tracheal occlusion on lung development in the sheep model of congenital diaphragmatic hernia: a morphological analysis of pulmonary structure and maturity. Pediatr Pulmonol. 1998;25:257–269. doi: 10.1002/(sici)1099-0496(199804)25:4<257::aid-ppul6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Liu C, Morrisey EE, Whitsett JA. GATA-6 is required for maturation of the lung in late gestation. Am J Physiol. 2002;283:L468–475. doi: 10.1152/ajplung.00044.2002. [DOI] [PubMed] [Google Scholar]
- Nardo L, Hooper SB, Harding R. Lung hypoplasia can be reversed by short-term obstruction of the trachea in fetal sheep. Pediatr Res. 1995;38:690–696. doi: 10.1203/00006450-199511000-00010. [DOI] [PubMed] [Google Scholar]
- Nardo L, Hooper SB, Harding R. Stimulation of lung growth by tracheal obstruction in fetal sheep: relation to luminal pressure and lung liquid volume. Pediatr Res. 1998;43:184–190. doi: 10.1203/00006450-199802000-00005. [DOI] [PubMed] [Google Scholar]
- Ramirez MI, Chung UI, Williams MC. Aquaporin-5 expression, but not other peripheral lung marker genes, is reduced in PTH/PTHrP receptor null mutant fetal mice. Am J Respir Cell Mol Biol. 2000;22:367–372. doi: 10.1165/ajrcmb.22.3.3923. [DOI] [PubMed] [Google Scholar]
- Ruddy MK, Drazen JM, Pitkanen OM, Rafii B, O'Brodovich HM, Harris HW. Modulation of aquaporin 4 and the amiloride-inhibitable sodium channel in perinatal rat lung epithelial cells. Am J Physiol. 1998;274:L1066–1072. doi: 10.1152/ajplung.1998.274.6.L1066. [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JK, Siddiqui AA, Modica LA, Dykes R, Simmons C, Schmidt J, Krishnaswamy GA, Berk SL. Interferon-alpha upregulates gene expression of aquaporin-5 in human parotid glands. J Interferon Cytokine Res. 1999;19:929–935. doi: 10.1089/107999099313479. [DOI] [PubMed] [Google Scholar]
- Tangalakis K, Lumbers ER, Moritz KM, Towstoless MK, Wintour EM. Effect of cortisol on blood pressure and vascular reactivity in the ovine fetus. Exp Physiol. 1992;77:709–717. doi: 10.1113/expphysiol.1992.sp003637. [DOI] [PubMed] [Google Scholar]
- Torday JS, Sun H, Wang L, Torres E, Sunday ME, Rubin LP. Leptin mediates the parathyroid hormone-related protein paracrine stimulation of fetal lung maturation. Am J Physiol. 2002;282:L405–410. doi: 10.1152/ajplung.2002.282.3.L405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne JE, Harrod KS, Krane CM, Menon AG. Decreased expression of aquaporin (AQP)1 and AQP5 in mouse lung after acute viral infection. Am J Respir Cell Mol Biol. 2000;22:34–44. doi: 10.1165/ajrcmb.22.1.3818. [DOI] [PubMed] [Google Scholar]
- Towne JE, Krane CM, Bachurski CJ, Menon AG. Tumor necrosis factor-alpha inhibits aquaporin 5 expression in mouse lung epithelial cells. J Biol Chem. 2001;276:18657–18664. doi: 10.1074/jbc.M100322200. [DOI] [PubMed] [Google Scholar]
- Tschumperlin DJ, Shively JD, Swartz MA, Silverman ES, Haley KJ, Raab G, Drazen JM. Bronchial epithelial compression regulates MAP kinase signaling and HB-EGF-like growth factor expression. Am J Physiol Lung Cell Mol Physiol. 2002;282:L904–911. doi: 10.1152/ajplung.00270.2001. [DOI] [PubMed] [Google Scholar]
- Umenishi F, Carter EP, Yang B, Oliver B, Matthay MA, Verkman AS. Sharp increase in rat lung water channel expression in the perinatal period. Am J Respir Cell Mol Biol. 1996;15:673–679. doi: 10.1165/ajrcmb.15.5.8918374. [DOI] [PubMed] [Google Scholar]
- Verkman AS, Matthay MA, Song Y. Aquaporin water channels and lung physiology. Am J Physiol Lung Cell Mol Physiol. 2000;278:L867–879. doi: 10.1152/ajplung.2000.278.5.L867. [DOI] [PubMed] [Google Scholar]
- Wade JD, Layden SS, Lambert PF, Kakouris H, Tregear GW. Primate relaxin: synthesis of gorilla and rhesus monkey relaxins. J Protein Chem. 1994;13:315–321. doi: 10.1007/BF01901564. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Hooper SB, Harding R. Effects of elevated fetal cortisol concentrations on the volume, secretion and reabsorption of lung liquid. Am J Physiol. 1995;269:R881–887. doi: 10.1152/ajpregu.1995.269.4.R881. [DOI] [PubMed] [Google Scholar]
- Wintour EM, Earnest L, Alcorn D, Butkus A, Shandley L, Jeyaseelan K. Ovine AQP1: cDNA cloning, ontogeny, and control of renal gene expression. Pediatr Nephrol. 1998;12:545–553. doi: 10.1007/s004670050502. [DOI] [PubMed] [Google Scholar]
- Yasui M, Serlachius E, Lofgren M, Belusa R, Nielsen S, Aperia A. Perinatal changes in expression of aquaporin-4 and other water and ion transporters in rat lung. J Physiol. 1997;505:3–11. doi: 10.1111/j.1469-7793.1997.003bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenin S, Gunnarson E, Alikina T, Bondar A, Aperia A. Identification of a new form of AQP4 mRNA that is developmentally expressed in mouse brain. Pediatr Res. 2000;48:335–339. doi: 10.1203/00006450-200009000-00012. [DOI] [PubMed] [Google Scholar]


